1. Introduction
Bladder cancer (BC) is the 7th most commonly diagnosed cancer in males, with 600,000 patients being diagnosed in 2020 with BC worldwide [
1]. In non muscle invasive BC (NMIBC), approximately 75% of patients present with disease confined to the mucosa (stage Ta), carcinoma in situ (CIS) or submucosa (stage T1), although most of the cancer-specific mortality is associated with T2–T4 tumors, classified as muscle invasive BC (MIBC). NMIBC can progress to muscle invasive or metastatic disease in approximately 15% of patients, however high-grade NMIBC cases can reach a 45% rate of MI progression within 5 years, associated with a 50% 5-year survival rate, despite aggressive treatments such as radical cystectomy (RC) [
1,
2,
3,
4,
5]. Nowadays, further approaches are needed to not only refine risk stratification of muscle invasion but also to guide personalized therapeutic strategies according to it, and therefore improve overall outcomes for patients with BC, addressing a critical gap in uro-oncological care.
The standard method used to determine stage of neoplasia in BC is transurethral resection of the bladder tumour (TURBT); this procedure provides treatment for patients with NMIBC and staging information for those with MIBC through histopathological biopsy analyses. However, the inter-observer difference between pathologists can vary between 27% and 74% [
6,
7,
8,
9,
10] In BC, staging of the primary lesion by TURBT is fundamental to a rational therapeutic approach; the absence of detrusor muscle and high risk tumors in the TURBT derived specimen is associated with a high risk of residual disease, early recurrence and tumour understaging. Therefore, in these cases, a second TURBT should be performed to achieve a precise diagnosis, exposing the patient to a new invasive procedure and its associated complications [
11,
12,
13]. Although much work has been done in the areas of detecting early cancer, and only few studies have focused on accurately identifying muscle invasion in BC cases [
14,
15,
16,
17]. The search for new methods that could improve the detection of MIBC in patients is necessary to improve the concordance between pathologists, diminish the number of second resections needed that could lead to fewer complications, better quality of life, and less economic burden.
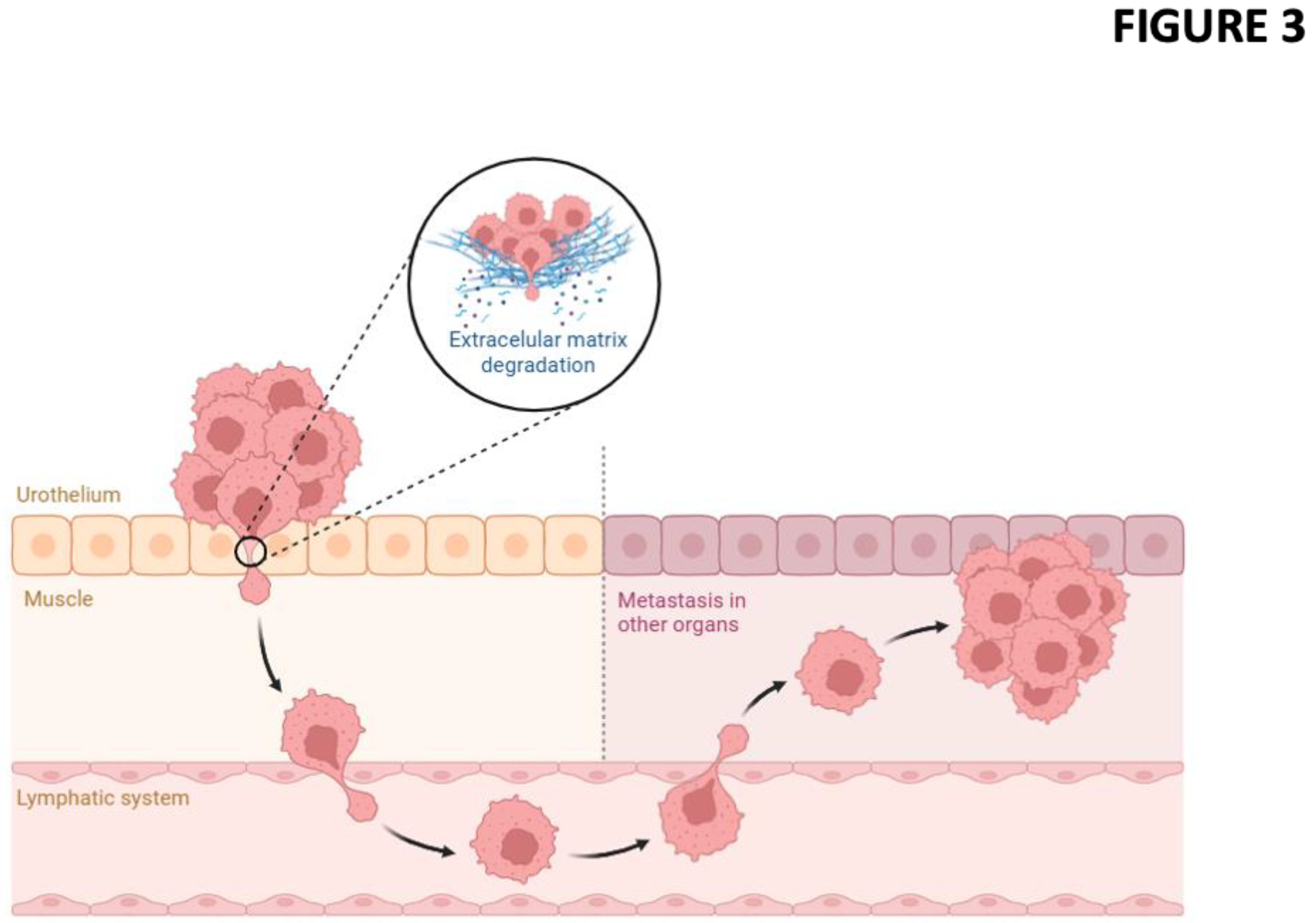
Muscle invasion requires more aggressive therapeutic approaches as it involves a complex interaction between tumor cells and their microenvironment for overcoming surrounding matrix and tissue barriers, colonizing adjacent structures through migration. In BC, this phenomenon represents not only a morphological change, but also a molecular reprogramming orchestrated by a network of biological signals that includes modifications in gene expression, activation of oncogenic signaling pathways, and remodeling of the extracellular microenvironment. Regarding the tumor microenvironment, the extracellular matrix (ECM) is a dynamic and highly structured component that modulates not only tissue support, but also cancer cell behavior, influencing invasion, differentiation, and angiogenesis [
