Submitted:
12 December 2025
Posted:
15 December 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
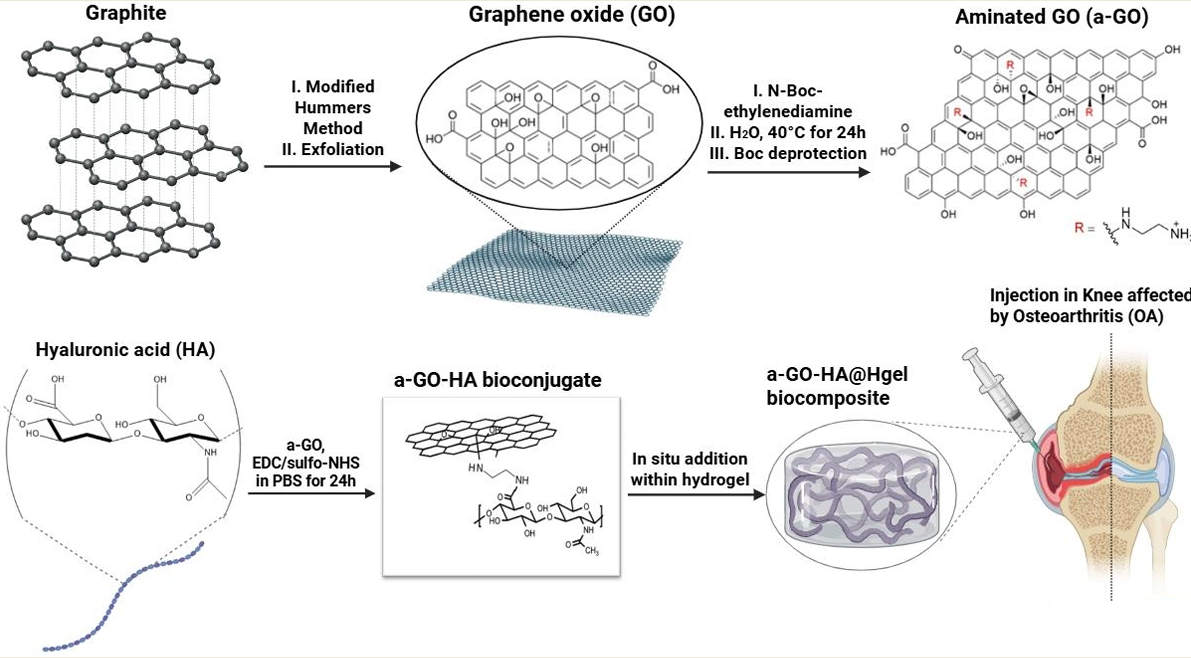
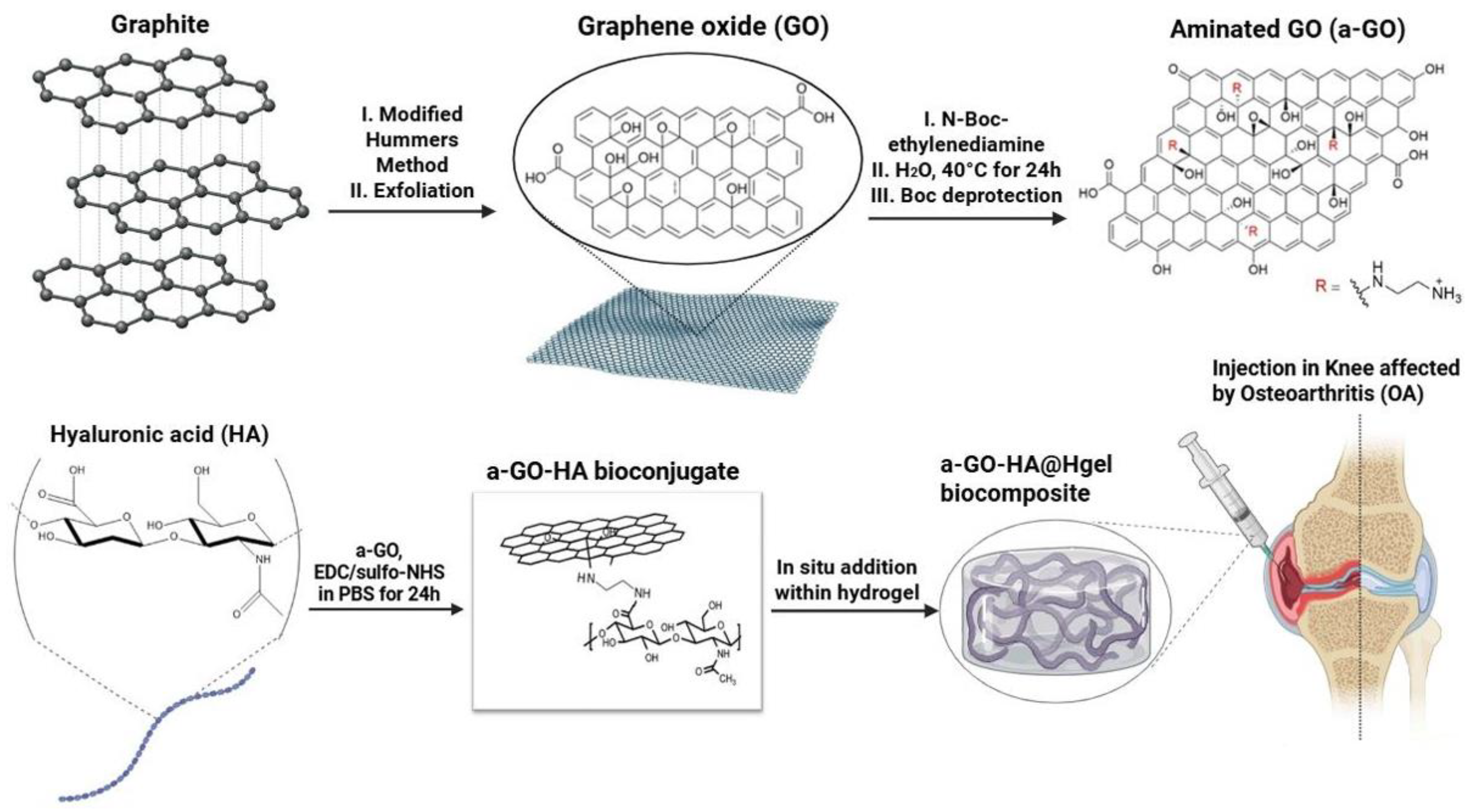
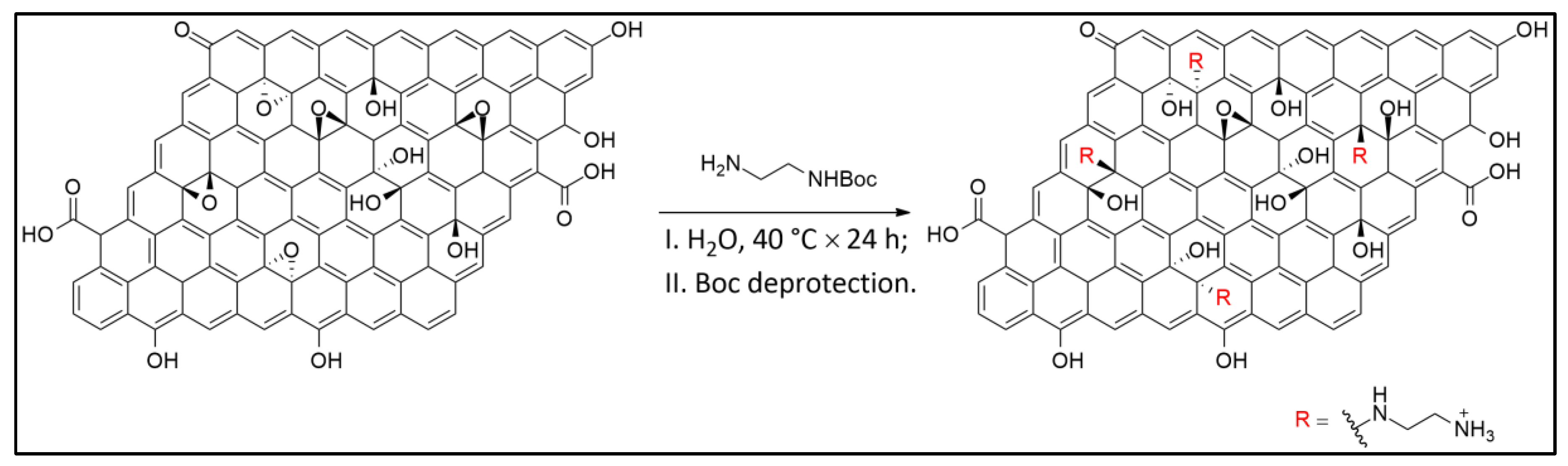
2.2. Synthesis of Graphene Oxide (GO) and Functionalization with Amine Groups (a-GO)
2.3. Preparation of a-GO–HA Conjugates
2.4. Preparation of a-GO-HA@Hgel Composite
2.5. Cell Culture and Viability Test
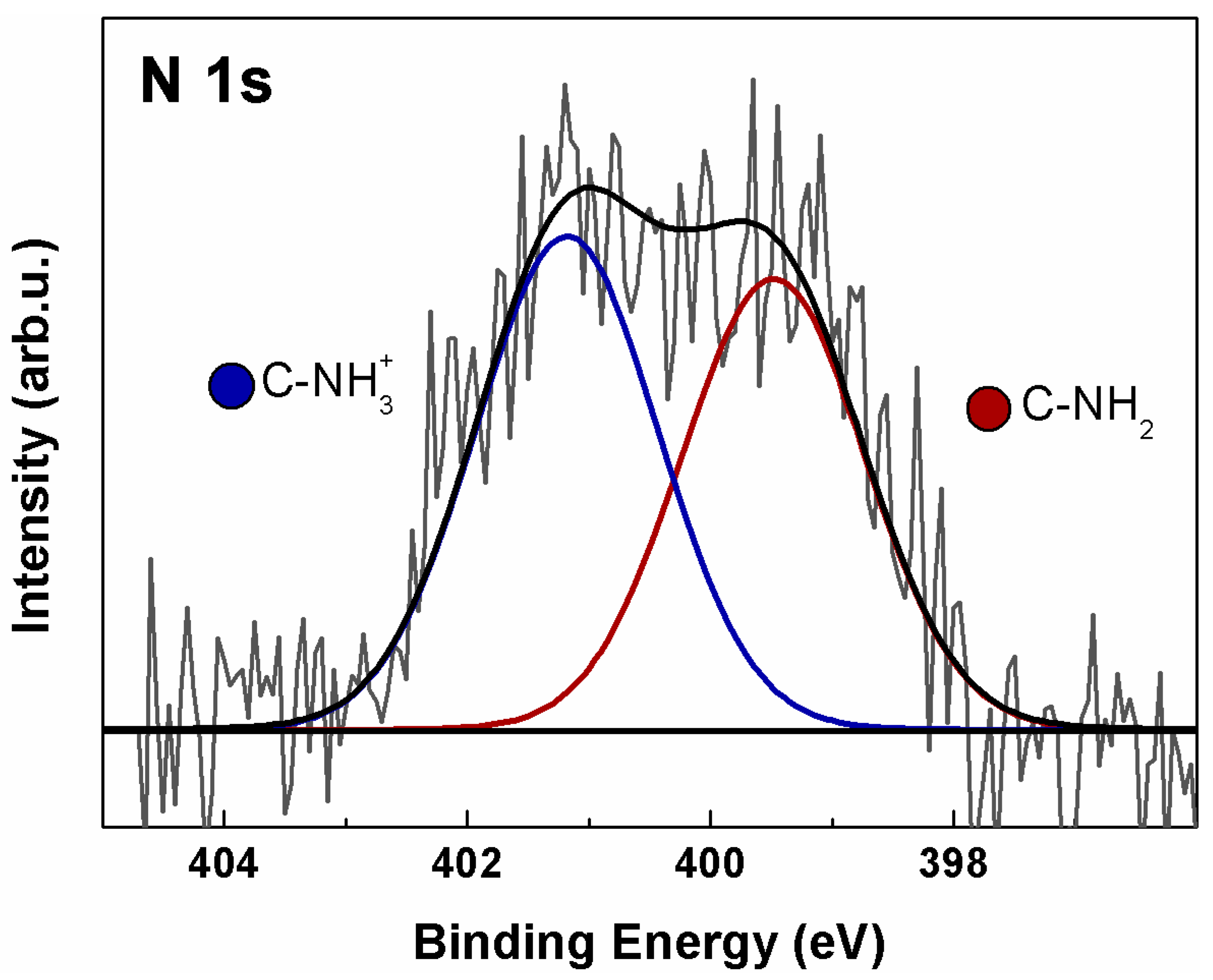
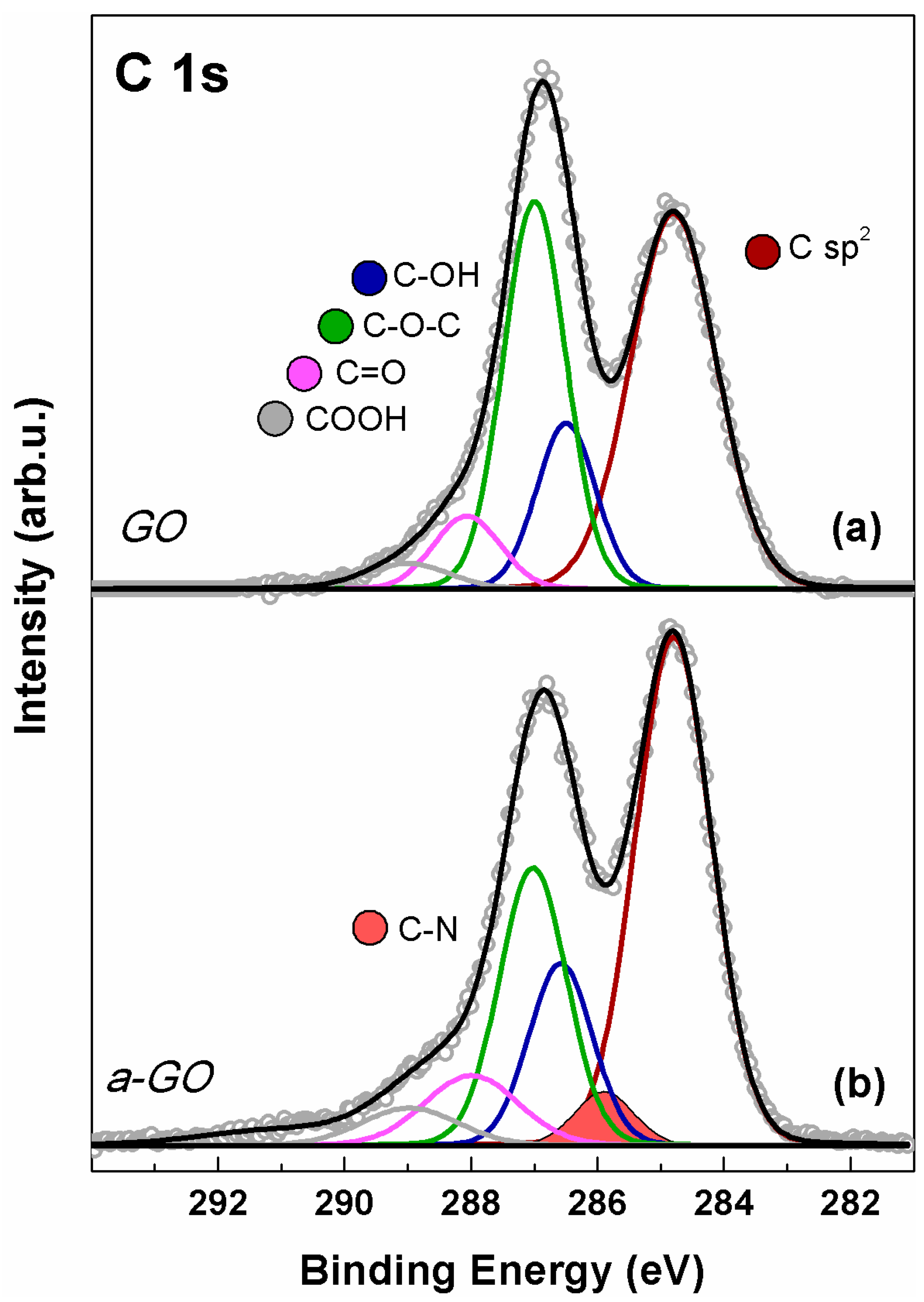
2.6. X-Ray Photoelectron Spectroscopy (XPS)
2.7. Kaiser Test for the Detection of Free Primary Amine Groups on Solid Phases
- A570 is the absorbance at 570 nm
- V is the final volume of the solution
- ε\epsilon is the molar extinction coefficient
- m is the mass of the sample in grams
2.8. HPLC
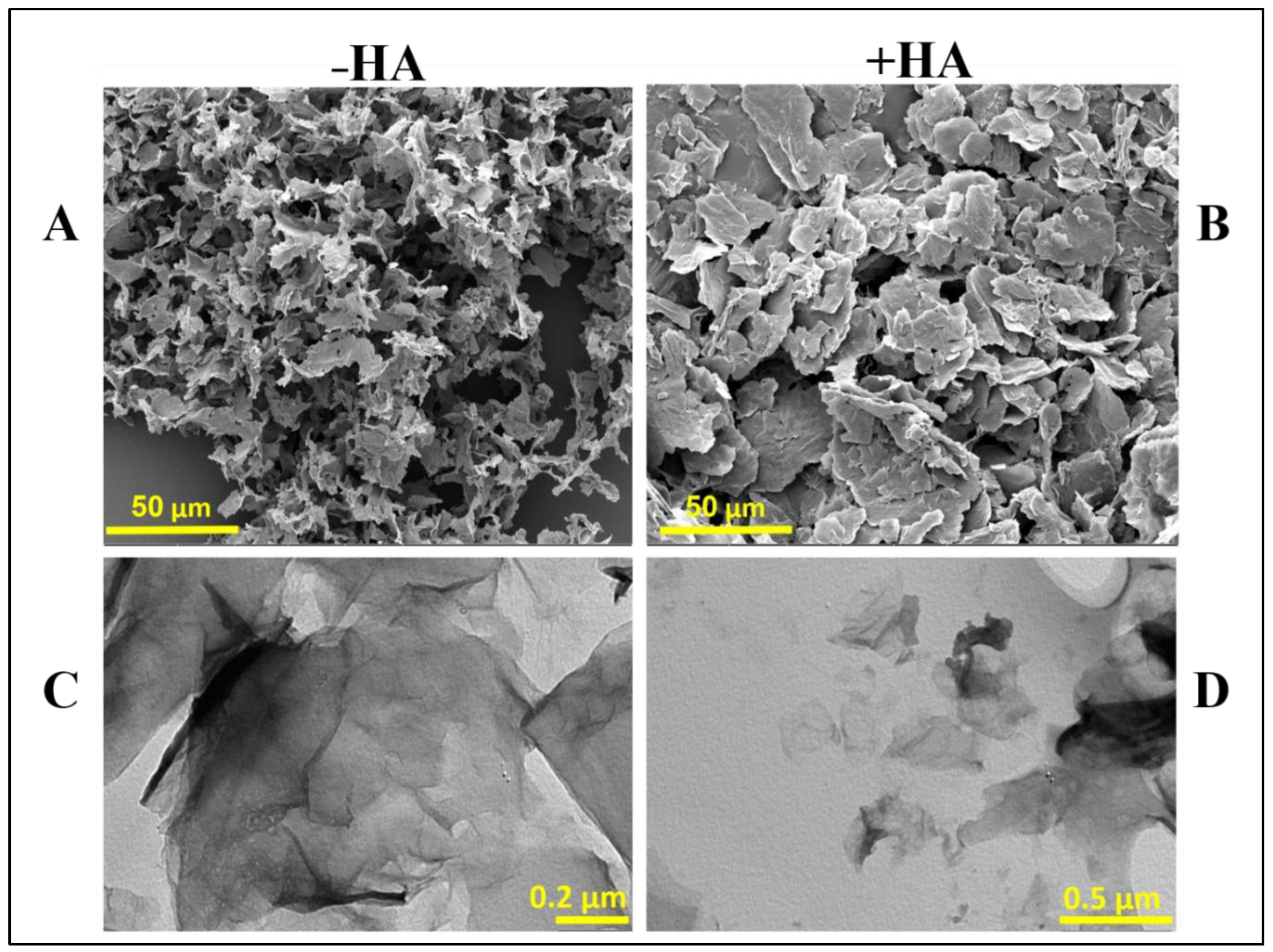
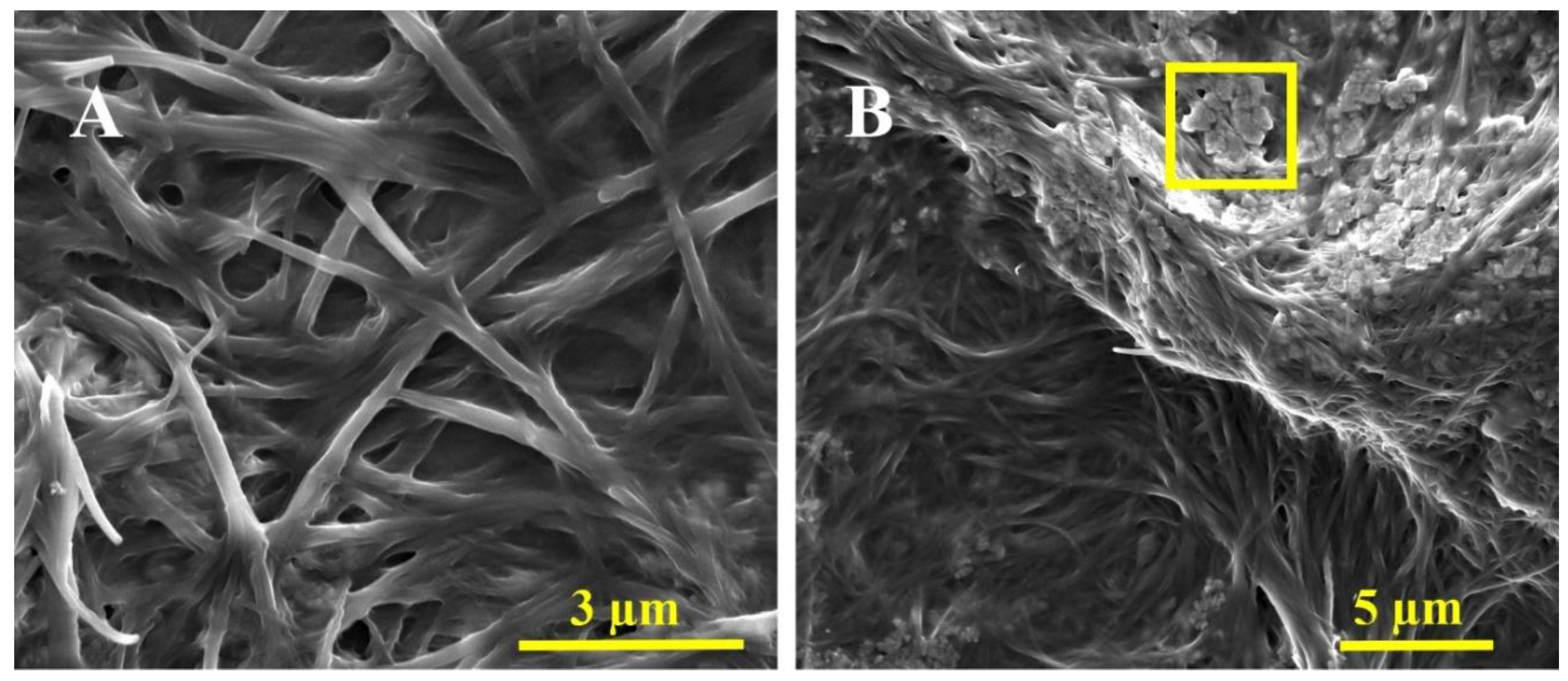
2.9. Electron Microscopy
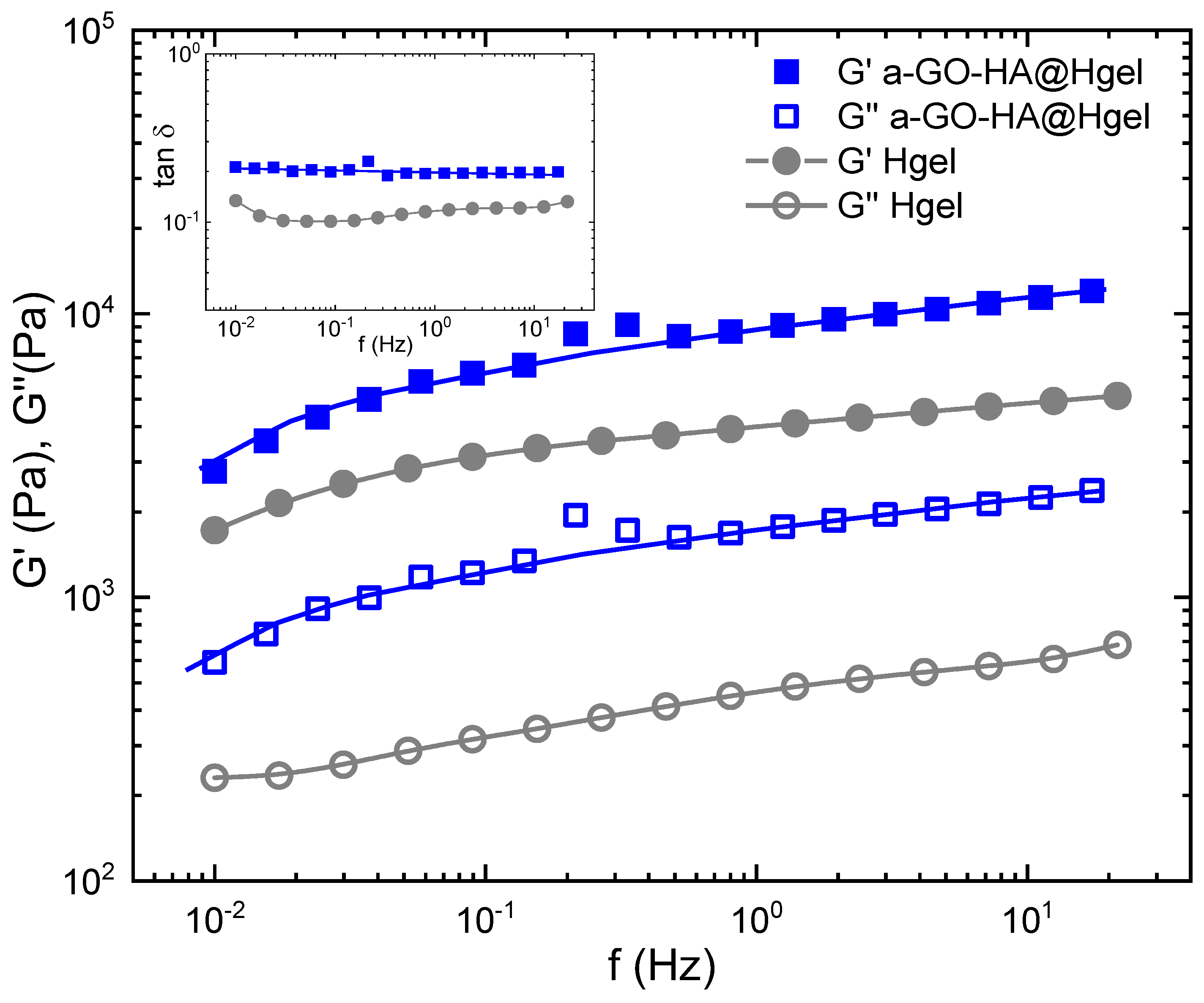
2.10. Rheology Measurement
2.11. Swelling Ability Studies
3. Results and Discussion
3.1. Graphene Oxide Functionalization Approach
3.2. Characterization of a-GO-HA Conjugate
3.3. Characterization of the a-GO-HA@Hgel Nanocomposite
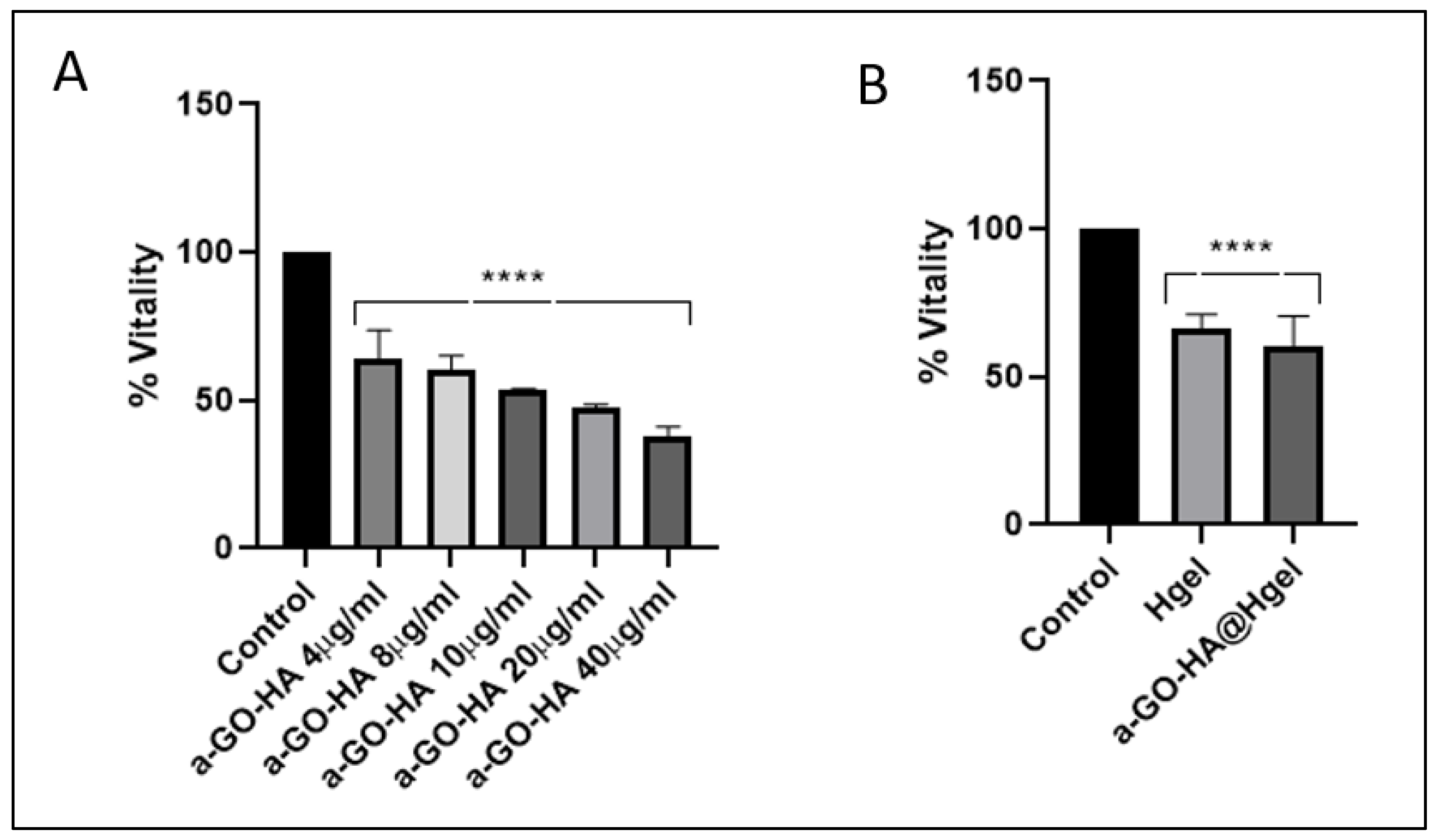
3.4. Cell Viability Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Grässel, S.; Aszodi, A. Osteoarthritis and Cartilage Regeneration: Focus on Pathophysiology and Molecular Mechanisms. Int. J. Mol. Sci. 2019, 20, 6156. [Google Scholar] [CrossRef]
