1. Introduction
Between 18 to 66% of stroke survivors suffer from aphasia in the United States of America [
1]. Aphasia significantly restricts participation in social, familial, and professional spheres, profoundly impacting the quality of life [
2,
3]. While Speech and Language Therapy (SLT) is the gold standard for aphasia recovery, it often does not lead to complete recovery [
4].
Non-invasive brain stimulations, such as transcranial Direct Current Stimulation (tDCS) or repetitive Transcranial Magnetic Stimulation (rTMS) are promising tools for enhancing post-stroke aphasia recovery [
5,
6]. Contrary to tDCS, rTMS alone has shown to be effective for post-stroke aphasia recovery with or without being associated with online language tasks or SLT [
5,
6]. Majority of rTMS studies in post-stroke aphasia following a left hemisphere stroke applied inhibitory stimulation to the right Inferior Frontal Gyrus (IFG). This approach is based on the theory of transcallosal interhemispheric imbalance, where a brain lesion leads to hyperactivation of the undamaged hemisphere and hypoactivation of the damaged hemisphere [
7,
8]. However, targeting the right IFG for all individuals with post-stroke aphasia may be inefficient [
9]. This method may overlook the complex organization of language, which involves a large, interconnected network, also linked to other cognitive functions such as executive functions and working memory [
10]. Stefaniak et al. [
9] indicated that people with different aphasia profiles may have varied recovery paths. In an rTMS study targeting the right IFG in people with post-stroke aphasia, Dresang et al. [
11] found that semantic and phonological baseline abilities [measured using the Philadelphia Naming Test error types] [
12] could predict rTMS outcomes. More specifically, they observed that higher baseline semantic scores were associated with greater naming improvement in the rTMS group compared to the sham group, whereas individuals with higher phonological scores appeared to show reduced benefit from rTMS [
11]. This suggests that a participant’s language profile may influence their response to rTMS. The precise clinical profile of participants can potentially inform the most effective cortical target to facilitate or inhibit. A study highlighted this approach, showing that participants with phonological impairments benefited from inhibitory rTMS targeting the right motor cortex of the lips [
13]. As rTMS not only modulates the targeted area but also influences the connected network through intra- and interhemispheric connection, improvements in accuracy and the reduction of phonological errors in a Picture Naming task following motor cortex stimulation may be attributed to the stimulation of the dorsal phonological stream [
10,
13,
14].
For those with lexicosemantic difficulties, post-stroke aphasia can result in anomia and various semantic errors (e.g., saying
wolf instead of
dog). These difficulties can result from a deficit in lexicon access or the semantic system itself [
15]. Lexicosemantic processes involve a complex brain network including several cortical areas [
16]. According to the hub-and-spokes theory, the Anterior Temporal Lobe (ATL) serves as a central amodal semantic hub, integrating information from various modalities [
17,
18]. Other theories emphasize its multimodal and potentially lexical sensibility [
16,
19].
Here we targeted, for the first time to the best of our knowledge, the right preserved ATL using inhibitory rTMS in persons with post-stroke aphasia characterized by lexicosemantic difficulties. We hypothesized that participants will improve naming abilities, as well as other lexicosemantic processes such as semantic judgment. We also hypothesized that this approach will improve communication abilities monitored using self-report questionnaire and a speech production task.
2. Materials and Methods
Participants
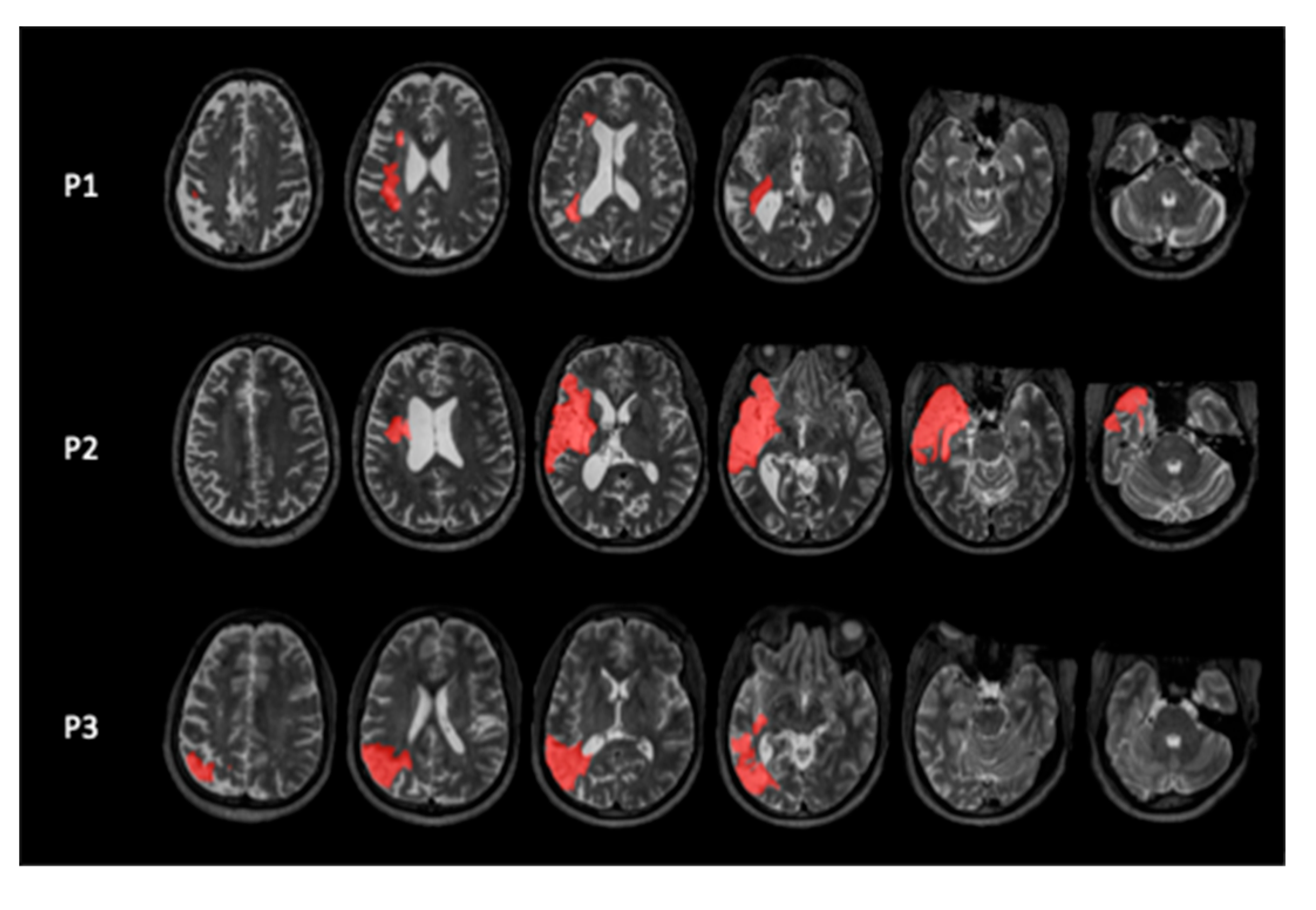
We included three participants with chronic mild to moderate chronic aphasia following a left hemispheric stroke (
Figure 1) at the University of South Carolina. The study was approved by the IRB of the University of South Carolina, and all participants signed an Informed Consent form. They received monetary compensation for their effort.
All participants were right-handed [Edinburgh questionnaire] [
20], fluent English speakers, had a single stroke event, and were diagnosed with aphasia by a Speech and Language Pathologist or a physician. We excluded participants with contraindications to rTMS such as uncontrolled epilepsy, intracranial ferromagnetic body, or factors that lower seizure threshold, following the safety recommendation of Rossi et al. [
21]. Non-inclusion criteria also concerned psychiatric history, illiteracy, known neurodegenerative disorders, or severe uncorrected visual or hearing impairment. Demographic characteristics of the participants are shown in
Table 1.
All participants underwent language assessment prior to the experiment conducted by a Speech and Language Pathologist, that included the bedside Western Aphasia Battery (WAB) [
22] and the short form of the Philadelphia Naming Test (PNT) [
23] (see
Table A1). They all presented with lexicosemantic impairment characterized by anomia and produced semantic errors. They had no major phonological deficit (i.e., preserved word repetition).
P1 presented with moderate aphasia characterized by long latencies and reduced spontaneous speech, primarily due to anomia. Picture and object naming tasks resulted mainly in nonresponses and long delays. Repetition was preserved for both single words and simple sentences, though he could not repeat longer sentences fully. Auditory comprehension was largely intact, although he made occasional errors on complex yes/no questions.
P2 exhibited mild fluent aphasia, with spontaneous speech marked by long latencies, semantic errors, and circumvolutions. Minor sequalae of apraxia of speech were observed such as disrupted prosody and initial phoneme repetition. Despite word-finding difficulties in spontaneous speech, he did not show anomia during the naming tasks. Repetition was preserved for words or short sentences but impaired for longer ones. Auditory comprehension was preserved for short sentences, though difficulties emerged with increased sentence length.
P3 presented mild aphasia with fluent speech, although frequent pauses were noted due to word-finding difficulties. She produced semantic errors both in spontaneous speech and during naming tasks. Repetition was preserved at the word level but showed errors at the sentence level. Auditory comprehension was intact for both simple and complex sentences.
Experimental Design
We used a randomized multiple-baseline design across participants following SCED protocols [
24,
25], which is suitable for interventions with potentially long-lasting effect. This design involves intrasubject analysis, repeated measures, and randomization of the baseline’s length.
