1. Introduction
The global burden of health complications following the SARS-CoV-2 pandemic has established post-acute sequelae of COVID-19 (or "long COVID") as a major public health concern. The clinical spectrum of long COVID is extensive, involving nearly all organ systems [
1,
2]; however, the precise pathogenetic mechanisms remain incompletely defined. Proposed contributors include viral persistence [
3,
4,
5,
6], systemic immune dysregulation, metabolic disturbances, endothelial dysfunction, and impaired tissue repair, which collectively may lead to cardiovascular, respiratory, endocrine, and immune disorders [
7,
8]. Investigating the molecular underpinnings of these post-viral syndromes is crucial for understanding fundamental disease pathophysiology and for identifying prognostic biomarkers and therapeutic targets.
Matrix metalloproteinases (MMPs), particularly the gelatinases MMP-2 and MMP-9, are central regulators of extracellular matrix (ECM) breakdown and remodeling and are implicated in both tissue repair and fibrogenesis. MMPs, calcium-dependent, zinc-containing neutral endopeptidases, possess broad substrate specificity. Their functions extend beyond ECM proteostasis to include the proteolytic activation or inactivation of cytokines, chemokines, and cell-surface receptors, thereby influencing immune cell recruitment, proliferation, and viability [
9,
10]. MMP-2 and MMP-9 are especially critical in alveolar and vascular remodeling due to their efficient hydrolysis of type IV collagen, a key structural component of basement membranes located at the epithelial/endothelial interface and the underlying connective tissue [
11]. While essential for normal turnover, their overexpression and dysregulated activity are hallmarks of pathological tissue destruction and fibrosis. For instance, elevated MMP-9 is strongly associated with the progression of idiopathic pulmonary fibrosis (IPF) and aortic aneurysm [
12,
13], while MMP-2 contributes to vascular dysfunction, atherosclerosis, and plaque instability [
14]. Both enzymes can degrade elastin and basement membranes, increasing vascular permeability, promoting leukocyte infiltration, and disrupting endothelial integrity. Conversely, MMPs also exhibit context-dependent anti-inflammatory roles; for example, MMP-9 activity is necessary for resolving inflammation after myocardial infarction [
15], and MMP-2 can proteolytically inactivate pro-inflammatory mediators [
16,
17].
MMPs also play a dual role in viral pathogenesis, possessing both antiviral properties and contributing to viral dissemination and persistence. Viruses such as HIV, HTLV-1, and hepatitis B stimulate hyperproduction of MMP-2, MMP-9, and MMP-14 in monocytes and T-lymphocytes, disrupting basement membranes and endothelial barriers – a process implicated in neuroinvasion and blood-brain barrier breakdown [
18,
19,
20,
21]. In COVID-19, plasma MMP-9 levels are significantly elevated, correlating with disease severity, leukocytosis, and the pro-inflammatory cytokine storm that drives acute respiratory distress syndrome (ARDS) and pulmonary fibrosis [
22,
23,
24]. MMP-9 further contributes to immunothrombosis via neutrophil and platelet activation [
25]. Interestingly, MMP-2 levels are often decreased in acute COVID-19 plasma, possibly due to systemic ACE imbalance or consumption [
26,
27], while lung tissues show significant increases in MMP-2, MMP-7, MMP-8, and MMP-14 [
28].
Notably, there is considerable evidence of striking clinical, histopathological, and molecular similarities between idiopathic pulmonary fibrosis (IPF) and COVID-induced pulmonary fibrosis; for a summary see the recent review [
29]. However, few studies have described common mechanisms and hallmarks for IPF and other chronic pulmonary diseases, such as post-COVID pulmonary fibrosis (PCPF) [
30]. IPF and PCPF are both progressive fibrotic lung diseases characterized by chronic inflammation, aberrant vascular remodeling, and immune dysregulation. While IPF is the prototypical progressive fibrosing interstitial lung disease, PCPF is an emerging entity following SARS-CoV-2 infection. They converge on key pro-fibrotic pathways – including TGF-β, IL-6, and TNF-α signaling – and systemic features such as endothelial injury and pro-thrombotic states [
29,
30]. This overlap suggests shared underlying mechanisms where dysregulated MMP activity may perpetuate a cycle of injury, failed repair, and fibrosis in both conditions.
