Submitted:
23 December 2025
Posted:
25 December 2025
Read the latest preprint version here
Abstract
Keywords:
Introduction
Modern Therapeutic Advances for Cryptococcal Meningitis
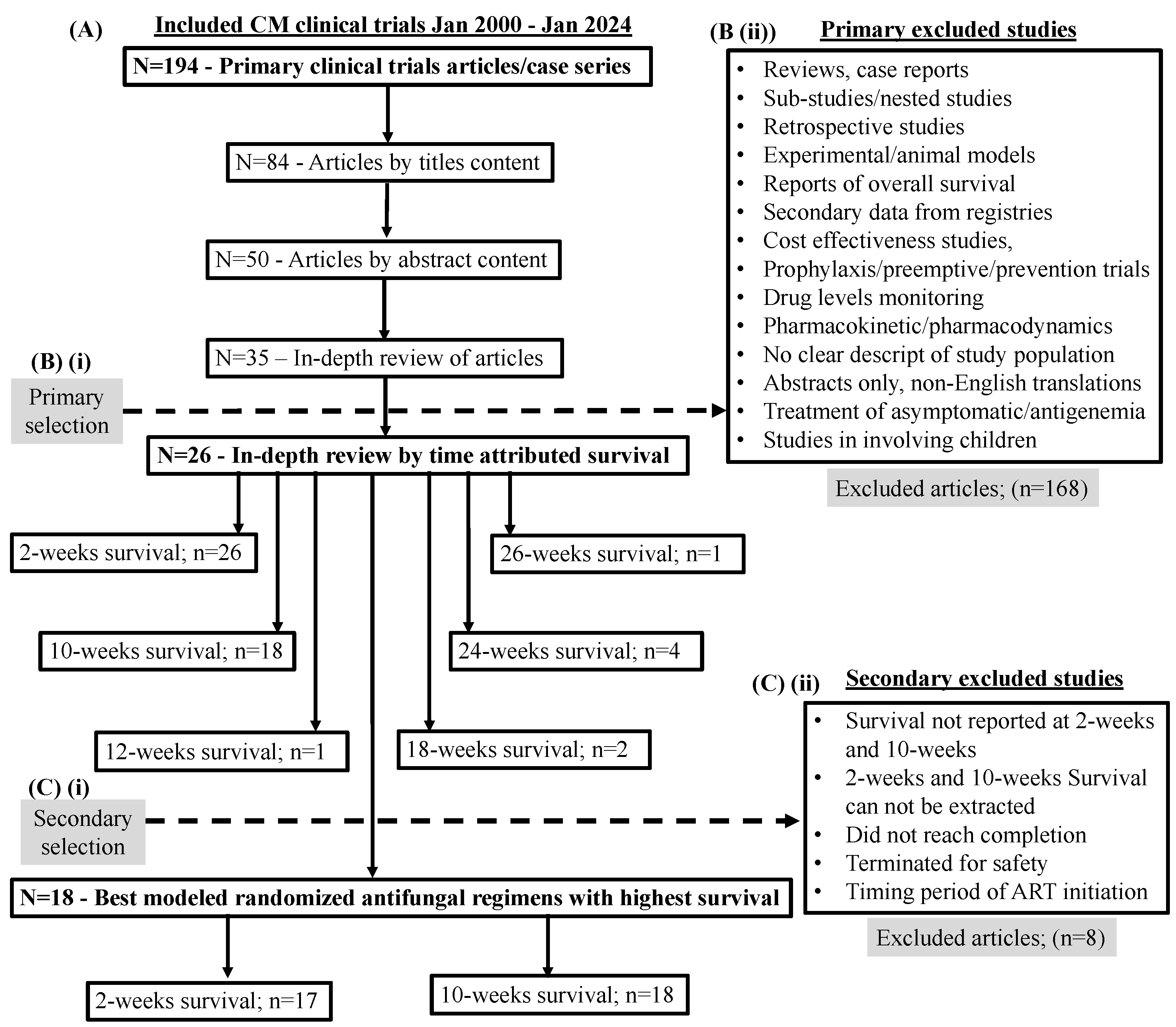
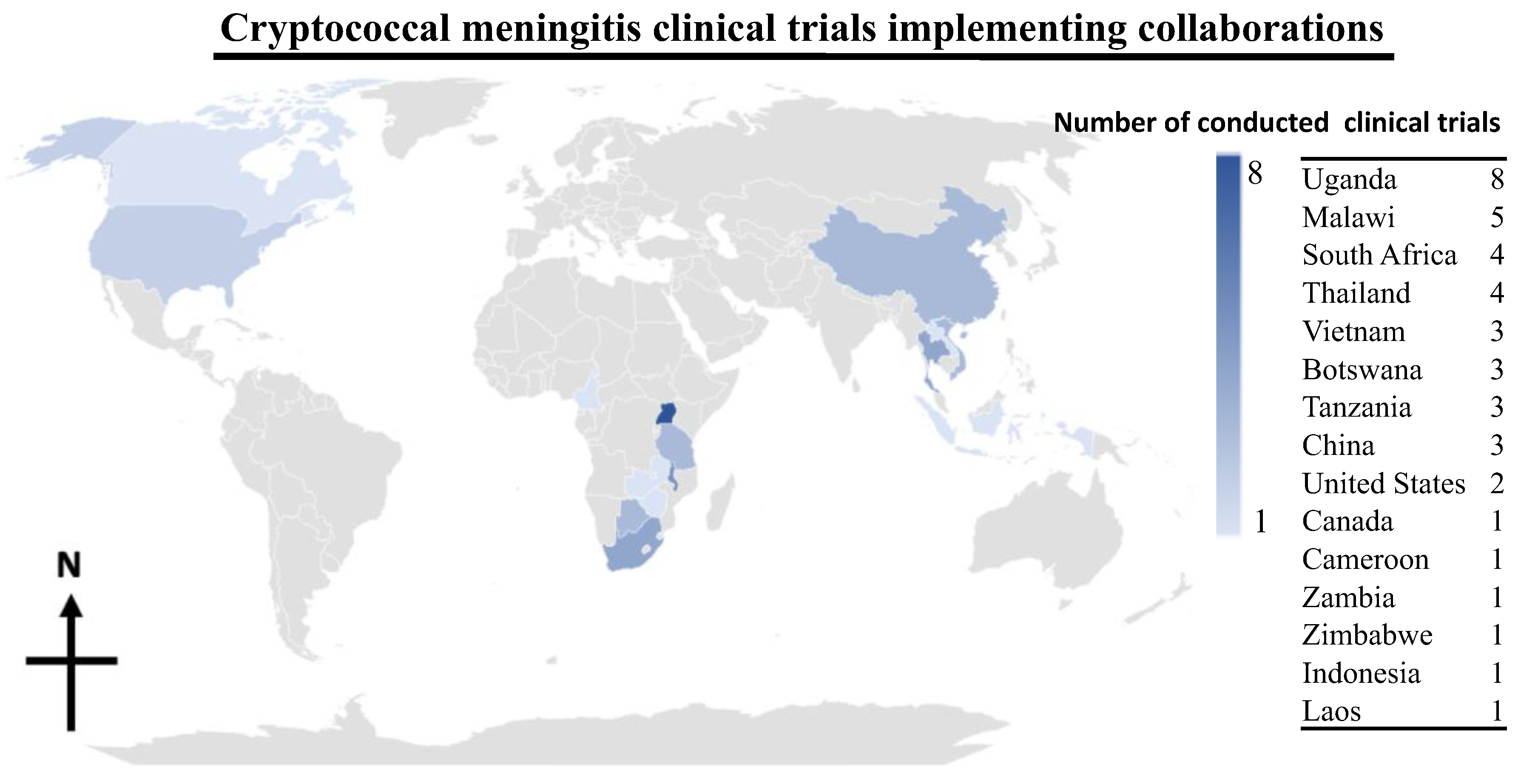
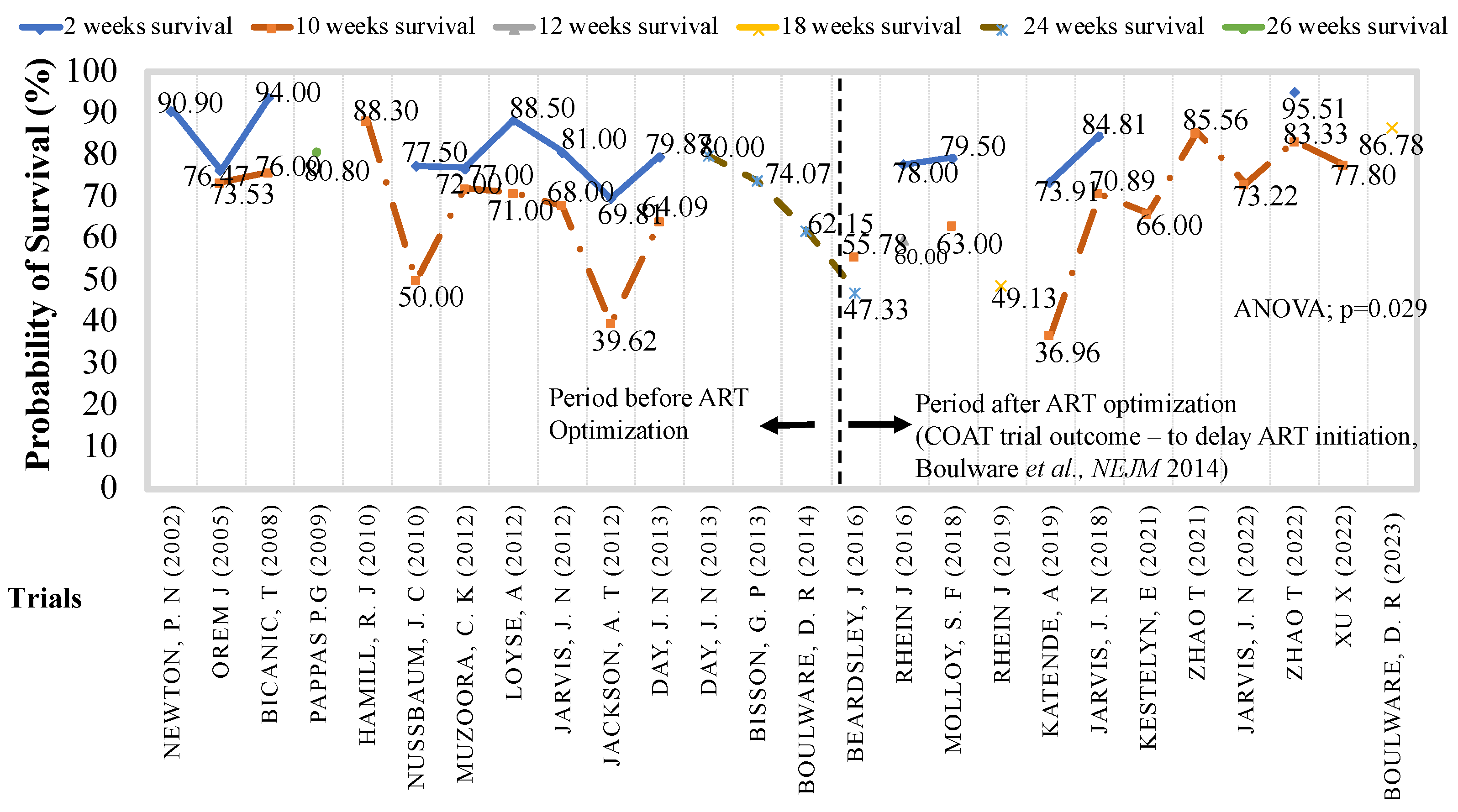
Evolution of Antifungal Clinical Trials to Improve Survival with Cryptococcal Meningitis
Trials of Antifungal Dosage Formulations to Improve Cryptococcal Meningitis Survival
Evolution of Promising Cryptococcal Meningitis Optimal Antifungal Therapy
Conclusion
Limitations
Author Contributions
Funding
Data Availability Statement
Ethics Approval and Consent to Participate:
Clinical Trial Number:
Consent for Publication
Acknowledgments
Conflicts of Interest
References
