Submitted:
02 January 2026
Posted:
05 January 2026
You are already at the latest version
Abstract
Keywords:
1. Introduction and Basic Principles
1.1. Single Pulse Measures
1.2. Paired Pulse Measures
1.3. TMS Neuroplasticity-Related Measures
1.4. Repetitive TMS Paradigms
1.5. TBS Paradigms
1.6. QPS Paradigm
2. Parkinson’s Disease Through the “Looking Glass” of TMS
2.1. Motor Threshold
2.2. MEP and Recruitment Curve
2.3. Central Motor Conduction Time
2.4. Contralateral and Ipsilateral Silent Period
2.5. Intracortical Inhibition
2.6. Intracortical Facilitation
2.7. Short-Latency Afferent Inhibition
2.8. TMS-Derived Neural Plasticity in PD
3. Experimental Role of rTMS, TBS, and QPS Protocols in PD
3.1. Repetitive TMS
3.2. Theta Burst Stimulation
3.3. Quadripulse Stimulation
4. TMS and Drugs in PD
5. TMS and Sleep Disorders in PD
6. TMS in PD-Associated Dementia
7. TMS in Atypical Parkinsonian Syndromes
7.1. Progressive Supranuclear Palsy
7.2. Multiple System Atrophy
7.3. Corticobasal Degeneration
7.4. Dementia with Lewy Bodies
8. TMS in Secondary Parkinsonisms
8.1. Vascular Parkinsonism
8.2. Other Secondary Parkinsonisms
8.3. Functional Movement Disorders
8. Conclusive Remarks and Future Outlooks
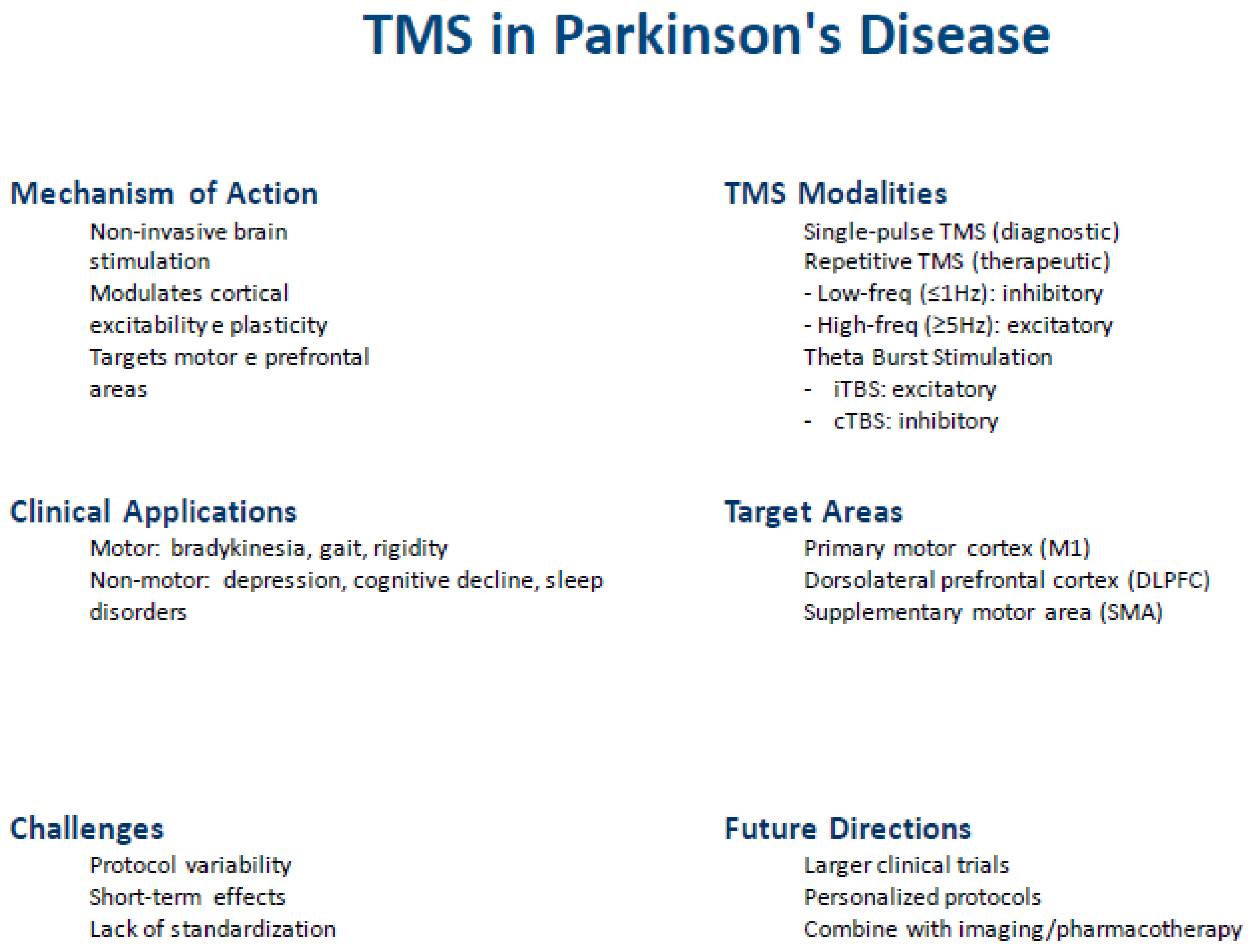
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barker, A.T.; Jalinous, R.; Freeston, I.L. Non-Invasive Magnetic Stimulation of Human Motor Cortex. Lancet Lond. Engl. 1985, 1, 1106–1107. [Google Scholar] [CrossRef]
- Bella, R.; Lanza, G.; Cantone, M.; Giuffrida, S.; Puglisi, V.; Vinciguerra, L.; Pennisi, M.; Ricceri, R.; D’Agate, C.C.; Malaguarnera, G.; et al. Effect of a Gluten-Free Diet on Cortical Excitability in Adults with Celiac Disease. PloS One 2015, 10, e0129218. [Google Scholar] [CrossRef]
- Lanza, G.; Fisicaro, F.; Dubbioso, R.; Ranieri, F.; Chistyakov, A.V.; Cantone, M.; Pennisi, M.; Grasso, A.A.; Bella, R.; Di Lazzaro, V. A Comprehensive Review of Transcranial Magnetic Stimulation in Secondary Dementia. Front. Aging Neurosci. 2022, 14, 995000. [Google Scholar] [CrossRef] [PubMed]
- Pennisi, M.; Lanza, G.; Cantone, M.; Ricceri, R.; Ferri, R.; D’Agate, C.C.; Pennisi, G.; Di Lazzaro, V.; Bella, R. Cortical Involvement in Celiac Disease before and after Long-Term Gluten-Free Diet: A Transcranial Magnetic Stimulation Study. PloS One 2017, 12, e0177560. [Google Scholar] [CrossRef]
- Vucic, S.; Stanley Chen, K.-H.; Kiernan, M.C.; Hallett, M.; Benninger, D.H.; Di Lazzaro, V.; Rossini, P.M.; Benussi, A.; Berardelli, A.; Currà, A.; et al. Clinical Diagnostic Utility of Transcranial Magnetic Stimulation in Neurological Disorders. Updated Report of an IFCN Committee. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2023, 150, 131–175. [Google Scholar] [CrossRef]
- Lefaucheur, J.-P. Transcranial Magnetic Stimulation. Handb. Clin. Neurol. 2019, 160, 559–580. [Google Scholar] [CrossRef]
- Rossini, P.M.; Burke, D.; Chen, R.; Cohen, L.G.; Daskalakis, Z.; Di Iorio, R.; Di Lazzaro, V.; Ferreri, F.; Fitzgerald, P.B.; George, M.S.; et al. Non-Invasive Electrical and Magnetic Stimulation of the Brain, Spinal Cord, Roots and Peripheral Nerves: Basic Principles and Procedures for Routine Clinical and Research Application. An Updated Report from an I.F.C.N. Committee. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2015, 126, 1071–1107. [Google Scholar] [CrossRef] [PubMed]
- Rossini, P.M.; Rossi, S. Transcranial Magnetic Stimulation: Diagnostic, Therapeutic, and Research Potential. Neurology 2007, 68, 484–488. [Google Scholar] [CrossRef]
- Chen, R. Studies of Human Motor Physiology with Transcranial Magnetic Stimulation. Muscle Nerve. Suppl. 2000, 9, S26–32. [Google Scholar] [CrossRef] [PubMed]
- Udupa, K.; Chen, R. Central Motor Conduction Time. Handb. Clin. Neurol. 2013, 116, 375–386. [Google Scholar] [CrossRef]
- Mills, K.R. Magnetic Brain Stimulation: A Review after 10 Years Experience. Electroencephalogr. Clin. Neurophysiol. Suppl. 1999, 49, 239–244. [Google Scholar]
- Chen, R.; Lozano, A.M.; Ashby, P. Mechanism of the Silent Period Following Transcranial Magnetic Stimulation. Evidence from Epidural Recordings. Exp. Brain Res. 1999, 128, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Cantello, R.; Gianelli, M.; Civardi, C.; Mutani, R. Magnetic Brain Stimulation: The Silent Period after the Motor Evoked Potential. Neurology 1992, 42, 1951–1959. [Google Scholar] [CrossRef]
- Siebner, H.R.; Dressnandt, J.; Auer, C.; Conrad, B. Continuous Intrathecal Baclofen Infusions Induced a Marked Increase of the Transcranially Evoked Silent Period in a Patient with Generalized Dystonia. Muscle Nerve 1998, 21, 1209–1212. [Google Scholar] [CrossRef]
- Kobayashi, M.; Pascual-Leone, A. Transcranial Magnetic Stimulation in Neurology. Lancet Neurol. 2003, 2, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Pennisi, G.; Bella, R.; Lanza, G. Motor Cortex Plasticity in Subcortical Ischemic Vascular Dementia: What Can TMS Say? Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2015, 126, 851–852. [Google Scholar] [CrossRef]
- Kujirai, T.; Caramia, M.D.; Rothwell, J.C.; Day, B.L.; Thompson, P.D.; Ferbert, A.; Wroe, S.; Asselman, P.; Marsden, C.D. Corticocortical Inhibition in Human Motor Cortex. J. Physiol. 1993, 471, 501–519. [Google Scholar] [CrossRef]
- Ziemann, U.; Rothwell, J.C.; Ridding, M.C. Interaction between Intracortical Inhibition and Facilitation in Human Motor Cortex. J. Physiol. 1996, 496 Pt 3, 873–881. [Google Scholar] [CrossRef]
- Di Lazzaro, V.; Pilato, F.; Dileone, M.; Ranieri, F.; Ricci, V.; Profice, P.; Bria, P.; Tonali, P.A.; Ziemann, U. GABAA Receptor Subtype Specific Enhancement of Inhibition in Human Motor Cortex. J. Physiol. 2006, 575, 721–726. [Google Scholar] [CrossRef]
- Ziemann, U. TMS and Drugs. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2004, 115, 1717–1729. [Google Scholar] [CrossRef]
- Valls-Solé, J.; Pascual-Leone, A.; Wassermann, E.M.; Hallett, M. Human Motor Evoked Responses to Paired Transcranial Magnetic Stimuli. Electroencephalogr. Clin. Neurophysiol. 1992, 85, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Wassermann, E.M.; Samii, A.; Mercuri, B.; Ikoma, K.; Oddo, D.; Grill, S.E.; Hallett, M. Responses to Paired Transcranial Magnetic Stimuli in Resting, Active, and Recently Activated Muscles. Exp. Brain Res. 1996, 109, 158–163. [Google Scholar] [CrossRef]
- McDonnell, M.N.; Orekhov, Y.; Ziemann, U. The Role of GABA(B) Receptors in Intracortical Inhibition in the Human Motor Cortex. Exp. Brain Res. 2006, 173, 86–93. [Google Scholar] [CrossRef]
- Tokimura, H.; Di Lazzaro, V.; Tokimura, Y.; Oliviero, A.; Profice, P.; Insola, A.; Mazzone, P.; Tonali, P.; Rothwell, J.C. Short Latency Inhibition of Human Hand Motor Cortex by Somatosensory Input from the Hand. J. Physiol. 2000, 523, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Di Lazzaro, V.; Oliviero, A.; Profice, P.; Pennisi, M.A.; Di Giovanni, S.; Zito, G.; Tonali, P.; Rothwell, J.C. Muscarinic Receptor Blockade Has Differential Effects on the Excitability of Intracortical Circuits in the Human Motor Cortex. Exp. Brain Res. 2000, 135, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Di Lazzaro, V.; Oliviero, A.; Pilato, F.; Saturno, E.; Dileone, M.; Marra, C.; Ghirlanda, S.; Ranieri, F.; Gainotti, G.; Tonali, P. Neurophysiological Predictors of Long Term Response to AChE Inhibitors in AD Patients. J. Neurol. Neurosurg. Psychiatry 2005, 76, 1064–1069. [Google Scholar] [CrossRef]
- Dubbioso, R.; Esposito, M.; Peluso, S.; Iodice, R.; De Michele, G.; Santoro, L.; Manganelli, F. Disruption of GABA(A)-Mediated Intracortical Inhibition in Patients with Chorea-Acanthocytosis. Neurosci. Lett. 2017, 654, 107–110. [Google Scholar] [CrossRef]
- R, D.; F, M.; Hr, S.; D.L., V. Fast Intracortical Sensory-Motor Integration: A Window Into the Pathophysiology of Parkinson’s Disease. Front. Hum. Neurosci. 2019, 13. [Google Scholar] [CrossRef]
- Martorana, A.; Mori, F.; Esposito, Z.; Kusayanagi, H.; Monteleone, F.; Codecà, C.; Sancesario, G.; Bernardi, G.; Koch, G. Dopamine Modulates Cholinergic Cortical Excitability in Alzheimer’s Disease Patients. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2009, 34, 2323–2328. [Google Scholar] [CrossRef]
- Sailer, A.; Molnar, G.F.; Paradiso, G.; Gunraj, C.A.; Lang, A.E.; Chen, R. Short and Long Latency Afferent Inhibition in Parkinson’s Disease. Brain J. Neurol. 2003, 126, 1883–1894. [Google Scholar] [CrossRef]