Despite extensive data on MMPs in acute COVID-19, their role in the chronic phase, particularly in post-COVID-19 syndrome with persistent respiratory sequelae, remains poorly understood. Evidence of prolonged SARS-CoV-2 antigen persistence [
3,
4,
5,
6] suggests that MMP dysregulation may continue well into the convalescent period, potentially driving ongoing inflammation and fibrotic remodeling.
This study aimed to analyze the expression of MMP-2 and MMP-9 genes in peripheral blood leukocytes and the levels of their encoded proteins in blood plasma of patients at 6 and 12 months after acute COVID-19. We hypothesize that sustained dysregulation of these MMPs is associated with persistent inflammatory and endothelial dysfunction, reflecting a pathophysiological continuum shared with IPF.
2. Materials and Methods
2.1. Ethics
This study was conducted in accordance with the ethical principles of the World Medical Association Declaration of Helsinki for medical research involving human subjects. The protocol was approved by the Medical Ethics Committee of Petrozavodsk State University and the Ministry of Health of the Republic of Karelia (ethical approval no. 2, dated 09.09.2024).
2.2. Study Participants and Materials
This case-control study included 86 patients (mean age 47.15 ± 0.84 years; 1:1 sex ratio) with a history of confirmed COVID-19 (diagnosed in 2020–2021). Post-COVID patients were stratified into four groups according to the presence or absence of acute-phase pulmonary manifestations on computed tomography (CT) and the time of sample collection after infection: (a) patients without pulmonary involvement at 6 months post-infection (n=30); (b) patients with lung lesions and subsequent development of pulmonary fibrosis at 6 months post-infection. (n=16); (c) patients without pulmonary involvement at 12 months post-infection (n=24); (d) patients with lung lesions and subsequent development of pulmonary fibrosis at 12 months post-infection (n=16). The control group consisted of 20 conditionally healthy individuals (mean age 43.42 ± 1.14 years) from whom blood samples were collected and processed in 2019, prior to the pandemic. To provide a specific comparator for COVID-19-induced pulmonary changes, a separate group of patients diagnosed with idiopathic pulmonary fibrosis (IPF) was also included (mean age 63.6 ± 3.44 years, n=10).
Exclusion criteria for all participants were: concomitant immunoinflammatory or chronic systemic diseases; acute infectious diseases (other than COVID 19) within the preceding 6 months; current tobacco use; alcohol abuse; body mass index (BMI) ≥ 28 kg·m⁻². Prior to the study, hematological and biochemical parameters were assessed for all enrolled donors using a BF-6800 hematological analyzer and a CS-300B automated biochemical analyzer (Dirui, China); the quality of results was ensured through daily internal quality control using control sera for normal and pathological levels. The assessed parameters included a complete blood count, erythrocyte sedimentation rate, and the levels/activities of total protein, albumin, glucose, direct bilirubin, creatinine, urea, total cholesterol, ALT, AST, and alkaline phosphatase to ensure cohort homogeneity based on values approaching the Biological Reference Interval; according to the observation of [
31] the values in COVID and post-COVID patients with comorbidity could substantially differ from reference interval [
31]. At the time of enrollment, all participants tested negative for SARS-CoV-2. Participants were recruited from the Respiratory Centre of the V.A. Baranov Republican Hospital (Petrozavodsk, Russia). Peripheral blood samples were collected by staff of the Centre for Biomedical Research, KarRC RAS. Written informed consent was obtained from every participant.
2.3. Enzyme-Linked Immunosorbent Assay (ELISA)
The plasma levels of matrix metalloproteinases and other markers were assayed by non-competitive enzyme-linked immunosorbent assay (ELISA) using Human ELISA kits for MMP-2, MMP-9, Endothelin-1, and E-Selectin (ELK Biotechnology, China); IL-6-IFA-BEST and TNFα-IFA-BEST kits (Vector-Best, Russia) according to the manufacturers’ protocols on a microplate reader AMR-100 (Allsheng, China). The number of technical replicates was at least two. We used blood plasma, rather than serum, to determine extracellular MMP concentrations and exclude MMP amounts released from cells during blood coagulation.
