Submitted:
08 January 2026
Posted:
08 January 2026
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Core Body Temperature Measurement Techniques
2.1. Invasive and Semi-Invasive Reference Measurements
2.2. Non-Invasive and Minimally Burdensome Measurement Approaches
2.2.1. In-Ear Temperature Sensors for Core Body Temperature Monitoring
2.2.2. Infrared Thermography for Non-Contact Temperature Assessment
2.2.3. Ingestible Telemetric Sensors for Core Body Temperature Measurement
2.2.4. Heat-Flux-Based Methods for Core Body Temperature Estimation
2.2.5. Model-Based and Multimodal Estimation of Core Body Temperature
3. Discussion and Future Perspectives
4. Limitation of This Review
5. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- K. J. Collins, “Regulation of Body Temperature. In: J. Tinker, W. M. Zapol (eds), Care of the Critically III Patient.,” Springer, London. [CrossRef]
- C. L. Lin, “Fundamental Concepts of Human Thermoregulation and Adaptation to Heat: A Review in the Context of Global Warming,” Int. J. Environ. Res. Public Health, vol. 17, no. 21, 7795, 2020. [CrossRef]
- M. N. Cramer, D. Gagnon, O. Laitano, and C. G. Crandall, “Human temperature regulation under heat stress in health, disease, and injury,” Physiol. Rev., vol. 102, no. 4, pp. 1907-1989, 2022. [CrossRef]
- T. Tamura, M. Huang, and T. Togawa, “Current Developments in Wearable Thermometer,” Adv. Biomed. Eng., vol. 7, pp. 88-99, 2018. [CrossRef]
- H. Ota, M. Chao, Y. Gao, E. Wu, L.-C. Tai, K. Chen, Y. Matsuoka, K. Iwai, H. M. Fahad, W. Gao, H. Y. Y. Nyein, L. Lin, and A. Javey, “3D Printed “Earable” Smart Devices for Real-Time Detection of Core Body Temperature,” ACS Sensors, vol. 2, no. 7, pp. 990-997, 2017. [CrossRef]
- Q. Wang, Y. Zhou, P. Ghassemi, D. McBride, J. P. Casamento, and T. Pfefer, “Infrared Thermography for Measuring Elevated Body Temperature: Clinical Accuracy, Calibration, and Evaluation,” Sensors, vol. 22, no. 1, 215, 2021. [CrossRef]
- C. Byrne and C. L. Lim, “The ingestible telemetric body core temperature sensor: a review of validity and exercise applications,” Br. J. Sports Med., vol. 41, no. 3, pp. 126-133, 2007. [CrossRef]
- H. C. Gunga, M. Sandsund, R. E. Reinertsen, F. Sattler, and J. Koch, “A non-invasive device to continuously determine heat strain in humans,” J. Therm. Biol., vol. 33, no. 5, pp. 297-307, 2008. [CrossRef]
- R. Niedermann, E. Wyss, S, Annaheim, A, Psikuta, S, Davey, and R. M. Rossi, “Prediction of human core body temperature using non-invasive measurement methods,” Int. J. Biometeorol., vol. 58, pp. 7-15, 2013. [CrossRef]
- D. S. Moran and L. Mendal, “Core Temperature Measurement: Methods and Current Insights,” Sports Med., vol. 32, no. 14, pp. 879-885, 2002. [CrossRef]
- A. Conway, M. Bittner, D. Phan, K. Chang, N. Kamboj, E, Tipton, and M. Parotto, “Accuracy and precision of zero-heat-flux temperature measurements with the 3M™ Bair Hugger™ Temperature Monitoring System: a systematic review and meta-analysis, J. Clin. Monit. Comput., vol. 35, pp. 39-49, 2021. [CrossRef]
- S. L. Cutuli, E. J. See, E. A. Osawa, P. Ancona, D. Marshall, G. M. Eastwood, N. J. Glassford, R. Bellomo, “Accuracy of non-invasive body temperature measurement methods in adult patients admitted to the intensive care unit: a systematic review and meta-analysis,” Crit. Care Resusc., vol. 1, pp. 6-13, 2023. [CrossRef]
- T. Falcone, F. Cordella, V. Molinaro, L. Zollo, and S. D. Ferraro, “Real-time human core temperature estimation methods and their application in the occupational field: A systematic review,” Measurement, vol. 183, 109776, 2021. [CrossRef]
- J. Foster, A. B. Lloyd, and G. Havenith, “Non-contact infrared assessment of human body temperature: The journal Temperature toolbox,” Temperature, vol. 8, no. 4, pp. 306-319, 2021. [CrossRef]
- C. M. Dolson, E. R. Harlow, D. M. Phelan, T. J. Gabbett, B. Gaal, C. McMellen, B. J. Geletka, J. G. Calcei, J. E. Voos, and D. R. Seshadri, “Wearable Sensor Technology to Predict Core Body Temperature: A Systematic Review,” Sensors, vol. 22, 7639, 2022. [CrossRef]
- Y. Zhao and H. M. Bergmann, “Non-Contact Infrared Thermometers and Thermal Scanners for Human Body Temperature Monitoring: A Systematic Review,” Sensors, vol. 23, no. 17, 7439, 2023. [CrossRef]
- D. I. Sessler, D. S. Warner, and M. A. Mark, “Temperature Monitoring and Perioperative Thermoregulation,” Anesthesiology, vol. 109, no. 2, pp. 318-338, 2008. [CrossRef]
- J.-Y. Lefrant, L. Muller, J. E. de La Coussaye, M. Benbabaali, C. Lebris, N. Zeitoun, C. Mari, G. Saïssi, J. Ripart, and J.-J. Eledjam, “Temperature measurement in intensive care patients: comparison of urinary bladder, oesophageal, rectal, axillary, and inguinal methods versus pulmonary artery core method,” Intensive Care Med., vol. 29, pp. 414-418, 2003. [CrossRef]
- D. C. Evans, V. A. Doraiswamy, M. P. Prosciak, M. Silviera, M. J. Seamon, V. Rodriguez Funes, J. Clipolla, C. F. Wang, S. Kavuturu, D. A. Torigian, C. H. Cook, D. E. Lindsey, S. M. Steinberg, and S. P. Stawicki, “Complications Associated with Pulmonary Artery Catheters: A Comprehensive Clinical Review,” Scand. J. Surg., vol. 98, no. 4, pp. 199-208, 2009. [CrossRef]
- H. Hymczak, A. Golab, K. Mendrala, D. Plicner, T. Darocha, P. Podsiadlo, D. Hudziak, R. Gocol, and S. Kosinski, “Core Temperature Measurement—Principles of Correct Measurement, Problems, and Complications,” Int. J. Environ. Public Health, vol. 18, 10606, 2021. [CrossRef]
- S. M. C. Lee, W. J. Williams, and S. M. F. Schneider, “Core Temperature Measurement During Supine Exercise: Esophageal, Rectal, and Intestinal Temperatures,” Aviation, Space, and Environmental Medicine, vol. 71, no. 9, pp. 939-945, 2000.
- Y. Sekiguchi, L. N. Belval, L. Stearns, and J. Casa, “Monitoring Internal Body Temperature,” Sports Science Exchange, vol. 29, No. 192, pp. 1-5, 2019.
- K. C. Miller and W. M. Adams, “Common Body Temperature Sites Provide Invalid Measures of Body Core Temperature in Hyperthermic Humans Wearing American Football Uniforms,” Temperature, vol. 8, no. 2, pp. 166-175, 2020. [CrossRef]
- M. J. Crowe, M. T. Meehan, and R. E. Jones, “Comparison of Rectal and Gastrointestinal Core Temperatures During Heat Tolerance Testing,” Medicina, vol. 61, no. 6, 1111, 2025. [CrossRef]
- P. Eggenberger, B. A. MacRae, S. Kemp, M. Bürgisser, R. M. Rossi, and S. Annaheim, “Prediction of Core Body Temperature Based on Skin Temperature, Heat Flux, and Heart Rate Under Different Exercise and Clothing Conditions in the Heat in Young Adult Males,” Front. Physiol., vol. 9, 1780, 2018. [CrossRef]
- R. W. Scott, and K. Fredriksen, “Barriers to body temperature monitoring among prehospital personnel: a qualitative study using the modified nominal group technique,” BMJ Open, vol. 12, no. 6, e058910, 2022. [CrossRef]
- J. K. Lilly, J. P. Boland, and S. Zekan, “Urinary Bladder Temperature Monitoring: A New Index of Body Core Temperature,” Crit. Care Med., vol. 8, no. 12, pp. 742-745, 1980.