18,
19]. However, tumors can also often induce changes in the ECM of the connective tissue, including alterations in collagen density, cross-linking, and degradation of it. ECM remodeling, often promoted by the secretion of matrix metalloproteinases (MMPs) by tumor and stromal cells, facilitates cell migration by generating pathways through which tumor cells can invade surrounding tissues [
20,
21]. The interaction between tumor cells and an unstructured ECM can favor muscle invasion, a critical marker of cancer progression and prognosis [
22]. The study of the state of the ECM in this context could allow us to evaluate its contribution to tumor migration and identify patterns that help in detecting muscle invasion.
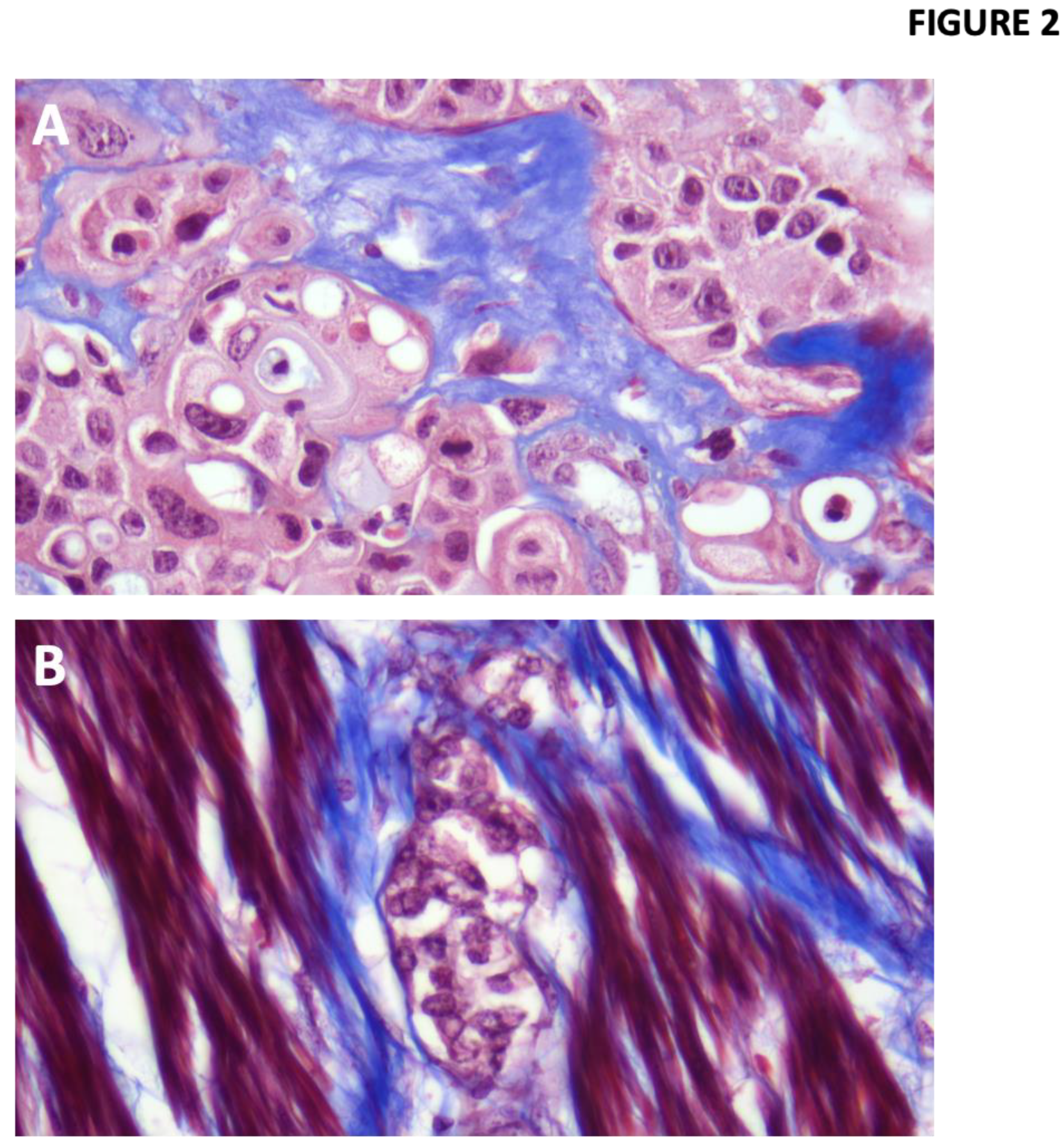
Imaging techniques and molecular assays targeting these ECM changes can provide valuable insight into the tumor microenvironment’s dynamics, aiding in the early detection of muscle invasion. MTS is a well-known histological technique widely used to evaluate the composition and organization of the ECM in tissues, due to its ability to differentiate collagen, muscle and cellular cytoplasm [
23,
24]. In the context of BC, this stain could offer significant diagnostic value by allowing clear visualization of the tumor cells and their environment, including potential muscle invasion associated with the ECM state. The ability to accurately identify the presence of unstructured or reorganized collagen fibers and changes in the integrity of the muscularis propria, could be useful in determining patterns associated with cell migration, facilitating the correlation between histological findings and underlying oncological mechanisms. However, traditional diagnostic methods including histopathological evaluation using staining techniques can be limited by subjectivity and inter-observer variability [
6,
7,
8,
9,
10].
Nowadays, Machine Learning (ML) is revolutionizing the field of medical diagnostics by enabling the analysis of complex datasets with unprecedented accuracy and efficiency [
25]. In the context of diagnosing MIBC, ML could offer a powerful tool to complement traditional histopathological techniques through algorithms capable of detecting patterns and features within biopsy images, enhancing diagnostic precision and reducing inter-observer variability inherent to manual assessments. The application of ML to analyze bladder biopsies stained with MTS represents a transformative step towards integrating computational intelligence with clinical pathology, with the potential to significantly improve patient diagnosis and outcomes.
