Submitted:
22 December 2025
Posted:
23 December 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
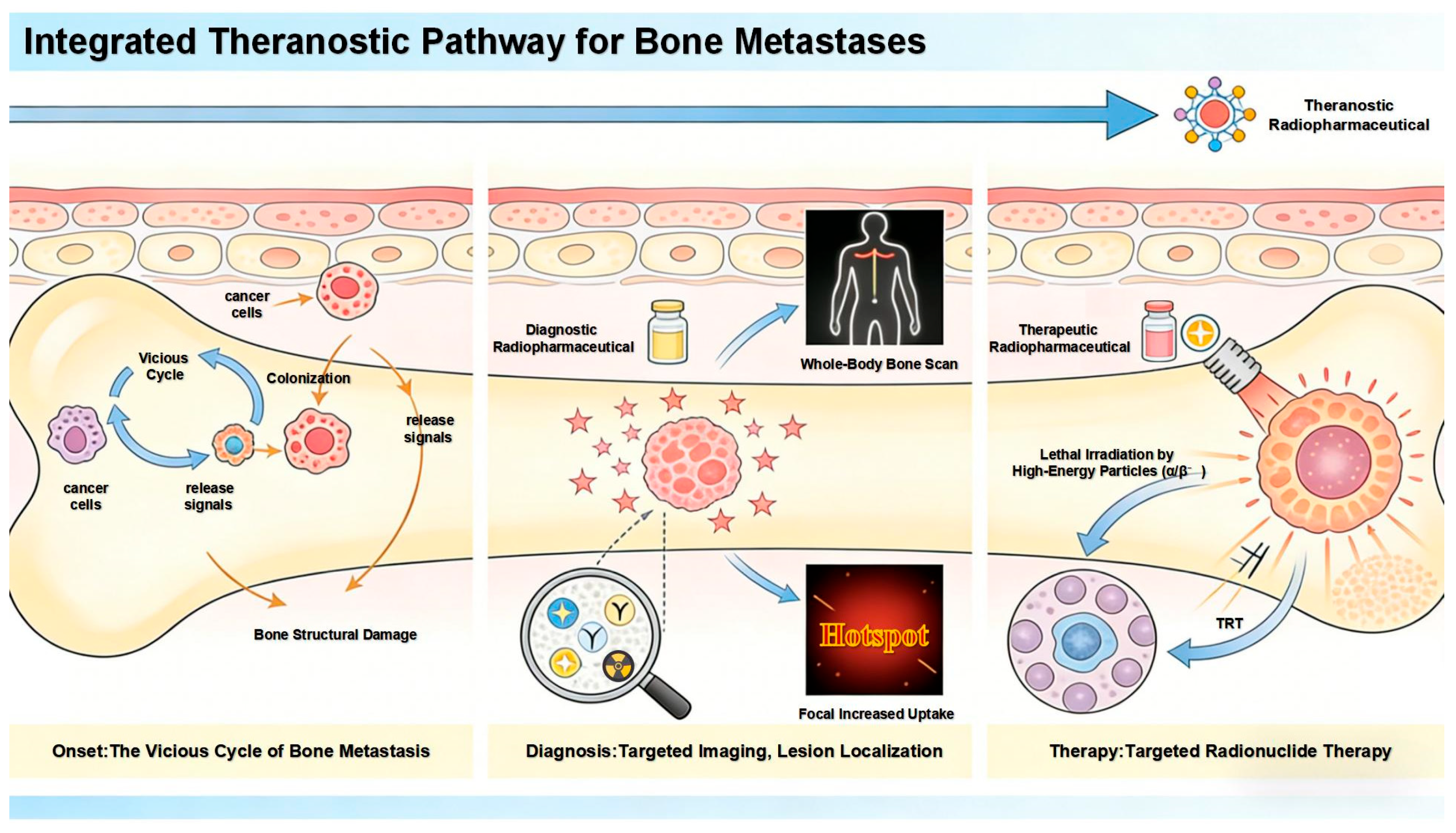
2. Bisphosphonate-Based Imaging Agents for Bone
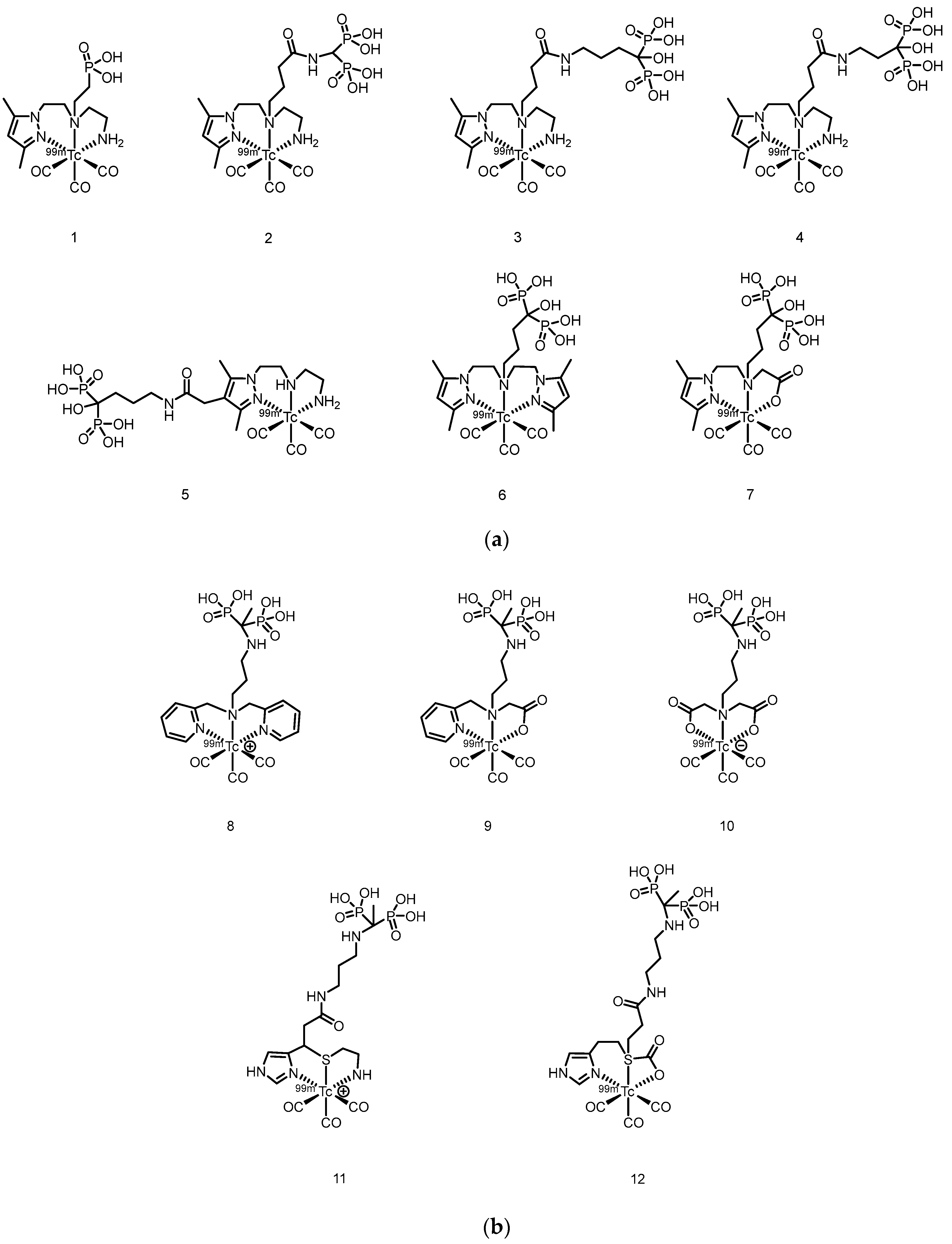
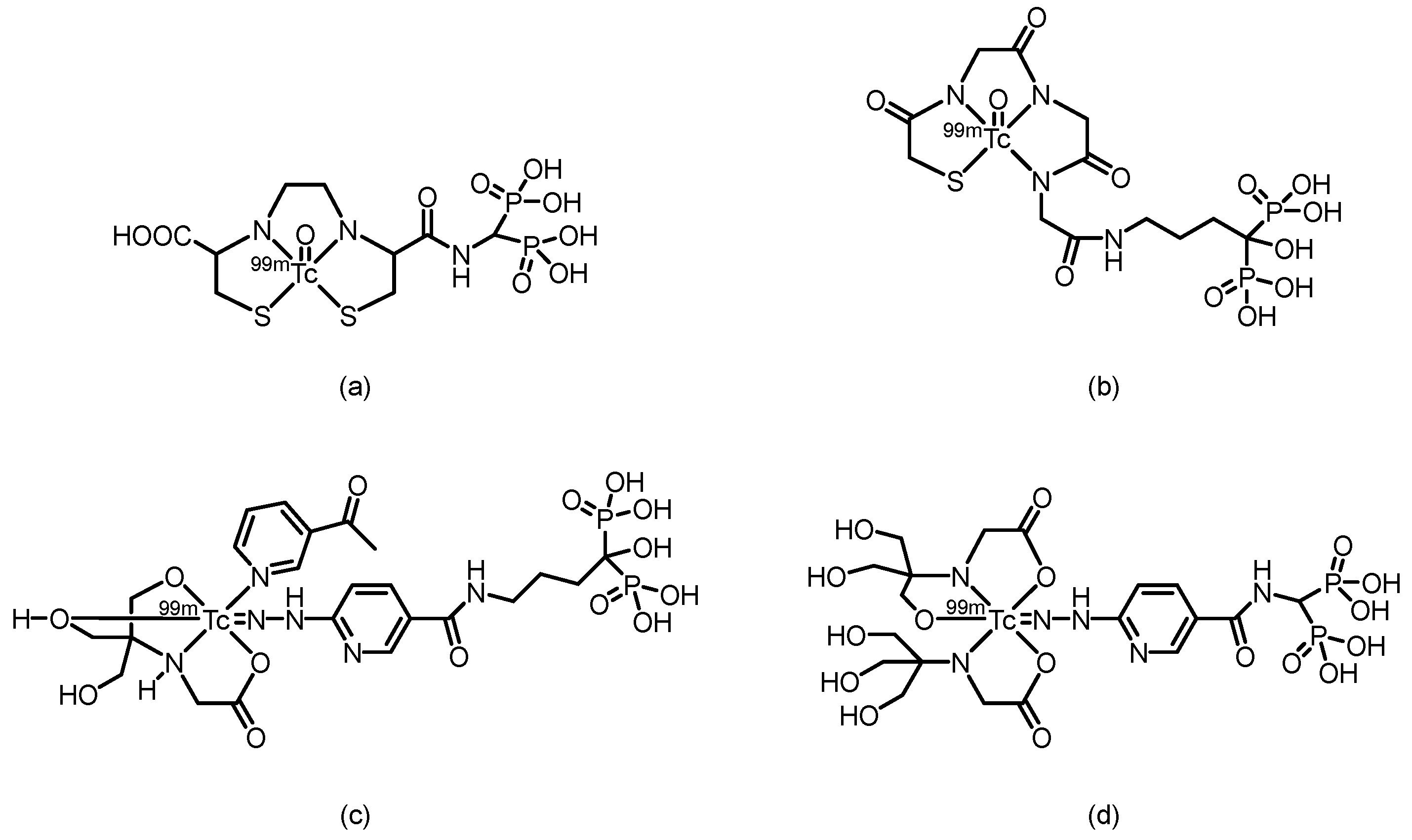
2.1. SPECT Tracers
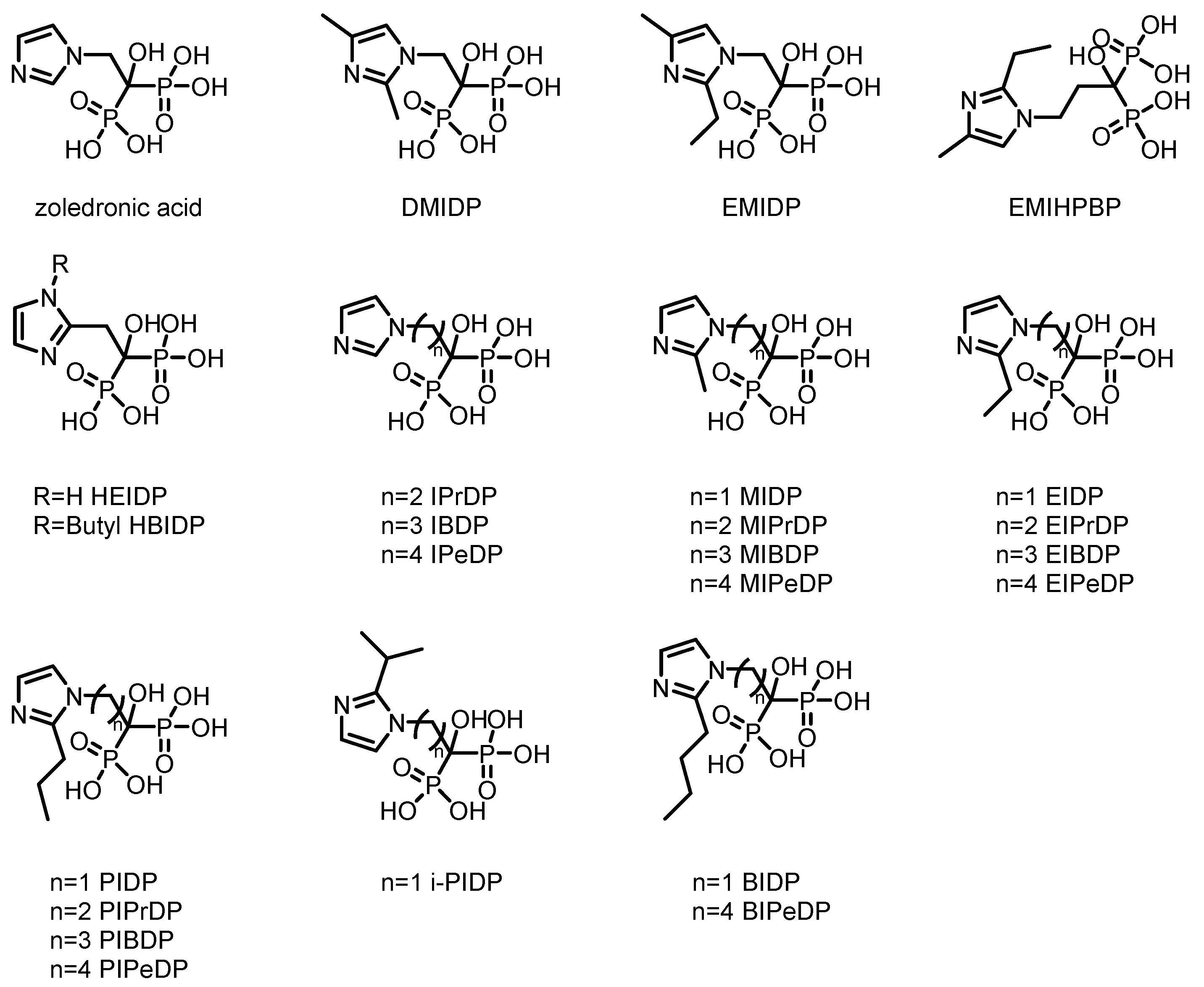
| Complex | Animal | Time p.i.(h) | Bone uptake(%ID/g) | Bone/muscle Ratio | Bone/Blood Ratio | Reference |
| 99ᵐTc-PIDP | Normal mice | 2 | 5.94 ± 0.75 | 41.54 | 28.29 | [50] |
| 99ᵐTc-EMIDP | Normal mice | 1 | 7.07 ± 0.59 | 29.79 | 6.93 | [51] |
| 99ᵐTc-i-PIDP | ICR mice | 1 | 9.63 ± 0.64 | 67.83 | 20.06 | [52] |
| 99ᵐTc-IPrDP | ICR mice | 1 | 11.14 ± 1.85 | 58.40 | 6.10 | [53] |
| 99ᵐTc-MIPrDP | ICR mice | 2 | 19.6 ± 0.87 | 196.0 | 150.8 | [54] |
| 99ᵐTc-MIBDP | ICR mice | 2 | 11.2 ± 0.13 | 140.0 | 58.9 | [54] |
| 99ᵐTc-MIPeDP | ICR mice | 2 | 17.6 ± 0.42 | 160.0 | 97.8 | [54] |
| 99ᵐTc-DMIDP | ICR mice | 1 | 10.68 ± 0.79 | 30.67 | 18.70 | [55] |
| 99ᵐTc-IBDP | ICR mice | 1 | 11.4 ± 0.38 | 51.82 | 15.20 | [56] |
| 99ᵐTc-IPeDP | ICR mice | 1 | 16.2 ± 1.75 | 51.14 | 12.97 | [56] |
| 99ᵐTc-PIPrDP | ICR mice | 1 | 8.42 ± 0.53 | 33.68 | 8.42 | [57] |
| 99ᵐTc-PIBDP | ICR mice | 1 | 9.08 ± 0.65 | 41.27 | 10.81 | [57] |
| 99ᵐTc-PIPeDP | ICR mice | 1 | 10.3 ± 0.61 | 41.2 | 20.20 | [57] |
| 99ᵐTc-HEIDP | ICR mice | 1 | 6.04 ± 0.80 | 32.79 | -- | [58] |
| 99ᵐTc-BIDP | ICR mice | 1 | 22.8 ± 2.32 | 37.37 | -- | [59] |
| 99ᵐTc-BIPeDP | ICR mice | 1 | 17.3 ± 0.14 | 82.38 | 32.04 | [60] |
| 99ᵐTc-EIPrDP | ICR mice | 1 | 13.3 ± 1.23 | 110.8 | 38.0 | [61] |
| 99ᵐTc-EIBDP | ICR mice | 1 | 11.7 ± 0.28 | 58.5 | 22.1 | [61] |
| 99ᵐTc-EIPeDP | ICR mice | 1 | 8.69 ± 0.04 | 37.8 | 13.8 | [61] |
| 99ᵐTc-HBIDP | ICR mice | 1 | -- | -- | -- | [62] |
| 99ᵐTc-EMIHPBP | Albino mice | 1 | 31.60 ± 0.15 | 16.90 | 23.76 | [63] |
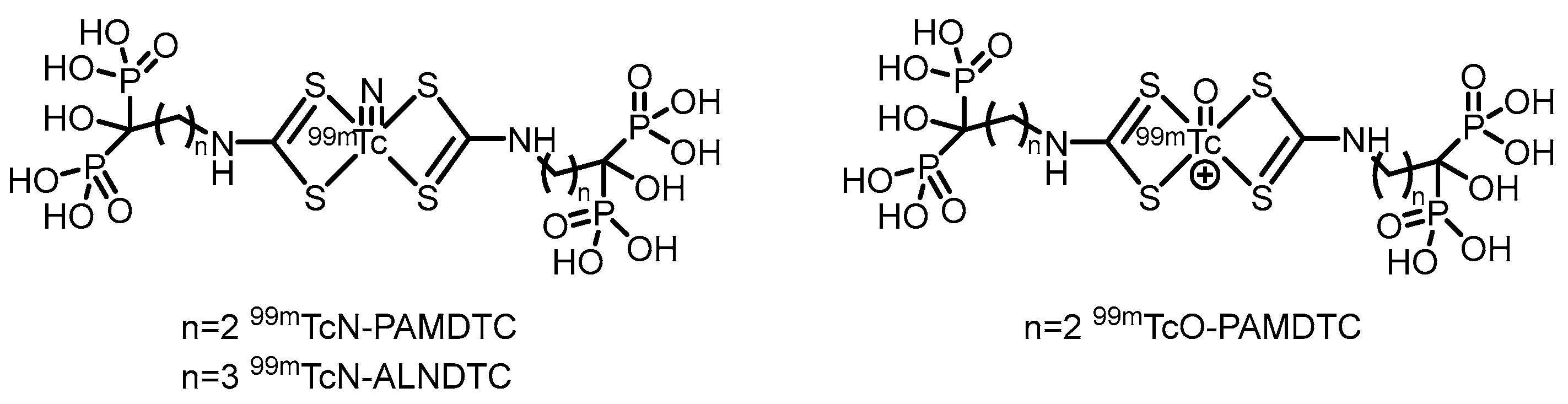
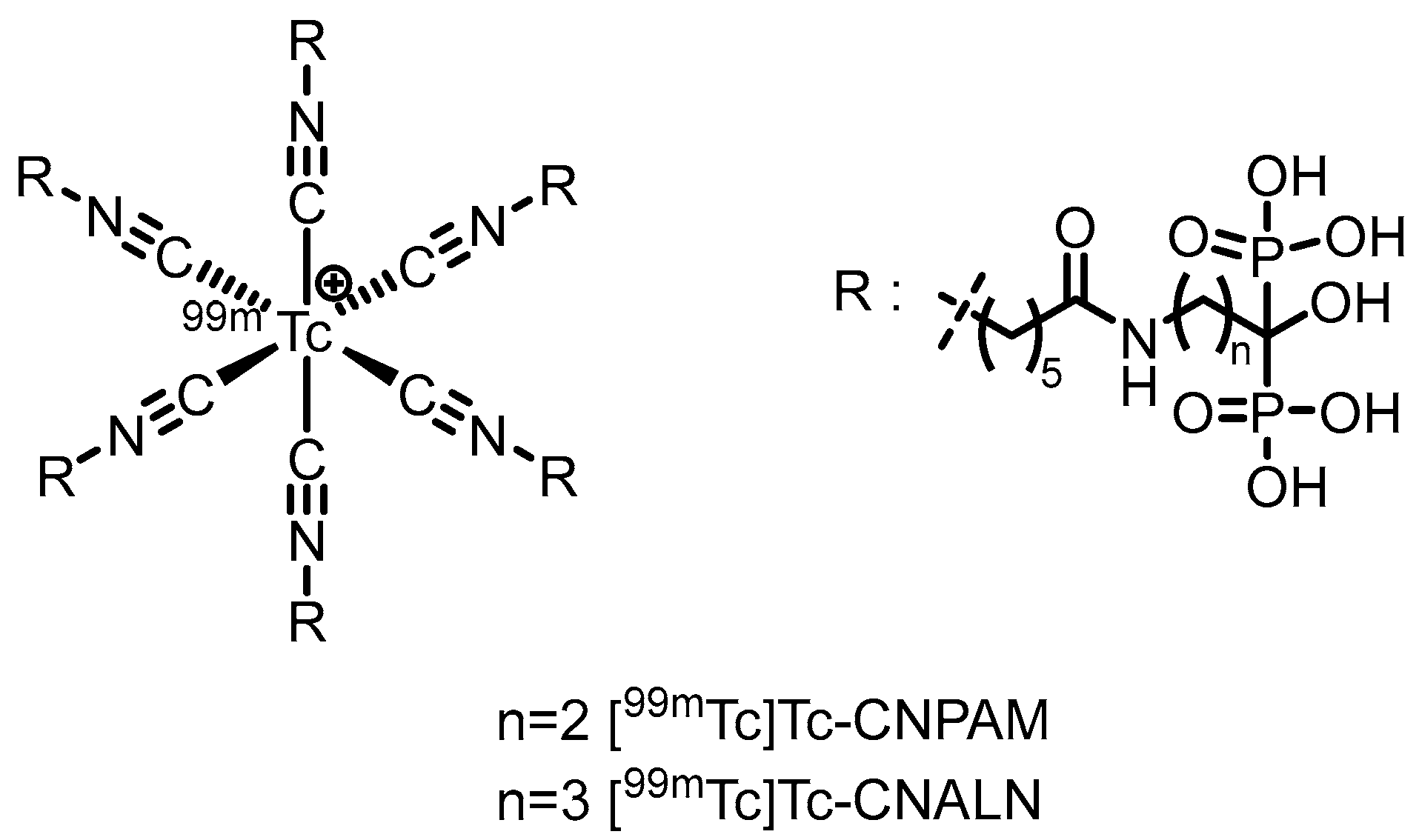
2.2. PET Tracers
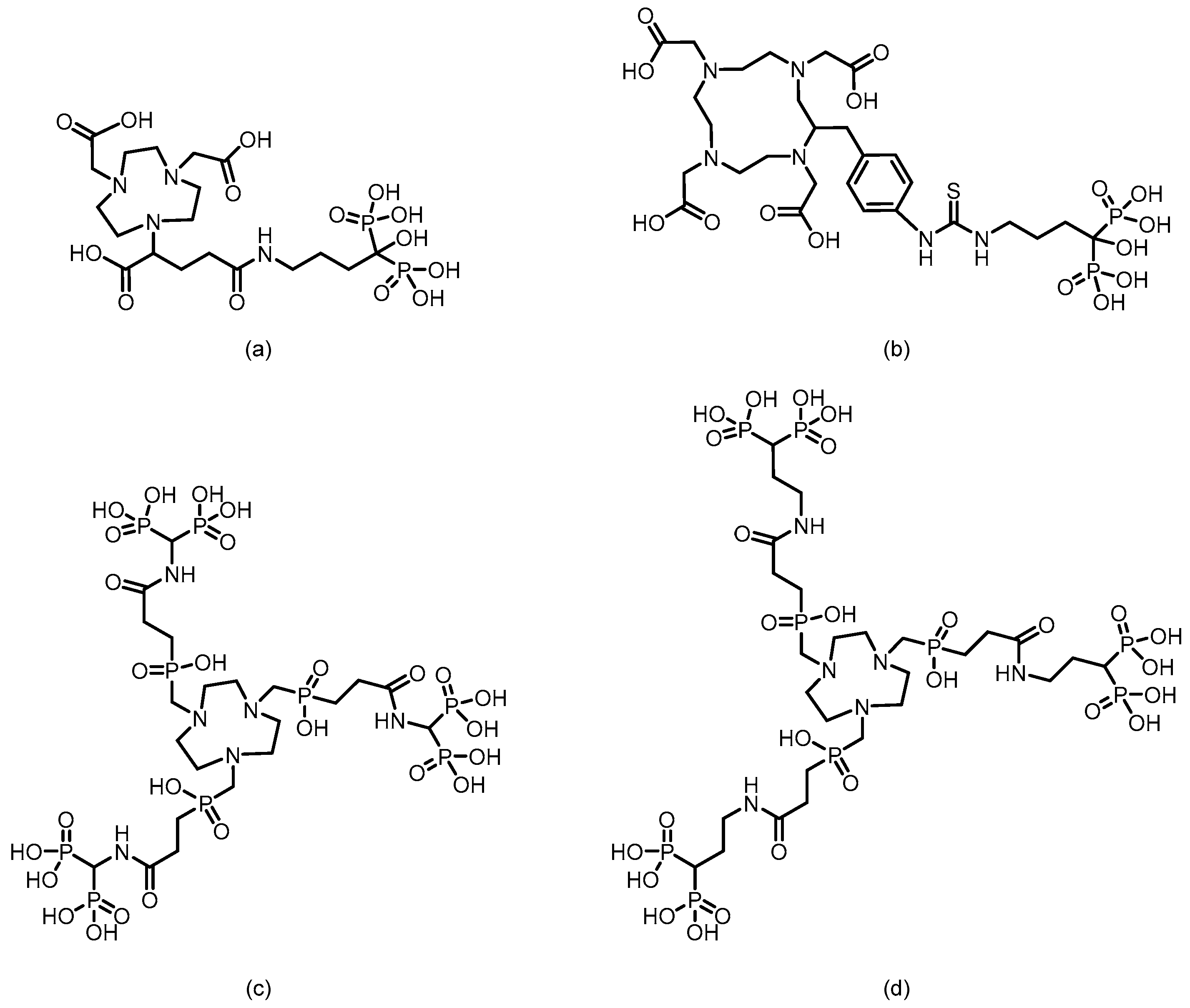
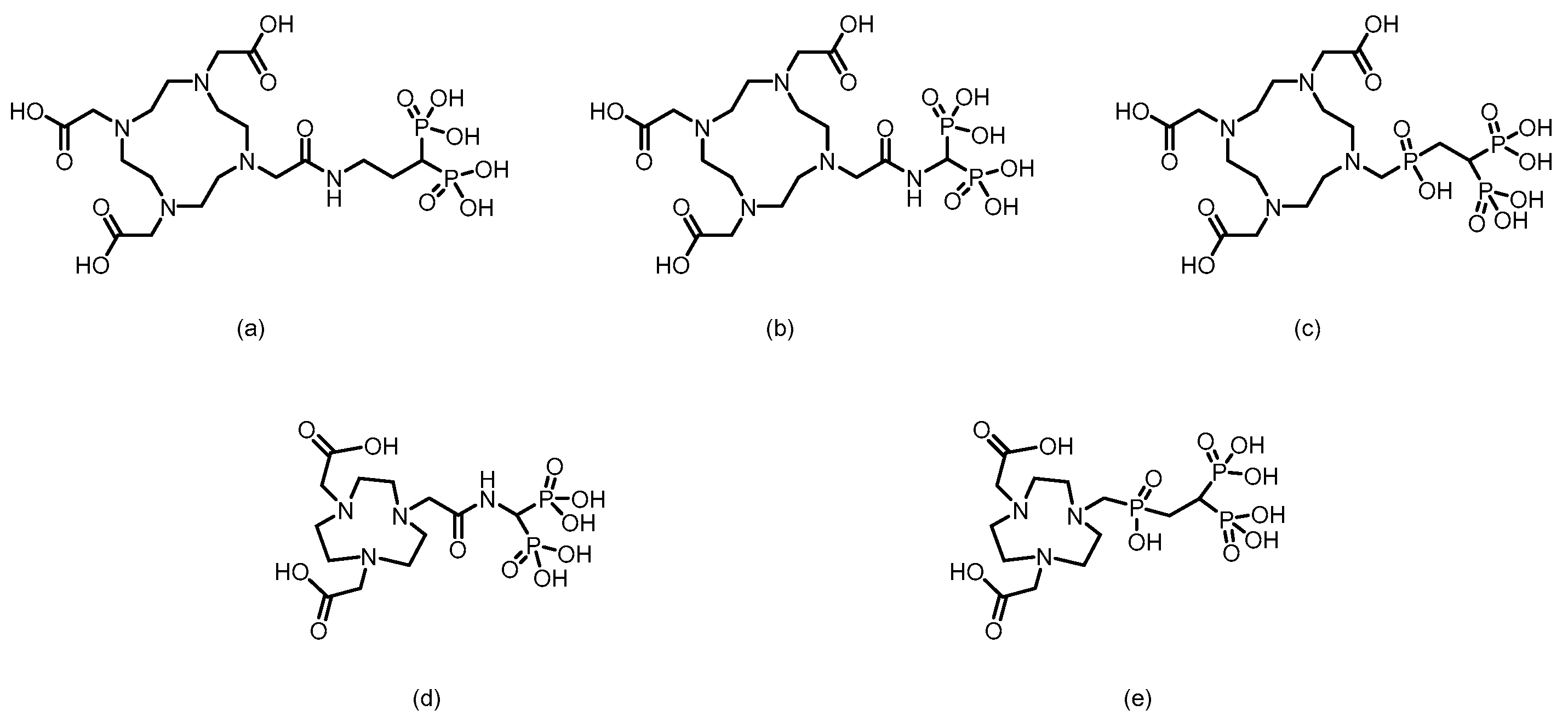
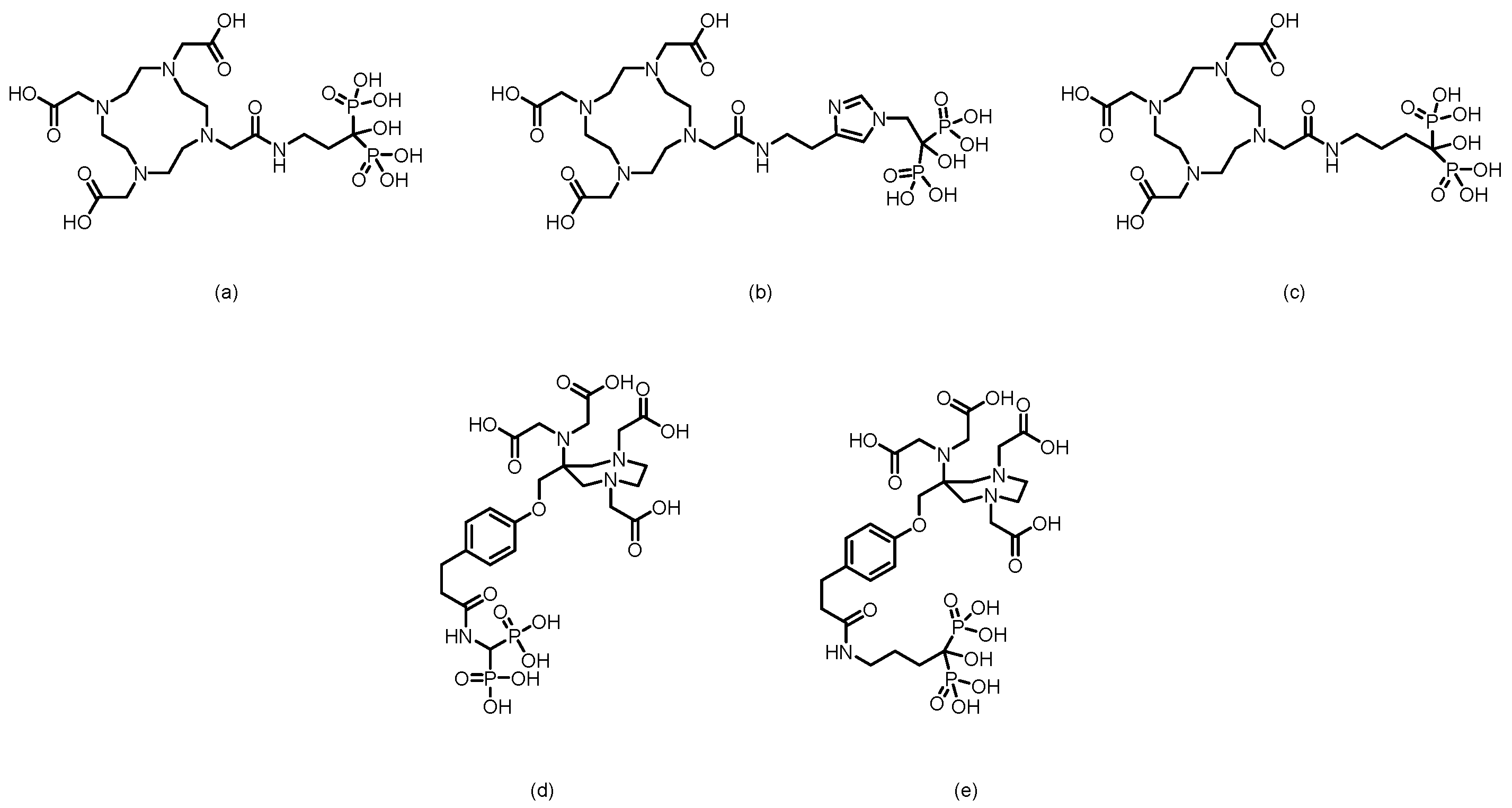
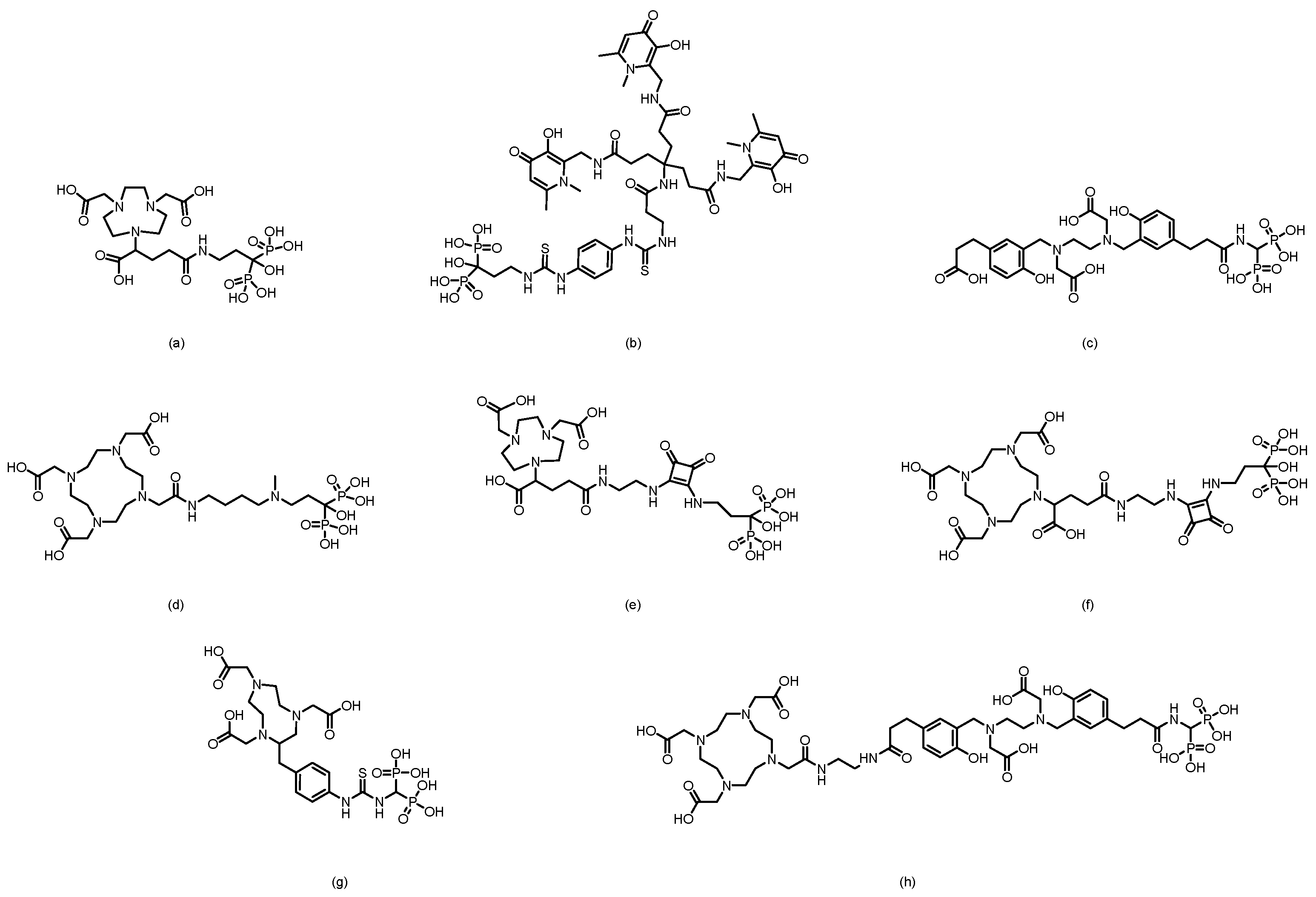
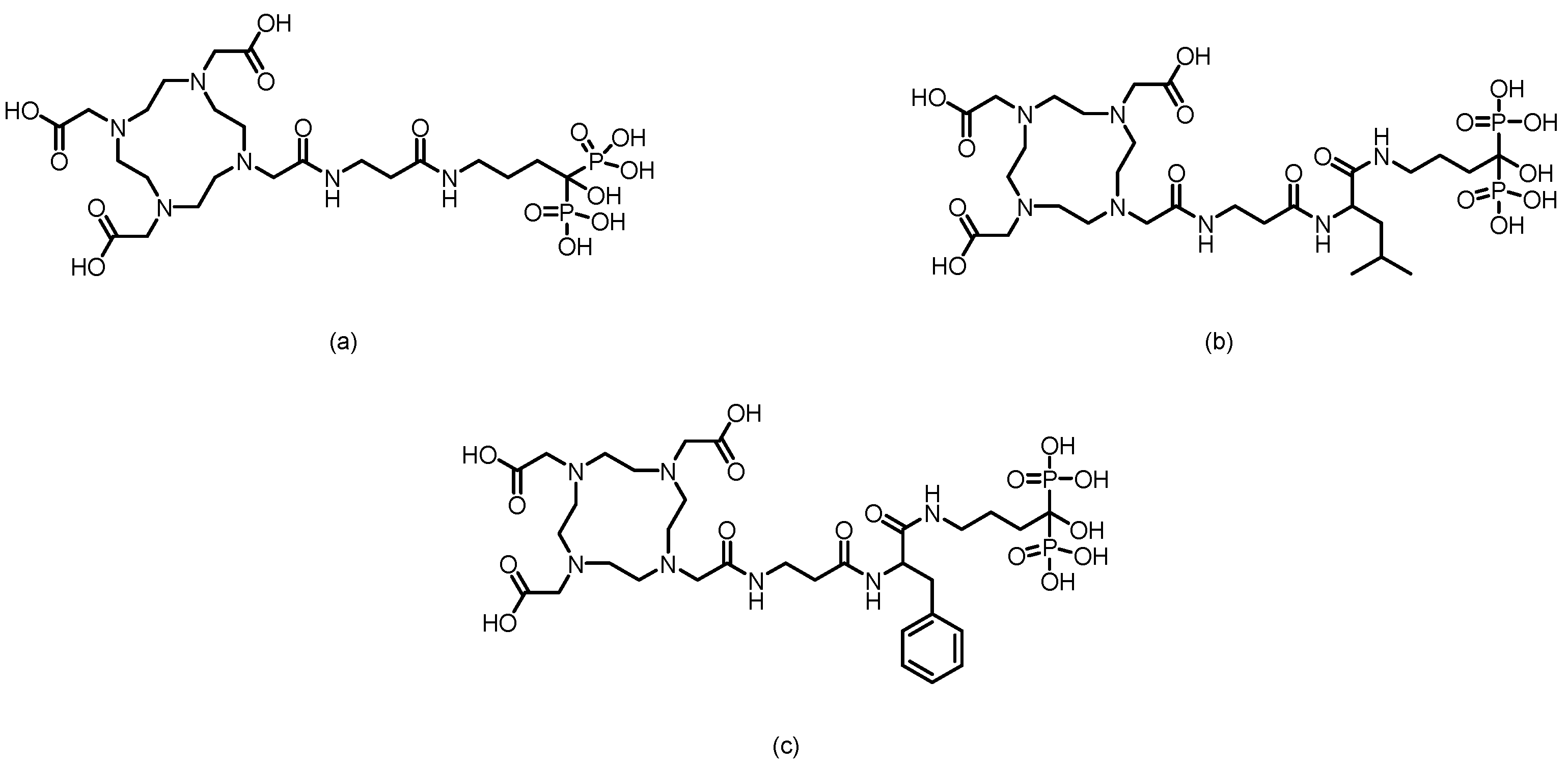
3. Bisphosphonate-Based Targeted Therapeutics for Bone Metastases
3.1. β-Emitter Radiopharmaceuticals
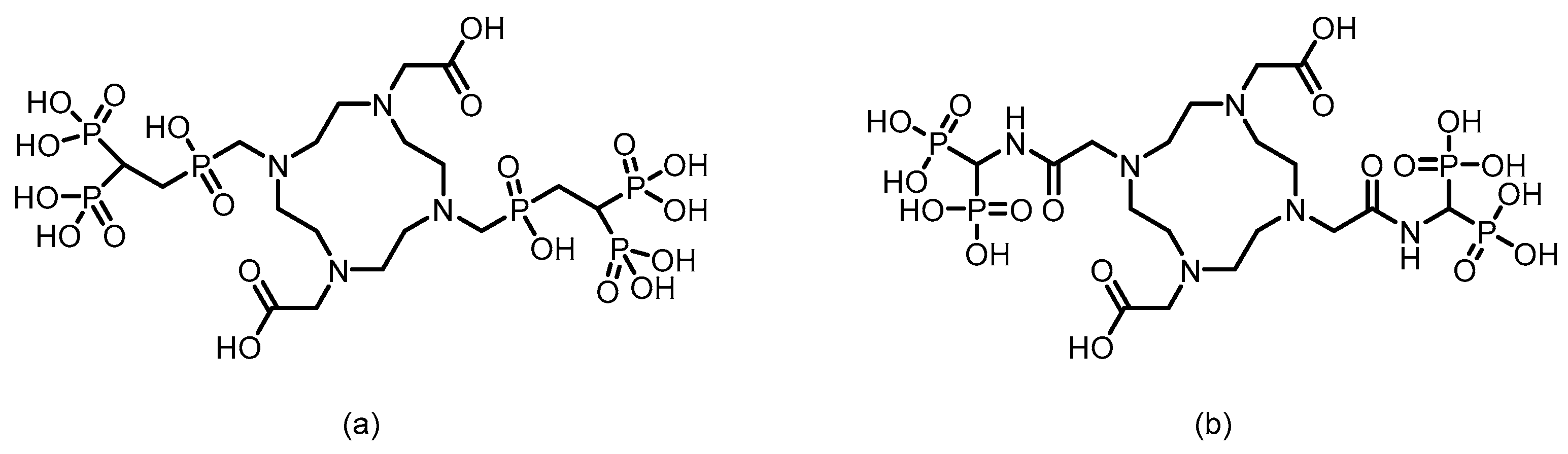
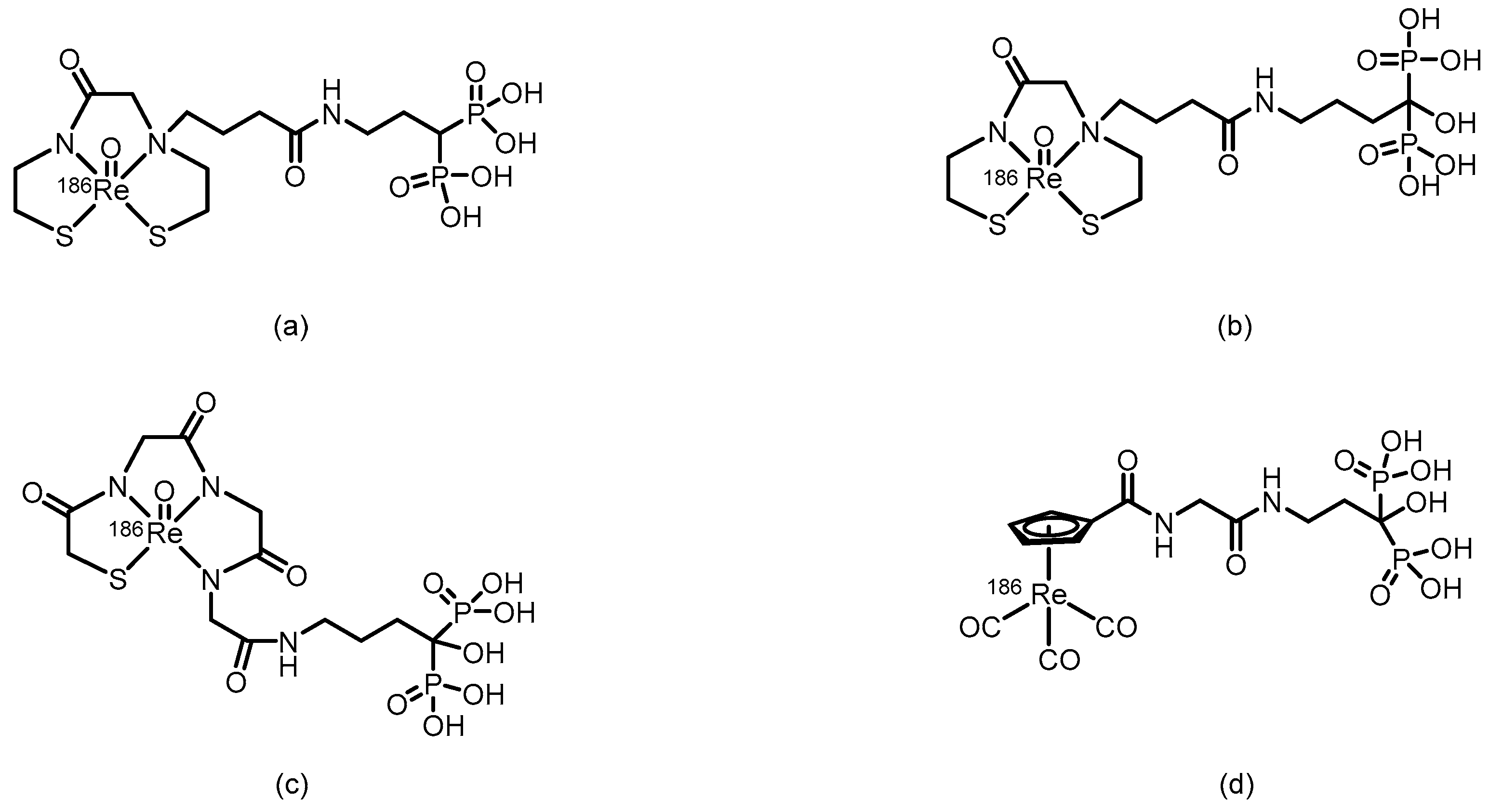
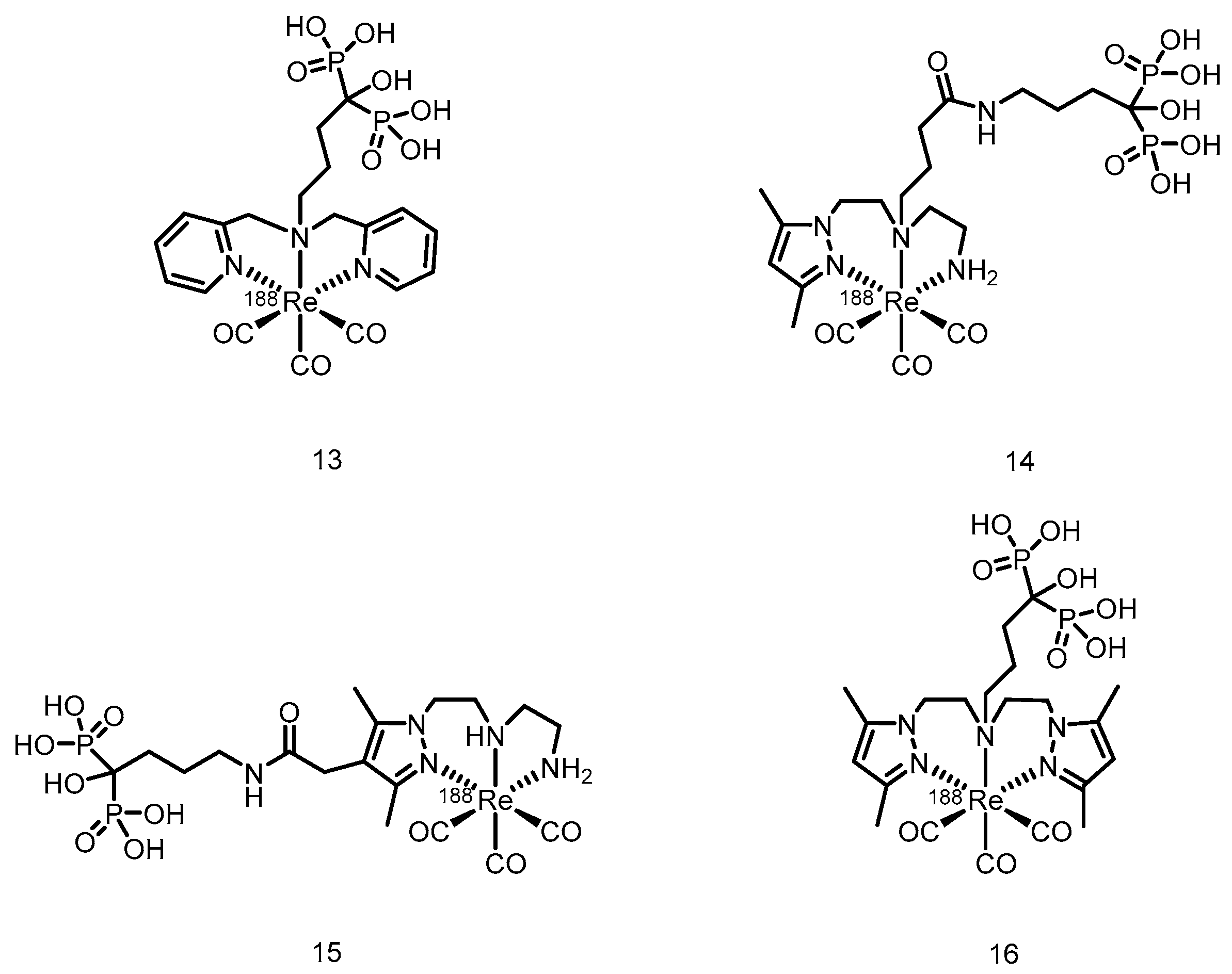
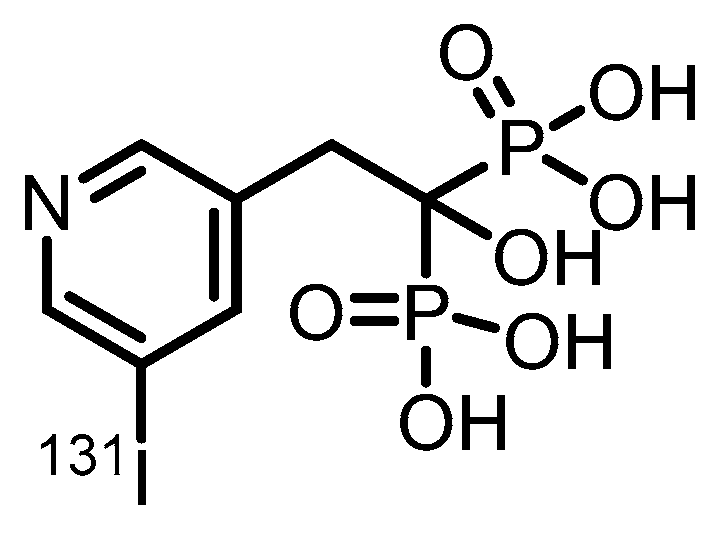
3.2. α-Emitter Radiopharmaceuticals
3.3. Auger Electron Emitters
4. Conclusion and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SPECT | Single Photon Emission Computed Tomography |
| PET | Positron Emission Tomography |
| RCP | Radiochemical Purity |
| RCY SUV DFT |
Radiochemical Yield standardized uptake value density functional theory |
References
- Baldessari, C.; Pipitone, S.; Molinaro, E.; Cerma, K.; Fanelli, M.; Nasso, C.; Oltrecolli, M.; Pirola, M.; D’Agostino, E.; Pugliese, G.; et al. Bone Metastases and Health in Prostate Cancer: From Pathophysiology to Clinical Implications. Cancers (Basel) 2023, 15. [Google Scholar] [CrossRef]
- Knapp, B.J.; Cittolin-Santos, G.F.; Flanagan, M.E.; Grandhi, N.; Gao, F.; Samson, P.P.; Govindan, R.; Morgensztern, D. Incidence and risk factors for bone metastases at presentation in solid tumors. Front Oncol 2024, 14, 1392667. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Pan, Q.; Huang, F.; Hu, H.; Shao, Z. Research progress of bone metastases: From disease recognition to clinical practice. Front Oncol 2022, 12, 1105745. [Google Scholar] [CrossRef]
- Chaffer, C.L.; Weinberg, R.A. A perspective on cancer cell metastasis. Science 2011, 331, 1559–1564. [Google Scholar] [CrossRef]
- Massagué, J.; Obenauf, A.C. Metastatic colonization by circulating tumour cells. Nature 2016, 529, 298–306. [Google Scholar] [CrossRef]
- Paget, S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev 1989, 8, 98–101. [Google Scholar]
- Yin, J.J.; Pollock, C.B.; Kelly, K. Mechanisms of cancer metastasis to the bone. Cell Res 2005, 15, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.K.; Dayyani, F.; Gallick, G.E. Steps in prostate cancer progression that lead to bone metastasis. Int J Cancer 2011, 128, 2545–2561. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Cai, D.; Hong, S. Prevalence and prognosis of bone metastases in common solid cancers at initial diagnosis: a population-based study. BMJ Open 2023, 13, e069908. [Google Scholar] [CrossRef]
- Ibrahim, T.; Flamini, E.; Mercatali, L.; Sacanna, E.; Serra, P.; Amadori, D. Pathogenesis of osteoblastic bone metastases from prostate cancer. Cancer 2010, 116, 1406–1418. [Google Scholar] [CrossRef] [PubMed]
