Introduction
Bipolar-OCD is among the most treatment-resistant conditions in clinical psychiatry. Lifetime prevalence of OCD in bipolar samples ranges from 15–30 %, with higher rates in early-onset illness [
1,
2]. Obsessions in this group are typically mood-congruent (aggressive, sexual, symmetry, or somatic themes), worsen during depressive episodes, and predict more frequent relapses, greater functional impairment, and elevated suicide risk [
3,
4]. Conventional OCD therapy—high-dose selective serotonin reuptake inhibitors (SSRIs)—carries a manic-switch risk of up to 60 % in bipolar patients when used without adequate mood-stabiliser cover [
3]. Mood stabilisers alone rarely address the obsessive-compulsive component, leaving many patients disabled despite optimised bipolar management.
Intravenous ketamine and other fast-acting glutamatergic drugs have shown promise in treating both bipolar depression and OCD by temporarily blocking NMDA receptors, causing a surge of glutamate, and promoting AMPA-dependent synaptogenesis [
5,
6,
7]. But logistical problems and side effects make it hard to use regularly. Cheung [
8] proposed a fully oral, low-cost alternative—the "Cheung Glutamatergic Regimen"—designed to replicate ketamine's neuroplasticity cascade using (1) dextromethorphan (DXM) for NMDA antagonism, (2) a CYP2D6 inhibitor to prolong DXM exposure, (3) piracetam as an AMPA positive allosteric modulator, and (4) optional L-glutamine for presynaptic glutamate replenishment. Initial uncontrolled reports from the same center indicate significant efficacy in treatment-resistant bipolar-OCD presentations when CYP2D6 inhibition is properly sustained [
9,
10].
We present a case of a young woman with previously stabilised bipolar disorder who, after treatment interruption, developed severe moral-scrupulosity OCD that responded only partially to DXM + piracetam until CYP2D6 inhibition was restored.
Methods
This retrospective case report was prepared from the electronic and handwritten clinical records of a patient treated in routine private outpatient practice at Cheung Ngo Medical, Hong Kong SAR, between April 2024 and December 2025. All interventions formed part of standard clinical care; no experimental procedures were introduced. Written informed consent for anonymised publication was obtained from the patient in accordance with local ethical guidelines for case reporting.
Diagnoses were made according to DSM-5 criteria by the treating psychiatrist. Follow-up occurred every 2–8 weeks with unstructured clinical interview, mental-state examination, and self-reported Patient Health Questionnaire-9 (PHQ-9) and Generalised Anxiety Disorder-7 (GAD-7) scales. No structured OCD instrument (e.g., Y-BOCS) was administered in routine practice.
Dose adjustments were guided solely by clinical response, tolerability, and early hypomanic warning signs, consistent with previously described optimisation principles for the Cheung Glutamatergic Regimen [
8].
Case Presentation
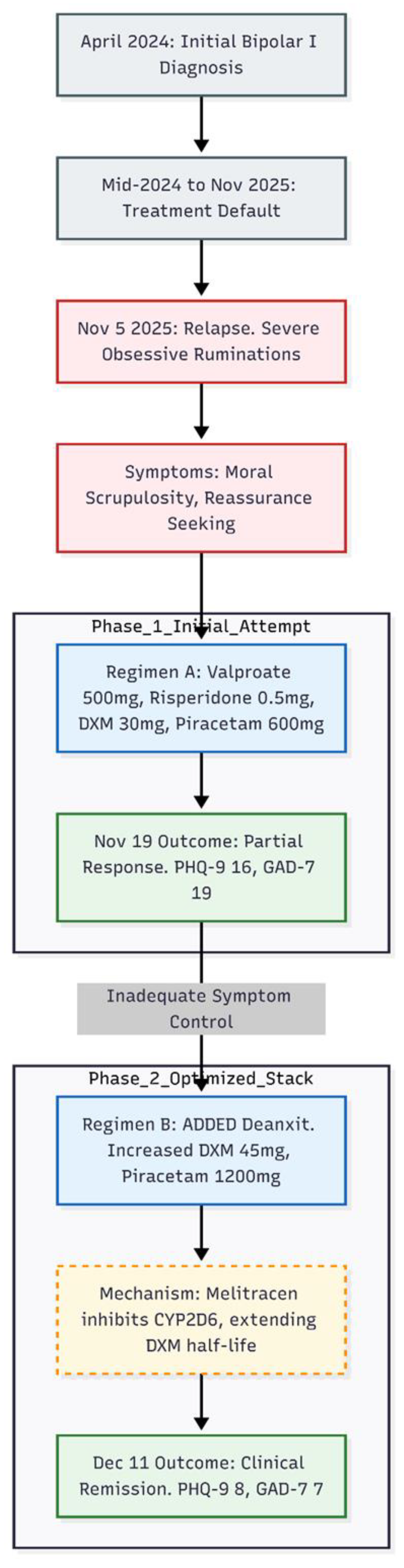
A 23-year-old woman was re-referred in November 2025 because unrelenting obsessive ruminations had begun to dominate her daily life. The thoughts—largely moral in theme—drove near-continuous reassurance-seeking from her partner and produced marked social and occupational impairment (
Figure 1).