- Ellis J, Bangdiwala AS, Cresswell FV, et al. The Changing Epidemiology of HIV-Associated Adult Meningitis, Uganda 2015–2017. Open Forum Infect Dis. 2019;6(10):ofz419. [CrossRef]
- Rajasingham R, Smith RM, Park BJ, et al. Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Lancet Infect Dis. 2017;17(8):873-881. [CrossRef]
- Kashef Hamadani BH, Franco-Paredes C, McCollister B, Shapiro L, Beckham JD, Henao-Martínez AF. Cryptococcosis and cryptococcal meningitis: New predictors and clinical outcomes at a United States academic medical centre. Mycoses. 2018;61(5):314-320. [CrossRef]
- Henao-Martínez AF, Gross L, Mcnair B, et al. Risk Factors for Cryptococcal Meningitis: A Single United States Center Experience. Mycopathologia. 2016;181(11-12):807-814. [CrossRef]
- Mugabi T, Namombwe S, Dai B, et al. Etiology and Outcomes of Meningitis among Adults in Three Ugandan Referral Hospitals, 2018–2023: A Prospective Cohort Study in a High-HIV Endemic Setting.
- Rajasingham R, Govender NP, Jordan A, et al. The global burden of HIV-associated cryptococcal infection in adults in 2020: a modelling analysis. Lancet Infect Dis. 2022;22(12):1748-1755. [CrossRef]
- Perfect JR. Efficiently Killing a Sugar-Coated Yeast. N Engl J Med. 2013;368(14):1354-1356. [CrossRef]
- Tenforde MW, Gertz AM, Lawrence DS, et al. Mortality from HIV-associated meningitis in sub-Saharan Africa: a systematic review and meta-analysis. J Int AIDS Soc. 2020;23(1):e25416. [CrossRef]
- Ellis J, Nsangi L, Bangdiwala A, et al. advanced HIV disease: a randomised strategy trial. Wellcome Open Res. Published online 2024.