- Dubbioso, R.; Manganelli, F.; Siebner, H.R.; Di Lazzaro, V. Fast Intracortical Sensory-Motor Integration: A Window Into the Pathophysiology of Parkinson’s Disease. Front. Hum. Neurosci. 2019, 13, 111. [Google Scholar] [CrossRef]
- Carson, R.G.; Kennedy, N.C. Modulation of Human Corticospinal Excitability by Paired Associative Stimulation. Front. Hum. Neurosci. 2013, 7, 823. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Huang, Y.; Bai, Y.; Tsai, S.; Su, T.; Cheng, C. Critical Role of Glutamatergic and GABAergic Neurotransmission in the Central Mechanisms of Theta-burst Stimulation. Hum. Brain Mapp. 2019, 40, 2001–2009. [Google Scholar] [CrossRef]
- Matsumoto, H.; Ugawa, Y. Quadripulse Stimulation (QPS). Exp. Brain Res. 2020, 238, 1619–1625. [Google Scholar] [CrossRef]
- Yger, P.; Gilson, M. Models of Metaplasticity: A Review of Concepts. Front. Comput. Neurosci. 2015, 9, 138. [Google Scholar] [CrossRef]
- Kirkovski, M.; Donaldson, P.H.; Do, M.; Speranza, B.E.; Albein-Urios, N.; Oberman, L.M.; Enticott, P.G. A Systematic Review of the Neurobiological Effects of Theta-Burst Stimulation (TBS) as Measured Using Functional Magnetic Resonance Imaging (fMRI). Brain Struct. Funct. 2023, 228, 717–749. [Google Scholar] [CrossRef] [PubMed]
- George, M.S.; Lisanby, S.H.; Avery, D.; McDonald, W.M.; Durkalski, V.; Pavlicova, M.; Anderson, B.; Nahas, Z.; Bulow, P.; Zarkowski, P.; et al. Daily Left Prefrontal Transcranial Magnetic Stimulation Therapy for Major Depressive Disorder: A Sham-Controlled Randomized Trial. Arch. Gen. Psychiatry 2010, 67, 507–516. [Google Scholar] [CrossRef]
- Bella, R.; Ferri, R.; Cantone, M.; Pennisi, M.; Lanza, G.; Malaguarnera, G.; Spampinato, C.; Giordano, D.; Raggi, A.; Pennisi, G. Motor Cortex Excitability in Vascular Depression. Int. J. Psychophysiol. Off. J. Int. Organ. Psychophysiol. 2011, 82, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Bella, R.; Ferri, R.; Lanza, G.; Cantone, M.; Pennisi, M.; Puglisi, V.; Vinciguerra, L.; Spampinato, C.; Mazza, T.; Malaguarnera, G.; et al. TMS Follow-up Study in Patients with Vascular Cognitive Impairment-No Dementia. Neurosci. Lett. 2013, 534, 155–159. [Google Scholar] [CrossRef]
- Nicoletti, V.G.; Fisicaro, F.; Aguglia, E.; Bella, R.; Calcagno, D.; Cantone, M.; Concerto, C.; Ferri, R.; Mineo, L.; Pennisi, G.; et al. Challenging the Pleiotropic Effects of Repetitive Transcranial Magnetic Stimulation in Geriatric Depression: A Multimodal Case Series Study. Biomedicines 2023, 11, 958. [Google Scholar] [CrossRef]
- Lefaucheur, J.-P.; André-Obadia, N.; Antal, A.; Ayache, S.S.; Baeken, C.; Benninger, D.H.; Cantello, R.M.; Cincotta, M.; de Carvalho, M.; De Ridder, D.; et al. Evidence-Based Guidelines on the Therapeutic Use of Repetitive Transcranial Magnetic Stimulation (rTMS). Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2014, 125, 2150–2206. [Google Scholar] [CrossRef]
- Lefaucheur, J.-P.; Aleman, A.; Baeken, C.; Benninger, D.H.; Brunelin, J.; Di Lazzaro, V.; Filipović, S.R.; Grefkes, C.; Hasan, A.; Hummel, F.C.; et al. Evidence-Based Guidelines on the Therapeutic Use of Repetitive Transcranial Magnetic Stimulation (rTMS): An Update (2014–2018). Clin. Neurophysiol. 2020, 131, 474–528. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Hui, Y.; Wu, Z.; Wang, L.; Wu, X.; Bai, Y.; Zhang, Q.; Li, L. Dose and Time-Dependence of Acute Intermittent Theta-Burst Stimulation on Hippocampus-Dependent Memory in Parkinsonian Rats. Front. Neurosci. 2023, 17, 1124819. [Google Scholar] [CrossRef]
- Zeljkovic Jovanovic, M.; Stanojevic, J.; Stevanovic, I.; Stekic, A.; Bolland, S.J.; Jasnic, N.; Ninkovic, M.; Zaric Kontic, M.; Ilic, T.V.; Rodger, J.; et al. Intermittent Theta Burst Stimulation Improves Motor and Behavioral Dysfunction through Modulation of NMDA Receptor Subunit Composition in Experimental Model of Parkinson’s Disease. Cells 2023, 12, 1525. [Google Scholar] [CrossRef]
- Hamada, M.; Terao, Y.; Hanajima, R.; Shirota, Y.; Nakatani-Enomoto, S.; Furubayashi, T.; Matsumoto, H.; Ugawa, Y. Bidirectional Long-Term Motor Cortical Plasticity and Metaplasticity Induced by Quadripulse Transcranial Magnetic Stimulation. J. Physiol. 2008, 586, 3927–3947. [Google Scholar] [CrossRef] [PubMed]
- Bloem, B.R.; Okun, M.S.; Klein, C. Parkinson’s Disease. The Lancet 2021, 397, 2284–2303. [Google Scholar] [CrossRef]
- Dickson, D.W. Neuropathology of Parkinson Disease. Parkinsonism Relat. Disord. 2018, 46 Suppl 1, S30–S33. [Google Scholar] [CrossRef]
- Marsili, L.; Rizzo, G.; Colosimo, C. Diagnostic Criteria for Parkinson’s Disease: From James Parkinson to the Concept of Prodromal Disease. Front. Neurol. 2018, 9, 156. [Google Scholar] [CrossRef]
- Jankovic, J.; McDermott, M.; Carter, J.; Gauthier, S.; Goetz, C.; Golbe, L.; Huber, S.; Koller, W.; Olanow, C.; Shoulson, I. Variable Expression of Parkinson’s Disease: A Base-Line Analysis of the DATATOP Cohort. The Parkinson Study Group. Neurology 1990, 40, 1529–1534. [Google Scholar] [CrossRef] [PubMed]
- Sauerbier, A.; Jenner, P.; Todorova, A.; Chaudhuri, K.R. Non Motor Subtypes and Parkinson’s Disease. Parkinsonism Relat. Disord. 2016, 22 Suppl 1, S41–46. [Google Scholar] [CrossRef] [PubMed]
- Shakya, S.; Prevett, J.; Hu, X.; Xiao, R. Characterization of Parkinson’s Disease Subtypes and Related Attributes. Front. Neurol. 2022, 13. [Google Scholar] [CrossRef]
- Fisicaro, F.; Lanza, G.; Cantone, M.; Ferri, R.; Pennisi, G.; Nicoletti, A.; Zappia, M.; Bella, R.; Pennisi, M. Clinical and Electrophysiological Hints to TMS in De Novo Patients with Parkinson’s Disease and Progressive Supranuclear Palsy. J. Pers. Med. 2020, 10, 274. [Google Scholar] [CrossRef] [PubMed]
- Lanza, G.; Cosentino, F.I.I.; Lanuzza, B.; Tripodi, M.; Aricò, D.; Figorilli, M.; Puligheddu, M.; Fisicaro, F.; Bella, R.; Ferri, R.; et al. Reduced Intracortical Facilitation to TMS in Both Isolated REM Sleep Behavior Disorder (RBD) and Early Parkinson’s Disease with RBD. J. Clin. Med. 2022, 11, 2291. [Google Scholar] [CrossRef] [PubMed]
- MacKinnon, C.D.; Gilley, E.A.; Weis-McNulty, A.; Simuni, T. Pathways Mediating Abnormal Intracortical Inhibition in Parkinson’s Disease. Ann. Neurol. 2005, 58, 516–524. [Google Scholar] [CrossRef]
- Ni, Z.; Bahl, N.; Gunraj, C.A.; Mazzella, F.; Chen, R. Increased Motor Cortical Facilitation and Decreased Inhibition in Parkinson Disease. Neurology 2013, 80, 1746–1753. [Google Scholar] [CrossRef]
- Ridding, M.C.; Inzelberg, R.; Rothwell, J.C. Changes in Excitability of Motor Cortical Circuitry in Patients with Parkinson’s Disease. Ann. Neurol. 1995, 37, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, F.; Tremblay, L.E. Cortico-Motor Excitability of the Lower Limb Motor Representation: A Comparative Study in Parkinson’s Disease and Healthy Controls. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2002, 113, 2006–2012. [Google Scholar] [CrossRef]
- Valls-Solé, J.; Pascual-Leone, A.; Brasil-Neto, J.P.; Cammarota, A.; McShane, L.; Hallett, M. Abnormal Facilitation of the Response to Transcranial Magnetic Stimulation in Patients with Parkinson’s Disease. Neurology 1994, 44, 735–741. [Google Scholar] [CrossRef]
- Cantello, R.; Gianelli, M.; Bettucci, D.; Civardi, C.; De Angelis, M.S.; Mutani, R. Parkinson’s Disease Rigidity: Magnetic Motor Evoked Potentials in a Small Hand Muscle. Neurology 1991, 41, 1449–1456. [Google Scholar] [CrossRef]
- Kolmancic, K.; Perellón-Alfonso, R.; Pirtosek, Z.; Rothwell, J.C.; Bhatia, K.; Kojovic, M. Sex Differences in Parkinson’s Disease: A Transcranial Magnetic Stimulation Study. Mov. Disord. Off. J. Mov. Disord. Soc. 2019, 34, 1873–1881. [Google Scholar] [CrossRef]
- Spagnolo, F.; Coppi, E.; Chieffo, R.; Straffi, L.; Fichera, M.; Nuara, A.; Gonzalez-Rosa, J.; Martinelli, V.; Comi, G.; Volontè, M.A.; et al. Interhemispheric Balance in Parkinson’s Disease: A Transcranial Magnetic Stimulation Study. Brain Stimulat. 2013, 6, 892–897. [Google Scholar] [CrossRef]
- Bologna, M.; Guerra, A.; Paparella, G.; Giordo, L.; Alunni Fegatelli, D.; Vestri, A.R.; Rothwell, J.C.; Berardelli, A. Neurophysiological Correlates of Bradykinesia in Parkinson’s Disease. Brain J. Neurol. 2018, 141, 2432–2444. [Google Scholar] [CrossRef] [PubMed]
