1. Introduction
Food safety assurance and clinical parameters monitoring are two critical aspects that intersect in safeguarding human health and well-being. Ensuring food safety is crucial for protecting public health and instilling consumer confidence. It involves implementing rigorous standards and control measures to prevent foodborne illnesses and hazards throughout the production chain. Regarding clinical parameters monitoring, they play a pivotal role in disease prevention, diagnosis, and management. Regular assessments of vital signs, blood chemistry, and other health indicators allow healthcare professionals to identify early warning signs of illness, track the progression of diseases, and evaluate the effectiveness of treatment interventions. By closely monitoring these parameters, individuals can take proactive steps to maintain their health and wellness make informed lifestyle choices and seek timely medical intervention when necessary. Multiple ways are being described to get the required clinical or food information. However, is vibrational spectroscopy one of the most powerful techniques that can give a real-time and/or non-invasive response to solve the analytical approach.
Over the last few decades, vibrational spectroscopy has established itself as a useful and applicable technology in a wide range of sectors including agri-food, pharmaceuticals, petrochemicals and healthcare. As the requirements of each sector change over time, it is recognised that these techniques have attracted even more interest as part of the suite of non-destructive spectral sensors (NDSS). These spectroscopic technologies coupled with data analysis and chemometrics have allowed the development of new trends towards non-target methods that can be used to analyse products and create a unique fingerprint to provide information on food safety and health parameters. Furthermore, vibrational spectroscopy, combined with data analysis, offers cost-effective, value-added solutions to a range of food industry and health problems. The most powerful and versatile vibrational tools are Near Infrared (NIR) and Raman Spectroscopy. Raman spectroscopy is based on scattering, whereas NIR spectrum is due to absorption of radiation. Both can be used as simple spectrum measures or acquiring images by scanning simultaneously image information and spectra.
Raman and NIRS are the most attractive techniques for the agri-food and health sector, due to their simplicity, ease of use and multi-parametric character, broadening the range of sample analysis to facilitate a comprehensive investigation. In this manuscript, the main trends in the development of methodologies based on vibrational spectroscopy (NIR and Raman spectroscopy) applied to food safety and medicine will be presented, including conventional spectroscopy, imaging and microscopy proposals, instrumental trends (focusing on miniaturized and portable instruments) as well as new strategies and trends in chemometric approaches.
2. Near Infrared Spectroscopy
In NIRS analysis polychromatic radiation is focused on the samples, which absorb particular frequencies, corresponding to its molecular transitions, overtone or combination vibrations. A major objective related to NIR analysis is the design and development of robust systems that can be used in harsh environments such as on field or production lines and be flexible to accept both in-situ, on-line and remote measurements wherever possible. There are two approaches typically adopted for achieving these objectives: miniaturizing spectrometers [
1,
2] and designing multimode and single mode fibre optics [
3].
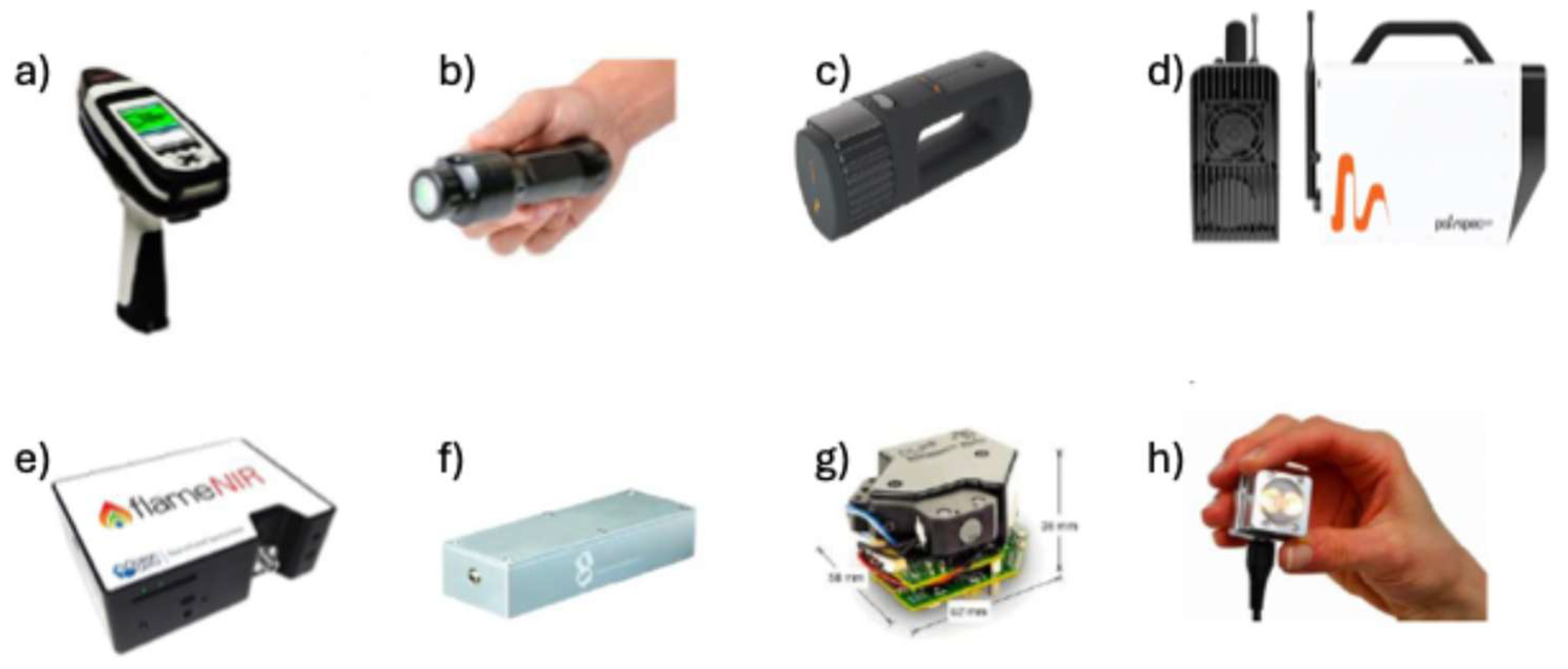
Miniaturised devices had external radiation sources and were mains-powered. They had a limited wavelength range and low resolution, and the spectra were not reproducible. The optical window was also limited in size. It was also necessary to connect to an external computer, so they could not be used in the field. With advancements in technology, medium-sized sensors that are handheld systems have been developed. These include all the necessary components to be used autonomously, such as battery or electronic control systems. The most representative and widely used instrument in miniaturized NIRS is the Phazir™ Instrument (currently distributed by Thermo Fisher Scientific). The rapid development of microelectronics as well as micro-electro-mechanical systems (MEMS) has allowed the transition from medium-sized equipment (around 1.5 kg) to micro spectrometers (over 100 g) in the last few years. In this case, the most used device is the MicroNIR 1700 (VIAVI Solutions Inc.).
Bec et al. [
4] examines the fundamental principles and diverse applications of miniaturized near-infrared (NIR) spectrometers. Of particular note is the broad and diverse range of applications, which span agriculture and the food industry, materials science, industrial processes and environmental monitoring. Unlike the relatively uniform and mature design of benchtop FT-NIR spectrometers, miniaturised instruments incorporate a variety of technological solutions that directly impact their operational performance. Steady technological progress has led to a continuous stream of new devices entering the market. This has shifted the current focus in analytical NIR spectroscopy towards systematically evaluating these instruments, refining associated methodologies and comprehensively characterising their performance profiles.
Figure 1 shows different miniaturised NIR instruments.
Characteristics of these NIR miniaturized instruments are listed in Tabla 1. Some manufacturers do not provide complete specifications for their instruments on their websites. The instruments are available in a range of models, which differ mainly in terms of technology, spectral resolution, spectral range, size and price. Although several spectrophotometers are small in size, they require external radiation sources and mains power, which limits their use in the field. Another group of handheld NIR spectrophotometers has an intermediate size and weight of up to 1.5 kg. Most of these use sensor arrays, while others use MEMS (microelectromechanical systems) devices as the intermediate spectral element. All of them include the necessary electronics, radiation source, battery, control electronics and displays to operate as autonomous units.
A few others portable instrument manufacturers took full advantage of the advances in microelectronics and MEMS or microfabrication processes, to produce tiny devices that are used to assemble complete instruments. These instruments are battery powered, have internal radiation sources (intended for reflectance measurements), and offer modern wireless digital data exchange technologies, such as Bluetooth®. They operate in a stand-alone mode and are referred to as “micro” instruments due their low weight (about 100 g), and compact size, approximating in average a 10 × 10 × 10 cm, or smaller box.
Table 1.
Main specifications of popular miniaturized NIR spectrometers.
Table 1.