- Okwir M, Link A, Rhein J, et al. High Burden of Cryptococcal Meningitis Among Antiretroviral Therapy–Experienced Human Immunodeficiency Virus–Infected Patients in Northern Uganda in the Era of “Test and Treat”: Implications for Cryptococcal Screening Programs. Open Forum Infect Dis. 2022;9(2):ofac004. [CrossRef]
- Okurut, S. (2023) B cell responses, immune modulation and survival among patients with HIV-associated cryptococcal meningitis. [PhD. Thesis]. [Kampala, Uganda]: Makerere University.
- Kimuda S, Kwizera R, Dai B, et al. Comparison of Early Fungicidal Activity and Mortality Between Daily Liposomal Amphotericin B and Daily Amphotericin B Deoxycholate for Cryptococcal Meningitis. Clin Infect Dis. 2025;80(1):153-159. [CrossRef]
- Bennett JE, Dismukes WE, Duma RJ, et al. A Comparison of Amphotericin B Alone and Combined with Flucytosine in the Treatment of Cryptoccal Meningitis. N Engl J Med. 1979;301(3):126-131. [CrossRef]
- Perfect JR. The antifungal pipeline: a reality check. Nat Rev Drug Discov. 2017;16(9):603-616. [CrossRef]
- Okurut S, Boulware DR, Manabe YC, et al. Impact of cerebrospinal fluid leukocyte infiltration and activated neuroimmune mediators on survival with HIV-associated cryptococcal meningitis. Rodrigues ML, ed. PLoS Negl Trop Dis. 2025;19(2):e0012873. [CrossRef]
- Okurut S, Boulware DR, Okafor E, et al. Divergent neuroimmune signatures in the cerebrospinal fluid predict differential gender-specific survival among patients with HIV-associated cryptococcal meningitis. Front Immunol.
- Han X, Liu H, Wang Y, et al. A nomogram for predicting paradoxical immune reconstitution inflammatory syndrome associated with cryptococcal meningitis among HIV-infected individuals in China. AIDS Res Ther. 2022;19(1):20. [CrossRef]
- Kambugu A, Meya DB, Rhein J, et al. Outcomes of cryptococcal meningitis in Uganda before and after the availability of highly active antiretroviral therapy. Clin Infect Dis. 2008;46(11):1694-1701. [CrossRef]
- Dutcher JD. The Discovery and Development of Amphotericin B. Dis Chest. 1968;54:296-298. [CrossRef]
- Sigera LSM, Denning DW. Flucytosine and its clinical usage. Ther Adv Infect Dis. 2023;10:20499361231161387. [CrossRef]
- Richardson K, Cooper K, Marriott MS, Tarbit MH, Troke F, Whittle PJ. Discovery of Fluconazole, a Novel Antifungal Agent. Clin Infect Dis. 1990;12(Supplement_3):S267-S271. [CrossRef]
- Boulware DR, Meya DB, Muzoora C, et al. Timing of Antiretroviral Therapy after Diagnosis of Cryptococcal Meningitis. N Engl J Med. 2014;370(26):2487-2498. [CrossRef]