- Liu, B.; Liu, T.; Li, Y.; Tan, C. Innovative Biotherapies and Nanotechnology in Osteoarthritis: Advancements in Inflammation Control and Cartilage Regeneration. Int. J. Mol. Sci. 2024, 25, 13384. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Zhang, Q.; Kuang, G.; Wang, X.; Fan, Q.; Ye, F. Functional Biomaterials for Osteoarthritis Treatment: From Research to Application. Smart Med. 2022, 1, e20220014. [Google Scholar] [CrossRef] [PubMed]
- Farinelli, L.; Riccio, M.; Gigante, A.; De Francesco, F. Pain Management Strategies in Osteoarthritis. Biomedicines 2024, 12, 805. [Google Scholar] [CrossRef] [PubMed]
- Naranda, J.; Bračič, M.; Vogrin, M.; Maver, U. Recent Advancements in 3D Printing of Polysaccharide Hydrogels in Cartilage Tissue Engineering. Materials 2021, 14, 3977. [Google Scholar] [CrossRef]
- Liang, J.; Liu, P.; Yang, X.; Liu, L.; Zhang, Y.; Wang, Q.; Zhao, H. Biomaterial-Based Scaffolds in Promotion of Cartilage Regeneration: Recent Advances and Emerging Applications. J. Orthop. Translat 2023, 41, 54–62. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, W. Recent Advances in Bionic Scaffolds for Cartilage Tissue Engineering. Front. Bioeng. Biotechnol. 2025, 13, 1625550. [Google Scholar] [CrossRef]
- Arslan, E.; Ekiz, M. S.; Cimenci, C. E.; Can, N.; Gemci, M. H.; Ozkan, H.; Guler, M. O.; Tekinay, A. B. Protective Therapeutic Effects of Peptide Nanofiber and Hyaluronic Acid Hybrid Membrane in in vivo Osteoarthritis Model. Acta Biomater. 2018, 73, 263–274. [Google Scholar] [CrossRef]
- Chen, N.; Li, S.; Miao, C.; Zhao, Q.; Dong, J.; Li, L.; Li, C. Polysaccharide-Based Hydrogels for Cartilage Regeneration. Front. Cell Dev. Biol. 2024, 12, 1444358. [Google Scholar] [CrossRef]