- Azad, G.K.; Taylor, B.; Rubello, D.; Colletti, P.M.; Goh, V.; Cook, G.J. Molecular and Functional Imaging of Bone Metastases in Breast and Prostate Cancers: An Overview. Clin Nucl Med 2016, 41, e44-50. [Google Scholar] [CrossRef]
- Huang, G.; Hou, T.; Song, D.; Meng, T. The regulatory networks and mechanisms of bone microenvironment in tumorigenesis and metastasis. J Bone Oncol 2025, 55, 100729. [Google Scholar] [CrossRef]
- Dyer, M.R.; Jing, Z.; Duncan, K.; Godbe, J.; Shokeen, M. Advancements in the development of radiopharmaceuticals for nuclear medicine applications in the treatment of bone metastases. Nucl Med Biol 2024, 130–131, 108879. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, K.; Ishizaki, A. Well-designed bone-seeking radiolabeled compounds for diagnosis and therapy of bone metastases. Biomed Res Int 2015, 2015, 676053. [Google Scholar] [CrossRef]
- Souche, C.; Fouillet, J.; Rubira, L.; Donzé, C.; Deshayes, E.; Fersing, C. Bisphosphonates as Radiopharmaceuticals: Spotlight on the Development and Clinical Use of DOTAZOL in Diagnostics and Palliative Radionuclide Therapy. Int J Mol Sci 2023, 25. [Google Scholar] [CrossRef]
- Domstad, P.A.; Coupal, J.J.; Kim, E.E.; Blake, J.S.; DeLand, F.H. 99mTc-hydroxymethane diphosphonate: a new bone imaging agent with a low tin content. Radiology 1980, 136, 209–211. [Google Scholar] [CrossRef] [PubMed]
- Mandegaran, R.; Dhillon, S.; Jen, H. Beyond the bones and joints: a review of ligamentous injuries of the foot and ankle on 99mTc-MDP-SPECT/CT. Br J Radiol 2019, 92, 20190506. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; He, Q.; Zhou, T.; Zhang, B.; Li, W.; Peng, H.; Zhong, X.; Ma, L.; Zhang, R. Accurate characterization of 99mTc-MDP uptake in extraosseous neoplasm mimicking bone metastasis on whole-body bone scan: contribution of SPECT/CT. BMC Med Imaging 2019, 19, 44. [Google Scholar] [CrossRef]
- Yang, H.L.; Liu, T.; Wang, X.M.; Xu, Y.; Deng, S.M. Diagnosis of bone metastases: a meta-analysis comparing 18FDG PET, CT, MRI and bone scintigraphy. Eur Radiol 2011, 21, 2604–2617. [Google Scholar] [CrossRef]
- Cook, G.J.; Goh, V. Functional and Hybrid Imaging of Bone Metastases. J Bone Miner Res 2018, 33, 961–972. [Google Scholar] [CrossRef]
- Cook, G.J.R. Imaging with radiolabelled bisphosphonates. Bone 2020, 137, 115372. [Google Scholar] [CrossRef] [PubMed]
- Liepe, K.; Kotzerke, J. A comparative study of 188Re-HEDP, 186Re-HEDP, 153Sm-EDTMP and 89Sr in the treatment of painful skeletal metastases. Nucl Med Commun 2007, 28, 623–630. [Google Scholar] [CrossRef]
- Lam, M.G.; Hoekstra, A.; de Klerk, J.M.; van Rijk, P.P.; Zonnenberg, B.A. Radiation safety considerations for the bone seeking radiopharmaceuticals. 89SrCl2, 186Re-HEDP and 153Sm-EDTMP. Nuklearmedizin 2009, 48, 37–43. [Google Scholar] [PubMed]
- Strigari, L.; Sciuto, R.; D’Andrea, M.; Pasqualoni, R.; Benassi, M.; Maini, C.L. Radiopharmaceutical therapy of bone metastases with 89SrCl2, 186Re-HEDP and 153Sm-EDTMP: a dosimetric study using Monte Carlo simulation. Eur J Nucl Med Mol Imaging 2007, 34, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Sciuto, R.; Festa, A.; Pasqualoni, R.; Semprebene, A.; Rea, S.; Bergomi, S.; Maini, C.L. Metastatic bone pain palliation with 89-Sr and 186-Re-HEDP in breast cancer patients. Breast Cancer Res Treat 2001, 66, 101–109. [Google Scholar] [CrossRef]
- Binnie, D.; Divoli, A.; McCready, V.R.; Dearnaley, D.; Flux, G. The potential use of 99mTc-MDP bone scans to plan high-activity 186Re-HEDP targeted therapy of bony metastases from prostate cancer. Cancer Biother Radiopharm 2005, 20, 189–194. [Google Scholar] [CrossRef]
- Das, T.; Shinto, A.; Kamaleshwaran, K.K.; Banerjee, S. 170Tm-EDTMP: A Prospective Alternative of 89SrCl2 for Theranostic Treatment of Metastatic Bone Pain. Clin Nucl Med 2017, 42, 235–236. [Google Scholar] [CrossRef]
- Ji, L.; Zhang, W.; Hao, S.; Wang, Z.; Zhang, G.; Wang, J. Clinical value of CT-guided radioactive 125I particle implantation combined with 89SrCl2 in relieving pain after failure of external irradiation in patients with prostate cancer bone metastases. J Contemp Brachytherapy 2025, 17, 322–332. [Google Scholar] [CrossRef]
- Henriksen, G.; Breistøl, K.; Bruland, S.; Fodstad; Larsen, R.H. Significant antitumor effect from bone-seeking, alpha-particle-emitting 223Ra demonstrated in an experimental skeletal metastases model. Cancer Res 2002, 62, 3120–3125. [Google Scholar]
- Iagaru, A.H.; Mittra, E.; Colletti, P.M.; Jadvar, H. Bone-Targeted Imaging and Radionuclide Therapy in Prostate Cancer. J Nucl Med 2016, 57, 19s–24s. [Google Scholar] [CrossRef]
- Parker, C.; Nilsson, S.; Heinrich, D.; Helle, S.I.; O’Sullivan, J.M.; Fosså, S.D.; Chodacki, A.; Wiechno, P.; Logue, J.; Seke, M.; et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med 2013, 369, 213–223. [Google Scholar] [CrossRef]
