Submitted:
22 December 2025
Posted:
23 December 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Experimental
2.1. Preparation of the Sample
2.2. Material Characterization
2.3. Electrochemical Measurements
3. Results and Discussion
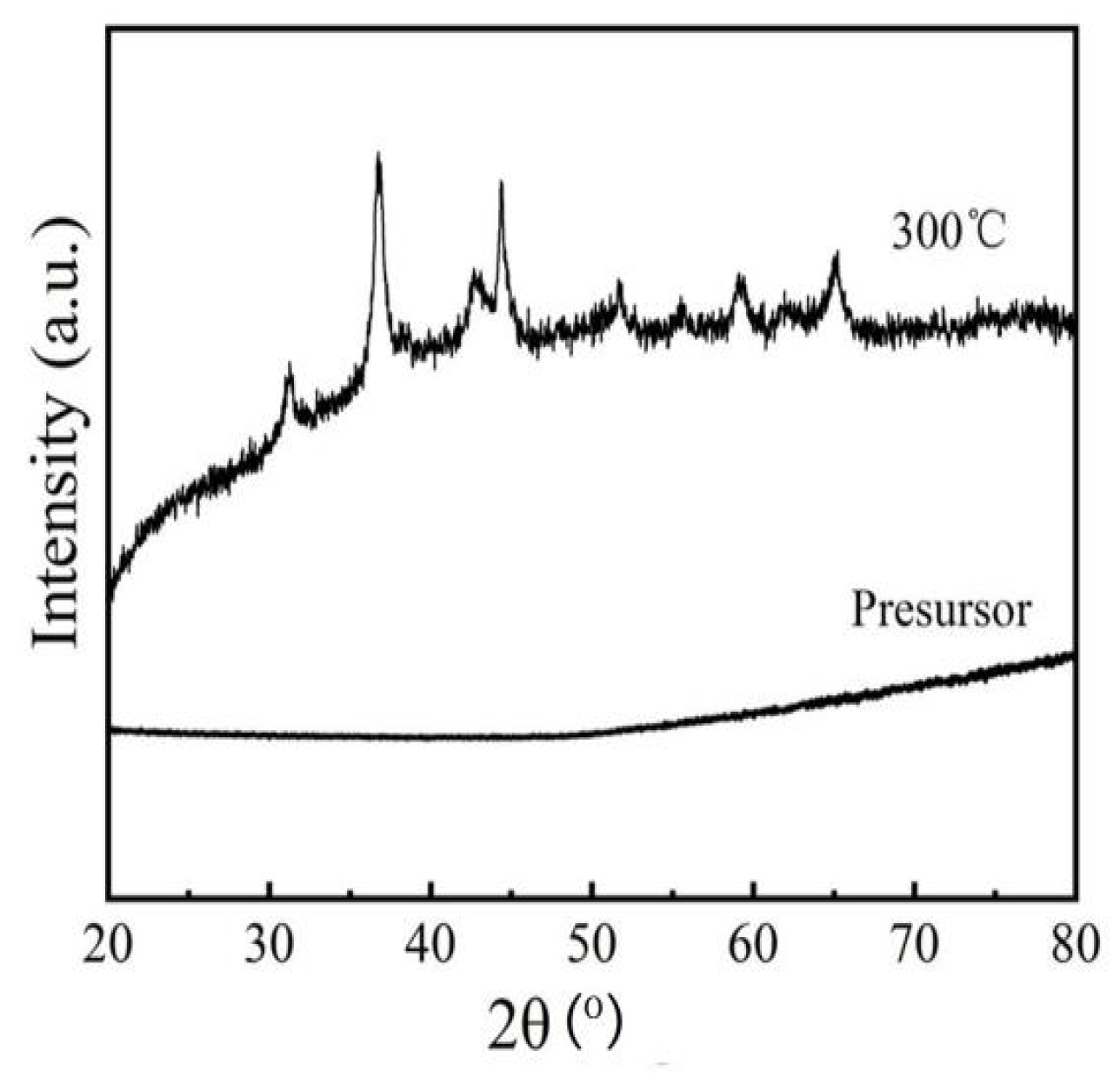
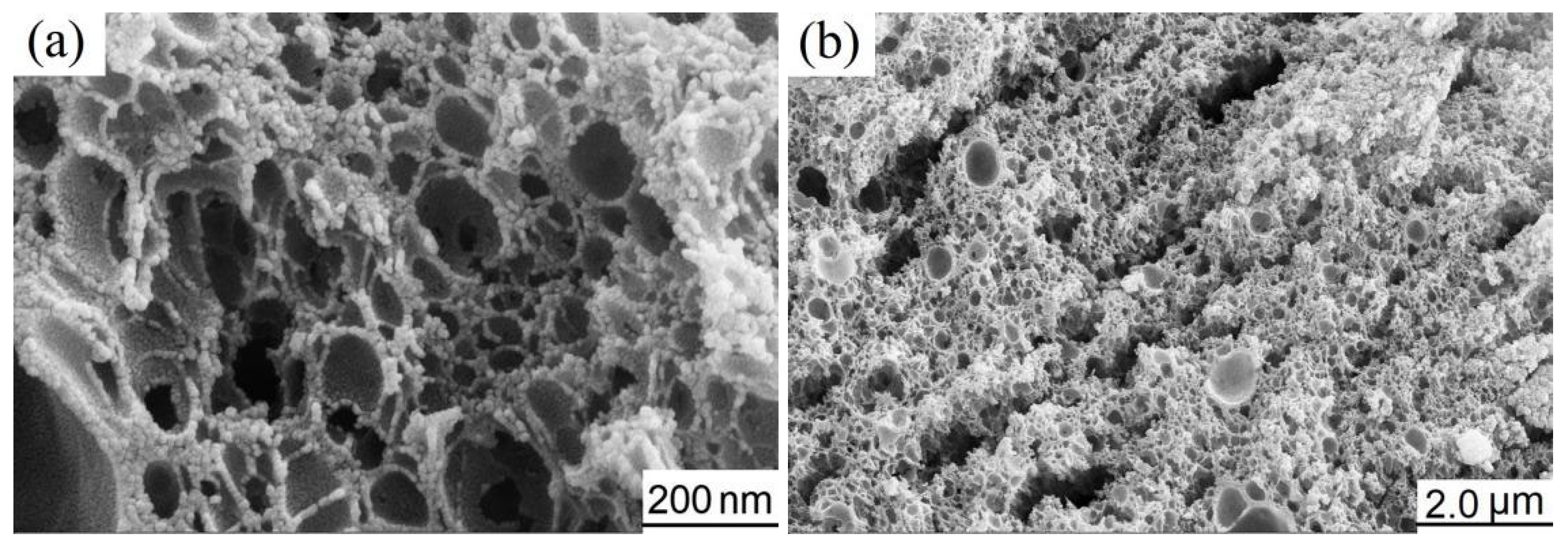
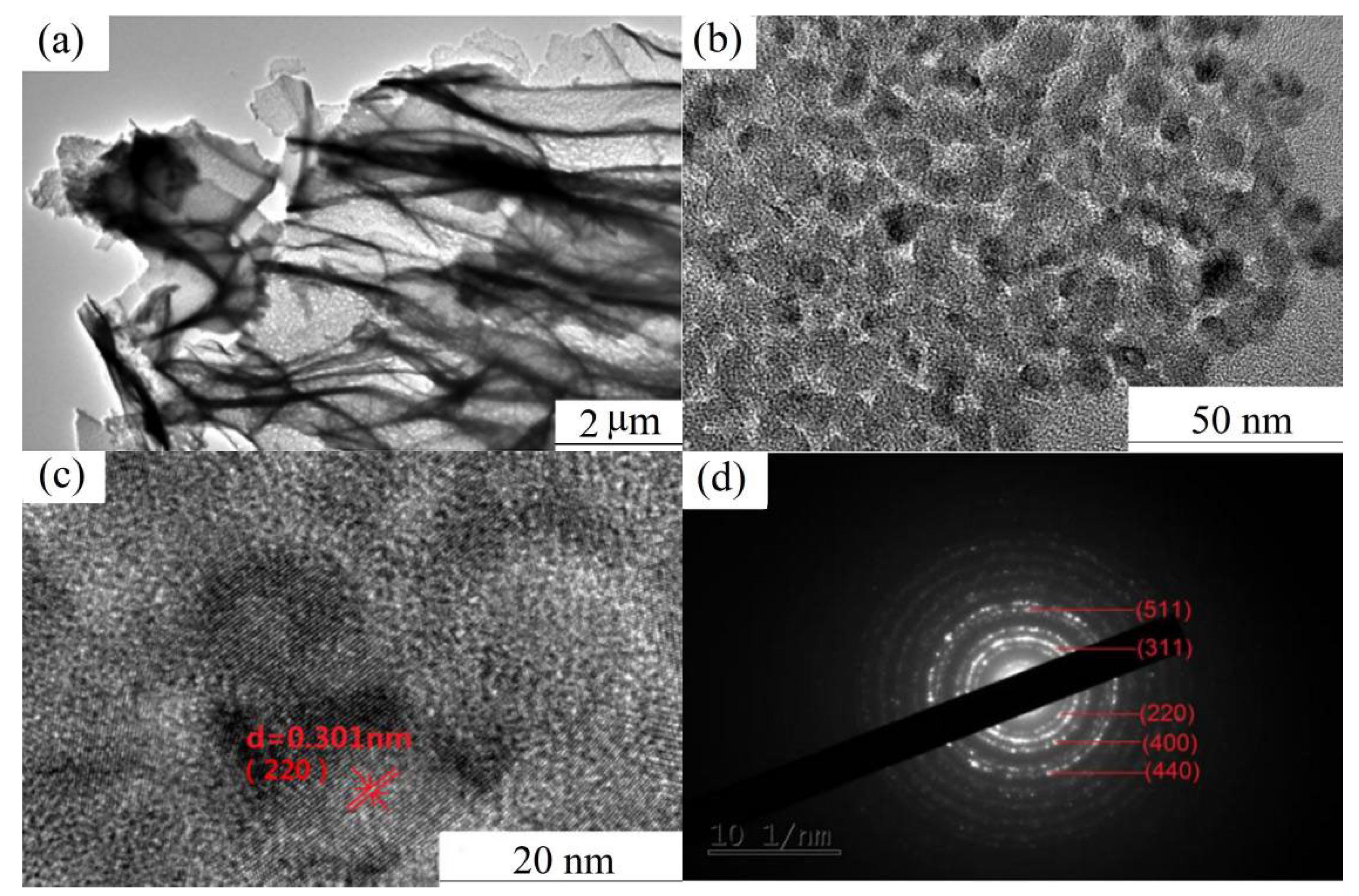
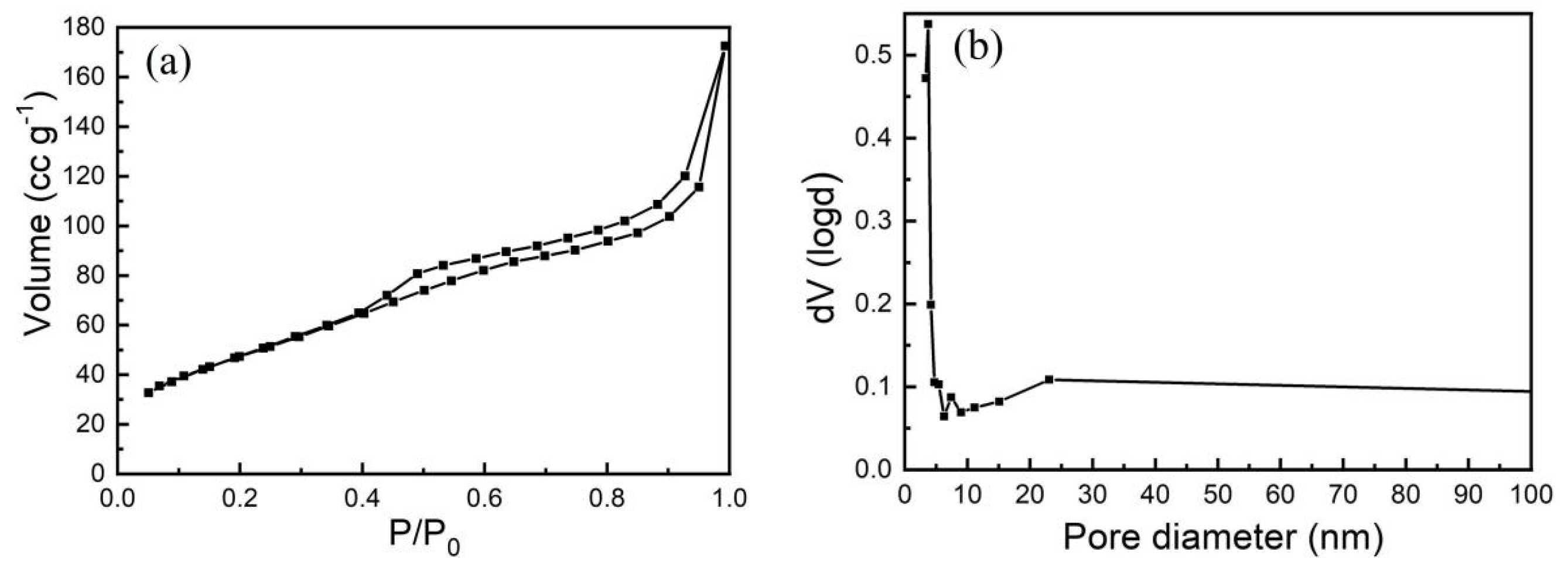
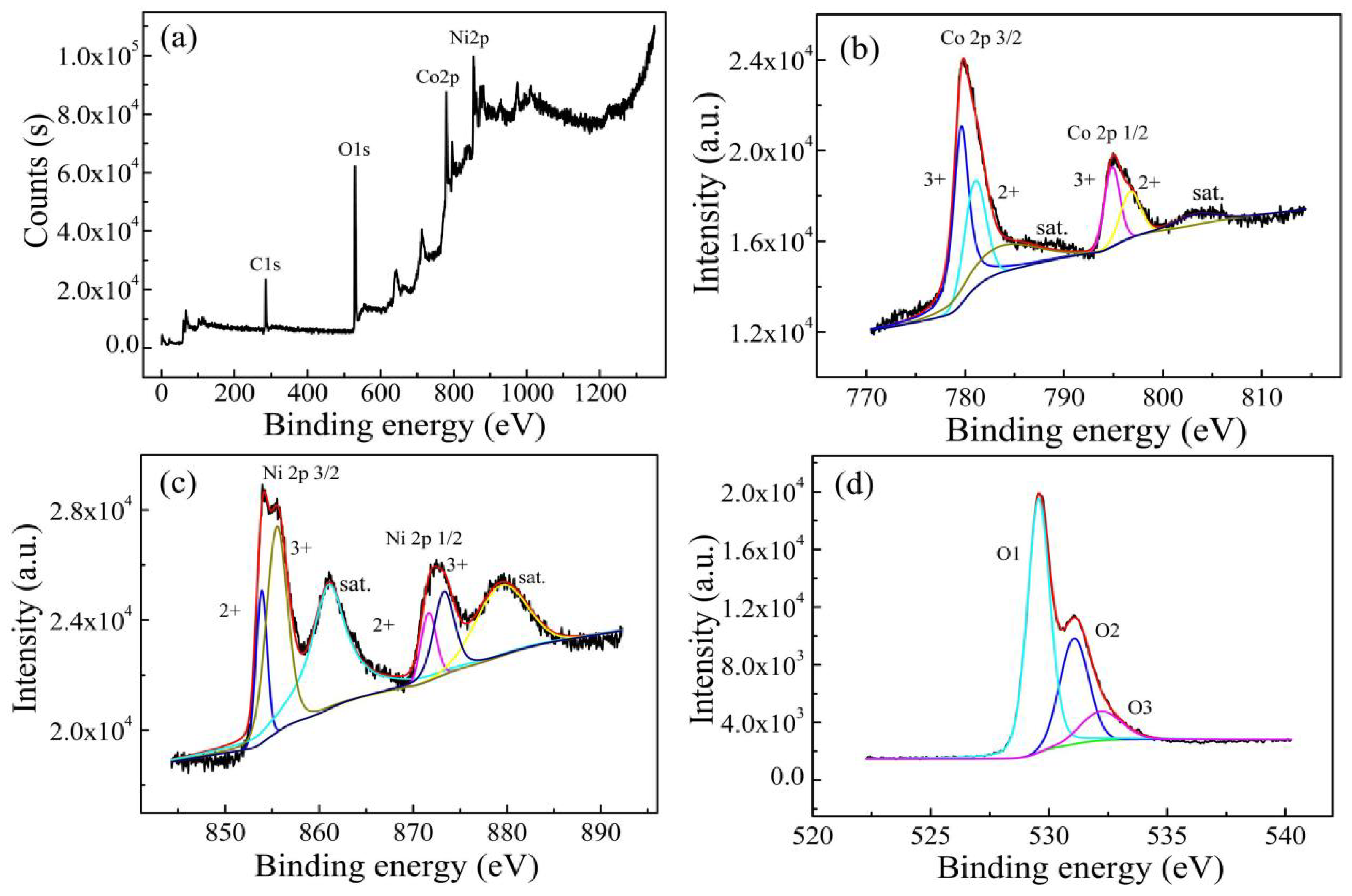
3.1. Characterization of as-Calcined NiCo2O4
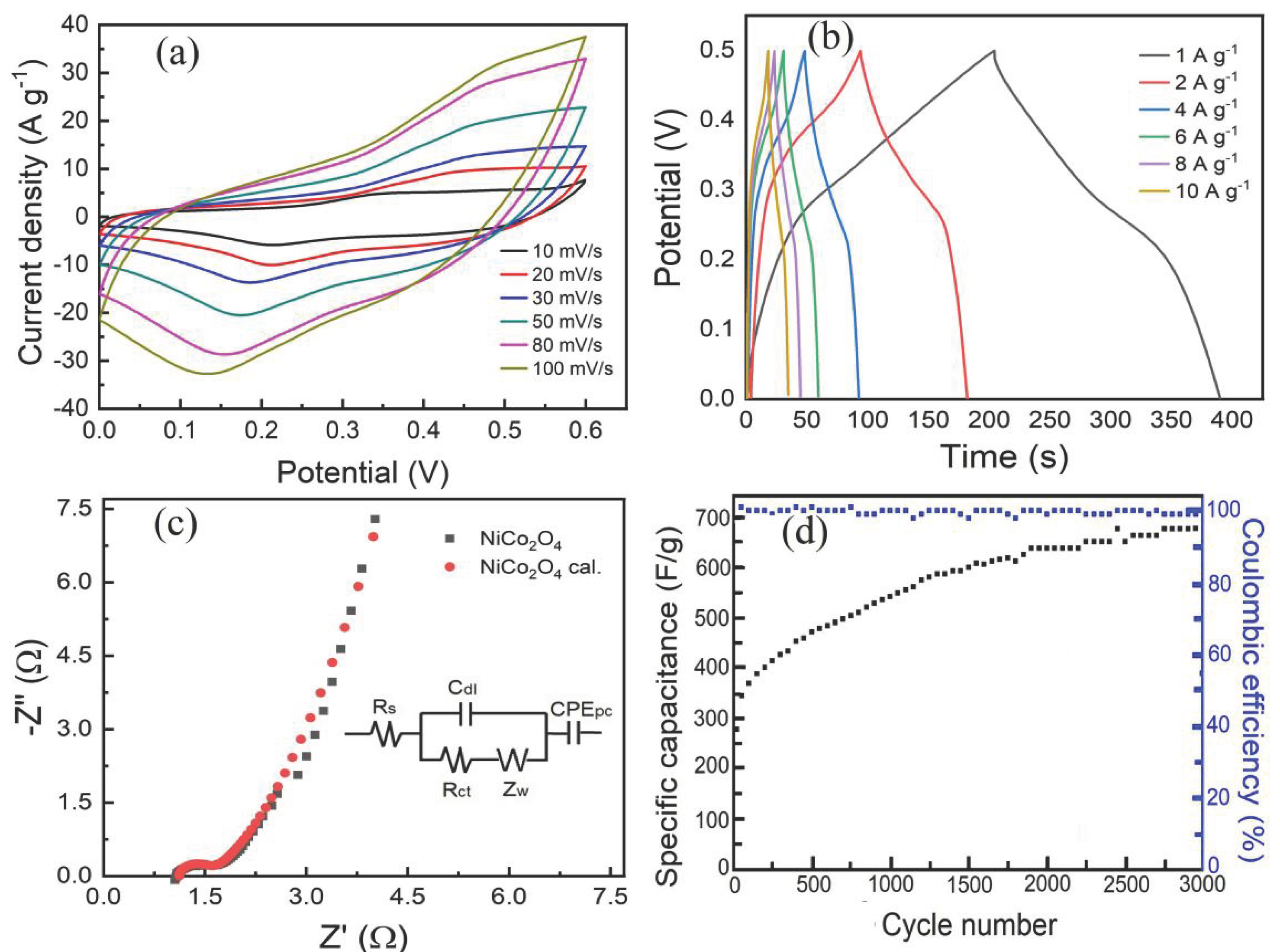
3.2. Electrochemical Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shen, L.; Che, Q.; Li, H.; Zhang, X. Mesoporous NiCo2O4 nanowire arrays grown on carbon textiles as binder-free flexible electrodes for energy storage. Adv Funct Mater 2014, 24, 2630–2637. [Google Scholar] [CrossRef]
- Wang, T.; Guo, Y.; Zhao, B.; Yu, S. NiCo2O4 nanosheets in-situ grown on three dimensional porous Ni film current collectors as integrated electrodes for high-performance supercapacitors. J Power Sources 2015, 286, 371–379. [Google Scholar] [CrossRef]