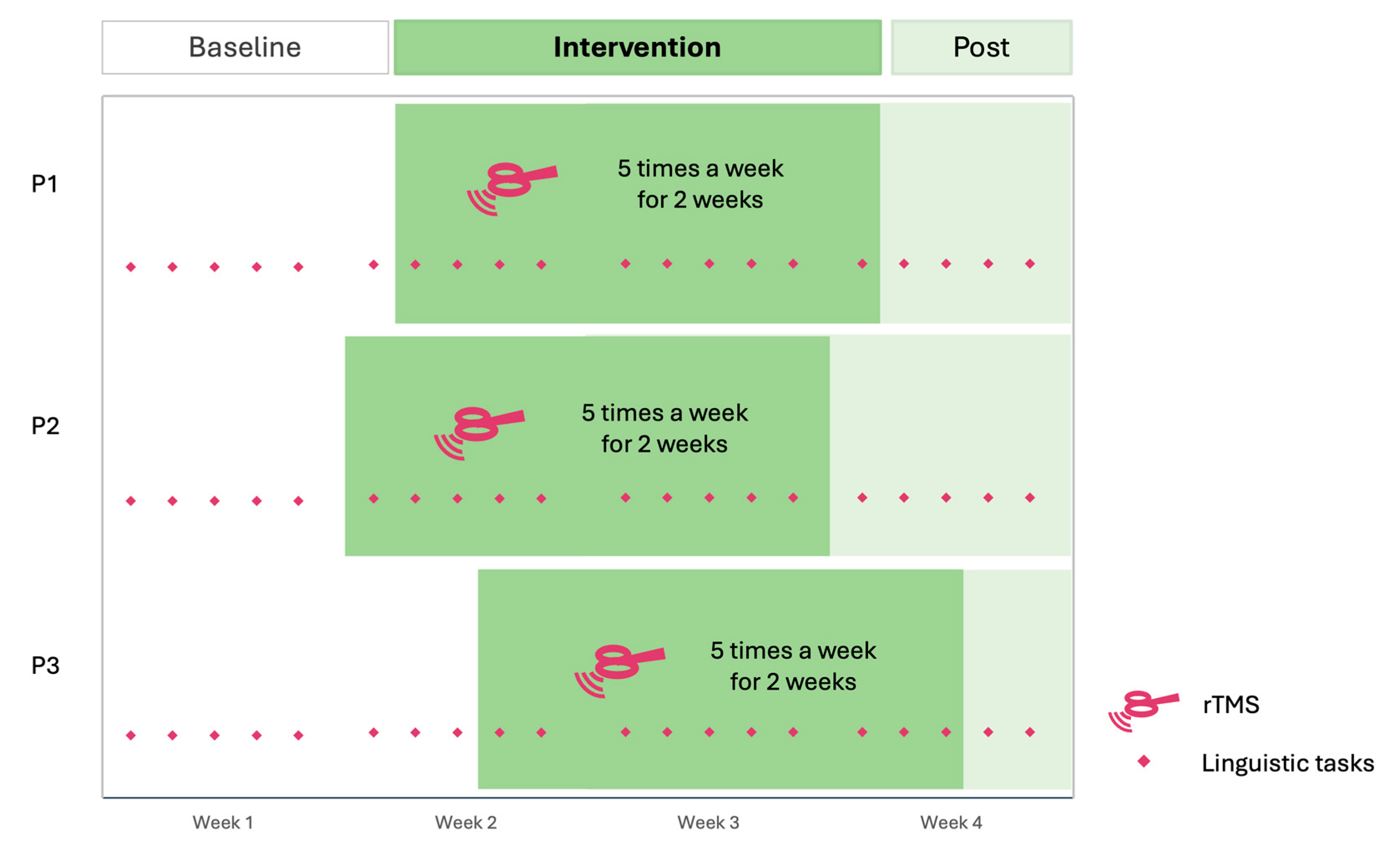
Each participant performed three linguistic tasks five times a week over four weeks (
Figure 2). These tasks included a Picture Naming task, an Auditory Naming task, and a Semantic Decision task. The length of the baseline phase varied among participants (pseudo-randomized between 5 to 9 baseline points), followed by a two-week period of rTMS administered five times a week (10 sessions in total). The rTMS targeted the right ATL using inhibitory stimulation. Participants underwent a post-intervention period extending to the end of the fourth week of inclusion (1 to 5 follow-up points). This results in 6 baseline points for P1, 5 for P2 and 8 for P3. Additionally, participants completed weekly the Stroke and Aphasia Quality of Life Scale-39 as self-report questionnaires of communication abilities [
26], as well as the Peer Conflict Resolution (PCR) task [
27], both measures accounted as generalization effects of the stimulation. A follow-up measure was recorded one month later with the same tasks.
Behavioral Measures
For all three linguistic tasks, the order of the trials was randomized and controlled by e-prime software 3.0. Before each task, the participants performed several trials with corrective feedback if necessary to ensure the good comprehension of the instructions.
Naming Tasks
The Naming tasks comprises one Picture Naming and one Auditory Naming task. Before starting the Auditory Naming task, the participants (with the exception of P1) underwent a familiarization phase where all auditory definitions were played, and the corrective feedback was given to the participant orally. As this familiarization phase was not administered for P1, their first session was excluded from the analysis.
The Auditory and Picture Naming tasks included the 36 pictures and 36 definitions from the Auditory and Visual Naming Test [
28]. Supplementary items were added to the task. For the Picture Naming task, we added 10 items with late age-of-acquisition words [
29]. For the Auditory Naming task, we added 10 abstract items [
30] with high frequency [
31] (
Table A2). Auditory definitions were generated by Chat GPT-4o using a prompt following Hamberger et al.’s guidelines [
28] (i.e., “give a short description for this word that can be presentable within 4 seconds, with low likelihood that the target word can be named before the final word”). All added items (pictures and definition) were tested on a group of 20 neurotypicals (mean age = 60.5, SD = 7.2), and we selected items with a naming agreement > 60% (mean = 83.0%, SD = 1.16). To maximize regularity between sessions, Auditory Naming items were produced using Typecast, an AI virtual actor service (character casted: Carl,
https://typecast.ai).
Semantic Decision Task
These tasks contain 82 stimuli, with half concrete (mean = 4.54; SD = 0.29) and half abstract (mean = 2.51; SD = 0.51) words that were presented to participants on the screen [
30]. They were asked to press the “k” key when the word was concrete and “j” if the word was abstract. Response accuracy and reaction time was automatically recorded.
Peer Conflict Resolution
Participants performed the Peer Conflict Resolution (PCR) task [
27,
32] as a measure of discourse production. First, they were asked to immediately recall a story in their own words, and then to answer questions about the story. To evaluate performance, we analyzed the transcription using the CLAN [
33,
34] and obtained various measures such as the mean length of utterance, the fluency, and frequency of words. We also assessed the semantic coherence and logical flow with Chat GPT-5, providing it with the PCR task instructions, including the story participants worked on [
27]. The model was then prompted with: “What is the coherence and logical flow of the following text sequences corresponding to the task provided earlier, on a scale of 0 to 100? Present a score for (1) the retelling part and (2) the questions part”. Semantic coherence scores were compared against those from neurotypical participants with high SES scores from the ABC database [
35]. High SES was defined as Hollingshead scores greater than 55 (n=10; 7 Female; mean age = 67.4 years; SD = 5.0). To rule out potential cognitive impairments, only participants with Montreal Cognitive Assessment scores ≥ 26 were included [
36]. In the neurotypical sample, mean semantic coherence was 67.4 for the retelling part (SD = 7.0) and 74.0 for the questions part (SD = 9.3).
SAQOL-39
The SAQOL-39 is a self-report quality of life questionnaire specifically adapted for people with aphasia [
26]. It comprises four subdomains (i.e., physical, psychosocial, communication, and energy) that assess areas of functioning potentially affected by stroke and aphasia.
rTMS
We applied rTMS with a 75mm figure-8 coil (Cool-B65 Butterfly Coil, MagVenture) connected to MagPro device. Participants received five sessions per week over two weeks targeting the right ATL using continuous Theta Burst Stimulation (600 pulses, burst frequency at 5 Hz, burst of three pulses at 50Hz). The stimulation intensity was set at 80-90% of the resting motor threshold (RMT). As rTMS targeting the ATL may cause discomfort due to facial nerve twitching, the stimulation intensity was varied from 90% to 80% of the RMT depending on participant comfort, resulting in the following stimulation intensity: P1: 80%, P2: 90%, P3: 80%. The target localization was facilitated by the neuronavigation software Brainsight®, which helped to localize the right ATL based on each participant’s anatomical T1 scan. The stimulation target was identified at 10 mm posteriorly from the tip of the middle temporal lobe.
Analysis
Data Analysis and Cleaning
For both Naming tasks, accuracy and reaction time (RT) were analyzed using the speech analysis software CheckVocal [
37]. For Picture Naming, RT was calculated starting from the display of each picture. For Auditory Naming, RT was calculated starting at the last phoneme of each description.
For the Semantic Decision, accuracy and RT were automatically recorded. To clean data, we deleted RTs < 200ms and > 3000ms.
Statistical Analysis
Statistical analysis followed SCED guidelines [
38,
39]. Initially, we calculated the monotonic trend of the baseline. If the baseline showed a significant trend, we adjusted the results using Kendall’s Tau rank correlation coefficient (Tarlow, 2016). This correction involved calculating a baseline corrected Tau-U if significant positive or negative trends are observed before intervention. The Tau-U calculation generates effect sizes with following results: < 0.2 corresponding to small effect, 0.2 - 0.6 moderate effect, 0.6 - 0.8 large effect and > 0.8 very large effect [
38,
39]. All data visualization aligned with SCED recommendations [
25] and were generated using RStudio (4.4.0).
