4. Materials and Methods
Solvents for extractions and chromatography were of technical grade and were distilled prior to use. Extracts were dried over technical grade anhydrous Na2SO4. Melting points were determined on a Kofler micro hot stage and on SRS OptiMelt MPA100 – Automated Melting Point System (Stanford Research Systems, Sunnyvale, California, United States). The NMR spectra were obtained on a Bruker UltraShield 500 plus spektrometer and on a BRUKER AVANCE NEO 600 MHz NMR spektrometer (Bruker, Billerica, Massachusetts, United States) at 500 and 600 MHz for 1H and 126 and 150 MHz for 13C nucleus, respectively, using DMSO-d6 and CDCl3 with TMS as the internal standard, as solvents. Mass spectra were recorded on an Agilent 6224 Accurate Mass TOF LC/MS and Agilent 6530 Q-TOF LC/MS coupled with Agilent 1260 Infinity2 HPLC (Agilent Technologies, Santa Clara, California, United States), IR spectra on a Perkin-Elmer Spectrum BX FTIR spectrophotometer (PerkinElmer, Waltham, Massachusetts, United States). Column chromatography (CC) was performed on silica gel (Silica gel 60, particle size: 0.035-0.070 mm (Sigma-Aldrich, St. Louis, Missouri, United States)). HPLC analyses were performed on an Agilent 1260 Infinity LC (Agilent Technologies, Santa Clara, California, United States) using CHIRALPAK IA-3 (0.46 cm ø × 25 cm), CHIRALPAK AD-H (0.46 cm ø × 25 cm), CHIRALCEL OD-H (0.46 cm ø × 25 cm), and CHIRALPAK AS-H (0.46 cm ø × 25 cm) as chiral column (CHIRAL TECHNOLOGIES, INC., West Chester, Pennsylvania, United States). All the commercially available chemicals used were purchased from Sigma-Aldrich (St. Louis, Missouri, United States).
Organocatalysts
I [
21],
II [
57],
III [
58],
IV [
58],
VI [
59],
VII [
60],
VIII [
61], and
IX [
62] were prepared following the literature procedures; organocatalyst
V was purchased from Sigma-Aldrich.
Synthesis of β-keto esters 2 from carboxylic acids 1 – General procedure 1 (GP1)
To a solution or suspension of carboxylic acid 1 (10 mmol) in anhydrous THF (50 mL), 1,1'-carbonyldiimidazole (CDI; 12 mmol, ω = 0.97, 1.672 g) was added under argon, and the resulting reaction mixture was stirred for 2 h at room temperature. A solid mixture of MgCl2 (9.8 mmol, ω = 0.98, 952 mg) and methyl potassium malonate (15 mmol, 2.343 g) or tert-butyl potassium malonate (15 mmol, ω = 0.95, 3.130 g) was then added. The reaction mixture was stirred for a further 24 hours under argon at room temperature. The volatiles were evaporated in vacuo, the residue was dissolved in EtOAc (150 mL) and washed with NaHSO4 (1 M in H2O, 3×50 mL), NaHCO3 (aq. sat., 2×20 mL), and NaCl (aq. sat., 2×50 mL). The organic phase was dried over anhydrous Na2SO4, filtered, and the volatiles evaporated in vacuo. If necessary, the residue was purified by column chromatography (CC, Silica gel 60). The fractions containing the product were combined and the volatiles were evaporated in vacuo.
Synthesis of methyl 4-((tert-butoxycarbonyl)amino)-3-oxobutanoate (2a) [22]
Following GP1. Prepared from (tert-butoxycarbonyl)glycine (1a) (10 mmol, 1.752 g), methyl potassium malonate (15 mmol, 2.343 g); isolation by extraction. Yield: 2.10 g (9.1 mmol, 91 %) of yellowish oil. 1H-NMR (500 MHz, DMSO-d6): δ 1.39 (s, 9H), 3.60 (s, 2H), 3.63 (s, 3H), 3.86 (d, J=5.9 Hz, 2H), 7.13 (t, J=5.9 Hz, 1H). 13C-NMR (126 MHz, DMSO-d6): δ 28.18, 45.91, 49.81, 51.93, 78.30, 155.81, 167.50, 200.67.
Synthesis of methyl 5-((tert-butoxycarbonyl)amino)-3-oxopentanoate (2b) [22]
Following GP1, prepared from Boc-β-alanine (1b) (10 mmol, 1.892 g), methyl potassium malonate (15 mmol, 2.343 g); isolation by extraction. Yield: 2.18 g (8.9 mmol, 89 %) of yellowish oil. 1H-NMR (500 MHz, DMSO-d6): δ 1.36 (s, 9H), 2.66 (t, J=6.9 Hz, 2H), 3.11 (q, J=6.9 Hz, 2H), 3.61 (s, 2H), 3.62 (s, 3H), 6.77 (t, J=5.6 Hz, 1H). 13C-NMR (126 MHz, DMSO-d6): δ 28.24, 34.88, 42.40, 48.68, 51.84, 77.72, 155.53, 167.71, 202.42.
Synthesis of methyl 4-((3-methylbut-2-en-1-yl)oxy)-3-oxobutanoate (2c) [63]
Following
GP1. Prepared from 2-((3-methylbut-2-en-1-yl)oxy)acetic acid (
1c) [
64] (10 mmol, 1.442 g), methyl potassium malonate (15 mmol, 2.343 g); isolation by extraction. Yield: 1.602 g (8.0 mmol, 80 %) of colorless oil.
1H-NMR (500 MHz, CDCl
3):
δ 1.69 (
s, 3H), 1.77 (
s, 3H), 3.55 (
s, 2H), 3.74 (
s, 3H), 4.04 (
d,
J=7.1 Hz, 2H), 4.09 (
s, 2H), 5.29 – 5.36 (
m, 1H).
13C-NMR (126 MHz, CDCl
3):
δ 18.11, 25.88, 45.77, 52.41, 67.89, 74.63, 119.97, 138.70, 167.62, 202.30.
Synthesis of methyl 3-oxooctadecanoate (2d) [65]
Following GP1. Prepared from palmitic acid (1d) (10 mmol, 2.564 g), methyl potassium malonate (15 mmol, 2.343 g); isolation by extraction and column chromatography (EtOAc/petroleum ether = 1:10). Yield: 2,717 g (8.70 mmol, 87 %) of white solid; m.p. = 49.2–50.5 °C. 1H-NMR (500 MHz, CDCl3): δ 0.88 (t, J=6.9 Hz, 3H), 1.19 – 1.35 (m, 24H), 1.53 – 1.65 (m, 2H), 2.53 (t, J=7.4 Hz, 2H), 3.45 (s, 2H), 3.74 (s, 3H). 13C-NMR (126 MHz, CDCl3): δ 14.26, 22.83, 23.60, 29.14, 29.49, 29.50, 29.58, 29.73, 29.77, 29.79, 29.80, 29.82, 29.83, 32.06, 43.23, 49.15, 52.46, 167.85, 203.01.
Synthesis of methyl 3-oxoicosanoate (2e) [65]
Following GP1. Prepared from stearic acid (1e) (10 mmol, 2.845 g), methyl potassium malonate (15 mmol, 2.343 g); isolation by extraction and column chromatography (EtOAc/petroleum ether = 1:10). Yield: 2.282 g (6.70 mmol, 67 %) of white solid; m.p. = 49.5 –51.2 °C. 1H-NMR (500 MHz, CDCl3): δ 0.88 (t, J=6.9 Hz, 3H), 1.18 – 1.34 (m, 28H), 1.55 – 1.63 (m, 2H), 2.53 (t, J=7.4 Hz, 2H), 3.45 (s, 2H), 3.74 (s, 3H). 13C-NMR (151 MHz, CDCl3): δ 14.25, 22.83, 23.60, 29.14, 29.49, 29.50, 29.58, 29.73, 29.78, 29.79, 29.80, 29.83, 32.06, 43.22, 49.14, 52.45, 167.84, 203.00 (3 signals missing due to overlapping).
Synthesis of tert-butyl 3-oxoicosanoate (2f)
Following GP1. Prepared from stearic acid (1e) (10 mmol, 2.845 g), tert-butyl potassium malonate (15 mmol, ω = 0.95, 3.130 g); isolation by extraction and column chromatography (EtOAc/petroleum ether = 1:15). Yield: 1.722 g (4.50 mmol, 45 %) of white solid; m.p. = 36.9–38.1 °C. EI-HRMS: m/z = 327.2885 (MH+-tBuOH); C20H39O3 requires: m/z = 327.2894 (MH+-tBuOH); νmax 2960, 2916, 2849, 1729, 1715, 1466, 1406, 1367, 1329, 1276, 1260, 1155, 1131, 1109, 1080, 947, 920, 842, 790, 723, 647 cm-1. 1H-NMR (500 MHz, CDCl3): δ 0.88 (t, J=6.9 Hz, 3H), 1.21 – 1.34 (m, 28H), 1.47 (s, 9H), 1.55 – 1.61 (m, 2H), 2.51 (t, J=7.4 Hz, 2H), 3.34 (s, 2H). 13C-NMR (151 MHz, CDCl3): δ 14.26, 22.83, 23.62, 28.10, 29.21, 29.50, 29.52, 29.59, 29.74, 29.78, 29.80, 29.81, 29.84, 32.07, 43.09, 50.81, 81.99, 166.69, 203.68 (3 signals missing due to overlapping).
Synthesis of methyl (11Z,14Z)-3-oxoicosa-11,14-dienoate (2g) [65]
Following GP1. Prepared from linoleic acid (1f) (10 mmol, 2.804 g), methyl potassium malonate (15 mmol, 2.343 g); isolation by extraction and column chromatography (EtOAc/petroleum ether = 1:5). Yield: 1.851 g (5.50 mmol, 55 %) of colorless oil. 1H-NMR (500 MHz, CDCl3): δ 0.89 (t, J=6.9 Hz, 3H), 1.23 – 1.40 (m, 14H), 1.54 – 1.64 (m, 2H), 1.97 – 2.09 (m, 4H), 2.53 (t, J=7.4 Hz, 2H), 2.77 (t, J=6.6 Hz, 2H), 3.45 (s, 2H), 3.74 (s, 3H), 5.28 – 5.43 (m, 4H). 13C-NMR (126 MHz, CDCl3): δ 14.21, 22.71, 23.57, 25.76, 27.31, 27.33, 29.10, 29.21, 29.39, 29.48, 29.72, 31.66, 43.20, 49.15, 52.46, 128.02, 128.19, 130.15, 130.35, 167.83, 202.95.
Synthesis of methyl (S)-4-((tert-butoxycarbonyl)amino)-3-oxo-5-phenylpentanoate (10a) [22]
Following GP1. Prepared from Boc-L-phenylalanine (9a) (10 mmol, 2.653 g), methyl potassium malonate (15 mmol, 2.343 g); isolation by extraction. Yield: 2.346 g (7.30 mmol, 73 %) of colorless oil. 1H-NMR (500 MHz, CDCl3): δ 1.40 (s, 9H), 2.98 (dd, J=7.5, 14.1 Hz, 1H), 3.14 (dd, J=6.2, 14.1 Hz, 1H), 3.46 (d, J=16.0 Hz, 1H), 3.52 (d, J=16.0 Hz, 1H), 3.71 (s, 3H), 4.56 (q, J=7.2 Hz, 1H), 4.96 – 5.07 (m, 1H), 7.14 – 7.20 (m, 2H), 7.21 – 7.35 (m, 3H).
Synthesis of methyl (S)-8-(((benzyloxy)carbonyl)amino)-4-((tert-butoxycarbonyl)amino)-3-oxooctanoate (10b) [22]
Following GP1. Prepared from Boc-Lys(Z)-OH (9b) (10 mmol, 3.804 g), methyl potassium malonate (15 mmol, 2.343 g); isolation by extraction. Yield: 3.317 g (7.60 mmol, 76 %) of colorless oil. 1H-NMR (500 MHz, CDCl3): δ 1.30 – 1.65 (m, 5H), 1.43 (s, 9H), 1.81 – 1.93 (m, 1H), 3.12 – 3.27 (m, 2H), 3.54 (d, J=15.7 Hz, 1H), 3.59 (d, J=16.0 Hz, 1H), 3.73 (s, 3H), 4.27 – 4.36 (m, 1H), 4.93 (t, J=6.0 Hz, 1H), 5.05 – 5.17 (m, 2H), 5.27 (br d, J=7.7 Hz, 1H), 7.28 – 7.39 (m, 5H).
Synthesis of 7-benzyl 1-methyl (S)-4-((tert-butoxycarbonyl)amino)-3-oxoheptanedioate (10c) [23]
Following GP1. Prepared from Boc-Glu(OBzl)-OH (9c) (10 mmol, 3.374g), methyl potassium malonate (15 mmol, 2.343 g); isolation by extraction. Yield: 2.675 g (6.80 mmol, 68 %) of colorless oil. 1H-NMR (500 MHz, CDCl3): δ 1.43 (s, 9H), 1.80 – 1.91 (m, 1H), 2.22 – 2.32 (m, 1H), 2.38 – 2.56 (m, 2H), 3.55 – 3.65 (m, 2H), 3.73 (s, 3H), 4.38 – 4.45 (m, 1H), 5.12 (s, 2H), 5.23 (br d, J=8.1 Hz, 1H), 7.29 – 7.41 (m, 5H).