Main specifications of popular miniaturized NIR spectrometers.
| Manufacturer |
Model |
Technology |
Spectral
Range (nm) |
Spectral
Resolution (nm) |
| Texas Instruments |
NIRscan Nano
EVM |
Grating–MEMS
DMD |
900–1700 |
10 |
| Viavi Solutions |
MicroNIR Pro 1700 |
LVF–Linear |
908–1676 |
12–25 |
Si-Ware
Systems |
NeoSpectra |
MEMS–FT |
1250–1700 |
8–16 |
| Ocean Optics |
Flame NIR |
Grating |
970–1700 |
~10.0 |
| ITPhotonics |
Polispec NIR |
x |
900-1700 |
3,2 |
| Thermo Fisher Scientific |
MicroPhazir |
MEMS |
1596-2396 |
11 |
| SouthNest Tecnology |
NanoFTIR |
MEMS-Michaelson Interferometer |
800-2600 |
2.5-13 |
| Spectral Engines |
NIROne Sensor |
MEMS Fabry-Pérot Interferometer |
1350-2500 |
16 |
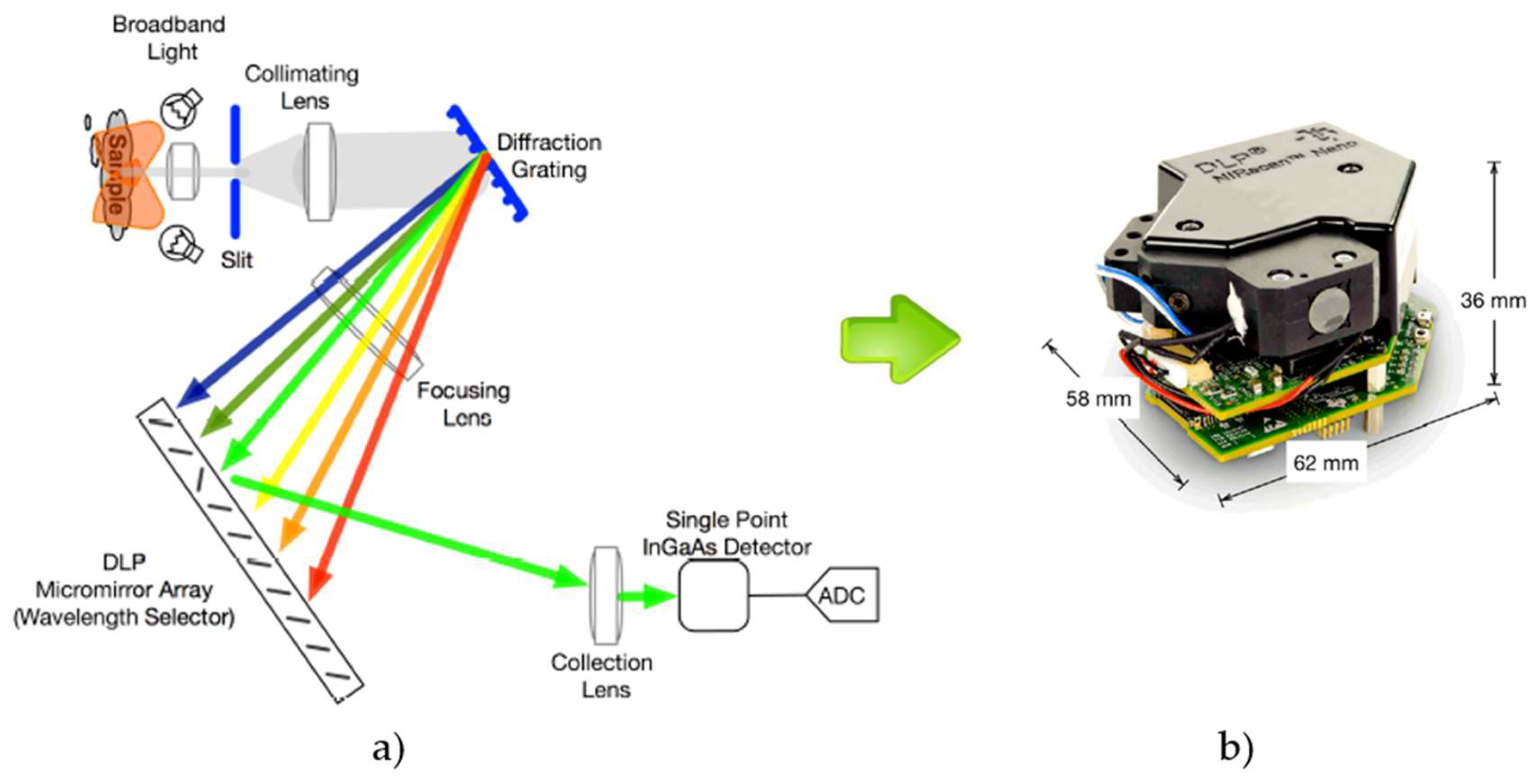
A notable example of miniaturized NIR devices is the DLP™ NIRscan Nano spec-trometer [
5]. This device is based on a Digital Micromirror Device (DMD™) designed by Texas Instruments, combining a light detector and a grating. It uses Digital Light Pro-cessing (DLP) technology according to the principle of a monochromator.
Figure 2 illustrates the architecture of the spectrometer based on DLP technology. The key component is the DMD microarray. This consists of a matrix of thousands of micromirrors, each of which has a surface angle that can be controlled by applying a voltage. Individual wavelengths are selected by turning columns of mirrors on or off to reflect only the desired wavelengths to the detector. This technology enables a larger detector area and the use of a single light detector.
While developing compact instruments is one of the main research lines, integrating micro spectrometers into smartphones is one of the latest and most common trends. In [
6] Huang
et al. describe the development of smartphone-based NIR fluorescent imaging technology and the quality and potential of point-of-care applications. Another key goal for the future is the promotion and application of cloud computing platforms (IoT technology), however, despite the development of a large number of new chemometric methods, instrument software is often not updated for the development of these methods. Wang et al. review various chemometric methods that have been applied in modern spectral analysis over the past ten years, particularly from a practical perspective [
7].
With cloud platforms, spectra data from different sources, such as different points on the production line, can be managed and stored. It would enable automatic optimisation processing and big data analysis [
8,
9]. Even with all the benefits of miniaturised devices, sampling can still be highly difficult in certain circumstances. One of the best instrumental solutions is to employ fiber optics either single or multiplexing fibres for parallel processing. Fiber optic probes have emerged as indispensable tools in modern spectroscopy, renowned for their versatility across the visible and near-infrared frequency ranges [
3]. Fibre optic probes typically consist of separate illumination and collection optical fibres connected to the light source and detector, respectively. They are bonded to the probe tip to allow simultaneous illumination of the sample and collection of the light. The geometry of the probe tip varies depending on the optimal coupling to the sample or the geometry of the light emission from the probe tip.
Optical fibers enable the transmission of light to and from biological tissues, achieving the measurement of various physiological parameters without the need for invasive procedures. NIRS, for example, can be employed to analyse the chemical composition of tissues and body fluids, providing valuable insights into blood oxygenation, tissue perfusion, and metabolic activity [
10]. When contrasting miniaturized equipment with fiber-optic probes and laboratory instruments, it has been observed that, despite the lower performance, the ability to conduct on-site and in-line analyses is of greater interest to the industry than the potential decrease in accuracy [
11]. The employment of optical fibers for non-invasive clinical analysis enhances patient comfort, reduces the risk of complications, and improves the efficiency and accuracy of medical diagnostics.