By integrating ML into the analysis of MTS of biopsy specimens, this study aims to enhance diagnostic precision of muscle invasion in BC, enabling a more standardized and reproducible assessment of tumor characteristics, improving the patient’s decision making process.
2. Results
A total of 32 patients with BC were included, 702 images were taken and submitted to the artificial intelligence model (
Figure 2), of which 22 were NMIBC and 10 MIBC: 12 were Ta, 11 T1, 7 T2 and 2 T3, mean age was 73 years for NMIBC, while for MIBC it was 66.3, the probability of detecting muscle invasion was 0.487 and 0.998 for NMIBC and MIBC images, respectively (p=0.001) (
Table 1).
Our model achieved an accuracy of 95% for the training process and up to 90.1% in the validation process. The probability generated by the model was correlated with tumour grade, tumour size, tumour weight, which had a clinically significant association (p= 0.005, 0.017 and 0.01, respectively), (
Table 2). In addition, sensitivity and specificity were calculated for diagnosis by AUC of ROC Curves obtaining a sensitivity of 90%, specificity of 91%, a positive predictive value of 82% and a negative predictive value of 95% (
Table 3).
3. Discussion
In BC, artificial intelligence (AI) has consolidated its role as a supportive tool at various levels of the diagnostic and therapeutic process, optimizing procedures such as cystoscopy, urinary cytology, and the prediction of clinical outcomes. AI has been utilized for the automated detection of tumor lesions in endoscopic images, increasing diagnostic sensitivity compared to conventional evaluation [
15]. Furthermore, models capable of predicting tumor recurrence and progression have been developed through the analysis of clinical and imaging data, giving strength to risk stratification [
15]. Also the use of AI in bladder biopsies has been investigated to improve the accuracy of the diagnosis and decrease the inter-observer variability.
The role of ECM remodeling and connective tissue status in tumor progression and muscle invasion in BC is still under investigation. Through the use of MTS, we demonstrated its utility not only in detecting muscle invasion but also in providing insights into the structural alterations of the ECM associated with tumor cell invasion. These observations underscore the interplay between tumor cells and their microenvironment, where ECM degradation and reorganization could facilitate invasive behavior and therefore serve as an indicator of muscle invasion (
Figure 3). Our work showed the ability of the MTS technique to distinguish key histological features of the ECM in tumor biopsies, which in addition to ML algorithms makes it a valuable tool in clinical diagnostics, particularly in identifying muscle invasion. Our model achieved an accuracy in the validation process of 90.1%, which reached 95.2% in the training to differentiate NMIBC from MIBC, reaching significant differences in tumour grade, tumour weight and tumour size.