3. Results
All participants completed 21 sessions, including the 1-month follow-up assessment. However, P1 rescheduled three consecutive sessions after the 7th session (the first rTMS session) due to being out of town. We also encountered a recording issue with the Semantic Decision task for Participant 3 in Session 9. All sessions were carried out without other interruptions.
3.1. Naming Tasks
3.1.1. Picture Naming
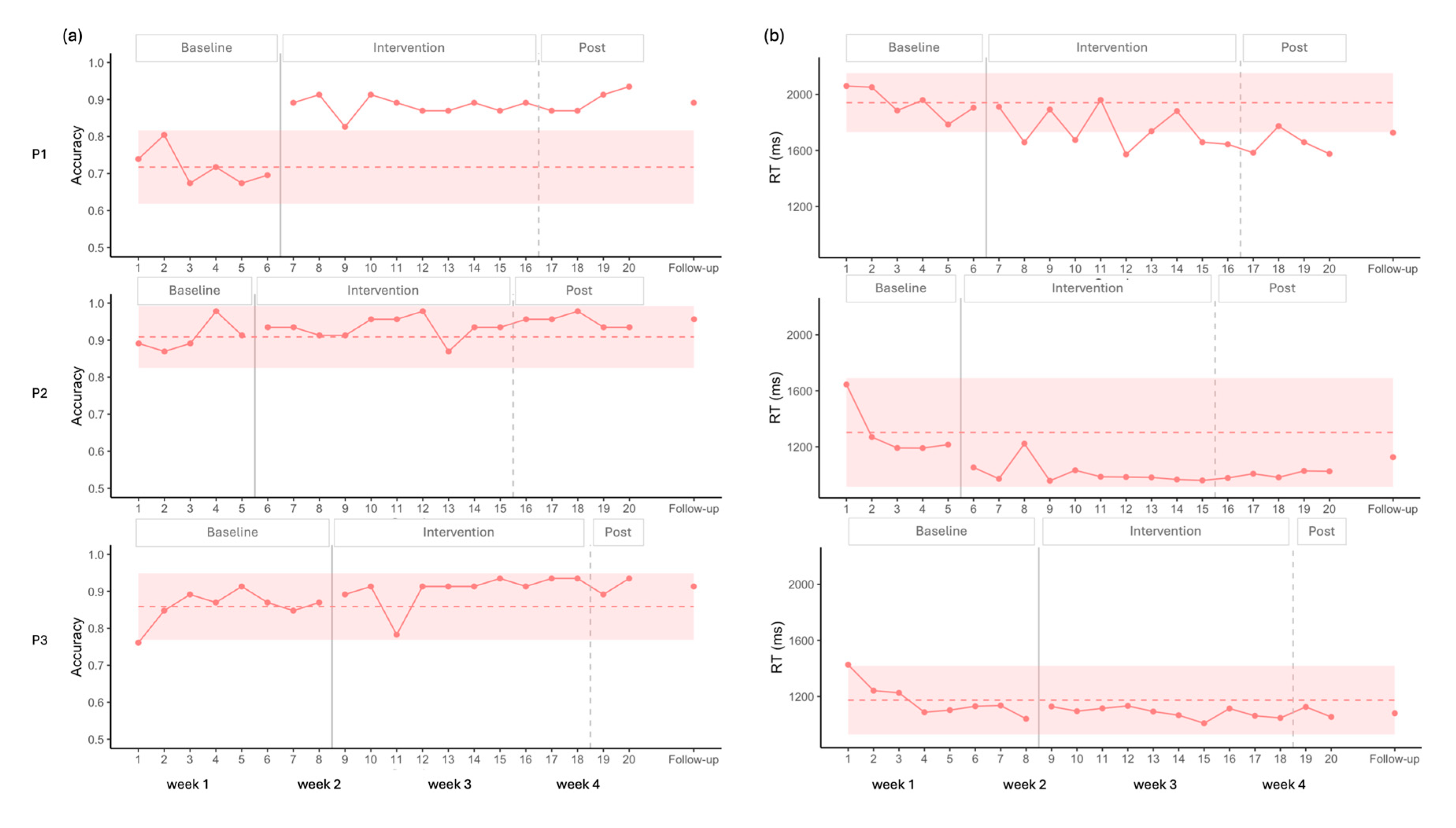
Visual analysis of the accuracy for P1 showed a stable baseline, followed by a clear increase after the onset of the intervention, with all data points falling above the 2SD band (
Figure 3). Statistical analysis supported these findings, indicating no baseline trend (p > 0.05), and a large positive effect size (
Table 2). This improvement was maintained at follow-up.
For P2, visual analysis indicated a stable baseline, with no observable effect of the stimulation on accuracy. A potential ceiling effect was noted, as most data points were above 90%. Statistical analysis confirmed these results, showing no significant effect of stimulation (p>0.05,
Table 2).
In the case of P3, visual analysis revealed no significant baseline trend for accuracy. However, the first baseline data point was lower than the others, resulting in a wider 2SD envelope. Consequently, all intervention points remained within the 2SD band, suggesting no clear visual effect of the intervention. In contrast, statistical analysis indicated a significant moderate effect size (
Table 2).
Regarding RT, a negative trend was observed across participants during the initial baseline points; however, statistical analysis of the complete baseline indicated no significant trend (
Table 2).
For P1, visual analysis revealed a significant decrease in RT (
Figure 3), with eight data points falling below the 2SD band, and a moderate effect size that sustained at follow-up. Statistical analysis supported these findings, showing a moderate decreasing effect of intervention (
Table 2).
For P2, visual analysis showed an initial decrease in RT followed by stability, with all points remaining within the 2SD band, suggesting a possible floor effect. Nevertheless, statistical analysis captured the decrease in RT, indicating a significant moderate decreasing effect size (
Table 2).
For P3, both visual and statistical analyses indicated no improvements.
3.1.2. Auditory Naming
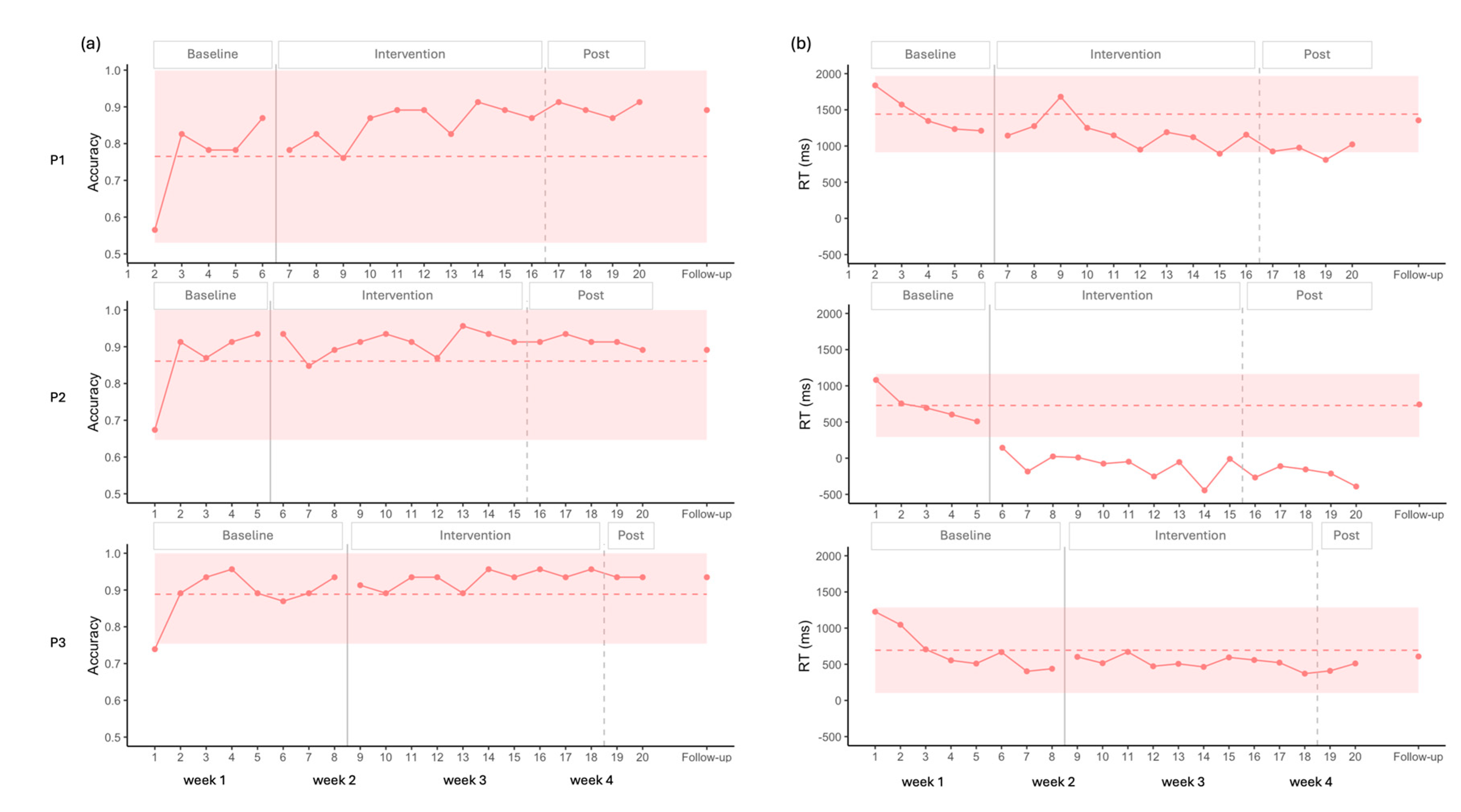
For accuracy, all participants displayed very low initial and/or second data points compared to the rest of the baseline, suggesting an initial learning effect. Subsequent baseline points were more stable, and statistical analysis did not indicate any significant trend.