- Liao, H-J.; Chen, H-T.; Chang, C-H. Peptides for Targeting Chondrogenic Induction and Cartilage Regeneration in Osteoarthritis. Cartilage 2024, 19476035241276406. [Google Scholar] [CrossRef]
- Gupta, R. C.; Lall, R.; Srivastava, A.; Sinha, A. Hyaluronic Acid: Molecular Mechanisms and Therapeutic Trajectory. Front. Vet. Sci. 2019, 6, 192. [Google Scholar] [CrossRef] [PubMed]
- Hummer, C. D.; Angst, F.; Ngai, W.; Whittington, C.; Yoon, S. S.; Duarte, L.; Manitt, C.; Schemitsch, E. High Molecular Weight Intraarticular Hyaluronic Acid for The Treatment of Knee Osteoarthritis: A Network Meta-Analysis. BMC Musculoskelet. Disord. 2020, 21, 702. [Google Scholar] [CrossRef] [PubMed]
- Di Francesco, M.; Fragassi, A.; Pannuzzo, M.; Ferreira, M.; Brahmachari, S.; Decuzzi, P. Management of Osteoarthritis: From Drug Molecules to Nano/Micromedicines. WIREs Nanomed. Nanobiotechnol 2022, 14, e1780. [Google Scholar] [CrossRef] [PubMed]
- Fallacara, A.; Baldini, E.; Manfredini, S.; Vertuani, S. Hyaluronic Acid in the Third Millennium. Polymers 2018, 10, 701. [Google Scholar] [CrossRef]
- Shi, L.; Zhang, Y.; Ossipov, D. Enzymatic Degradation of Hyaluronan Hydrogels with Different Capacity for in situ Bio-Mineralization. Biopolymers 2017, 109. [Google Scholar] [CrossRef]
- Chhillar, Anish; Jaiswal, Amit. Hyaluronic Acid-Based Self-Healing Hydrogels for Diabetic Wound Healing. Adv. Healthcare Mater 2024, 14, e2404255. [Google Scholar] [CrossRef]
- Yin, B.; Xu, J.; Lu, J.; Ou, C.; Zhang, K.; Gao, F.; Zhang, Y. Responsive Hydrogel-Based Drug Delivery Platform for Osteoarthritis Treatment. Gels 2024, 10, 696. [Google Scholar] [CrossRef]