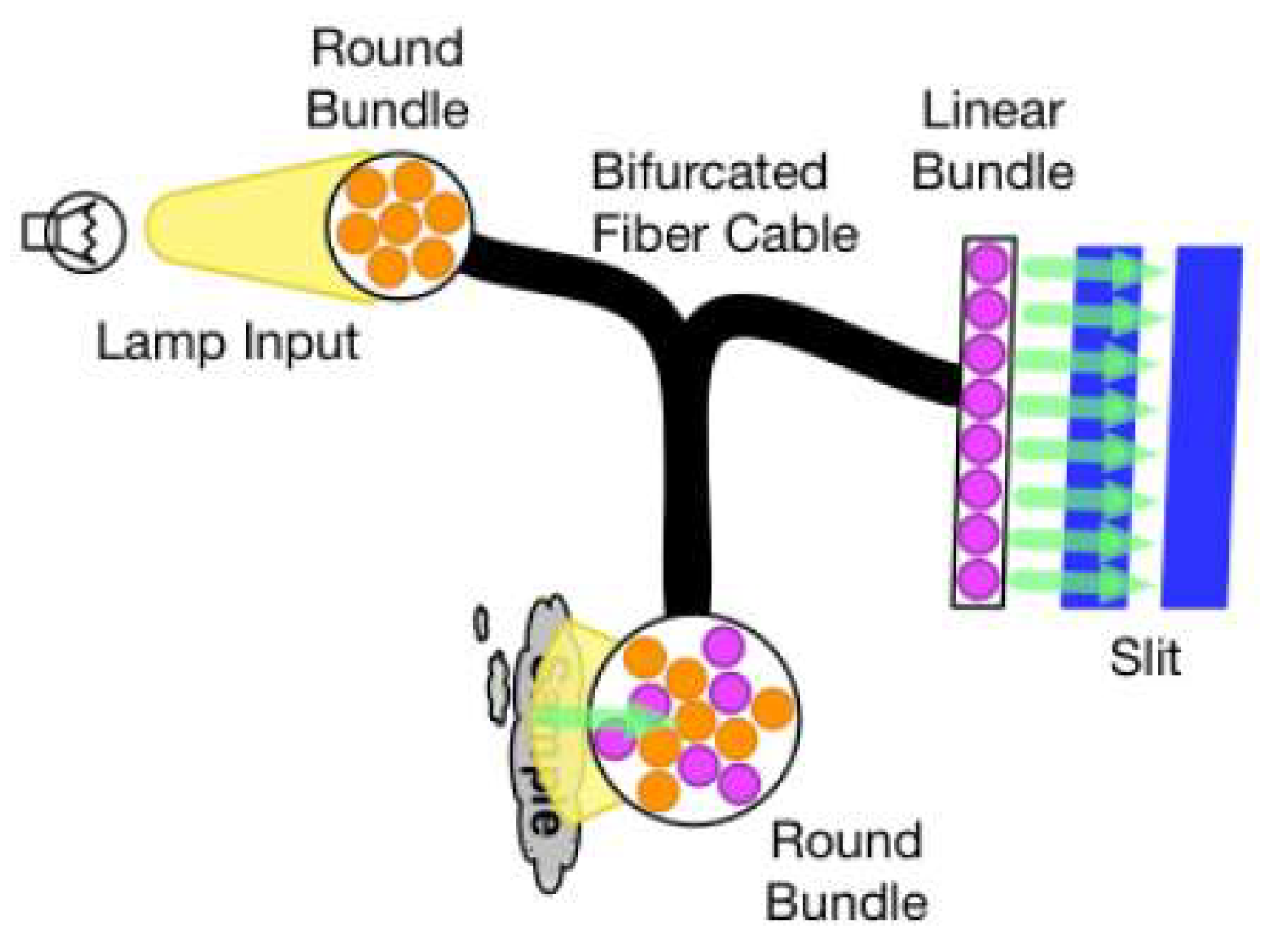
The most basic type of fibre optic probe is a reflectance probe. The fibre bundle is usually divided into two parts (see
Figure 3): one set of fibres is routed to a light source, while the other set of fibres captures the reflected light and directs it to the input slit. DLP Technology-based spectrometers have tall slits, thus, when combined with round-to-linear fiber bundles that fill the complete slit, the highest performance is obtained.Before reflection data can be collected by the spectrometer, the system must be calibrated by taking a reference scan. To take this reference scan, diffuse reflectance material such as Spectralon is placed in the same position relative to the probe as will be used for the actual measurement.
3. Raman Spectroscopy for Health and Food Safety Control
While the NIR spectrum is due to radiation absorption, the Raman spectrum is related to scattering effects. This type of spectroscopy uses a chromatic laser to excite molecules, and the Raman spectrum is observed when they return to their initial vibrational level through inelastic scattering. For highly hydrated samples, Raman spectroscopy has several advantages over NIRS: minimal scattering of the polar O-H group and more intense bands of homonuclear groups. However, one of the drawbacks of Raman spectroscopy is the low fraction of scattered photons (often lower than 0.0001%), which results in low signal-to-noise ratios and long acquisition times.
Raman techniques enable signals to be collected from food and clinical samples. In addition to standard spectral collection, advanced Raman technologies such as surface-enhanced Raman scattering (SERS), spatially offset Raman spectroscopy (SORS) and the emerging Raman hyperspectral imaging (RHSI) are now the most widely employed. In [
12] Sun et al. present advantages of Raman technologies and applications in food-production chain. SERS emerged as an alternative to the low cross-section of Raman scattering (10–28 cm
2 per molecule). It includes metallic nanostructures near or absorbed by the target analyte to enhance the Raman scattering signal. SORS is a technology that spatially separates the excitation laser and the signal detection, enabling it to probe signals from deeper layers of a sample. An alternative way to improve and increase the SORS spectra is to use hyperspectral Raman imaging, which allows spectral and spatial resolution to be obtained simultaneously. All of these Raman techniques can be used for qualitative and quantitative analysis.
One area of Raman analysis is the design of miniaturised devices to be used as detector in different miniaturized analytical techniques such as lab-on-a-chip (LOC) [
13], microfluidic [
14] or lateral flow [
15]. These analytical strategies often use nanomaterials to enhance Raman signal (SERS) and have all been developed as on-site sensor procedures for detecting target analytes in specific samples. They frequently require sample pre-treatment to extract the analyte prior to analysis, and this process is usually straightforward. The LOC is an emerging strategy because multiple components (i.e., sample extraction, preconcentration and detection) are integrated into a chip. The Raman signal can be used as a final signal in (bio)chemical sensors or a technique to detect unknown molecules or quantify specific compounds by combining spectra and chemometrics.
Similarly to NIRS, Raman can be used to scan samples on-site with portable, handheld instruments or a modular system. Raman miniaturization is not limited to reducing physical dimensions but must also ensure adequate performance for specific applications. Today, handheld Raman devices are used across diverse fields, including food safety, where they enable rapid detection of adulterants and counterfeit products, and medicine, where their integration with wearable SERS sensors supports non-invasive biomolecule detection and potential early disease diagnosis. Handheld Raman technologies have also expanded into dermatology, allowing in-skin compound analysis through confocal probes, and into public safety, where remote handheld systems can identify hazardous substances with precision comparable to benchtop instruments. Recently, handheld FT-Raman spectrometers have been deployed for on-site drug analysis. A growing trend is the integration of Raman spectroscopy with smartphones, either through embedded modules or detachable designs, improving portability and usability. When combined with SERS substrates and cloud-based processing, smartphone-based Raman systems can leverage deep learning to enhance spectral interpretation, mixture discrimination, and quantitative accuracy [
16].
With portable Raman spectrometers (sometimes called compact spectrometers), the user can streamline and customize a Raman system to fit their specific application, reducing size, weight, complexity, and cost in the process. The small size and robustness of these devices make them easy to move from one location to another, yet they are powerful enough to carry out analytical measurements on-site.
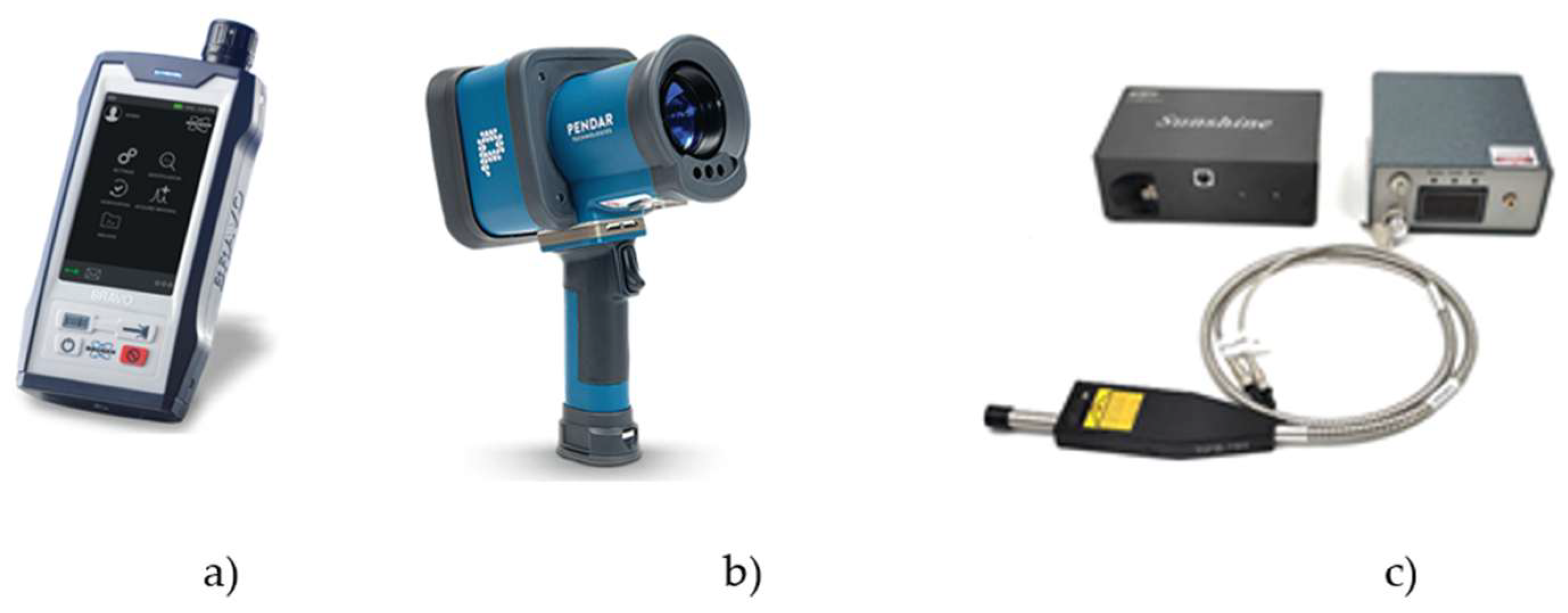
Handheld Raman instruments (see
Figure 4a and
Figure 4b) are designed for use in the field by non-experts, providing definitive identification of a substance with a simple point-and-click operation. They integrate a single-wavelength Raman system, sampling optics, data processing and a display screen/user interface into a lightweight, battery-powered unit robust enough for use by first responders and in manufacturing environments. They deliver rapid and reliable results but are limited to the installed libraries. The third option is the Raman modular system. These consist of a spectrometer, an external laser, flexible fibres and a Raman probe.