- Tang, X.; Ren, Q.; Yu, F.D.; Wang, Z.B. The improved cycling stability of nanostructured NiCo2O4 anodes for lithium and sodium ion batteries. Ionics 2023, 29, 3943–3954. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, K.; Li, W.; Xu, K. Structure-designed synthesis of hierarchical NiCo2O4@NiO composites for high-performance supercapacitors. J Colloid & Interface Sci 2019, 556, 386–391. [Google Scholar]
- Chen, D.; Pang, D.; Zhang, S.; Song, H.; Zhu, W.; Zhu, J. Synergistic coupling of NiCo2O4 nanorods onto porous Co3O4 nanosheet surface for tri-functional glucose, hydrogen-peroxide sensors and supercapacitor. Electrochim Acta 2020, 330, pp. 135326. [Google Scholar] [CrossRef]
- Yuan, Y.; Wang, W.; Yang, J.; Tang, H.; Ye, Z.; Zeng, Y.; Lu, J. Three-dimensional NiCo2O4@MnMoO4 core-shell nanoarrays for high-performance asymmetric supercapacitors. Langmuir ACS J Surf Colloids 2017, 33, 10446–10454. [Google Scholar] [CrossRef]
- Zeng, W.; Wang, L.; Shi, H.; et al. Metal–organic- framework-derived ZnO@C@NiCo2O4 core–shell structures as an advanced electrode for high-performance supercapacitors. J Mater Chem A 2016, 4, 8233–8241. [Google Scholar] [CrossRef]
- Zou, R.; Xu, K.; Wang, T.; He, G.; Liu, Q.; Liu, X. Chain-like NiCo2O4 nanowires with different exposed reactive planes for high-performance supercapacitors. J Mater Chem A 2013, 1, 8560–8566. [Google Scholar] [CrossRef]
- Jiang, H.; Ma, J.; Li, C. Hierarchical porous NiCo2O4 nanowires for high-rate supercapacitors. Chem Commun 2012, 48, 4465–4467. [Google Scholar] [CrossRef]
- Yuan, C.; Li, J.; Hou, L.; Zhang, X.; Shen, L.; Lou, X.W. Ultrathin mesoporous NiCo2O4 nanosheets supported on Ni foam as advanced electrodes for supercapacitors. Adv Funct Mater 2012, 22, 4592–4597. [Google Scholar] [CrossRef]
- Zhang, G.; Lou, X.W. General solution growth of mesoporous NiCo2O4 nanosheets on various conductive substrates as high-performance electrodes for supercapacitors. Adv Mater 2013, 25, 976–979. [Google Scholar] [CrossRef]