2.4. RNA Extraction, Quantitative Real-Time PCR
Total RNA was isolated from peripheral blood leukocytes (PBLs), which were obtained by lysing whole blood with a 0.86% ammonium chloride solution, using MagZole reagent (Magen, China). Complementary DNA (cDNA) was synthesized from the RNA template using an MMLV RT kit (Eurogen, Russia). Prior to reverse transcription, the RNA was treated with 1 unit of DNase (Syntol, Russia) to remove genomic DNA contamination.
The mRNA expression levels were quantified by real-time PCR using a C1000 Touch thermal cycler equipped with a CFX96 optical reaction module (BioRad, USA). Primer annealing specificity was verified by analyzing the melting curves of the PCR amplicons. All reactions were performed with a minimum of three replicates.
The relative expression of the target genes was calculated using the ΔΔCt method [
32], with normalization to the geometric mean of two reference genes,
18S rRNA and
RPL19. Primer sequences were sourced from [
33] and are detailed in
Table 1.
2.5. Statistical Analysis
Experimental data were processed using Microsoft Excel and Statgraphics Centurion XVI software. According to the Shapiro-Wilk test, the distribution of the studied parameters was non-normal. Differences in gene expression levels and biochemical indices were evaluated using the Mann-Whitney U test. Correlation analysis was used to assess the relationships between parameters. Data are presented as median (Me) with 25th and 75th percentiles (Q1; Q3). The age of individuals included in the study is presented as mean ± standard error (M ± SE). Differences were considered statistically significant at p < 0.05.
3. Results
3.1. MMP-2 and MMP-9 Plasma Levels and Gene Expression
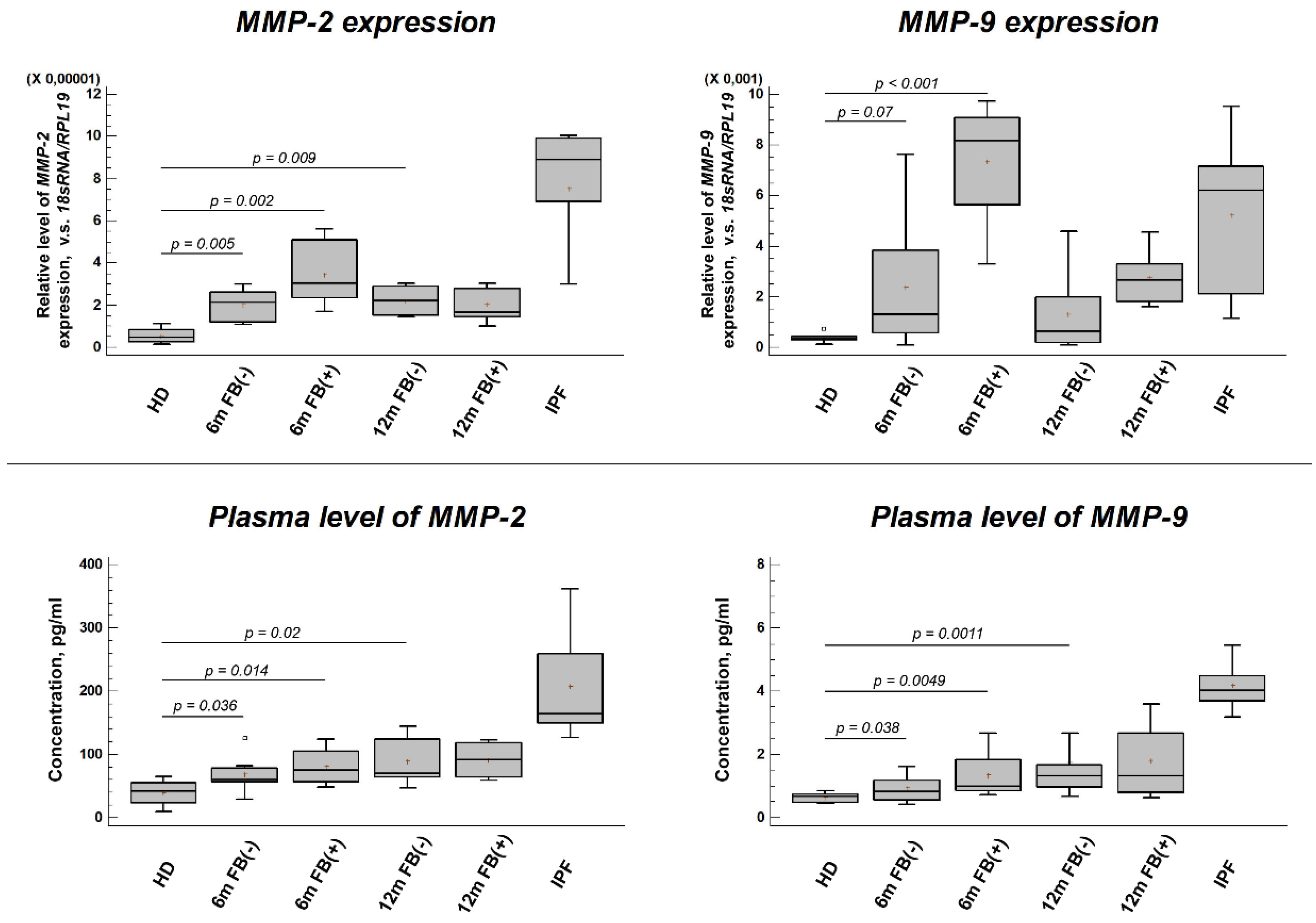
The results of the study are presented in
Figure 1 (A-D). At 6- and 12-months post-SARS-CoV-2 infection, a significant increase in the plasma levels and leukocyte gene expression of both MMP-2 and MMP-9 was observed compared to pre-pandemic control samples. This increase was statistically significant in patient groups regardless of whether the acute infection was associated with lung damage and subsequent fibrosis (FB+) or not (FB–). However, in both post-COVID-19 groups, MMP-2 and MMP-9 levels remained lower than in patients with idiopathic pulmonary fibrosis (IPF), who, as expected, demonstrated substantially elevated levels of both MMPs compared to healthy donors.
3.2. Plasma Concentration of Pro-inflammatory Cytokines