- Ellaway, P.H.; Davey, N.J.; Maskill, D.W.; Dick, J.P. The Relation between Bradykinesia and Excitability of the Motor Cortex Assessed Using Transcranial Magnetic Stimulation in Normal and Parkinsonian Subjects. Electroencephalogr. Clin. Neurophysiol. 1995, 97, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Khedr, E.M.; Lefaucheur, J.-P.; Hasan, A.M.; Osama, K. Are There Differences in Cortical Excitability between Akinetic-Rigid and Tremor-Dominant Subtypes of Parkinson’s Disease? Neurophysiol. Clin. Clin. Neurophysiol. 2021, 51, 443–453. [Google Scholar] [CrossRef]
- Topka, M.; Schneider, M.; Zrenner, C.; Belardinelli, P.; Ziemann, U.; Weiss, D. Motor Cortex Excitability Is Reduced during Freezing of Upper Limb Movement in Parkinson’s Disease. NPJ Park. Dis. 2022, 8, 161. [Google Scholar] [CrossRef]
- Sciacca, G.; Mostile, G.; Disilvestro, I.; Donzuso, G.; Nicoletti, A.; Zappia, M. Long-Duration Response to Levodopa, Motor Learning, and Neuroplasticity in Early Parkinson’s Disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2023, 38, 626–635. [Google Scholar] [CrossRef] [PubMed]
- Perretti, A.; De Rosa, A.; Marcantonio, L.; Iodice, V.; Estraneo, A.; Manganelli, F.; Garavaglia, B.; Filla, A.; Santoro, L.; De Michele, G. Neurophysiological Evaluation of Motor Corticospinal Pathways by TMS in Idiopathic Early-Onset Parkinson’s Disease. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2011, 122, 546–549. [Google Scholar] [CrossRef]
- Soysal, A.; Sobe, I.; Atay, T.; Sen, A.; Arpaci, B. Effect of Therapy on Motor Cortical Excitability in Parkinson’s Disease. Can. J. Neurol. Sci. J. Can. Sci. Neurol. 2008, 35, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Diószeghy, P.; Hidasi, E.; Mechler, F. Study of Central Motor Functions Using Magnetic Stimulation in Parkinson’s Disease. Electromyogr. Clin. Neurophysiol. 1999, 39, 101–105. [Google Scholar]
- Mochizuki, Y.; Oishi, M.; Hara, M.; Takasu, T. Central Motor Conduction Time in Parkinson’s Disease. J. Clin. Neurosci. Off. J. Neurosurg. Soc. Australas. 1999, 6, 17–19. [Google Scholar] [CrossRef]
- De Rosa, A.; Volpe, G.; Marcantonio, L.; Santoro, L.; Brice, A.; Filla, A.; Perretti, A.; De Michele, G. Neurophysiological Evidence of Corticospinal Tract Abnormality in Patients with Parkin Mutations. J. Neurol. 2006, 253, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Schneider, S.A.; Talelli, P.; Cheeran, B.J.; Khan, N.L.; Wood, N.W.; Rothwell, J.C.; Bhatia, K.P. Motor Cortical Physiology in Patients and Asymptomatic Carriers of Parkin Gene Mutations. Mov. Disord. Off. J. Mov. Disord. Soc. 2008, 23, 1812–1819. [Google Scholar] [CrossRef] [PubMed]
- Cantello, R.; Tarletti, R.; Civardi, C. Transcranial Magnetic Stimulation and Parkinson’s Disease. Brain Res. Brain Res. Rev. 2002, 38, 309–327. [Google Scholar] [CrossRef]
- Priori, A.; Berardelli, A.; Inghilleri, M.; Accornero, N.; Manfredi, M. Motor Cortical Inhibition and the Dopaminergic System. Pharmacological Changes in the Silent Period after Transcranial Brain Stimulation in Normal Subjects, Patients with Parkinson’s Disease and Drug-Induced Parkinsonism. Brain J. Neurol. 1994, 117 Pt 2, 317–323. [Google Scholar] [CrossRef]
- Chen, R.; Garg, R.R.; Lozano, A.M.; Lang, A.E. Effects of Internal Globus Pallidus Stimulation on Motor Cortex Excitability. Neurology 2001, 56, 716–723. [Google Scholar] [CrossRef]
- Ammann, C.; Dileone, M.; Pagge, C.; Catanzaro, V.; Mata-Marín, D.; Hernández-Fernández, F.; Monje, M.H.G.; Sánchez-Ferro, Á.; Fernández-Rodríguez, B.; Gasca-Salas, C.; et al. Cortical Disinhibition in Parkinson’s Disease. Brain J. Neurol. 2020, 143, 3408–3421. [Google Scholar] [CrossRef] [PubMed]
- Guerra, A.; Colella, D.; Giangrosso, M.; Cannavacciuolo, A.; Paparella, G.; Fabbrini, G.; Suppa, A.; Berardelli, A.; Bologna, M. Driving Motor Cortex Oscillations Modulates Bradykinesia in Parkinson’s Disease. Brain J. Neurol. 2022, 145, 224–236. [Google Scholar] [CrossRef]
- Cunic, D.; Roshan, L.; Khan, F.I.; Lozano, A.M.; Lang, A.E.; Chen, R. Effects of Subthalamic Nucleus Stimulation on Motor Cortex Excitability in Parkinson’s Disease. Neurology 2002, 58, 1665–1672. [Google Scholar] [CrossRef]
- Kojovic, M.; Kassavetis, P.; Bologna, M.; Pareés, I.; Rubio-Agusti, I.; Berardelli, A.; Edwards, M.J.; Rothwell, J.C.; Bhatia, K.P. Transcranial Magnetic Stimulation Follow-up Study in Early Parkinson’s Disease: A Decline in Compensation with Disease Progression? Mov. Disord. Off. J. Mov. Disord. Soc. 2015, 30, 1098–1106. [Google Scholar] [CrossRef]
- Chu, J.; Wagle-Shukla, A.; Gunraj, C.; Lang, A.E.; Chen, R. Impaired Presynaptic Inhibition in the Motor Cortex in Parkinson Disease. Neurology 2009, 72, 842–849. [Google Scholar] [CrossRef]
- Pierantozzi, M.; Palmieri, M.G.; Marciani, M.G.; Bernardi, G.; Giacomini, P.; Stanzione, P. Effect of Apomorphine on Cortical Inhibition in Parkinson’s Disease Patients: A Transcranial Magnetic Stimulation Study. Exp. Brain Res. 2001, 141, 52–62. [Google Scholar] [CrossRef]
- Valzania, F.; Strafella, A.P.; Quatrale, R.; Santangelo, M.; Tropeani, A.; Lucchi, D.; Tassinari, C.A.; De Grandis, D. Motor Evoked Responses to Paired Cortical Magnetic Stimulation in Parkinson’s Disease. Electroencephalogr. Clin. Neurophysiol. 1997, 105, 37–43. [Google Scholar] [CrossRef]
- Berardelli, A.; Inghilleri, M.; Priori, A.; Marchetti, P.; Curra, A.; Rona, S.; Manfredi, M. Inhibitory Cortical Phenomena Studied with the Technique of Transcranial Stimulation. Electroencephalogr. Clin. Neurophysiol. Suppl. 1996, 46, 343–349. [Google Scholar]
- Bares, M.; Kanovský, P.; Klajblová, H.; Rektor, I. Intracortical Inhibition and Facilitation Are Impaired in Patients with Early Parkinson’s Disease: A Paired TMS Study. Eur. J. Neurol. 2003, 10, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Strafella, A.P.; Valzania, F.; Nassetti, S.A.; Tropeani, A.; Bisulli, A.; Santangelo, M.; Tassinari, C.A. Effects of Chronic Levodopa and Pergolide Treatment on Cortical Excitability in Patients with Parkinson’s Disease: A Transcranial Magnetic Stimulation Study. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2000, 111, 1198–1202. [Google Scholar] [CrossRef] [PubMed]
- Berardelli, A.; Rona, S.; Inghilleri, M.; Manfredi, M. Cortical Inhibition in Parkinson’s Disease. A Study with Paired Magnetic Stimulation. Brain J. Neurol. 1996, 119 Pt 1, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Shirota, Y.; Ohminami, S.; Tsutsumi, R.; Terao, Y.; Ugawa, Y.; Tsuji, S.; Hanajima, R. Increased Facilitation of the Primary Motor Cortex in de Novo Parkinson’s Disease. Parkinsonism Relat. Disord. 2019, 66, 125–129. [Google Scholar] [CrossRef]
- Guerra, A.; Suppa, A.; D’Onofrio, V.; Di Stasio, F.; Asci, F.; Fabbrini, G.; Berardelli, A. Abnormal Cortical Facilitation and L-Dopa-Induced Dyskinesia in Parkinson’s Disease. Brain Stimulat. 2019, 12, 1517–1525. [Google Scholar] [CrossRef]
- Celebi, O.; Temuçin, C.M.; Elibol, B.; Saka, E. Short Latency Afferent Inhibition in Parkinson’s Disease Patients with Dementia. Mov. Disord. Off. J. Mov. Disord. Soc. 2012, 27, 1052–1055. [Google Scholar] [CrossRef]
- Nardone, R.; Bergmann, J.; Brigo, F.; Christova, M.; Kunz, A.; Seidl, M.; Tezzon, F.; Trinka, E.; Golaszewski, S. Functional Evaluation of Central Cholinergic Circuits in Patients with Parkinson’s Disease and REM Sleep Behavior Disorder: A TMS Study. J. Neural Transm. Vienna Austria 1996 2013, 120, 413–422. [Google Scholar] [CrossRef]
- Rochester, L.; Yarnall, A.J.; Baker, M.R.; David, R.V.; Lord, S.; Galna, B.; Burn, D.J. Cholinergic Dysfunction Contributes to Gait Disturbance in Early Parkinson’s Disease. Brain J. Neurol. 2012, 135, 2779–2788. [Google Scholar] [CrossRef]
- Yarnall, A.J.; Rochester, L.; Baker, M.R.; David, R.; Khoo, T.K.; Duncan, G.W.; Galna, B.; Burn, D.J. Short Latency Afferent Inhibition: A Biomarker for Mild Cognitive Impairment in Parkinson’s Disease? Mov. Disord. Off. J. Mov. Disord. Soc. 2013. [Google Scholar] [CrossRef]
- Manganelli, F.; Vitale, C.; Santangelo, G.; Pisciotta, C.; Iodice, R.; Cozzolino, A.; Dubbioso, R.; Picillo, M.; Barone, P.; Santoro, L. Functional Involvement of Central Cholinergic Circuits and Visual Hallucinations in Parkinson’s Disease. Brain J. Neurol. 2009, 132, 2350–2355. [Google Scholar] [CrossRef]