- Buck, A.K.; Serfling, S.E.; Lindner, T.; Hänscheid, H.; Schirbel, A.; Hahner, S.; Fassnacht, M.; Einsele, H.; Werner, R.A. CXCR4-targeted theranostics in oncology. Eur J Nucl Med Mol Imaging 2022, 49, 4133–4144. [Google Scholar] [CrossRef]
- Bednarz, B. Theranostics and Patient-Specific Dosimetry. Semin Radiat Oncol 2023, 33, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Mallak, N.; O’Brien, S.R.; Pryma, D.A.; Mittra, E. Theranostics in Neuroendocrine Tumors. Cancer J 2024, 30, 185–193. [Google Scholar] [CrossRef]
- Baum, R.P.; Novruzov, E.; Zhao, T.; Greifenstein, L.; Jakobsson, V.; Perrone, E.; Mishra, A.; Eismant, A.; Ghai, K.; Klein, O.; et al. Radiomolecular Theranostics With Fibroblast-Activation-Protein Inhibitors and Peptides. Semin Nucl Med 2024, 54, 537–556. [Google Scholar] [CrossRef]
- Zhang, H.; Koumna, S.; Pouliot, F.; Beauregard, J.M.; Kolinsky, M. PSMA Theranostics: Current Landscape and Future Outlook. Cancers (Basel) 2021, 13. [Google Scholar] [CrossRef]
- Ashhar, Z.; Ahmad Fadzil, M.F.; Hassan, H.; Othman, M.F.; Md Hassan, M.B.; Chun Vui, V.Y.; Guat Choo, C.; Yusof, N.A. The Potential of Radiolabeled Bisphosphonates in SPECT and PET for Bone Imaging. Curr Med Imaging 2024, 20, e15734056270935. [Google Scholar] [CrossRef]
- Liu, H.; Wang, X.; Shi, Y.; Yang, Y.; Li, P.; Chen, Q.; Hu, Y.; Wang, J.; Gu, B. Dual effects of bisphosphonates on bone metabolism and implant osseointegration: mechanisms, risks and clinical strategies. J Stomatol Oral Maxillofac Surg 2025, 126, 102534. [Google Scholar] [CrossRef]
- Roelofs, A.J.; Thompson, K.; Ebetino, F.H.; Rogers, M.J.; Coxon, F.P. Bisphosphonates: molecular mechanisms of action and effects on bone cells, monocytes and macrophages. Curr Pharm Des 2010, 16, 2950–2960. [Google Scholar] [CrossRef] [PubMed]
- Gałęzowska, J. Interactions between Clinically Used Bisphosphonates and Bone Mineral: from Coordination Chemistry to Biomedical Applications and Beyond. ChemMedChem 2018, 13, 289–302. [Google Scholar] [CrossRef] [PubMed]
- Young, R.N.; Grynpas, M.D. Targeting therapeutics to bone by conjugation with bisphosphonates. Curr Opin Pharmacol 2018, 40, 87–94. [Google Scholar] [CrossRef]
- Barbosa, J.S.; Almeida Paz, F.A.; Braga, S.S. Bisphosphonates, Old Friends of Bones and New Trends in Clinics. J Med Chem 2021, 64, 1260–1282. [Google Scholar] [CrossRef] [PubMed]
- Papagiannopoulou, D. Technetium-99m radiochemistry for pharmaceutical applications. J Labelled Comp Radiopharm 2017, 60, 502–520. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, K.; Saji, H. Advances in drug design of radiometal-based imaging agents for bone disorders. Int J Mol Imaging 2011, 2011, 537687. [Google Scholar] [CrossRef]
- Love, C.; Din, A.S.; Tomas, M.B.; Kalapparambath, T.P.; Palestro, C.J. Radionuclide bone imaging: an illustrative review. Radiographics 2003, 23, 341–358. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Kumar, V.; Little, D.G.; Howman-Giles, R.B.; Wong, E.; Ali, S.O. Evaluation of biodistribution by local versus systemic administration of 99mTc-labeled pamidronate. J Orthop Sci 2006, 11, 512–520. [Google Scholar] [CrossRef]
- Arteaga de Murphy, C.; Meléndez-Alafort, L.; Montoya-Molina, C.E.; Sepúlveda-Méndez, J. Radiopharmacokinetic data for 99mTc-ABP--a new radiopharmaceutical for bone scanning: comparison with 99mTc-MDP. Nucl Med Biol 1997, 24, 27–33. [Google Scholar] [CrossRef]
- Ekinci, M.; İlem Özdemir, D.; Özgenç, E.; Gündoğdu, E.; Aşıkoğlu, M. Radiolabeling, Quality Control, and Cell Binding Studies of New 99mTc-Labeled Bisphosphonates: 99mTc-Ibandronate Sodium. Turk J Pharm Sci 2023, 20, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Motaleb, M.A.; Adli, A.S.; El-Tawoosy, M.; Sanad, M.H.; AbdAllah, M. An easy and effective method for synthesis and radiolabelling of risedronate as a model for bone imaging. J Labelled Comp Radiopharm 2016, 59, 157–163. [Google Scholar] [CrossRef]
- Chen, C.Q.; Luo, S.N.; Lin, J.G.; Yang, M.; Ye, W.Z.; Qiu, L.; Sang, G.M.; Xia, Y.M. Preparation and biodistribution of 99Tcm-PIDP as bone imaging agent. Nuclear Science and Techniques 2009, 20, 302–306. [Google Scholar]
- Lin, J.; Luo, S.; Chen, C.; Qiu, L.; Wang, Y.; Cheng, W.; Ye, W.; Xia, Y. Preparation and preclinical pharmacological study on a novel bone imaging agent 99mTc-EMIDP. Applied Radiation and Isotopes 2010, 68, 1616–1622. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, S.; Lin, J.; Qiu, L.; Cheng, W.; Zhai, H.; Nan, B.; Ye, W.; Xia, Y. Animal studies of 99mTc–i-PIDP: A new bone imaging agent. Applied Radiation and Isotopes 2011, 69, 1169–1175. [Google Scholar] [CrossRef]