ELISA revealed a decrease in plasma levels of TNF-α and IL-6 at 6- and 12-months post-COVID-19 in patients whose acute infection did not involve lung damage according to CT (FB– group;
Table 2). In contrast, patients diagnosed with post-COVID pulmonary fibrosis (FB+ group) exhibited a significant increase in TNF-α and IL-6 levels at both time points compared to the pre-pandemic healthy donor (HD) control group and the FB– group.
3.3. Plasma Concentration of Endothelial Dysfunction Markers
Analysis of endothelial dysfunction markers revealed a distinct pattern of late elevation (
Table 3). At 12 months post-infection, significant increases in Endothelin-1 (ET-1) and soluble intercellular adhesion molecule-1 (sICAM-1) were observed in patients with pulmonary fibrosis (FB+), with ET-1 reaching an exceptionally high concentration. E-selectin was also elevated in the FB+ group at this time point, whereas soluble vascular cell adhesion molecule-1 (sVCAM-1) levels did not change significantly. At the 6-month time point, marker levels did not differ significantly from healthy donor (HD) controls, except for sICAM-1, which was elevated in the FB+ group.
3.4. Correlation of Plasma Levels of MMPs, Pro-inflammatory Cytokines, and Endothelial Dysfunction Markers
Correlation analysis revealed a positive, moderate association between plasma MMP-2 and MMP-9 levels and Endothelin-1 (ET-1) (rₛ = 0.65, p = 0.004 and rₛ = 0.49, p = 0.009, respectively). ET-1 and soluble intercellular adhesion molecule-1 (sICAM-1) levels were also positively correlated (rₛ = 0.68, p = 0.01). This pattern of correlations suggests a potential link between extracellular matrix remodeling (reflected by MMPs), endothelial dysfunction, and systemic inflammation in the post-COVID-19 state.
4. Discussion
This study demonstrates a distinct and sustained dysregulation of the systemic inflammatory and endothelial milieu in patients after COVID-19, with a critical divergence based on the presence of post-infection pulmonary fibrosis (PCPF). Our key findings reveal: (1) a persistent pro-inflammatory state (elevated TNF-α and IL-6) specifically in patients with fibrosis (FB+) at 6 and 12 months; (2) a delayed but marked increase in endothelial dysfunction markers (ET-1, sICAM-1, E-selectin) in the FB+ group at 12 months; and (3) significant positive correlations between markers of extracellular matrix (ECM) remodeling (MMP-2, MMP-9), the potent vasoconstrictor ET-1, and the adhesion molecule sICAM-1. Together, these data suggest a pathophysiological triad linking persistent inflammation, aberrant endothelial activation, and dysregulated fibrosis in post-COVID pulmonary sequelae.