AI-driven image analysis has the potential to significantly reduce variability in cancer muscle invasion diagnosis and improve patient outcomes in oncology. By the support of advanced algorithms, AI enhances the precision of diagnostic imaging, which is crucial for accurately assessing muscle invasiveness in cancers such as BC [
26]. AI algorithms can achieve comparable or even superior performance to human experts in detecting and classifying cancer, thereby reducing diagnostic variability [
27,
28]. In this context, inter-observer variability between pathologists in the MI varies between 27% and up to 74% depending on the studies, of which up to 28% of the cases even may require some change in their treatment [
6,
7,
8,
9,
10] These differences in the pathological interpretation of TURBT specimens include the presence of technical artefacts, distinction between muscularis mucosae and muscularis propria, absence of muscularis propria, distinction between stromal desmoplasia and muscular invasion, tangential sectioning and lack of spatial orientation caused by random inclusion of bladder tissue, which leads to inter-observer variability, but also to risk of under- or overstaging and may lead to inappropriate therapeutic decisions [
7,
8,
9].
For the management of NMIBC, TURBT is a procedure with low associated morbidity and mortality. Although life threatening events are rare, complications include tumor cell dissemination, hematuria, bladder spasms, urinary retention, urinary tract infection, and bladder perforation. Complication rates reported in different studies range from 4% to 6%, with urinary tract infection and hematuria being the most common, while bladder perforation requiring surgical repair occurs in 0.5% to 8.3% of complications [
29,
30,
31]. On the other hand, for patients with MIBC, the standard treatment is RC, however, despite management, the overall 5-year survival rate is around 60%, and the decision to perform RC must be weighed against the risks, the impact on quality of life and the patient’s preferences [
32,
33]. Early complications, such as infectious, genitourinary, gastrointestinal, and wound-related complications, occur in up to 58% of patients with MIBC, and perioperative mortality has been reported at 3.2% at 30 days and 5.2% at 90 days. Additionally, patients’ independence, sexual, social, and psychological function, body image, and economic status are impaired, significantly impacting their quality of life [
4,
34,
35,
36].
A study built a model to evaluate the economic impact of BC in the USA, where they observed that the average cost per patient for NMIBC was
$31,375 USD, while for MIBC it was
$56,247 USD[
37], however, these costs could vary depending on the therapy used, a high-risk NMIBC treated with BCG could bring costs exceeding
$200,000 USD after 5 years of management, and an MIBC on trimodality therapy can exceed
$200,000 USD for each year of treatment [
38]. All these differences in the morbimortality and costs between the treatment in NMI and MI disease highlight the importance to properly distinguish the level of invasion.
This instrument could also reduce the need for re-TURBT when there is diagnostic uncertainty, which in the literature is described as varying from 17% to 71% [
16,
17,
39]. This could also reduce the costs associated with a second TURBT, which varies from 1,700 to 2,900 euros per patient, which could increase substantially if it is associated with complications and requires prolonged hospitalization [
40,
41,
42].
4. Materials and Methods
Study: Retrospective, observational, analytical study
Patients: A retrospective cohort of BC patients from the Dr. Franco Ravera Zunino’s Hospital who underwent TURBT and RC between January 2022 and October 2024 was included. A total of 32 patients were considered (11 RC and 21 TURBT) Patients with non-urothelial tumors, CIS or metastatic disease at diagnosis were excluded.
Clinical data: Patient clinical information was obtained from the pathological biopsies reports (T, tumour grade, muscle invasion, specimen weight) and the hospital clinical electronic record system.
This study was approved by the Scientific Ethical Committee of Servicio de Salud Metropolitano Oriente, the waiver of informed consent was approved by the committee.
Tumor samples and Image processing: Biopsies were obtained by TURBT or RC procedures and formalin embedded and paraffin fixed (FEPF). From the selected cases, 3-4 µm sections of FEPF samples were obtained and collected on positively charged slides. Sections stained with Hematoxylin Eosin (HE) using the ST Infinity HE Staining System kit (Leica Biosystems 3801698) in an automated mode in Autostainer ST5020 Leica equipment following the manufacturer’s instructions. MTS was then performed using the Trichrome Staining Kit (Roche 860-031) in an automated process on Ventana BenchMark Special Stains equipment following the manufacturer’s instructions.
Photographs with a total magnification of 400X were taken with a Leica FLEXACAM C1 camera, 10 to 15 photographs per biopsy (Leica DM2500 microscope, 400X (40X objective and a 10X eyepiece).
One slide per biopsy was selected that had several areas of non-disaggregated stroma associated with neoplastic urothelium, invasive or non-invasive, without sectioning or staining artifacts. In biopsies with little stroma per slide and more than one slide, areas from two or more slides were photographed.