The patient's first contact with the clinic dated to April 2024. At that time she had endured two months of low mood, insomnia, racing thoughts, pressured speech and intrusive, pre-sleep rumination after a coercive relationship. Past episodes of elevated mood with inflated self-confidence were also reported, and bipolar I disorder was diagnosed. She began night-time treatment with sodium valproate 500 mg (Epilim Chrono), risperidone 1 mg, flupentixol–melitracen 1 tablet (Deanxit), lemborexant 2.5–5 mg, zolazepam 0.5 mg as required and clonazepam 0.5–1 mg as required. By May 2024 mood and sleep had normalised, irritability had eased and hypomanic features were absent; anxiety, though still present, was tolerable. She remained on this regimen until mid-2024, then defaulted and saw another psychiatrist who labelled an "anxiety disorder" and stopped all medication except occasional benzodiazepines (details unknown).
Eighteen months later, on 5 November 2025, she returned to the original clinic with severe obsessive ruminations—"Am I a bad person?"—accompanied by repetitive confession and reassurance requests. Moral scrupulosity and fear of harming others were prominent. Scores on 19 November were PHQ-9 = 16 (moderately severe depression) and GAD-7 = 19 (severe anxiety). The presentation was re-framed as bipolar disorder with comorbid, treatment-resistant obsessive–compulsive disorder. Night-time medication was restarted with valproate 300 mg (quickly raised to 500 mg) and risperidone 0.5 mg. An oral glutamatergic "stack" was added: dextromethorphan 30 mg (2 × 15 mg) plus piracetam 600 mg (½ tablet of a 1 200 mg formulation).
After two weeks she reported a modest easing of low mood and some reduction in rumination intensity, yet obsessive thoughts and reassurance-seeking remained frequent. The regimen was changed on November 19, 2025. Deanxit 1 tablet was given every night to provide melitracen-mediated CYP2D6 inhibition. Dextromethorphan was raised to 45 mg (3 × 15 mg) and piracetam to 1,200 mg (1 tablet). Valproate 500 mg and risperidone 0.5 mg were continued unchanged.
By 11 December 2025—three-and-a-half weeks after Deanxit was reinstated—both patient and boyfriend described marked improvement. Reassurance-seeking had dwindled and ruminations were "much less." PHQ-9 had fallen to 8 (mild depression) and GAD-7 to 7 (mild anxiety). Obsessive–compulsive symptoms were now occasional and low-grade. The stabilised night-time regimen comprised valproate 500 mg, Deanxit 1 tablet, dextromethorphan 45 mg, piracetam 1 200 mg and risperidone 0.5 mg (with a planned switch to aripiprazole 2.5 mg to address menstrual irregularity).
In summary, reinstating valproate alongside a dextromethorphan–piracetam base yielded only partial benefit, but full remission of obsessive–compulsive, depressive and anxious symptoms emerged after Deanxit—providing CYP2D6 inhibition—prolonged dextromethorphan exposure. Improvements persisted at one-month follow-up without hypomanic activation or significant adverse effects.