Synthesis of methyl (S)-5-((tert-butoxycarbonyl)amino)-2-stearamidopentanoate (4)
To a solution of stearic acid (1e) (20 mmol, 5.690 g) in anhydrous THF (50 mL) was added 1,1’-carbonyldiimidazole (CDI; 22 mmol, ω = 0.97, 3.678 g) under argon and the resulting reaction mixture was stirred for 2 h at room temperature. Then H-Orn(Boc)-OMe×HCl (3) (22 mmol, ω = 0.96, 6.480 g) and Et3N (22 mmol, 3.07 mL) were added. The reaction mixture was stirred for a further 24 hours under argon at room temperature. The volatiles were evaporated in vacuo, the residue was dissolved in CH2Cl2 (150 mL) and washed with NaHSO4 (1 M in H2O, 4×50 mL), NaHCO3 (aq. sat., 3×20 mL) and NaCl (aq. sat., 2×50 mL). The organic phase was dried over anhydrous Na2SO4, filtered and the volatiles evaporated in vacuo. Yield: 7.076 g (13.8 mmol, 69 %) of white solid; m.p. = 86.0–88.0 °C. EI-HRMS: m/z = 513.4272 (MH+); C29H57N2O5 requires: m/z = 513.4262 (MH+); νmax 3345, 2915, 2847, 1762, 1684, 1648, 1526, 1473, 1462, 1369, 1285, 1252, 1212, 1171, 1143, 1047, 994, 950, 871, 754, 729, 719, 654 cm-1. 1H-NMR (500 MHz, DMSO-d6): δ 0.85 (t, J=6.8 Hz, 3H), 1.14 – 1.31 (m, 30H), 1.37 (s, 9H), 1.42 – 1.57 (m, 3H), 1.61 – 1.69 (m, 1H), 2.09 (t, J=7.1 Hz, 2H), 2.89 (q, J=6.8 Hz, 2H), 3.60 (s, 3H), 4.14 – 4.23 (m, 1H), 6.79 (t, J=5.7 Hz, 1H), 8.14 (d, J=7.5 Hz, 1H). 13C-NMR (126 MHz, DMSO-d6): δ 13.98, 22.11, 25.23, 26.02, 28.20, 28.26, 28.55, 28.72, 28.78, 28.97, 29.02, 29.05, 31.31, 34.94, 51.65, 51.70, 77.40, 155.58, 172.43, 172.78 (7 signals missing due to overlapping).
Synthesis of (S)-5-((tert-butoxycarbonyl)amino)-2-stearamidopentanoic acid (5)
To a solution/suspension of methyl (S)-5-((tert-butoxycarbonyl)amino)-2-stearamidopentanoate (4) (1.70 mmol, 872 mg) in a mixture of H2O (3.0 mL) and THF (3.0 mL) was added NaOH (15.0 mmol, 600 mg). The reaction mixture was stirred for 3 hours at room temperature. The mixture was acidified with HCl (aq. 1 M) to pH < 3 and extracted with EtOAc (3×30 mL). The combined organic layers were washed with brine (1×10 mL), dried over Na2SO4, filtered and the volatiles evaporated in vacuo. The residue was azeotropically evaporated with CHCl3 (3×30 mL) to give the anhydrous product 5. Yield: 763 mg (1.53 mmol, 90 %) of white solid; m.p. = 82.0–84.3 °C. EI-HRMS: m/z = 499.4113 (MH+); C28H55N2O5 requires: m/z = 499.4105 (MH+); νmax 3359, 2955, 2916, 2849, 1738, 1682, 1605, 1525, 1465, 1454, 1388, 1365, 1290, 1274, 1244, 1210, 1170, 1112, 1043, 1019, 957, 890, 860, 783, 727, 637 cm-1. 1H-NMR (500 MHz, CDCl3): δ 0.88 (t, J=6.9 Hz, 3H), 1.17 – 1.36 (m, 28H), 1.44 (s, 9H), 1.52 – 1.67 (m, 4H), 1.68 – 1.79 (m, 1H), 1.87 – 1.98 (m, 1H), 2.25 (t, J=7.8 Hz, 2H), 3.04 – 3.27 (m, 2H), 4.60 (td, J=4.9, 7.5 Hz, 1H), 4.86 (t, J=6.4 Hz, 1H), 6.73 (br d, J=7.4 Hz, 1H), 9.64 (br s, 1H). 13C-NMR (126 MHz, CDCl3): δ 14.27, 22.84, 25.79, 26.56, 28.52, 29.06, 29.42, 29.49, 29.51, 29.67, 29.80, 29.83, 29.86, 32.07, 36.57, 39.88, 52.22, 79.99, 156.91, 174.55, 174.80 (5 signals missing due to overlapping). 1H-NMR (500 MHz, DMSO-d6): δ 0.85 (t, J=6.9 Hz, 3H), 1.06 – 1.31 (m, 29H), 1.37 (s, 9H), 1.32 – 1.56 (m, 4H), 1.61 – 1.71 (m, 1H), 2.04 – 2.15 (m, 2H), 2.84 – 2.94 (m, 2H), 4.13 (td, J=5.0, 8.5 Hz, 1H), 6.77 (t, J=5.8 Hz, 1H), 7.98 (d, J=7.8 Hz, 1H), 12.40 (br s, 1H). 13C-NMR (126 MHz, DMSO-d6): δ 13.91, 22.06, 25.24, 26.15, 28.23, 28.43, 28.57, 28.66, 28.77, 28.93, 28.97, 29.00, 31.26, 35.02, 51.54, 77.33, 155.54, 172.23, 173.71 (7 signals missing due to overlapping).
Synthesis of methyl (S)-7-((tert-butoxycarbonyl)amino)-3-oxo-4-stearamidoheptanoate (2h)
Following GP1, prepared from (S)-5-((tert-butoxycarbonyl)amino)-2-stearamidopentanoic acid (5) (1.5 mmol, 748 mg), CDI (1.8 mmol, ω = 0.97, 301 mg), MgCl2 (1.47 mmol, ω = 0.98, 143 mg), methyl potassium malonate (2.25 mmol, 351 mg); isolation by extraction and column chromatography (EtOAc/petroleum ether = 1:1). Yield: 591 mg (1.065 mmol, 71 %) of white solid; m.p. = 62.3–64.9 °C. EI-HRMS: m/z = 555.4384 (MH+); C31H59N2O6 requires: m/z = 555.4368 (MH+); νmax 3341, 2915, 2848, 1747, 1711, 1681, 1638, 1524, 1438, 1390, 1365, 1316, 1251, 1168, 1016, 886, 769, 719, 643 cm-1. 1H-NMR (500 MHz, CDCl3): δ 0.88 (t, J=6.9 Hz, 3H), 1.18 – 1.35 (m, 28H), 1.44 (s, 9H), 1.47 – 1.68 (m, 5H), 1.90 – 2.00 (m, 1H), 2.21 – 2.26 (m, 2H), 3.15 (q, J=6.7 Hz, 2H), 3.58 (s, 2H), 3.74 (s, 3H), 4.66 (br s, 1H), 4.68 – 4.74 (m, 1H), 6.43 (br d, J=7.4 Hz, 1H). 13C-NMR (126 MHz, CDCl3): δ 14.27, 22.83, 25.73, 26.44, 27.58, 28.52, 29.44, 29.47, 29.50, 29.63, 29.77, 29.80, 29.84, 32.06, 36.62, 39.87, 46.26, 52.66, 58.11, 79.56, 156.40, 167.45, 173.54, 201.86 (6 signals missing due to overlapping). 1H-NMR (500 MHz, DMSO-d6): δ 0.85 (t, J=6.9 Hz, 3H), 1.08 – 1.30 (m, 29H), 1.37 (s, 9H), 1.31 – 1.54 (m, 4H), 1.62 – 1.75 (m, 1H), 2.12 (t, J=7.4 Hz, 2H), 2.89 (q, J=6.2 Hz, 2H), 3.58 (s, 2H), 3.61 (s, 3H), 4.23 (ddd, J=4.5, 7.3, 9.5 Hz, 1H), 6.78 (t, J=5.9 Hz, 1H), 8.16 (d, J=7.4 Hz, 1H). 13C-NMR (126 MHz, DMSO-d6): δ 13.90, 22.05, 25.10, 25.88, 26.25, 28.22, 28.57, 28.65, 28.71, 28.89, 28.96, 28.99, 31.25, 34.91, 45.49, 51.76, 57.75, 77.36, 155.57, 167.46, 172.67, 202.73 (7 signals missing due to overlapping).
Synthesis of (S)-5-methoxy-5-oxo-4-stearamidopentan-1-aminium 2,2,2-trifluoroacetate (6)
Methyl (S)-5-((tert-butoxycarbonyl)amino)-2-stearamidopentanoate (4) (10 mmol, 5.128 g) was dissolved in a 1:1 mixture of CF3COOH and anhydrous CH2Cl2 (60 mL) under argon, and the reaction mixture was stirred for 3 hours at room temperature. Volatile components were evaporated in vacuo, and the residue was azeotropically evaporated with anhydrous toluene (3×100 mL) to give ammonium salt 6. Yield: 5.00 g (9.50 mmol, 95%) of white solid; m.p. = 93.0–95.7 °C. EI-HRMS: m/z = 413.3726 (MH+); C24H49N2O3+ requires: m/z = 413.3738 (MH+); νmax 3318, 2915, 2848, 1752, 1671, 1645, 1528, 1474, 1462, 1430, 1400, 1381, 1358, 1276, 1237, 1207, 1173, 1127, 1067, 1003, 970, 955, 893, 839, 800, 768, 747, 723, 668, 613 cm-1. 1H-NMR (500 MHz, CDCl3 (700 μL) + TFA (20 μL)): δ 0.88 (t, J=6.9 Hz, 3H), 1.09 – 1.35 (m, 26H), 1.53 – 1.63 (m, 2H), 1.69 – 1.85 (m, 3H), 1.91 – 2.01 (m, 1H), 2.23 – 2.33 (m, 2H), 3.01 – 3.20 (m, 2H), 3.76 (s, 3H), 4.48 – 4.57 (m, 1H), 6.86 (d, J=7.5 Hz, 1H), 7.61 (br s, 3H). 13C-NMR (126 MHz, CDCl3 (700 μL) + TFA (20 μL)): δ 14.25, 22.84, 23.37, 25.78, 29.22, 29.28, 29.31, 29.51, 29.59, 29.74, 29.80, 29.81, 29.86, 32.07, 36.22, 39.62, 51.65, 53.08, 115.46 (q, J=287.8 Hz), 161.08 (q, J=39.3 Hz), 172.10, 176.30 (3 signals missing due to overlapping).
Synthesis of methyl (S)-2,5-distearamidopentanoate (7)
To a solution of stearic acid (1e) (8.5 mmol, 2.416 g) in anhydrous THF (30 mL), 1,1’-carbonyldiimidazole (CDI; 9.35 mmol, ω = 0.97, 1.563 g) was added under argon, and the resulting reaction mixture was stirred for 2 h at room temperature. The resulting activated acid was transferred to a suspension of (S)-5-methoxy-5-oxo-4-stearamidopentan-1-aminium 2,2,2-trifluoroacetate (6) (9.35 mmol, 4.924 g) in anhydrous THF (30 mL) under argon. Et3N (9.35 mmol, 1.303 mL) was then added to the reaction mixture at room temperature. The reaction mixture was stirred at 40 °C for 12 hours. Volatile components were evaporated in vacuo. The residue was extracted with CH2Cl2 (100 mL), EtOAc (100 mL), Et2O (100 mL), and n-hexane (100 mL) using a laboratory ultrasonic bath (5 minutes each), followed by decanting, respectively, to remove unreacted starting material and small portions of the product. H2O (150 mL) was added to the residue, followed by ultrasonic bath treatment (15 minutes). The resulting precipitate was collected by filtration and thoroughly washed with H2O (3×70 mL). The residue was dried under high vacuum at 40 °C for 12 hours to give product 7. Yield: 3.476 g (5.270 mmol, 62 %) of white solid; m.p. = 106.7–108.4 °C. EI-HRMS: m/z = 679.6336 (MH+); C42H83N2O4 requires: m/z = 679.6347 (MH+); νmax 3305, 2914, 2848, 1742, 1639, 1542, 1470, 1420, 1385, 1277, 1259, 1239, 1205, 1173, 980, 717 cm-1. 1H-NMR (500 MHz, CDCl3 (700 μL) + TFA (20 μL)): δ 0.88 (t, J=6.9 Hz, 6H), 1.18 – 1.36 (m, 56H), 1.54 – 1.77 (m, 7H), 1.87 – 1.97 (m, 1H), 2.26 – 2.38 (m, 4H), 3.23 – 3.34 (m, 1H), 3.34 – 3.47 (m, 1H), 3.79 (s, 3H), 4.57 – 4.64 (m, 1H), 6.70 (t, J=6.1 Hz, 1H), 6.79 (d, J=7.7 Hz, 1H). 13C-NMR (126 MHz, CDCl3 (700 μL) + TFA (20 μL)): δ 14.25, 22.84, 24.97, 25.87, 25.99, 29.20, 29.23, 29.28, 29.51, 29.56, 29.72, 29.78, 29.81, 29.83, 29.85, 30.00, 32.07, 36.28, 36.35, 39.58, 52.17, 53.15, 172.43, 176.50, 177.11 (17 signals missing due to overlapping).