Dysregulated extracellular matrix remodeling, driven by the hyperactivation of matrix metalloproteinases (MMPs), is a hallmark of fibrotic lung diseases, including post-COVID-19 complications [
27,
28]. Given evidence of persistent SARS-CoV-2 components in patient blood long after acute infection [
3,
4,
5,
6] we hypothesized that such viral persistence could contribute to sustained systemic alterations, including elevated MMP levels. Supporting this, we found significantly increased MMP-2 and MMP-9 gene expression in PBLs and corresponding plasma protein levels at 6- and 12-months post-infection in all patients, regardless of fibrotic status (FB+ or FB–). However, MMP levels in post-COVID-19 cohorts remained lower than those in patients with idiopathic pulmonary fibrosis (IPF), a canonical fibrotic disease. While elevated MMPs in early convalescence may reflect tissue repair [
34], their sustained elevation at 6-12 months aligns with models linking prolonged MMP dysregulation to chronic viral antigenemia and inflammatory sequelae [
27], suggesting a shift from transient repair to persistent pathological remodeling.
The immunological profiles diverged sharply based on fibrotic outcome. In patients without fibrosis (FB–), plasma levels of TNF-α and IL-6 decreased significantly by 6 months, indicating resolution of the acute hyperinflammatory state. In stark contrast, the FB+ group exhibited a significant and persistent increase in these cytokines at both time points. This sustained pro-inflammatory state is consistent with the established role of TNF-α and IL-6 in driving fibroblast activation and progressive fibrogenesis [
30,
35], and suggests the presence of an unresolved inflammatory circuit, potentially fueled by viral persistence or autoimmune mechanisms [
3,
6].
The most novel finding is the delayed, profound endothelial dysfunction observed specifically in the FB+ group at 12 months. Markers of endothelial activation (sICAM-1, E-selectin) were elevated, but the most striking increase was in ET-1—a potent vasoconstrictor and pro-fibrotic mediator—which reached concentrations an order of magnitude above controls. This late-phase surge suggests a process of cumulative microvascular injury that becomes biochemically manifest during the tissue remodeling phase [
8,
36]. The concurrent rise in adhesion molecules confirms widespread endothelial activation, facilitating leukocyte recruitment and perpetuating local injury [
10].
Crucially, correlation analysis suggests these pathways are interconnected. We found positive, moderate correlations between plasma MMP-2/MMP-9 and ET-1, and between ET-1 and sICAM-1. This points to a potential feed-forward loop: persistent inflammation (TNF-α/IL-6) may drive endothelial dysfunction and MMP activation; MMPs can then process and activate latent ET-1 and cleave adhesion molecules like ICAM-1 from the endothelial surface [
14,
37,
38]; and ET-1, in turn, can upregulate MMP expression and promote further fibrosis and inflammation [
36,
39]. Thus, we propose a vicious cycle where sustained inflammation, endothelial dysfunction, and MMP-mediated ECM remodeling reinforce each other, driving the progression and persistence of post-COVID pulmonary fibrosis.
5. Conclusions
In conclusion, our results identify a specific biochemical signature associated with post-COVID pulmonary fibrosis, characterized by the convergence of sustained systemic inflammation, severe late-phase endothelial dysfunction (dominated by ET-1), and evidence of persistent ECM remodeling. The correlative links between MMPs, ET-1, and inflammatory markers suggest these pathways are interconnected, potentially forming a self-perpetuating cycle that drives injury and fibrotic progression. The primary limitations of this study are its observational design and modest sample size, particularly in the fibrosis group, which preclude definitive causal inferences. Furthermore, the lack of a mechanistic model limits our ability to dissect the precise interactions within the proposed MMP-ET-1-inflammation axis. Despite these limitations, our findings highlight novel therapeutic targets. Pharmacological intervention within this axis—for instance, using endothelin receptor antagonists or selective MMP modulators—may represent a promising strategy for preventing or mitigating the progression of post-COVID pulmonary fibrosis. Future longitudinal studies and mechanistic investigations are warranted to validate this pathogenic loop and explore its therapeutic potential.