Discussion
The clinical trajectory of this 23-year-old woman with bipolar disorder and subsequent severe, treatment-resistant obsessive-compulsive symptoms illustrates two persistent challenges in modern psychiatry: (1) the management of obsessive-compulsive disorder (OCD) when it complicates bipolar affective disorder (bipolar-OCD phenotype), and (2) the essential pharmacokinetic reliance of oral dextromethorphan-based glutamatergic regimens on sufficient CYP2D6 inhibition.
Difficulties in Managing OCD in Individuals with Bipolar Affective Disorder
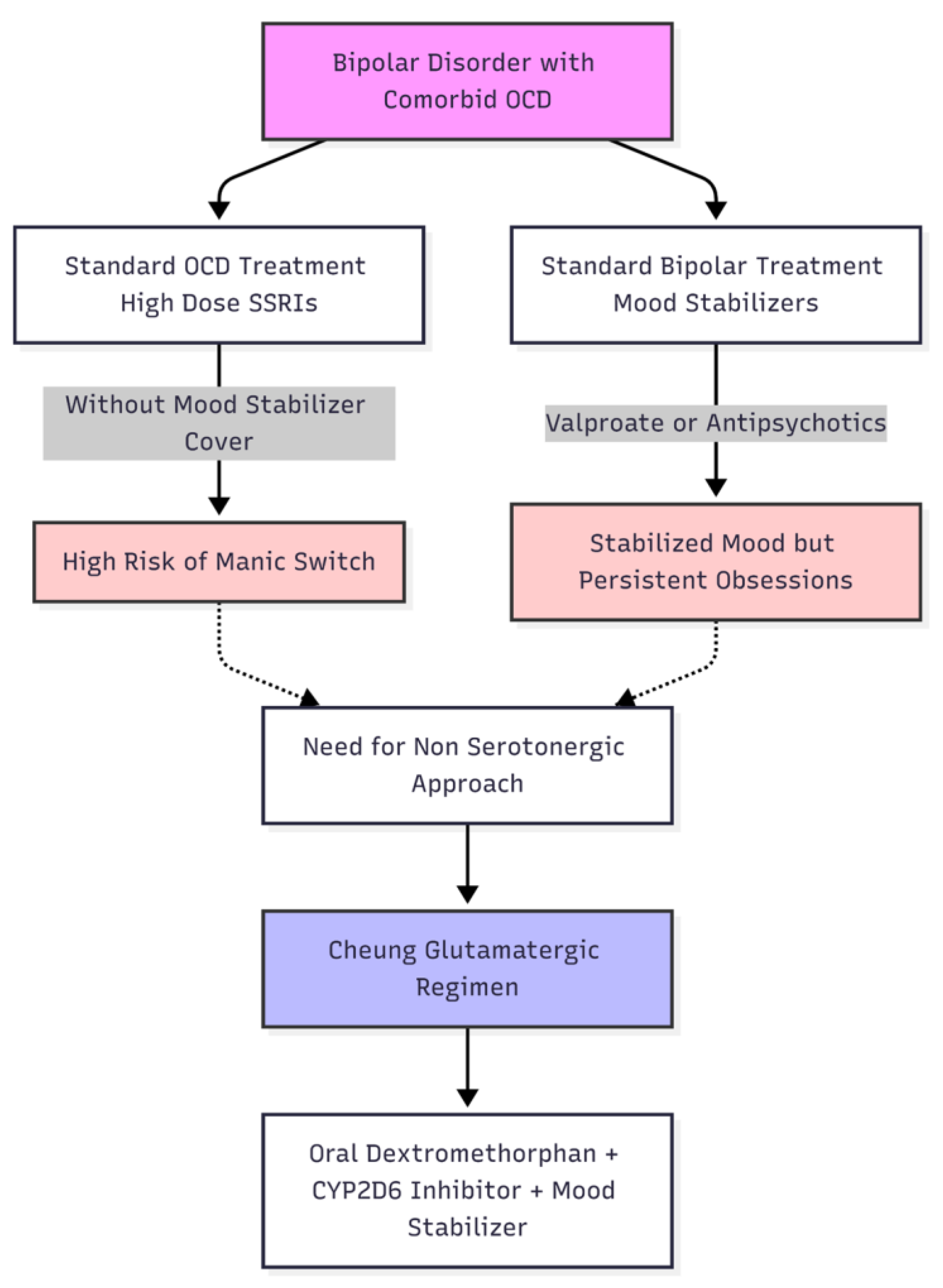
Comorbid OCD affects 15–30% of individuals with bipolar disorder, significantly deteriorating prognosis, heightening depressive recurrence, and increasing suicide risk [
1,
2,
4]. Obsessions in this population are frequently mood-congruent (aggressive, sexual, symmetry, or somatic themes), intensify during depressive episodes, and respond poorly to conventional mood stabilisers alone [
3]. High-dose selective serotonin reuptake inhibitors (SSRIs)—the cornerstone of OCD monotherapy—carry a documented manic-switch risk of up to 60 % in bipolar patients when used without robust mood-stabiliser cover [
3]. In the present case, earlier successful stabilisation of bipolar symptomatology with valproate and low-dose antipsychotics left prominent moral-scrupulosity ruminations and compulsive reassurance-seeking untouched, a pattern repeatedly observed in the bipolar-OCD subgroup [
1].