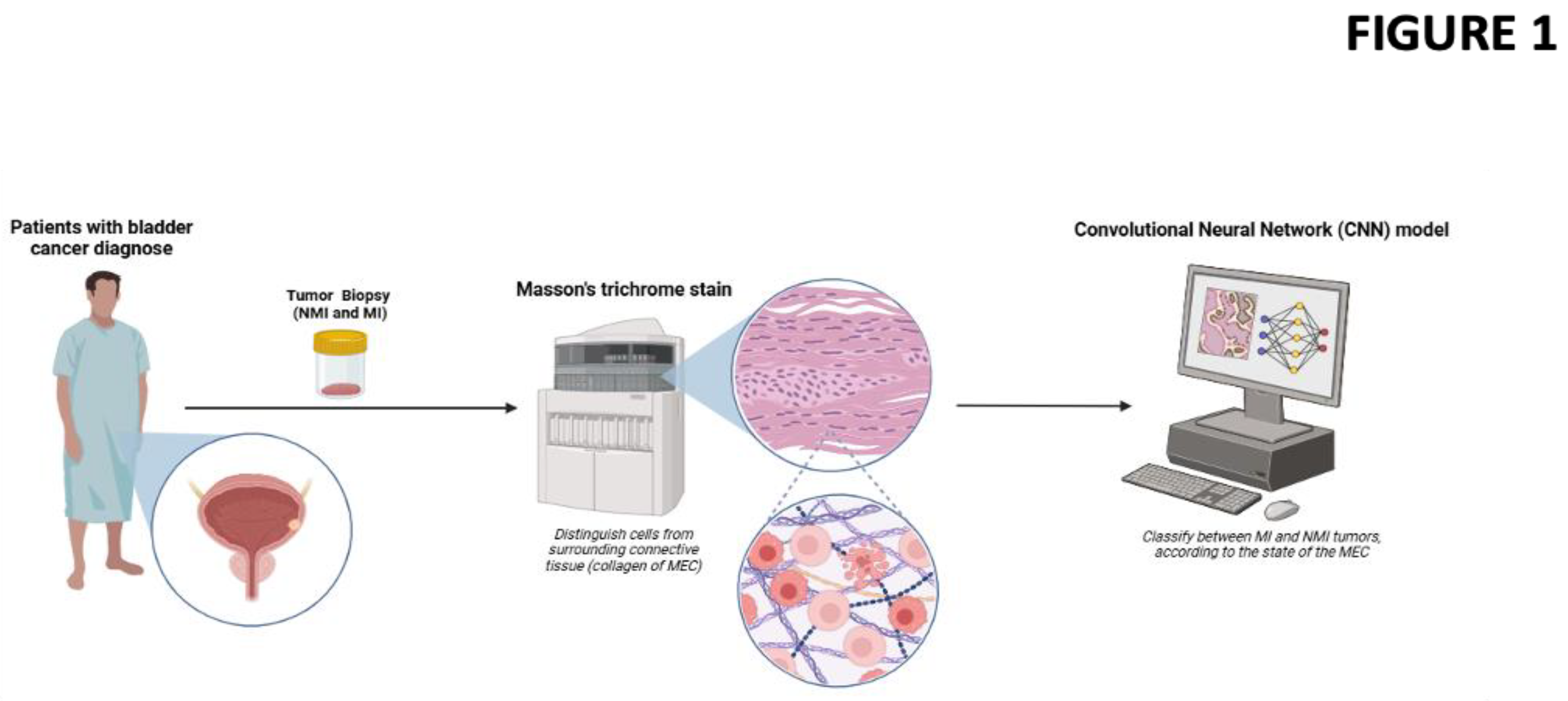
Machine Learning: A total of 702 histopathological images of BC tumors were collected for this study. The images were annotated by expert pathologists and categorized into two classes: NMIBC, which includes stages Ta and T1, and MIBC tumors. The dataset was randomly split into training and validation sets with a ratio of 75:25 respectively. Data augmentation techniques, including rotation, flipping, and zooming, were applied to the training set to increase variability and prevent overfitting. A Convolutional Neural Network (CNN) was employed for the classification task. Given the limited size of the dataset, Transfer Learning was utilized to leverage the pre-trained VGG16 model, which was initially trained on the ImageNet dataset. The fully connected layers of the pre-trained model were replaced with custom layers tailored to the binary classification problem NMIBC (Ta and T1) vs. MIBC.
The model was fine-tuned to adapt it to the specific task of histopathological image classification. During the initial phase, the convolutional layers of VGG16 were frozen, and only the newly added fully connected layers were trained. In the subsequent phase, the entire model, including the convolutional layers, was fine-tuned with a low learning rate to further optimize performance. The model was trained using the Adam optimizer with a categorical cross-entropy loss function. The model’s performance was evaluated using the validation set during training to monitor overfitting and guide hyperparameter tuning. After training, the model was tested on the validation set to assess its generalization ability. Performance metrics, including accuracy, precision, recall, F1-score, and the area under the receiver operating characteristic curve (AUC-ROC), were calculated to comprehensively evaluate the model.
Data analysis:
Finally, the model probabilities were correlated with the aforementioned clinical variables of the patients. Statistical analysis was performed with IBM SPSS Statistics v25 using chi square (χ2) or Fisher exact test as appropriate for association analyses. Spearman correlation analysis was performed to evaluate correlation between quantitative variables and the probabilities of the model. Statistical significance was set at p < 0.05.
Figure 1 graphically summarizes the methodology used.