- Ni, F.; Chen, Y.; Wang, Z.; Zhang, X.; Gao, F.; Shao, Z.; Wang, H. Graphene Derivative Based Hydrogels in Biomedical Applications. J. Tissue Eng. 2024, 15, 20417314241282131. [Google Scholar] [CrossRef]
- Gostaviceanu, A.; Gavrilaş, S.; Copolovici, L.; Copolovici, D.M. Graphene-Oxide Peptide-Containing Materials for Biomedical Applications. Int. J. Mol. Sci. 2024, 25, 10174. [Google Scholar] [CrossRef]
- Sittisanguanphan, N.; Paradee, N.; Sirivat, A. Hyaluronic Acid and Graphene Oxide-incorporated Hyaluronic Acid Hydrogels for Electrically Stimulated Release of Anticancer Tamoxifen Citrate. J. Pharm. Sci. 2022, 111, 1633−1641. [Google Scholar] [CrossRef]
- Fuster-Gómez, S.; Campillo-Fernández, A.J. Hyaluronic Acid Ultra-Porous Scaffolds Reinforced with Low Quantities of Graphene Oxide: Influence on the Delivery of Curcumin and Bacterial Inhibition. Nanomaterials 2025, 15, 735. [Google Scholar] [CrossRef]
- Chronopoulou, L.; Margheritelli, S.; Toumia, Y.; Paradossi, G.; Bordi, F.; Sennato, S.; Palocci, C. Biosynthesis and Characterization of Cross-Linked Fmoc Peptide-Based Hydrogels for Drug Delivery Applications. Gels 2015, 1, 179–193. [Google Scholar] [CrossRef] [PubMed]
- Binaymotlagh, R.; Chronopoulou, L.; Palocci, C. Peptide-Based Hydrogels: Template Materials for Tissue Engineering. J. Funct. Biomater. 2023, 14, 233. [Google Scholar] [CrossRef] [PubMed]
- Chronopoulou, L.; Lorenzoni, S.; Masci, G.; Dentini, M.; Togna, A.R.; Togna, G.; Bordi, F.; Palocci, C. Lipase-Supported Synthesis of Peptidic Hydrogels. Soft Matter 2010, 6, 2525–2532. [Google Scholar] [CrossRef]
