Submitted:
01 January 2026
Posted:
02 January 2026
You are already at the latest version
Abstract
Keywords:
1. Introduction
1.1. Malnutrition, Dehydration, and Outcomes in Dementia
1.2. Limitations of Conventional Nutrition Education and Dietary Interventions
2. Constraints in Dietary and Nutritional Interventions
2.1. Cognitive Constraints: Difficulties in Judgment, Choice, and Self-Management
2.2. Behavioral and Environmental Constraints: Instability of Eating Behavior
2.3. Nutritional Interventions as Implementation Failure
3. Separating Active Ingredients from Excessive Burden
3.1. Core Active Ingredients in Dietary and Nutritional Interventions
- Securing adequate energy and protein
- Sustained fluid intake
- Prevention of acute deterioration due to malnutrition and dehydration
3.2. Excessive Implementation Burdens to Be Removed
- Detailed management at the level of individual nutrients
- Judgment and choice at every meal
- Self-recording of food intake
- Evaluation of achievement or intake levels
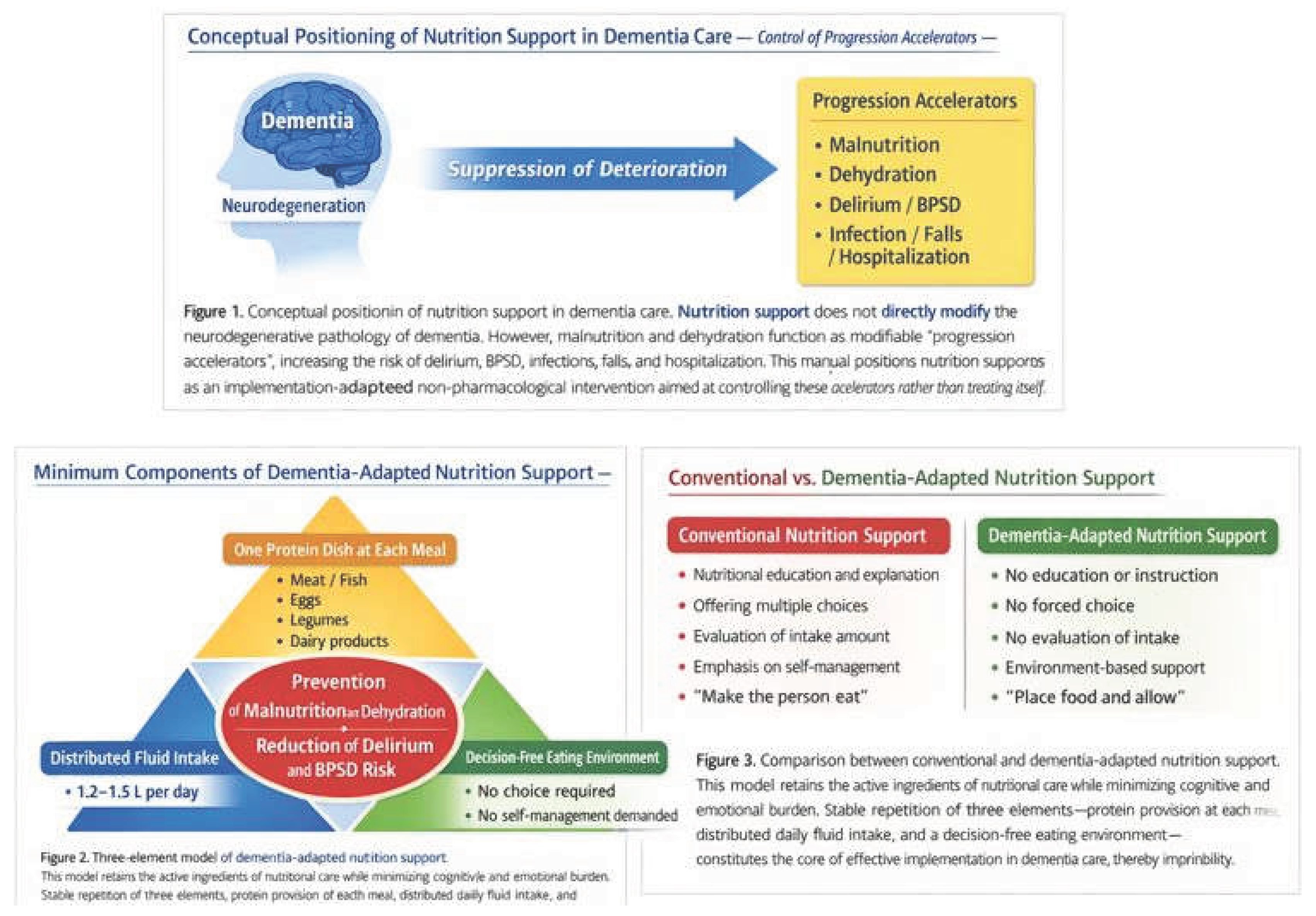
4. Dementia-Adapted Dietary and Nutrition Intervention Model
4.1. Minimal Component ①: One Protein Item at Each Meal
4.2. Minimal Component ②: Daily Fluid Intake of 1.2–1.5 L
4.3. Minimal Component ③: A Decision-Free Eating Environment
5. Implementation Protocol (Summary)
- Duration: Continuous
- Decision-maker: Caregivers and environment
- Recording: Simple checklist only
- Progression: Do not increase complexity
6. Implications for Research, Practice, and Policy
7. Conclusion
Appendix
- Purpose and Positioning
- Malnutrition
- Dehydration
- Delirium and BPSD
- Infection, falls, and hospitalization
- Care staff working in long-term care facilities or home care settings
- Family caregivers supporting people with dementia
- Medical and welfare professionals who wish to integrate dietary and hydration management into daily care
- This manual is not a clinical trial protocol, but an implementation guide for routine care.
- It does not claim therapeutic effects of specific nutritional therapies or foods.
- 2.
- Target Population
- People with mild to moderate dementia
- Individuals capable of oral intake