- Lee, K.D.; Koo, J.H.; Song, S.H.; Jo, K.D.; Lee, M.K.; Jang, W. Central Cholinergic Dysfunction Could Be Associated with Oropharyngeal Dysphagia in Early Parkinson’s Disease. J. Neural Transm. Vienna Austria 1996 2015, 122, 1553–1561. [Google Scholar] [CrossRef] [PubMed]
- Oh, E.; Park, J.; Youn, J.; Kim, J.S.; Park, S.; Jang, W. Olfactory Dysfunction in Early Parkinson’s Disease Is Associated with Short Latency Afferent Inhibition Reflecting Central Cholinergic Dysfunction. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2017, 128, 1061–1068. [Google Scholar] [CrossRef]
- Versace, V.; Langthaler, P.B.; Sebastianelli, L.; Höller, Y.; Brigo, F.; Orioli, A.; Saltuari, L.; Nardone, R. Impaired Cholinergic Transmission in Patients with Parkinson’s Disease and Olfactory Dysfunction. J. Neurol. Sci. 2017, 377, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Pelosin, E.; Ogliastro, C.; Lagravinese, G.; Bonassi, G.; Mirelman, A.; Hausdorff, J.M.; Abbruzzese, G.; Avanzino, L. Attentional Control of Gait and Falls: Is Cholinergic Dysfunction a Common Substrate in the Elderly and Parkinson’s Disease? Front. Aging Neurosci. 2016, 8, 104. [Google Scholar] [CrossRef] [PubMed]
- Bharti, K.; Suppa, A.; Tommasin, S.; Zampogna, A.; Pietracupa, S.; Berardelli, A.; Pantano, P. Neuroimaging Advances in Parkinson’s Disease with Freezing of Gait: A Systematic Review. NeuroImage Clin. 2019, 24, 102059. [Google Scholar] [CrossRef]
- Picillo, M.; Dubbioso, R.; Iodice, R.; Iavarone, A.; Pisciotta, C.; Spina, E.; Santoro, L.; Barone, P.; Amboni, M.; Manganelli, F. Short-Latency Afferent Inhibition in Patients with Parkinson’s Disease and Freezing of Gait. J. Neural Transm. Vienna Austria 1996 2015, 122, 1533–1540. [Google Scholar] [CrossRef]
- Wang, L.; Ji, M.; Sun, H.; Gan, C.; Zhang, H.; Cao, X.; Yuan, Y.; Zhang, K. Reduced Short-Latency Afferent Inhibition in Parkinson’s Disease Patients with L-Dopa-Unresponsive Freezing of Gait. J. Park. Dis. 2022, 12, 2507–2518. [Google Scholar] [CrossRef]
- Kačar, A.; Filipović, S.R.; Kresojević, N.; Milanović, S.D.; Ljubisavljević, M.; Kostić, V.S.; Rothwell, J.C. History of Exposure to Dopaminergic Medication Does Not Affect Motor Cortex Plasticity and Excitability in Parkinson’s Disease. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2013, 124, 697–707. [Google Scholar] [CrossRef]
- Morgante, F.; Espay, A.J.; Gunraj, C.; Lang, A.E.; Chen, R. Motor Cortex Plasticity in Parkinson’s Disease and Levodopa-Induced Dyskinesias. Brain J. Neurol. 2006, 129, 1059–1069. [Google Scholar] [CrossRef]
- Ueki, Y.; Mima, T.; Kotb, M.A.; Sawada, H.; Saiki, H.; Ikeda, A.; Begum, T.; Reza, F.; Nagamine, T.; Fukuyama, H. Altered Plasticity of the Human Motor Cortex in Parkinson’s Disease. Ann. Neurol. 2006, 59, 60–71. [Google Scholar] [CrossRef]
- Eggers, C.; Fink, G.R.; Nowak, D.A. Theta Burst Stimulation over the Primary Motor Cortex Does Not Induce Cortical Plasticity in Parkinson’s Disease. J. Neurol. 2010, 257, 1669–1674. [Google Scholar] [CrossRef]
- Suppa, A.; Marsili, L.; Belvisi, D.; Conte, A.; Iezzi, E.; Modugno, N.; Fabbrini, G.; Berardelli, A. Lack of LTP-like Plasticity in Primary Motor Cortex in Parkinson’s Disease. Exp. Neurol. 2011, 227, 296–301. [Google Scholar] [CrossRef]
- Zamir, O.; Gunraj, C.; Ni, Z.; Mazzella, F.; Chen, R. Effects of Theta Burst Stimulation on Motor Cortex Excitability in Parkinson’s Disease. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2012, 123, 815–821. [Google Scholar] [CrossRef]
- Kishore, A.; Joseph, T.; Velayudhan, B.; Popa, T.; Meunier, S. Early, Severe and Bilateral Loss of LTP and LTD-like Plasticity in Motor Cortex (M1) in de Novo Parkinson’s Disease. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2012, 123, 822–828. [Google Scholar] [CrossRef] [PubMed]
- Kojovic, M.; Bologna, M.; Kassavetis, P.; Murase, N.; Palomar, F.J.; Berardelli, A.; Rothwell, J.C.; Edwards, M.J.; Bhatia, K.P. Functional Reorganization of Sensorimotor Cortex in Early Parkinson Disease. Neurology 2012, 78, 1441–1448. [Google Scholar] [CrossRef] [PubMed]
- Kishore, A.; James, P.; Krishnan, S.; Yahia-Cherif, L.; Meunier, S.; Popa, T. Motor Cortex Plasticity Can Indicate Vulnerability to Motor Fluctuation and High L-DOPA Need in Drug-Naïve Parkinson’s Disease. Parkinsonism Relat. Disord. 2017, 35, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Belvisi, D.; Fabbrini, A.; De Bartolo, M.I.; Costanzo, M.; Manzo, N.; Fabbrini, G.; Defazio, G.; Conte, A.; Berardelli, A. The Pathophysiological Correlates of Parkinson’s Disease Clinical Subtypes. Mov. Disord. Off. J. Mov. Disord. Soc. 2021, 36, 370–379. [Google Scholar] [CrossRef]
- Latorre, A.; Rocchi, L.; Berardelli, A.; Bhatia, K.P.; Rothwell, J.C. The Use of Transcranial Magnetic Stimulation as a Treatment for Movement Disorders: A Critical Review. Mov. Disord. Off. J. Mov. Disord. Soc. 2019, 34, 769–782. [Google Scholar] [CrossRef]
- Strafella, A.P.; Paus, T.; Fraraccio, M.; Dagher, A. Striatal Dopamine Release Induced by Repetitive Transcranial Magnetic Stimulation of the Human Motor Cortex. Brain J. Neurol. 2003, 126, 2609–2615. [Google Scholar] [CrossRef]
- Kim, J.Y.; Chung, E.J.; Lee, W.Y.; Shin, H.Y.; Lee, G.H.; Choe, Y.-S.; Choi, Y.; Kim, B.J. Therapeutic Effect of Repetitive Transcranial Magnetic Stimulation in Parkinson’s Disease: Analysis of [11C] Raclopride PET Study. Mov. Disord. Off. J. Mov. Disord. Soc. 2008, 23, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Filipović, S.R.; Rothwell, J.C.; van de Warrenburg, B.P.; Bhatia, K. Repetitive Transcranial Magnetic Stimulation for Levodopa-Induced Dyskinesias in Parkinson’s Disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2009, 24, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.H.; Kim, M.S.; Park, E.; Cho, J.W.; Youn, J.; Kim, Y.K.; Kim, Y.-H. Effect of Dual-Mode and Dual-Site Noninvasive Brain Stimulation on Freezing of Gait in Patients With Parkinson Disease. Arch. Phys. Med. Rehabil. 2017, 98, 1283–1290. [Google Scholar] [CrossRef]
- González-García, N.; Armony, J.L.; Soto, J.; Trejo, D.; Alegría, M.A.; Drucker-Colín, R. Effects of rTMS on Parkinson’s Disease: A Longitudinal fMRI Study. J. Neurol. 2011, 258, 1268–1280. [Google Scholar] [CrossRef] [PubMed]
- Maruo, T.; Hosomi, K.; Shimokawa, T.; Kishima, H.; Oshino, S.; Morris, S.; Kageyama, Y.; Yokoe, M.; Yoshimine, T.; Saitoh, Y. High-Frequency Repetitive Transcranial Magnetic Stimulation over the Primary Foot Motor Area in Parkinson’s Disease. Brain Stimulat. 2013, 6, 884–891. [Google Scholar] [CrossRef]
- Potvin-Desrochers, A.; Paquette, C. Potential Non-Invasive Brain Stimulation Targets to Alleviate Freezing of Gait in Parkinson’s Disease. Neuroscience 2021, 468, 366–376. [Google Scholar] [CrossRef]
- Rektorova, I.; Sedlackova, S.; Telecka, S.; Hlubocky, A.; Rektor, I. Repetitive Transcranial Stimulation for Freezing of Gait in Parkinson’s Disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2007, 22, 1518–1519. [Google Scholar] [CrossRef]
- Rossi, S.; Ferro, M.; Cincotta, M.; Ulivelli, M.; Bartalini, S.; Miniussi, C.; Giovannelli, F.; Passero, S. A Real Electro-Magnetic Placebo (REMP) Device for Sham Transcranial Magnetic Stimulation (TMS). Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2007, 118, 709–716. [Google Scholar] [CrossRef]
- Brys, M.; Fox, M.D.; Agarwal, S.; Biagioni, M.; Dacpano, G.; Kumar, P.; Pirraglia, E.; Chen, R.; Wu, A.; Fernandez, H.; et al. Multifocal Repetitive TMS for Motor and Mood Symptoms of Parkinson Disease: A Randomized Trial. Neurology 2016, 87, 1907–1915. [Google Scholar] [CrossRef]
- Makkos, A.; Pál, E.; Aschermann, Z.; Janszky, J.; Balázs, É.; Takács, K.; Karádi, K.; Komoly, S.; Kovács, N. High-Frequency Repetitive Transcranial Magnetic Stimulation Can Improve Depression in Parkinson’s Disease: A Randomized, Double-Blind, Placebo-Controlled Study. Neuropsychobiology 2016, 73, 169–177. [Google Scholar] [CrossRef]
- Chou, Y.; Hickey, P.T.; Sundman, M.; Song, A.W.; Chen, N. Effects of Repetitive Transcranial Magnetic Stimulation on Motor Symptoms in Parkinson Disease: A Systematic Review and Meta-Analysis. JAMA Neurol. 2015, 72, 432–440. [Google Scholar] [CrossRef]
- Li, S.; Jiao, R.; Zhou, X.; Chen, S. Motor Recovery and Antidepressant Effects of Repetitive Transcranial Magnetic Stimulation on Parkinson Disease. Medicine (Baltimore) 2020, 99, e19642. [Google Scholar] [CrossRef]