- Boulware DR, Atukunda M, Kagimu E, et al. Oral Lipid Nanocrystal Amphotericin B for Cryptococcal Meningitis: A Randomized Clinical Trial. Clin Infect Dis. 2023;77(12):1659-1667. [CrossRef]
- Nussbaum JC, Jackson A, Namarika D, et al. Combination Flucytosine and High-Dose Fluconazole Compared with Fluconazole Monotherapy for the Treatment of Cryptococcal Meningitis: A Randomized Trial in Malawi. Clin Infect Dis. 2010;50(3):338-344. [CrossRef]
- Meya DB, Manabe YC, Boulware DR, Janoff EN. The immunopathogenesis of cryptococcal immune reconstitution inflammatory syndrome: understanding a conundrum. Curr Opin Infect Dis. 2016;29(1):10-22. [CrossRef]
- Van Wyk M, Govender NP, Mitchell TG, Litvintseva AP. Multilocus sequence typing of serially collected isolates of cryptococcus from HIV-infected patients in South Africa. J Clin Microbiol. 2014;52(6):1921-1931. [CrossRef]
- Hakyemez IN, Erdem H, Beraud G, et al. Prediction of unfavorable outcomes in cryptococcal meningitis: results of the multicenter Infectious Diseases International Research Initiative (ID-IRI) cryptococcal meningitis study. Eur J Clin Microbiol Infect Dis. 2018;37(7):1231-1240. [CrossRef]
- Ding Y, Li P, He Q, et al. The CD4+ T-lymphocyte count is an important predictor for the prognosis of cryptococcosis. Eur J Clin Microbiol Infect Dis. 2017;36(5):897-904. [CrossRef]
- Bahr NC, Skipper CP, Huppler-Hullsiek K, et al. Recurrence of Symptoms Following Cryptococcal Meningitis: Characterizing a Diagnostic Conundrum With Multiple Etiologies. Clin Infect Dis. 2023;76(6):1080-1087. [CrossRef]
- Khawcharoenporn T, Damronglerd P, Chunloy K, Sha BE. Enhanced inpatient rounds, appointment reminders, and patient education improved HIV care engagement following hospital discharge. Int J STD AIDS. 2018;29(7):641-649. [CrossRef]
- Beardsley J, Wolbers M, Kibengo FM, et al. Adjunctive Dexamethasone in HIV-Associated Cryptococcal Meningitis. N Engl J Med. 2016;374(6):542-554. [CrossRef]
- Day J, Imran D, Ganiem AR, et al. CryptoDex: A randomised, double-blind, placebo-controlled phase III trial of adjunctive dexamethasone in HIV-infected adults with cryptococcal meningitis: study protocol for a randomised control trial. Trials. 2014;15(1):441. [CrossRef]
- Rhein J, Huppler Hullsiek K, Tugume L, et al. Adjunctive sertraline for HIV-associated cryptococcal meningitis: a randomised, placebo-controlled, double-blind phase 3 trial. Lancet Infect Dis. 2019;19(8):843-851. [CrossRef]