- Individuals for whom eating and drinking support can be provided in daily life
- Severe dysphagia requiring specialized nutritional management
- Medically unstable acute conditions (e.g., severe infection, acute delirium)
- Cases in which specific food-related stimuli provoke marked refusal or agitation
- Final decisions should always prioritize clinical judgment.
- Even in advanced dementia, this approach may be flexibly applied as supportive eating care that respects the individual’s presence, without evaluating intake volume or reactions.
- 3.
- Fundamental Design Principles
- Do not require nutritional knowledge or understanding
- Do not ask the individual to judge intake quantity or nutritional balance
- Do not require food selection by the individual
- Do not evaluate meal quantity or eating behavior
- Do not reprimand, correct, or persuade
- If anxiety, confusion, or refusal is observed, immediately pause or scale down support
- Fix meal content, timing, and provision method as much as possible
- Avoid making meals “special events”
- Reduce statements such as “Today is special”
- Do not assume motivation, judgment, or self-management by the individual
- Value support that “remains valid even if the person cannot eat”
- 4.
- Overall Structure of Implementation
- Meals: Three meals per day (frequency may be adjusted according to lifestyle)
- Fluid intake: Approximately 1.2–1.5 L per day, divided across time
- Verbal prompting and monitoring: As needed at each meal
- Familiar daily living environment
- Calm locations (avoid excessive stimulation and noise)
- Minimize movement, preparation, and choice requirements
- 5.
- Core Support Components (Three Mandatory Elements)
- Prevention of malnutrition
- Suppression of muscle weakness and susceptibility to infection
- Meat or fish
- Eggs
- Legumes or soy products
- Dairy products
- Quantity, cooking method, and nutritional calculations are not required.
- Failure to finish the meal is not considered failure.
- “I’ll just leave a little here for you.”
- “It’s okay to eat as much as you can.”
- Prevention of dehydration
- Suppression of delirium, constipation, and infection
- Use water, tea, soup, jelly drinks, etc.