5. Conclusions
In summary, AI has been used for various applications, both in the bladder and other organs, so it is a tool that could potentially help improve diagnostic accuracy when used as an aid to medical assessment. Thus, our study suggests that CNN-based MTS stain histological images could help identify NMIBC from MIBC and therefore reduce interobserver variability among pathologists, preventing the under or overtreatment, having crucial relevance the first sample for the future management, helping to avoid a second TURBT or even preventing a RC when the muscularis mucosae can produce a challenging interpretation against the muscularis propria. This would allow for significant savings in morbidity and mortality, and reduce burden associated with the disease, and would be especially useful in centers without trained uropathologists, which could have greater interobserver variability in the diagnosis and staging of BC. Furthermore, its incorporation into digital histopathology platforms has allowed for the validation of clinically relevant morphological biomarkers in urological oncology [
43]. These findings support the relevance of MTS not only as a descriptive technique but also as a basis for the development of quantitative algorithms that integrate histological analyses with advanced predictive models, as well as a star point to future research that relate microenvironment with the oncological features and outcomes.
Author Contributions
D.P.: Conceptualization - Writing— original draft - review & editing - Investigation - Formal analysis. H.G.: Methodology - Software - Formal analysis - Validation. K.R.: Methodology - Resources. R.L.: Writing - original draft - investigation. C.S.: Writing - original draft - Investigation. R.G.: Writing - original draft - Investigation. M.L.: Writing - original draft - Investigation.. C.B.L.: Conceptualization —writing—original draft. J.C.B.: Conceptualization - Project administration - Formal analysis - Writing - review & editing - supervisor - . All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board and Ethics Committee of Servicio de Salud Metropolitano Oriente.
Informed Consent Statement
Patient consent was waived due to patient identification or personal data will remain anonymous in any presentation or communication generated by the study; furthermore, this work is retrospective and only involves clinical records or databases.
Data Availability Statement
Datasets analyzed or generated during the study are unavailable due to privacy restrictions.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
BC
NMIBC
MIBC
MTS
ECM
CNN
TURBT
RC
ML
AI
MMPs
FEPF
HE
CIS
AUC-ROC |
Bladder Cancer
Non-Muscle Invasive Bladder Cancer
Muscle Invasive Bladder Cancer
Masson’s Trichrome Staining
Extracellular Matrix
Convolutional Neural Network
Transurethral Resection of Bladder Tumor
Radical Cystectomy
Machine Learning
Artificial Intelligence
Matrix Metalloproteinases
Formalin-Embedded and Paraffin-Fixed
Hematoxylin Eosin
Carcinoma in situ
Area Under the Receiver Operating Characteristic curve
|
References
- Dyrskjøt, L; Hansel, DE; Efstathiou, JA; Knowles, MA; Galsky, MD; Teoh, J; et al. Bladder cancer. Nat Rev Dis Primers 2023, 9, 58. [Google Scholar] [CrossRef] [PubMed]
- Holzbeierlein, JM; Bixler, BR; Buckley, DI; Chang, SS; Holmes, R; James, AC; et al. Diagnosis and Treatment of Non-Muscle Invasive Bladder Cancer: AUA/SUO Guideline: 2024 Amendment. Journal of Urology 2024, 211, 533–8. [Google Scholar] [CrossRef] [PubMed]
- Halaseh, SA; Halaseh, S; Alali, Y; Ashour, ME; Alharayzah, MJ. A Review of the Etiology and Epidemiology of Bladder Cancer: All You Need To Know. Cureus 2022. [Google Scholar] [CrossRef] [PubMed]
- Parrao, D; Lizana, N; Saavedra, C; Fernández, V; Lindsay, CB; Larrañaga, M; et al. Bladder preservation alternatives in non-metastatic muscle-invasive bladder tumor: A systematic review and meta-analysis. Asian J Urol 2025, 12, 309–19. [Google Scholar] [CrossRef]
- Parrao, D; Lizana, N; Saavedra, C; Larrañaga, M; Lindsay, CB; San Francisco, IF; et al. Active Surveillance in Non-Muscle Invasive Bladder Cancer, the Potential Role of Biomarkers: A Systematic Review. Current Oncology 2024, 31, 2201–20. [Google Scholar] [CrossRef]
- Campbell, RA; Wood, A; Michael, PD; Shin, D; Pramod, N; Haywood, SC; et al. Impact of pathologic re-review on grade, clinical stage, and risk stratification for patients with nonmuscle invasive bladder cancer. Urologic Oncology: Seminars and Original Investigations 2024, 42, 372.e21–372.e27. [Google Scholar] [CrossRef]