This allows us to build a ‘tailor-made’ system by choosing the laser wavelength, probe type, spectral range, resolution and detector type. This gives researchers, laboratories and industries great flexibility.
Figure 4c shows a low-cost modular system from CNI, and
Table 2 provides the specifications of some popular Raman spectrometers.
These instruments can be used to analyse bulk samples in-field, and real time without sample pre-treatments. The most emerging instrumentation development is focused on the integration of Raman device with a smartphone connected to a cloud that can be used to integrate the math calculation that identifies qualitative or quantitatively sample characteristics.
It is important to note that Raman scattering, and fluorescence often coexist as competing effects, making it challenging to distinguish weak Raman signals from the fluorescence background. This necessitates the development of instrumentation capable of distinguishing between Raman and fluorescence events. The selection of the excitation laser and imaging spectrometer is critical to the success of the final application.
4. Hyperspectral imaging for Health and Food Safety Control
HSI uses a combination of imaging and spectroscopy to collect spatial and spectral information from materials simultaneously. This enables detailed chemical and structural analysis. Although HSI was initially developed for remote sensing, it is now increasingly being used in biomedical diagnostics, clinical surgery, pharmaceutical inspection, agricultural monitoring, and food safety control. This technology is highly effective in detecting subtle biochemical differences, measuring physical properties and identifying contaminants without destroying the sample. Pallua
et al. [
17] present new applications of hyperspectral imaging in clinical research.
HSI systems generate rich datasets known as ‘hypercubes’, comprising two spatial dimensions and one spectral dimension. Each pixel contains a full spectrum of reflectance, absorbance or fluorescence, enabling researchers and practitioners to accurately classify materials, measure chemical concentrations, detect diseases and identify contaminants. HSI has quickly started using real-world health and food safety applications, having initially started with laboratory demonstrations. The most significant trends are: (1) the use of deep learning and transformer-style models for tasks involving both spectrum and space; (2) miniaturisation, with the development of portable and handheld devices enabling on-site screening; (3) a push towards in-line/real-time HSI on production lines, via push-broom and snapshot designs, as well as edge inference; (4) increased interest in SWIR and multimodal fusion, when chemical or penetrative information is required; (5) a focus on domain shift, few-shot learning, transfer learning and explainability. These approaches are being developed to address variability across different sites and seasons. Nevertheless, cost, standardisation, data volume, calibration/illumination dependency and regulatory validation continue to be the main barriers to the widespread adoption of this technology in industry and clinical practice. Kumar et al. provided a thorough survey of DL for HSI classification [
18].
Modern systems typically employ convolutional neural networks, spectral-spatial architectures and, increasingly, transformer-based models to extract subtle features from cubes for classification, segmentation and regression. Although these models significantly improve detection and quantification performance compared to classical chemometrics (PLS and SVM), they require larger labelled datasets and more computing power. Recent surveys suggest that transformer and contrastive transfer approaches are increasingly popular ways of addressing spectral complexity and domain shifts. On the other hand, several studies and reviews demonstrate the effectiveness of portable HSI technology for on-site meat authentication, screening snacks and produce, and testing grain quality. These studies often use push-scan sensors and lightweight embedded processors. This makes it possible to carry out field and plant inspections, as well as in situ factory checks, for which laboratory spectroscopy was previously required. Bruno et al. demonstrate that portable and handheld HSI systems can be used as a rapid, non-destructive technique to predict the acrylamide content of potato crisps [
19].
Push-broom line-scan setups remain dominant for high throughput. However, snapshot imagers combined with optimised inference (quantised models, GPU/FPGA/edge inference) are enabling real-time foreign object detection and composition mapping (fat/moisture), and sorting on conveyor belts. Several groups have reported improvements in production rates in agriculture and food processing by using AI-assisted push-broom systems. Neri
et al. present the development and implementation of a real-time, AI-assisted, push-broom, hyperspectral system for identifying plants [
20].
When surface colour alone is insufficient for identification purposes (e.g., for detecting internal defects, polymers or chemicals, or mycotoxin proxies), SWIR (1000–2500 nm) provides more direct compositional contrast. Snapshot spectral imagers are ideal for applications where motion or speed is critical. Reviews emphasise the importance of matching the spectral range and sensor architecture to the chemical target. Thomas et al. identifies several trends in snapshot spectral imaging [
21]. Combining HSI with RGB, thermal, Raman, fluorescence or chemical/targeted sensors can make it possible to distinguish between cases where spectral signatures are difficult to separate (e.g., microbial and chemical contaminants, or visually similar adulterants). Fusion strategies, such as early and late fusion, as well as learned fusion, are a current area of research. Medina-García et al. explore strategies for analysing hyperspectral imaging (HSI) data to identify issues relating to the quality and safety of food [
22].
Real-world deployments have demonstrated that models trained in one season, factory or instrument tend to perform poorly in others. Recent work has focused on transfer learning, contrastive and few-shot methods, domain adaptation, and instrument standardisation procedures. The aim is to reduce the need for re-labelling. Cross-domain hyperspectral imaging (HSI) classification and few-shot learning are active and practically relevant topics. Jiang
et al. discuss the task of cross-domain HSI classification in great detail. [
23]. However, high-performance HSI cameras with a broad SWIR range and high spectral resolution remain more expensive than RGB/mono cameras. The cost is increased by the need for specialised optics, stable illumination systems and robust housings, particularly for SWIR and cooled detectors. Cost is repeatedly cited as the main barrier to adoption in reviews and application papers. In [
24], Li
et al. present the latest advances and challenges in the use of hyperspectral imaging (HSI) to detect foreign matter in food products, and outline future directions in this field..
HSI cubes contain hundreds of bands and have a high spatial resolution, meaning they are orders of magnitude larger than RGB frames. This creates bottlenecks in terms of storage, transfer and computing, particularly for long-term production logging and traceability. Heavy pre-processing is required, including calibration, dark/current correction, and reflectance conversion. HSI-derived features are sensitive to the spectrum, angle, and environmental conditions of the illumination. Without strict spectral and radiometric calibration and controlled lighting, the accuracy of the models deteriorates rapidly. Many practical deployments fail to allocate sufficient funds for regular spectral reference checks and maintenance. Spectral signatures may differ due to variations in instrumentation, ageing, surface wetness, cultivar/variety, and seasonal changes. Cross-domain failure is well documented. While model transfer methods are necessary, they are not infallible. Building robust classifiers often requires chemically verified ground truth, such as laboratory assays, microbiology and GC/MS analysis of contaminants. However, these assays are expensive and time-consuming, which limits the amount of labelled data available and slows down model development. Several food safety reviews have highlighted the lack of labels as a bottleneck. Guo
et al. conducted a thorough investigation into the use of HSI technologies for detecting fungal and mycotoxin contamination in grains and oilseeds [
25].
Although clinical HSI research is promising, regulatory approvals and large prospective trials remain scarce. Standardised clinical endpoints, safety profiles and acceptance criteria are under development. Standardised test protocols for food safety are still being developed across vendors. In their review, Ali et al. demonstrate the significant potential of HSI as an intraoperative guidance tool for assisting surgeons during tumour resection by generating detailed tissue density maps [
26]. Even a low rate of false positives or false negatives can result in significant operational costs in production lines (e.g., wasted product, rework, line stoppage). Integrating HSI requires production engineers to co-design optics, mechanical positioning, preprocessing and AI thresholds [
20].