For P1, visual analysis showed an increase in accuracy alongside a ceiling effect, as the 2SD band approached the maximum score, leaving little room for improvement. Nevertheless, complementary statistical analysis detected a moderate positive effect size (
Figure 4 and
Table 2).
P2 and P3 exhibited similar patterns, with no changes induced by stimulation according to both visual and statistical analyses. A ceiling effect was also observed in these cases, with the 2SD band encompassing the maximum value (
Figure 4 and
Table 2).
A common decrease in RT was observed across all three participants during the baseline, which was statistically significant in each case (P1: Tau = -1.000, p = 0.027; P2: Tau = -1.000, p = 0.027; P3: Tau = -0.786, p = 0.009).
For P1, visual analysis showed two points under the 2SD band in RT, suggesting improvement (
Figure 4). However, statistical analysis did not confirm this trend. When accounting for the baseline trend in the Tau calculation, the decrease observed during baseline was no longer present after the onset of the stimulation, resulting in a significant Baseline Corrected Tau, indicating a stabilization of performance after the onset of the stimulation (
Table 2).
For P2, all intervention points fell below the 2SD band in the visual analysis for RT, again suggesting improvement (
Figure 4). However, statistical analysis did not confirm this effect. Moreover, this participant showed negative RT values, reflecting responses given before the end of the auditory description (
Table 2).
For P3, visual analysis did not show any improvement for RT (
Figure 4). Baseline corrected statistical analysis confirmed a pattern similar to that of P1, with the baseline trend discontinuing after stimulation (
Table 2).
Overall, the results suggest a possible floor effect across participants, which appears more pronounced for P1 and P3 who may have been waiting for the end of the definition before responding (see Limitation section).
3.2. Semantic Decision
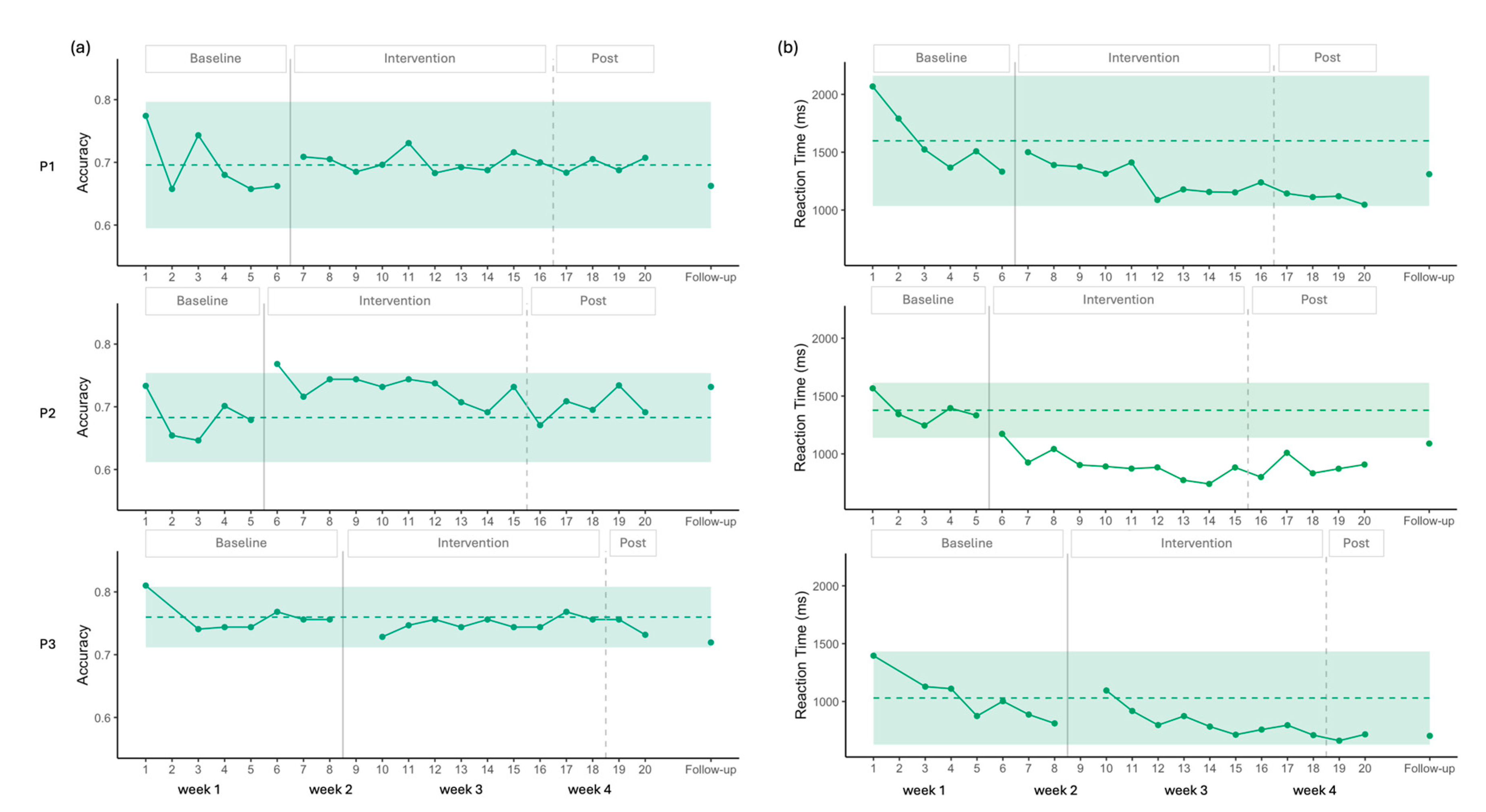
Visual analysis showed a stable baseline for P1 and P3 in accuracy. The 2 SD band encompassed all data points following the introduction of the intervention, indicating no change in accuracy (
Figure 5). Statistical analysis was consistent with this observation, showing no baseline trend (p > 0.05) and no significant change after the intervention was introduced (
Table 2).
For P2, the baseline was also stable, and only one data point during the intervention phase exceeded the 2SD band, suggesting a small improvement in accuracy. Statistical analysis confirmed the absence of a baseline trend (p > 0.05) and indicated a positive trend with a moderate effect size following the introduction of the intervention (
Table 2). However, this improvement was not maintained post-intervention, and no gains were evident at follow-up (
Figure 5).
For P1, visual analysis showed no baseline trend and then a decrease in RT; however, all intervention data points remained withing the 2 SD band, indicating no meaningful change (
Figure 5). Statistical analysis confirmed no baseline trend (p > 0.05) and identified a moderate decrease in RT following the introduction of stimulation (
Table 2). At follow-up, the improvements observed were not maintained.
For P2, visual analysis showed a clearer improvement in RT. Following the first stimulation session, all intervention data points fell below the 2SD band (
Figure 5). Statistical analysis indicated no baseline trend (p > 0.05) and demonstrated a large effect size for the intervention (
Table 2). At follow-up, RT scores remained below the 2SD envelope, suggesting maintenance of effect.
For P3, visual analysis showed a decreasing trend in RT across the eight-session baseline. This decrease was statistically significant, with a very large effect size (Tau = -0.810, p = 0.016). During the intervention phase, all data points remained within the 2SD band. After correcting for the baseline trend, statistical analysis indicated a positive effect size for RT (
Table 2), indicating the RT decrease observed during the baseline was no longer present, likely reflecting a floor effect, with the lowest mean reaction time observed at session 19 (661 ms). Thus, the apparent negative effect of the intervention can be explained by the floor level already reached during baseline.