- A. Bräuer, A. Fazliu, T. Perl, D. Heise, K. Meissner, and I. F. Brandes, “Accuracy of zero-heat-flux thermometry and bladder temperature measurement in critically ill patients,” Sci. Rep., vol. 10, 21746, 2020. [CrossRef]
- J. Langenhorst, A. Benkert, S. Peterss, M. Feuerecker, T. Scheiermann, P. Scheiermann, M. Witte, A. Benkert, A. Bayer, S. Prueckner, M. Pichlmaier, and R. Schniepp, “Agreement of in-ear temperature to core body temperature measures during invasive whole-body cooling for hypothermic circulatory arrest in aortic arch surgery,” Sci. Rep., vol. 14, 27607, 2024. [CrossRef]
- F. Pompei, M. Pompei, “Noninvasive temporal artery thermometry: physics, physiology, and clinical accuracy,” Thermosense XXVI, vol. 5405, pp. 61-67, 2004. [CrossRef]
- K. D. Olson, P. O’Brien, A. S. Lin, D. A. Fabry, S. Hanke, and M. J. Schroeder, “A Continuously Worn Dual Temperature Sensor System for Accurate Monitoring of Core Body Temperature from the Ear Canal,” Sensors, vol. 23, no. 17, 7323, 2023. [CrossRef]
- S. C. C. Tan, T. C. K. Tan, C. Y. N. Chiang, J. Pan, and I. C. C. Low, “External auricle temperature enhances ear-based wearable accuracy during physiological strain monitoring in the heat,” Sci. Rep., vol. 14, 12418, 2024. [CrossRef]
- J. S. E. Chaglla, N. Celik, and W. Balachandran, “Measurement of Core Body Temperature Using Graphene-Inked Infrared Thermopile Sensor,” Sensors, vol. 18, no. 10, 3315, 2018. [CrossRef]
- Y. Ko, J. Y. Jung, H.-T. Kim, and J.-Y. Lee, “Auditory canal temperature measurement using a wearable device during sleep: Comparisons with rectal temperatures at 6, 10, and 14 cm depths,” J. Therm. Biol., vol. 85, 102410, 2019. [CrossRef]
- C. C. Roossien, A. P. Hodselmans, R. Heus, M. F. Reneman, and G. J. Verkerke, “Evaluation of a Wearable Non-Invasive Thermometer for Monitoring Ear Canal Temperature during Physically Demanding (Outdoor) Work, Int. J. Environ. Res. Public Health, vol. 18, no. 9, 4896, 2021. [CrossRef]
- I. Kato, H. Watanabe, K. Nagashima, “Evaluation of newly developed wearable ear canal thermometer, mimicking the application to activities on sports and labor fields,” J. Physiol. Sci., vol. 73, 15, 2023. [CrossRef]
- K. D. Olson, P. O’Brien, A. S. Lin, D. A. Fabry, S. Hanke, and M. J. Schroeder, “A Continuously Worn Dual Temperature Sensor System for Accurate Monitoring of Core Body Temperature from the Ear Canal,” Sensors, vol. 23, no. 17, 7323, 2023. [CrossRef]
- W. Zhang, L. Li, Y. Wang, X. Dong, C. Liu, L. Sun, Q. Guan, F. Zhang, and S. Xu, “Continuous Core Body Temperature Monitoring for Heatstroke Alert via a Wearable In-Ear Thermometer,” ACS Sens., vol. 10, no. 2, 1440-1449, 2025. [CrossRef]
- D. B. Ellebrecht, D. Gola, and M. Kaschwich, “Evaluation of a Wearable in-Ear Sensor for Temperature and Heart Rate Monitoring: A Pilot Study,” J. Med. Syst., vol. 46, 91, 2022. [CrossRef]
- S. Hasting, S. W. Kim, and R. D. Brown, “Face Temperature as an Indicator of Thermal Stress in Outdoor Work Environments,” Atmosphere, vol. 11, no. 6, 627, 2020. [CrossRef]
- U. Riaz, M. Idris, M. Ahmed, F. Ali, and L. Yang, “Infrared Thermography as a Potential Non-Invasive Tool for Estrus Detection in Cattle and Buffaloes,” Animals, vol. 13, no. 8, 1425, 2023. [CrossRef]
- G. Hayward, J. Y. Verbkel, F. A. Ismail, G. Edwards, K. Wang, S. Fleming, G. A. Holtman, M. Glogowska, E. Morris, K. Curtis, and A. V. D. Bruel, “Non-contact infrared versus axillary and tympanic thermometers in children attending primary care: a mixed-methods study of accuracy and acceptability,” Br. J. Gen. Pract., vol. 70, no. 693, pp. e236-e244, 2020. [CrossRef]
- D. Perpetuini, C. Filippini, D. Cardone, and A. Merla, “An Overview of Thermal Infrared Imaging-Based Screenings during Pandemic Emergencies,” Int. J. Environ. Res. Public Health, vol. 18, no. 6, 3286, 2021. [CrossRef]
- Z. Cai, J. Cui, H. Yuan, and M. Cheng, “Application and research progress of infrared thermography in temperature measurement of livestock and poultry animals: A review,” Comput. Electron. Agric., vol. 205, 107586, 2023. [CrossRef]
- C. Hildebrandt, C. Raschner, and K. Ammer, “An Overview of Recent Application of Medical Infrared Thermography in Sports Medicine in Austria,” Sensors, vol. 10, no. 5, pp. 4700-4715, 2010. [CrossRef]
- C. J. Coehoom, P. S. Martin, J. Teran, H. Cowart, L. Waite, and S. Newman, “Firefighter uncompensable heat stress results in excessive upper body temperatures measured by infrared thermography: Implications for cooling strategies,” Appl. Ergon., vol. 120, 104342, 2024. [CrossRef]
- A. Cabizosu, C. M. Pagan, P. E. Alcaraz, and F. J. M. Noguera, “Infrared Thermography Sensor in the Analysis of Acute Metabolic Stress Response during Race Walking Competition,” Biosensors, vol. 14, no. 10, 478, 2024. [CrossRef]
- E. F. J. Ring and K. Ammer, “Infrared thermal imaging in medicine,” Physiol. Meas., vol. 33, no. 3, pp. R33-R46, 2012. [CrossRef]
- K. C. Miller and W. M. Adams, “Common body temperature sites provide invalid measures of body core temperature in hyperthermic humans wearing American football uniforms,” Temperature, vol. 8, no. 2, pp. 166-175, 2020. [CrossRef]
- L. P. J. Teunissen and H. A. M. Daanen, “Infrared thermal imaging of the inner canthus of the eye as an estimator of body core temperature,” J. Med. Eng. Technol., vol. 35, no. 3-4, pp. 134-138, 2011. [CrossRef]
- A. A. Fernandes, D. G. Moreira, C. J. Brito, C. D. da Silva, M. Sillero-Quintana, E. M. Pimenta, A. J. E. Bach, E. S. Garcia, and J. C. B. Marins, “Validity of inner canthus temperature recorded by infrared thermography as a non-invasive surrogate measure for core temperature at rest, during exercise and recovery,” J. Therm. Biol., vol. 62, part A, pp. 50-55, 2016. [CrossRef]
- H. Nishimura and K. Kamiya, “Fever screening during the influenza (H1N1-2009) pandemic at Narita International Airport, Japan,” BMC Infect Dis., vol. 11, 111, 2011. [CrossRef]
- K. Khaksari, T. Nguyen, B. Hill, T. Quang, J. Perreault, V. Gorti, R. Malpani, E. Blick, T. G. Cano, B. Shadgan, and A. H. Grandjbakhche,” J. Med. Imaging, vol. 8, no. S1, 010901, 2021. [CrossRef]
- P. Dollard, I. Griffin, A. Berro, N. J. Cohen, K. Singler, Y. Hader, C. de la Motte Hurst, A. Stolp, S. Atti, and L. Hausman, C. E. Shockey, S. Roohi, C. M. Brown, L. D. Rotz, M. S. Cetron, and F. Alvarado-Ramy, “Risk Assessment and Management of COVID-19 Among Travelers Arriving at Designated U.S. Airports, January 17–September 13, 2020, MMWR. Morb. Mortal. Wkly. Rep., vol. 69, pp. 1681-1685, 2020.