- Karmakar, S.; Varma, S.; Behera, D. Investigation of structural and electrical transport properties of nano-flower shaped NiCo2O4 supercapacitor electrode materials. J Alloys & Comp 2018, 757, 49–59. [Google Scholar]
- Pang, M.J.; Jiang, S.; Long, G.H.; Ji, Y.; Han, W.; Wang, B.; et al. Mesoporous NiCo2O4 nanospheres with a high specific surface area as electrode materials for high-performance supercapacitors. RSC Adv 2016, 6, 67839–67848. [Google Scholar] [CrossRef]
- Xue, W.; Wang, W.; Fu, Y.; He, D.; Zeng, F.; Zhao, R. Rational synthesis of honeycomb-like NiCo2O4@NiMoO4 core/shell nanofilm arrays on Ni foam for high-performance supercapacitors. Mater Lett 2017, 186, 34–37. [Google Scholar] [CrossRef]
- Sun, S.; Li, S.; Wang, S.; Li, Y.; Wang, P. Fabrication of hollow NiCo2O4 nanoparticle /graphene composite for supercapacitor electrode. Mater Lett 2016, 182, 23–26. [Google Scholar] [CrossRef]
- Tomboc, G.M.; Kim, H. Derivation of both EDLC and pseudocapacitance characteristics based on synergistic mixture of NiCo2O4 and hollow carbon nanofiber: An efficient electrode towards high energy density supercapacitor. Electrochim Acta 2019, 318, 392–404. [Google Scholar] [CrossRef]
- Zhang, D.; Yan, H.; Lu, Y.; et al. Hierarchical mesoporous nickel cobaltite nanoneedle/carbon cloth arrays as superior flexible electrodes for supercapacitors. Nanoscale Res Lett 2014, 9, 139–148. [Google Scholar] [CrossRef]
- Guo, D.; Zhang, L.; Song, X.; Tan, L. NiCo2O4 nanosheets grown on interconnected honeycomb-like porous biomass carbon for high performance asymmetric supercapacitor. New J Chem 2018, 42, 8478–8484. [Google Scholar] [CrossRef]
- Li, C.; Liu, Y.; Li, G.; Ren, R. Preparation and electrochemical properties of nanostructured porous spherical NiCo2O4 materials. RSC Adv 2020, 10, 9438–9443. [Google Scholar] [CrossRef]
- Wu, Y.Q.; Chen, X.Y.; Ji, P.T.; Zhou, Q.Q. Sol–gel approach for controllable synthesis and electrochemical properties of NiCo2O4 crystals as electrode materials for application in supercapacitors. Electrochim Acta 2011, 56, 7517–7522. [Google Scholar] [CrossRef]