3.3. Peer Conflict Resolution
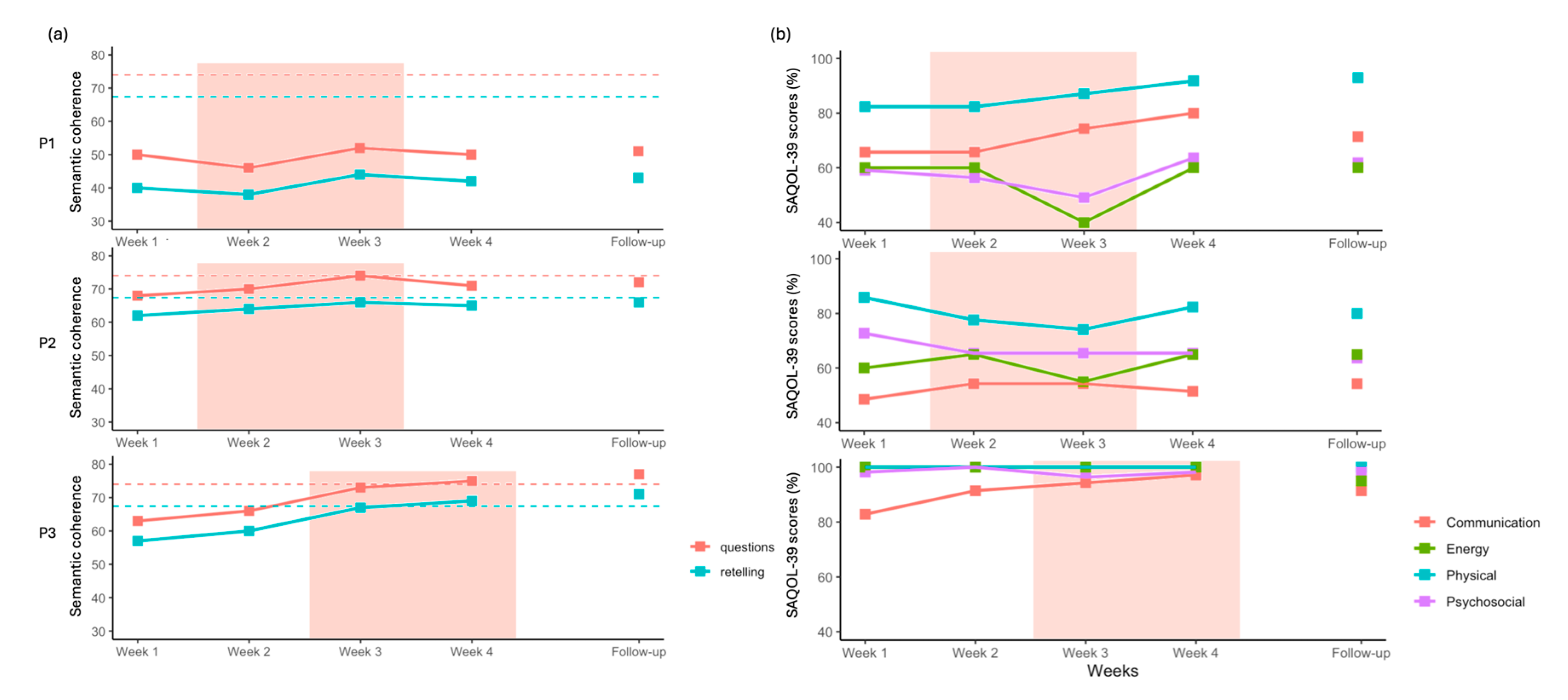
For P1, semantic coherence scores for both story retelling and questions answering showed a small increase at week 3 but remained below the neurotypical range (
Figure 6). For P2, a slight increase was observed during the two weeks of stimulation; however, this improvement was not fully sustained at week 4 or at follow-up. For P3, semantic coherence scores showed a larger increase after the onset of stimulation at week 3. This improvement was maintained at follow-up, with scores exceeding the neurotypical mean for both measures.
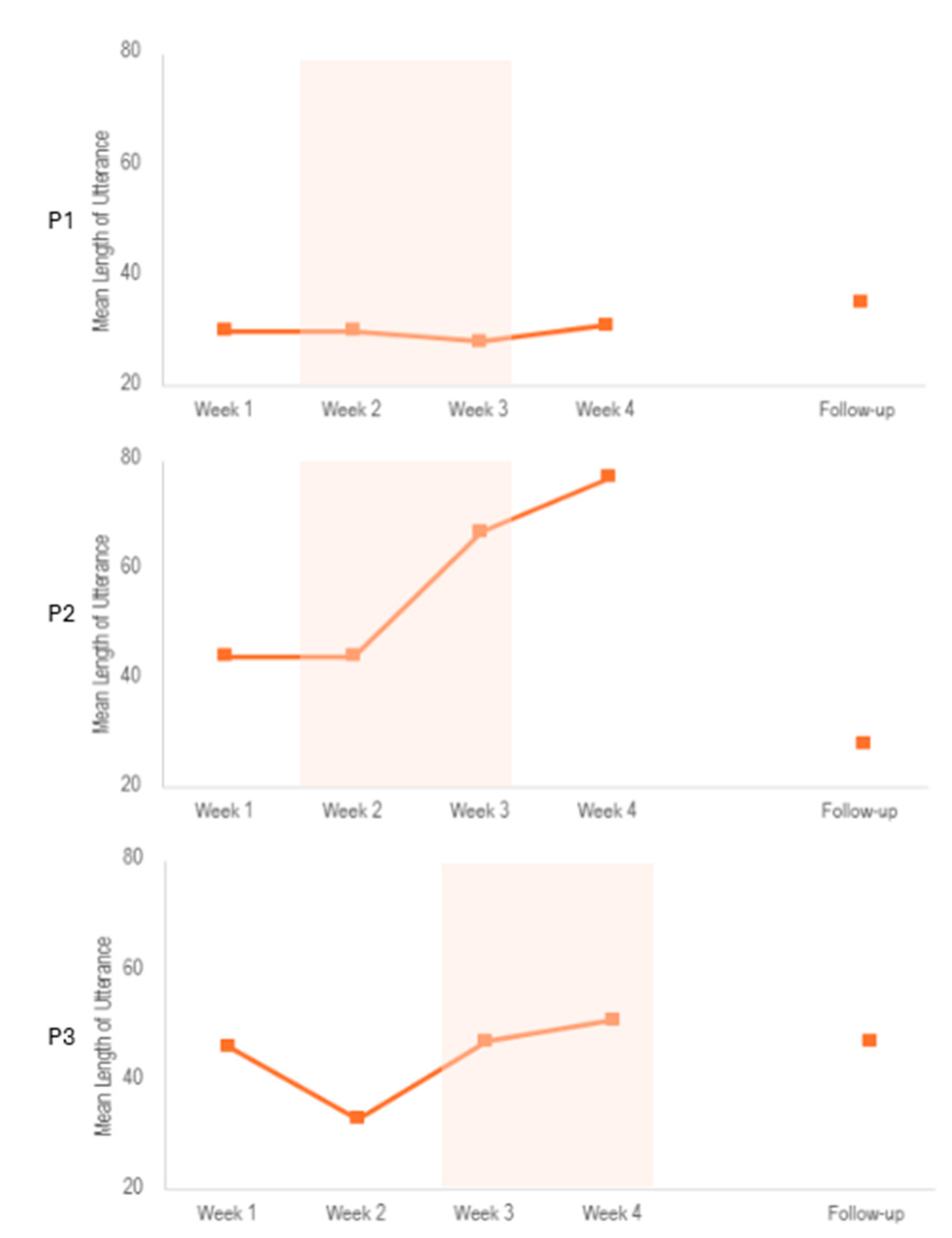
Regarding the other measures, we found an increase in the mean length of utterance after the stimulation period for P2, which was not sustained at follow-up (see
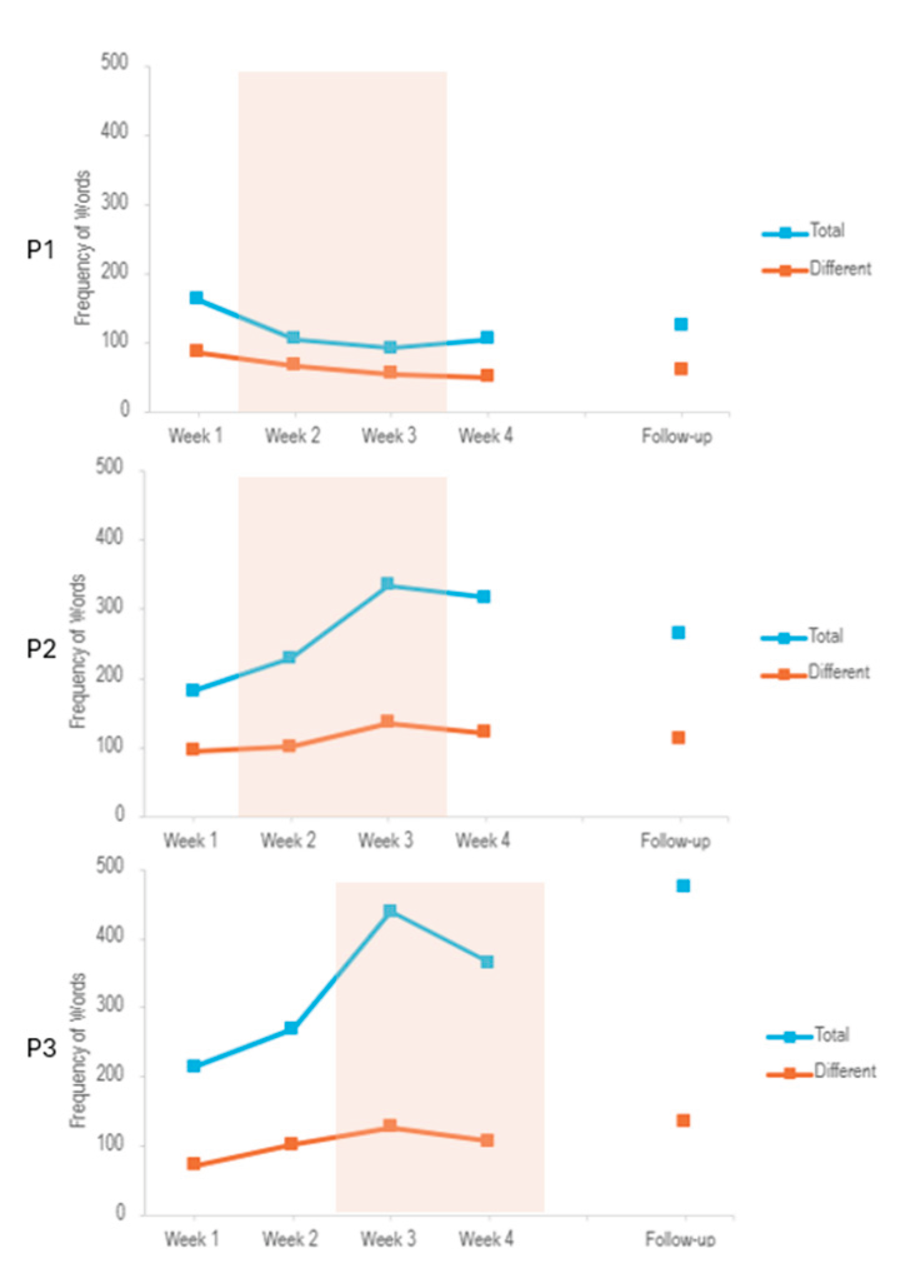
Figure A1). Other linguistic measures, such as fluency and word frequency, were not associated with the stimulation (See
Figure A1 and
Figure A2, and
Table A3 and
Table A4).