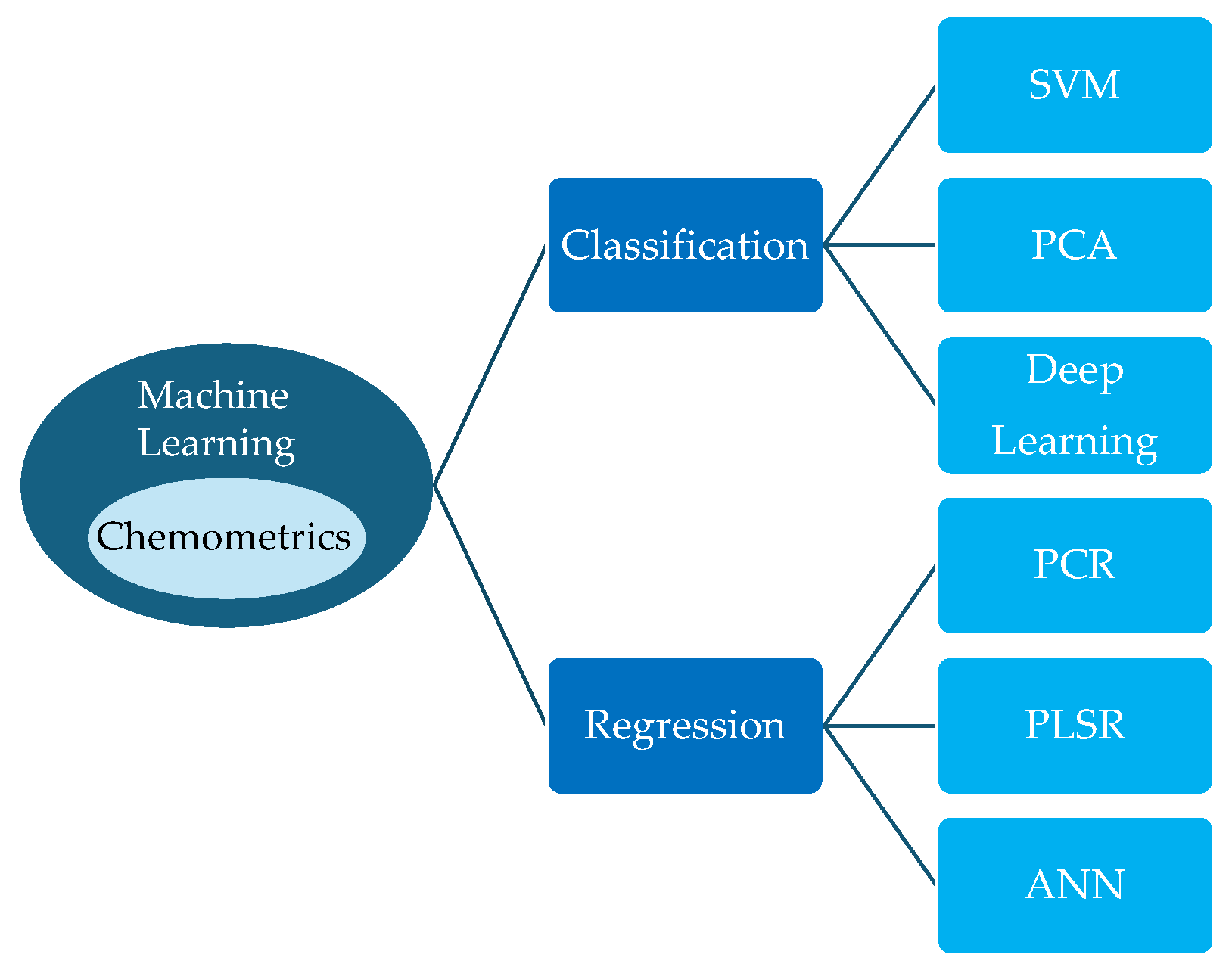
5. Combination of Vibrational Spectroscopy and chemometric analysis
Vibrational spectroscopy generates huge amounts of spectral data. Processing this data is a critical step involving pre-processing, calibration, and analysis. Advanced algorithms, including chemometrics and machine learning, are used to extract meaningful information (qualitative and/or quantitative) such as nutrients, moisture, and disease. Chemometrics involves analysing chemical data using specialised statistical tools. Machine learning, on the other hand, is a general-purpose pattern-learning framework that is used across many domains, including chemistry.
Figure 5 shows some of the classification and regression techniques that are used in food safety and health control.
The accuracy and reliability of the information derived from the spectral data is increased through this processing. Integrating this technology into the agri-food and medical sectors requires developing accurate models with high inter-instrument reproducibility, which currently necessitates investment in development and maintenance over time of robust chemometric models. Nowadays, to develop and maintain the calibration models requires specialized personnel, which significantly limits their practical utility. For this reason, one of the future research trends is focused on the development of new algorithms addressed to reduce the workload associated with model establishment and maintenance.
As previously mentioned, vibrational spectroscopy generates a large-scale database. Typically, in common supervised learning strategies, each spectrum is labelled with reference data (e.g., parameter concentration or sample type). However, one of the main emerging trends from the large amount of vibrational spectroscopy data is the development of semi- or unsupervised models that can extract relevant information for future applications [
7].
One of the most remarkable aspects of current trends is that it is becoming more and more common to use two or more techniques in order to obtain the maximum information. This means that classical chemometric methods such as Principal Component Analysis (PCA) and Partial Least Squares Regression (PLS) sometimes fail to produce satisfactory models. In recent years, multiblock methods have been developed to perform these analyses. Multi-block analysis enables complementary information to be obtained from different techniques. Mishra et al. provide an overview of the multi-block data analysis concept in [
27], while Smilde et al. provide a framework for this subfield of data fusion [
28]. This type of analysis identifies common and distinct information from data obtained from different sensors. In [
29], Song
et al. propose a model for addressing the issue of mixed data types in multiple datasets. It is important to note that machine learning algorithms are being applied in the development of new and robust calibration models. Going to the most actual deep learning approaches, especially convolutional neural networks (CNN) have started to be applied because it does not require to select variables before model development. It uses multiple convolution layers and pooling layers to extract the relevant information. Zhang et al. discuss the challenges currently associated with conventional chemometric methods and DL to spectral analysis [
30].
To add to the development of robust calibrations, it would be a challenge the development of learning strategies able to offer the possibility of transferring calibrations from one to another instrument, moving vibrational calibration models to a universality, not depending on the instrument employed to analyse the sample. According to Mishra
et al. in [
31], translation of NIR technology into commercial slaughterhouse conditions is challenging. Yang et al. demonstrate that combining HSI and DL increases the accuracy and efficiency of inspections of fruit and vegetables [
32]. The future research should, however, emphasize cost reduction, equipment optimization, feature extraction personalization, and model generalizability.
The adoption of ML models in the industrial sector for food safety purposes is still in its early stages. This is because there are some issues with how these models are designed and how they are used in practice. PLS-based models are reliable, but their robustness for industrial deployment is lacking. The methods for analyzing samples and categorizing them also need to be improved. Benelli et al. HSI was adopted to evaluate the ripeness degree of kiwifruit [
33]. To address these issues, there is a pressing need for industrial-focused research on ML model adaptations, particularly models for staple foods which are integral to food security. Implementing kernel functions within these models and focusing on more specialized, food-specific algorithms would bridge existing research gaps and make ML models more applicable to industrial food safety contexts.
Looking to the future, chemometrics and machine learning offer clear benefits for food safety tasks, such as faster pathogen detection, predictive outbreak forecasting, automated visual inspection and richer supply chain traceability. However, progress is hindered by inconsistent data, poor model interpretability and validation, limitations in sensors and integration, and regulatory and operational barriers. In order to progress from promising pilots to reliable, trusted systems, we require standards for data and evaluation, privacy-preserving distributed learning and multimodal models that are linked to domain knowledge. Furthermore, regulatory pathways are needed for the deployment of these systems to be validated. Zhang
et al. review the transformative role of ML and DL in enabling intelligent food safety management through the efficient analysis of high-quality, nonlinear data [
34].
6. Chemometrics Software Tools
The number of chemometrics software tools available for developing the trained model is just as numerous as the mathematical methods. Depending on the needs (spectroscopy analysis, calibration, data exploration, regression, classification, etc.), there are several widely used chemometrics tools, both commercial and open source.
Table 3 shows some of the most popular and well-established chemometrics software packages.
7. Vibrational Spectroscopy Applications
Miniaturized and low cost NIR sensors have been widely employed for quality and safety controls in a wide variability of foods, such as vegetables [
35], meat [
36], milk [
37], fish [
38], etc [
39]. The most recent applications combine portable instrumentation and chemometrics to extract relevant and safe information and remark the importance of optimizing the new generation of NIR instruments for on-site and on-line NIRS analysis.
Microbiological control is of paramount importance in ensuring food safety. Bacteria, viruses, parasites, and fungi can contaminate food at any stage of production, posing serious health risks to consumers if ingested. Through rigorous microbiological testing and monitoring, food producers can identify and mitigate potential hazards, ensuring that food products are safe for consumption. In essence, microbiological control plays a crucial role in preventing foodborne diseases and protecting the well-being of individuals and communities. However, non-invasive and real time analysis strategies need to be developed to increase sampling and improve food safety tools.