- R. K. Kakulu, E. G. Kimaro, and E. A. Mpolya, “Effectiveness of Point of Entry Health Screening Measures among Travelers in the Detection and Containment of the International Spread of COVID-19: A Review of the Evidence,” Int. J. Environ. Res. Public Health, vol. 21, no. 4, 410, 2024. [CrossRef]
- A. S. Hussain, H. S. Hussain, N. Betcher, R. Behm, and B. Cagir, “Proper use of noncontact infrared thermometry for temperature screening during COVID-19,” Sci. Rep., vol. 11, 11832, 2021. [CrossRef]
- S. J. L. Sullivan, J. E. Rinaldi, P. Hariharan, J. P. Casamento, S. Baek, N. Seay, O. Vesnovsky, and L. D. T. Topoleski, “Clinical evaluation of non-contact infrared thermometers,” Sci. Rep., vol. 11, no. 1, 22079, 2021. [CrossRef]
- C.-C. Liu, R.-E. Chang, and W.-C. Chang, “Limitations of Forehead Infrared Body Temperature Detection for Fever Screening for Severe Acute Respiratory Syndrome,” Infect. Control Hosp. Epidemiol., vol. 25, no. 12, pp. 1109-1111, 2004. [CrossRef]
- K. Ronneberg, W. O. Roberts, A. D. McBean, and B. A. Center, “Temporal artery temperature measurements do not detect hyperthermic marathon runners, Med. Sci. Sport Exerc., vol. 40, no. 8, pp. 1373-1375, 2008. [CrossRef]
- D. J. Casa, S. M. Becker, M. S. Ganio, C. M. Brown, S. W. Yeargin, M. W. Roti, J. Siegler, J. A. Blower, N. R. Glaviano, R. A. Huggins, L. E. Armstrong, and C. M. Maresh, “Validity of Devices That Assess Body Temperature During Outdoor Exercise in the Heat,” J. Athl. Train., vol. 42, no. 3, pp. 333-342, 2007.
- K. C. Miller and W. M. Adams, “Common body temperature sites provide invalid measures of body core temperature in hyperthermic humans wearing American football uniforms,” Temperature, vol. 8, no. 2, pp. 166-175, 2021. [CrossRef]
- T. C. Hower and K. D. Blehm, “Infrared Thermometry in the Measurement of Heat Stress in Firefighters Wearing Protective Clothing,” Appl. Occup. Environ. Hyg., vol. 5, no. 11, pp. 782-786, 1990. [CrossRef]
- M. S. Ganio, C. M. Brown, D. J. Casa, S. M. Becker, S. W. Yeargin, B. P. Mcdermott, L. M. Boots, P. W. Boyd, L. E. Armstrong, and C. M. Maresh, “Validity and Reliability of Devices That Assess Body Temperature During Indoor Exercise in the Heat,” J. Athl. Train., vol. 44, no. 2, pp. 124-135, 2009. [CrossRef]
- D. A. Low, A. Vu, M. Brown, S. L. Davis, D. M. Keller, B. D. Levine, amd C. G. Crandall, “Temporal Thermometry Fails to Track Body Core Temperature during Heat Stress”, Med. Sci. Sport Exerc., vol. 39, no. 7, pp. 1029-1035, 2007.
- F.-K. Wang, J.-Y. Shih, P.-H. Juan, Y.-C. Su, and Y. C. Wang, “Non-Invasive Cattle Body Temperature Measurement Using Infrared Thermography and Auxiliary Sensors,” Sensors, vol. 21, no. 7, 425, 2021. [CrossRef]
- C. Shan, J. Hu, T. Zhou, and J. Li, “Accurate Core Body Temperature Prediction for Infrared Thermography Considering Ambient Temperature and Personal Features,” IEEE J. Biomed. Health Inform., vol. 29, no. 7, pp. 5016-5027, 2025.
- A. Abdigazy, M. Arfan, G. Lazzi, C. Sideris, A. Abramson, and Y. Khan, “End-to-end design of ingestible electronics,” Nature Electronics, vol. 7, pp. 102–118, 2024. [CrossRef]
- M. M. Mau, S. Sarker, and B. S. Terry, “Ingestible devices for long-term gastrointestinal residency: a review,” Progress in Biomedical Engineering, vol. 3, no. 4, p. 042001, 2021. . [CrossRef]
- K. Kalantar-zadeh, N. Ha, J. Z. Ou, and K. J. Berean, “Ingestible sensors,” ACS Sensors, vol. 2, no. 4, pp. 468–483, 2017. [CrossRef]
- R. C. Eberhart and A. F. Hogrefe, “A commandable ingestible temperature monitor,” Proc. IEEE EMBS, 1988. [CrossRef]
- B. B. Mittal, V. Sathiaseelan, A. W. Rademaker, M. C. Pierce, P. M. Johnson, and W. N. Brand, “Evaluation of an ingestible telemetric temperature sensor for deep hyperthermia applications,” Int. J. Radiat. Oncol. Biol. Phys., vol. 21, no. 5, pp. 1353-1361, 1991. [CrossRef]
- H.-J. Engels, H. N. Yarandi, and J. E. Davis, “Utility of an ingestible capsule for core temperature measurements during body warming,” J. Exxerc. Physiol. Online, vol. 12, no. 1, pp. 1–9, 2009.
- A. D. Ruddock, G. A. Tew, and A. J. Purvis, “Reliability of intestinal temperature using an ingestible telemetry pill system during exercise in a hot environment,” J. Strength Cond. Res., vol. 28, no. 3, pp. 861–869, 2014. [CrossRef]
- C. C. W. G. Bongers, M. T. E. Hopman, and T. M. H. Eijsvogels, “Validity and reliability of the myTemp ingestible temperature capsule,” J. Sci. Med. Sport, vol. 21, no. 3, pp. 322-326, 2018. [CrossRef]
- O. C. Koumar, R. Beaufils, C. Chesneau, H. Normand, and N. Bessot, “Validation of e-Celsius gastrointestinal telemetry system as measure of core temperature,” J. Therm. Biol., vol. 112, 103471, 2023. [CrossRef]
- C. R. Monnard, E.-J. Fares, J. Calonne, J. L. Miles-Chan, J.-P. Montani, D. Durrer, Y. Schutz, and A. G. Dulloo, “Issues in continuous 24-h core body temperature monitoring in humans using an ingestible capsule telemetric sensor,” Front. Endocrinol., vol. 8, 130, 2017. [CrossRef]
- M. A. Kolka, L. Levine, and L. A. Stephenson, “Use of an ingestible telemetry sensor to measure core temperature under chemical protective clothing,” J. Therm. Biol., vol. 22, nos. 4–5, pp. 343–349, 1997. [CrossRef]
- D. A. Goodman, R. W. Kenefick, B. S. Cadarette, and S. N. Cheuvront, ”Influence of sensor ingestion timing on consistency of temperature measures,” Med. Sci. Sports Exerc., vol. 41, no. 3, pp. 597–602, 2009. [CrossRef]
- G. J. S. Travers, D. S. Nichols, A. Farooq, S. Racinais, and J. D. Periard, “Validation of an ingestible temperature data logging and telemetry system during exercise in the heat,” Temperature, vol. 3, no. 2, pp. 208-219, 2016. [CrossRef]
- H.Fanyu, C. Magnin, and P. Brouqui, “Ingestible sensors correlate closely with peripheral temperature measurements in febrile patients,” J. Infect., vol. 80, no. 2, pp. 161-166, 2020. [CrossRef]
- S. Osinchuk, S. M. Taylor, C. L. Shmon, J. Pharr, and J. Campbell, “Comparison between core temperatures measured telemetrically using the CorTemp® ingestible temperature sensor and rectal temperature in healthy Labrador retrievers,” Can, Vet. J., vol. 55, no. 10, pp. 939-945, 2014.