- Chronopoulou, L.; Binaymotlagh, R.; Cerra, S.; Haghighi, F.H.; Di Domenico, E.G.; Sivori, F.; Fratoddi, I.; Mignardi, S.; Palocci, C. Preparation of Hydrogel Composites Using a Sustainable Approach for In Situ Silver Nanoparticles Formation. Materials 2023, 16, 2134. [Google Scholar] [CrossRef]
- Mitrovic, J.; Richey, G.; Kim, S.; Guler, M. O. Peptide Hydrogels and Nanostructures Controlling Biological Machinery. Langmuir 2023, 39, 11935–11945. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, Q.; Zhu, S.; Liu, H.; Chen, J. Preparation and Applications of Peptide-Based Injectable Hydrogels. RSC Adv. 2019, 9, 28299–28311. [Google Scholar] [CrossRef]
- Chronopoulou, L.; Toumia, Y.; Cerroni, B.; Gentili, A.; Paradossi, G.; Palocci, C. Biosynthesis and Characterization of a Novel Fmoc-Tetrapeptide-Based Hydrogel for Biotechnological Applications. Colloids Surf. A. Physicochem. Eng. Asp. 2017, 532, 535–540. [Google Scholar] [CrossRef]
- Bakhtiary, N.; Ghalandari, B.; Ghorbani, F.; Varma, S.N.; Liu, C. Advances in Peptide-Based Hydrogel for Tissue Engineering. Polymers 2023, 15, 1068. [Google Scholar] [CrossRef]
- Gan, X.; Wang, X.; Huang, Y.; Li, G.; Kang, H. Applications of Hydrogels in Osteoarthritis Treatment. Biomedicines 2024, 12, 923. [Google Scholar] [CrossRef]
- Chronopoulou, L.; Sennato, S.; Bordi, F.; Giannella, D.; Di Nitto, A.; Barbetta, A.; Dentini, M.; Togna, A.R.; Togna, G.I.; Moschini, S.; Palocci, C. Designing Unconventional Fmoc-Peptide-Based Biomaterials: Structure and Related Properties. Soft Matter 2014, 10, 1944. [Google Scholar] [CrossRef] [PubMed]