3.4. SAQOL-39
For P1, we observed an increase in the Communication and Physical subscores (
Figure 6). A decrease was noted at Week 3 for both Psychological and Energy subscores, while these remained stable during the other weeks. At follow-up, only the Physical subscore remained higher compared to pre-intervention level. Qualitatively, P1 reported greater ease in communication within professional settings, particularly in group contexts.
For P2, no clear trends were observed across subscores, except for a small decrease in the Energy subscore in Week 3. Qualitatively, P2 reported improvements in communication with his young children.
For P3, the Communication subscore increased steadily from the first week of stimulation to the end of the intervention, although this improvement was not maintained at follow-up. Additionally, P3 reported improvements in reading, which were not formally assessed by the measures included in this study.
4. Discussion
This study aimed to improve lexicosemantic impairments in three persons with post-stroke aphasia using inhibitory rTMS targeting the right ATL. The findings can be summarized as follows: (1) in the Picture Naming task, all participants improved in accuracy (P1 and P3) and/or RT (P1 and P2), while only P1 showed improvement in accuracy for the Auditory Naming task; (2) in the Semantic Decision task, P2 showed improvement in both accuracy and RT, while P1 showed improvement in RT alone and P3 showed no improvement; (3) the PCR task showed a clear improvement for P3 alone; and (4) P1 and P3 reported changes in daily life communication that were not sustained at follow-up.
To the best of our knowledge, this is the first study to show the potential of the ATL as a cortical target to improve lexicosemantic impairments in post-stroke population. Since rTMS induces effects not only on the targeted area but also on connected networks, we hypothesize that language improvement could be associated with the modulation of the ventral pathway via ATL stimulation, leading to specific improvement in lexical retrieval processes [
10,
14]. These results are consistent with several studies implicating ATL in tasks with a lexical component [
16,
19]. For example, a lesion-symptom mapping study of picture naming [
40] identified the left ATL as critical but not the left IFG. Another study in neurotypical population using inhibitory rTMS targeting the left ATL showed specific impairments in picture naming RT [
41]. The present study did not directly target the left ATL but instead applied inhibitory stimulation to the right ATL, following the principle of interhemispheric imbalance [
7,
8]. While our behavioral results are consistent with this theory, further work is needed to test whether the benefits are indeed due to suppression of an overactive RH, or due to a different mechanism, as some discrepancies have been noted in the literature. For instance, Lin et al. [
42] used inhibitory rTMS targeting the pars triangularis of the right Inferior Frontal Gyrus in persons with post-stroke aphasia and reported language improvements associated with both decreased and increased right hemisphere connectivity, as measured by resting state fMRI. Another point to consider is the effect of inhibitory vs. facilitatory rTMS on brain connectivity, while taking into account different types of aphasia, and lesion profiles [
9].
Predicting rTMS efficacy, and non-invasive brain stimulation more broadly, in post-stroke aphasia is challenging. Several studies have highlighted the potential influence of aphasia type and lesion localization on treatment response [
43,
44]. Furthermore, the ATL is a key region involved in lexicosemantic processes, partly due to its extensive connectivity. First, both ATLs are interconnected. Moreover, superior, middle, and ventral ATL are connected to the angular gyrus, posterior MTG, IFG and medial prefrontal cortex functionally [
45], and through several white matter tracts, including the uncinate, inferior longitudinal, middle longitudinal, and arcuate fasciculi [
46]. These regions are central parts of the lexical semantic processing system [
16,
19]. Preservation of ATL connectivity may also predict the effectiveness of rTMS, as stimulation can influence the entire connected network [
10,
14]. The present study included participants with high SES scores, which occurred by chance. This unexpected factor may have influenced the results. First, our participants may have had higher expectations for language fluency and accuracy, making them more likely to present floor/ceiling effects. Moreover, even mild deficit had a strong impact on quality of life for P1 and P2, and they both did not return to work after their stroke. When comparing with a similar high SES neurotypical population for the PCR task, we found improvement for P3 only. Finally, high SES may itself be a positive factor for improved rehabilitation response [
47].
In conjunction with a previous study focusing on motor cortex stimulation in people with post-stroke aphasia suffering from phonological impairments [
13], the present study highlights the possibility of selecting cortical targets for rTMS based on a participant’s clinical profile. Here, we stimulated the ATL in participants with lexicosemantic impairments. This approach could make rTMS more tailored to the patient’s profile and more easily accessible to clinicians [
13]. Incorporating rTMS into clinical practice could rely on a combination of published evidence (cortical target selection, stimulation parameters) and practitioner expertise (contraindications, side effects management). This project aims to advance the implementation of rTMS in the rehabilitation of people with post-stroke aphasia [
48]. It could also be used as an adjuvant to SLT, with cortical targets chosen in parallel with therapeutic goals. While the present study reported improvements for all participants, several gains were not sustained at follow-up, which contrasts with most findings on rTMS in chronic post-stroke aphasia [
1]. This may be attributable to the fact that rTMS was administered without SLT, which may have enhanced and prolonged its effects. Future studies could therefore combine this approach with SLT.
One important limitation of this study concerns the calculation of the RMT. One participant’s hair affected the RMT measurement by increasing the distance to the target site. This issue is particularly relevant for ATL stimulation, since RMT is measured in regard to the motor cortex (closer to the top of the head) where hair is usually thicker, while the ATL target is typically on the temple (where hair is thinner or absent). This led to variations in RMT estimates and applied strength. The Sol Braiding Method might have provided more accurate RMT measure [
49]. Another limitation is that several participants showed a floor effect for RT in the Auditory Naming task. This was mainly due to the inclusion of participants with mild aphasia, making improvements harder to detect. In addition, one participant began responding before the end of the stimuli during the rTMS intervention period. While this cannot be attributed to the intervention or repetition effects (see Results
Section 3.1.2, b), it may have introduced differences compared with P1 and P3, who likely waited for the end of the description before answering. This issue should be considered when using an Auditory Naming task in a SCED model, where the same items are presented multiple times. Finally, as is inherent to the SCED model, each participant is compared to themselves and there is no sham control. Future studies with a randomized sham-controlled trial to rule out a placebo effect would be informative.
5. Conclusions
This study highlighted the potential of ATL stimulation in the recovery of post-stroke aphasia. More precisely, we hypothesized that stimulating the ATL modulates interconnected networks, particularly the ventral semantic pathway, leading to improvements in lexicosemantic processes. Each of the three participants with mild to moderate aphasia demonstrated improvement in at least one of the lexicosemantic tasks. This approach is suitable for clinical practice, providing clinicians with a framework for selecting the most relevant target based on each person with aphasia’s specific impairments and rehabilitation goals.
Author Contributions
Conceptualization, Sophie Arheix-Parras and Rutvik H. Desai; methodology, Sophie Arheix-Parras and Rutvik H. Desai; formal analysis, Sophie Arheix-Parras and Sophia Moore; resources, Rutvik H. Desai; writing—original draft preparation, Sophie Arheix-Parras; writing—review and editing, Sophia Moore and Rutvik H. Desai; resources, Rutvik H. Desai; visualization, Sophie Arheix-Parras and Sophia Moore; supervision, Rutvik H. Desai; Project administration, Sophie Arheix-Parras and Sophia Moore; funding acquisition, Sophie Arheix-Parras and Rutvik H. Desai. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Barbara Martin Aphasia Award, also co-sponsored by the Academy of Aphasia. The study was also supported by the NIH/NIDCD R01DC017162.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board the University of South Carolina (protocol code Pro00138658 and date 08/28/2024).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
This work used data from the ABC@USC initiative under Data Use Agreement [#88] to compare the post-stroke participants scores to neurotypicals. The authors thank the ABC@USC Data Use Committee for its review and approval of this research.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AI |
Artificial Intelligence |
| ATL |
Anterior Temporal Lobe |
| fMRI |
Functional Magnetic Resonance Imaging |
| IFG |
Inferior Frontal Gyrus |
| MTG |
Middle Temporal Gyrus |
| PCR |
Peer Conflict Resolution |
| PNT |
Philadelphia Naming Test |
| PWA |
People With Aphasia |
| RH |
Right Hemisphere |
| RMT |
Resting Motor Threshold |
| RT |
Reaction Time |
| rTMS |
Repetitive Transcranial Magnetic Stimulation |
| SAQOL-39 |
Stroke and Aphasia Quality of Life Scale – 39 items |
| SCED |
Single-Case Experimental Design |
| SES |
Socioeconomic Status |
| SLT |
Speech and Language Therapy |
| tDCS |
Transcranial Direct Current Stimulation |
| WAB |
Western Aphasia Battery |
Appendix A
Table A1.
Language score for the WAB and the PNT.
Table A1.