Vibrational spectroscopy is a good alternative to classical methods of identification and control of microbiological growth, which are usually expensive, require skilled personnel and are difficult or not possible to carry out in situ. In their literature review, Tian et al. [
40] describe the different ways of applying near-infrared spectroscopy for microbiological control, including examples of identification and classification of pathogenic bacteria in foodstuffs as well as monitoring systems. HSI combined with machine learning has allowed to obtain a lot of information about products with complex matrices such as vegetables and cereals [
41].
One of the advantages of HSI technique is its capability to provide both spectral and spatial information. This feature enables the analysis of complex and non-homogeneous samples. For instance, in a study by Hardy et al. [
42], salmon fillets were examined using absorption spectroscopy with a hyperspectral camera over four storage days. Classification methods such as K-Nearest Neighbors (K-NN) and PCA were utilized, achieving accuracies of 77.0% and 73.7%, respectively, in predicting storage day. The use of hyperspectral imaging unveiled increased spoilage in tail bottom fillet sections as the fillets aged, with the dampening of an absorbance band around 600 nm identified as the primary indicator distinguishing between fresh and spoiled samples. This highlights the significance of considering spatial inhomogeneity in studies on fish freshness, with HSI proving to be a valuable tool for such analyses.
In addition to microbiological contamination and the sale of adulterated or non-fresh foods, the presence of microplastics in food poses a significant threat to food safety. This study adopts an analytical approach to confirm the internalization of microplastics in cryogenic cross-sections of muscle tissue. Utilizing 3D Raman confocal microscopy with chemometrics, microplastics measuring 1 μm were successfully identified. Optical and fluorescence microscopy further validated the findings. The investigation unveiled evidence of microplastics internalization in the digestive epithelial tissues of exposed mussels (
Mytilus galloprovincialis) [
43].
Raman is a very useful alternative for food safety too. It can be a tool to guarantee food safety for inspection services or consumers. It has been used to detect fungi, toxins or frauds among others. Raman spectra combined with proper chemometrics provides a fingerprint detection of mycotoxins, and using specific antibodies or aptamers the selective quantification can be carried out by SERS. Future work should be developed to implement SERS detection in smartphones integrating the spectrum collection, processing and results [
44]. Using a handheld Raman spectrometer and Partial Least squares Discriminant Analysis (PLS-DA), Logan et al. [
45] demonstrated the viability of the Raman spectrum to correctly classify Australian beef; differences in spectra were observed predominantly at 1301, 1440 and 1658 cm
-1.
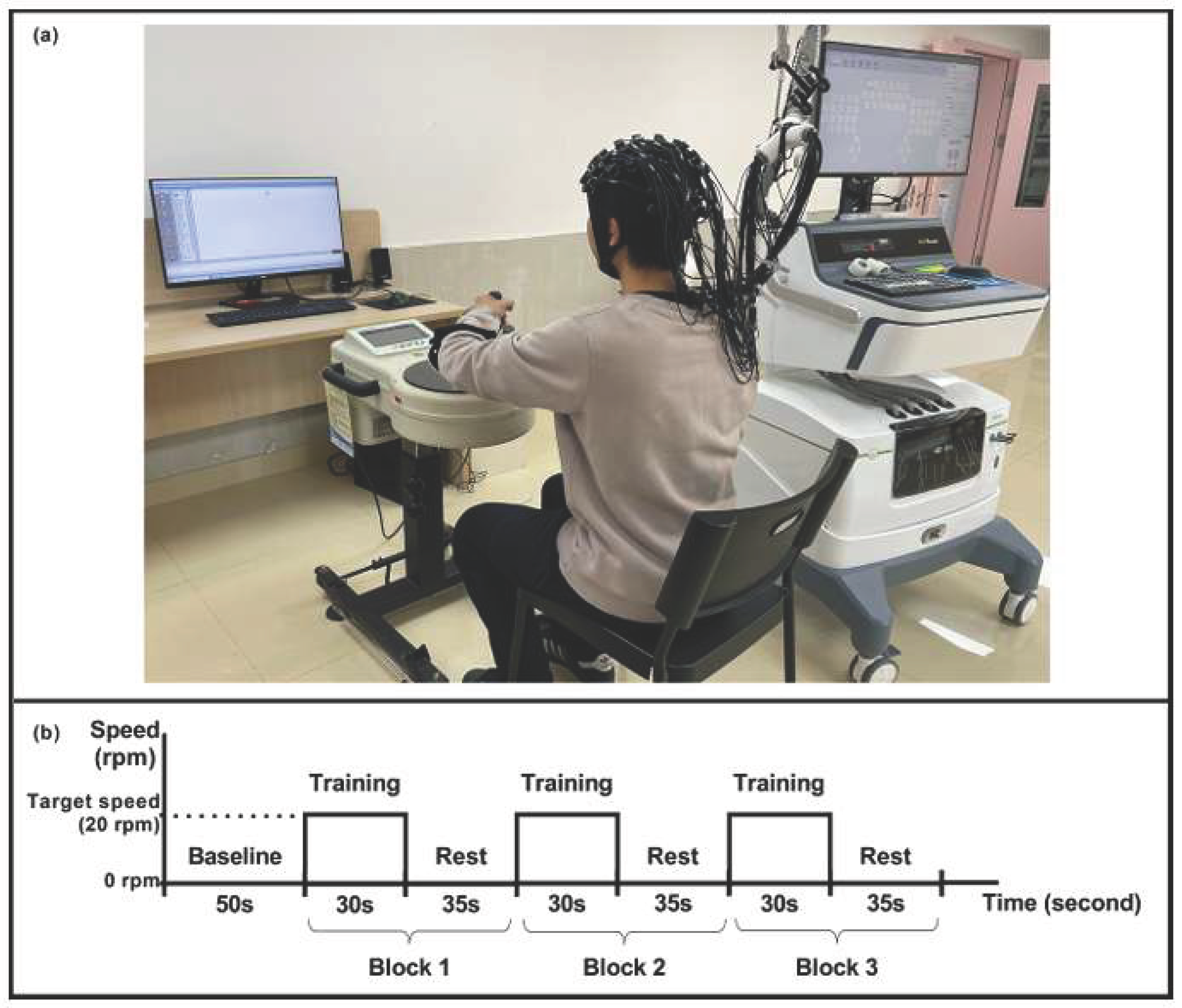
Concerning the use of these techniques in the field of health, one of the most established uses of vibrational spectroscopy is in the area of neurology. Such as the central nervous system is difficult to access and, in many cases, information is required from complex areas, invasive interventions would not be possible, and the use of sensors of this type (see
Figure 6) allows for non-invasive tests.
Figure 6 shows the use of functional near-infrared spectroscopy (fNIRS), a noninvasive neuroimaging method used to study cortical activity by tracking hemodynamic responses during human movement. Its portability and low sensitivity to motion artifacts have made it widely used for assessing cortical responses in motor tasks. In
Figure 6 using fNIRS, is examined sensorimotor cortical activation during active upper-limb movement (with and without visual feedback on motor performance) and during passive movement [
46].
Phillips, Z.
et al. [
47] in their literature review article report on some of the latest applications of NIRS as a tool for monitoring brain activity. The authors point out that the use of these techniques allows multi-parametric monitoring, which enables clinicians to relate cerebral haemodynamic to other measured phenomena. They also highlight the application of these sensors during dynamic activities of patients, which allows a more comprehensive monitoring of brain activity.
As mentioned previously, one of the qualities that make these non-invasive and non-destructive techniques so interesting is that they allow for ex vivo analysis. Yim et al. [
48] like in this study assessed how well near-infrared spectroscopy (NIR) can distinguish between benign and malignant bladder conditions right after surgery, considering the severity and stage of cancer. They analysed 355 spectra from 71 bladder samples taken during tumour removal surgeries. Using a portable NIR device, they scanned the samples within 10 minutes of removal. After routine lab analysis, machine learning models were applied to develop diagnostic tools based on the spectra data. Results showed high accuracy in identifying low- and high-grade cancers (97% sensitivity, 99% specificity) and predicting different cancer stages (97% sensitivity, 92% specificity). This suggests NIRS’s potential for quick assessment of bladder tissues post-surgery, which could help guide treatment decisions and reduce the need for further procedures [
48]. Authors agreed that further research with fiber-optic probes may enhance real-time tissue evaluation.
Vibrational radiation has also demonstrated excellent potential in the therapeutic field. In recent research work Yu
et al. [
49] has described an innovative application to treat wounds and other cutaneous diseases, using an e-bandage device by combining smartphone and NIRS. The energy collected using NIR light emitting diodes (LEDs) with a central wavelength at about 940 nm and the temperature sensors that monitor the healing process by comparing temperature in the middle of the wound and in healthy regions become the e-banding in this system.