- Ligorio, C.; Vijayaraghavan, A.; Hoyland, J. A.; Saiani, A. Acidic and Basic Self-Assembling Peptide and Peptide-Graphene Oxide Hydrogels: Characterisation and Effect on Encapsulated Nucleus Pulposus Cells. Acta Biomaterial 2022, 143, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Mo, Y.; Zhou, T.; Li, W.; Niu, Y.; Sheu, C. Advances in Nanohybrid Hydrogels for Wound Healing: From Functional Mechanisms to Translational Prospects. Gels 2025, 11, 483. [Google Scholar] [CrossRef] [PubMed]
- Sauce-Guevara, M.A.; García-Schejtman, S.D.; Alarcon, E.I.; Bernal-Chavez, S.A.; Mendez-Rojas, M.A. Development and Characterization of an Injectable Alginate/Chitosan Composite Hydrogel Reinforced with Cyclic-RGD Functionalized Graphene Oxide for Potential Tissue Regeneration Applications. Pharmaceuticals 2025, 18, 616. [Google Scholar] [CrossRef]
- Patil, R.; Kansara, V.; Ray, D.; Aswal, V. K.; Jha, P. K.; Bahadur, P.; Tiwari, S. Slow Degrading Hyaluronic Acid Hydrogel Reinforced with Cationized Graphene Nanosheets. Int. J. Biol. Macromol. 2019, 141, 232–239. [Google Scholar] [CrossRef]
- Amato, F.; Fazi, M.; Giaccari, L.; Colecchia, S.; Perini, G.; Palmieri, V.; Papi, M.; Altimari, P.; Motta, A.; Giustini, M. Isolation by Dialysis and Characterization of Luminescent Oxidized Carbon Nanoparticles from Graphene Oxide Dispersions: A Facile Novel Route towards a More Controlled and Homogeneous Substrate with a Wider Applicability. Nanotechnology 2025, 36, 185602. [Google Scholar] [CrossRef]