- Wu, S.; Liu, Q.; Yan, Q.; Jiang, H.; wang, Q. Synthesis and electrochemical properties of NiCo2O4 nanoparticles. J Chin Ceram Soc 2017, 45, 504–509. [Google Scholar]
- Lin, W.C.; Guo, W.G.; Wang, L.H. Synthesis crystal structure and characterization of a new zinc citrate complex. Chin J Struc Chem 2014, 33, 591–596. [Google Scholar]
- Tang, X.; Ren, Q.; Yu, F.D.; Wang, Z.B. The improved cycling stability of nanostructured NiCo2O4 anodes for lithium and sodium ion batteries. Ionics 2023, 29, 3943–3954. [Google Scholar] [CrossRef]
- Hao, P.; Tian, J.; Sang, Y.; et al. 1D Ni-Co oxide and sulfide nanoarray/carbon aerogel hybrid nanostructures for asymmetric supercapacitors with high energy density and excellent cycling stability. Nanoscale 2019, 8, 16292–16301. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.F.; Wu, D.J.; Li, R.Z.; Li, Q.; Tong, Y.X.; Li, G.R. Hierarchical NiCo2O4 nanosheets@hollow microrod arrays for high-performance asymmetric supercapacitors. J Mater Chem A 2014, 2, 4706–4713. [Google Scholar] [CrossRef]