Portable Raman devices have been also investigated as an alternative for in vivo diagnosis of skin cancer. A large database including 615 patients and PLS-DA as chemometric tool, were employed to correctly classify in vivo Raman spectra. Prior to scanning skin, in this assay, a specialized oncologist examined patients and after selecting the damaged area proposed the scanning zone of the tissue skin collection spectra [
50].
8. Conclusion and Outlook
Vibrational spectroscopy has emerged as a versatile and valuable technology in many sectors, including food, pharmaceuticals, petrochemicals and healthcare. Its application as a tool for rapid decision-making in the areas of food safety and health is linked to the development of instrumentation and chemometrics. The latest technology in this field focuses on miniaturising spectrometers to increase robustness and reduce costs.
Alternatively, another point of interest in vibrational spectroscopy methodologies is sampling strategy. For the successful development of methods, portable instrumentation, fibre optic probes and spatial spectra collection (HSI) procedures are key approaches to providing innovative solutions and enhancing the sensitivity and accuracy of analyses in food safety and health.
On the other hand, instrumentation development and sampling in vibrational spectroscopy require chemometric and machine learning procedures. The complexity of the vibrational spectrum necessitates the use of mathematical strategies (such as chemometric and machine learning techniques) to extract relevant information that can be related to the target that needs to be detected or quantified. Specific mathematical procedures must be applied to each problem to be solved [
51].
It is evident that the successful and widespread use of NIR technology will go hand-in-hand with the ability to transfer calibration models between different vibrational instruments and sampling strategies. It is well established that developing vibrational methodologies to solve a problem is not a simple task. It requires a large library of spectra in order to identify common or differing patterns between scanned samples. However, if this methodology could be easily transferred between instruments, the applicability and effectiveness of vibrational spectroscopy would increase.
Author Contributions
Conceptualization, C.M.; methodology, A.S, J.M.C.F.; validation, C.M. and A.S.; formal analysis, J.M.C.F. and J.C.C.; investigation, C.M. and J.A.M.; resources, F.F.L. and J.M.C.F.; data curation, M.V. and J.C.C.; writing—original draft preparation, C.M., A.S. and F.F.; writing—review and editing, C.M., A.S. and F.F.; visualization, F.F.L. and M.V.; supervision, A.S. and J.M.C.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Spanish Ministry of Science and Innovation PID2022-142323NB-I00 and PDI2020-117282RB-I00 funded by MCIN/AEI/10.13039/501100011033/ and by “ERDF A way of making Europe. Candela Melendreras acknowledges her PhD grant (BP22-115) from the Asturias Regional Government (Spain).
Data Availability Statement
Data will be made available on request.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Beć, K. B.; Grabska, J.; Huck, C. W. Principles and Applications of Miniaturized Near-Infrared (NIR) Spectrometers. Chemistry – A European Journal 2021, 27(5), 1514–1532. [Google Scholar] [CrossRef]
- Yang, Z.; Albrow-Owen, T.; Cai, W.; Hasan, T. Miniaturization of optical spectrometers. Science 2021, 371(6528). [Google Scholar] [CrossRef]
- Leone, M.; Consales, M.; Passeggio, G.; Buontempo, S.; Zaraket, H.; Youssef, A.; Persiano, G.V.; Cutolo, A.; Cusano, A. Fiber optic soil water content sensor for precision farming. Optics & Laser Technology 2022, 149–107816. [Google Scholar] [CrossRef]
- Beć, K. B.; Grabska, J.; Huck, C.K.; Chem. Principles and Applications of Miniaturized Near-Infrared (NIR) Spectrometers. Eur. J. 2021, 27, 1514. [Google Scholar] [CrossRef]
- Texas Instruments. DLP NIRscan Nano EVM User’s Guide; Texas Instruments: Dallas, TX, USA, 2017. [Google Scholar]
- Huang, W.; Luo, S.; Yang, D.; Zhang, S. Applications of smartphone-based near-infrared (NIR) imaging, measurement, and spectroscopy technologies to point-of-care (POC) diagnostics. J. Zhejiang Univ Sci B 2021, 22(3), 171–189. [Google Scholar] [CrossRef]
- Wang, H.-P.; Chen, P.; Dau, J.W.; Liu, D.; Li, J.Y.; Xu, Y.P.; Chu, X.L. Recent advances of chemometric calibration methods in modern spectroscopy: Algorithms, strategy, and related issues. TrAC Trends in Analytical Chemistry 2022, 153, 116648. [Google Scholar] [CrossRef]
- Xia, J.; Zhang, W.; Zhang, W.; Yang, Y.; Hu, G.; Ge, D.; Liu, H.; Cao, H. A cloud computing-based approach using the visible near-infrared spectrum to classify greenhouse tomato plants under water stress. Comput. Electron. Agr. 2021, 181, 105966. [Google Scholar] [CrossRef]
- Rego, C.; Ferrero, F; Valledor, M.; Campo, J.C.; Forcada, S.; Royo, L.; Soldado, A. A portable IoT NIR spectroscopic system to analyze the quality of dairy farm forage. Computers and Electronics in Agriculture 2020, 175, 105578. [Google Scholar] [CrossRef]
- Perezcampos Mayoral, C.; Gutiérrez Gutiérrez, J.; Cano Pérez, J.L.; Vargas Treviño, M.; Gallegos Velasco, I.B.; Hernández Cruz, P.A.; Torres Rosas, R.; Tepech Carrillo, L.; Arnaud Ríos, J.; Apreza, E.L.; et al. Fiber Optic Sensors for Vital Signs Monitoring. A Review of Its Practicality in the Health Field. Biosensors 2021, 11, 58. [Google Scholar] [CrossRef] [PubMed]
- Pasquini, C. Near infrared spectroscopy: A mature analytical technique with new perspectives – A review. Anal. Chim. Acta 2018, 1026, 8–36. [Google Scholar] [CrossRef]
- Sun, Y.; Tang, H.; Zou, X.; Meng, G.; Wu, N. Raman spectroscopy for food quality assurance and safety monitoring: a review. Current Opinion in Food Science 2022, 47, 100910. [Google Scholar] [CrossRef]
- Surappa, S.; Multani, P.; Ugur Parlatan, U.; Sinawang, P.D.; Kaifi, J.; Akinac de, D.; Demirci, U. Integrated “lab-on-a-chip” microfluidic systems for isolation, enrichment, and analysis of cancer biomarkers. Lab Chip 2023, 23, 2942–2958. [Google Scholar] [CrossRef]
- Nie, Ch; Shaw, I.; Chen, C. Application of microfluidic technology based on surface-enhanced Raman scattering in cancer biomarker detection: A review. Journal of Pharmaceutical Analysis 2023, Volume 13(Issue 12), Pages 1429–1451. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, H.; Zhong, W.; et al. The development and application of SERS-based lat-eral flow immunochromatography in the field of food safety. Microchim Acta 2025, 192, 246. [Google Scholar] [CrossRef]
- Bai, J.; Dong, D. Observing and analyzing living plants using handheld and miniaturized Raman spectrometers: A review. Trends in Analytical Chemistry 2025, 193, 118437. [Google Scholar] [CrossRef]
- Pallua, J. D.; Brunner, A.; Zelger, B.; Huck, C.W.; Schirmer, M.; Laimer, J.; Putzer, D.; Thaler, M.; Zelger, B. New perspectives of hyperspectral imaging for clinical research. NIR news 2021, 32(3-4), 5–13. [Google Scholar] [CrossRef]
- Kumar, V.; Shankar, R.; Rambabu, M.; Dua, Y. Deep learning for hyperspectral image classification: A survey. Computer Science Review 2024, Volume 53, 100658. [Google Scholar] [CrossRef]
- Bruno, F.; Mishra, P.; Davies, B.; Sturrock, K.; Fiore, A. Portable and handheld hyperspectral imaging for non-destructive acrylamide prediction in ground potato crisps. Food Control 2025, Volume 178, 111512. [Google Scholar] [CrossRef]