- Rhein J, Morawski BM, Hullsiek KH, et al. Efficacy of adjunctive sertraline for the treatment of HIV-associated cryptococcal meningitis: an open-label dose-ranging study. Lancet Infect Dis. 2016;16(7):809-818. [CrossRef]
- Jarvis JN, Meintjes G, Rebe K, et al. Adjunctive interferon-γ immunotherapy for the treatment of HIV-associated cryptococcal meningitis: a randomized controlled trial. AIDS. 2012;26(9):1105-1113. [CrossRef]
- Ngan NTT, Thanh Hoang Le N, Vi Vi NN, et al. An open label randomized controlled trial of tamoxifen combined with amphotericin B and fluconazole for cryptococcal meningitis. eLife. 2021;10:e68929. [CrossRef]
- Newton PN, Short JM, Chierakul W, et al. A Randomized, Double-Blind, Placebo- Controlled Trial of Acetazolamide for the Treatment of Elevated Intracranial Pressure in Cryptococcal Meningitis.
- Orem J, Tindyebwa L, Twinoweitu O, Mukasa B, Tomberland M, Mbidde EK. Feasibility study of serial lumbar puncture and acetazolamide combination in the management of elevated cerebrospinal fluid pressure in AIDS patients with cryptococcal meningitis in Uganda. Trop Doct. 2005;35(1):19-21. [CrossRef]
- Zhao T, Xu X, Wu Y, et al. Comparison of amphotericin B deoxycholate in combination with either flucytosine or fluconazole, and voriconazole plus flucytosine for the treatment of HIV-associated cryptococcal meningitis: a prospective multicenter study in China. BMC Infect Dis. 2022;22(1):677. [CrossRef]
- Pappas PG, Chetchotisakd P, Larsen RA, et al. A Phase II Randomized Trial of Amphotericin B Alone or Combined with Fluconazole in the Treatment of HIV-Associated Cryptococcal Meningitis. Clin Infect Dis. 2009;48(12):1775-1783. [CrossRef]
- Jackson AT, Nussbaum JC, Phulusa J, et al. A phase II randomized controlled trial adding oral flucytosine to high-dose fluconazole, with short-course amphotericin B, for cryptococcal meningitis. AIDS. 2012;26(11):1363-1370. [CrossRef]
- Pappas PG, Chetchotisakd P, Larsen RA, et al. A Phase II Randomized Trial of Amphotericin B Alone or Combined with Fluconazole in the Treatment of HIV-Associated Cryptococcal Meningitis. Clin Infect Dis. 2009;48(12):1775-1783. [CrossRef]
- Day JN, Chau TTH, Wolbers M, et al. Combination Antifungal Therapy for Cryptococcal Meningitis. N Engl J Med. 2013;368(14):1291-1302. [CrossRef]