- Amato, F.; Motta, A.; Giaccari, L.; Di Pasquale, R.; Scaramuzzo, F.A.; Zanoni, R.; Marrani, A.G. One-Pot Carboxyl Enrichment Fosters Water-Dispersibility of Reduced Graphene Oxide: A Combined Experimental and Theoretical Assessment. Nanoscale Adv. 2023, 5, 893–906. [Google Scholar] [CrossRef]
- Wu, H.; Shi, H.; Wang, Y.; Jia, X.; Tang, C.; Zhang, J.; Yang, S. Hyaluronic Acid Conjugated Graphene Oxide for Targeted Drug Delivery. Carbon 2013, 69, 379–389. [Google Scholar] [CrossRef]
- Sciandra, F.; Bottoni, P.; De Leo, M.; Braca, A.; Brancaccio, A.; Bozzi, M. Verbascoside Elicits Its Beneficial Effects by Enhancing Mitochondrial Spare Respiratory Capacity and the Nrf2/HO-1 Mediated Antioxidant System in a Murine Skeletal Muscle Cell Line. Int. J. Mol. Sci. 2023, 24, 15276. [Google Scholar] [CrossRef]
- Scofield, J.H. Hartree–Slater Subshell Photoionization Cross-Sections at 1254 and 1487 eV. J. Electron Spectrosc. Relat. Phenom. 1976, 8, 129–137. [Google Scholar] [CrossRef]
- Filippini, G.; Amato, F.; Rosso, C.; Ragazzon, G.; Vega-Peñaloza, A.; Companyó, X.; Dell’Amico, L.; Bonchio, M.; Prato, M. Mapping the Surface Groups of Amine-Rich Carbon Dots Enables Covalent Catalysis in Aqueous Media. Chem 2020, 6, 3022–3037. [Google Scholar] [CrossRef]
- Chernyshova, I.V.; Hanumantha Rao, K. Mechanism of Coadsorption of Long-Chain Alkylamines and Alcohols on Silicates: Fourier Transform Spectroscopy and X-ray Photoelectron Spectroscopy Studies. Langmuir 2001, 17, 2711–2719. [Google Scholar] [CrossRef]
- Beamson, G.; Briggs, D. High Resolution XPS of Organic Polymers: The Scienta ESCA300 Database; John Wiley & Sons Ltd.: Chichester, UK, 1992. [Google Scholar]