Language score for the WAB and the PNT.
| Subtest |
P1 |
P2 |
P3 |
| Bedside Language Score (WAB) |
88.75 |
92 |
91.25 |
| Spontaneous Speech: Content |
8 |
10 |
10 |
| Spontaneous Speech: Fluency |
8 |
8 |
8 |
| Auditory Verbal Comprehension |
9 |
9 |
10 |
| Sequential Commands |
10 |
8 |
10 |
| Repetition |
9 |
7 |
7 |
| Object Naming |
9 |
10 |
8 |
| Reading |
10 |
10 |
10 |
| Writing |
8 |
10 |
10 |
| PNT 30 |
28 |
30 |
30 |
Table A2.
Mean values and standard deviations of variables for Picture and Auditory Naming tasks in Original and Supplementary items.
Table A2.
Mean values and standard deviations of variables for Picture and Auditory Naming tasks in Original and Supplementary items.
| |
Picture Naming |
Auditory Naming |
| |
Original |
Supplementary |
Original |
Supplementary |
| Age of Acquisition |
4.93 (0.94) |
10.12 (1.12) |
5.98 (1.23) |
7.27 (2.98) |
| Concreteness |
4.88 (0.13) |
4.87 (0.10) |
4.77 (0.23) |
2.17 (0.45) |
| Frequency |
2.55 (0.56) |
2.09 (0.34) |
2.54 (0.48) |
3.62 (0.88) |
Figure A1.
Mean length of utterance (total number of utterances) during the PCR task. Utterances are a string of words that must follow three rules, which is be followed by at least a one second pause, has proper grammatical structure, or ends with a terminal intonation contour. Shaded orange areas indicate stimulation period.
Figure A1.
Mean length of utterance (total number of utterances) during the PCR task. Utterances are a string of words that must follow three rules, which is be followed by at least a one second pause, has proper grammatical structure, or ends with a terminal intonation contour. Shaded orange areas indicate stimulation period.
Table A3.
Fluency (P1, P2 and P3) in raw counts and percentages during the PCR task. Percentage is error over intended words in the sample. Typical disfluencies include phonological fragments, phrase revisions, pauses, and filled pauses. Stimulation period include Weeks 2-3 for P1 and P2, Weeks 3-4 for P3.
Table A3.
Fluency (P1, P2 and P3) in raw counts and percentages during the PCR task. Percentage is error over intended words in the sample. Typical disfluencies include phonological fragments, phrase revisions, pauses, and filled pauses. Stimulation period include Weeks 2-3 for P1 and P2, Weeks 3-4 for P3.
| P1 |
|
|
|
|
|
|
|
| |
Phonological Fragments |
Phrase Revisions |
Pauses |
Filled Pauses |
Typical Disfluencies |
Total Disfluencies |
% Total Disfluencies |
| Week 1 |
0 |
0 |
19 |
16 |
35 |
35 |
23.19 |
| Week 2 |
0 |
1 |
10 |
0 |
11 |
11 |
9.09 |
| Week 3 |
0 |
0 |
12 |
0 |
12 |
12 |
9.45 |
| Week 4 |
0 |
0 |
12 |
0 |
12 |
12 |
9.45 |
| Follow-up |
0 |
0 |
9 |
0 |
9 |
9 |
5.81 |
| P2 |
|
|
|
|
|
|
|
| Week 1 |
0 |
3 |
0 |
26 |
29 |
29 |
12.89 |
| Week 2 |
0 |
1 |
5 |
27 |
33 |
33 |
11.54 |
| Week 3 |
2 |
2 |
4 |
20 |
28 |
29 |
6.87 |
| Week 4 |
3 |
0 |
16 |
36 |
55 |
55 |
13.72 |
| Follow-up |
0 |
0 |
2 |
0 |
2 |
2 |
0.60 |
| P3 |
|
|
|
|
|
|
|
| Week 1 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
| Week 2 |
0 |
0 |
15 |
0 |
15 |
16 |
4.73 |
| Week 3 |
0 |
0 |
9 |
0 |
9 |
9 |
2.08 |
| Week 4 |
0 |
0 |
9 |
0 |
9 |
9 |
2.08 |
| Follow-up |
0 |
0 |
1 |
0 |
1 |
1 |
0.17 |
Table A4.
Total number of words, Number of Different words, and Type/Token Ratio (TTR) during the PCR task. “Tokens” are total number of items (words), and the ratio is a measure of lexical diversity. Number ranges .001 to 1.0. A low value indicates a lot of repetition and a high value means each word in the sample was different. Stimulation period include Weeks 2-3 for P1 and P2, Weeks 3-4 for P3.
Table A4.
Total number of words, Number of Different words, and Type/Token Ratio (TTR) during the PCR task. “Tokens” are total number of items (words), and the ratio is a measure of lexical diversity. Number ranges .001 to 1.0. A low value indicates a lot of repetition and a high value means each word in the sample was different. Stimulation period include Weeks 2-3 for P1 and P2, Weeks 3-4 for P3.
| |
Different words |
Total words |
TTR |
| P1 |
|
|
|
| Week 1 |
87 |
163 |
0.534 |
| Week 2 |
67 |
106 |
0.632 |
| Week 3 |
56 |
92 |
0.609 |
| Week 4 |
51 |
106 |
0.481 |
| Follow-up |
61 |
124 |
0.492 |
| P2 |
|
|
|
| Week 1 |
95 |
182 |
0.522 |
| Week 2 |
101 |
229 |
0.441 |
| Week 3 |
136 |
334 |
0.407 |
| Week 4 |
122 |
317 |
0.385 |
| Follow-up |
113 |
265 |
0.426 |
| P3 |
|
|
|
| Week 1 |
73 |
215 |
0.340 |
| Week 2 |
103 |
270 |
0.381 |
| Week 3 |
128 |
439 |
0.292 |
| Week 4 |
107 |
365 |
0.293 |
| Follow-up |
136 |
475 |
0.286 |
Figure A2.
Frequency of Words out of all words in each session during the PCR task. Lines show Total number of words and Number of Different words. Shaded orange area indicates stimulation period.
Figure A2.
Frequency of Words out of all words in each session during the PCR task. Lines show Total number of words and Number of Different words. Shaded orange area indicates stimulation period.
References
- Frederick, A.; Jacobs, M.; Adams, -.M.C.J.; Ellis, C. The Global Rate of Post-Stroke Aphasia. Perspectives of the ASHA Special Interest Groups 2022, 7, 1567-1572. [CrossRef]
- Lam, J.M.C.; Wodchis, W.P. The relationship of 60 disease diagnoses and 15 conditions to preference-based health-related quality of life in Ontario hospital-based long-term care residents. Medical Care 2010, 48, 380-387. [CrossRef]
- Lee, H.; Lee, Y.; Choi, H.; Pyun, S.B. Community Integration and Quality of Life in Aphasia after Stroke. Yonsei Med J 2015, 56, 1694-1702. [CrossRef]
- Brady, M.C.; Ali, M.; VandenBerg, K.; Williams, L.J.; Williams, L.R.; Abo, M.; Becker, F.; Bowen, A.; Brandenburg, C.; Breitenstein, C.; et al. Precision rehabilitation for aphasia by patient age, sex, aphasia severity, and time since stroke? A prespecified, systematic review-based, individual participant data, network, subgroup meta-analysis. International Journal of Stroke 2022, 17474930221097477. [CrossRef]
- Arheix-Parras, S.; Barrios, C.; Python, G.; Cogné, M.; Sibon, I.; Engelhardt, M.; Dehail, P.; Cassoudesalle, H.; Moucheboeuf, G.; Glize, B. A systematic review of repetitive transcranial magnetic stimulation in aphasia rehabilitation: Leads for future studies. Neuroscience and Biobehavioral Reviews 2021, 127, 212-241. [CrossRef]
- Biou, E.; Cassoudesalle, H.; Cogné, M.; Sibon, I.; De Gabory, I.; Dehail, P.; Aupy, J.; Glize, B. Transcranial direct current stimulation in post-stroke aphasia rehabilitation: A systematic review. Annals of Physical and Rehabilitation Medicine 2019, 62, 104-121. [CrossRef]
- Carson, R.G. Inter-hemispheric inhibition sculpts the output of neural circuits by co-opting the two cerebral hemispheres. The Journal of Physiology 2020, 598, 4781-4802. [CrossRef]
- Stefaniak, J.D.; Halai, A.D.; Lambon Ralph, M.A. The neural and neurocomputational bases of recovery from post-stroke aphasia. Nature Reviews. Neurology 2020, 16, 43-55. [CrossRef]
- Stefaniak, J.D.; Geranmayeh, F.; Lambon Ralph, M.A. The multidimensional nature of aphasia recovery post-stroke. Brain 2022, 145, 1354-1367. [CrossRef]
- Duffau, H.; Moritz-Gasser, S.; Mandonnet, E. A re-examination of neural basis of language processing: proposal of a dynamic hodotopical model from data provided by brain stimulation mapping during picture naming. Brain and Language 2014, 131, 1-10. [CrossRef]
- Dresang, H.C.; Harvey, D.Y.; Vnenchak, L.; Parchure, S.; Cason, S.; Twigg, P.; Faseyitan, O.; Maher, L.M.; Hamilton, R.H.; Coslett, H.B. Semantic and Phonological Abilities Inform Efficacy of Transcranial Magnetic Stimulation on Sustained Aphasia Treatment Outcomes | Neurobiology of Language | MIT Press. Neurobiology of Language 2025, 6. [CrossRef]
- Roach, A.; Schwartz, M.F.; Martin, N.; Grewal, R.S.; Brecher, A. The Philadelphia naming test: Scoring and rationale. Clinical Aphasiology 1996, 24, 121-134.