- Lin, J.; Qiu, L.; Cheng, W.; Luo, S.; Ye, W. Preparation and in vivo biological investigations on a novel radioligand for bone scanning: technetium-99m-labeled zoledronic acid derivative. Nuclear Medicine and Biology 2011, 38, 619–629. [Google Scholar] [CrossRef]
- Qiu, L.; Cheng, W.; Lin, J.; Luo, S.; Xue, L.; Pan, J. Synthesis and Biological Evaluation of Novel 99mTc-Labelled Bisphosphonates as Superior Bone Imaging Agents. Molecules 2011, 16, 6165–6178. [Google Scholar] [CrossRef]
- Qiu, L.; Lin, J.; Luo, S.; Wang, Y.; Cheng, W.; Zhang, S. A novel 99mTc-labeled dimethyl-substituted zoledronic acid (DMIDP) with improved bone imang efficiency. Radiochimica Acta 2012, 100, 463–471. [Google Scholar] [CrossRef]
- Lin, J.; Qiu, L.; Cheng, W.; Luo, S.; Xue, L.; Zhang, S. Development of superior bone scintigraphic agent from a series of 99mTc-labeled zoledronic acid derivatives. Appl Radiat Isot 2012, 70, 848–855. [Google Scholar] [CrossRef]
- Qiu, L.; Cheng, W.; Lin, J.G.; Chen, L.P.; Yao, J.; Luo, S.N. Synthesis and biological evaluation of a series of 99mTc-labeled diphosphonates as novel radiotracers with improved bone imaging. Journal of Labelled Compounds & Radiopharmaceuticals 2012, 55, 429–435. [Google Scholar] [CrossRef]
- Qiu, L.; Xue, L.; Chen, Y.S.; Lin, J.G.; Nan, B.B.; Cheng, W.; Luo, S.N. Preparation and in vivo biological investigation of 99mTc-HEIDP as a novel radioligand for bone scanning. Journal of Labelled Compounds & Radiopharmaceuticals 2012, 55, 307–314. [Google Scholar] [CrossRef]
- Qiu, L.; Lin, J.G.; Cheng, W.; Wang, Y.; Luo, S.N. 99mTc-labeled butyl-substituted zoledronic acid as a novel potential SPECT imaging agent: preparation and preclinical pharmacology study. Medicinal Chemistry Research 2013, 22, 6154–6162. [Google Scholar] [CrossRef]
- Qiu, L.; Cheng, W.; Lin, J.G.; Zhang, S.; Luo, S.N. Synthesis and pharmacological evaluation of 99mTc-labeled imidazolyl-containing diphosphonic acid as a novel bone imaging agent. Journal of Labelled Compounds & Radiopharmaceuticals 2013, 56, 573–580. [Google Scholar] [CrossRef]
- Qiu, L.; Cheng, W.; Lin, J.G.; Chen, L.P.; Yao, J.; Pu, W.W.; Luo, S.N. Synthesis and evaluation of a series of 99mTc-labelled zoledronic acid derivatives as potential bone seeking agents. Journal of Radioanalytical and Nuclear Chemistry 2013, 295, 545–552. [Google Scholar] [CrossRef]
- Qiu, L.; Lin, J.G.; Nan, B.B.; Lv, G.C.; Cheng, W. Pharmacokinetic and imaging evaluation of 99mTc-HBIDP as a potential bone imaging agent. Pakistan Journal of Pharmaceutical Sciences 2015, 28, 815–818. [Google Scholar] [PubMed]
- Motaleb, H.A.; Ibrahim, I.T.; El-Tawoosy, M.; Mohamed, M.I. Synthesis, preclinical, and pharmacokinetic evaluation of a new zoledronate derivative as a promising antiosteoporotic candidate using radiolabeling technique. Journal of Labelled Compounds & Radiopharmaceuticals 2017, 60, 542–549. [Google Scholar] [CrossRef]
- Palma, E.; Oliveira, B.L.; Correia, J.D.; Gano, L.; Maria, L.; Santos, I.C.; Santos, I. A new bisphosphonate-containing 99mTc(I) tricarbonyl complex potentially useful as bone-seeking agent: synthesis and biological evaluation. J Biol Inorg Chem 2007, 12, 667–679. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, F.L.; Zhang, J.B.; Yang, S.Y.; Guo, H.X.; Wang, X.B. Synthesis, characterization and biodistribution of 99mTc(CO)3-ABP and comparison with 99mTc-ABP. Journal of Labelled Compounds & Radiopharmaceuticals 2007, 50, 1243–1247. [Google Scholar] [CrossRef]
- Palma, E.; Correia, J.D.; Oliveira, B.L.; Gano, L.; Santos, I.C.; Santos, I. 99mTc(CO)3-labeled pamidronate and alendronate for bone imaging. Dalton Trans 2011, 40, 2787–2796. [Google Scholar] [CrossRef]
- Fernandes, C.; Monteiro, S.; Mendes, P.; Gano, L.; Marques, F.; Casimiro, S.; Costa, L.; Correia, J.D.G.; Santos, I. Biological assessment of novel bisphosphonate-containing 99mTc/Re-organometallic complexes. Journal of Organometallic Chemistry 2014, 760, 197–204. [Google Scholar] [CrossRef]
- Makris, G.; Tseligka, E.D.; Pirmettis, I.; Papadopoulos, M.S.; Vizirianakis, I.S.; Papagiannopoulou, D. Development and Pharmacological Evaluation of New Bone-Targeted 99mTc-Radiolabeled Bisphosphonates. Mol Pharm 2016, 13, 2301–2317. [Google Scholar] [CrossRef]
- Verbeke, K.; Rozenski, J.; Cleynhens, B.; Vanbilloen, H.; de Groot, T.; Weyns, N.; Bormans, G.; Verbruggen, A. Development of a conjugate of 99mTc-EC with aminomethylenediphosphonate in the search for a bone tracer with fast clearance from soft tissue. Bioconjug Chem 2002, 13, 16–22. [Google Scholar] [CrossRef]
- Ogawa, K.; Mukai, T.; Inoue, Y.; Ono, M.; Saji, H. Development of a novel 99mTc-chelate-conjugated bisphosphonate with high affinity for bone as a bone scintigraphic agent. J Nucl Med 2006, 47, 2042–2047. [Google Scholar] [PubMed]