- Zanjani, A.; Zakzanis, K.K.; Daskalakis, Z.J.; Chen, R. Repetitive Transcranial Magnetic Stimulation of the Primary Motor Cortex in the Treatment of Motor Signs in Parkinson’s Disease: A Quantitative Review of the Literature. Mov. Disord. Off. J. Mov. Disord. Soc. 2015, 30, 750–758. [Google Scholar] [CrossRef]
- Deng, S.; Dong, Z.; Pan, L.; Liu, Y.; Ye, Z.; Qin, L.; Liu, Q.; Qin, C. Effects of Repetitive Transcranial Magnetic Stimulation on Gait Disorders and Cognitive Dysfunction in Parkinson’s Disease: A Systematic Review with Meta-Analysis. Brain Behav. 2022, 12, e2697. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.L.-H.; Mak, M.K.-Y.; Hallett, M. Transcranial Magnetic Stimulation Promotes Gait Training in Parkinson Disease. Ann. Neurol. 2020, 88, 933–945. [Google Scholar] [CrossRef]
- Hamada, M.; Ugawa, Y.; Tsuji, S. Effectiveness of rTMS on Parkinson’s Disease Study Group, Japan High-Frequency rTMS over the Supplementary Motor Area for Treatment of Parkinson’s Disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2008, 23, 1524–1531. [Google Scholar] [CrossRef] [PubMed]
- Shirota, Y.; Ohtsu, H.; Hamada, M.; Enomoto, H.; Ugawa, Y. Research Committee on rTMS Treatment of Parkinson’s Disease Supplementary Motor Area Stimulation for Parkinson Disease: A Randomized Controlled Study. Neurology 2013, 80, 1400–1405. [Google Scholar] [CrossRef]
- Sayın, S.; Cakmur, R.; Yener, G.G.; Yaka, E.; Uğurel, B.; Uzunel, F. Low-Frequency Repetitive Transcranial Magnetic Stimulation for Dyskinesia and Motor Performance in Parkinson’s Disease. J. Clin. Neurosci. Off. J. Neurosurg. Soc. Australas. 2014, 21, 1373–1376. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Paeng, S.H.; Kang, S.Y. Stimulation in Supplementary Motor Area Versus Motor Cortex for Freezing of Gait in Parkinson’s Disease. J. Clin. Neurol. Seoul Korea 2018, 14, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Bhat, P.; Kumaran, S.S.; Goyal, V.; Srivastava, A.K.; Behari, M. Effect of rTMS at SMA on Task-Based Connectivity in PD. Behav. Brain Res. 2023, 452, 114602. [Google Scholar] [CrossRef]
- Chi, S.; Wen, X.; Yu, Y.; Wang, G.; Zhang, J.; Xue, C.; Zhang, X.; Wang, Z.; Gesang, M.; Chen, J.; et al. Sensorimotor Network Connectivity Correlates with Motor Improvement after Repetitive Transcranial Magnetic Stimulation in Patients with Parkinson’s Disease. Parkinsonism Relat. Disord. 2023, 106, 105218. [Google Scholar] [CrossRef]
- Lefaivre, S.C.; Brown, M.J.N.; Almeida, Q.J. Cerebellar Involvement in Parkinson’s Disease Resting Tremor. Cerebellum Ataxias 2016, 3, 13. [Google Scholar] [CrossRef]
- Minks, E.; Mareček, R.; Pavlík, T.; Ovesná, P.; Bareš, M. Is the Cerebellum a Potential Target for Stimulation in Parkinson’s Disease? Results of 1-Hz rTMS on Upper Limb Motor Tasks. Cerebellum Lond. Engl. 2011, 10, 804–811. [Google Scholar] [CrossRef]
- Fregni, F.; Santos, C.M.; Myczkowski, M.L.; Rigolino, R.; Gallucci-Neto, J.; Barbosa, E.R.; Valente, K.D.; Pascual-Leone, A.; Marcolin, M.A. Repetitive Transcranial Magnetic Stimulation Is as Effective as Fluoxetine in the Treatment of Depression in Patients with Parkinson’s Disease. J. Neurol. Neurosurg. Psychiatry 2004, 75, 1171–1174. [Google Scholar] [CrossRef]
- Pal, E.; Nagy, F.; Aschermann, Z.; Balazs, E.; Kovacs, N. The Impact of Left Prefrontal Repetitive Transcranial Magnetic Stimulation on Depression in Parkinson’s Disease: A Randomized, Double-Blind, Placebo-Controlled Study. Mov. Disord. Off. J. Mov. Disord. Soc. 2010, 25, 2311–2317. [Google Scholar] [CrossRef] [PubMed]
- Hai-Jiao, W.; Ge, T.; Li-Na, Z.; Deng, C.; Da, X.; Shan-Shan, C.; Liu, L. The Efficacy of Repetitive Transcranial Magnetic Stimulation for Parkinson Disease Patients with Depression. Int. J. Neurosci. 2020, 130, 19–27. [Google Scholar] [CrossRef]
- Randver, R. Repetitive Transcranial Magnetic Stimulation of the Dorsolateral Prefrontal Cortex to Alleviate Depression and Cognitive Impairment Associated with Parkinson’s Disease: A Review and Clinical Implications. J. Neurol. Sci. 2018, 393, 88–99. [Google Scholar] [CrossRef]
- Zheng, H.-B.; Liu, B.; Shen, J.; Xie, F.; Ji, Q.-M.; Zhu, X.-Y. Non-Invasive Brain Stimulation for Treating Psychiatric Symptoms in Parkinson’s Disease: A Systematic Review and Meta-Analysis. J. Clin. Neurosci. Off. J. Neurosurg. Soc. Australas. 2022, 106, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Yokoe, M.; Mano, T.; Maruo, T.; Hosomi, K.; Shimokawa, T.; Kishima, H.; Oshino, S.; Morris, S.; Kageyama, Y.; Goto, Y.; et al. The Optimal Stimulation Site for High-Frequency Repetitive Transcranial Magnetic Stimulation in Parkinson’s Disease: A Double-Blind Crossover Pilot Study. J. Clin. Neurosci. Off. J. Neurosurg. Soc. Australas. 2018, 47, 72–78. [Google Scholar] [CrossRef]
- Cacace, F.; Mineo, D.; Viscomi, M.T.; Latagliata, E.C.; Mancini, M.; Sasso, V.; Vannelli, A.; Pascucci, T.; Pendolino, V.; Marcello, E.; et al. Intermittent Theta-Burst Stimulation Rescues Dopamine-Dependent Corticostriatal Synaptic Plasticity and Motor Behavior in Experimental Parkinsonism: Possible Role of Glial Activity. Mov. Disord. Off. J. Mov. Disord. Soc. 2017, 32, 1035–1046. [Google Scholar] [CrossRef]
- Zeljkovic Jovanovic, M.; Stanojevic, J.; Stevanovic, I.; Ninkovic, M.; Nedeljkovic, N.; Dragic, M. Sustained Systemic Antioxidative Effects of Intermittent Theta Burst Stimulation beyond Neurodegeneration: Implications in Therapy in 6-Hydroxydopamine Model of Parkinson’s Disease. Antioxid. Basel Switz. 2024, 13, 218. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, M.Z.; Stanojevic, J.; Stevanovic, I.; Ninkovic, M.; Ilic, T.V.; Nedeljkovic, N.; Dragic, M. Prolonged Intermittent Theta Burst Stimulation Restores the Balance between A2AR- and A1R-Mediated Adenosine Signaling in the 6-Hydroxidopamine Model of Parkinson’s Disease. Neural Regen. Res. 2025, 20, 2053–2067. [Google Scholar] [CrossRef]
- Jin, Z.-H.; Wang, Y.-X.; Meng, D.-T.; Qin, Y.; Duan, Y.-N.; Fang, J.-P.; Wang, R.-D.; Liu, Y.-J.; Liu, C.; Wang, P.; et al. Intermittent Theta-Burst Stimulation Combined with Physical Therapy as an Optimal Rehabilitation in Parkinson’s Disease: Study Protocol for a Randomised, Double-Blind, Controlled Trial. Trials 2023, 24, 410. [Google Scholar] [CrossRef] [PubMed]
- Rashid-López, R.; Macías-García, P.; Sánchez-Fernández, F.L.; Cano-Cano, F.; Sarrias-Arrabal, E.; Sanmartino, F.; Méndez-Bértolo, C.; Lozano-Soto, E.; Gutiérrez-Cortés, R.; González-Moraleda, Á.; et al. Neuroimaging and Serum Biomarkers of Neurodegeneration and Neuroplasticity in Parkinson’s Disease Patients Treated by Intermittent Theta-Burst Stimulation over the Bilateral Primary Motor Area: A Randomized, Double-Blind, Sham-Controlled, Crossover Trial Study. Front. Aging Neurosci. 2023, 15, 1258315. [Google Scholar] [CrossRef]
- Cheng, B.; Zhu, T.; Zhao, W.; Sun, L.; Shen, Y.; Xiao, W.; Zhang, S. Effect of Theta Burst Stimulation-Patterned rTMS on Motor and Nonmotor Dysfunction of Parkinson’s Disease: A Systematic Review and Metaanalysis. Front. Neurol. 2021, 12, 762100. [Google Scholar] [CrossRef]
- Chung, S.W.; Hill, A.T.; Rogasch, N.C.; Hoy, K.E.; Fitzgerald, P.B. Use of Theta-Burst Stimulation in Changing Excitability of Motor Cortex: A Systematic Review and Meta-Analysis. Neurosci. Biobehav. Rev. 2016, 63, 43–64. [Google Scholar] [CrossRef]
- Vanbellingen, T.; Wapp, M.; Stegmayer, K.; Bertschi, M.; Abela, E.; Kübel, S.; Nyffeler, T.; Müri, R.; Walther, S.; Nef, T.; et al. Theta Burst Stimulation over Premotor Cortex in Parkinson’s Disease: An Explorative Study on Manual Dexterity. J. Neural Transm. Vienna Austria 1996 2016, 123, 1387–1393. [Google Scholar] [CrossRef]
- Brugger, F.; Wegener, R.; Baty, F.; Walch, J.; Krüger, M.T.; Hägele-Link, S.; Bohlhalter, S.; Kägi, G. Facilitatory rTMS over the Supplementary Motor Cortex Impedes Gait Performance in Parkinson Patients with Freezing of Gait. Brain Sci. 2021, 11, 321. [Google Scholar] [CrossRef]
- Benninger, D.H.; Berman, B.D.; Houdayer, E.; Pal, N.; Luckenbaugh, D.A.; Schneider, L.; Miranda, S.; Hallett, M. Intermittent Theta-Burst Transcranial Magnetic Stimulation for Treatment of Parkinson Disease. Neurology 2011, 76, 601–609. [Google Scholar] [CrossRef]
- Degardin, A.; Devos, D.; Defebvre, L.; Destée, A.; Plomhause, L.; Derambure, P.; Devanne, H. Effect of Intermittent Theta-Burst Stimulation on Akinesia and Sensorimotor Integration in Patients with Parkinson’s Disease. Eur. J. Neurosci. 2012, 36, 2669–2678. [Google Scholar] [CrossRef]
- Eggers, C.; Günther, M.; Rothwell, J.; Timmermann, L.; Ruge, D. Theta Burst Stimulation over the Supplementary Motor Area in Parkinson’s Disease. J. Neurol. 2015, 262, 357–364. [Google Scholar] [CrossRef]
- Ji, G.-J.; Liu, T.; Li, Y.; Liu, P.; Sun, J.; Chen, X.; Tian, Y.; Chen, X.; Dahmani, L.; Liu, H.; et al. Structural Correlates Underlying Accelerated Magnetic Stimulation in Parkinson’s Disease. Hum. Brain Mapp. 2021, 42, 1670–1681. [Google Scholar] [CrossRef]
- Rashid-López, R.; Macías-García, P.; Cruz-Gómez, Á.J.; Sánchez-Fernández, F.L.; Cano-Cano, F.; Sanmartino, F.; Sarrias-Arrabal, E.; Lozano-Soto, E.; Méndez-Bértolo, C.; López-Sosa, F.; et al. Bilateral Primary Motor Area Intermittent Theta-Burst Stimulation May Alleviate Gait and Postural Disturbances in Parkinson’s Disease Patients by Astrocytic Modulation, Caudate Volume Changes, and Increased Functional Neuroplasticity. Parkinsonism Relat. Disord. 2024, 123, 106074. [Google Scholar] [CrossRef]
- Pastore-Wapp, M.; Kaufmann, B.C.; Nyffeler, T.; Wapp, S.; Bohlhalter, S.; Vanbellingen, T. Feasibility of a Combined Intermittent Theta-Burst Stimulation and Video Game-Based Dexterity Training in Parkinson’s Disease. J. Neuroengineering Rehabil. 2023, 20, 2. [Google Scholar] [CrossRef]
- Li, P.; Luo, N.; Sun, S.; Li, Y.; Shen, D.; Zhu, X.; Zhou, L.; Zhou, H.; Liu, J. Neuroprotective Effects of Intermittent Theta Burst Stimulation in Parkinson’s Disease (NET-PD): A Study Protocol for a Delayed-Start Randomized Double-Blind Sham-Controlled Trial. J. Clin. Med. 2022, 11, 4972. [Google Scholar] [CrossRef] [PubMed]
- Koch, G.; Brusa, L.; Carrillo, F.; Lo Gerfo, E.; Torriero, S.; Oliveri, M.; Mir, P.; Caltagirone, C.; Stanzione, P. Cerebellar Magnetic Stimulation Decreases Levodopa-Induced Dyskinesias in Parkinson Disease. Neurology 2009, 73, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Sanna, A.; Follesa, P.; Puligheddu, M.; Cannas, A.; Serra, M.; Pisu, M.G.; Dagostino, S.; Solla, P.; Tacconi, P.; Marrosu, F. Cerebellar Continuous Theta Burst Stimulation Reduces Levodopa-Induced Dyskinesias and Decreases Serum BDNF Levels. Neurosci. Lett. 2020, 716, 134653. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, H.; Terao, Y.; Kadowaki, S.; Nakamura, K.; Moriya, A.; Nakatani-Enomoto, S.; Kobayashi, S.; Yoshihara, A.; Hanajima, R.; Ugawa, Y. Effects of L-Dopa and Pramipexole on Plasticity Induced by QPS in Human Motor Cortex. J. Neural Transm. Vienna Austria 1996 2015, 122, 1253–1261. [Google Scholar] [CrossRef]
- Enomoto, H.; Ugawa, Y. Abnormal plasticity and drug effect in Parkinson’s disease. Rinsho Shinkeigaku 2012, 52, 1204–1206. [Google Scholar] [CrossRef]
- Koch, G.; Brusa, L.; Caltagirone, C.; Peppe, A.; Oliveri, M.; Stanzione, P.; Centonze, D. rTMS of Supplementary Motor Area Modulates Therapy-Induced Dyskinesias in Parkinson Disease. Neurology 2005, 65, 623–625. [Google Scholar] [CrossRef]
- Bagnato, S.; Agostino, R.; Modugno, N.; Quartarone, A.; Berardelli, A. Plasticity of the Motor Cortex in Parkinson’s Disease Patients on and off Therapy. Mov. Disord. Off. J. Mov. Disord. Soc. 2006, 21, 639–645. [Google Scholar] [CrossRef]
- Kishore, A.; Popa, T.; Balachandran, A.; Chandran, S.; Pradeep, S.; Backer, F.; Krishnan, S.; Meunier, S. Cerebellar Sensory Processing Alterations Impact Motor Cortical Plasticity in Parkinson’s Disease: Clues from Dyskinetic Patients. Cereb. Cortex N. Y. N 1991 2014, 24, 2055–2067. [Google Scholar] [CrossRef]
- Kishore, A.; Popa, T.; James, P.; Yahia-Cherif, L.; Backer, F.; Varughese Chacko, L.; Govind, P.; Pradeep, S.; Meunier, S. Age-Related Decline in the Responsiveness of Motor Cortex to Plastic Forces Reverses with Levodopa or Cerebellar Stimulation. Neurobiol. Aging 2014, 35, 2541–2551. [Google Scholar] [CrossRef]
- Enomoto, H.; Terao, Y.; Kadowaki, S.; Nakamura, K.; Moriya, A.; Nakatani-Enomoto, S.; Kobayashi, S.; Yoshihara, A.; Hanajima, R.; Ugawa, Y. Effects of L-Dopa and Pramipexole on Plasticity Induced by QPS in Human Motor Cortex. J. Neural Transm. Vienna Austria 1996 2015, 122, 1253–1261. [Google Scholar] [CrossRef]
- Suppa, A.; Bologna, M.; Conte, A.; Berardelli, A.; Fabbrini, G. The Effect of L-Dopa in Parkinson’s Disease as Revealed by Neurophysiological Studies of Motor and Sensory Functions. Expert Rev. Neurother. 2017, 17, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Moriyasu, S.; Shimizu, T.; Honda, M.; Ugawa, Y.; Hanajima, R. Motor Cortical Plasticity and Its Correlation with Motor Symptoms in Parkinson’s Disease. eNeurologicalSci 2022, 29, 100422. [Google Scholar] [CrossRef] [PubMed]
- Kolmančič, K.; Zupančič, N.K.; Trošt, M.; Flisar, D.; Kramberger, M.G.; Pirtošek, Z.; Kojović, M. Continuous Dopaminergic Stimulation Improves Cortical Maladaptive Changes in Advanced Parkinson’s Disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2022, 37, 1465–1473. [Google Scholar] [CrossRef]
- Antelmi, E.; Lanza, G.; Mogavero, M.P.; Mingolla, G.P.; Plazzi, G.; Ferini-Strambi, L.; Ferri, R.; Tinazzi, M. Intersection of Sleep Disorders and Parkinson Disease: Unveiling the Bidirectional Relationship. Mov. Disord. Clin. Pract. 2025, 12, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Donzuso, G.; Cicero, C.E.; Giuliano, L.; Squillaci, R.; Luca, A.; Palmucci, S.; Basile, A.; Lanza, G.; Ferri, R.; Zappia, M.; et al. Neuroanatomical Findings in Isolated REM Sleep Behavior Disorder and Early Parkinson’s Disease: A Voxel-Based Morphometry Study. Brain Imaging Behav. 2024, 18, 83–91. [Google Scholar] [CrossRef]
- Joza, S.; Hu, M.T.; Jung, K.-Y.; Kunz, D.; Stefani, A.; Dušek, P.; Terzaghi, M.; Arnaldi, D.; Videnovic, A.; Schiess, M.C.; et al. Progression of Clinical Markers in Prodromal Parkinson’s Disease and Dementia with Lewy Bodies: A Multicentre Study. Brain J. Neurol. 2023, 146, 3258–3272. [Google Scholar] [CrossRef]
- Figorilli, M.; Meloni, M.; Lanza, G.; Casaglia, E.; Lecca, R.; Saibene, F.L.; Congiu, P.; Puligheddu, M. Considering REM Sleep Behavior Disorder in the Management of Parkinson’s Disease. Nat. Sci. Sleep 2023, 15, 333–352. [Google Scholar] [CrossRef]
- Figorilli, M.; Meloni, F.; Lecca, R.; Tamburrino, L.; Mascia, M.G.; Cocco, V.; Meloni, M.; Marques, A.R.; Vidal, T.; Congiu, P.; et al. Severity of REM Sleep without Atonia Correlates with Measures of Cognitive Impairment and Depressive Symptoms in REM Sleep Behaviour Disorder. J. Sleep Res. 2023, 32, e13880. [Google Scholar] [CrossRef] [PubMed]
- Mogavero, M.P.; Ferri, R.; Marelli, S.; Lanza, G.; Terzaghi, M.; Castelnuovo, A.; DelRosso, L.M.; Schenck, C.H.; Ferini-Strambi, L. Polysomnographic Features Associated with Clonazepam and Melatonin Treatment in Isolated REM Sleep Behavior Disorder: Time for New Therapeutic Approaches? CNS Neurosci. Ther. 2024, 30, e14569. [Google Scholar] [CrossRef] [PubMed]
- Figorilli, M.; Lanza, G.; Congiu, P.; Lecca, R.; Casaglia, E.; Mogavero, M.P.; Puligheddu, M.; Ferri, R. Neurophysiological Aspects of REM Sleep Behavior Disorder (RBD): A Narrative Review. Brain Sci. 2021, 11, 1588. [Google Scholar] [CrossRef] [PubMed]
- Nardone, R.; Bergmann, J.; Kunz, A.; Christova, M.; Brigo, F.; Tezzon, F.; Trinka, E.; Golaszewski, S. Cortical Afferent Inhibition Is Reduced in Patients with Idiopathic REM Sleep Behavior Disorder and Cognitive Impairment: A TMS Study. Sleep Med. 2012, 13, 919–925. [Google Scholar] [CrossRef]
- Lanza, G.; Aricò, D.; Lanuzza, B.; Cosentino, F.I.I.; Tripodi, M.; Giardina, F.; Bella, R.; Puligheddu, M.; Pennisi, G.; Ferri, R.; et al. Facilitatory/Inhibitory Intracortical Imbalance in REM Sleep Behavior Disorder: Early Electrophysiological Marker of Neurodegeneration? Sleep 2020, 43, zsz242. [Google Scholar] [CrossRef]
- Peever, J.; Luppi, P.-H.; Montplaisir, J. Breakdown in REM Sleep Circuitry Underlies REM Sleep Behavior Disorder. Trends Neurosci. 2014, 37, 279–288. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Kamble, N.; Yadav, R.; Stezin, A.; Pal, P.K. Abnormal Intracortical Functions in Parkinson’s Disease with Rapid Eye Movement Sleep Behaviour Disorder. Can. J. Neurol. Sci. J. Can. Sci. Neurol. 2022, 49, 672–677. [Google Scholar] [CrossRef]
- Khedr, E.M.; Ahmed, G.K.; Korayem, M.A.; Elamary, S.A.S.H.; El-Kholy, M.M.; Haridy, N.A. Short-Term Therapeutic Effect of Repetitive Transcranial Magnetic Stimulations of Sleep Disorders in Parkinson’s Disease: A Randomized Clinical Trial (Pilot Study). Brain Sci. 2024, 14, 556. [Google Scholar] [CrossRef]
- Arias-Carrion, O.; Ortega-Robles, E.; Ortuno-Sahagun, D.; Ramirez-Bermudez, J.; Hamid, A.; Shalash, A. Sleep-Related Disorders in Parkinson’s Disease: Mechanisms, Diagnosis, and Therapeutic Approaches. CNS Neurol. Disord. Drug Targets 2025, 24, 132–143. [Google Scholar] [CrossRef]
- van Dijk, K.D.; Møst, E.I.S.; Van Someren, E.J.W.; Berendse, H.W.; van der Werf, Y.D. Beneficial Effect of Transcranial Magnetic Stimulation on Sleep in Parkinson’s Disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2009, 24, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Lanza, G.; Fisicaro, F.; Cantone, M.; Pennisi, M.; Cosentino, F.I.I.; Lanuzza, B.; Tripodi, M.; Bella, R.; Paulus, W.; Ferri, R. Repetitive Transcranial Magnetic Stimulation in Primary Sleep Disorders. Sleep Med. Rev. 2023, 67, 101735. [Google Scholar] [CrossRef]
- Jellinger, K.A. Pathobiology of Cognitive Impairment in Parkinson Disease: Challenges and Outlooks. Int. J. Mol. Sci. 2023, 25, 498. [Google Scholar] [CrossRef]
- Nardone, R.; Florio, I.; Lochner, P.; Tezzon, F. Cholinergic Cortical Circuits in Parkinson’s Disease and in Progressive Supranuclear Palsy: A Transcranial Magnetic Stimulation Study. Exp. Brain Res. Exp. Hirnforsch. Expérimentation Cérébrale 2005, 163, 128–131. [Google Scholar] [CrossRef] [PubMed]
- Nardone, R.; Bergmann, J.; Brigo, F.; Christova, M.; Kunz, A.; Seidl, M.; Tezzon, F.; Trinka, E.; Golaszewski, S. Functional Evaluation of Central Cholinergic Circuits in Patients with Parkinson’s Disease and REM Sleep Behavior Disorder: A TMS Study. J. Neural Transm. Vienna Austria 1996 2013, 120, 413–422. [Google Scholar] [CrossRef]
- Khedr, E.M.; Mohamed, K.O.; Ali, A.M.; Hasan, A.M. The Effect of Repetitive Transcranial Magnetic Stimulation on Cognitive Impairment in Parkinson’s Disease with Dementia: Pilot Study. Restor. Neurol. Neurosci. 2020, 38, 55–66. [Google Scholar] [CrossRef]
- Trung, J.; Hanganu, A.; Jobert, S.; Degroot, C.; Mejia-Constain, B.; Kibreab, M.; Bruneau, M.-A.; Lafontaine, A.-L.; Strafella, A.; Monchi, O. Transcranial Magnetic Stimulation Improves Cognition over Time in Parkinson’s Disease. Parkinsonism Relat. Disord. 2019, 66, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Buard, I.; Sciacca, D.M.; Martin, C.S.; Rogers, S.; Sillau, S.H.; Greher, M.R.; Chen, R.; Kluger, B.M. Transcranial Magnetic Stimulation Does Not Improve Mild Cognitive Impairment in Parkinson’s Disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2018, 33, 489–491. [Google Scholar] [CrossRef]
- Nagao, S.; Yokota, O.; Nanba, R.; Takata, H.; Haraguchi, T.; Ishizu, H.; Ikeda, C.; Takeda, N.; Oshima, E.; Sakane, K.; et al. Progressive Supranuclear Palsy Presenting as Primary Lateral Sclerosis but Lacking Parkinsonism, Gaze Palsy, Aphasia, or Dementia. J. Neurol. Sci. 2012, 323, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Kühn, A.A.; Grosse, P.; Holtz, K.; Brown, P.; Meyer, B.-U.; Kupsch, A. Patterns of Abnormal Motor Cortex Excitability in Atypical Parkinsonian Syndromes. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2004, 115, 1786–1795. [Google Scholar] [CrossRef]
- Wittstock, M.; Pohley, I.; Walter, U.; Grossmann, A.; Benecke, R.; Wolters, A. Interhemispheric Inhibition in Different Phenotypes of Progressive Supranuclear Palsy. J. Neural Transm. Vienna Austria 1996 2013, 120, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Wolters, A.; Classen, J.; Kunesch, E.; Grossmann, A.; Benecke, R. Measurements of Transcallosally Mediated Cortical Inhibition for Differentiating Parkinsonian Syndromes. Mov. Disord. Off. J. Mov. Disord. Soc. 2004, 19, 518–528. [Google Scholar] [CrossRef]
- Brusa, L.; Ponzo, V.; Mastropasqua, C.; Picazio, S.; Bonnì, S.; Di Lorenzo, F.; Iani, C.; Stefani, A.; Stanzione, P.; Caltagirone, C.; et al. Theta Burst Stimulation Modulates Cerebellar-Cortical Connectivity in Patients with Progressive Supranuclear Palsy. Brain Stimulat. 2014, 7, 29–35. [Google Scholar] [CrossRef]
- Conte, A.; Belvisi, D.; Bologna, M.; Ottaviani, D.; Fabbrini, G.; Colosimo, C.; Williams, D.R.; Berardelli, A. Abnormal Cortical Synaptic Plasticity in Primary Motor Area in Progressive Supranuclear Palsy. Cereb. Cortex N. Y. N 1991 2012, 22, 693–700. [Google Scholar] [CrossRef]
- Shirota, Y; M, H.; R, H.; Y, T.; H, M.; S, O.; S, T.; Y, U. Cerebellar Dysfunction in Progressive Supranuclear Palsy: A Transcranial Magnetic Stimulation Study. Mov. Disord. Off. J. Mov. Disord. Soc. 2010, 25. [Google Scholar] [CrossRef]
- Dale, M.L.; DeVries, W.H.; Mancini, M.; George, M.S. Cerebellar rTMS for Motor Control in Progressive Supranuclear Palsy. Brain Stimulat. 2019, 12, 1588–1591. [Google Scholar] [CrossRef] [PubMed]
- Fichera, M.; Volonté, M.A.; Comi, G.; Leocani, L. Repetitive Transcranial Magnetic Stimulation with H Coil in Progressive Supranuclear Palsy: A Double-Blind, Placebo-Controlled, Crossover Study (P3.8-001). Neurology 2019, 92, P3.8–001. [Google Scholar] [CrossRef]
- Papp, M.I.; Lantos, P.L. The Distribution of Oligodendroglial Inclusions in Multiple System Atrophy and Its Relevance to Clinical Symptomatology. Brain J. Neurol. 1994, 117 Pt 2, 235–243. [Google Scholar] [CrossRef]
- Abbruzzese, G.; Marchese, R.; Trompetto, C. Sensory and Motor Evoked Potentials in Multiple System Atrophy: A Comparative Study with Parkinson’s Disease. Mov. Disord. Off. J. Mov. Disord. Soc. 1997, 12, 315–321. [Google Scholar] [CrossRef]
- Tabaraud, F.; Hugon, J.; Salle, J.Y.; Boulesteix, J.M.; Rigaud, M.; Vallat, J.M.; Dumas, M. Central Motor Conduction and Glutamate Deshydrogenase: Activity in Olivo-Ponto-Cerebellar Atrophy. Neurophysiol. Clin. Clin. Neurophysiol. 1989, 19, 433–441. [Google Scholar] [CrossRef]
- Abele, M.; Schulz, J.B.; Bürk, K.; Topka, H.; Dichgans, J.; Klockgether, T. Evoked Potentials in Multiple System Atrophy (MSA). Acta Neurol. Scand. 2000, 101, 111–115. [Google Scholar] [CrossRef]
- Eusebio, A.; Azulay, J.-P.; Witjas, T.; Rico, A.; Attarian, S. Assessment of Cortico-Spinal Tract Impairment in Multiple System Atrophy Using Transcranial Magnetic Stimulation. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2007, 118, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Marchese, R.; Trompetto, C.; Buccolieri, A.; Abbruzzese, G. Abnormalities of Motor Cortical Excitability Are Not Correlated with Clinical Features in Atypical Parkinsonism. Mov. Disord. Off. J. Mov. Disord. Soc. 2000, 15, 1210–1214. [Google Scholar] [CrossRef]
- Suppa, A.; Marsili, L.; Di Stasio, F.; Latorre, A.; Parvez, A.K.; Colosimo, C.; Berardelli, A. Primary Motor Cortex Long-Term Plasticity in Multiple System Atrophy. Mov. Disord. Off. J. Mov. Disord. Soc. 2014, 29, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Mascia, M.M.; Valls-Solé, J.; Martí, M.J.; Salazar, G. Sensorimotor Integration in Patients with Parkinsonian Type Multisystem Atrophy. J. Neurol. 2005, 252, 473–481. [Google Scholar] [CrossRef]
- Löscher, W.N.; Stampfer-Kountchev, M.; Sawires, M.; Seppi, K.; Mueller, J.; Szubski, C.; Hirnsperger, K.; Brenneis, C.; Poewe, W.; Wenning, G.K. Abnormal Responses to Repetitive Transcranial Magnetic Stimulation in Multiple System Atrophy. Mov. Disord. Off. J. Mov. Disord. Soc. 2007, 22, 174–178. [Google Scholar] [CrossRef]
- Pan, J.; Mi, T.-M.; Ma, J.-H.; Sun, H.; Chan, P. High-Frequency Repetitive Transcranial Magnetic Stimulation Over the Left Dorsolateral Prefrontal Cortex Shortly Alleviates Fatigue in Patients With Multiple System Atrophy: A Randomized Controlled Trial. Front. Neurol. 2021, 12, 755352. [Google Scholar] [CrossRef]
- Lee, S.E.; Rabinovici, G.D.; Mayo, M.C.; Wilson, S.M.; Seeley, W.W.; DeArmond, S.J.; Huang, E.J.; Trojanowski, J.Q.; Growdon, M.E.; Jang, J.Y.; et al. Clinicopathological Correlations in Corticobasal Degeneration. Ann. Neurol. 2011, 70, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Rankin, K.P.; Mayo, M.C.; Seeley, W.W.; Lee, S.; Rabinovici, G.; Gorno-Tempini, M.L.; Boxer, A.L.; Weiner, M.W.; Trojanowski, J.Q.; DeArmond, S.J.; et al. Behavioral Variant Frontotemporal Dementia with Corticobasal Degeneration Pathology: Phenotypic Comparison to bvFTD with Pick’s Disease. J. Mol. Neurosci. MN 2011, 45, 594–608. [Google Scholar] [CrossRef]