- Neri, I.; Caponi, S.; Bonacci, F.; Clementi, G.; Cottone, F.; Gammaitoni, L.; Figorilli, S.; Ortenzi, L.; Aisa, S.; Pallottino, F.; Mattarelli, M. Real-Time AI-Assisted Push-Broom Hyperspectral System for Precision Agriculture. Sensors 2023, 24(2), 344. [Google Scholar] [CrossRef]
- Thomas, J.; Lapray, P.; Le Moan, S. Trends in Snapshot Spectral Imaging: Systems, Processing, and Quality. Sensors 2024, 25(3), 675. [Google Scholar] [CrossRef]
- Medina–García, M.; Amigo, J.M.; Martínez-Domingo, M.A.; Valero, E.M.; Jiménez–Carvelo, A.M. Strategies for analysing hyperspectral imaging data for food quality and safety issues – A critical review of the last 5 years. Microchemical Journal 214, 113994. [CrossRef]
- Jiang, Z.; Li, J.; Xu, S.; Liu, Z.; Ma, D.; Wang, Q.; Yuan, Y. Cross-domain hyperspectral image classification. Pattern Recognition 2025, Volume 168(2025), 111836. [Google Scholar] [CrossRef]
- Li, W.; Wu, Y.; Du, L.; Shang, X.; Shi, J. Hyperspectral Imaging for Foreign Matter Detection in Foods: Advances, Challenges, and Future Directions. Foods 2024, 14(17), 3026. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, J.; Wang, H.; Li, S.; Shao, X.; Xia, L.; Darwish, I.A.; Guo, Y.; Sun, X. Advancing detection of fungal and mycotoxins contamination in grains and oilseeds: Hyperspectral imaging for enhanced food safety. Food Chemistry 2025, 470, 142689. [Google Scholar] [CrossRef]
- Ali, H.M.; Xiao, Y.; Kersten-Oertel, M. Surgical hyperspectral imaging: a systematic review. Comput Assist Surg (Abingdon) 2025, 30(1), 2546819. [Google Scholar] [CrossRef]
- Mishra, P.; Roger, J.M.; Jouan-Rimbaud-Bouveresse, D.; Biancolillo, A.; Marini, F.; Nordon, A.; Rutledge, D.N. Recent trends in multi-block data analysis in chemometrics for multi-source data integration. TrAC Trends in Analytical Chemistry 2021, 137, 116206. [Google Scholar] [CrossRef]
- Smilde, A. K.; Mage, I.; Naes, T.; Hankemeier, T.; Lips, M. A.; Kiers, H.A-L-; Acar, E.; Bro, R. Common and distinct components in data fusion. Journal of Chemometrics 2017, 31(7), p. e2900. [Google Scholar] [CrossRef]
- Song, Y.; Westerhuis, J. A.; Smilde, A. K. Separating common (global and local) and distinct variation in multiple mixed types data sets. Journal of Chemometrics 2020, 34(1), e3197. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, J.; Lin, T.; Ying, Y. Food and agro-product quality evaluation based on spectroscopy and deep learning: A review. Trends in Food Science & Technology 2021, 112, 431–441. [Google Scholar] [CrossRef]
- Mishra, P.; Klont, R.; Verkleij, T.; Wisse, S. Translating near-infrared spectroscopy from laboratory to commercial slaughterhouse: Existing challenges and solutions. Infrared Physics & Technology 2021, 119, 103918. [Google Scholar] [CrossRef]
- Yang, C.; Guo, Z.; Barbin, D. F.; Dai, Z.; Watson, N.; Povey, M.; Zou, X. Hyperspectral Imaging and Deep Learning for Quality and Safety Inspection of Fruits and Vegetables: A Review. Journal of Agricultural and Food Chemistry 2025, 73(17), 10019–10035. [Google Scholar] [CrossRef]
- Benelli, A.; Cevoli, C.; Fabbri, A.; Ragni, L. Ripeness evaluation of kiwifruit by hyperspectral imaging. Biosyst. Eng. 2022, 42–52. [Google Scholar] [CrossRef]
- Zhang, Q.; Lu, Z.; Liu, Z.; Li, J.; Chang, M.; Zuo, M. Application of Machine Learning in Food Safety Risk Assessment. Foods 2024, 14(23), 4005. [Google Scholar] [CrossRef]
- Entrenas, J.-A.; Pérez-Marín, D.; Torres, I.; Garrido-Varo, A.; Sánchez, M.-T. Simultaneous detection of quality and safety in spinach plants using a new generation of NIRS sensors. Postharvest Biology and Technology 2020, 160, 111026. [Google Scholar] [CrossRef]
- Parastar, H.; van Kollenburg, G.; Weesepoel, Y.; van den Doel, A.; Buydens, L.; Jansen, J. Integration of handheld NIR and machine learning to “Measure & Monitor” chicken meat authenticity. Food Control 2020, 112, 107149. [Google Scholar] [CrossRef]
- de la Roza-Delgado, B.; Garrido-Varo, A.; Soldado, A.; González Arrojo, A.; Cuevas Valdés, M.; Maroto, F.; Pérez Marín, D. Matching portable NIRS instruments for in situ monitoring indicators of milk composition. Food Control 2017, 76, 74–81. [Google Scholar] [CrossRef]
- Yakes, B. J.; Ellsworth, Z.; Karunathilaka, S. R.; Crump, E. Evaluation of Portable Sensor and Spectroscopic Devices for Seafood Decomposition Determination. Food Anal. Methods 2021, 14(11), 2346–2356. [Google Scholar] [CrossRef]
- Beć, K. B.; Grabska, J.; Huck, C. W. Miniaturized NIR Spectroscopy in Food Analysis and Quality Control: Promises, Challenges, and Perspectives. Foods 2022, 11(10). [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Gao, X.; Qi, W-L.; QWang, Y.; Wang, X.; Zhou, J.; Lu, D.; Chen, B. Advances in differentiation and identification of foodborne bacteria using near infrared spectroscopy. Anal. Methods 2021, 13(23), 2558–2566. [Google Scholar] [CrossRef]
- Soni, A.; Dixit, Y.; Reis, M. M.; Brightwell, G. Hyperspectral imaging and machine learning in food microbiology: Developments and challenges in detection of bacterial, fungal, and viral contaminants. Comprehensive Reviews in Food Science and Food Safety 2022, 21(4), 3717–3745. [Google Scholar] [CrossRef]
- Hardy, M.; Moser, B.; Haughey, S. A.; Elliott, C. T. Does the fish rot from the head? Hyperspectral imaging and machine learning for the evaluation of fish freshness. Chemometrics and Intelligent Laboratory Systems 2024, 245, 105059. [Google Scholar] [CrossRef]
- Aramendia, J.; García-Velasco, N.; Amigo, J.M.; Izagirre, U.; Seifert, A.; Soto, M.; Cstro, K. Evidence of internalized microplastics in mussel tissues detected by volumetric Raman imaging. Science of The Total Environment 2024, 914, 169960. [Google Scholar] [CrossRef]
- Wu, Z.; Pu, H.; Sun, D.-W. Fingerprinting and tagging detection of mycotoxins in agri-food products by surface-enhanced Raman spectroscopy: Principles and recent applications. Trends in Food Science & Technology 2021, 393–404. [Google Scholar] [CrossRef]
- Logan, B. G.; Hopkins, D. L.; Schmidtke, L. M.; Fowler, S. M. Authenticating common Australian beef production systems using Raman spectroscopy. Food Control 2021, 121, 107652. [Google Scholar] [CrossRef]
- Xia, W.; Dai, R.; Xu, X.; Huai, B.; Bai, Z.; Zhang, J.; Jin, M.; Niu, W. Cortical mapping of active and passive upper limb training in stroke patients and healthy people: A functional near-infrared spectroscopy study. Brain Research 2022, 1788, 147935. [Google Scholar] [CrossRef]
- Phillips, Z.; Canoy, R. J.; Paik, S.; Lee, S.H.; Kim, B.M. Functional Near-Infrared Spectroscopy as a Personalized Digital Healthcare Tool for Brain Monitoring. J. Clin Neurol. 2023, 19(2), 115–124. [Google Scholar] [CrossRef]
- Yim, A.; Alberto, M.; Sharma, V.; Gree, A.; Mclean, A.; Plessis, J.; Wong, L.M.; Wood, B.; Ischia, J.; Raman, J.; Bolton, D. Near-infrared spectroscopy as a novel method of ex vivo bladder cancer tissue characterisation. BJU International 2024. [Google Scholar] [CrossRef] [PubMed]
- Yu M.; Yi C.; Lin S.; Ye H., Xue J.; Yin J.; Zhong R.; Yao H., Liao H.; Zhang Y.; Yang J.; Guo Y.; Song X-J; Ye T-T. e-Bandage: Exploiting Smartphone as a Therapeutic Device for Cutaneous Wound Treatment. Advanced Intelligent Systems 2024, 6(3), 2300494. [CrossRef]
- Bratchenko, I. A.; Bratchenko, L.A.; Moryatov, A. A.; Khristoforova, Y.A.; Artemyev, D.N.; Myakinin, O.O.; Orlov, A.E.; Kozlov, S.V.; Zakharov, V.P. In vivo diagnosis of skin cancer with a portable Raman spectroscopic device. Experimental Dermatology 2021, 30(5), 652–663. [Google Scholar] [CrossRef]
- Yang, G.; Wang, Z. A Deep Transfer Contrastive Learning Network for Few-Shot Hyperspectral Image Classification. Remote Sensing 2024, 17(16), 2800. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).