Synthesis of (S)-2,5-distearamidopentanoic acid (8)
To a suspension of methyl (S)-2,5-distearamidopentanoate (7) (5 mmol, 3.393 g) in a mixture of H2O (20 mL) and THF (5 mL), KOH (powder for synthesis, 50 mmol, 2.810 g) was added, and the reaction mixture was stirred at 90 °C for 12 hours. The mixture was cooled to room temperature, and HCl (aq., 2 M) was added under stirring until the pH reached 1–2. The precipitate was collected by filtration and thoroughly washed with H2O (3×100 mL). The residue was dried under high vacuum at 40 °C for 12 hours to give acid 8. Yield: 2.792 g (4.20 mmol, 84 %) of white solid; m.p. = 108.8–110.1 °C. EI-HRMS: m/z = 665.6191 (MH+); C41H81N2O4 requires: m/z = 665.6191 (MH+); νmax 3310, 2955, 2916, 2849, 1736, 1639, 1586, 1545, 1466, 1446, 1418, 1372, 1275, 1245, 1211, 1181, 1128, 970, 829, 720, 685, 633 cm-1. 1H-NMR (500 MHz, CDCl3 (700 μL) + TFA (20 μL)): δ 0.88 (t, J=6.8 Hz, 6H), 1.09 – 1.40 (m, 56H), 1.52 – 1.73 (m, 6H), 1.75 – 1.89 (m, 1H), 1.92 – 2.11 (m, 1H), 2.27 – 2.45 (m, 4H), 3.13 – 3.49 (m, 2H), 4.61 (q, J=6.7, 1H), 6.87 (s, 1H), 6.98 (d, J=7.3 Hz, 1H), 11.12 (br s, 1H). 13C-NMR (126 MHz, CDCl3 (700 μL) + TFA (20 μL)): δ 14.26, 22.84, 24.97, 25.88, 25.96, 29.14, 29.19, 29.21, 29.25, 29.27, 29.52, 29.55, 29.57, 29.73, 29.79, 29.82, 29.84, 29.86, 32.08, 36.09, 36.13, 39.82, 52.36, 175.92, 177.30, 177.72 (15 signals missing due to overlapping).
Synthesis of methyl (S)-3-oxo-4,7-distearamidoheptanoate (2i)
To a suspension of (S)-2,5-distearamidopentanoic acid (8) (2.5 mmol, 1.662 g) in anhydrous THF (25 mL), 1,1'-carbonyldiimidazole (CDI; 5 mmol, ω = 0.97, 836 mg) was added under argon, and the reaction mixture was stirred for 15 minutes at room temperature, 30 minutes at 90 °C, and 60 minutes at 50 °C. Then, a solid mixture of MgCl2 (2.5 mmol, ω = 0.98, 243 mg) and methyl potassium malonate (7.5 mmol, 1.171 g) was carefully added at 50 °C. The reaction mixture was stirred for 15 minutes at 50 °C, 30 minutes at 90 °C, and 24 hours at room temperature. The volatiles were evaporated in vacuo, and NaHSO4 (1 M in H2O, 100 mL) was added to the residue. The mixture was stirred at room temperature for 30 minutes. The precipitate was collected by filtration and thoroughly washed with H2O (3×100 mL). The residue was dried under high vacuum at 40 °C for 12 hours to give β-keto ester 2i. Yield: 1.099 g (1.525 mmol, 61 %) of white solid; m.p. = 92.1–94.7 °C. EI-HRMS: m/z = 721.6456 (MH+); C44H85N2O5 requires: m/z = 721.6453 (MH+); νmax 3306, 2916, 2849, 1748, 1717, 1638, 1539, 1463, 1378, 1328, 1258, 1239, 1223, 1204, 1147, 1013, 719 cm-1. 1H-NMR (600 MHz, CDCl3): δ 0.88 (t, J=6.9 Hz, 6H), 1.18 – 1.39 (m, 56H), 1.51 – 1.67 (m, 7H), 1.88 – 1.97 (m, 1H), 2.17 (t, J=7.7 Hz, 2H), 2.25 (t, J=7.7 Hz, 2H), 3.22 – 3.38 (m, 2H), 3.58 (d, J=1.7 Hz, 2H), 3.74 (s, 3H), 4.65 – 4.72 (m, 1H), 5.87 (t, J=6.0 Hz, 1H), 6.67 (d, J=7.5 Hz, 1H). 13C-NMR (151 MHz, CDCl3): δ 14.26, 22.83, 25.72, 25.94, 25.98, 27.70, 29.45, 29.50, 29.52, 29.66, 29.78, 29.80, 29.81, 29.85, 32.07, 36.56, 36.96, 38.86, 46.22, 52.66, 58.21, 167.58, 173.81, 173.95, 201.90 (19 signals missing due to overlapping).
Synthesis of pyrrolones 11 from β-keto esters 10 – General procedure 2 (GP2)
To a solution of β-keto ester 10 (1.0 mmol) in anhydrous toluene (5 mL), DMFDMA (3 mmol, ω = 0.94, 424 μL) was added under argon and the resulting reaction mixture was stirred at 70 °C under argon until completion of the reaction, as judged by TLC analysis (1–3 hours). The volatiles were evaporated in vacuo and the residue was purified as quickly as possible by column chromatography (CC, Silica gel 60). The fractions containing the product 11 were combined and the volatiles were evaporated in vacuo. The product was immediately used for the following transformation or/and stored under argon at –20 °C.
Synthesis of 1-(tert-butyl) 3-methyl 5-benzyl-4-oxo-4,5-dihydro-1H-pyrrole-1,3-dicarboxylate (11a) and 1-(tert-butyl) 3-methyl 5-benzyl-4-hydroxy-1H-pyrrole-1,3-dicarboxylate (11a’) [22]
Following GP2. Prepared from methyl (S)-4-((tert-butoxycarbonyl)amino)-3-oxo-5-phenylpentanoate (10a) (1 mmol, 321.4 mg), 45 minutes; CC (EtOAc/petroleum ether = 1:1). 11a/11a’ = 45:55 (in DMSO-d6). Yield: 268 mg (0.81 mmol, 81 %) of colorless oil. 1H-NMR (500 MHz, DMSO-d6) for 11a: δ 1.55 (s, 9H), 3.22 (dd, J=2.7, 13.8 Hz, 1H), 3.38 (dd, J=6.4, 13.8 Hz, 1H), 3.61 (s, 3H), 4.58 (dd, J=2.6, 6.3 Hz, 1H), 6.91 – 6.97 (m, 2H), 8.71 (s, 1H). 1H-NMR (500 MHz, DMSO-d6) for 11a': δ 1.34 (s, 9H), 3.76 (s, 3H), 4.14 (s, 2H), 6.98 – 7.04 (m, 2H), 7.11 – 7.28 (m, 3H), 7.57 (s, 1H), 8.26 (s, 1H).
Synthesis of 1-(tert-butyl) 3-methyl 5-(4-(((benzyloxy)carbonyl)amino)butyl)-4-oxo-4,5-dihydro-1H-pyrrole-1,3-dicarboxylate (11b) and 1-(tert-butyl) 3-methyl 5-(4-(((benzyloxy)carbonyl)amino)butyl)-4-hydroxy-1H-pyrrole-1,3-dicarboxylate (11b’) [22]
Following GP2. Prepared from methyl (S)-8-(((benzyloxy)carbonyl)amino)-4-((tert-butoxycarbonyl)amino)-3-oxooctanoate (10b) (1 mmol, 436.5 mg), 1 hour; CC (EtOAc/petroleum ether = 1:1). 11b/11b’ = 37:63 (in DMSO-d6). Yield: 336 mg (0.82 mmol, 82 %) of colorless oil. 1H-NMR (500 MHz, DMSO-d6) for 11b: δ 0.98 – 1.08 (m, 1H), 1.08 – 1.19 (m, 1H), 1.50 (s, 9H), 1.83 – 1.93 (m, 1H), 1.97 – 2.08 (m, 1H), 2.93 (q, J=6.7 Hz, 2H), 3.68 (s, 3H), 4.30 (dd, J=3.1, 6.5 Hz, 1H), 8.98 (s, 1H). 1H-NMR (500 MHz, DMSO-d6) for 11b’: δ 1.28 – 1.46 (m, 4H), 1.54 (s, 9H), 2.70 (t, J=6.9 Hz, 2H), 2.98 (q, J=6.3 Hz, 2H), 3.73 (s, 3H), 4.99 (s, 2H), 7.23 (t, J=5.8 Hz, 1H), 7.26 – 7.41 (m, 5H), 7.49 (s, 1H), 7.95 (s, 1H).
Synthesis of 1-(tert-butyl) 3-methyl 5-(3-(benzyloxy)-3-oxopropyl)-4-oxo-4,5-dihydro-1H-pyrrole-1,3-dicarboxylate (11c) and 1-(tert-butyl) 3-methyl 5-(3-(benzyloxy)-3-oxopropyl)-4-hydroxy-1H-pyrrole-1,3-dicarboxylate (11c’) [23]
Following GP2. Prepared from 7-benzyl 1-methyl (S)-4-((tert-butoxycarbonyl)amino)-3-oxoheptanedioate (10c) (1 mmol, 393.4 mg), 45 minutes; CC (EtOAc/petroleum ether = 1:1). 11c/11c’ = 42:58 (in DMSO-d6). Yield: 343 mg (0.85 mmol, 85 %) of colorless oil. 1H-NMR (500 MHz, DMSO-d6) for 11c: δ 1.50 (s, 9H), 3.68 (s, 3H), 4.35 – 4.40 (m, 1H), 5.06 (d, J=5.4 Hz, 2H), 8.92 (s, 1H). 1H-NMR (500 MHz, DMSO-d6) for 11c’: δ 1.53 (s, 9H), 2.51 – 2.57 (m, 2H), 2.99 – 3.05 (m, 2H), 3.73 (s, 3H), 5.08 (s, 2H), 7.30 – 7.40 (m, 5H), 7.49 (s, 1H), 8.11 (s, 1H).
Synthesis of hexadecan-1-ol (13) [54]
Palmitic acid (11d) (20 mmol, 5.128 g) was dissolved in anhydrous THF (80 mL) under argon and the solution was cooled in an ice bath (0 ºC). While stirring in the ice bath, LiAlH4 (2.4 M in THF, 80 mmol, 33.3 mL) was added and the reaction mixture was allowed to warm to room temperature over 1 hour. The reaction mixture was stirred for a further 24 hours under argon at room temperature, and then quenched by careful addition of NaOH (1 M in H2O, 60 mL). The reaction mixture was extracted with diethyl ether (2×70 mL). The organic phase was dried over anhydrous Na2SO4, filtered, and the volatile components evaporated in vacuo. Yield: 3.957 g (16.3 mmol, 81 %) of a white solid. 1H-NMR (500 MHz, CDCl3): δ 0.88 (t, J=6.9 Hz, 3H), 1.19 – 1.39 (m, 27H), 1.52 – 1.61 (m, 2H), 3.64 (t, J=6.6 Hz, 2H).
Synthesis of palmitaldehyde (14) [55]
To a solution of hexadecan-1-ol (13) (16.3 mmol, 3.951 g) in anhydrous CH2Cl2 (100 mL), pyridinium chlorochromate (PCC, 24.5 mmol, ω = 0.98, 5.389 g) was added at room temperature and the reaction mixture was stirred for 16 h at room temperature. The solution was filtered through a plaque of Celite®, washed with CH2Cl2 and the volatiles evaporated in vacuo. The residue was purified by column chromatography (Silica gel 60, petroleum ether/ethyl acetate = 10:1). The fractions containing the pure product 14 were combined and the volatile components were evaporated in vacuo. Yield: 2.940 g (12.23 mmol, 75 %) of a colorless oil. 1H-NMR (500 MHz, CDCl3): δ 0.88 (t, J=6.9 Hz, 3H), 1.26 (s, 24H), 1.58 – 1.67 (m, 2H), 2.42 (td, J=1.9, 7.4 Hz, 2H), 9.76 (t, J=1.9 Hz, 1H).
Synthesis of 1-nitroheptadecan-2-ol (15)
Prepared according to the literature procedure [
56]. Palmitaldehyde (
14) (11.44 mmol, 2.751 g) was dissolved in a mixture of anhydrous THF and anhydrous
t-butanol in a 1:1 ratio (50 mL) under argon. Nitromethane (17.16 mmol, 930 μL) was then added at room temperature. The mixture was cooled to 0 °C,
tBuOK (1.144 mmol, 128 mg) was added, and the reaction mixture was allowed to warm to room temperature over 1 hour. After 16 h at room temperature under argon, the reaction mixture was diluted with H
2O (300 mL) and the product was extracted with diethyl ether (2×100 mL). The organic phase was washed with NaCl (aq. sat., 2×50 mL), dried over anhydrous Na
2SO
4, filtered, and the volatiles evaporated
in vacuo. The crude product
15 was used for the following transformation without further purification. Yield: 2.794 g (9.267 mmol, 81 %) of a yellowish oil.
1H-NMR (500 MHz, CDCl
3):
δ 0.88 (
t,
J=6.9 Hz, 3H), 1.18 – 1.42 (
m, 26H), 1.44 – 1.57 (
m, 2H), 2.61 (br
s, 1H), 4.28 – 4.34 (
m, 1H), 4.38 (
dd,
J=8.5, 13.0 Hz, 1H), 4.43 (
dd,
J=2.7, 13.0 Hz, 1H).