- Cox, L.E.; Jack, J.J.; Hercules, D.M. Study of Monoprotonated Nitrogen Bases Using X-ray Photoelectron Spectroscopy (ESCA). J. Am. Chem. Soc. 1972, 94, 6575–6578. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Cheng, C.-E.; Wang, S.; Shiu, H.W.; Chang, L.Y.; Chen, C.-H.; Lin, T.-W.; Chang, C.-S.; Chien, F.S. Synchrotron Radiation Soft X-ray Induced Reduction in Graphene Oxide Characterized by Time-Resolved Photoelectron Spectroscopy. J. Phys. Chem. C 2015, 119, 12910–12915. [Google Scholar] [CrossRef]
- Vacchi, I.A.; Spinato, C.; Raya, J.; Bianco, A.; Ménard-Moyon, C. Chemical Reactivity of Graphene Oxide Towards Amines Elucidated by Solid-State NMR. Nanoscale 2016, 8, 13714–13721. [Google Scholar] [CrossRef]
- Sarkar, K.; Bank, S.; Chatterjee, A.; Dutta, K.; Das, A.; Chakraborty, S.; Paul, N.; Sarkar, J.; De, S.; Ghosh, S.; Acharyya, K.; Chattopadhyay, D.; Das, M. Hyaluronic Acid-Graphene Oxide Quantum Dots Nanoconjugate as Dual Purpose Drug Delivery and Therapeutic Agent in Meta-Inflammation. J. Nanobiotechnol. 2023, 21, 246. [Google Scholar] [CrossRef]
- Winter, H.H.; Chambon, F. Analysis of Linear Viscoelasticity of a Crosslinking Polymer at The Gel Point. J. Rheol. 1986, 30, 367–382. [Google Scholar] [CrossRef]
- Pang, K.-L.; Chow, Y.Y.; Leong, L.M.; Law, J.X.; Ghafar, N.A.; Soelaiman, I.N.; Chin, K.-Y. Establishing SW1353 Chondrocytes as a Cellular Model of Chondrolysis. Life 2021, 11, 272. [Google Scholar] [CrossRef] [PubMed]
| Name of the compound | q |
|---|---|
| Pristine Hgel | 78.90 ± 0.22 |
| a-GO-HA-Hgel | 57.05 ± 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