- Lei, Y.; Li, J.; Wang, Y.; Gu, L.; Chang, Y.; Yuan, H.; Xiao, D. Rapid microwave-assisted green synthesis of 3D hierarchical flower-shaped NiCo2O4 microsphere for high-performance supercapacitor. ACS Appl Mater & Interfaces 2014, 6, pp. 1773−1780. [Google Scholar]
- Li, J.; Xiong, S.; Liu, Y.; Ju, Z.; Qian, Y. High electrochemical performance of monodisperse NiCo2O4 mesoporous microspheres as an anode material for li-ion batteries. ACS Appl Mater & Interfaces 2013, 5, 981–988. [Google Scholar]
- Gao, S.; Liao, F.; Ma, S.; Zhu, L.; Shao, M. Network-like mesoporous NiCo2O4 grown on carbon cloth for high-performance pseudocapacitors. J Mater Chem A 2015, 3, 16520–16527. [Google Scholar] [CrossRef]
- Liu, W.W.; Lu, C.; Liang, K.; Tay, B.K. A three dimensional vertically aligned multiwall carbon nanotube/NiCo2O4 core/shell structure for novel high-performance supercapacitors. J Mater Chem A 2014, 2, 5100–5107. [Google Scholar] [CrossRef]
- Nwanya, A.C.; Offiah, S.U.; Amaechi, I.C.; et al. Electrochromic and electrochemical supercapacitive properties of room temperature PVP capped Ni(OH)2/NiO thin films. Electrochim Acta 2015, 171, 128–141. [Google Scholar] [CrossRef]
- Xie, X.; Chang, Z.; Wu, M.; Tao, Y.; Lv, W.; Yang, Q.H. Porous MnO2 for use in a high performance supercapacitor: replication of a 3D graphene network as a reactive template. Chem Commun 2013, 49, 11092–11094. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.; Liu, B.; Wang, D.; et al. Construction of unique NiCo2O4 nanowire@CoMoO4 nanoplate core/shell arrays on Ni foam for high areal capacitance supercapacitors. J Mater Chem A 2014, 2, 4954–4960. [Google Scholar] [CrossRef]
- Padmanathan, N.; Selladurai, S. Controlled growth of spinel NiCo2O4 nanostructures on carbon cloth as a superior electrode for supercapacitors. RSC Adv 2014, 4, 8341–8349. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.