Recent findings suggest that glutamatergic dysregulation in cortico-striato-thalamo-cortical circuits plays a role in the pathogenesis of OCD, irrespective of serotonergic deficits [
11]. Rapid-acting agents like intravenous ketamine or memantine have shown effectiveness in reducing obsessive thoughts by temporarily blocking NMDA receptors, causing a compensatory increase in glutamate levels, and promoting AMPA-dependent synaptogenesis [
6,
12]. These results elucidate a mechanistic justification for focusing on the NMDA–AMPA axis in treatment-resistant OCD, especially when serotonergic enhancement is inadvisable, as frequently observed in bipolar disorder (
Figure 2).
Pivotal Role of CYP2D6 Inhibition in Achieving Therapeutic Efficacy
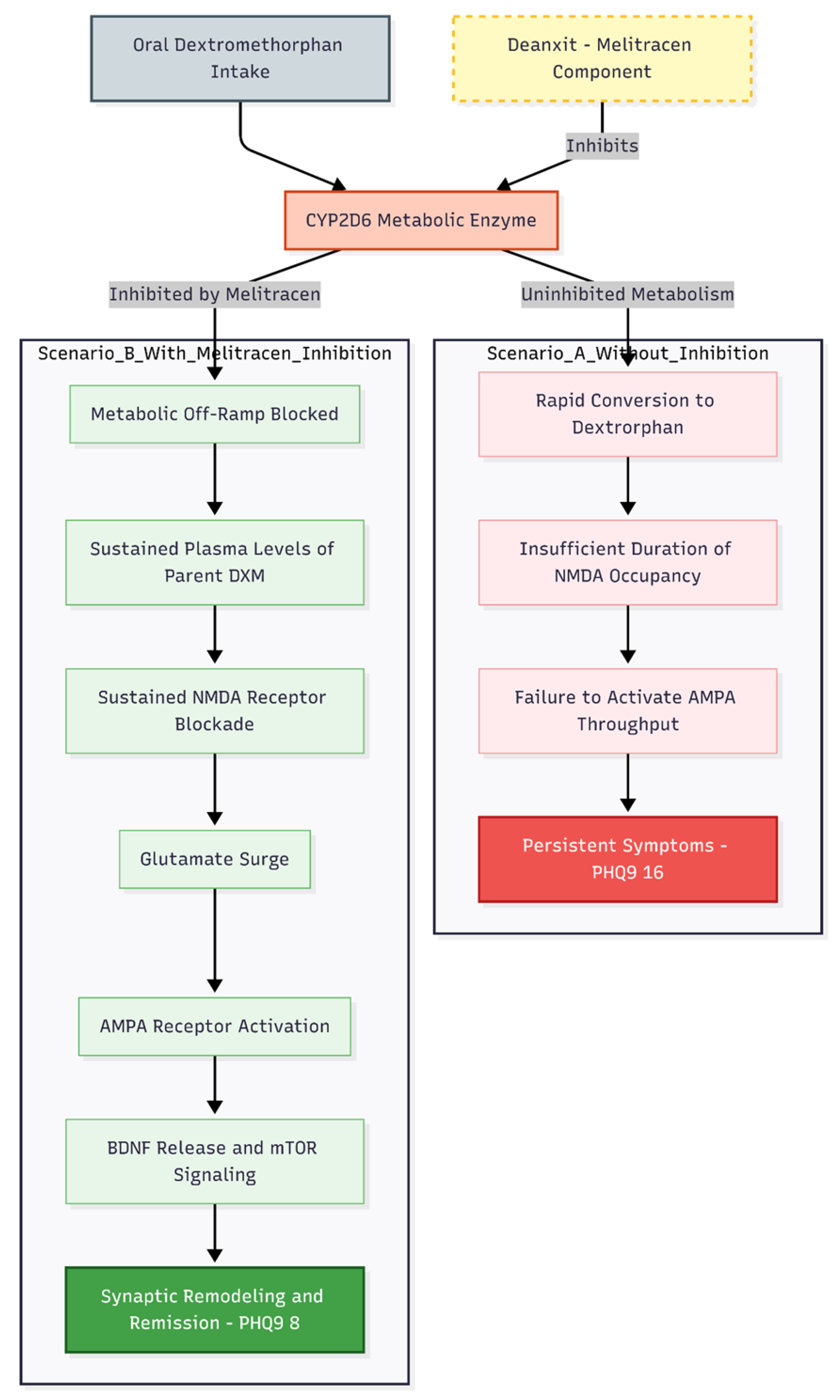
The most instructive aspect of this case is the clear temporal relationship between restoration of CYP2D6 inhibition and full remission of obsessive-compulsive symptoms (
Figure 3).
Dextromethorphan 30–45 mg nocte combined with piracetam 600–1200 mg and valproate 500 mg produced only modest attenuation of ruminations. Dramatic improvement—reduction of PHQ-9 from 16 to 8, GAD-7 from 19 to 7, and near-elimination of reassurance-seeking—occurred only after re-introduction of Deanxit 1 tablet nocte (supplying the moderate CYP2D6 inhibitor melitracen). This observation directly parallels patterns documented across Cheung's uncontrolled series [
8,
9,
10].
As Cheung [
8] explicitly states:
> "the simplest way to keep dextromethorphan (DXM) from being chewed up too quickly is to pair it with another psychotropic that blocks the same metabolic 'off-ramp,' CYP2D6… Fluoxetine and paroxetine stand out because they are high-affinity, mechanism-based inhibitors that can push extensive metabolizers into the poor-metabolizer range within days" [
13,
14].
Without CYP2D6 inhibition, dextromethorphan quickly becomes dextrorphan. This means that the parent drug doesn't stay in the body long enough to keep the NMDA receptors occupied long enough to cause the glutamate surge needed to activate the AMPA receptors, release BDNF, and cause synaptic remodeling through mTOR [