- Hamill RJ, Sobel JD, El-Sadr W, et al. Comparison of 2 Doses of Liposomal Amphotericin B and Conventional Amphotericin B Deoxycholate for Treatment of AIDS-Associated Acute Cryptococcal Meningitis: A Randomized, Double-Blind Clinical Trial of Efficacy and Safety. Clin Infect Dis. 2010;51(2):225-232. [CrossRef]
- Loyse A, Wilson D, Meintjes G, et al. Comparison of the Early Fungicidal Activity of High-Dose Fluconazole, Voriconazole, and Flucytosine as Second-Line Drugs Given in Combination With Amphotericin B for the Treatment of HIV-Associated Cryptococcal Meningitis. Clin Infect Dis. 2012;54(1):121-128. [CrossRef]
- Muzoora CK, Kabanda T, Ortu G, et al. Short course amphotericin B with high dose fluconazole for HIV-associated cryptococcal meningitis. J Infect. 2012;64(1):76-81. [CrossRef]
- Jarvis JN, Leeme TB, Molefi M, et al. Short-course High-dose Liposomal Amphotericin B for Human Immunodeficiency Virus–associated Cryptococcal Meningitis: A Phase 2 Randomized Controlled Trial. Clin Infect Dis. 2019;68(3):393-401. [CrossRef]
- Katende A, Mbwanji G, Faini D, et al. Short-course amphotericin B in addition to sertraline and fluconazole for treatment of HIV-associated cryptococcal meningitis in rural Tanzania. Mycoses. 2019;62(12):1127-1132. [CrossRef]
- Jarvis JN, Lawrence DS, Meya DB, et al. Single-Dose Liposomal Amphotericin B Treatment for Cryptococcal Meningitis. N Engl J Med. 2022;386(12):1109-1120. [CrossRef]
- Zhao T, Xu XL, Lu YQ, et al. The Effect of Early vs. Deferred Antiretroviral Therapy Initiation in HIV-Infected Patients With Cryptococcal Meningitis: A Multicenter Prospective Randomized Controlled Analysis in China. Front Med. 2021;8(November):1-12. [CrossRef]
- Xu X, Lu Y, Harypursat V, et al. The Optimal Timing of Antiretroviral Therapy Initiation in HIV-Infected Patients with Cryptococcal Meningitis: A Multicenter Prospective Randomized Controlled Trial. Acta Med Okayama. 2021;75(1).
- Molloy SF, Kanyama C, Heyderman RS, et al. Antifungal Combinations for Treatment of Cryptococcal Meningitis in Africa. N Engl J Med. 2018;378(11):1004-1017. [CrossRef]
- Newton PN, Thai LH, Tip NQ, et al. A Randomized, Double-Blind, Placebo-Controlled Trial of Acetazolamide for the Treatment of Elevated Intracranial Pressure in Cryptococcal Meningitis. Clin Infect Dis. 2002;35(6):769-772. [CrossRef]
- Bicanic T, Wood R, Meintjes G, et al. High-Dose Amphotericin B with Flucytosine for the Treatment of Cryptococcal Meningitis in HIV-Infected Patients: A Randomized Trial. Clin Infect Dis. 2008;47(1):123-130. [CrossRef]