- D. Darwent, X. Zhou, C. van den Heuvel, C. Sargent, and G. D. Roach, “The Validity of Temperature-Sensitive Ingestible Capsules for Measuring Core Body Temperature in Laboratory Protocols,” vol. 28, no. 8, pp. 719-726, 2011. [CrossRef]
- C. W. G. Bongers, M. T. E. Hopman, and T. M. H. Eijsvogels, “Using an ingestible telemetric temperature pill to assess gastrointestinal temperature during exercise,” J. Vis. Exp., no. 104, 53258, 2015. [CrossRef]
- J. W. Domitrovich, J. S. Cuddy, and B. C. Ruby, “Core-temperature sensor ingestion timing and measurement variability,” J. Athl. Train., vol. 45, no. 6, pp. 594–600, 2010. [CrossRef]
- D. A. Goodman, R. W. Kenefick, B. S. Cadarette, and S. N. Cheuvront, ”Influence of sensor ingestion timing on consistency of temperature measures,” Med. Sci. Sports Exerc., vol. 41, no. 3, pp. 597–602, 2009. [CrossRef]
- A. P. Hunt, A. J. E. Bach, D. N. Borg, J. T. Costello, and I. B. Stewart, “The Systematic Bias of Ingestible Core Temperature Sensors Requires a Correction by Linear Regression,” Front. Physiol., vol. 8, 260, 2017. [CrossRef]
- C. C. W. G. Bongers, H. A. M. Daanen, C. P. Bogerd, M. T. E. Hopman, and T. M. H. Eijsvogels, “Validity, reliability, and inertia of four different temperature capsule systems,” Med. Sci. Sports Exerc., vol. 50, no. 1, pp. 169–175, 2018. . [CrossRef]
- S. Yoshida, H. Miyaguchi, T. Nakamura, “Development of Ingestible Thermometer With Built-in Coil Antenna Charged by Gastric Acid Battery and Demonstration of Long-Time in Vivo Telemetry,” IEEE Access, vol. 9, pp. 102368-102377, 2021. [CrossRef]
- R. H. Fox and A. J. Solman, “A new technique for monitoring the deep body temperature in man from the intact skin surface.”, J. Physiol., vol. 212, no. 2, pp. 8-10, 1971.
- R.H. Fox, A.J. Solman, R. Issacs, A.J. Fry, I.C. MacDonald, “A new method for monitoring deep body temperature from the skin surface,” Clin. Sci., vol. 44, pp. 81-86, 1973. [CrossRef]
- K.-I. Kitamura, X. Zhu, W. Chen, and T. Nemoto, “Development of a new method for the noninvasive measurement of deep body temperature without a heater,” Medical Engineering & Physics, vol. 32, no. 1, pp. 1–6, 2010. [CrossRef]
- T. Togawa, T. Nemoto, T. Tsuji, and K. Suma, “Deep temperature monitoring in intensive care,” Resuscitation, vol. 7, no. 1, pp. 53-57, 1979. [CrossRef]
- T. Tsuji, “Patient monitoring during and after open heart surgery by an improved deep body thermometer,” In: Atsumi, K., Kajiya, F., Tsuji, T., Tsujioka, K. (eds) Medical Progress through Technology. Springer, Dordrecht, 1987. [CrossRef]
- L. P. J. Teunissen, J. Klewer, A. de Haan, J. J. de Koning, and H. A. M. Daanen, “Non-invasive continuous core temperature measurement by zero heat flux,” Physiological Measurement, vol. 32, no. 5, pp. 559–570, 2011. [CrossRef]
- M.-T. Mäkinen, A. Pesonen, I. Jousela, J. Päivärinta, S. Poikajärvi, A. Albäck, U.-S. Salminen, and E. Pesonen, “Novel Zero-Heat-Flux Deep Body Temperature Measurement in Lower Extremity Vascular and Cardiac Surgery,” Journal of Cardiothoracic and Vascular Anesthesia, vol. 30, no. 4, pp. 973–978, 2016. [CrossRef]
- C. Dahyot-Fizelier, S. Lamarche, T. Kerforne, T. Bénard, B. Giraud, R. Bellier, E. Carise, D. Frasca, and O. Mimoz, “Accuracy of Zero-Heat-Flux Cutaneous Temperature in Intensive Care Adults,” Critical Care Medicine, vol. 45, no. 7, pp. e715–e717, 2017. [CrossRef]
- H. Lu, S. Aratake, H. Naito, M. Nogawa, T. Nemoto, T. Togawa, and S. Tanaka, “Development of a Core Body Thermometer Applicable for High-Temperature Environment Based on the Zero-Heat-Flux Method,” Sensors, vol. 23, no. 4, 1970, 2023. [CrossRef]
- H.-C. Gunga, A. Werner, A. Stahn, M. Steinach, T. Schlabs, E. Koralewski, D. Kunz, D. L. Belavý, D. Felsenberg, F. Sattler, and J. Koch, “The Double Sensor—A non-invasive device to continuously monitor core temperature in humans on earth and in space,” Respiratory Physiology & Neurobiology, vol. 169, Supplement, pp. S63–S68, 2009. [CrossRef]
- O. Opatz, A. Stahn, A. Werner, and H.-C. Gunga, “Determining core body temperature via heat flux - a new promising approach,” Resuscitation, vol. 81, no. 11, pp. 1588–1589, 2010. [CrossRef]
- D. Matsunaga, Y. Tanaka, M. Seyama, and K. Nagashima, “Non-invasive and wearable thermometer for continuous monitoring of core body temperature under various convective conditions,” in Proc. IEEE Engineering in Medicine and Biology Society (EMBC), 2020, pp. 4377–4380, 2020. [CrossRef]
- Y. Hashimoto, S. Tada, and Y. Nishida, “Improvement of environmental robustness in non-invasive core body temperature sensor studied numerically and experimentally,” Sensors and Actuators A: Physical, vol. 368, 115136, 2024. [CrossRef]
- Y. Hashimoto, S. Tada, and Y. Nishida, “Reference-Free Calibration for Wearable Core Body Temperature Sensor Based on Single-Heat-Flux Method,” IEEE Sensors Letters, vol. 8, no. 9, pp. 1–4, 2024. [CrossRef]
- Y. Tanaka, D. Matsunaga, T. Tajima, M. Seyama, I. Kato, and K. Nagashima, “Skin-Attachable Sensor for Continuous Core Body Temperature Monitoring,” IEEE Sensors Journal, vol. 24, no. 23, pp. 38708–38714, 2024. [CrossRef]
- Y. Hashimoto, S. Tada, and Y. Nishida, “Rapid Dataset-Free Self-Calibration for Heat Flux-Based Core Body Temperature Sensors,” IEEE Access, vol. 13, pp. 61048–61055, 2025. [CrossRef]
- Y. Hashimoto, S. Tada, and Y. Nishida, “Lightweight Probe Cover for Wearable Thermal Device Designed Through Topology Optimization,” IEEE Access, vol. 13, pp. 69081–69089, 2025. [CrossRef]
- Y. Hashimoto, K. Noto, S. Tada, and Y. Nishida, “Wearable Multimodal Sensor Probe for Monitoring Core Body Temperature, Electrocardiogram, Heart Rate, and Sweat Rate,” IEEE Access, vol. 13, pp. 70769–70778, 2025. [CrossRef]
- P. S. R. Goods, P. Maloney, J. Miller, D. Jennings, J. Fahey-Gilmour, P. Peeling, and B. Galna, “Concurrent validity of the CORE wearable sensor with BodyCap temperature pill to assess core body temperature during an elite women’s field hockey heat training camp,” Eur. J. Sport Sci., vol. 23, no. 8, pp. 1509–1517, 2023. [CrossRef]