15,
16,
17]. There is both preclinical and clinical evidence that AMPA throughput, not NMDA blockade itself, is the main signal for the immediate and long-lasting antidepressant and anti-obsessional effects of ketamine-class drugs [
16,
18].
The present case extends Cheung's earlier findings by demonstrating that even milder inhibitors (melitracen component of Deanxit) can suffice when dosed consistently, reinforcing the principle that pharmacokinetic protection of dextromethorphan is a non-negotiable component of the regimen rather than an optional adjunct.
Clinical Implications and Integration with the Cheung Glutamatergic Regimen
The trajectory—partial response to dextromethorphan + piracetam, full remission only after CYP2D6 inhibition—is now a recurring motif across approximately 25 published cases from a single centre employing the "Cheung Glutamatergic Regimen" [
8,
9,
10]. Mood stabiliser cover (valproate) prevented hypomanic activation, while sequential addition of NMDA antagonism, AMPA potentiation, and pharmacokinetic protection mirrors the mechanistic sequence of intravenous ketamine but in an entirely oral, low-cost format.
These naturalistic data, though preliminary and uncontrolled, suggest that oral dextromethorphan-based glutamatergic augmentation—when correctly implemented with CYP2D6 inhibition—may offer a viable therapeutic bridge for the difficult bipolar-OCD subgroup in which conventional serotonergic strategies are either ineffective or destabilising.
Randomised controlled trials are urgently needed to confirm efficacy, define optimal CYP2D6 inhibitor choice and dosing, and establish long-term safety in this vulnerable population.
Conflicts of Interest and Source of Funding Statement
None declared.
Funding Declaration
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics Declaration
Not applicable.