- Boulware DR, Meya DB, Muzoora C, et al. Timing of Antiretroviral Therapy after Diagnosis of Cryptococcal Meningitis. N Engl J Med. 2014;370(26):2487-2498. [CrossRef]
- Molloy SF, Kanyama C, Heyderman RS, et al. Antifungal combinations for treatment of cryptococcal meningitis in Africa. N Engl J Med. 2018;378(11):1004-1017. [CrossRef]
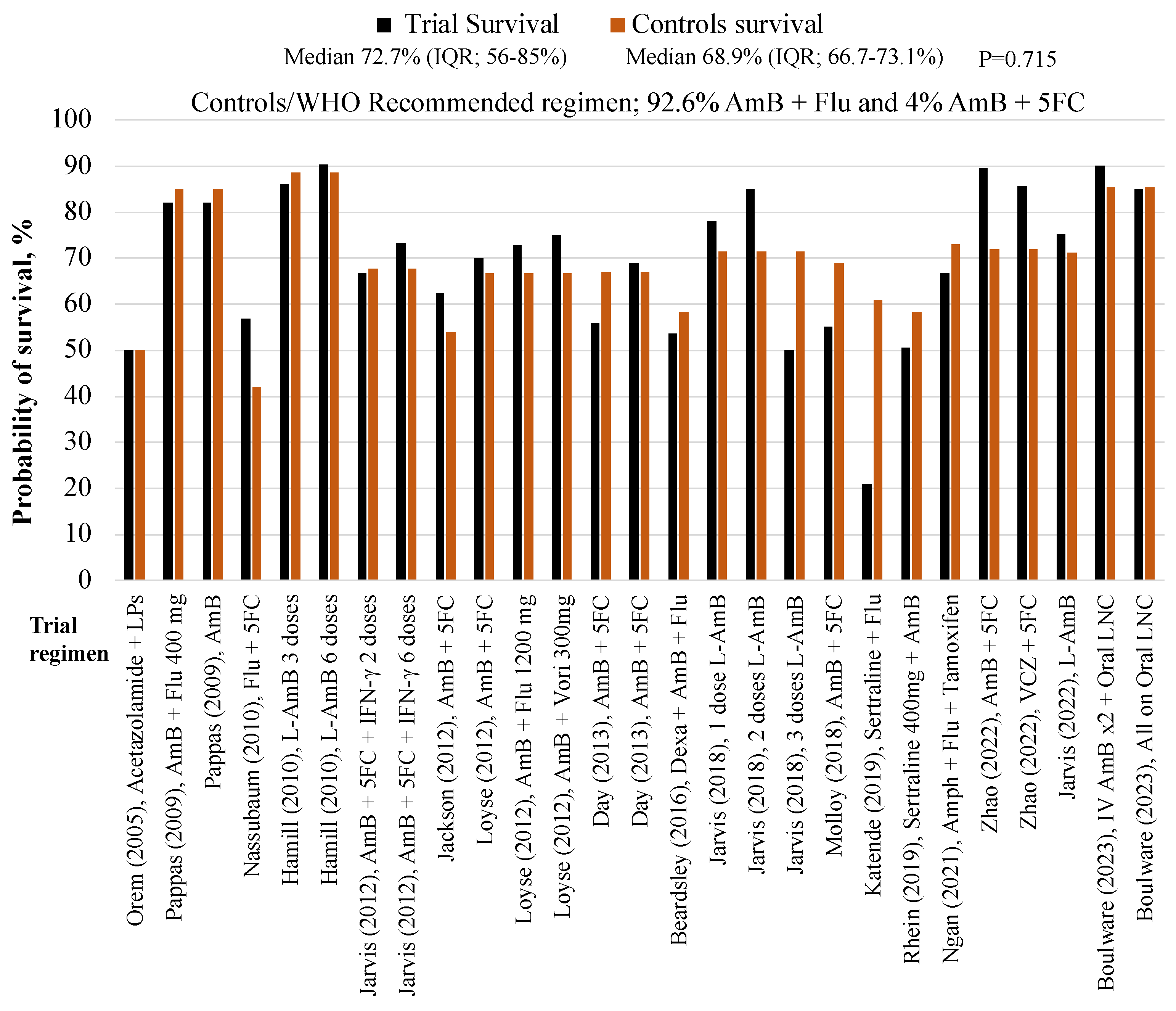
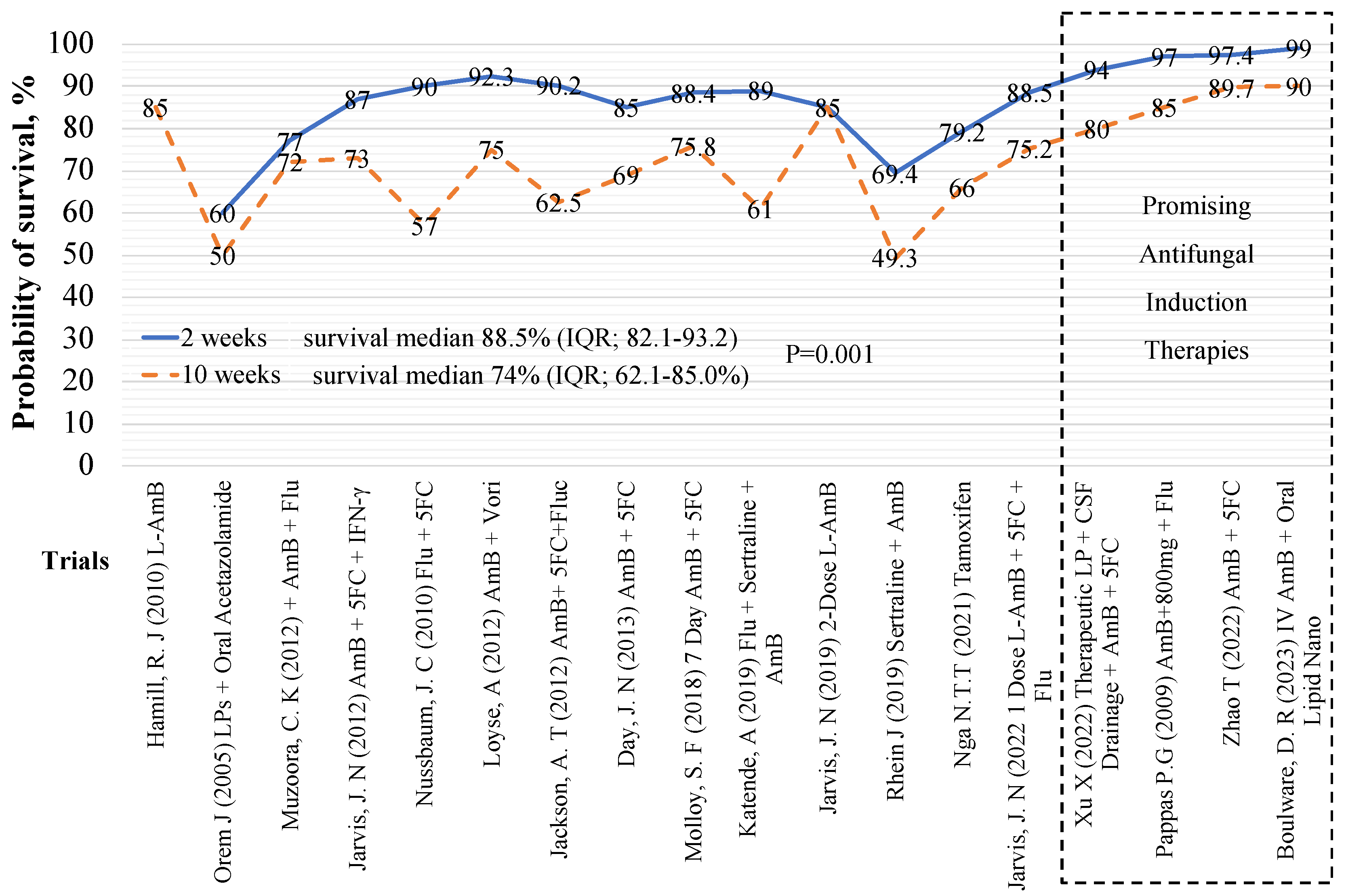
| Author | Trial regimen | Trial arm, N | 10-week, survival, n (%) |
Control arm, N, AmB + Flu, |
10-week survival, n (%) |
| Boulware (2023) | IV AmB x2 + Oral AmB Nanocrystals | 40 | 35 (90%) | 41** | 35 (85.4%) |
| Boulware (2023) | All - Oral AmB Nanocrystals | 40 | 34 (85%) | 41** | 35 (85.4%) |
| Zhao (2022) | 10-week AmB + 5FC | 78 | 70 (89.7%) | 50 | 36 (72%) |
| Zhao (2022) | 10-week VCZ + 5FC | 28 | 24 (85.7%) | 50 | 36 (72%) |
| Jarvis (2022) | L-AmB 10 mg/kg x1 | 101 | 76 (75.2%) | 117** | 117 (71.3%) |
| Ngan NTT, (2021) | AmB + Flu + Tamoxifen | 24 | 16 (66.7%) | 26 | 19 (73.1%) |
| Rhein, (2019) | AmB + Fluconazole + Sertraline 400mg | 229 | 113 (50.7%) | 231 | 135 (58.4%) |
| Katende, (2019) | Sertraline + Fluconazole | 28 | 6 (21%) | 18 | 11 (61%) |
| Jarvis, (2018) | Single dose L-AmB + fluconazole | 18 | 14 (78%) | 21 | 15 (71.4%) |
| Jarvis, (2018) | 2 doses L-AmB + fluconazole | 20 | 17 (85%) | 21 | 15 (71.4%) |
| Jarvis, (2018) | 3 doses L-AmB + fluconazole | 20 | 10 (50%) | 21 | 15 (71.4%) |
| Molloy (2018) | AmB + 5FC | 225 | 124 (55%) | 228 | 157 (68.9%) |
| Beardsley (2016) | Dexa + AmB + Flu | 224 | 120 (53.6%) | 226 | 132 (58.4%) |
| Day J (2013) | AmB | 99 | 55 (56%) | 99 | 66 (67%) |
| Day J (2013) | AmB + 5FC | 100 | 70 (69%) | 99 | 66 (67%) |
| Jarvis (2012) | AmB + 5FC + IFN-γ | 57 | 40 (70.2%) | 62 | 42 (67.7%) |
| Jackson T (2012) | AmB + 5FC | 40 | 25 (62.5%) | 39 | 21 (53.8%) |
| Loyse (2012) | AmB + 5FC | 20 | 14 (70%) | 21 | 14 (66.7%) |
| Loyse (2012) | AmB + Flu 1200 mg | 22 | 16 (72.7%) | 21 | 14 (66.7%) |
| Loyse (2012) | AmB + Vori 300mg | 12 | 9 (75%) | 21 | 14 (66.7%) |
| Nussbaum, 2010 | Flu + 5FC | 21 | 12 (57%) | 19 | 8 (42%) |
| Hamill J.R, (2010) | L-AmB 3 mg/kg | 74 | 64 (86%) | 77 | 68 (88.5%) |
| Hamill J.R, (2010) | L-AmB 6 mg/kg | 85 | 77 (90.4%) | 77 | 68 (88.5%) |
| Pappas (2009) | AmB + Flu 400 mg | 48 | 41 (85%) | 45 | 44 (97%) |
| Pappas (2009) | AmB | 47 | 40 (85%) | 45 | 44 (97%) |
| Orem J (2005) | Acetazolamide + LPs | 10 | 5 (50%) | 8* | 4 (50%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.