- H. A. M. Daanen, V. Kohlen, and L. P. J. Teunissen, “Heat flux systems for body core temperature assessment during exercise,” J. Therm. Biol., vol. 112, p. 103480, 2023. [CrossRef]
- X. Xu, G. Wu, Z. Lian, and H. Xu, “Feasibility analysis of applying non-invasive core body temperature measurement in sleep research,” Energy Build., vol. 303, p. 113827, 2024. [CrossRef]
- W. M. Januário, N. F. Lessa, A. J. O. Schittine, E. R. B. A. Prata, J. C. B. Marins, A. J. Natali, S. P. Wanner, and T. N. Prímola-Gomes, “Validity and reproducibility of the CALERA Research Sensor to estimate core temperature at different intensities of a cycling exercise in the heat,” J. Therm. Biol., vol. 123, 103907, 2024. [CrossRef]
- A. Kaltsatou, M. Anifanti, A. D. Flouris, G. Xiromerisiou, and E. Kouidi, “Validity of the CALERA research sensor to assess body core temperature during maximum exercise in patients with heart failure,” Sensors, vol. 24, no. 3, 807, 2024. [CrossRef]
- U. E. Ehlers, J. Ulmer, M. Keller, C. Klein, and U. Pietsch, “Comparison of continuous temperature measurement methods in the intensive care unit: standard bladder catheter measurements versus non-invasive transcutaneous sensors,” J. Clin. Monit. Comput., vol. 39, pp. 193–203, 2025. [CrossRef]
- J. I. Priego-Quesada, N. MacKay, D. C. Adejuwon, and D. A. Keir, “Effect of aerobic fitness on the validity of the Calera Research™ sensor to estimate core temperature during exercise,” J. Therm. Biol., vol. 127, p. 104067, 2025. [CrossRef]
- B. C. McLaughlin, J. T. Aguilera, and A. C. D’Lugos, “Validity of the CORE wearable sensor during constant-load cycling exercise in the heat,” J. Therm. Biol., vol. 132, 104241, 2025. [CrossRef]
- H. Xu, Y. Xu, H. Wang, T.-H. Lei, and F. Wang, “Validity of the wearable CORE temperature sensor during 8-hour indoor heat exposure with and without electric fan use,” Build. Environ., vol. 282, p. 113288, 2025. [CrossRef]
- M. Huang and W. Chen, “Theoretical simulation of the dual-heat-flux method in deep body temperature measurements,” 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology, Buenos Aires, Argentina, 2010, pp. 1–4. [CrossRef]
- M. Huang, W. Chen, K. Kitamura, T. Nemoto, and T. Tamura, “Improvement of the dual-heat-flux method for deep body temperature measurement based on a finite element model,” 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 2013, pp. 1–4. [CrossRef]
- M. Huang, T. Tamura, W. Chen, and S. Kanaya, “Evaluation of structural and thermophysical effects on the measurement accuracy of deep body thermometers based on dual-heat-flux method,” Journal of Thermal Biology, vol. 47, pp. 26–31, 2015. [CrossRef]
- J. Feng, C. Zhou, C. He, Y. Li, and X. Ye, “Development of an improved wearable device for core body temperature monitoring based on the dual heat flux principle,” Physiological Measurement, vol. 38, no. 4, pp. 652–668, 2017. [CrossRef]
- M. Huang, T. Tamura, Z. Tang, W. Chen, and S. Kanaya, “A wearable thermometry for core body temperature measurement and its experimental verification,” IEEE Journal of Biomedical and Health Informatics, vol. 21, no. 3, pp. 708–714, 2017. [CrossRef]
- J. Fang, C. Zhou, and X. Ye, “Optimization of a Wearable Device for Core Body Temperature Monitoring Based on the Dual-Heat-Flux Model,” IOP Conference Series: Materials Science and Engineering, vol. 677, no. 3, p. 032006, 2019. [CrossRef]
- A. Yoshida, R. Kamon, T. Naka, N. Chigusa, S. Kinoshita, and T. Kawabata, “Evaluation of conventional invasive measurements and examination of non-invasive measurement technique on human body core temperature,” IOP Conference Series: Materials Science and Engineering, vol. 1137, no. 1, 012038, 2021. [CrossRef]
- K. Tokizawa, T. Shimuta, and H. Tsuchimoto, “Validity of a wearable core temperature estimation system in heat using patch-type sensors on the chest,” Journal of Thermal Biology, vol. 108, 103294, 2022. [CrossRef]
- T. Tamura, M. Huang, T. Yoshimura, S. Umezu, and T. Ogata, “An Advanced Internet of Things System for Heatstroke Prevention with a Noninvasive Dual-Heat-Flux Thermometer,” Sensors, vol. 22, no. 24, 9985, 2022. [CrossRef]
- J. Żmigrodzki, S. Cygan, J. Łusakowski, and P. Lamprecht, “Analytical Analysis of Factors Affecting the Accuracy of a Dual-Heat Flux Core Body Temperature Sensor,” Sensors, vol. 24, no. 6, 1887, 2024. [CrossRef]
- X. Xu, A. J. Karis, M. J. Buller, and W. R. Santee, “Relationship between core temperature, skin temperature, and heat flux during exercise in heat,” European Journal of Applied Physiology, vol. 113, no. 9, pp. 2381–2389, 2013. [CrossRef]
- C. Shan, J. Hu, J. Zou, and A. Zhang, “Wearable Personal Core Body Temperature Measurement Considering Individual Differences and Dynamic Tissue Blood Perfusion,” IEEE Journal of Biomedical and Health Informatics, vol. 26, no. 5, pp. 2158–2168, 2022. [CrossRef]
- Mark J. Buller, William J. Tharion, Reed W. Hoyt and Odest C. Jenkins, Estimation of human internal temperature from wearable physiological sensors, Proceedings of the AAAI Conference on Artificial Intelligence, vol. 24, no. 2, pp. 1763-1768, 2010. [CrossRef]
- A. P. Hunt, M. J. Buller, M. J. Maley, J. T. Costello and I. B. Stewart, Validity of a noninvasive estimation of deep body temperature when wearing personal protective equipment during exercise and recovery, Mil. Med. Res., vol. 6, 20, 2019. [CrossRef]
- Y. Zhao and J. H. M. Bergmann, Core body temperature estimation from heart rate via multi-model Kalman filtering and variance-based fusion, Physiol. Meas., vol. 46, no. 10, article 105002, 2025. [CrossRef]
- M. J. Buller, W. J. Tharion, S. N. Cheuvront, S. J. Montain, R. W. Kenefick, J. Castellani, W. A. Latzka, W. S. Roberts, M. Richter, O. C. Jenkins and R. W. Hoyt, Estimation of human core temperature from sequential heart rate observations, Physiological Measurement, vol. 34, no. 7, pp. 781-798, 2013. [CrossRef]
- T. Hamatani, A. Uchiyama and T. Higashino, Estimating core body temperature based on human thermal model using wearable sensors, Proceedings of the 30th Annual ACM Symposium on Applied Computing, pp. 521-526, 2015. [CrossRef]