- Abe, K.; Sasaki, M.; Kuwabara, Y.; Koga, H.; Baba, S.; Hayashi, K.; Takahashi, N.; Honda, H. Comparison of 18FDG-PET with 99mTc-HMDP scintigraphy for the detection of bone metastases in patients with breast cancer. Ann Nucl Med 2005, 19, 573–579. [Google Scholar] [CrossRef]
- Liu, L.Q.; Zhong, G.R.; Wei, Y.Q.; Zhang, M.; Wang, X.B. Synthesis and biological evaluation of a novel 99mTc complex of HYNIC-conjugated aminomethylenediphosphonate as a potential bone imaging agent. Journal of Radioanalytical and Nuclear Chemistry 2011, 288, 467–473. [Google Scholar] [CrossRef]
- Song, X.; Wang, Y.; Zhang, J.; Jin, Z.; Zhang, W.; Zhang, Y. Synthesis and evaluation of a novel 99mTc nitrido radiopharmaceutical with alendronate dithiocarbamate as a potential bone-imaging agent. Chem Biol Drug Des 2018, 91, 545–551. [Google Scholar] [CrossRef]
- Song, X.Q.; Wang, Y.; Zhang, J.B. Influence of different 99mTc cores on the physicochemical and biodistribution behaviours of 99mTc-labelled complexes of pamidronate dithiocarbamate. Journal of Radioanalytical and Nuclear Chemistry 2018, 316, 313–319. [Google Scholar] [CrossRef]
- Xiao, D.; Jiang, Y.; Feng, J.; Ruan, Q.; Wang, Q.; Yin, G.; Zhang, J. Novel 99mTc labeled complexes with bisphosphonate isocyanide: Radiosynthesis and evaluation as potential bone-seeking agents. Bioorg Med Chem Lett 2022, 73, 128918. [Google Scholar] [CrossRef] [PubMed]
- Beheshti, M.; Rezaee, A.; Geinitz, H.; Loidl, W.; Pirich, C.; Langsteger, W. Evaluation of Prostate Cancer Bone Metastases with 18F-NaF and 18F-Fluorocholine PET/CT. J Nucl Med 2016, 57, 55s–60s. [Google Scholar] [CrossRef]
- Fan, Z.; Wang, T.; Zou, L.; Liu, D. Comparison of the diagnostic value of 18F-NaF PET/CT and 99mTc-MDP SPECT for bone metastases: a systematic review and meta-analysis. Transl Cancer Res 2023, 12, 3166–3178. [Google Scholar] [CrossRef] [PubMed]
- Donners, R.; Tunariu, N.; Tovey, H.; Hall, E.; Chua, S.; Cook, G.; Du, Y.; Blackledge, M.D.; Parker, C.C.; Koh, D.M. The value of baseline 18F-sodium fluoride and 18F-choline PET activity for identifying responders to radium-223 treatment in castration-resistant prostate cancer bone metastases. Eur Radiol 2024, 34, 1146–1154. [Google Scholar] [CrossRef]
- Suzuki, K.; Satake, M.; Suwada, J.; Oshikiri, S.; Ashino, H.; Dozono, H.; Hino, A.; Kasahara, H.; Minamizawa, T. Synthesis and evaluation of a novel 68Ga-chelate-conjugated bisphosphonate as a bone-seeking agent for PET imaging. Nucl Med Biol 2011, 38, 1011–1018. [Google Scholar] [CrossRef]
- Ogawa, K.; Takai, K.; Kanbara, H.; Kiwada, T.; Kitamura, Y.; Shiba, K.; Odani, A. Preparation and evaluation of a radiogallium complex-conjugated bisphosphonate as a bone scintigraphy agent. Nucl Med Biol 2011, 38, 631–636. [Google Scholar] [CrossRef]
- Notni, J.; Plutnar, J.; Wester, H.J. Bone-seeking TRAP conjugates: surprising observations and their implications on the development of gallium-68-labeled bisphosphonates. EJNMMI Res 2012, 2, 13. [Google Scholar] [CrossRef] [PubMed]
- Fellner, M.; Biesalski, B.; Bausbacher, N.; Kubícek, V.; Hermann, P.; Rösch, F.; Thews, O. 68Ga-BPAMD: PET-imaging of bone metastases with a generator based positron emitter. Nucl Med Biol 2012, 39, 993–999. [Google Scholar] [CrossRef]
- Meckel, M.; Fellner, M.; Thieme, N.; Bergmann, R.; Kubicek, V.; Rösch, F. In vivo comparison of DOTA based 68Ga-labelled bisphosphonates for bone imaging in non-tumour models. Nucl Med Biol 2013, 40, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Holub, J.; Meckel, M.; Kubíček, V.; Rösch, F.; Hermann, P. Gallium(III) complexes of NOTA-bis (phosphonate) conjugates as PET radiotracers for bone imaging. Contrast Media Mol Imaging 2015, 10, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Meckel, M.; Bergmann, R.; Miederer, M.; Roesch, F. Bone targeting compounds for radiotherapy and imaging: *Me(III)-DOTA conjugates of bisphosphonic acid, pamidronic acid and zoledronic acid. EJNMMI Radiopharm Chem 2017, 1, 14. [Google Scholar] [CrossRef]
- Fakhari, A.; Jalilian, A.R.; Johari-Daha, F.; Shafiee-Ardestani, M.; Khalaj, A. Preparation and Biological Study of 68Ga-DOTA-alendronate. Asia Ocean J Nucl Med Biol 2016, 4, 98–105. [Google Scholar] [CrossRef]
- Wu, Z.; Zha, Z.; Choi, S.R.; Plössl, K.; Zhu, L.; Kung, H.F. New 68Ga-PhenA bisphosphonates as potential bone imaging agents. Nucl Med Biol 2016, 43, 360–371. [Google Scholar] [CrossRef]
- Ashhar, Z.; Yusof, N.A.; Ahmad Saad, F.F.; Mohd Nor, S.M.; Mohammad, F.; Bahrin Wan Kamal, W.H.; Hassan, M.H.; Ahmad Hassali, H.; Al-Lohedan, H.A. Preparation, Characterization, and Radiolabeling of [68Ga]Ga-NODAGA-Pamidronic Acid: A Potential PET Bone Imaging Agent. Molecules 2020, 25. [Google Scholar] [CrossRef]