- Do not request large volumes at once
- Provide fluids divided across meals and between meals
- Do not assume awareness of thirst.
- Accurate recording of intake volume is not mandatory.
- “Shall we take just a little sip?”
- “I’ll leave this here for you.”
- Prevention of food refusal and confusion
- Prevention of BPSD triggers
- Standardize menus
- Do not present choices
- Keep meal time and location as consistent as possible
- Asking “Which would you like?”
- Presenting intake targets
- Instructing how to eat
- 6.
- Explicitly Excluded Interventions
- Nutrition education or explanatory guidance
- Interventions aimed at evaluating intake volume or nutritional balance
- Treating meals as “rehabilitation tasks”
- Engagement aimed at achieving meal completion or independent eating
- 7.
- Adjustment and Discontinuation Criteria
- Meal quantity (reduction is acceptable)
- Number of meals
- Provision method (texture, temperature, etc.)
- Clear anxiety, refusal, or agitation
- Worsening of BPSD during meal situations
- Increased risk of aspiration
- 8.
- Safety and Burden Management
- Physical risks are relatively low
- The greatest risk is over-intervention
- Minimize caregiver guilt
- A day without eating is not considered failure
- 9.
- Consistency with Existing Evidence
- Associations between malnutrition/dehydration and mortality, hospitalization, and functional decline
- Nutrition and hydration management guidelines for people with dementia
- Evidence that dehydration triggers delirium and BPSD
- 10.
- Use in Research and Practice
- Supplementary materials describing intervention content
- Clarification of implementation methods and fidelity
- Simplified manuals for care staff
- Handout materials for family caregivers
- Shared policy documents for dietary support
References
- El-Sharkawy, A. M.; Watson, P.; Neal, K. R.; Ljungqvist, O.; Maughan, R. J.; Lobo, D. N. Hydration and outcome in older patients admitted to hospital (The HOOP study). Age and Ageing 2015, 44(6), 943–947. [Google Scholar] [CrossRef] [PubMed]
- Livingston, G.; Huntley, J.; Sommerlad, A.; et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. The Lancet 2020, 396(10248), 413–446. [Google Scholar] [CrossRef] [PubMed]
- Oh, E. S.; Fong, T. G. Delirium in older persons: Advances in diagnosis and treatment. JAMA 2017, 318(12), 1161–1174. [Google Scholar] [CrossRef] [PubMed]
- Parkinson, E.; Crichton, M.; Horne, R.; et al. Low-intake dehydration and clinical outcomes in older adults: A systematic review and meta-analysis. Clinical Nutrition 2023, 42(4), 728–738. [Google Scholar] [CrossRef]
- Soininen, H.; Solomon, A.; Visser, P. J.; Hendrix, S. B.; Blennow, K.; Kivipelto, M.; LipiDiDiet Clinical Study Group. 24-month intervention with a specific multinutrient in people with prodromal Alzheimer’s disease (LipiDiDiet): A randomised, double-blind, controlled trial. The Lancet Neurology 2017, 16(12), 965–975. [Google Scholar] [CrossRef] [PubMed]
- Soininen, H.; Kivipelto, M.; Solomon, A.; et al. Long-term effects of the LipiDiDiet intervention in people with prodromal Alzheimer’s disease. Alzheimer’s & Dementia 2021, 17(4), 1–11. [Google Scholar] [CrossRef]
- Volkert, D.; Beck, A. M.; Cederholm, T.; Cereda, E.; Cruz-Jentoft, A.; Goisser, S.; Bischoff, S. C. ESPEN guideline on clinical nutrition and hydration in geriatrics. Clinical Nutrition 2019, 38(1), 10–47. [Google Scholar] [CrossRef] [PubMed]
- Volkert, D.; Chourdakis, M.; Faxen-Irving, G.; Frühwald, T.; Landi, F.; Suominen, M. H.; ESPEN. ESPEN guideline on nutrition and hydration in dementia. Clinical Nutrition 2024, 43(2), 159–190. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