- Victoria L. Richmond, Sarah Davey, Katy Griggs and George Havenith, Prediction of core body temperature from multiple variables, Annals of Work Exposures and Health, vol. 59, no. 9, pp. 1168-1178, 2015. [CrossRef]
- Srinivas Laxminarayan, Vineet Rakesh, Tatsuya Oyama, Josh B Kazman, Ran Yanovich, Itay Ketko, Yoram Epstein, Shawnda Morrison and Jaques Reifman, Individualized estimation of human core body temperature using noninvasive measurements, Journal of Applied Physiology, vol. 124, no. 6, pp. 1387-1402, 2017. [CrossRef]
- S. Y. Sim, K. M. Joo, H. B. Kim, S. Jang, B. Kim, S. Hong, S. Kim and K. S. Park, Estimation of circadian body temperature rhythm based on heart rate in healthy, ambulatory subjects, IEEE J. Biomed. Health Inform., vol. 21, no. 2, pp. 407-415, 2017. [CrossRef]
- D. P. Looney, M. J. Buller, A. V. Gribok, J. L. Leger, A. W. Potter, W. V. Rumpler, W. J. Tharion, A. P. Welles, K. E. Friedl and R. W. Hoyt, Estimating resting core temperature using heart rate, Journal for the Measurement of Physical Behaviour, vol. 1, no. 2, pp. 79-85, 2018. [CrossRef]
- Alexander P. Welles, Xiaojiang Xu, William R. Santee, David P. Looney, Mark J. Buller, Adam W. Potter and Reed W. Hoyt, Estimation of core body temperature from skin temperature, heat flux, and heart rate using a Kalman filter, Computers in Biology and Medicine, vol. 99, pp. 1-6, 2018. [CrossRef]
- P. Eggenberger, B. A. MacRae, S. Kemp, M. Burgisser, R. M. Rossi and S. Annaheim, Prediction of core body temperature based on skin temperature, heat flux, and heart rate under different exercise and clothing conditions in the heat in young adult males, Frontiers in Physiology, vol. 9, article 1780, 2018. [CrossRef]
- Joshua Hagen, Aaron Himmler, Joseph Clark, Jad Ramadan, Jason Stone, Jon Divine and Robert Mangine, Test and evaluation of heart rate derived core temperature algorithms for use in NCAA Division I football athletes, Journal of Functional Morphology and Kinesiology, vol. 5, no. 3, 46, 2020. [CrossRef]
- Akimasa Hirata, Taiki Miyazawa, Ryota Uematsu, Sachiko Kodera, Yuki Hashimoto, Kazuhiko Takagahara, Yuichi Higuchi, Hiroyoshi Togo, Takashi Kawahara and Hideto Tanaka, Body Core Temperature Estimation Using New Compartment Model With Vital Data From Wearable Devices, IEEE Access, vol. 9, pp. 124452-124462, 2021. [CrossRef]
- N. E. Moyen, R. C. Bapat, B. Tan, L. A. Hunt, O. Jay and T. Mundel, Accuracy of algorithm to non-invasively predict core body temperature using the Kenzen wearable device, Int. J. Environ. Res. Public Health, vol. 18, no. 24 13126, 2021. [CrossRef]
- I. H. Rizvi and U. Raj, A modified Kalman filter based model for core temperature estimation during exercise and recovery with/without personal cooling interventions, Journal of Thermal Biology, vol. 109, 103307, 2022. [CrossRef]
- S. J. Pearson, B. Highlands, R. Jones and M. J. Matthews, Comparisons of core temperature between a telemetric pill and heart rate estimated core temperature in firefighters, Safety and Health at Work, vol. 13, no. 1, pp. 99-103, 2022. [CrossRef]
- C. Kurosaka, T. Maruyama, S. Yamada, Y. Hachiya, Y. Ueta and T. Higashi, “Estimating core body temperature using electrocardiogram signals,” PLoS ONE, vol. 17, no. 6, p. e0270626, 2022. [CrossRef]
- J. Q. de Korte, B. J. Veenstra, M. van Rijswick, E. J. K. Derksen, M. T. E. Hopman, C. C. W. G. Bongers and T. M. H. Eijsvogels, A heart rate based algorithm to estimate core temperature responses in elite athletes exercising in the heat, Front. Sports Act. Living, vol. 4, 882254, 2022. [CrossRef]
- S. Etienne, R. Oliveras, G. Schiboni, L. Durrer, F. Rochat, P. Eib, M. Zahner, M. Osthoff, S. Bassetti and J. Eckstein, Free-living core body temperature monitoring using a wrist-worn sensor after COVID-19 booster vaccination a pilot study, Biomed. Eng. Online, vol. 22, 25, 2023. [CrossRef]
- Mandy A. G. Peggen, Coen C. W. G. Bongers, Johannus Q. de Korte, Bertil J. Veenstra, Koen Levels, Maria T. E. Hopman and Thijs M. H. Eijsvogels, Validity of the estimated core temperature algorithm during real-world prolonged walking exercise under warm ambient conditions, Journal of Thermal Biology, vol. 125, article 103982, 2024. [CrossRef]
- T. Falcone, S. Del Ferraro, V. Molinaro, L. Zollo and P. Lenzuni, A real-time biphasic Kalman filter based model for estimating human core temperature from heart rate measurements for application in the occupational field, Front. Public Health, vol. 12, 1219595, 2024.
- Y. Zhao and J. H. M. Bergmann, “Estimating core body temperature from heart rate using a residual-compensated adaptive Kalman filter,” Biocybernetics and Biomedical Engineering, vol. 45, no. 4, pp. 617–629, 2025. [CrossRef]
- Y. Zhao and J. Bergmann, “A Hybrid Core Temperature Estimation Method Integrating Physiological and Environmental Factors,” IEEE Sensors Letters, vol. 9, no. 7, pp. 1-4, 2025. [CrossRef]
- A. Mah, L. G. Zadeh, M. K. Tehrani, S. Askari, and B. Shadgan, “Studying the accuracy of infrared thermography for measuring core body temperature,” Proc. SPIE 11956, Biophotonics in Exercise Science, Sports Medicine, Health Monitoring Technologies, and Wearables III, 119560E, 2022. [CrossRef]
| Review (Year) |
In-ear sensors | Ingestible sensors | Infrared thermography | Heat flux based merhod | HR-based method | Mult-sensor fusion | ||
|---|---|---|---|---|---|---|---|---|
| Zero-heat-flux method | Single-heat-flux method | Dual-heat-flux method | ||||||
| Moran et al. (2002) [10] | ✓ | — | — | — | — | — | — | — |
| Byrne et al. (2007) [7] | — | ✓ | — | — | — | — | — | — |
| Conway et al. (2020) [11] | — | — | — | ✓ | — | — | — | — |
| Cutuli et al. (2021) [12] | ✓ | — | — | ✓ | — | — | — | — |
| Falcone et al. (2021) [13] | — | — | — | ✓ | ✓ | — | ✓ | ✓ |
| Foster et al. (2021) [14] | — | — | ✓ | — | — | — | — | — |
| Dolson et al. (2022) [15] | — | — | — | — | — | — | ✓ | ✓ |
| Zhao et al. (2023) [16] | — | — | ✓ | — | — | — | — | — |
|
This study |
✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Method | Advantages | Limitations | Representative application scenarios |
|---|---|---|---|
| Invasive measurement (pulmonary artery, esophageal, rectal) |
Highest accuracy Closest to physiological definition Gold standard |