- Keeling, G.P.; Sherin, B.; Kim, J.; San Juan, B.; Grus, T.; Eykyn, T.R.; Rösch, F.; Smith, G.E.; Blower, P.J.; Terry, S.Y.A.; et al. [68Ga]Ga-THP-Pam: A Bisphosphonate PET Tracer with Facile Radiolabeling and Broad Calcium Mineral Affinity. Bioconjug Chem 2021, 32, 1276–1289. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.; Chen, Z.; Yang, J.; Liu, H.; Peng, D.; Lei, L.; Liu, L.; Wang, L.; Xing, N.; et al. Preparation, biological characterization and preliminary human imaging studies of 68Ga-DOTA-IBA. Front Oncol 2022, 12, 1027792. [Google Scholar] [CrossRef]
- Greifenstein, L.; Engelbogen, N.; Máthé, D.; Grus, T.; Rösch, F.; Bergmann, R. Squaric Acid Bisphposphonates for Theranostics of Bone Metastasis—the Easy DOTA-Zoledronate. Front Nucl Med 2022, 2, 870910. [Google Scholar] [CrossRef]
- Chakraborty, S.; Chatterjee, S.; Chakravarty, R.; Sarma, H.D.; Nanabala, R.; Goswami, D.; Joy, A.; Pillai, M.R.A. Synthesis, Quality Control, and Bench-to-Bed Translation of a New [68Ga]Ga-Labeled NOTA-Conjugated Bisphosphonate for Imaging Skeletal Metastases by Positron Emission Tomography. Cancer Biother Radiopharm 2024, 39, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Zhao, R.; Wang, R.; Choi, S.R.; Ploessl, K.; Alexoff, D.; Wu, Z.; Zhu, L.; Kung, H.F. Theranostic Agent Targeting Bone Metastasis: A Novel [68Ga]Ga/[177Lu]Lu-DOTA-HBED-bisphosphonate. J Med Chem 2024, 67, 4793–4803. [Google Scholar] [CrossRef]
- Lin, Y.; Pan, Y.; Zhang, J.; Zhou, B.; Hou, G.; Gao, F. Preparation and preclinical evaluation of 68Ga-labeled alendronate analogs for diagnosis of bone metastases. Dalton Trans 2025, 54, 2886–2895. [Google Scholar] [CrossRef]
- Vos van Avezathe, A.; Brandhoff, P.N.; van Bourgondiën, M.J.; Krijger, G.C. Rapid screening methods for beta-emitters in food samples. J Environ Radioact 2015, 141, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Stokke, C.; Kvassheim, M.; Blakkisrud, J. Radionuclides for Targeted Therapy: Physical Properties. Molecules 2022, 27. [Google Scholar] [CrossRef]
- Humm, J.L.; Chin, L.M. A model of cell inactivation by alpha-particle internal emitters. Radiat Res 1993, 134, 143–150. [Google Scholar] [CrossRef]
- Buchegger, F.; Perillo-Adamer, F.; Dupertuis, Y.M.; Delaloye, A.B. Auger radiation targeted into DNA: a therapy perspective. Eur J Nucl Med Mol Imaging 2006, 33, 1352–1363. [Google Scholar] [CrossRef] [PubMed]
- O’Donoghue, J.A.; Wheldon, T.E. Targeted radiotherapy using Auger electron emitters. Phys Med Biol 1996, 41, 1973–1992. [Google Scholar] [CrossRef]
- Pirovano, G.; Wilson, T.C.; Reiner, T. Auger: The future of precision medicine. Nucl Med Biol 2021, 96-97, 50–53. [Google Scholar] [CrossRef]
- Bergmann, R.; Meckel, M.; Kubíček, V.; Pietzsch, J.; Steinbach, J.; Hermann, P.; Rösch, F. 177Lu-labelled macrocyclic bisphosphonates for targeting bone metastasis in cancer treatment. EJNMMI Res 2016, 6, 5. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, J.; Wang, Y.; Liu, H.; Feng, Y.; Qiu, L.; Chen, Y. Lutetium177-Labeled DOTA-Ibandronate: A Novel Radiopharmaceutical for Targeted Treatment of Bone Metastases. Mol Pharm 2023, 20, 1788–1795. [Google Scholar] [CrossRef]
- Uehara, T.; Jin, Z.L.; Ogawa, K.; Akizawa, H.; Hashimoto, K.; Nakayama, M.; Arano, Y. Assessment of 186Re chelate-conjugated bisphosphonate for the development of new radiopharmaceuticals for bones. Nucl Med Biol 2007, 34, 79–87. [Google Scholar] [CrossRef]
- Giannakenas, C.; Kalofonos, H.P.; Apostolopoulos, D.J.; Zarakovitis, J.; Kosmas, C.; Vassilakos, P.J. Preliminary results of the use of Re-186-HEDP for palliation of pain in patients with metastatic bone disease. Am J Clin Oncol 2000, 23, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Bucerius, J.; Wallny, T.; Brackmann, H.H.; Joe, A.Y.; Roedel, R.; Biersack, H.J.; Palmedo, H. Rhenium-186 hydroxyethylidenediphosphonate (186Re HEDP) for the treatment of hemophilic arthropathy: first results. Clin J Pain 2007, 23, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Denis-Bacelar, A.M.; Chittenden, S.J.; Dearnaley, D.P.; Divoli, A.; O’Sullivan, J.M.; McCready, V.R.; Johnson, B.; Du, Y.; Flux, G.D. Phase I/II trials of 186Re-HEDP in metastatic castration-resistant prostate cancer: post-hoc analysis of the impact of administered activity and dosimetry on survival. Eur J Nucl Med Mol Imaging 2017, 44, 620–629. [Google Scholar] [CrossRef]
- Kothari, K.; Samuel, G.; Banerjee, S.; Unni, P.R.; Sarma, H.D.; Chaudhari, P.R.; Unnikrishnan, T.P.; Pillai, M.R. 186Re-1,4,8,11-tetraaza cyclotetradecyl-1,4,8,11-tetramethylene phosphonic acid: a novel agent for possible use in metastatic bone-pain palliation. Nucl Med Biol 2001, 28, 709–717. [Google Scholar] [CrossRef]