- Robesti, D; Moschini, M; Pio Tenace, N; Burgio, G; Re, C; Leni, R; et al. The Impact of Second Opinion Expert Pathology Review in Patient Management at the Time of Transurethral Resection of the Bladder. Eur Urol Focus 2024, 10, 1043–8. [Google Scholar] [CrossRef]
- Giunchi, F; Panzacchi, R; Capizzi, E; Schiavina, R; Brunocilla, E; Martorana, G; et al. Role of Inter-Observer Variability and Quantification of Muscularis Propria in the Pathological Staging of Bladder Cancer. Clin Genitourin Cancer 2016, 14, e307–12. [Google Scholar] [CrossRef]
- Traboulsi, SL; Brimo, F; Yang, Y; Maedler, C; Prévost, N; Tanguay, S; et al. Pathology review impacts clinical management of patients with T1‒T2 bladder cancer. Canadian Urological Association Journal 2017, 11, 188. [Google Scholar] [CrossRef]
- Taraz Jamshidi, S; Shahraki, M; Kalantari, MR; Farzadnia, M; Jafarian, A; Morovatdar, N. Comparison of Interobserver Variability Between Two Grading Systems Used for Urothelial Carcinoma. Nephrourol Mon 2024, 16. [Google Scholar] [CrossRef]
- Chang, SS; Boorjian, SA; Chou, R; Clark, PE; Daneshmand, S; Konety, BR; et al. Diagnosis and Treatment of Non-Muscle Invasive Bladder Cancer: AUA/SUO Guideline. Journal of Urology 2016, 196, 1021–9. [Google Scholar] [CrossRef]
- Durant, AM; Nguyen, M; Choudry, MM; Mi, L; Andrews, JR; Tyson, MD. Repeat TURBT in large volume high-grade non-invasive bladder cancer. Bladder Cancer 2024, 10, 270–7. [Google Scholar] [CrossRef] [PubMed]
- Shim, JS; Choi, H; Il, Noh T; Tae, JH; Yoon, SG; Kang, SH; et al. The clinical significance of a second transurethral resection for T1 high-grade bladder cancer: Results of a prospective study. Korean J Urol 2015, 56, 429. [Google Scholar] [CrossRef] [PubMed]
- Ma, X; Zhang, Q; He, L; Liu, X; Xiao, Y; Hu, J; et al. Artificial intelligence application in the diagnosis and treatment of bladder cancer: advance, challenges, and opportunities. Front Oncol 2024, 14. [Google Scholar] [CrossRef] [PubMed]
- Ferro, M; Falagario, UG; Barone, B; Maggi, M; Crocetto, F; Busetto, GM; et al. Artificial Intelligence in the Advanced Diagnosis of Bladder Cancer-Comprehensive Literature Review and Future Advancement. Diagnostics 2023, 13, 2308. [Google Scholar] [CrossRef]
- Cumberbatch, MGK; Foerster, B; Catto, JWF; Kamat, AM; Kassouf, W; Jubber, I; et al. Repeat Transurethral Resection in Non–muscle-invasive Bladder Cancer: A Systematic Review. Eur Urol 2018, 73, 925–33. [Google Scholar] [CrossRef]
- Vianello, A; Costantini, E; Del Zingaro, M; Bini, V; Herr, HW; Porena, M. Repeated White Light Transurethral Resection of the Bladder in Nonmuscle-Invasive Urothelial Bladder Cancers: Systematic Review and Meta-Analysis. J Endourol 2011, 25, 1703–12. [Google Scholar] [CrossRef]
- Witjes, JA. Follow-up in non-muscle invasive bladder cancer: facts and future. World J Urol 2021, 39, 4047–53. [Google Scholar] [CrossRef]
- Brunner, A; Tzankov, A. The Role of Structural Extracellular Matrix Proteins in Urothelial Bladder Cancer (Review). Biomark Insights 2007, 2, BMI.S294. [Google Scholar] [CrossRef]
- Hu, X; Sun, C; Ren, X; Ge, S; Xie, C; Li, X; et al. Contrast-enhanced Ultrasound Combined With Elastography for the Evaluation of Muscle-invasive Bladder Cancer in Rats. Journal of Ultrasound in Medicine 2023, 42, 1999–2011. [Google Scholar] [CrossRef]
- Dozmorov, MG; Kyker, KD; Saban, R; Knowlton, N; Dozmorov, I; Centola, MB; et al. Analysis of the interaction of extracellular matrix and phenotype of bladder cancer cells. BMC Cancer 2006, 6, 12. [Google Scholar] [CrossRef] [PubMed]
- Alfano, M; Nebuloni, M; Allevi, R; Zerbi, P; Longhi, E; Lucianò, R; et al. Linearized texture of three-dimensional extracellular matrix is mandatory for bladder cancer cell invasion. Sci Rep 2016, 6, 36128. [Google Scholar] [CrossRef] [PubMed]
- Bauman, TM; Nicholson, TM; Abler, LL; Eliceiri, KW; Huang, W; Vezina, CM; et al. Characterization of Fibrillar Collagens and Extracellular Matrix of Glandular Benign Prostatic Hyperplasia Nodules. PLoS One 2014, 9, e109102. [Google Scholar] [CrossRef] [PubMed]
- Rani, S; Pervaiz, N; Parsad, D; Kumar, R. Differential expression of extracellular matrix proteins in the lesional skin of vitiligo patients. Arch Dermatol Res 2023, 315, 2393–402. [Google Scholar] [CrossRef]