Highly invasive Low usability Unsuitable for long-term or field use |
Surgery and anesthesia management Intensive care Physiological reference measurements |
| In-ear sensors | Non-invasive Good wearability Suitable for continuous monitoring |
Sensitive to placement and ambient conditions Limited accuracy |
Daily health monitoring Sports and occupational safety Wearable healthcare |
| Infrared thermography | Non-contact Rapid measurement Suitable for mass screening |
Low individual accuracy Strongly affected by environment Indirect estimation |
Fever screening during pandemics Public health surveillance Disaster response |
| Ingestible sensors | High accuracy close to invasive methods | Single-use Limited monitoring duration Cost Recovery issues |
Sports science Military and firefighting training Validation studies |
| Zero-heat-flux (ZHF) method | High accuracy Clinically validated Continuous monitoring |
Requires active heating High power consumption Bulky sensor |
Perioperative monitoring Intensive care Clinical research |
| Single-heat-flux (SHF) method | Simple structure Low power consumption Wearable potential |
Sensitive to skin thermal properties and environment Limited robustness |
Wearable prototypes Moderate-accuracy field monitoring |
| Dual-heat-flux (DHF) method | Improved robustness to individual variability Better balance of accuracy and wearability |
Increased complexity Calibration and motion artifacts |
Advanced wearable core temperature sensors Field and occupational monitoring |
| Heart rate–based estimation | Extremely high usability Uses existing wearables Low cost |
Model dependency Training data required Generalization issues Strong individual dependence |
Heat strain screening Large-scale population monitoring |
| Heart rate + other sensors (skin temperature, acceleration, etc.) |
Higher accuracy than single-sensor methods Scalable Adaptive models |
Model dependency Training data required Generalization issues |
Industrial safety Sports monitoring Continuous daily-life assessment |
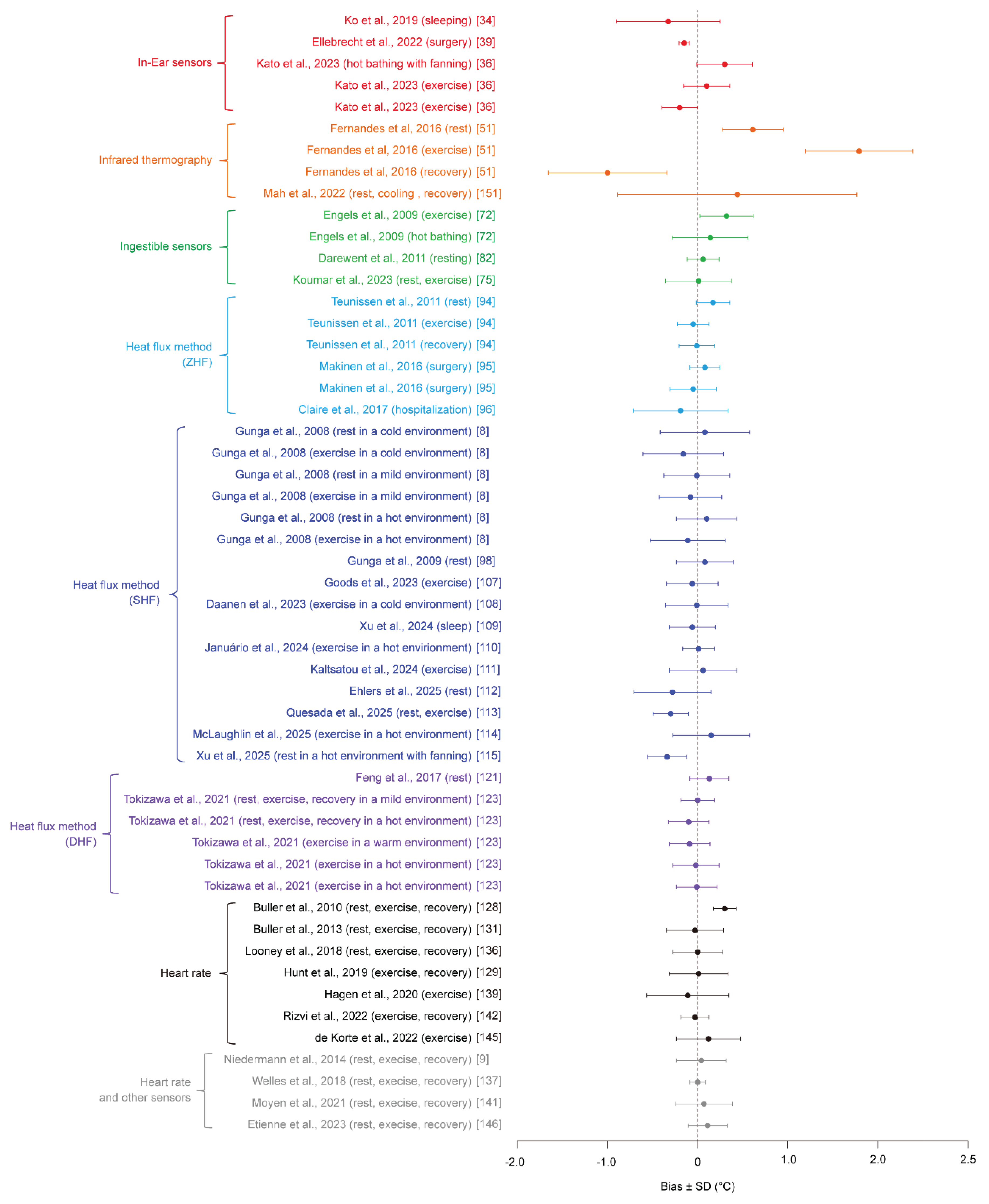
| Measurement technique |
Paper | Subject types | Number of Validation subjects, n | Environmental conditions | Exercise types | Reference for core body temperature | Bias ± SD [°C] |
|---|---|---|---|---|---|---|---|
| In-Ear sensors | Ko et al. 2019 [34] | Healthy females | 9 | 27 °C, 50%RH | Sleep | Rectal | -0.32 ± 0.58 |
| Ellebrecht et al. 2022 [39] | Patients | 10 | N/A | Surgery | Bladder | -0.15 ± 0.06 | |
| Kato et al. 2023 [36] | Healthy adults (23 ± 2 years) | 9 (6 males, 3 females) | 28 °C, 50%RH | Lower-limb warm-water immersion with 2 m/s airflow | Rectal | 0.30 ± 0.31 | |
| Kato et al. 2023 [36] | Healthy adult males (22 ± 1 years) | 11 | 35 °C, 50%RH | Walking at 4 km/h | Rectal | 0.10 ± 0.26 | |
| Kato et al. 2023 [36] | Healthy adults (23 ± 3 years) | 9 | 35 °C, 65%RH | Walking at 4 km/h with a 5% incline | Rectal | -0.20 ± 0.20 | |
| Infrared thermography | Fernandes et al. 2016 [51] | Healthy, physically active adult males | 12 | 24.9 ± 0.6 °C, 62.3 ± 5.7%RH | Rest | Gastrointestinal | 0.61 ± 0.31 |
| Fernandes et al. 2016 [51] | Healthy, physically active adult males | 12 | 24.9 ± 0.6 °C, 62.3 ± 5.7%RH | Exercise (60%VO2max) | Gastrointestinal | 1.79 ± 0.60 | |
| Fernandes et al. 2016 [51] | Healthy, physically active adult males | 12 | 24.9 ± 0.6 °C, 62.3 ± 5.7%RH | Recovery | Gastrointestinal | -1.00 ± 0.66 | |
| Mah et al. 2022 [151] | Healthy adults (28.3 ± 9.4 years) | 30 (14 males, 16 females) | 23 °C, 55%RH | Rest, Lower-limb cold-water immersion, recovery | Oral | 0.44 ± 1.33 | |
| Ingestible sensors | Engels et al. 2009 [72] | Healthy adult females (55.3 ± 5.9 years) | 8 | N/A | 70% HRmax exercise | Rectal | 0.32 ± 0.30 |
| Engels et al. 2009 [72] | Healthy adult females (55.3 ± 5.9 years) | 8 | N/A | 40 °C whole-body warm-water immersion | Rectal | 0.14 ± 0.42 | |
| Darewent et al. 2011 [82] | Healthy adult males (22.4 ± 2.4 years) | 11 | 21.0 ± 1.0 °C | Rest | Rectal | 0.06 ± 0.18 | |
| Koumar et al. 2023 [77] | Healthy adults (18-59 years) | 23 (13 males, 10 females) | N/A | In hospital (24 h), fasting, light activity, normal sleep | Rectal | 0.01 ± 0.37 | |
| Heat flux method (ZHF) | Teunissen et al. 2011 [94] | Healthy adults (28.3 ± 5.3 years) | 7 (4 males, 3 females) | 35 °C, 50%RH | Rest | Esophageal | 0.17 ± 0.19 |
| Teunissen et al. 2011 [94] | Healthy adults (28.3 ± 5.3 years) | 7 (4 males, 3 females) | 35 °C, 50%RH | Exercise | Esophageal | -0.05 ± 0.18 | |
| Teunissen et al. 2011 [94] | Healthy adults (28.3 ± 5.3 years) | 7 (4 males, 3 females) | 35 °C, 50%RH | Recovery | Esophageal | -0.01 ± 0.20 | |
| Makinen et al. 2016 [95] | Vascular surgery patients | 15 (11 males, 4 females) | N/A | Vascular surgery | Esophageal | 0.08 ± 0.17 | |
| Makinen et al. 2016 [95] | Cardiac surgery patients | 15 (11 males, 4 females) | N/A | Cardiac surgery | Pulmonary artery | -0.05 ± 0.26 | |
| Clare et al. 2017 [96] | ICU patients | 52 | N/A | Rest | Esophageal | -0.19 ± 0.53 | |
| Heat flux method (SHF) | Gunga et al. 2008 [8] | Healthy adult males (39.5 ± 10.2 years) | 20 | 10 °C | Rest | Rectal | 0.08 ± 0.50 |
| Gunga et al. 2008 [8] | Healthy adult males (39.5 ± 10.2 years) | 20 | 10 °C | Exercise at 35%, 45%, and 55% VO2max) |
Rectal | -0.16 ± 0.45 | |
| Gunga et al. 2008 [8] | Healthy adult males (39.5 ± 10.2 years) | 20 | 25 °C | Rest | Rectal | -0.01 ± 0.37 | |
| Gunga et al. 2008 [8] | Healthy adult males (39.5 ± 10.2 years) | 20 | 25 °C | Exercise at 25%, 45%, and 55% VO2max) |
Rectal | -0.08 ± 0.35 | |
| Gunga et al. 2008 [8] | Healthy adult males (39.5 ± 10.2 years) | 20 | 40 °C | Rest | Rectal | 0.10 ± 0.34 | |
| Gunga et al. 2008 [8] | Healthy adult males (39.5 ± 10.2 years) | 20 | 40 °C | Exercise at 25%, 35%, and 45% VO2max) |
Rectal | -0.11 ± 0.42 | |
| Gunga et al. 2009 [98] | Healthy adult males (31.9 ± 8.0 years) | 7 | N/A | Rest | Rectal | 0.08 ± 0.32 | |
| Goods et al. 2023 [107] | Athlete females (26 ± 4 years) | 19 | 31.0 °C, 38.5%RH | Exercise (Training) | Gastrointestinal | -0.06 ± 0.29 | |