- Fujimoto, K.; Sayama, S.; Shizuma, N.; Kano, M.; Nakano, I. Intracranial inhibitory mechanisms in clinically diagnosed corticobasal degeneration: a study of a silent period followed by transcranial magnetic stimulation. Rinsho Shinkeigaku 2000, 40, 701–706. [Google Scholar]
- Leiguarda, R.C.; Merello, M.; Nouzeilles, M.I.; Balej, J.; Rivero, A.; Nogués, M. Limb-Kinetic Apraxia in Corticobasal Degeneration: Clinical and Kinematic Features. Mov. Disord. Off. J. Mov. Disord. Soc. 2003, 18, 49–59. [Google Scholar] [CrossRef]
- Lu, C.S.; Ikeda, A.; Terada, K.; Mima, T.; Nagamine, T.; Fukuyama, H.; Kohara, N.; Kojima, Y.; Yonekura, Y.; Chen, R.S.; et al. Electrophysiological Studies of Early Stage Corticobasal Degeneration. Mov. Disord. Off. J. Mov. Disord. Soc. 1998, 13, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Alberici, A.; Bonato, C.; Calabria, M.; Agosti, C.; Zanetti, O.; Miniussi, C.; Padovani, A.; Rossini, P.M.; Borroni, B. The Contribution of TMS to Frontotemporal Dementia Variants. Acta Neurol. Scand. 2008, 118, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Trompetto, C.; Buccolieri, A.; Marchese, R.; Marinelli, L.; Michelozzi, G.; Abbruzzese, G. Impairment of Transcallosal Inhibition in Patients with Corticobasal Degeneration. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2003, 114, 2181–2187. [Google Scholar] [CrossRef]
- Frasson, E.; Bertolasi, L.; Bertasi, V.; Fusina, S.; Bartolomei, L.; Vicentini, S.; Rizzuto, N.; Priori, A. Paired Transcranial Magnetic Stimulation for the Early Diagnosis of Corticobasal Degeneration. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2003, 114, 272–278. [Google Scholar] [CrossRef]
- Okuma, Y.; Urabe, T.; Mochizuki, H.; Miwa, H.; Shimo, Y.; Mori, H.; Mizuno, Y. Asymmetric Cortico-Cortical Inhibition in Patients with Progressive Limb-Kinetic Apraxia. Acta Neurol. Scand. 2000, 102, 244–248. [Google Scholar] [CrossRef]
- Lanza, G.; Bella, R.; Giuffrida, S.; Cantone, M.; Pennisi, G.; Spampinato, C.; Giordano, D.; Malaguarnera, G.; Raggi, A.; Pennisi, M. Preserved Transcallosal Inhibition to Transcranial Magnetic Stimulation in Nondemented Elderly Patients with Leukoaraiosis. BioMed Res. Int. 2013, 2013, 351680. [Google Scholar] [CrossRef]
- Valls-Solé, J. Neurophysiological Characterization of Parkinsonian Syndromes. Neurophysiol. Clin. Clin. Neurophysiol. 2000, 30, 352–367. [Google Scholar] [CrossRef] [PubMed]
- McKeith, I.; Cummings, J. Behavioural Changes and Psychological Symptoms in Dementia Disorders. Lancet Neurol. 2005, 4, 735–742. [Google Scholar] [CrossRef]
- Takahashi, R.; Ishii, K.; Shimada, K.; Ohkawa, S.; Nishimura, Y. Hypoperfusion of the Motor Cortex Associated with Parkinsonism in Dementia with Lewy Bodies. J. Neurol. Sci. 2010, 288, 88–91. [Google Scholar] [CrossRef] [PubMed]
- Di Lazzaro, V.; Pilato, F.; Dileone, M.; Saturno, E.; Profice, P.; Marra, C.; Daniele, A.; Ranieri, F.; Quaranta, D.; Gainotti, G.; et al. Functional Evaluation of Cerebral Cortex in Dementia with Lewy Bodies. NeuroImage 2007, 37, 422–429. [Google Scholar] [CrossRef]
- Tiraboschi, P.; Hansen, L.A.; Alford, M.; Sabbagh, M.N.; Schoos, B.; Masliah, E.; Thal, L.J.; Corey-Bloom, J. Cholinergic Dysfunction in Diseases with Lewy Bodies. Neurology 2000, 54, 407–411. [Google Scholar] [CrossRef]
- Marra, C.; Quaranta, D.; Profice, P.; Pilato, F.; Capone, F.; Iodice, F.; Di Lazzaro, V.; Gainotti, G. Central Cholinergic Dysfunction Measured “in Vivo” Correlates with Different Behavioral Disorders in Alzheimer’s Disease and Dementia with Lewy Body. Brain Stimulat. 2012, 5, 533–538. [Google Scholar] [CrossRef]
- Taylor, J.-P.; Firbank, M.; Barnett, N.; Pearce, S.; Livingstone, A.; Mosimann, U.; Eyre, J.; McKeith, I.G.; O’Brien, J.T. Visual Hallucinations in Dementia with Lewy Bodies: Transcranial Magnetic Stimulation Study. Br. J. Psychiatry J. Ment. Sci. 2011, 199, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Petsani, C.; Aloizou, A.-M.; Siokas, V.; Messinis, L.; Peristeri, E.; Bakirtzis, C.; Nasios, G.; Dardiotis, E. Therapeutic Application of rTMS in Atypical Parkinsonian Disorders. Behav. Neurol. 2021, 2021, 3419907. [Google Scholar] [CrossRef]
- Lanza, G.; Papotto, M.; Pennisi, G.; Bella, R.; Ferri, R. Epileptic Seizure as a Precipitating Factor of Vascular Progressive Supranuclear Palsy: A Case Report. J. Stroke Cerebrovasc. Dis. Off. J. Natl. Stroke Assoc. 2014, 23, e379-381. [Google Scholar] [CrossRef] [PubMed]
- Lanza, G.; Bramanti, P.; Cantone, M.; Pennisi, M.; Pennisi, G.; Bella, R. Vascular Cognitive Impairment through the Looking Glass of Transcranial Magnetic Stimulation. Behav. Neurol. 2017, 2017, 1421326. [Google Scholar] [CrossRef]
- Zijlmans, J.C.M.; Katzenschlager, R.; Daniel, S.E.; Lees, A.J.L. The L-Dopa Response in Vascular Parkinsonism. J. Neurol. Neurosurg. Psychiatry 2004, 75, 545–547. [Google Scholar] [CrossRef]
- Liepert, J.; Bär, K.J.; Meske, U.; Weiller, C. Motor Cortex Disinhibition in Alzheimer’s Disease. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2001, 112, 1436–1441. [Google Scholar] [CrossRef]
- Agrawal, A.; Bhattacharya, A.; Kamble, N.; Mailankody, P.; Yadav, R.; Pal, P.K. Cortical Excitability Changes in Patients of Vascular Parkinsonism with Cognitive Impairment. Parkinsonism Relat. Disord. 2023, 116, 105869. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Bhattacharya, A.; Kamble, N.; Mailankody, P.; Yadav, R.; Pal, P.K. Cortical Excitability Changes in Patients of Vascular Parkinsonism with Cognitive Impairment. Parkinsonism Relat. Disord. 2023, 116, 105869. [Google Scholar] [CrossRef]
- Di Lazzaro, V.; Oliviero, A.; Mazzone, P.; Pilato, F.; Saturno, E.; Insola, A.; Visocchi, M.; Colosimo, C.; Tonali, P.A.; Rothwell, J.C. Direct Demonstration of Long Latency Cortico-Cortical Inhibition in Normal Subjects and in a Patient with Vascular Parkinsonism. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2002, 113, 1673–1679. [Google Scholar] [CrossRef]
- Antczak, J.; Rusin, G.; Słowik, A. Transcranial Magnetic Stimulation as a Diagnostic and Therapeutic Tool in Various Types of Dementia. J. Clin. Med. 2021, 10, 2875. [Google Scholar] [CrossRef] [PubMed]
- Yip, C.W.; Cheong, P.W.T.; Green, A.; Prakash, P.K.; Fook-Cheong, S.K.; Tan, E.K.; Lo, Y.L. A Prospective Pilot Study of Repetitive Transcranial Magnetic Stimulation for Gait Dysfunction in Vascular Parkinsonism. Clin. Neurol. Neurosurg. 2013, 115, 887–891. [Google Scholar] [CrossRef]
- del Toro-Pérez, C.; Guevara-Sánchez, E.; Martínez-Sánchez, P. Treatment of Vascular Parkinsonism: A Systematic Review. Brain Sci. 2023, 13, 489. [Google Scholar] [CrossRef]
- Masur, H.; Althoff, S.; Erim, Y.; Oberwittler, C.; Hornung, W.P. Postexcitatory Inhibition after Transcranial Magnetic Stimulation of the Motor Cortex in Patients with Drug-Induced Parkinsonism and in Healthy Individuals. Int. Clin. Psychopharmacol. 1998, 13, 79–82. [Google Scholar] [CrossRef]
- Priori, A.; Berardelli, A.; Inghilleri, M.; Accornero, N.; Manfredi, M. Motor Cortical Inhibition and the Dopaminergic System. Pharmacological Changes in the Silent Period after Transcranial Brain Stimulation in Normal Subjects, Patients with Parkinson’s Disease and Drug-Induced Parkinsonism. Brain J. Neurol. 1994, 117 Pt 2, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Liepert, J.; Hassa, T.; Tüscher, O.; Schmidt, R. Electrophysiological Correlates of Motor Conversion Disorder. Mov. Disord. Off. J. Mov. Disord. Soc. 2008, 23, 2171–2176. [Google Scholar] [CrossRef]
- Garcin, B.; Mesrati, F.; Hubsch, C.; Mauras, T.; Iliescu, I.; Naccache, L.; Vidailhet, M.; Roze, E.; Degos, B. Impact of Transcranial Magnetic Stimulation on Functional Movement Disorders: Cortical Modulation or a Behavioral Effect? Front. Neurol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Gonsalvez, I.; Spagnolo, P.; Dworetzky, B.; Baslet, G. Neurostimulation for the Treatment of Functional Neurological Disorder: A Systematic Review. Epilepsy Behav. Rep. 2021, 16, 100501. [Google Scholar] [CrossRef] [PubMed]
- Oriuwa, C.; Mollica, A.; Feinstein, A.; Giacobbe, P.; Lipsman, N.; Perez, D.L.; Burke, M.J. Neuromodulation for the Treatment of Functional Neurological Disorder and Somatic Symptom Disorder: A Systematic Review. J. Neurol. Neurosurg. Psychiatry 2022, 93, 280–290. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