- Ogawa, K.; Mukai, T.; Arano, Y.; Hanaoka, H.; Hashimoto, K.; Nishimura, H.; Saji, H. Design of a radiopharmaceutical for the palliation of painful bone metastases: rhenium-186-labeled bisphosphonate derivative. Journal of Labelled Compounds & Radiopharmaceuticals 2004, 47, 753–761. [Google Scholar] [CrossRef]
- Ogawa, K.; Mukai, T.; Arano, Y.; Otaka, A.; Ueda, M.; Uehara, T.; Magata, Y.; Hashimoto, K.; Saji, H. Rhemium-186-monoaminemonoamidedithiol-conjugated bisphosphonate derivatives for bone pain palliation. Nucl Med Biol 2006, 33, 513–520. [Google Scholar] [CrossRef]
- Ogawa, K.; Mukai, T.; Arano, Y.; Ono, M.; Hanaoka, H.; Ishino, S.; Hashimoto, K.; Nishimura, H.; Saji, H. Development of a rhenium-186-labeled MAG3-conjugated bisphosphonate for the palliation of metastatic bone pain based on the concept of bifunctional radiopharmaceuticals. Bioconjug Chem 2005, 16, 751–757. [Google Scholar] [CrossRef]
- Pillai, M.R.; Dash, A.; Knapp, F.F., Jr. Rhenium-188: availability from the 188W/188Re generator and status of current applications. Curr Radiopharm 2012, 5, 228–243. [Google Scholar] [CrossRef]
- Dasha, A.; Knapp, F.F.R. An overview of radioisotope separation technologies for development of 188W/188Re radionuclide generators providing 188Re to meet future research and clinical demands. Rsc Advances 2015, 5, 39012–39036. [Google Scholar] [CrossRef]
- Scheffler, J.; Derejko, M.; Bandurski, T.; Romanowicz, G. Application of rhenium-188 HEDP in bone metastases therapy. Nucl Med Rev Cent East Eur 2003, 6, 55–57. [Google Scholar]
- Zhang, H.; Tian, M.; Li, S.; Liu, J.; Tanada, S.; Endo, K. Rhenium-188-HEDP therapy for the palliation of pain due to osseous metastases in lung cancer patients. Cancer Biother Radiopharm 2003, 18, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, J.; Zhang, H.; Tian, M.; Wang, J.; Zheng, X. Rhenium-188 HEDP to treat painful bone metastases. Clin Nucl Med 2001, 26, 919–922. [Google Scholar] [CrossRef] [PubMed]
- Arteaga de Murphy, C.; Ferro-Flores, G.; Pedraza-López, M.; Meléndez-Alafort, L.; Croft, B.Y.; Ramírez, F.M.; Padilla, J. Labelling of Re-ABP with 188Re for bone pain palliation. Appl Radiat Isot 2001, 54, 435–442. [Google Scholar] [CrossRef]
- Qingnuan, L.; Xiaodong, Z.; Rong, S.; Wenxin, L. Preparation of [188Re] Re-AEDP and its biodistribution studies. Appl Radiat Isot 2000, 53, 993–997. [Google Scholar] [CrossRef]
- El-Mabhouh, A.; Mercer, J.R. 188Re-labeled bisphosphonates as potential bifunctional agents for therapy in patients with bone metastases. Appl Radiat Isot 2005, 62, 541–549. [Google Scholar] [CrossRef]
- Ranjbar, H.; Bagheri, R.; Miremad, S.M. Evaluation of 188Re- IBA as a novel radiopharmaceutical for bone marrow ablation. Appl Radiat Isot 2024, 208, 111300. [Google Scholar] [CrossRef]
- Xu, T.; Wang, Y.; Chen, Z.; Liu, H.; Yang, S.; Liu, G.; Zhao, Y.; Fu, W.; Liu, L.; Xiang, K.; et al. Preparation, Characterization, and Preliminary Imaging Study of [188Re]Re-Ibandronate as a Novel Theranostic Radiopharmaceutical for Bone Metastasis. Contrast Media Mol Imaging 2022, 2022, 7684076. [Google Scholar] [CrossRef]
- Erfani, M.; Rahmani, N.; Doroudi, A.; Shafiei, M. Preparation and evaluation of rhenium-188-pamidronate as a palliative treatment in bone metastasis. Nucl Med Biol 2017, 49, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lisic, E.C.; Phillips, M.; Ensor, D.; Nash, K.L.; Beets, A.; Knapp, F.F. Synthesis of a new bisphosphonic acid ligand (SEDP) and preparation of a 188Re-(Sn)SEDP bone seeking radiotracer. Nucl Med Biol 2001, 28, 419–424. [Google Scholar] [CrossRef]
- Erfani, M.; Tabatabaei, M.; Doroudi, A.; Shafiei, M. Radiolabeling of zoledronic acid with 188Re as a new palliative agent radiotracer in treatment of bone tumors. Journal of Radioanalytical and Nuclear Chemistry 2018, 316, 491–500. [Google Scholar] [CrossRef]
- Torres Martin de Rosales, R.; Finucane, C.; Foster, J.; Mather, S.J.; Blower, P.J. 188Re(CO)3-dipicolylamine-alendronate: a new bisphosphonate conjugate for the radiotherapy of bone metastases. Bioconjug Chem 2010, 21, 811–815. [Google Scholar] [CrossRef]
- Fernandes, C.; Monteiro, S.; Belchior, A.; Marques, F.; Gano, L.; Correia, J.D.; Santos, I. Novel 188Re multi-functional bone-seeking compounds: Synthesis, biological and radiotoxic effects in metastatic breast cancer cells. Nucl Med Biol 2016, 43, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, K.; Kawashima, H.; Shiba, K.; Washiyama, K.; Yoshimoto, M.; Kiyono, Y.; Ueda, M.; Mori, H.; Saji, H. Development of [90Y]DOTA-conjugated bisphosphonate for treatment of painful bone metastases. Nucl Med Biol 2009, 36, 129–135. [Google Scholar] [CrossRef]