| Goods et al. 2023 [107] | Athlete females (26 ± 4 years) | 19 | 32.2 °C, 50%RH | Exercise (Match) | Gastrointestinal | -0.10 ± 0.29 | |
| Goods et al. 2023 [107] | Athlete females (26 ± 4 years) | 19 | 27.6 °C, 80%RH | Exercise (Match) | Gastrointestinal | -0.17 ± 0.27 | |
| Goods et al. 2023 [107] | Athlete females (26 ± 4 years) | 19 | 27.4 °C, 74%RH | Exercise (Match) | Gastrointestinal | -0.34 ± 0.20 | |
| Daanen et al. 2023 [108] | Healthy adults (24.3 ± 1.2 years) | 9 (4 males, 5 females) | 18 °C, 50%RH | Rest, exercise (cycling), recovery | Rectal | -0.01 ± 0.35 | |
| Xu et al. 2024 [109] | Healthy adults (20-25 years) | 14 (7 males, 7 females) | 16, 20, 24 °C | Rest, sleep | Gastrointestinal | -0.06 ± 0.26 | |
| Januario et al. 2024 [110] | Healthy adults (33.4 ± 8.2 years) | 15 (7 males, 8 females) | 32 °C, 60%RH | Rest, exercise (cycling), recovery | Gastrointestinal | 0.01 ± 0.18 | |
| Kaltsatou et al. 2024 [111] | Chronic heart failure patients (53.5 ± 8.5 years) | 12 (8 males, 4 females) | 28 °C, 39%RH | Rest | Gastrointestinal | -0.14 ± 0.80 | |
| Kaltsatou et al. 2024 [111] | Chronic heart failure patients (53.5 ± 8.5 years) | 12 (8 males, 4 females) | 28 °C, 39%RH | Exercise (Bruce protocol) | Gastrointestinal | 0.06 ± 0.38 | |
| Kaltsatou et al. 2024 [111] | Chronic heart failure patients (53.5 ± 8.5 years) | 12 (8 males, 4 females) | 28 °C, 39%RH | Recovery | Gastrointestinal | 0.11 ± 0.28 | |
| Ehler et al. 2025 [111] | ICU patients (63.3 ± 15.1 years) | 112 (64 males, 48 females) | N/A | N/A | Bladder | -0.38 ± 0.43 | |
| Quesada et al. 2025 [112] | Healthy adults (23 ± 4 years) | 20 (10 males, 10 females) | 22.2 ± 0.5 °C, 37 ± 10%RH | Rest, exercise (cycling), recovery | Gastrointestinal | -0.3 ± 0.2 | |
| McLaughlin et al. 2025 [113] | Healthy adults | 24 (13 males, 11 females) | 35.9 ± 0.3 °C, 20.7 ± 3.3%RH | Exercise (cycling) | Rectal | 0.15 ± 0.43 | |
| Xu et al. 2025 [1014] | Healthy males | 24 | 40 °C, 57-58%RH, 0.15±0.05 m/s | Free-living conditions ranging from rest to low-intensity daily activities (8 h) | Rectal | -0.19 ± 0.52 | |
| Xu et al. 2025 [114] | Healthy females | 14 | 40 °C, 57-58%RH, 0.15±0.05 m/s | Free-living conditions ranging from rest to low-intensity daily activities (8 h) | Rectal | 0.06 ± 0.50 | |
| Xu et al. 2025 [114] | Healthy males | 24 | 40 °C, 57-58%RH, 0.15±3.2±0.4 m/s | Free-living conditions ranging from rest to low-intensity daily activities (8 h) | Rectal | -0.34 ± 0.22 | |
| Xu et al. 2025 [114] | Healthy females | 14 | 40 °C, 57-58%RH, 0.15±3.2±0.4 m/s | Free-living conditions ranging from rest to low-intensity daily activities (8 h) | Rectal | -0.27 ± 0.19 | |
| Heat flux method (DHF) | Feng et al. 2017 [121] | Healthy adults (26.8 ± 2.1 years) | 34 (30 males, 4 females) | 26 °C, 50-60%RH | Rest | Sublingual | 0.13 ± 0.22 |
| Tokizawa et al. 2021 [123] | Healthy adults (37 ± 7 years) | 21 (15 males, 6 females) | 25 °C, 35 °C | Rest, exercise, recovery | Esophageal | 0.00 ± 0.19 | |
| Tokizawa et al. 2021 [123] | Healthy adult males (36 ± 8 years) | 9 | 35 °C | Rest, exercise, recovery | Esophageal | -0.10 ± 0.23 | |
| Tokizawa et al. 2021 [123] | Healthy adult males (36 ± 11 years) | 11 | 30 °C | Exercise | Esophageal | -0.09 ± 0.23 | |
| Tokizawa et al. 2021 [123] | Healthy adult males (36 ± 11 years) | 11 | 40 °C | Exercise | Esophageal | -0.02 ± 0.26 | |
| Tokizawa et al. 2021 [123] | Healthy adult males (30 ± 6 years) | 8 | 35 °C | Exercise | Esophageal | -0.01 ± 0.23 | |
| Heart rate (Kalman filter) | Buller et al. 2010 [128] | Soldiers, runners | 25 | 20-40 °C | Exercise (Low-to-moderate-intensity exercise, 2–8 h, intermittent) | Rectal, esophageal | 0.30 ± 0.13 |
| Heart rate (Extended Kalman filter) | Buller et al. 2013 [131] | Healthy adults (20-30 years) | 83 (82 males, 1 females) | 9-45 °C, 9-97%RH, 0-4 m/s | Treadmill walking/running, cycling, long-distance marching/patrolling; ~1–24 h | Rectal, esophageal, gastrointestinal | -0.03 ± 0.32 |
| Looney et al. 2018 [136] | Healthy adults (24 ± 3 years) | 8 (6 males, 2 females) | 18-22 °C | Sleep and light seated activities (≈16 h) | Gastrointestinal | 0.00 ± 0.28 | |
| Hunt et al. 2019 [129] | Healthy adult males (26.4 ± 6.0 years) | 8 | 24 °C, 50%RH; 32 °C, 60%RH | Treadmill walking at 2.5–5.5 km/h with recovery periods | Gastrointestinal | 0.01 ± 0.33 | |
| Hagen et al. 2020 [139] | Healthy adult male athletes (American football) | 13 | N/A | Sports training (5-min × 18–22 sets) | Gastrointestinal | -0.11 ± 0.46 | |
| Rizvi et al. 2022 [142] | Healthy adults (21-23 years) | 16 | 36±0.5 °C, 59±5%RH, 0.17±0.05 m/s | Treadmill walking at 4.5 km/h with recovery periods | Gastrointestinal | -0.03 ± 0.16 | |
| de Korte et al. 2022 [145] | Elite athletes (26 ± 5 years) | 101 (49 males, 52 females) | 31.6 ± 1.0 °C, 74 ± 5%RH | Graded exercise | Gastrointestinal | 0.15 ± 0.36 | |
| Paggen et al. 2024 [147] | General adults (some on cardiovascular/psychotropic medications) | 18 | N/A | Free-living conditions ranging from rest to low-intensity daily activities (≈26 h) | Gastrointestinal | -0.03 ± 0.16 | |
| Heart rate and other sensors | Niedermann et al. 2014 [9] | Healthy adult males (23.0 ± 3.9 years) | 10 | 30±0.2 °C, 42.9±1.1%RH, <0.3 m/s | Rest, exercise at 40% and 60% VO2max, recovery | Gastrointestinal | -0.17 ± 0.14 |
| Niedermann et al. 2014 [9] | Healthy adult males (24.6 ± 2.0 years) | 10 | 10.1±0.2 °C, 49.5±4.9%RH, 0.5±0.1 m/s | Rest, exercise at 60% VO2max, recovery | Gastrointestinal | 0.04 ± 0.28 | |
| Welles et al. 2018 [137] | Healthy adult soldiers (22 ± 4 years) | 8 | 25 °C, 50%RH; 35 °C, 70%RH; 40 °C, 20%RH | Rest, exercise, recovery | Gastrointestinal | 0.00 ± 0.09 | |
| Moyen et al. 2021 [141] | Healthy adults (28.9 ± 7.8 years) | 27 (19 males, 8 females) | 13.4-43.2 °C, 11-75%RH | Rest, exercise, recovery | Rectal, gastrointestinal | 0.07 ± 0.32 | |
| Etienne et al. 2023 [146] | Post-vaccination healthy adults (35.8 ± 8.2 years) | 17 | N/A | Free-living conditions ranging from rest to low-intensity daily activities (≈26 h) | Gastrointestinal | 0.09 ± 0.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