- Rana, M; Bhushan, M. Machine learning and deep learning approach for medical image analysis: diagnosis to detection. Multimed Tools Appl 2023, 82, 26731–69. [Google Scholar] [CrossRef]
- Hassane, M. Artificial-Intelligence-Driven Precision Medicine in Cancer Treatment. Premier Journal of Science 2024. [Google Scholar] [CrossRef]
- Yang, Y; Guan, S; Ou, Z; Li, W; Yan, L; Situ, B. Advances in AI-based Cancer Cytopathology (3/2023). Interdisciplinary Medicine 2023, 1. [Google Scholar] [CrossRef]
- Prabhua, S; Prasada, K; Robels-Kelly, A; Lu, X. AI-based Carcinoma Detection and Classification Using Histopathological Images: A Systematic Review. 2022. [Google Scholar] [CrossRef]
- Sheybaee Moghaddam, F; Dwabe, S; Mar, N; Safdari, L; Sabharwal, N; Goldberg, H; et al. The Role of Maximal TURBT in Muscle-Invasive Bladder Cancer: Balancing Benefits in Bladder Preservation and Beyond. Cancers (Basel) 2024, 16, 3361. [Google Scholar] [CrossRef]
- Pereira, JF; Pareek, G; Mueller-Leonhard, C; Zhang, Z; Amin, A; Mega, A; et al. The Perioperative Morbidity of Transurethral Resection of Bladder Tumor: Implications for Quality Improvement. Urology 2019, 125, 131–7. [Google Scholar] [CrossRef]
- Jindal, T; Sarwal, A; Jain, P; Koju, R; Mukherjee, S. A retrospective analysis of the factors associated with increased risk of readmission within 30 days after primary transurethral resection of bladder tumor. Curr Urol 2023, 17, 257–61. [Google Scholar] [CrossRef]
- Yang, X; Zhang, S; Cui, Y; Li, Y; Song, X; Pang, J. Efficacy and safety of transurethral resection of bladder tumour combined with chemotherapy and immunotherapy in bladder-sparing therapy in patients with T1 high-grade or T2 bladder cancer: a protocol for a randomized controlled trial. BMC Cancer 2023, 23, 320. [Google Scholar] [CrossRef]
- Hautmann, RE; de Petriconi, RC; Pfeiffer, C; Volkmer, BG. Radical Cystectomy for Urothelial Carcinoma of the Bladder Without Neoadjuvant or Adjuvant Therapy: Long-Term Results in 1100 Patients. Eur Urol 2012, 61, 1039–47. [Google Scholar] [CrossRef] [PubMed]
- Maibom, SL; Joensen, UN; Poulsen, AM; Kehlet, H; Brasso, K; Røder, MA. Short-term morbidity and mortality following radical cystectomy: a systematic review. BMJ Open 2021, 11, e043266. [Google Scholar] [CrossRef] [PubMed]
- Tyson, MD; Barocas, DA. Quality of Life After Radical Cystectomy. Urologic Clinics of North America 2018, 45, 249–56. [Google Scholar] [CrossRef] [PubMed]
- Yang, LS; Shan, BL; Shan, LL; Chin, P; Murray, S; Ahmadi, N; et al. A systematic review and meta-analysis of quality of life outcomes after radical cystectomy for bladder cancer. Surg Oncol 2016, 25, 281–97. [Google Scholar] [CrossRef]
- Clark, O; Sarmento, T; Eccleston, A; Brinkmann, J; Picoli, R; Daliparthi, V; et al. Economic Impact of Bladder Cancer in the USA. Pharmacoecon Open 2024, 8, 837–45. [Google Scholar] [CrossRef]
- Scilipoti, P; Moschini, M; Li, R; Lerner, SP; Black, PC; Necchi, A; et al. The Financial Burden of Localized and Metastatic Bladder Cancer. Eur Urol 2025, 87, 536–50. [Google Scholar] [CrossRef]
- Gendy, R; Delprado, W; Brenner, P; Brooks, A; Coombes, G; Cozzi, P; et al. Repeat transurethral resection for non-muscle-invasive bladder cancer: a contemporary series. BJU Int 2016, 117, 54–9. [Google Scholar] [CrossRef]
- Contieri, R; Lughezzani, G; Buffi, NM; Taverna, G; Giacobbe, A; Micheli, E; et al. Could We Safely Avoid a Second Resection in Selected Patients With T1 Non-Muscle-Invasive Bladder Cancer? Preliminary Results of Cost-Effectiveness Study From HUmanitas New Indications for ReTUR (HuNIRe) Multicenter Prospective Trial. Front Oncol 2022, 12. [Google Scholar] [CrossRef]
- Zhang, JH; Joyce, DD; Shan, Y; Fadel, A; Liao, B; Boorjian, SA; et al. National complication and cost burden of transurethral resection of bladder tumor for bladder cancer. Urologic Oncology: Seminars and Original Investigations 2025, 43, 469.e1–469.e11. [Google Scholar] [CrossRef]
- Pattou, M; Peyrottes, A; Neuzillet, Y; Lebret, T. Outpatient transurethral resection of bladder tumors: A systematic review of oncologic and safety outcomes. World J Urol 2025, 43, 359. [Google Scholar] [CrossRef]
- Levy, JJ; Azizgolshani, N; Andersen, MJ; Suriawinata, A; Liu, X; Lisovsky, M; et al. A large-scale internal validation study of unsupervised virtual trichrome staining technologies on nonalcoholic steatohepatitis liver biopsies. Modern Pathology 2021, 34, 808–22. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).