- Rabiei, A.; Shamsaei, M.; Yousefnia, H.; Zolghadri, S.; Jalilian, A.R.; Enayati, R. Development and biological evaluation of 90Y-BPAMD as a novel bone seeking therapeutic agent. Radiochimica Acta 2016, 104, 727–734. [Google Scholar] [CrossRef]
- Lin, Z.; Hai, W.; Pan, P.; Lin, J.H.; Li, B.; Xiao, J.C. Synthesis and Biological Evaluation of 131I-Risedronate with Bone Targeting Activity. Mol Pharm 2025, 22, 3508–3514. [Google Scholar] [CrossRef] [PubMed]
- Radchenko, V.; Engle, J.; Wilson, J.J.; Maassen, J.; Nortier, M.; Taylor, W.; Birnbaum, E.; Hudston, L.; John, K.; Fassbender, M. A new strategy for isolation of Ac from proton-irradiated thorium. Journal of Labelled Compounds & Radiopharmaceuticals 2015, 58, S27–S27. [Google Scholar]
- Radchenko, V.; Engle, J.W.; Wilson, J.J.; Maassen, J.R.; Nortier, F.M.; Taylor, W.A.; Birnbaum, E.R.; Hudston, L.A.; John, K.D.; Fassbender, M.E. Application of ion exchange and extraction chromatography to the separation of actinium from proton-irradiated thorium metal for analytical purposes. J Chromatogr A 2015, 1380, 55–63. [Google Scholar] [CrossRef]
- Li, C.; Zhang, Y.L.; Wei, Z.; Sun, Y.; Yang, L.; Gao, Y.C.; Wang, W.; Zhang, Y.S. Predictions of 225Ac and 223Ra production cross sections from p, α and heavy ion irradiated thorium and uranium targets. Appl Radiat Isot 2025, 225, 111981. [Google Scholar] [CrossRef]
- Xu, T.; Qu, G.; Liu, G.; Wang, L.; Chen, Y. A New Radiopharmaceutical 225Ac-DOTA-IBA in the Treatment of a Case of Bone Metastases. Clin Nucl Med 2023, 48, 650–652. [Google Scholar] [CrossRef]
- Pfannkuchen, N.; Bausbacher, N.; Pektor, S.; Miederer, M.; Rosch, F. In vivo Evaluation of [225Ac]Ac-DOTAZOL for α-Therapy of Bone Metastases. Curr Radiopharm 2018, 11, 223–230. [Google Scholar] [CrossRef]
- Müller, C.; van der Meulen, N.P.; Schibli, R. Opportunities and potential challenges of using terbium-161 for targeted radionuclide therapy in clinics. Eur J Nucl Med Mol Imaging 2023, 50, 3181–3184. [Google Scholar] [CrossRef] [PubMed]
- Alcocer-Ávila, M.E.; Ferreira, A.; Quinto, M.A.; Morgat, C.; Hindié, E.; Champion, C. Radiation doses from 161Tb and 177Lu in single tumour cells and micrometastases. EJNMMI Phys 2020, 7, 33. [Google Scholar] [CrossRef]
- Trejtnar, F.; Bárta, P.; Kozempel, J.; Vlk, M.; Ďurinová, A.; Kuchařová, M.; Pávek, P. Terbium-161 in nuclear medicine: Preclinical and clinical progress in comparison with lutetium-177. Nucl Med Biol 2025, 144–145, 108998. [Google Scholar] [CrossRef] [PubMed]
- Sitarica, P.; Vukadinović, A.; Marić, M.; Vranješ-Đurić, S.; Stanković, D.; Perić, M.; Janković, D.; Stanković, D.; Mirković, M.; Radović, M. Evaluation of 161Tb-Labeled Diphosphonates as Potential Bone-Targeting Agents. Int J Mol Sci 2025, 26. [Google Scholar] [CrossRef]
- Zhang, X. Interactions between cancer cells and bone microenvironment promote bone metastasis in prostate cancer. Cancer Commun (Lond) 2019, 39, 76. [Google Scholar] [CrossRef]
- DiNatale, A.; Fatatis, A. The Bone Microenvironment in Prostate Cancer Metastasis. Adv Exp Med Biol 2019, 1210, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Fornetti, J.; Welm, A.L.; Stewart, S.A. Understanding the Bone in Cancer Metastasis. J Bone Miner Res 2018, 33, 2099–2113. [Google Scholar] [CrossRef]
- Silberstein, E.B. Teletherapy and radiopharmaceutical therapy of painful bone metastases. Semin Nucl Med 2005, 35, 152–158. [Google Scholar] [CrossRef]
- Mahajan, S.; Gavane, S.; Pandit-Taskar, N. Targeted Radiopharmaceutical Therapy for Bone Metastases. Semin Nucl Med 2024, 54, 497–512. [Google Scholar] [CrossRef]
- Crișan, G.; Moldovean-Cioroianu, N.S.; Timaru, D.G.; Andrieș, G.; Căinap, C.; Chiș, V. Radiopharmaceuticals for PET and SPECT Imaging: A Literature Review over the Last Decade. Int J Mol Sci 2022, 23. [Google Scholar] [CrossRef]
- Pisaneschi, F.; Viola, N.T. Development and Validation of a PET/SPECT Radiopharmaceutical in Oncology. Mol Imaging Biol 2022, 24, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kreppel, B.; Gaertner, F.C.; Ahmadzadehfar, H.; Khawar, A.; Roesch, F.; Kürpig, S.; Meisenheimer, M.; Essler, M.; Bundschuh, R.A. [177Lu]Lu-DOTA-zoledronate therapy—first application in a patient with primary osseous metastatic bronchial carcinoma. Nuklearmedizin 2020, 59, 281–283. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.P.; Ballal, S.; Meckel, M.; Roesch, F.; Bal, C. [177Lu]Lu-DOTA-ZOL bone pain palliation in patients with skeletal metastases from various cancers: efficacy and safety results. EJNMMI Res 2020, 10, 130. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Yang, J.; Cheng, Z.X.; Chen, Y. Treatment of Bone Metastases of Breast Cancer With 177Lu-DOTA-IBA. Clinical Nuclear Medicine 2024, 49, 659–661. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