Synthesis of (E)-1-nitroheptadec-1-ene (16) [66]
Prepared according to the literature procedure [
56]. 1-Nitroheptadecan-2-ol (
15) (9.12 mmol, 2.75 g) was dissolved in anhydrous CH
2Cl
2 (25 mL) and cooled to –10 °C. With stirring, trifluoroacetic anhydride (TFAA, 9.12 mmol, 1.269 mL) was added dropwise and the cooled mixture (–10 °C) was stirred for another 2 minutes. Over the next 10 minutes, triethylamine (2.532 mL, 18.24 mmol) was added dropwise and the reaction mixture was stirred at –10 °C for another 30 minutes. The reaction mixture was then diluted with CH
2Cl
2 (100 mL) and washed with NaHSO
4 (aq., 1 M, 200 mL). The aqueous phase was extracted with CH
2Cl
2 (2×40 mL). The combined organic phase was dried over anhydrous Na
2SO
4, filtered, and the volatiles evaporated
in vacuo. The residue was purified by column chromatography (Silica gel 60; petroleum ether/EtOAc = 40:1). The fractions containing the pure product
16 were combined and the volatile components were evaporated
in vacuo. Product
16 was stored under argon at 5 °C. Yield: 2.016 g (7.114 mmol, 78 %) of a white solid; m.p. = 25.0–25.7 °C. EI-HRMS:
m/z = 306.2395 (MNa
+); C
17H
34NNaO
2 requires:
m/z = 306.2404 (MNa
+);
νmax 2922, 2853, 1650, 1526, 1465, 1350, 960, 835, 723 cm
-1.
1H-NMR (500 MHz, CDCl
3):
δ 0.88 (
t,
J=6.9 Hz, 3H), 1.19 – 1.37 (
m, 24H), 1.51 (
p,
J=7.3 Hz, 2H), 2.27 (
qd,
J=1.5, 7.4 Hz, 2H), 6.98 (
dt,
J=1.6, 13.4 Hz, 1H), 7.23 – 7.33 (
m, 1H).
13C-NMR (126 MHz, CDCl
3):
δ 14.27, 22.84, 27.86, 28.61, 29.24, 29.40, 29.51, 29.59, 29.72, 29.77, 29.80, 29.82, 29.84, 32.07, 139.69, 143.01 (1 signal missing due to overlapping).
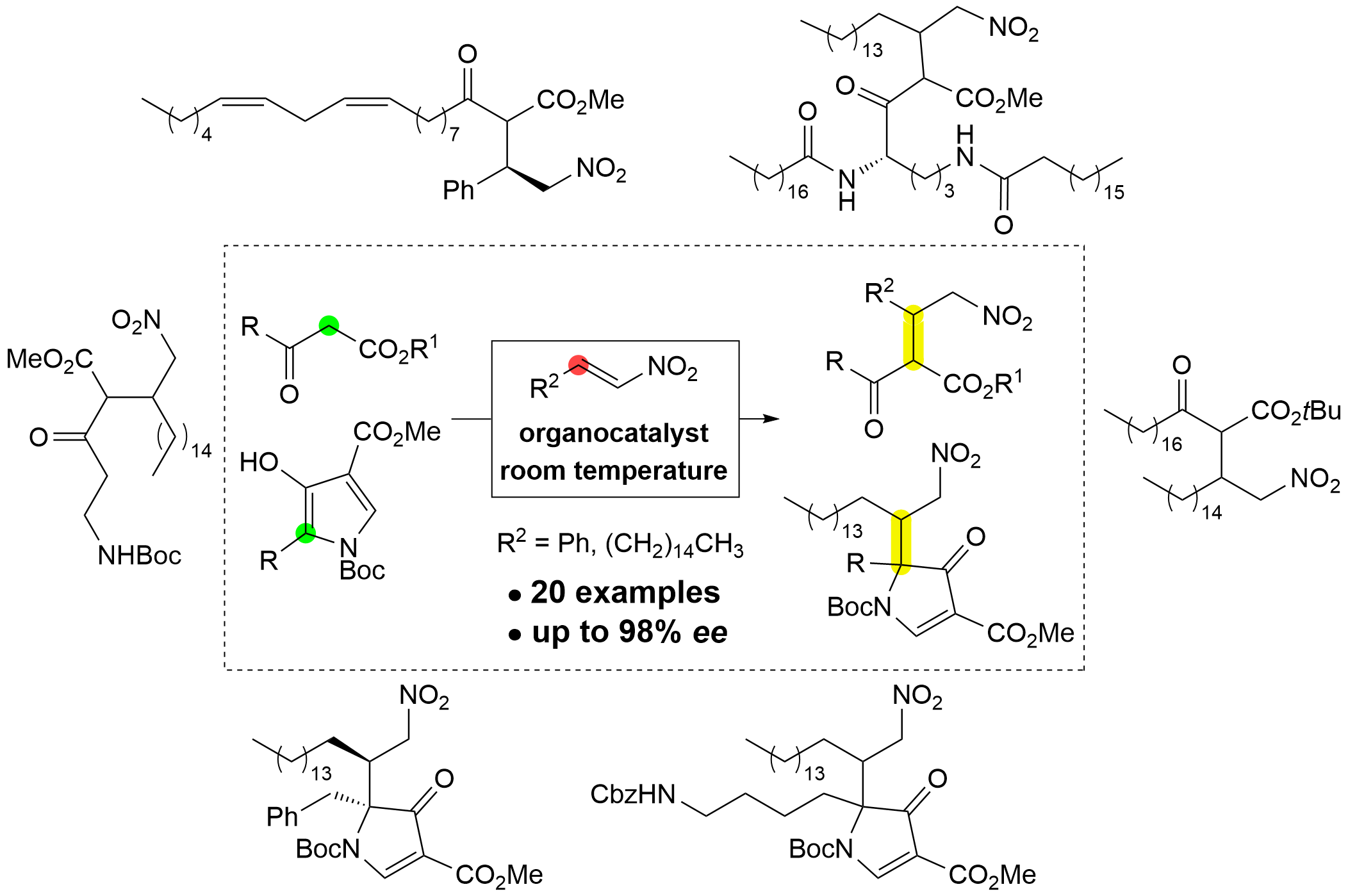
Organocatalyzed Michael addition of β-keto esters 2 to nitroalkenes – General procedure for the preparation of racemic products rac-17 – General procedure 3 (GP3)
To a solution/suspension of nitroalkene 12 or 16 (0.2 mmol, 1.0 equivalent or 0.3 mmol, 1.5 equivalents) and the achiral organocatalyst X (0.04 mmol, 0.2 equivalents, 16.4 mg) in anhydrous CH2Cl2 (1 mL) under argon at room temperature, β-keto ester 2 (0.3 mmol, 1.5 equivalents or 0.2 mmol, 1.0 equivalent) was added and the resulting reaction mixture was stirred at room temperature for 24–72 hours. The volatiles were evaporated in vacuo and the residue was purified by column chromatography (Silica gel 60, mobile phase). The fractions containing the pure racemic product rac-17 were combined and the volatiles were evaporated in vacuo. The product rac-17 was fully characterized and analyzed by HPLC.
Organocatalyzed Michael addition of β-keto esters 2 to nitroalkenes – General procedure for the organocatalyzed asymmetric addition – General procedure 4 (GP4)
To a solution/suspension of nitroalkene 12 or 16 (0.2 mmol, 1.0 equivalent or 0.3 mmol, 1.5 equivalents) and the chiral organocatalyst VIII (0.02 mmol, 0.1 equivalents, 12.3 mg) or I (0.02 mmol, 0.1 equivalents, 10.9 mg) in anhydrous CH2Cl2 (1 mL) under argon at room temperature, β-keto ester 2 (0.3 mmol, 1.5 equivalents or 0.2 mmol, 1.0 equivalent) was added and the resulting reaction mixture was stirred at room temperature for 24–72 hours. The volatiles were evaporated in vacuo and the residue was purified by column chromatography (Silica gel 60, mobile phase). The fractions containing the pure chiral nonracemic product 17 were combined the volatiles were evaporated in vacuo. The product 17 was fully characterized and analyzed by HPLC.
Synthesis of methyl 4-((tert-butoxycarbonyl)amino)-2-((R)-2-nitro-1-phenylethyl)-3-oxobutanoate (17a)
Following GP3 and GP4. Prepared from methyl 4-((tert-butoxycarbonyl)amino)-3-oxobutanoate (2a) (0.2 mmol, 46.3 mg) and trans-β-nitrostyrene (12) (0.3 mmol, 44.7 mg), organocatalyst VIII, 24 h; isolation by column chromatography (EtOAc/petroleum ether = 1:4). rac-17a Yield: 41.8 mg (0.110 mmol, 55%, two diastereomers in a ratio of 59:41 in DMSO-d6) of white solid. 17a Yield: 63.9 mg (0.168 mmol, 84%, two diastereomers in a ratio of 56:44 in DMSO-d6) of white solid; m.p. = 116–122 °C. EI-HRMS: m/z = 381.1641 (MH+); C18H25N2O7 requires: m/z = 381.1656 (MH+); νmax 3378, 2981, 1751, 1714, 1555, 1497, 1455, 1429, 1367, 1252, 1154, 1020, 974, 895, 858, 764, 699, 637 cm-1. 1H-NMR (500 MHz, DMSO-d6) for major diastereomer: δ 1.39 (s, 9H), 3.37 (s, 3H), 3.95 (dd, J=3.5, 5.8 Hz, 2H), 4.06 – 4.13 (m, 1H), 4.40 (d, J=10.7 Hz, 1H), 4.87 – 4.96 (m, 2H), 7.18 (t, J=5.9 Hz, 1H), 7.22 – 7.34 (m, 5H). 1H-NMR (500 MHz, DMSO-d6) for minor diastereomer: δ 1.33 (s, 9H), 3.70 (s, 3H), 3.50 (dd, J=5.9, 18.8 Hz, 1H), 3.80 (dd, J=5.8, 18.8 Hz, 1H), 4.46 (d, J=10.2 Hz, 1H), 4.75 (dd, J=4.3, 13.2 Hz, 1H), 4.85 (d, J=10.8 Hz, 1H), 7.06 (t, J=5.8 Hz, 1H). 13C-NMR (126 MHz, DMSO-d6) for both diastereomers: δ 28.10, 28.14, 42.35, 42.65, 50.18, 50.25, 52.45, 52.84, 57.28, 57.93, 59.78, 77.74, 78.22, 78.24, 78.44, 127.79, 127.85, 128.18, 128.25, 128.49, 128.64, 136.85, 136.92, 155.47, 155.81, 167.33, 170.36, 199.88, 200.62 (3 signals missing due to overlapping). HPLC: Chiralpak IA-3, n-Hexane/i-PrOH = 80:20, flow rate 1.0 mL/min, λ = 210 nm, T = 20°C. Diastereomer 1: tR = 9.24 minutes (minor); 17.62 minutes (major) – 91% ee. Diastereomer 2: tR = 14.12 minutes (major); 23.56 minutes (minor) – 93% ee.
Synthesis of methyl 5-((tert-butoxycarbonyl)amino)-2-((R)-2-nitro-1-phenylethyl)-3-oxopentanoate (17b) [22]
Following GP3 and GP4. Prepared from methyl 5-((tert-butoxycarbonyl)amino)-3-oxopentanoate (2b) (0.2 mmol, 49.1 mg) and trans-β-nitrostyrene (12) (0.3 mmol, 44.7 mg), organocatalyst VIII, 24 h; isolation by column chromatography (EtOAc/petroleum ether = 1:4). rac-17b Yield: 69.4 mg (0.176 mmol, 88%, two diastereomers in a ratio of 53:47 in DMSO-d6) of white solid. 17b Yield: 67.1 mg (0.170 mmol, 85%, two diastereomers in a ratio of 53:47 in DMSO-d6) of white solid; m.p. = 96.1–98.4 °C. EI-HRMS: m/z = 417.1618 (MNa+); C19H27N2NaO7 requires: m/z = 417.1632 (MNa+); νmax 3424, 2978, 1743, 1707, 1553, 1506, 1455, 1434, 1366, 1246, 1164, 1082, 966, 859, 756, 701 cm-1. 1H-NMR (500 MHz, DMSO-d6) for both diastereomers: δ 1.34 (s, 4.5H), 1.37 (s, 4.5H), 2.24 – 2.34 (m, 0.5H), 2.58 – 2.68 (m, 0.5H), 2.74 (t, J=6.8 Hz, 1H), 2.76 – 2.91 (m, 1H), 3.15 (q, J=6.4 Hz, 1H), 3.35 (s, 1.5H), 3.70 (s, 1.5H), 4.00 – 4.09 (m, 1H), 4.37 (dd, J=6.0, 10.5 Hz, 1H), 4.81 (d, J=7.5 Hz, 1H), 4.87 – 4.99 (m, 1H), 6.58 (t, J=5.7 Hz, 0.5H), 6.85 (t, J=5.7 Hz, 0.5H), 7.21 – 7.35 (m, 5H). 13C-NMR (126 MHz, DMSO-d6) for both diastereomers: δ 28.19, 28.22, 34.52, 34.79, 42.30, 42.36, 42.66, 42.89, 52.43, 52.83, 60.00, 60.84, 77.69, 77.78, 78.00, 78.15, 127.79, 127.86, 128.27, 128.46, 128.63, 136.80, 136.96, 155.33, 155.53, 166.88, 167.72, 201.83 (6 signals missing due to overlapping). HPLC: Chiralpak IA-3, n-Hexane/i-PrOH = 80:20, flow rate 1.0 mL/min, λ = 210 nm, T = 20°C. Diastereomer 1: tR = 7.22 minutes (minor); 8.98 minutes (major) – 95% ee. Diastereomer 2: tR = 12.53 minutes (minor); 20.94 minutes (major) – 95% ee.
Synthesis of methyl 2-(3-((tert-butoxycarbonyl)amino)propanoyl)-3-(nitromethyl)octadecanoate (rac-17c)
Following GP3. Prepared from methyl 5-((tert-butoxycarbonyl)amino)-3-oxopentanoate (2b) (0.3 mmol, 73.6 mg) and (E)-1-nitroheptadec-1-ene (16) (0.2 mmol, 56.7 mg), organocatalyst X, 24 h; isolation by column chromatography (EtOAc/petroleum ether = 1:5). rac-17c Yield: 55.0 mg (0.104 mmol, 52%, two diastereomers in a ratio of 53:47 in CDCl3) of colorless oil. EI-HRMS: m/z = 429.3313 (MH+-Boc); C23H45N2O5 requires: m/z = 429.3323 (MH+-Boc); νmax 3413, 2923, 2853, 1743, 1712, 1552, 1505, 1436, 1366, 1248, 1168, 1084, 966, 911, 863, 781, 733 cm-1. 1H-NMR (600 MHz, CDCl3) for the major diastereomer: δ 0.88 (t, J=6.9 Hz, 3H), 1.19 – 1.40 (m, 28H), 1.43 (s, 9H), 2.71 – 2.79 (m, 1H), 2.82 – 2.96 (m, 2H), 3.30 – 3.45 (m, 2H), 3.76 (s, 3H), 3.73 – 3.83 (m, 1H), 4.60 (dd, J=5.0, 12.9 Hz, 1H), 4.65 (dd, J=4.5, 13.2 Hz, 1H), 4.86 – 4.96 (m, 1H). 1H-NMR (600 MHz, CDCl3) for the minor diastereomer: δ 3.76 (s, 3H), 4.52 (dd, J=5.9, 13.1 Hz, 2H). 13C-NMR (151 MHz, CDCl3) for both diastereomers: δ 14.27, 22.83, 26.82, 26.88, 28.50, 29.42, 29.43, 29.49, 29.50, 29.63, 29.65, 29.74, 29.79, 29.81, 29.83, 29.84, 30.28, 32.06, 35.17, 36.64, 36.66, 43.35, 43.56, 52.94, 53.08, 59.31, 59.76, 76.02, 76.47, 79.58, 79.63, 155.95, 168.46, 203.83 (18 signals missing due to overlapping).
Synthesis of methyl 4-((3-methylbut-2-en-1-yl)oxy)-2-((R)-2-nitro-1-phenylethyl)-3-oxobutanoate (17d)
Following GP3 and GP4. Prepared from methyl 4-((3-methylbut-2-en-1-yl)oxy)-3-oxobutanoate (2c) (0.2 mmol, 40.0 mg) and trans-β-nitrostyrene (12) (0.3 mmol, 44.7 mg), organocatalyst VIII, 24 h; isolation by column chromatography (EtOAc/petroleum ether = 1:5). rac-17d Yield: 54.5 mg (0.156 mmol, 78%, two diastereomers in a ratio of 60:40 in CDCl3) of colorless oil. 17d Yield: 62.2 mg (0.178 mmol, 89%, two diastereomers in a ratio of 60:40 in CDCl3) of colorless oil. EI-HRMS: m/z = 367.1858 (M+NH4+); C18H27N2O6 requires: m/z = 367.1864 (M+NH4+); νmax 3033, 2954, 2916, 1746, 1724, 1552, 1496, 1434, 1378, 1247, 1199, 1169, 1092, 1034, 981, 942, 893, 766, 700, 618 cm-1. 1H-NMR (500 MHz, CDCl3) for the major diastereomer: δ 1.61 (s, 3H), 1.74 (s, 3H), 3.73 (s, 3H), 3.76 – 3.90 (m, 2H), 4.21 – 4.32 (m, 3H), 4.79 – 4.95 (m, 3H), 5.20 – 5.24 (m, 1H), 7.18 – 7.24 (m, 2H), 7.25 – 7.33 (m, 3H). 1H-NMR (500 MHz, CDCl3) for the minor diastereomer: δ 1.66 (s, 3H), 1.75 (s, 3H), 3.52 (s, 3H), 3.98 (d, J=7.0 Hz, 2H), 4.02 (d, J=17.5 Hz, 1H), 4.10 (d, J=17.4 Hz, 1H). 13C-NMR (126 MHz, CDCl3): δ 18.12, 18.16, 25.90, 25.93, 42.13, 42.35, 52.80, 52.96, 56.60, 57.33, 67.75, 67.94, 74.72, 77.22, 77.77, 119.78, 119.80, 128.05, 128.22, 128.43, 128.50, 129.10, 129.22, 136.39, 136.41, 138.70, 138.84, 167.43, 167.90, 202.15, 202.77 (1 signal missing due to overlapping). HPLC: Chiralpak AS-H, n-Hexane/EtOH = 90:10, flow rate 1.0 mL/min, λ = 210 nm, T = 20 °C. Minor diastereomer: enantiomers: tR = 14.066 minutes (minor); 17.773 minutes (major) – 94% ee. Major diastereomer: enantiomers: tR = 17.164 minutes (major); 19.406 minutes (minor) – 91% ee.
Synthesis of methyl 2-(2-((3-methylbut-2-en-1-yl)oxy)acetyl)-3-(nitromethyl)octadecanoate (rac-17e)
Following GP3. Prepared from methyl 4-((3-methylbut-2-en-1-yl)oxy)-3-oxobutanoate (2c) (0.3 mmol, 60.1 mg) and (E)-1-nitroheptadec-1-ene (16) (0.2 mmol, 56.7 mg), organocatalyst X, 24 h; isolation by column chromatography (EtOAc/petroleum ether = 1:5). rac-17e Yield: 52.2 mg (0.108 mmol, 54%, two diastereomers in a ratio of 51:49 in CDCl3) of colorless oil. EI-HRMS: m/z = 501.3887 (M+NH4+); C27H53N2O6 requires: m/z = 501.3898 (M+NH4+); νmax 2923, 2853, 1726, 1553, 1435, 1379, 1250, 1199, 1158, 1092, 1000, 780, 722 cm-1. 1H-NMR (600 MHz, CDCl3) for both diastereomers: δ 0.88 (t, J=7.0 Hz, 3H), 1.18 – 1.51 (m, 28H), 1.68 (dd, J=1.4, 4.8 Hz, 3H), 1.77 (dd, J=1.2, 4.1 Hz, 3H), 2.85 – 2.93 (m, 1H), 3.73 (s, 1.5H), 3.74 (s, 1.5H), 3.94 – 4.15 (m, 5H), 4.46 (dd, J=7.1, 13.3 Hz, 0.5H), 4.53 (dd, J=5.7, 13.2 Hz, 0.5H), 4.59 – 4.69 (m, 1H), 5.27 – 5.34 (m, 1H). 13C-NMR (151 MHz, CDCl3) for both diastereomers: δ 14.28, 18.19, 18.21, 22.84, 25.96, 26.88, 27.05, 29.38, 29.45, 29.51, 29.66, 29.75, 29.80, 29.83, 29.85, 30.29, 32.07, 36.10, 36.30, 52.67, 52.82, 54.92, 55.32, 67.99, 68.00, 74.63, 74.73, 76.40, 76.75, 119.82, 119.92, 138.71, 138.89, 168.55, 168.56, 203.55, 203.88 (17 signals missing due to overlapping).
Synthesis of methyl 2-((R)-2-nitro-1-phenylethyl)-3-oxooctadecanoate (17f)
Following GP3 and GP4. Prepared from methyl 3-oxooctadecanoate (2d) (0.3 mmol, 93.7 mg) and trans-β-nitrostyrene (12) (0.2 mmol, 29.8 mg), organocatalyst VIII, 24 h; isolation by column chromatography (EtOAc/petroleum ether = 1:10). rac-17f Yield: 34.2 mg (0.074 mmol, 37%, two diastereomers in a ratio of 56:44 in CDCl3) of white solid. 17f Yield: 55.4 mg (0.120 mmol, 60%, two diastereomers in a ratio of 50:50 in CDCl3) of white solid; m.p. = 60.0–61.2 °C. EI-HRMS: m/z = 462.3214 (MH+); C27H44NO5 requires: m/z = 462.3214 (MH+); νmax 2915, 2850, 1742, 1711, 1550, 1496, 1471, 1455, 1438, 1383, 1334, 1281, 1245, 1208, 1173, 1128, 1092, 1072, 1033, 1004, 983, 918, 892, 853, 765, 718, 700, 616 cm-1. 1H-NMR (500 MHz, CDCl3) for the major diastereomer: δ 0.88 (t, J=6.9 Hz, 3H), 0.94 – 1.04 (m, 2H), 1.06 – 1.39 (m, 22H), 1.51 – 1.60 (m, 2H), 2.13 (dt, J=7.2, 17.8, 1H), 2.38 – 2.50 (m, 1H), 3.76 (s, 3H), 4.03 (d, J=10.0 Hz, 1H), 4.19 – 4.28 (m, 1H), 4.75 – 4.89 (m, 2H), 7.16 – 7.22 (m, 2H), 7.24 – 7.34 (m, 3H). 1H-NMR (500 MHz, CDCl3) for the minor diastereomer: δ 2.61 (dt, J=7.4, 17.7 Hz, 1H), 3.52 (s, 3H), 4.13 (d, J=9.5 Hz, 1H). 13C-NMR (126 MHz, CDCl3) for both diastereomers: δ 14.27, 22.83, 23.05, 23.39, 28.74, 29.00, 29.33, 29.45, 29.48, 29.50, 29.56, 29.68, 29.73, 29.76, 29.78, 29.80, 29.83, 32.06, 42.53, 42.86, 43.50, 43.84, 52.87, 53.05, 60.97, 61.34, 77.67, 77.93, 127.94, 128.11, 128.42, 128.50, 129.15, 129.26, 136.44, 136.63, 167.62, 168.14, 202.76, 203.81 (10 signals missing due to overlapping). HPLC: Chiralpak IA-3, n-Hexane/EtOH = 95:5, flow rate 1.0 mL/min, λ = 210 nm, T = 25 °C. Minor diastereomer: enantiomers: tR = 7.287 minutes (minor); 22.511 minutes (major) – 95% ee. Major diastereomer: enantiomers: tR = 8.963 minutes (major); 13.215 minutes (minor) – 96% ee.
Synthesis of methyl 2-(1-nitroheptadecan-2-yl)-3-oxooctadecanoate (rac-17g)
Following GP3. Prepared from methyl 3-oxooctadecanoate (2d) (0.3 mmol, 93.7 mg) and (E)-1-nitroheptadec-1-ene (16) (0.2 mmol, 56.7 mg), organocatalyst X, 24 h; isolation by column chromatography (EtOAc/petroleum ether = 1:10). rac-17g Yield: 78.7 mg (0.132 mmol, 66%, two diastereomers in a ratio of 68:32 in CDCl3) of white solid; m.p. = 40.0–40.9 °C. EI-HRMS: m/z = 594.5108 (M-H+)-; C36H68NO5 requires: m/z = 594.5103 (M-H+)-; νmax 2955, 2914, 2849, 1730, 1707, 1556, 1543, 1470, 1435, 1402, 1380, 1243, 1204, 1128, 1073, 1000, 863, 719 cm-1. 1H-NMR (600 MHz, CDCl3) for both diastereomers: δ 0.88 (t, J=7.0, 6H), 1.11 – 1.46 (m, 52H), 1.55 – 1.62 (m, 2H), 2.47 – 2.55 (m, 1H), 2.57 – 2.66 (m, 1H), 2.79 – 2.90 (m, 1H), 3.75 (s, 2.04H), 3.75 (s, 0.96H), 3.76 – 3.81 (m, 1H), 4.49 – 4.57 (m, 1H), 4.59 – 4.67 (m, 1H). 13C-NMR (151 MHz, CDCl3) for both diastereomers: δ 14.28, 22.85, 23.46, 23.50, 26.80, 26.91, 29.08, 29.10, 29.42, 29.45, 29.48, 29.50, 29.52, 29.60, 29.65, 29.67, 29.75, 29.81, 29.83, 29.85, 30.22, 32.08, 36.72, 36.77, 43.43, 43.60, 52.79, 52.91, 59.33, 59.72, 76.16, 76.57, 168.77, 168.81, 204.33, 204.46 (36 signals missing due to overlapping).
Synthesis of methyl 2-((R)-2-nitro-1-phenylethyl)-3-oxoicosanoate (17h)
Following GP3 and GP4. Prepared from methyl 3-oxoicosanoate (2e) (0.3 mmol, 102.2 mg) and trans-β-nitrostyrene (12) (0.2 mmol, 29.8 mg), organocatalyst VIII, 24 h; isolation by column chromatography (EtOAc/petroleum ether = 1:10). rac-17h Yield: 43.1 mg (0.088 mmol, 44%, two diastereomers in a ratio of 54:46 in CDCl3) of white solid. 17h Yield: 52.9 mg (0.108 mmol, 54%, two diastereomers in a ratio of 48:52 in CDCl3) of white solid; m.p. = 42.2–44.7 °C. EI-HRMS: m/z = 490.3524 (MH+); C29H48NO5 requires: m/z = 490.3527 (MH+); νmax 2914, 2849, 1737, 1712, 1555, 1496, 1471, 1455, 1433, 1404, 1378, 1271, 1198, 1168, 1113, 1082, 982, 891, 765, 716, 699 cm-1. 1H-NMR (500 MHz, CDCl3) for the major diastereomer: δ 0.88 (t, J=6.9 Hz, 3H), 1.06 – 1.37 (m, 30H), 2.38 – 2.50 (m, 1H), 2.61 (dt, J=7.4, 17.7 Hz, 1H), 3.52 (s, 3H), 4.13 (d, J=9.4 Hz, 1H), 4.19 – 4.28 (m, 1H), 4.74 – 4.89 (m, 2H), 7.16 – 7.22 (m, 2H), 7.24 – 7.33 (m, 3H). 1H-NMR (500 MHz, CDCl3) for the minor diastereomer: δ 0.94 – 1.04 (m, 2H), 1.51 – 1.60 (m, 2H), 2.13 (dt, J=7.1, 17.7 Hz, 1H), 3.75 (s, 3H), 4.03 (d, J=10.0 Hz, 1H). 13C-NMR (126 MHz, CDCl3) for both diastereomers: δ 14.25, 22.82, 23.03, 23.37, 28.72, 28.99, 29.32, 29.43, 29.47, 29.49, 29.55, 29.67, 29.72, 29.75, 29.77, 29.79, 29.82, 32.05, 42.51, 42.86, 43.48, 43.83, 52.84, 53.02, 60.94, 61.33, 77.65, 77.92, 127.93, 128.10, 128.39, 128.47, 129.13, 129.24, 136.44, 136.63, 167.61, 168.12, 202.74, 203.79 (14 signals missing due to overlapping). HPLC: Chiralpak IA-3, n-Hexane/EtOH = 95:5, flow rate 1.0 mL/min, λ = 210 nm, T = 25 °C. Major diastereomer: enantiomers: tR = 8.838 minutes (minor); 26.885 minutes (major) – 96% ee. Minor diastereomer: enantiomers: tR = 10.913 minutes (major); 15.371 minutes (minor) – 96% ee.
Synthesis of methyl 2-(1-nitroheptadecan-2-yl)-3-oxoicosanoate (rac-17i)
Following GP3. Prepared from methyl 3-oxoicosanoate (2e) (0.3 mmol, 102.2 mg) and (E)-1-nitroheptadec-1-ene (16) (0.2 mmol, 56.7 mg), organocatalyst X, 24 h; isolation by column chromatography (EtOAc/petroleum ether = 1:10). rac-17i Yield: 52.4 mg (0.084 mmol, 42%, two diastereomers in a ratio of 50:50 in CDCl3) of white solid; m.p. = 42.0–43.9 °C. EI-HRMS: m/z = 622.5416 (M-H+)-; C38H73NO5 requires: m/z = 622.5421 (M-H+)-; νmax 2956, 2915, 2848, 1731, 1706, 1556, 1542, 1470, 1435, 1402, 1379, 1349, 1241, 1205, 1128, 1108, 1078, 1001, 719 cm-1. 1H-NMR (500 MHz, CDCl3) for both diastereomers: δ 0.87 (t, J=6.9 Hz, 6H), 1.08 – 1.44 (m, 56H), 1.53 – 1.61 (m, 2H), 2.45 – 2.55 (m, 1H), 2.56 – 2.66 (m, 1H), 2.78 – 2.89 (m, 1H), 3.74 (s, 1.5H), 3.74 (s, 1.5H), 3.75 (d, J=6.9 Hz, 0.5H), 3.79 (d, J=7.6 Hz, 0.5H), 4.45 – 4.55 (m, 1H), 4.62 (td, J=4.6, 13.5 Hz, 1H). 13C-NMR (126 MHz, CDCl3) for both diastereomers: δ 14.25, 22.83, 23.43, 23.48, 26.77, 26.88, 29.06, 29.08, 29.40, 29.43, 29.46, 29.48, 29.50, 29.59, 29.64, 29.71, 29.74, 29.79, 29.82, 29.84, 30.20, 32.06, 36.68, 36.74, 43.38, 43.56, 52.74, 52.86, 59.30, 59.69, 76.13, 76.54, 168.74, 168.78, 204.28, 204.41 (40 signals missing due to overlapping).
Synthesis of tert-butyl 2-((R)-2-nitro-1-phenylethyl)-3-oxoicosanoate (17j)
Following GP3 and GP4. Prepared from tert-butyl 3-oxoicosanoate (2f) (0.3 mmol, 114.8 mg) and trans-β-nitrostyrene (12) (0.2 mmol, 29.8 mg), organocatalyst VIII, 24 h; isolation by column chromatography (EtOAc/petroleum ether = 1:10). rac-17j Yield: 42.5 mg (0.080 mmol, 40%, two diastereomers in a ratio of 61:39 in CDCl3) of white solid. 17j Yield: 50.0 mg (0.094 mmol, 47%, two diastereomers in a ratio of 36:64 in CDCl3) of white solid; m.p. = 54.0–56.4 °C. EI-HRMS: m/z = 549.4273 (M+NH4+); C32H57N2O5 requires: m/z = 549.4262 (M+NH4+); νmax 2916, 2849, 1731, 1712, 1553, 1496, 1468, 1434, 1394, 1378, 1284, 1250, 1148, 1126, 1092, 1062, 982, 914, 838, 770, 751, 720, 700 cm-1. 1H-NMR (500 MHz, CDCl3) for the major diastereomer: δ 0.88 (t, J=6.9 Hz, 3H), 0.98 – 1.07 (m, 2H), 1.09 – 1.39 (m, 28H), 1.46 (s, 9H), 1.55 – 1.63 (m, 1H), 2.14 (dt, J=7.1, 17.5 Hz, 1H), 2.38 – 2.52 (m, 1H), 3.91 (d, J=9.9 Hz, 1H), 4.12 – 4.23 (m, 1H), 4.65 – 4.76 (m, 1H), 7.16 – 7.34 (m, 5H). 1H-NMR (500 MHz, CDCl3) for the minor diastereomer: δ 2.62 (dt, J=7.4, 17.4 Hz, 1H), 4.01 (d, J=10.1 Hz, 1H), 4.77 – 4.90 (m, 2H). 13C-NMR (126 MHz, CDCl3) for both diastereomers: δ 14.26, 22.83, 23.18, 23.53, 27.52, 27.98, 28.90, 29.15, 29.38, 29.48, 29.50, 29.56, 29.69, 29.73, 29.76, 29.80, 29.83, 32.06, 42.54, 42.79, 42.87, 43.59, 62.16, 62.51, 78.06, 78.40, 82.95, 83.40, 128.18, 128.29, 128.32, 128.36, 128.95, 129.14, 136.81, 136.84, 166.04, 166.75, 203.13, 203.98 (16 signals missing due to overlapping). HPLC: Chiralpak IA-3, n-Hexane/EtOH = 95:5, flow rate 1.0 mL/min, λ = 210 nm, T = 25 °C. Minor diastereomer: enantiomers: tR = 6.051 minutes (minor); 8.241 minutes (major) – 93% ee. Major diastereomer: enantiomers: tR = 6.768 minutes (minor); 7.403 minutes (major) – 98% ee.
Synthesis of tert-butyl 2-(1-nitroheptadecan-2-yl)-3-oxoicosanoate (rac-17k)
Following GP3. Prepared from tert-butyl 3-oxoicosanoate (2f) (0.3 mmol, 114.8 mg) and (E)-1-nitroheptadec-1-ene (16) (0.2 mmol, 56.7 mg), organocatalyst X, 24 h; isolation by column chromatography (first CC: CH2Cl2/petroleum ether = 1:3; second CC: EtOAc/petroleum ether = 1:10). rac-17k Yield: 51.9 mg (0.079 mmol, 39%, two diastereomers in a ratio of 66:34 in CDCl3) of white solid; m.p. = 41.2–45.0 °C. EI-HRMS: m/z = 664.5894 (M-H+)-; C41H78NO5 requires: m/z = 664.5886 (M-H+)-; νmax 2916, 2848, 1726, 1703, 1556, 1545, 1466, 1370, 1256, 1210, 1157, 1047, 845, 720, 618 cm-1. 1H-NMR (500 MHz, CDCl3) for both diastereomers: δ 0.88 (t, J=7.0 Hz, 6H), 1.12 – 1.44 (m, 56H), 1.47 (s, 9H), 1.55 – 1.63 (m, 2H), 2.45 – 2.54 (m, 1H), 2.56 – 2.67 (m, 1H), 2.76 – 2.86 (m, 1H), 3.63 (d, J=6.9, 0.34H), 3.66 (d, J=7.5 Hz, 0.66H), 4.48 – 4.56 (m, 1H), 4.57 – 4.67 (m, 1H). 13C-NMR (126 MHz, CDCl3) for both diastereomers: δ 14.26, 22.84, 23.55, 23.60, 26.79, 26.88, 28.03, 28.07, 29.23, 29.49, 29.51, 29.54, 29.60, 29.67, 29.76, 29.81, 29.83, 29.85, 30.19, 32.08, 36.66, 36.77, 43.26, 43.47, 60.43, 60.98, 76.40, 76.77, 83.01, 83.07, 167.40, 167.43, 204.68, 204.83 (44 signals missing due to overlapping).
Synthesis of methyl (11Z,14Z)-2-((R)-2-nitro-1-phenylethyl)-3-oxoicosa-11,14-dienoate (17l)
Following GP3 and GP4. Prepared from methyl (11Z,14Z)-3-oxoicosa-11,14-dienoate (2g) (0.3 mmol, 101.0 mg) and trans-β-nitrostyrene (12) (0.2 mmol, 29.8 mg), organocatalyst VIII, 24 h; isolation by column chromatography (EtOAc/petroleum ether = 1:20). rac-17l Yield: 58 mg (0.120 mmol, 60%, two diastereomers in a ratio of 52:48 in CDCl3) of colorless oil. 17l Yield: 60.2 mg (0.124 mmol, 62%, two diastereomers in a ratio of 51:49 in CDCl3) of colorless oil. EI-HRMS: m/z = 508.3032 (MNa+); C29H43NO5Na requires: m/z = 508.3032 (MNa+); νmax 3009, 2925, 2855, 1744, 1717, 1554, 1496, 1455, 1434, 1377, 1243, 1168, 981, 914, 765, 699 cm-1. 1H-NMR (600 MHz, CDCl3) for both diastereomers: δ 0.89 (td, J=1.5, 7.0 Hz, 3H), 0.95 – 1.05 (m, 1H), 1.10 – 1.16 (m, 1H), 1.16 – 1.21 (m, 1H), 1.23 – 1.40 (m, 12H), 1.52 – 1.60 (m, 1H), 1.97 – 2.07 (m, 4H), 2.13 (dt, J=7.2, 17.8 Hz, 0.5H), 2.39 – 2.49 (m, 1H), 2.61 (dt, J=7.4, 17.7 Hz, 0.5H), 2.77 (q, J=6.5 Hz, 2H), 3.52 (s, 1.5H), 3.76 (s, 1.5H), 4.03 (d, J=10.0 Hz, 0.5H), 4.13 (d, J=9.4 Hz, 0.5H), 4.19 – 4.27 (m, 1H), 4.74 – 4.88 (m, 2H), 5.28 – 5.43 (m, 4H), 7.17 – 7.21 (m, 2H), 7.22 – 7.34 (m, 3H). 13C-NMR (151 MHz, CDCl3) for both diastereomers: δ 14.22, 22.71, 23.03, 23.37, 25.76, 27.30, 27.31, 27.34, 28.70, 28.97, 29.13, 29.20, 29.24, 29.36, 29.48, 29.67, 29.72, 31.66, 42.53, 42.87, 43.48, 43.83, 52.87, 53.05, 60.98, 61.35, 77.67, 77.93, 127.94, 128.00, 128.02, 128.12, 128.20, 128.23, 128.43, 128.51, 129.16, 129.26, 130.13, 130.38, 136.45, 136.64, 168.14, 202.70, 203.76 (9 signals missing due to overlapping). HPLC: Chiralpak IA-3, n-Hexane/EtOH = 95:5, flow rate 1.0 mL/min, λ = 210 nm, T = 25 °C. Minor diastereomer: enantiomers: tR = 7.439 minutes (minor); 19.360 minutes (major) – 96% ee. Major diastereomer: enantiomers: tR = 9.402 minutes (major); 12.992 minutes (minor) – 96% ee.
Synthesis of methyl (4S)-7-((tert-butoxycarbonyl)amino)-2-(2-nitro-1-phenylethyl)-3-oxo-4-stearamidoheptanoate (17m)
Following GP3. Prepared from methyl (S)-7-((tert-butoxycarbonyl)amino)-3-oxo-4-stearamidoheptanoate (2h) (0.2 mmol, 111.0 mg) and trans-β-nitrostyrene (12) (0.3 mmol, 44.7 mg), organocatalyst X, 48 h; isolation by column chromatography (1. EtOAc/petroleum ether = 1:2; 1. EtOAc/petroleum ether = 1:1). 17m Yield: 100.0 mg (0.142 mmol, 71%, 4 diastereomers in a ratio of 27:21:24:28 in CDCl3) of white semisolid. EI-HRMS: m/z = 704.4827 (MH+); C39H66N3O8 requires: m/z = 704.4844 (MH+); νmax 3375, 2920, 2851, 1739, 1720, 1687, 1648, 1551, 1518, 1455, 1436, 1365, 1247, 1214, 1168, 1089, 1005, 872, 764, 720, 701, 617 cm-1. 1H-NMR (500 MHz, CDCl3) for 4 diastereomers: δ 0.88 (t, J=6.9, 3H), 1.05 – 1.39 (m, 29H), 1.42 – 1.45 (m, 9H), 1.46 – 1.68 (m, 4H), 1.71 – 1.86 (m, 1H), 2.02 – 2.28 (m, 2H), 2.77 – 3.22 (m, 2H), 3.48, 3.49, 3.74, 3.77 (4 × s, 3H), 4.22 – 4.96 (m, 6H), 5.83 – 6.68 (m, 1H), 7.18 – 7.35 (m, 5H). 13C-NMR (126 MHz, CDCl3) for 4 diastereomers: δ 14.24, 22.80, 25.55, 25.62, 25.67, 25.90, 26.01, 26.20, 26.30, 26.90, 26.95, 27.05, 27.20, 28.48, 28.49, 29.38, 29.40, 29.43, 29.45, 29.47, 29.60, 29.61, 29.63, 29.73, 29.75, 29.77, 29.81, 32.03, 36.04, 36.40, 36.44, 36.50, 39.56, 39.71, 39.75, 42.54, 42.63, 43.10, 43.25, 52.84, 53.02, 53.11, 53.36, 57.89, 58.13, 58.32, 58.44, 58.62, 58.97, 59.18, 77.27, 77.36, 77.58, 77.70, 79.48, 79.52, 79.67, 79.70, 128.09, 128.14, 128.27, 128.36, 128.42, 128.50, 129.04, 129.18, 129.21, 136.21, 136.27, 136.50, 136.55, 156.26, 156.41, 156.55, 156.59, 166.95, 167.15, 167.30, 167.66, 173.10, 173.47, 173.85, 174.11, 200.93, 201.85, 202.18, 202.94 (69 signals missing due to overlapping).
Synthesis of methyl 2-((S)-5-((tert-butoxycarbonyl)amino)-2-stearamidopentanoyl)-3-(nitromethyl)octadecanoate (17n)
Following GP3 and GP4. Prepared from methyl (S)-7-((tert-butoxycarbonyl)amino)-3-oxo-4-stearamidoheptanoate (2h) (0.2 mmol, 111.0 mg) and (E)-1-nitroheptadec-1-ene (16) (0.3 mmol, 85.0 mg), organocatalyst X, 48 h; isolation by column chromatography (1. EtOAc/petroleum ether = 1:3; 1. EtOAc/petroleum ether = 1:2); 17n Yield: 129.1 mg (0.154 mmol, 77%, 4 diastereomers in a ratio of 26:28:25:21 in CDCl3) of white solid. Organocatalyst VIII, 48 h; isolation by column chromatography (1. EtOAc/petroleum ether = 1:3; 1. EtOAc/petroleum ether = 1:2); 17n Yield: 147.5 mg (0.176 mmol, 88%, 4 diastereomers in a ratio of 45:11:8:36 in CDCl3) of white solid. EI-HRMS: m/z = 838.6860 (MH+); C48H92N3O8 requires: m/z = 838.6879 (MH+); νmax 3361, 2917, 2850, 1741, 1718, 1687, 1644, 1553, 1524, 1467, 1366, 1250, 1222, 1171, 1039, 1011, 869, 721, 646 cm-1. 1H-NMR (500 MHz, CDCl3) for 4 diastereomers: δ 0.88 (t, J=6.9 Hz, 6H), 1.10 – 1.39 (m, 57H), 1.44 (s, 9H), 1.48 – 1.58 (m, 2H), 1.58 – 1.67 (m, 2H), 1.87 – 1.99 (m, 1H), 2.20 – 2.27 (m, 2H), 2.80 – 2.93 (m, 1H), 3.05 – 3.24 (m, 2H), 3.73, 3.74, 3.76, 3.77 (4 × s, 3H), 3.99 (d, J=6.3 Hz, 0.206H), 4.04 (d, J=6.9 Hz, 0.255H), 4.09 (d, J=5.2 Hz, 0.284H), 4.15 (d, J=7.0 Hz, 0.255H), 4.40 – 4.80 (m, 4H), 6.42 – 6.58 (m, 1H). 13C-NMR (126 MHz, CDCl3) for 4 diastereomers: δ 14.23, 22.80, 25.67, 25.70, 26.36, 26.74, 26.77, 26.83, 26.87, 27.08, 27.43, 28.48, 29.42, 29.44, 29.46, 29.48, 29.64, 29.66, 29.72, 29.73, 29.75, 29.78, 29.82, 30.43, 30.52, 32.04, 36.37, 36.43, 36.53, 36.60, 37.22, 37.42, 39.76, 39.79, 39.82, 39.83, 52.77, 52.92, 53.00, 53.15, 55.92, 56.15, 56.47, 58.05, 58.26, 58.39, 58.62, 76.25, 76.43, 76.48, 77.36, 79.56, 156.45, 168.14, 168.38, 168.43, 168.46, 173.58, 173.64, 173.71, 173.80, 203.24, 203.40, 203.67 (128 signals missing due to overlapping).
Synthesis of methyl (4S)-2-(2-nitro-1-phenylethyl)-3-oxo-4,7-distearamidoheptanoate (17o)
Following GP3. Prepared from methyl (S)-3-oxo-4,7-distearamidoheptanoate (2i) (0.2 mmol, 144.1 mg) and trans-β-nitrostyrene (12) (0.3 mmol, 44.7 mg), organocatalyst X, 48 h; isolation by column chromatography (first column chromatography: EtOAc/petroleum ether = 1:1; second column chromatography: CH2Cl2/MeOH = 75:1). 17o Yield: 50.5 mg (0.058 mmol, 29%, 4 diastereomers in a ratio of 24:22:30:24 in CDCl3) of light orange solid. EI-HRMS: m/z = 870.6936 (MH+); C52H92N3O7 requires: m/z = 870.6930 (MH+); νmax 3292, 2916, 2848, 1745, 1720, 1638, 1552, 1462, 1434, 1377, 1274, 1240, 1221, 1205, 1168, 1114, 755, 719, 700 cm-1. 1H-NMR (500 MHz, CDCl3) for 4 diastereomers: δ 0.88 (t, J=6.9 Hz, 6H), 0.99 – 1.42 (m, 56H), 1.42 – 1.68 (m, 6H), 1.69 – 1.86 (m, 1H), 2.05 – 2.29 (m, 4H), 2.91 – 3.35 (m, 3H), 3.47, 3.48, 3.74, 3.77 (4 × s, 3H), 4.22 – 4.65 (m, 3H), 4.72 – 4.95 (m, 2H), 5.57 – 5.85 (m, 1H), 6.14 (d, J=7.9 Hz, 0.244H), 6.56 (d, J=7.9 Hz, 0.296H), 6.85 (br s, 0.225H), 6.93 (d, J=7.1 Hz, 0.235H), 7.18 – 7.34 (m, 5H). 13C-NMR (126 MHz, CDCl3) for 4 diastereomers: δ 14.24, 22.81, 25.55, 25.60, 25.63, 25.67, 25.77, 25.91, 26.08, 26.27, 26.57, 26.77, 27.03, 27.27, 29.41, 29.43, 29.48, 29.50, 29.64, 29.66, 29.78, 29.80, 29.83, 32.04, 36.02, 36.38, 36.46, 36.89, 36.91, 38.52, 38.61, 38.68, 38.71, 42.54, 42.64, 43.09, 43.31, 52.83, 52.99, 53.14, 53.32, 58.08, 58.17, 58.19, 58.25, 58.27, 58.82, 58.95, 59.10, 77.29, 77.36, 77.56, 77.65, 77.69, 128.11, 128.16, 128.26, 128.36, 128.42, 128.49, 129.03, 129.17, 136.21, 136.33, 136.52, 136.60, 167.03, 167.21, 167.40, 167.69, 173.31, 173.74, 173.80, 174.07, 174.08, 174.21, 201.14, 201.89, 202.14, 203.10 (128 signals missing due to overlapping).
Synthesis of methyl 2-((S)-2,5-distearamidopentanoyl)-3-(nitromethyl)octadecanoate (17p)
Following GP3 and GP4. Prepared from methyl (S)-3-oxo-4,7-distearamidoheptanoate (2i) (0.2 mmol, 144.1 mg) and (E)-1-nitroheptadec-1-ene (16) (0.3 mmol, 85.0 mg), organocatalyst X, 48 h; isolation by column chromatography (1. EtOAc/petroleum ether = 1:3; 1. EtOAc/petroleum ether = 1:1); 17p Yield: 50.2 mg (0.05 mmol, 25%, 4 diastereomers in a ratio of 25:25:27:23 in CDCl3) of light orange solid. Organocatalyst VIII, 48 h; isolation by column chromatography (1. EtOAc/petroleum ether = 1:3; 1. EtOAc/petroleum ether = 1:1); 17p Yield: 46.2 mg (0.046 mmol, 23%, 4 diastereomers in a ratio of 39:11:12:38 in CDCl3) of light orange solid. EI-HRMS: m/z = 1004.8949 (MH+); C61H118N3O7 requires: m/z = 1004.8964 (MH+); νmax 3293, 2916, 2849, 1746, 1721, 1639, 1552, 1463, 1378, 1274, 1257, 1240, 1222, 1204, 719, 615 cm-1. 1H-NMR (500 MHz, CDCl3) for 4 diastereomers: δ 0.88 (t, J=6.9, 9H), 1.07 – 1.46 (m, 88H), 1.48 – 1.70 (m, 7H), 1.83 – 1.98 (m, 1H), 2.14 – 2.21 (m, 2H), 2.21 – 2.30 (m, 2H), 2.81 – 2.92 (m, 1H), 3.17 – 3.28 (m, 1H), 3.28 – 3.40 (m, 1H), 3.73, 3.74, 3.76, 3.77 (4 × s, 3H), 3.98 (d, J=5.9 Hz, 0.235H), 4.03 (d, J=7.0 Hz, 0.270H), 4.08 (d, J=5.2 Hz, 0.249H), 4.14 (d, J=7.0 Hz, 0.246H), 4.37 – 4.78 (m, 3H), 5.79 – 5.89 (m, 1H), [6.72 (d, J=7.6 Hz), 6.76 (d, J=7.7 Hz), 6.77 (d, J=7.8 Hz), 6.89 (d, J=7.6 Hz); 1H]. 13C-NMR (126 MHz, CDCl3) for 4 diastereomers: δ 22.81, 25.65, 25.68, 25.69, 25.91, 25.93, 26.35, 26.41, 26.45, 26.49, 26.75, 26.79, 26.85, 26.89, 27.11, 27.40, 27.57, 29.44, 29.45, 29.49, 29.52, 29.57, 29.66, 29.67, 29.69, 29.71, 29.74, 29.76, 29.79, 29.81, 29.83, 30.44, 30.59, 32.05, 36.34, 36.39, 36.46, 36.48, 36.56, 36.92, 37.19, 37.40, 38.78, 38.81, 52.80, 52.94, 52.97, 53.16, 55.83, 55.89, 56.43, 56.55, 58.28, 58.41, 58.46, 58.56, 76.25, 76.50, 76.58, 77.36, 168.25, 168.36, 168.52, 173.80, 173.90, 173.94, 173.95, 174.02, 203.12, 203.63, 203.70 (173 signals missing due to overlapping).
Organocatalyzed Michael addition of pyrrolones 11 to nitroalkene 16 – General procedure for the preparation of racemic mixtures – General procedure 5 (GP5)
To a solution/suspension of (E)-1-nitroheptadec-1-ene (16) (0.2 mmol, 56.7 mg; 1.0 equivalent) and the achiral organocatalyst X (0.04 mmol, 0.2 equivalents, 16.4 mg) in anhydrous toluene (1 mL) under argon at room temperature, pyrrolone 11 (0.3 mmol, 1.5 equivalents) was added and the resulting reaction mixture was stirred at room temperature for 48–72 hours. The volatiles were evaporated in vacuo and the residue was purified by column chromatography (Silica gel 60, mobile phase). The fractions containing the pure racemic product rac-18 were combined and the volatiles were evaporated in vacuo. The product rac-18 was fully characterized and analyzed by HPLC.
Organocatalyzed Michael addition of pyrrolones 11 to nitroalkene 16 – General procedure for the organocatalyzed asymmetric addition – General procedure 6 (GP6)
To a solution/suspension of (E)-1-nitroheptadec-1-ene (16) (0.2 mmol, 56.7 mg; 1.0 equivalent) and the chiral organocatalyst I (0.02 mmol, 0.1 equivalents, 10.9 mg) in anhydrous toluene (1 mL) under argon at room temperature, pyrrolone 11 (0.3 mmol, 1.5 equivalents) was added and the resulting reaction mixture was stirred at room temperature for 48–72 hours. The volatiles were evaporated in vacuo and the residue was purified by column chromatography (Silica gel 60, mobile phase). The fractions containing the pure chiral nonracemic product 18 were combined the volatiles were evaporated in vacuo. The product 18 was fully characterized and analyzed by HPLC.
Synthesis of 1-(tert-butyl) 3-methyl (S)-5-benzyl-5-((S)-1-nitroheptadecan-2-yl)-4-oxo-4,5-dihydro-1H-pyrrole-1,3-dicarboxylate (18a)
Following GP5 and GP6. Prepared from 1-(tert-butyl) 3-methyl 5-benzyl-4-oxo-4,5-dihydro-1H-pyrrole-1,3-dicarboxylate (11a) (0.3 mmol, 99.4 mg) and (E)-1-nitroheptadec-1-ene (16) (0.2 mmol, 56.7 mg), organocatalyst I, 48 h; isolation by column chromatography (EtOAc/petroleum ether = 1:5). rac-18a Yield: 94.7 mg (0.154 mmol, 77%, diastereomer 1/diastereomer 2 = 97:3 in CDCl3) of colorless oil. 18a Yield: 84.8 mg (0.138 mmol, 69%, diastereomer 1/diastereomer 2 = 97:3 in CDCl3) of colorless oil. EI-HRMS: m/z = 615.3970 (MH+); C35H55N2O7 requires: m/z = 615.4004 (MH+); νmax 2923, 2853, 1732, 1713, 1581, 1554, 1497, 1456, 1438, 1370, 1295, 1224, 1143, 1089, 1063, 992, 914, 876, 843, 760, 725, 703, 628 cm-1. 1H-NMR (500 MHz, CDCl3) for diastereomer 1: δ 0.88 (t, J=6.9 Hz, 3H), 1.07 – 1.43 (m, 26H), 1.44 – 1.85 (m, 11H), 3.26 – 3.54 (m, 3H), 3.77 (s, 3H), 4.31 (dd, J=5.1, 13.7 Hz, 1H), 4.51 (br s, 1H), 6.95 – 7.03 (m, 2H), 7.11 – 7.21 (m, 3H), 8.60 (br s, 1H). 1H-NMR (500 MHz, CDCl3) for diastereomer 2: δ 3.71 (s, 3H). 13C-NMR (126 MHz, CDCl3) for diastereomer 1: δ 14.26, 22.83, 27.55, 27.74, 28.10, 29.50, 29.52, 29.61, 29.63, 29.73, 29.78, 29.79, 29.82, 29.83, 32.06, 39.94, 43.20, 51.92, 76.56, 85.81, 112.39, 127.69, 128.43, 129.63, 133.25, 147.60, 161.78, 164.91, 196.43 (2 signals missing due to overlapping). HPLC: Chiralpak IA-3, n-Hexane/iPrOH = 95:5, flow rate 1.0 mL/min, λ = 210 nm, T = 25 °C. Major diastereomer: enantiomers: tR = 9.350 minutes (major); 13.217 minutes (minor) – 82% ee.
Synthesis of 1-(tert-butyl) 3-methyl 5-(4-(((benzyloxy)carbonyl)amino)butyl)-5-(1-nitroheptadecan-2-yl)-4-oxo-4,5-dihydro-1H-pyrrole-1,3-dicarboxylate (18b)
Following GP5 and GP6. Prepared from 1-(tert-butyl) 3-methyl 5-(4-(((benzyloxy)carbonyl)amino)butyl)-4-oxo-4,5-dihydro-1H-pyrrole-1,3-dicarboxylate (11b) (0.3 mmol, 134.0 mg) and (E)-1-nitroheptadec-1-ene (16) (0.2 mmol, 56.7 mg), organocatalyst I, 72 h; isolation by column chromatography (EtOAc/petroleum ether = 1:2). rac-18b Yield: 94.9 mg (0.130 mmol, 65%, diastereomer 1/diastereomer 2 = 90:10 in CDCl3) of colorless oil. 18b Yield: 26.3 mg (0.036 mmol, 18%, diastereomer 1/diastereomer 2 = 26:74 in CDCl3) of colorless oil. EI-HRMS: m/z = 730.4644 (MH+); C40H64N3O9 requires: m/z = 730.4637 (MH+); νmax 3675, 3369, 2923, 2854, 1711, 1582, 1553, 1455, 1438, 1394, 1371, 1280, 1226, 1140, 1067, 846, 763, 697 cm-1. 1H-NMR (500 MHz, CDCl3) for diastereomer 1: δ 0.88 (t, J=6.9 Hz, 3H), 0.91 – 1.03 (m, 2H), 1.06 – 1.34 (m, 27H), 1.35 – 1.49 (m, 4H), 1.56 (s, 9H), 1.96 – 2.07 (m, 1H), 2.10 – 2.22 (m, 1H), 3.03 – 3.17 (m, 3H), 3.84 (s, 3H), 4.22 (dd, J=5.3, 13.8 Hz, 1H), 4.30 – 4.41 (m, 1H), 4.71 (t, J=6.2 Hz, 1H), 5.06 (s, 2H), 7.27 – 7.39 (m, 5H). 1H-NMR (500 MHz, CDCl3) for diastereomer 2: δ 1.58 (s, 9H), 1.87 – 1.96 (m, 1H), 3.28 – 3.36 (m, 1H), 3.83 (s, 3H), 4.28 (dd, J=5.3, 14.6 Hz, 1H), 5.18 (dd, J=5.5, 14.7 Hz, 1H), 9.19 (s, 1H). 13C-NMR (126 MHz, CDCl3) for diastereomer 1: δ 14.26, 20.18, 22.82, 27.41, 27.49, 28.02, 29.49, 29.55, 29.62, 29.71, 29.77, 29.78, 29.81, 29.82, 32.05, 33.87, 40.61, 43.36, 52.03, 66.78, 76.35, 77.36, 86.22, 112.22, 128.24, 128.64, 136.59, 147.42, 156.40, 161.95, 165.24, 196.41 (4 signals missing due to overlapping). 13C-NMR (126 MHz, CDCl3) for diastereomer 1 and diastereomer 2 (diastereomer 1/diastereomer 2 = 26:74): δ 14.26, 20.19, 20.45, 22.82, 27.18, 27.50, 27.91, 28.03, 28.12, 29.44, 29.49, 29.50, 29.56, 29.63, 29.67, 29.72, 29.75, 29.79, 29.82, 29.83, 32.05, 33.89, 40.66, 41.98, 43.37, 52.03, 52.05, 66.79, 74.38, 76.11, 76.36, 77.40, 86.21, 112.23, 112.31, 128.25, 128.65, 136.59, 147.43, 156.39, 161.92, 165.29, 196.41, 197.67 (28 signals missing due to overlapping). HPLC: Chiralpak IA-3, n-Hexane/iPrOH = 90:10, flow rate 1.0 mL/min, λ = 210 nm, T = 25 °C. Major diastereomer: enantiomers: tR = 24.088 minutes (minor); 31.095 minutes (major) – 57% ee. Minor diastereomer: enantiomers: tR = 26.634 minutes (minor); 30.099 minutes (major) – 11% ee.
Synthesis of 1-(tert-butyl) 3-methyl 5-(3-(benzyloxy)-3-oxopropyl)-5-(1-nitroheptadecan-2-yl)-4-oxo-4,5-dihydro-1H-pyrrole-1,3-dicarboxylate (18c)
Following GP5 and GP6. Prepared from 1-(tert-butyl) 3-methyl 5-(3-(benzyloxy)-3-oxopropyl)-4-oxo-4,5-dihydro-1H-pyrrole-1,3-dicarboxylate (11c) (0.3 mmol, 121.0 mg) and (E)-1-nitroheptadec-1-ene (16) (0.2 mmol, 56.7 mg), organocatalyst I, 48 h; isolation by column chromatography (EtOAc/petroleum ether = 1:5). rac-18c Yield: 92.0 mg (0.134 mmol, 67%, diastereomer 1/diastereomer 2 = 93:7 in CDCl3) of colorless oil. 18c Yield: 42.6 mg (0.062 mmol, 31%, diastereomer 1/diastereomer 2 = 31:69 in CDCl3) of colorless oil. EI-HRMS: m/z = 687.4224 (MH+); C38H59N2O9 requires: m/z = 687.4215 (MH+); νmax 3675, 2923, 2854, 1735, 1713, 1582, 1554, 1439, 1393, 1371, 1279, 1256, 1227, 1141, 1077, 846, 800, 752, 698 cm-1. 1H-NMR (600 MHz, CDCl3) for diastereomer 1: δ 0.88 (t, J=6.9 Hz, 3H), 1.17 – 1.34 (m, 27H), 1.35 – 1.46 (m, 1H), 1.56 (s, 9H), 1.98 – 2.06 (m, 1H), 2.09 – 2.17 (m, 1H), 2.37 – 2.45 (m, 1H), 2.48 – 2.55 (m, 1H), 3.13 – 3.20 (m, 1H), 3.83 (s, 3H), 4.25 (dd, J=5.6, 13.8 Hz, 1H), 4.30 – 4.39 (m, 1H), 5.07 (s, 2H), 7.28 – 7.39 (m, 5H), 9.08 (s, 1H). 1H-NMR (500 MHz, CDCl3) for diastereomer 2: δ 1.58 (s, 9H), 2.26 – 2.33 (m, 1H), 2.54 – 2.62 (m, 1H), 3.35 – 3.41 (m, 1H), 3.82 (s, 3H), 5.06 (s, 2H), 5.19 (dd, J=5.1, 14.7 Hz, 1H), 9.17 (s, 1H). 13C-NMR (151 MHz, CDCl3) for diastereomer 1: δ 14.26, 22.83, 27.48, 27.60, 28.01, 29.13, 29.49, 29.50, 29.54, 29.62, 29.72, 29.77, 29.79, 29.82, 29.84, 32.06, 43.15, 52.06, 66.88, 75.92, 76.42, 86.62, 112.16, 128.44, 128.47, 128.56, 128.75, 135.56, 147.29, 161.76, 165.19, 171.48, 195.61 (1 signal missing due to overlapping). 13C-NMR (126 MHz, CDCl3) for diastereomer 1 and diastereomer 2 (diastereomer 1/diastereomer 2 = 31:69): δ 14.28, 22.84, 27.17, 27.49, 28.02, 28.07, 28.11, 28.37, 29.28, 29.44, 29.50, 29.56, 29.64, 29.68, 29.73, 29.76, 29.80, 29.83, 29.85, 32.07, 41.85, 43.16, 52.06, 52.08, 66.89, 66.93, 74.46, 75.26, 75.93, 76.42, 77.39, 86.67, 112.15, 112.36, 128.45, 128.48, 128.58, 128.77, 135.51, 135.56, 147.29, 161.72, 161.77, 165.20, 165.34, 171.18, 171.49, 195.63, 196.88 (19 signal missing due to overlapping). HPLC: Chiralpak IA-3, n-Hexane/iPrOH = 90:10, flow rate 1.0 mL/min, λ = 280 nm, T = 25 °C. Major diastereomer: enantiomers: tR = 10.649 minutes (major); 12.074 minutes (minor) – 68% ee. Minor diastereomer: enantiomers: tR = 9.991 minutes (minor); 12.514 minutes (major) – 38% ee.
Synthesis of 4-hydroxy-3-(1-nitroheptadecan-2-yl)furan-2(5H)-one (20)
To a solution of (E)-1-nitroheptadec-1-ene (16) (0.2 mmol, 56.7 mg) and the achiral organocatalyst X (0.04 mmol, 0.2 equivalents, 16.4 mg) in anhydrous CH2Cl2 (1 mL) under argon at room temperature, tetronic acid (19) (0.3 mmol, 30.0 mg) was added and the resulting reaction mixture was stirred at room temperature for 72 hours. The volatiles were evaporated in vacuo and the residue was purified by column chromatography (Silica gel 60, EtOAc/petroleum ether = 1:1). The fractions containing the pure product 20 were combined and the volatiles were evaporated in vacuo. Yield: 33.8 mg (0.088 mmol, 44%) of white solid; m.p. = 86.8-88.2 °C. EI-HRMS: m/z = 384.2741 (MH+); C21H38NO5 requires: m/z = 384.2744 (MH+); νmax 2918, 2850, 1714, 1611, 1547, 1428, 1381, 1348, 1279, 1260, 1129, 1097, 1044, 971, 954, 780, 720, 682, 658 cm-1. 1H-NMR (500 MHz, CDCl3): δ 0.88 (t, J=6.9 Hz, 3H), 1.09 – 1.35 (m, 26H), 1.50 – 1.60 (m, 1H), 1.65 – 1.77 (m, 1H), 3.37 (tt, J=5.6, 9.6 Hz, 1H), 4.53 (dd, J=5.6, 12.4 Hz, 1H), 4.71 – 4.81 (m, 3H), 10.97 (br s, 1H). 13C-NMR (126 MHz, CDCl3): δ 14.27, 22.84, 27.28, 29.44, 29.51, 29.62, 29.76, 29.79, 29.81, 29.84, 29.86, 29.99, 32.07, 32.87, 68.14, 76.88, 99.10, 177.35, 177.91 (2 signals missing due to overlapping).
X-ray Crystallography. Single-crystal X-ray diffraction data was collected on Agilent Technologies SuperNova Dual diffractometer with an Atlas detector using monochromated Mo-K
α radiation (λ = 0.71073 Å) at 150 K. The data was processed using CrysAlis PRO [
67]. Using Olex2.1.2. [
68], the structure was solved by direct methods implemented in SHELXS [
69] or SHELXT [
70] and refined by a full-matrix least-squares procedure based on F
2 with SHELXT-2014/7 [
71]. All nonhydrogen atoms were refined anisotropicallly. Hydrogen atoms were placed in geometrically calculated positions and were refined using a riding model. The drawing and the analysis of bond lengths, angles and intermolecular interactions were carried out using Mercury [
72] and Platon [
73]. Structural and other crystallographic details on data collection and refinement for compound
17a have been deposited with the Cambridge Crystallographic Data Centre as supplementary publication number CCDC Deposition Number 2513139. These data can be obtained free of charge via
www.ccdc.cam.ac.uk/conts/retrieving.html (or from the CCDC, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336033; e-mail: deposit@ccdc.cam.ac.uk).