- Arheix-Parras, S.; Franco, J.; Siklafidou, I.; Villain, M.; Rogue, C.; Python, G.; Glize, B. Neuromodulation of the Right Motor Cortex of the Lips With Repetitive Transcranial Magnetic Stimulation to Reduce Phonological Impairment and Improve Naming in Three Persons With Aphasia: A Single-Case Experimental Design. American journal of speech-language pathology 2024. [CrossRef]
- Ridding, M.C.; Rothwell, J.C. Is there a future for therapeutic use of transcranial magnetic stimulation? Nature Reviews Neuroscience 2007, 8, 559-567. [CrossRef]
- Hillis, A.E.; Caramazza, A. Converging evidence for the interaction of semantic and sublexical phonological information in accessing lexical representations for spoken output. Cognitive neuropsychology 1995, 12, 187-227. [CrossRef]
- Binder, J.R.; Desai, R.H. The neurobiology of semantic memory. Trends Cogn Sci 2011, 15, 527-536. [CrossRef]
- Lambon Ralph, M.A.; Jefferies, E.; Patterson, K.; Rogers, T.T. The neural and computational bases of semantic cognition. Nat Rev Neurosci 2017, 18, 42-55. [CrossRef]
- Patterson, K.; Kopelman, M.D.; Woollams, A.M.; Brownsett, S.L.; Geranmayeh, F.; Wise, R.J. Semantic memory: Which side are you on? Neuropsychologia 2015, 76, 182-191. [CrossRef]
- Binder, J.R.; Desai, R.H.; Graves, W.W.; Conant, L.L. Where Is the Semantic System? A Critical Review and Meta-Analysis of 120 Functional Neuroimaging Studies. Cerebral Cortex (New York, NY) 2009, 19, 2767-2796. [CrossRef]
- Oldfield, R.C. The Assessment And Analysis Of Handedness: The Edinburgh Inventory. Neuropsychologia 1971, 9, 97-113. [CrossRef]
- Rossi, S.; Antal, A.; Bestmann, S.; Bikson, M.; Brewer, C.; Brockmöller, J.; Carpenter, L.L.; Cincotta, M.; Chen, R.; Daskalakis, J.D.; et al. Safety and recommendations for TMS use in healthy subjects and patient populations, with updates on training, ethical and regulatory issues: Expert Guidelines. Clinical neurophysiology: official journal of the International Federation of Clinical Neurophysiology 2021, 132, 269-306. [CrossRef]
- Kertesz, A. Western Aphasia Battery--Revised. APA Psyc Net 2006. [CrossRef]
- Walker, G.M.; Schwartz, M.F. Short-form Philadelphia naming test: rationale and empirical evaluation. Am J Speech Lang Pathol 2012, 21, S140-153. [CrossRef]
- Krasny-Pacini, A.; Evans, J. Single-case experimental designs to assess intervention effectiveness in rehabilitation: A practical guide. Annals of Physical and Rehabilitation Medicine 2018, 61, 164-179. [CrossRef]
- Kratochwill, T.R.; Hitchcock, J.H.; Horner, R.H.; Levin, J.R.; Odom, S.L.; Rindskopf, D.M.; Shadish, W.R. Single-Case Intervention Research Design Standards. Remedial and Special Education 2013, 34, 26-38. [CrossRef]
- Hilari, K.; Byng, S.; Lamping, D.L.; Smith, S.C. Stroke and Aphasia Quality of Life Scale-39 (SAQOL-39): Evaluation of Acceptability, Reliability, and Validity. 2003. [CrossRef]
- Nippold, M.A.; Mansfield, T.C.; Billow, J.L. Peer Conflict Explanations in Children, Adolescents, and Adults: Examining the Development of Complex Syntax. 2007. [CrossRef]
- Hamberger, M.J.; Heydari, N.; Caccappolo, E.; Seidel, W.T. Naming in Older Adults: Complementary Auditory and Visual Assessment | Journal of the International Neuropsychological Society | Cambridge Core. 2022. [CrossRef]
- Kuperman, V.; Stadthagen-Gonzalez, H.; Brysbaert, M. Age-of-acquision ratings for 30 thousand English words. Behavior research methods 2012, 44, 987-990. [CrossRef]
- Brysbaert, M.; Warriner, A.; Kuperman, V. Concreteness ratings for 40 thousand generally known English word lemmas. Behavior research methods 2014, 46. [CrossRef]
- Brysbaert, M.; New, B. Moving beyond Kucera and Francis: a critical evaluation of current word frequency norms and the introduction of a new and improved word frequency measure for American English. Behav Res Methods 2009, 41, 977-990. [CrossRef]
- Wilson, S.C.; Teghipco, A.; Sayers, S.; Newman-Norlund, R.; Newman-Norlund, S.; Fridriksson, J. Story Recall in Peer Conflict Resolution Discourse Task to Identify Older Adults Testing Within Range of Cognitive Impairment. 2024. [CrossRef]
- Macwhinney, B.; Fromm, D.; Forbes, M.; Holland, A. AphasiaBank: Methods for Studying Discourse. Aphasiology 2011, 25, 1286-1307. [CrossRef]
- Macwhinney, B. The CHILDES project: tools for analyzing talk. Edition. Mahwah, NJ: Lawrence Erlbaum Associates 2000. [CrossRef]
- Newman-Norlund, R.D.; Newman-Norlund, S.E.; Sayers, S.; Nemati, S.; Riccardi, N.; Rorden, C.; Fridriksson, J. The Aging Brain Cohort (ABC) repository: The University of South Carolina’s multimodal lifespan database for studying the relationship between the brain, cognition, genetics and behavior in healthy aging. 1. Neuroimage: Reports; 2021. [CrossRef]
- Nasreddine, Z.S.; Phillips, N.A.; Bedirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005, 53, 695-699. [CrossRef]
- Protopapas, A. CheckVocal: a program to facilitate checking the accuracy and response time of vocal responses from DMDX. Behavior Research Methods 2007, 39, 859-862. [CrossRef]
- Tate, R.; Perdices, M. Single-Case Experimental Designs for Clinical Research and Neurorehabilitation Settings: Planning, Conduct, Analysis and Reporting; Routledge: London, 2018; p. 292.
- Vannest, K.J.; Ninci, J. Evaluating Intervention Effects in Single-Case Research Designs. Journal of Counseling & Development 2015, 93, 403-411. [CrossRef]
- Piai, V.; Eikelboom, D. Brain Areas Critical for Picture Naming: A Systematic Review and Meta-Analysis of Lesion-Symptom Mapping Studies. Neurobiology of Language 2023, 4, 280-296. [CrossRef]
- Pobric, G.; Jefferies, E.; Ralph, M.A. Anterior temporal lobes mediate semantic representation: mimicking semantic dementia by using rTMS in normal participants. Proc Natl Acad Sci U S A 2007, 104, 20137-20141. [CrossRef]
- Lin, B.-F.; Yeh, S.-C.; Kao, Y.-C.J.; Lu, C.-F.; Tsai, P.-Y. Functional Remodeling Associated With Language Recovery After Repetitive Transcranial Magnetic Stimulation in Chronic Aphasic Stroke. Frontiers in Neurology 2022, 13, 809843. [CrossRef]
- Campana, S.; Caltagirone, C.; Marangolo, P. Combining Voxel-based Lesion-symptom Mapping (VLSM) With A-tDCS Language Treatment: Predicting Outcome of Recovery in Nonfluent Chronic Aphasia. Brain stimulation 2015, 8. [CrossRef]
- Bonilha, L.; Rorden, C.; Roth, R.; Sen, S.; George, M.; Fridriksson, J. Improved naming in patients with Broca’s aphasia with tDCS. Journal of neurology, neurosurgery, and psychiatry 2024, 95. [CrossRef]
- Jackson, R.L.; Hoffman, P.; Pobric, G.; Lambon Ralph, M.A. The Semantic Network at Work and Rest: Differential Connectivity of Anterior Temporal Lobe Subregions. J Neurosci 2016, 36, 1490-1501. [CrossRef]
- Papinutto, N.; Galantucci, S.; Mandelli, M.L.; Gesierich, B.; Jovicich, J.; Caverzasi, E.; Henry, R.G.; Seeley, W.W.; Miller, B.L.; Shapiro 1, K.A.; et al. Structural connectivity of the human anterior temporal lobe: A diffusion magnetic resonance imaging study. Human Brain Mapping 2016, 37, 2210. [CrossRef]
- Anthony, S.R.; Babu, P.; Paplikar, A. Aphasia severity and factors predicting language recovery in the chronic stage of stroke - Anthony - 2025 - International Journal of Language & Communication Disorders - Wiley Online Library. Int J Lang Commun Disord. 2025, 60. [CrossRef]
- Georgiou, A.M. Perspective on the clinical management of post-stroke aphasia and dysphagia using repetitive transcranial magnetic stimulation (rTMS). Frontiers in Neurology 2024. [CrossRef]
- Archer, Q.; Brenya, J.; Chavaria, K.; Friest, A.; Ahmad, N.; Zorns, S.; Vaidya, S.; Shelanskey, T.; Sierra, S.; Ash, S.; et al. The Sol Braiding Method for Handling Thick Hair During Transcranial Magnetic Stimulation: An Address for Potential Bias in Brain Stimulation. Journal of visualized experiments: JoVE 2024. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |