Submitted:
17 December 2025
Posted:
19 December 2025
You are already at the latest version
Abstract
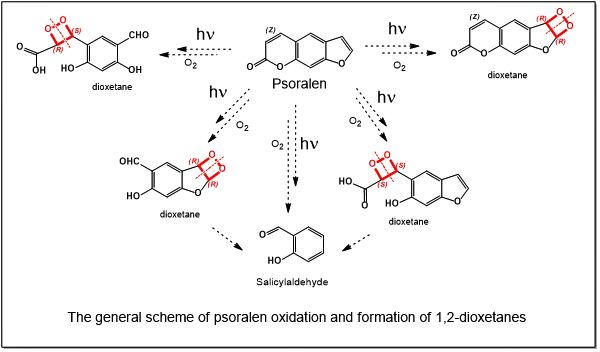
Sosnovsky’s hogweed (Heracleum sosnowskyi Manden.) is an invasive plant species widely distributed across Eastern Europe and Russia that poses a serious threat to human health due to its pronounced phototoxic properties. Contact with the plant sap, followed by exposure to ultraviolet radiation, frequently results in phytophotodermatitis characterized by erythema, blistering, ulceration, and long-lasting hyperpigmentation. The photochemical injuries are primarily attributed to highly oxygenated secondary metabolites, notably furanocoumarins, which act as potent photosensitizers and induce cellular and DNA damage upon UV activation. This review provides a comprehensive overview of the botanical distribution and invasiveness of H. sosnowskyi, the chemical composition of its biologically active metabolites, and the molecular mechanisms underlying hogweed-induced skin injuries. Particular emphasis is placed on the photochemical transformations of furanocoumarins, including psoralens and their photooxidation products, such as 1,2-dioxetanes, which generate reactive oxygen species and DNA crosslinks. In addition, the review discusses other compounds derived from hogweed biomass, including furan derivatives, aromatic compounds, fatty acids, sterols, and their oxidative products, which may contribute to phototoxic and cytotoxic effects. Clinical manifestations of hogweed burns, their classification, symptomatology, and current therapeutic approaches are critically analyzed, highlighting the lack of standardized treatment guidelines. By integrating chemical, biological, and clinical data, this review aims to elucidate the mechanisms of photochemical skin injury caused by H. sosnowskyi and to support the development of more effective preventive and therapeutic strategies.
Keywords:
1. Introduction

2. Furanocoumarins Produced by Hogweed
3. Photochemical Burns Caused by Hogweed Juice
4. Furan, Aromatic, and Other Compounds Derived from H. sosnowskyi Biomass
5. Aromatic, Lipid, and Oxidation-Derived Compounds from H. sosnowskyi

| No. | Fatty Acids | Roots | Stems | Leaves | Flowers |
|---|---|---|---|---|---|
| 62 | 8:0 | 0.7 | 15.0 | 0.1 | 0.7 |
| 63 | Succinic acid | 0.3 | 3.3 | 1.6 | 0.3 |
| 64 | 12:0 | 0.4 | 1.7 | 4.1 | 1.5 |
| 65 | 14:0 | 1.6 | 19.8 | 33.1 | 17.2 |
| 66 | Adipic acid | 1.2 | 14.5 | 5.8 | 1.5 |
| 67 | 9-oxo-9:0 | 1.4. | 23.3 | 3.3 | 0.7 |
| 68 | 15:0 | 2.8 | 18.8 | 7.9 | 2.4 |
| 69 | Azelaic acid | 1.6 | 10.2 | 4.8 | 1.2 |
| 70 | 16:0 | 60.1 | 884.1 | 615.8 | 173.3 |
| 71 | Iso-17:0 | 0.1 | 0.5 | 0.1 | 0.1 |
| 72 | 17:0 | 1.5 | 15.1 | 16.9 | 3.7 |
| 73 | 18:0 | 5.3 | 45.4 | 84.8 | 18.8 |
| 74 | 22:0 | 2.8 | 23.6 | 24.4 | 6.6 |
| 75 | 23:0 | 1.2 | 17.0 | 13.4 | 3.3 |
| 76 | 24:0 | 5.2 | 59.4 | 61.9 | 13.6 |
| 77 | 25:0 | 11.2 | 5.6 | 39.9 | 0.9 |
| No. | Fatty Acids | Roots | Stems | Leaves | Flowers |
|---|---|---|---|---|---|
| 78 | 16:1n-11 (Δ5c) | 0.6 | 0.1 | 1.8 | 0.5 |
| 79 | 16:1n-9 (Δ7c) | 0.8 | 22.6 | 2.8 | 7.1 |
| 80 | 16:1n-7 (Δ9c) | 0.5 | 2.6 | 7.0 | 0.6 |
| 81 | 16:1n-5 (Δ11c) | 0.4 | 0.5 | 0.1 | 0.3 |
| 82 | 16:1n-3 (Δ12c) | 0.3 | 6.8 | 86.0 | 1.1 |
| 83 | 17:1n-7 (Δ10c) | 0.3 | 0.1 | 0.1 | 3.9 |
| 84 | 18:1n-9 (Δ9t) | 9.5 | 15.2 | 77.2 | 56.1 |
| 85 | 18:1n-7 (Δ11t) | 2.4 | 42.7 | 9.5 | 10.4 |
| 86 | 18:1n-6 (Δ12c) | 7.6 | 6.5 | 15.9 | 1.6 |
| 87 | 18:1n-3 (Δ14t) | 1.2 | 2.6 | 2.2 | 0.5 |
| 88 | 18:1n-2 (Δ15t) | 30.0 | 5.0 | 35.7 | 2.6 |
| 89 | 20:1n-6 (Δ14t) 20:1n-9 (Δ11t) |
0.5 | 28.3 | 3.2 | 6.8 |
| 90 | 20:1n-11 (Δ9c) | 0.1 | 0.1 | 0.1 | 0.1 |
| 91 | 20:1n-9 (Δ11c) | 0.7 | 2.8 | 11.0 | 7.1 |
| 92 | 20:1n-7 (Δ13c) | 0.1 | 1.9 | 1.4 | 5.6 |
| No. | Fatty Acids | Roots | Stems | Leaves | Flowers |
|---|---|---|---|---|---|
| 93 | 16:2n-6 (Δ7c,10c) | 0.2 | 3.9 | 5.4 | 0.9 |
| 94 | 18:2n-6 (Δ9c,12t) | 5.5 | 600.6 | 10.0 | 2.7 |
| 95 | 18:2n-6 (Δ9c,12c) | 185.0 | 0.1 | 856.1 | 230.5 |
| 96 | 18:2n-6 (Δ10t,12t)(oxo-9) | 0.1 | 4.2 | 0.1 | 0.1 |
| 97 | 18:2n-6 (Δ10t,12c) | 0.1 | 0.1 | 0.1 | 0.1 |
| 98 | 16:3n-3 (Δ7c,10c,13c) | 0.9 | 55.0 | 633.2 | 6.3 |
| 99 | 18:3n-3 (Δ9c,12c,15c) | 23.2 | 71.4 | 1687.4 | 135.5 |
6. Hogweed Burns and Their Possible Treatment
6.1. Classification of Burns Caused by Hogweed
6.2. Symptoms of Hogweed Burn
6.3. Treatment for Hogweed Burns
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Grzędzicka, E. Invasion of the giant hogweed and the Sosnowsky’s hogweed as a multidisciplinary problem with unknown future—A review. Earth 2022, 3, 287–312. [Google Scholar] [CrossRef]
- Matarrese, E.; Renna, M. Prospects of hogweed (Heracleum sphondylium L.) as a new horticultural crop for food and non-food uses: A review. Horticulturae 2023, 9, 246. [Google Scholar] [CrossRef]
- Baker, B.G.; Bedford, J.; Kanitkar, S. Keeping pace with the media; Giant Hogweed burns—A case series and comprehensive review. Burns 2017, 43(5), 933–938. [Google Scholar] [CrossRef] [PubMed]
- Bahadori, M.B.; Dinparast, L.; Zengin, G. The genus Heracleum: A comprehensive review on its phytochemistry, pharmacology, and ethnobotanical values as a useful herb. Comprehen. Rev. Food Sci. Food Safety 2016, 15(6), 1018–1039. [Google Scholar] [CrossRef]
- Annenkov, N.I. Heracleum. Botanical Dictionary; Type. Imp. Academy of Science: St. Petersburg, 1878; p. XXI + 645 p. [Google Scholar]
- Liu, M.; Downie, S.R. The phylogenetic significance of fruit anatomical and micromorphological structures in Chinese Heracleum species and related taxa (Apiaceae). Systematic Botany 2017, 42(2), 313–325. [Google Scholar] [CrossRef]
- Logacheva, M.; Valiejo-Roman, C.; Pimenov, M. ITS phylogeny of west Asian Heracleum species and related taxa of Umbelliferae–Tordylieae WDJ Koch, with notes on evolution of their psbA-trnH sequences. Plant Syst. Evol. 2008, 270, 139–157. [Google Scholar] [CrossRef]
- Yu, Y.; Downie, S.R.; He, X.; Deng, X.; Yan, L. Phylogeny and biogeography of Chinese Heracleum (Apiaceae tribe Tordylieae) with comments on their fruit morphology. Plant Syst. Evol. 2011, 296, 179–203. [Google Scholar] [CrossRef]
- Kozhevnikov, A.E.; Probatova, N.S.; Tsvelev, N.N. Heracleum lanatum Michx. Vascular plants of the Soviet Far East; Kharkevich, S.S., Ed.; Nauka, 1987; Volume 2, pp. 269–270. [Google Scholar]
- Berkutenko, A.N.; Virek, E.G. Woolly hogweed - Heracleum lanatum. In Medicinal and food plants of Alaska and the Russian Far East; Publishing House of the Far Eastern University: Vladivostok, 1995; pp. P. 105–106. [Google Scholar]
- Satsyperova, I.F. Hogweeds of the USSR flora – new forage plants: Prospects for use in the national economy; Nauka Publishin House: Leningrad, 1984; p. 114. [Google Scholar]
- Tkachenko, K.G. Sosnowsky’s hogweed (Heracleum sosnowskyi Manden). Recommendations and methods for combating it; Publishing House "First IPH": Saint Petersburg, 2021; p. 68. [Google Scholar]
- Taysumov, M.A.; Astamirova, M.M.; Umarov, R.M.; Abdurzakova, A.S.; Magomadova, R.S.; Israilova, S.A.; Khasueva, B.A. Forage plants of Chechnya and classification of natural forage lands. International scientific and practical conference" Agro-SMART-Smart solutions for agriculture"(Agro-SMART 2018), December 2018; Atlantis Press: Russia; pp. 952–957. [Google Scholar]
- Paramonova, K.; Chaloupková, V.; Ivanova, T.A. Invasive Heracleum sosnowskyi as a potential feedstock for biorefineries: A review. Industrial Crops and Prod. 2024, 216, 118754. [Google Scholar] [CrossRef]
- Sefidkon, F.; Dabiri, M.; Mohammad, N. Analysis of the oil of Heracleum persicum L. (leaves and flowers). J. Essential Oil Res. 2002, 14(4), 295–297. [Google Scholar] [CrossRef]
- Osipova, E.S.; Gladkov, E.A. Heracleum Sosnowskyi Manden. as a Source of Valuable Chemicals (Elimination with Utility). Chem. Methodol. 2024, 8(12), 944–956. [Google Scholar]
- Voznyakovsky, A.P.; Karmanov, A.P.; Neverovskaya, A.Y.; Voznyakovsky, A.A.; Kocheva, L.S.; Kidalov, S.V. Biomass of Heracleum sosnowskyi as a raw material for obtaining 2D carbon nanostructures. Chem. Plant Raw Material 2020, (4), 83–92. [Google Scholar] [CrossRef]
- Kulikov, O.A.; Shlyapkina, V.I.; Brodovskaya, E.P.; Aioub, A.M.A.K.; Ageev, V.P.; Zharkov, M.N.; Sukhorukov, G.B. Phototoxicity in vitro and safety in vivo of the emulsion photosensitizer based on furanocoumarins of Heracleum sosnowskyi. European J. Pharmac. Biopharm. 2024, 198, 114257. [Google Scholar] [CrossRef] [PubMed]
- Frumin, G.T. Toxicity of juice of Heracleum sosnowskyi. Russ. J. General Chem. 2023, 93(13), 3483–3487. [Google Scholar] [CrossRef]
- Bruni, R.; Barreca, D.; Protti, M.; Brighenti, V.; Righetti, L.; Anceschi, L.; Mercolini, L.; Benvenuti, S.; Gattuso, G.; Pellati, F. Botanical sources, chemistry, analysis, and biological activity of furanocoumarins of pharmaceutical interest. Molecules 2019, 24, 2163. [Google Scholar] [CrossRef]
- Sarker, S.D.; Nahar, L. Progress in the chemistry of naturally occurring coumarins. In Progress in the Chemistry of Organic Natural Products; Kinghorn, A.D., Ed.; Springer International Publishing, 2017; Volume 106, p. 241. [Google Scholar]
- Klimkina, E.A.; Okolelova, M.S.; Smirnova, E.S. Analysis of data on phytophotodermatitis caused by contact with the sap of plants of the genus Hogweed (Heracleum L.). Bull. Russ. Military Med. Acad. 2024, 43, 183–192. [Google Scholar] [CrossRef]
- Shtratnikova, V.Y. Furanocoumarins: History of research, diversity, synthesis, physiological role in the plant, and medical application. Russian J. Plant Physiol. 2023, 70(7), 169. [Google Scholar] [CrossRef]
- Schulzová, V.; Hajšlová, J.; Botek, P.; Peroutka, R. Furanocoumarins in vegetables: Influence of farming system and other factors on levels of toxicants. J. Sci. Food Agricul 2007, 87(15), 2763–2767. [Google Scholar] [CrossRef]
- Vickackaite, V.; Pilaityte, K.; Poskus, V. Extraction, isolation, and purification of furanocoumarins from invasive Heracleum sosnowskyi. Separations 2025, 12(7), 175. [Google Scholar] [CrossRef]
- Bespalov, D.S.; Egorov, D.M. Study of the sum of furanocoumarins of Heracleum sosnowskyi obtained by alkaline extraction method. Scientific creativity of youth to the forest complex of Russia. In Proceedings of the XXI All-Russian Scientific and Technical Conference of Undergraduate and Postgraduate Students, Ekaterinburg, Russia, USFEU, 2025; pp. 891–896. [Google Scholar]
- Poliyoeicz, J.; Gebarowska, E.; Prockow, J.; Pietr, S.J.; Szumny, A. Antimicrobial activity of essential oil and furanocoumarin fraction of three Heracleum species. Acta Poloniae Pharm. 2017, 74(2), 723–728. [Google Scholar]
- Tkachenko, K.G. Genus Hogweed (Heraclium L.) – economically useful plants. Bulletin of Udmurt University. Biol. Ser. Earth Sciences (Russia) 2014, (4), 27–33. [Google Scholar]
- Luneva, N.N. Sosnowsky’s hogweed in Russia: Current status and relevance of its prompt suppression. Bull. Plant Protection (Russia) 2013, (1), 29–43. [Google Scholar]
- Tkachenko, K.G.; Krasnov, A.A. Sosnowsky’s hogweed: An ecological problem or an agricultural crop of the future? Bull. Botanical Garden-Institute Far Eastern Branch Russian Acad. Sci. (Russia) 2018, (20), 1–22. [Google Scholar]
- Rogozhnikova, D.R.; Abramova, L.M. Some information on the biology of Sosnowsky’s hogweed in Bashkortostan. Izvestia Ufa Scientific Center (Russia) 2018, (3), 94–98. [Google Scholar]
- Simonova, A.Y.; Belova, M.V.; Ilyashenko, K.K.; Pidchenko, N.E.; Potskhveria, M.M.; Sachkov, A.V. Photochemical dermatitis due to contact with Sosnowsky’s hogweed juice. Sklifosovsky J. Emergency Medical Care (Russia) 2020, 9(4), 653–658. [Google Scholar] [CrossRef]
- Simonova, A.Y.; Belova, M.V.; Ilyashenko, K.K.; Pidchenko, N.E.; Potskhveriya, M.M.; Sachkov, A.V.; Ponomarev, I.N. Photochemical dermatitis due to contact with Sosnovsky hogweed. Sklifosovsky J. Emergency Medical Care (Russia) 2021, 9(4), 653–658. [Google Scholar] [CrossRef]
- Klepov, I.D. Vesicular dermatitis of the meadow plant hogweed. Bull. Dermatol. Venereol. (Russia) 1960, (3), 34. [Google Scholar]
- Vinokurov, G.I. On dermatitis caused by sweet hogweed plants. Russ. Military Medical J. 1965, (7), 34. [Google Scholar]
- Komissarenko, N.F.; Zoz, I.G.; Chernobai, V.T.; Kolesnikov, V.G. Coumarins of hogweed flowers and taxonomy. Biochemistry (Moscow) 1961, 26(6), 980–983. [Google Scholar]
- Bruno, R.; Barreca, D.; Protti, M. Botanical sources, chemistry, analysis, and biological activity of furanocoumarins of pharmaceutical interest. Molecules 2019, 24(11), 2163. [Google Scholar] [CrossRef]
- Ozek, G.; Yur, S.; Goger, F. Furanocoumarin content, antioxidant activity, and inhibitory potential of Heracleum verticillatum, Heracleum sibiricum, Heracleum angustisectum, and Heracleum ternatum extracts against enzymes involved in alzheimer’s disease and type II diabetes. Chem. Biodiver 2019, 16(4), 1–25. [Google Scholar] [CrossRef]
- Patocka, J.; Cupalova, K. Giant Hogweed and photodermatitis. Mil. Med. Sci. Lett. (Voj Zdrav Listy) (Chechia) 2017, 86(3), 135–138. [Google Scholar] [CrossRef]
- Adam, W. The chemistry of 1, 2-dioxetanes. Adv. Heterocyclic Chem. 1977, 21, 437–481. [Google Scholar]
- Dembitsky, V.M. Highly oxygenated cyclobutane ring in biomolecules: Insights into structure and activity. Oxygen 2024, 4(2), 181–235. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Vil, V.A. Medicinal chemistry of stable and unstable 1,2-dioxetanes: Origin, formation, and biological activities. Science of Synthesis 2019, 3, 333–381. [Google Scholar]
- Vil, V.; Terent’ev, A.O.; Al Quntar, A.A.A.; Gloriozova, T.A.; Savidov, N.; Dembitsky, V.M. Oxetane-containing metabolites: Origin, structures, and biological activities. Appl. Microbiol. Biotechnol. 2019, 103, 2449–2467. [Google Scholar] [CrossRef]
- Adam, W.; Andler, S.; Ballmaier, D.; Emmert, S.; Epe, B.; Grimm, G.; Stopper, H. Oxidative DNA damage induced by dioxetanes, photosensitizing ketones, and photo-fenton reagents. Risk and Progression Factors in Carcinogenesis 1997, (2), 21–34. [Google Scholar]
- Epe, B.; Müller, E.; Adam, W.; Saha-Möller, C.R. Photochemical DNA modifications induced by 1,2-dioxetanes. Chemico-Biological Interact. 1992, 85(2-3), 265–281. [Google Scholar] [CrossRef]
- Adam, W.; Beinhauer, A.; Mosandl, T.; Saha-Möller, C.; Vargas, F.; Epe, B.; Wild, D. Photobiological studies with dioxetanes in isolated DNA, bacteria, and mammalian cells. Environ. Health Perspectives 1990, 88, 89–97. [Google Scholar] [CrossRef]
- Nevezhin, E.V.; Vlasova, N.V.; Pyatnitskiy, I.A.; Lysenko, E.P.; Malakhov, M.V. Mini-Review: On the mechanism of erythrocyte hemolysis induced by photooxidized psoralen. Biochemistry (Moscow) 2015, 80(6), 763–768. [Google Scholar] [CrossRef]
- Logani, M.K.; Austin, W.A.; Shah, B.; Davies, R.E. Photooxidation of 8-MOP with singlet oxygen. Photochem. Photobiol. 1982, 35, 569–573. [Google Scholar] [CrossRef]
- Wasserman, H.H.; Berdahl, D.R. The photooxidation of 8-methoxypsoralen. Photochem. Photobiol. 1982, 35, 565–567. [Google Scholar] [CrossRef]
- Caffieri, S. Furocoumarin photolysis: Chemical and biological aspects. Photochem. Photobiol. Sci. 2002, 1, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Marley, K.A.; Larson, R.A.; Davenport, R. Redox mechanisms of furocoumarin phototoxicity. The Spectrum 1995, 8, 9–14. [Google Scholar]
- Dall’Acqua, F.; Magno, S.M.; Zambon, F.; Rodighiero, G. Kinetic analysis of the photoreaction (365 nm) between psoralen and DNA. Photochem. Photobiol. 1979, 29(3), 489–495. [Google Scholar] [CrossRef]
- Gervais, J.; Schryver, F.D. Photochemistry of some furo-(3,2-g)-coumarin and 2,3-dihydrofuro-(3,2-g)-coumarin derivatives. Photochem. Photobiol. 1975, 21(2), 71–75. [Google Scholar] [CrossRef]
- Song, P.S.; Tapley, K.J. Photochemistry and photobiology of psoralens. Photochem. Photobiol. 1979, 29(6), 1177–1197. [Google Scholar] [CrossRef]
- Polina, I.N.; Mironov, M.V.; Belyy, V.A.; Brovarova, O.V. Investigation of the component composition of the oxidative thermal degradation products of fuel pellets fromthe Heracleum sosnowskyi Manden biomass by chromatography-mass-spectrometry. Izv. Vyssh. Uchebn. Zaved. Khim. Khim. Tekhnol. (Russia) 2022, 65, 68–76. [Google Scholar]
- Eseyin, A.E.; Steele, P.H. An overview of the applications of furfural and its derivatives. Inter. J. Adv. Chem. 2015, 3(2), 42–47. [Google Scholar] [CrossRef]
- Wieczorek, M.N.; Drabińska, N.; Jeleń, H.H. Thermal processing-induced changes in volatilome and metabolome of Brussels sprouts: Focus on glucosinolate metabolism. European Food Res. Technol. 2023, 249(8), 2165–2174. [Google Scholar] [CrossRef]
- Chen, W.; Hu, D.; Miao, A.; Qiu, G.; Qiao, X.; Xia, H.; Ma, C. Understanding the aroma diversity of Dancong tea (Camellia sinensis) from the floral and honey odors: Relationship between volatile compounds and sensory characteristics by chemometrics. Food Control 2022, 140, 109103. [Google Scholar] [CrossRef]
- Chen, L.; Li, D.; Ren, L.; Song, S.; Ma, X.; Rong, Y. Effects of simultaneous and sequential cofermentation of Wickerhamomyces anomalus and Saccharomyces cerevisiae on physicochemical and flavor properties of rice wine. Food Sci. Nutrition 2021, 9(1), 71–86. [Google Scholar] [CrossRef] [PubMed]
- Sisay, M.A.; Mammo, W.; Yaya, E.E. Phytochemical studies of Melilotus officinalis. Bull. Chem Soc. Ethiopia 2021, 35(1), 141–150. [Google Scholar] [CrossRef]
- Yu, S.; Wang, L.; Li, Q.; Zhang, Y.; Zhou, H. Sustainable carbon materials from the pyrolysis of lignocellulosic biomass. Materials Today Sustainab 2022, 19, 100209. [Google Scholar] [CrossRef]
- Kushakova, A.S.; Tkachenko, K.G.; Zenkevich, I.G. Determination of the component composition of the essential oils of hogweed heraculum using the chromatic-distribution method. Khim. Rastit. Syr’ya (Russia) 2010, (4), 111–114. [Google Scholar]
- Gianturco, M.A.; Giammarino, A.S.; Pitcher, R.G. The structures of five cyclic diketones isolated from coffee. Tetrahedron 1963, 19(12), 2051–2059. [Google Scholar] [CrossRef]
- Costa, D.C.; Costa, H.S.; Albuquerque, T.G.; Ramos, F.; Castilho, M.C.; Sanches-Silva, A. Advances in phenolic compounds analysis of aromatic plants and their potential applications. Trends Food Sci. Technol. 2015, 45(2), 336–354. [Google Scholar] [CrossRef]
- Biniecka, M.; Caroli, S. Analytical methods for the quantification of volatile aromatic compounds. TrAC Trends Anal. Chem. 2011, 30(11), 1756–1770. [Google Scholar] [CrossRef]
- Christaki, E.; Bonos, E.; Giannenas, I.; Florou-Paneri, P. Aromatic plants as a source of bioactive compounds. Agriculture 2012, 2(3), 228–243. [Google Scholar] [CrossRef]
- Shelepova, O.V.; Baranova, E.N.; Tkacheva, E.V.; Evdokimenkova, Y.B.; Ivanovskii, A.A.; Konovalova, L.N.; Gulevich, A.A. Aromatic Plants Metabolic Engineering: A Review. Agronomy 2022, 12(12), 3131. [Google Scholar] [CrossRef]
- Coutts, I.G.C. Aromatic compounds. Annual Reports Section (Organic Chemistry) 1991, 88, 131–148. [Google Scholar] [CrossRef]
- Chorlton, A.P. Aromatic compounds. Annual Reports Section" B"(Organic Chemistry) 1994, 91, 165–206. [Google Scholar] [CrossRef]
- Abdel-Shafy, H.I.; Mansour, M.S. A review on polycyclic aromatic hydrocarbons: Source, environmental impact, effect on human health and remediation. Egyptian J. Petroleum 2016, 25(1), 107–123. [Google Scholar] [CrossRef]
- Juana, F.L.; Angel, P.A.J.; Manuel, V.M. Beneficial health effects of bioactive compounds present in spices and aromatic herbs. Studies in Natural Products Chem. 2012, 37, 115–134. [Google Scholar]
- Kozlova, S.V.; Tsvetov, N.S. Optimization of conditions for ultrasonic water-ethanol extraction of polyphenolic components from Heracleum sosnowskyi Manden. Inflorescences. Bull. State Nikita Botan. Gard. (Russia) 2025, 154, 78–86. [Google Scholar]
- Ušjak, L.; Sofrenić, I.; Tešević, V.; Drobac, M.; Niketić, M.; Petrović, S. Fatty acids, sterols, and triterpenes of the fruits of 8 Heracleum taxa. Nat. Prod. Commun. 2019, 14(6), 1934578–19856788. [Google Scholar] [CrossRef]
- Kaur, R.; Sood, A.; Kanotra, M.; Arora, S.; Subramaniyan, V.; Bhatia, S.; Behl, T. Pertinence of nutriments for a stalwart body. Environ. Sci. Pollution Res. 2021, 28(39), 54531–54550. [Google Scholar] [CrossRef]
- Hilditch, T.P.; Jones, E.E. Seed fats of the Umbelliferae: Heracleum sphondylium and Angelica sylvestris. Biochemical J. 1928, 22(2), 326. [Google Scholar] [CrossRef]
- Borska, E.; Kviesis, J.; Ramata-Stunda, A.; Nikolajeva, V.; Ansone-Bertina, L.; Boroduskis, M.; Klavins, M. Bioactive lipids and allelopathic potential of the invasive plant Heracleum sosnowskyi: Insights into its fatty acid composition, antimicrobial and cytotoxic effects. Front. Pharmacol. 2025, 16, 1582694. [Google Scholar] [CrossRef]
- Zi, X.; Zhou, S.; Wu, B. Alpha-linolenic acid mediates diverse drought responses in maize (Zea mays L.) at seedling and flowering stages. Molecules 2022, 27(3), 771. [Google Scholar] [CrossRef]
- Mititelu, M.; Lupuliasa, D.; Neacsu, S.M.; Olteanu, G.; Busnatu, S.S.; Mihai, A. Polyunsaturated fatty acids and human health: A key to modern nutritional balance in association with polyphenolic compounds from food sources. Foods 2025, 14(1), 46. [Google Scholar] [CrossRef]
- Weber, H.; Vick, B.A.; Farmer, E.E. Dinor-oxo-phytodienoic acid: A new hexadecanoid signal in the jasmonate family. Proc. Natl. Acad. Sci. U. S. A 1997, 94, 10473–10478. [Google Scholar] [CrossRef]
- Watanebe, J.; Kawabata, J.; Kasai, T. 9-Oxooctadeca-10,12-dienoic acids as Acetyl-CoA carboxylase inhibitors from red pepper (Capsicum annuum L.). Biosci. Biotechnol. Biochem. 1999, 63(3), 489–493. [Google Scholar] [CrossRef] [PubMed]
- Tejero, I.; González-Lafont, A.; Lluch, J.M.; Eriksson, L.A. Photo-oxidation of lipids by singlet oxygen: A theoretical study. Chem. Physics Lett. 2004, 398(4-6), 336–342. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Goldshlag, P.; Srebnik, M. Occurrence of dicarboxylic (dioic) acids in some Mediterranean nuts. Food Chem. 2002, 76(4), 469–473. [Google Scholar] [CrossRef]
- Baker, B.G.; Bedford, J.; Kanitkar, S. Keeping pace with the media; Giant Hogweed burns—A case series and comprehensive review. Burns 2017, 43(5), 933–938. [Google Scholar] [CrossRef]
- Pfurtscheller, K.; Trop, M. Phototoxic plant burns: Report of a case and review of topical wound treatment in children. Pediatric Dermatol 2014, 31(6), e156–e159. [Google Scholar] [CrossRef]
- Zangerl, A.R.; Berenbaum, M.R. Furanocoumarins in wild parsnip: Effects of photosynthetically active radiation, ultraviolet light, and nutrients. Ecology 1987, 68(3), 516–520. [Google Scholar] [CrossRef]
- Kasperkiewicz, K.; Erkiert-Polguj, A.; Budzisz, E. Sunscreening and photosensitizing properties of coumarins and their derivatives. Letters Drug Design & Discovery 2016, 13(5), 465–474. [Google Scholar]
- Sumorek-Wiadro, J.; Zając, A.; Maciejczyk, A.; Jakubowicz-Gil, J. Furanocoumarins in anticancer therapy–For and against. Fitoterapia 2020, 142, 104492. [Google Scholar] [CrossRef]
- Janusz, S.C.; Schwartz, R.A. Botanical briefs: Phytophotodermatitis is an occupational and recreational dermatosis in the limelight. Cutis 2021, 107(4), 187–189. [Google Scholar] [CrossRef]
- Rzymski, P.; Klimaszyk, P.; Poniedziałek, B. Invasive giant hogweeds in Poland: Risk of burns among forestry workers and plant distribution. Burns 2015, 41(8), 1816–1822. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.C.; Sullivan, P.J.; O’Sullivan, M.J.; Eadie, P.A. Full thickness burn caused by exposure to giant hogweed: Delayed presentation, histological features and surgical management. J. Plastic, Reconstruct. Aesthetic Surg. 2011, 64(1), 128–130. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, K.E.; Blankenship, K.; Houk, L. Botanical Briefs: Phytophotodermatitis caused by giant hogweed (Heracleum mantegazzianum). Cutis 2021, 108, 5. [Google Scholar] [CrossRef] [PubMed]
- Kelly, A. Phyto-photo dermatitis. The Ulster Medical J. 1969, 38(1), 51. [Google Scholar]
- Morris, A.J.; Rueckeis, C.A. Sap and sun: A case of phytophotodermatitis. Wilderness & Environ. Med. 2023, 34(4), 532–535. [Google Scholar]
- Downs, J.W.; Cumpston, K.L.; Feldman, M.J. Giant hogweed phytophotodermatitis. Clinical Toxicology 2019, 57(9), 822–823. [Google Scholar] [CrossRef]
- King, A.; Pope, E. Dermatitis versus nonaccidental trauma: A systematic review of initial pediatric misdiagnoses. Pediatric Dermatology 2024, 41(2), 215–220. [Google Scholar] [CrossRef]
- Xu, Y.; Luan, X.; He, P.; Zhu, D.; Mu, R.; Wang, Y.; Wei, G. Fabrication and functional regulation of biomimetic interfaces and their antifouling and antibacterial applications: A review. Small 2024, 20(21), 2308091. [Google Scholar] [CrossRef]
- Grosu, C.; Jîjie, A.R.; Manea, H.C.; Moacă, E.A.; Iftode, A.; Minda, D.; Vlad, C.S. New insights concerning phytophotodermatitis induced by phototoxic plants. Life 2024, 14(8), 1019. [Google Scholar] [CrossRef]
- Panahi, Y.; Dadjo, Y.; Pishgoo, B.; Akbari, A.; Sahebkar, A. Clinical evaluation of the anti-inflammatory effects of Heracleum persicum fruits. Comp. Clinical Pathology 2015, 24(4), 971–974. [Google Scholar] [CrossRef]
- Piersiala, K.; Loroch, A.; Kaik, J.; Dadej, D.; Kierepa, A.; Mozer-Lisewska, I. Burn caused by exposure to giant hogweed (Heracleum sosnowskyi, Sosnowsky’s hogweed) and delayed wound healing in a 46 years old HIV and HCV positive patient-a case report. Polish Annals of Medicine 2019, 26((1).). [Google Scholar] [CrossRef]
- Dar, N.A.; Raja, W.Y.; Tewari, D.; Bhat, Z.A. Pharmacognostic study of roots and aerial parts of less explored Heracleum candicans Wall. ex DC. from Betaab Valley, Pahalgam, Kashmir, India. Indian J. Nat. Prod. Resour. 2022, 13(3), 362–373. [Google Scholar] [CrossRef]
- Patocka, J.; Cupalova, K. Giant Hogweed and photodermatitis. Mil. Med. Sci. Lett. (Voj Zdrav Listy) 2017, 86(3), 135–138. [Google Scholar] [CrossRef]
- Agrawal, D.; Singh, M.P.; Sharma, G.K. Pharmacodynamic approaches of phytoconstitutents in wound healing mechanisms. Pharmacological Research-Natural Prod. 2024, 5, 100119. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.