References
- Amerio, A.; Stubbs, B.; Odone, A.; et al. The prevalence and predictors of comorbid bipolar disorder and obsessive-compulsive disorder: A systematic review and meta-analysis. J Affect Disord 2015, 186, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Ferentinos, P.; Preti, A.; Veroniki, A.A.; et al. Comorbidity of obsessive-compulsive disorder in bipolar spectrum disorders: Systematic review and meta-analysis of its prevalence. J Affect Disord 2020, 263, 193–208. [Google Scholar] [CrossRef] [PubMed]
- de Filippis, R.; Aguglia, A.; Costanza, A.; et al. Obsessive-compulsive disorder as an epiphenomenon of comorbid bipolar disorder? An updated systematic review. J Clin Med 2024, 13, 1230. [Google Scholar] [CrossRef] [PubMed]
- De Prisco, M.; Tapoi, C.; Oliva, V.; et al. Clinical features in co-occurring obsessive-compulsive disorder and bipolar disorder: A systematic review and meta-analysis. Eur Neuropsychopharmacol 2024, 80, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Berman, R.M.; Cappiello, A.; Anand, A.; et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 2000, 47, 351–354. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, C.I.; Kegeles, L.S.; Levinson, A.; et al. Randomized controlled crossover trial of ketamine in obsessive-compulsive disorder: Proof-of-concept. Neuropsychopharmacology 2013, 38, 2475–2483. [Google Scholar] [CrossRef] [PubMed]
- Zarate, C.A., Jr.; Brutsche, N.E.; Ibrahim, L.; et al. Replication of ketamine's antidepressant efficacy in bipolar depression: A randomized controlled add-on trial. Biol Psychiatry 2012, 71, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Cheung, N. DXM, CYP2D6-inhibiting antidepressants, piracetam, and glutamine: Proposing a ketamine-class antidepressant regimen with existing drugs. Preprints 2025. [Google Scholar] [CrossRef]
- Cheung, N. Case Series: Marked Improvement in Treatment-Resistant Obsessive–Compulsive Symptoms with Over-the-Counter Glutamatergic Augmentation in Routine Clinical Practice. Preprints 2025. [Google Scholar] [CrossRef]
- Cheung, N. Oral NMDA and AMPA Modulation for Refractory Obsessive-Compulsive Symptoms in Bipolar Disorder: A Case Series of Routine Clinical Outcomes. Preprints 2025. [Google Scholar] [CrossRef]
- Pittenger, C.; Bloch, M.H.; Williams, K. Glutamate abnormalities in obsessive compulsive disorder: Neurobiology, pathophysiology, and treatment. Pharmacol Ther 2011, 132, 314–332. [Google Scholar] [CrossRef] [PubMed]
- Ghaleiha, A.; Entezari, N.; Modabbernia, A.; et al. Memantine add-on in moderate to severe obsessive-compulsive disorder: Randomized double-blind placebo-controlled study. J Psychiatr Res 2013, 47, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Crewe, H.K.; Lennard, M.S.; Tucker, G.T.; et al. The effect of selective serotonin re-uptake inhibitors on cytochrome P4502D6 (CYP2D6) activity in human liver microsomes. Br J Clin Pharmacol 1992, 34, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Preskorn, S.H.; Shah, R.; Neff, M.; et al. The potential for clinically significant drug-drug interactions involving the CYP 2D6 system: Effects with fluoxetine and paroxetine versus sertraline. J Psychiatr Pract 2007, 13, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Lee, B.; Liu, R.J.; et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 2010, 329, 959–964. [Google Scholar] [CrossRef] [PubMed]
- Maeng, S.; Zarate, C.A., Jr.; Du, J.; et al. Cellular mechanisms underlying the antidepressant effects of ketamine: Role of AMPA receptors. Biol Psychiatry 2008, 63, 349–352. [Google Scholar] [CrossRef] [PubMed]
- Koike, H.; Iijima, M.; Chaki, S. Involvement of AMPA receptor in both the rapid and sustained antidepressant-like effects of ketamine in animal models. Behav Brain Res 2011, 224, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Zanos, P.; Moaddel, R.; Morris, P.J.; et al. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature 2016, 533, 481–486. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).