Author Contributions
Conceptualization, O.V.B. and I.E.M.; methodology, O.V.B., I.E.M., and E.L.T.; investigation, O.V.B., I.E.M., and E.L.T.; data curation, I.E.M.; formal analysis, I.E.M.; medical observation, patient examination, and cohort formation, E.L.T.; writing—original draft preparation, O.V.B.; writing—review and editing, L.A.L.; visualization, I.E.M.; resources and funding acquisition, L.A.L.; supervision, O.V.B.; project administration, O.V.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the state task for KarRC RAS No. FMEN-2022-0017. The study was conducted using the equipment of the Core Facility of KarRC RAS.
Institutional Review Board Statement
The study was conducted in accordance with the ethical principles of the World Medical Association’s Declaration of Helsinki. The study protocol was reviewed and approved by the Medical Ethics Committee of Petrozavodsk State University and the Ministry of Health of the Republic of Karelia (Protocol no. 2, dated 09 September 2024).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
All data generated or analyzed during this study are shown in this article.
Acknowledgments
During the preparation of this work, the authors used DeepSeek AI to improve language and readability of the manuscript. After using this tool, the authors reviewed and edited the content as needed and take full responsibility for the content of this publication.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ARDS |
acute respiratory distress syndrome |
| COVID-19 |
coronavirus disease 2019 |
| CT |
computed tomography |
| ET-1 |
endothelin-1 |
| IL |
interleukin |
| IPF |
idiopathic pulmonary fibrosis |
| MMP |
matrix metalloproteinase |
| PBLs |
peripheral blood leucocytes |
| PCPF |
post-COVID-19 pulmonary fibrosis |
| SARS-CoV-2 |
severe acute respiratory syndrome coronavirus 2 |
| sICAM |
soluble intercellular cell adhesion molecule-1 |
| sVCAM |
soluble vascular cell adhesion molecule-1 |
| TNFα |
tumor necrosis factor-alpha |
References
- Al-Aly, Z.; Bowe, B.; Xie, Y. Long COVID after breakthrough SARS-CoV-2 infection. Nature Medicine 2022, 28, 1461–1467. [CrossRef]
- Schiff, H.; Boudreau, E.A.; Smith, J.M.; Nguyen, T.; Alavi, A.; Patel, Y.; Brown, E.M.; Langlois, J.P. Long-term symptoms following COVID-19: A 12-month follow-up study. Journal of Clinical Investigation 2023, 133, e154987. [CrossRef]
- Tejerina, F.; Catalan, P.; Rodriguez-Grande, C.; Adan, J.; Rodriguez-Gonzalez, C.; Muñoz, P.; Aldamiz, T.; Diez, C.; Perez, L.; Fanciulli, C.; et al. Post-COVID-19 syndrome. SARS-CoV-2 RNA detection in plasma, stool, and urine in patients with persistent symptoms after COVID-19. BMC Infectious Diseases 2022, 22, 211. [CrossRef]
- Topchieva, L.V.; Balan, O.V.; Men’shenin, A.V.; Malysheva, I.E.; Tikhonovich, E.L. Quantitative assay of SARS-CoV-2 RNA and level of proinflammatory protein gene transcripts in peripheral blood leukocytes after a novel coronavirus infection. Bulletin of Experimental Biology and Medicine 2022, 173, 740–744. [CrossRef]
- Swank, Z.; Senussi, Y.; Manickas-Hill, Z.; Yu, X.G.; Li, J.Z.; Alter, G.; Walt, D.R. Persistent circulating severe acute respiratory syndrome coronavirus 2 spike is associated with post-acute coronavirus disease 2019 sequelae. Clinical Infectious Diseases 2023, 76, e487–e490. [CrossRef]
- Zuo, W.; He, D.; Liang, C.; Du, S.; Hua, Z.; Nie, Q.; Zhou, X.; Yang, M.; Tan, H.; Xu, J.; et al. The persistence of SARS-CoV-2 in tissues and its association with long COVID symptoms: a cross-sectional cohort study in China. The Lancet Infectious Diseases 2024, 24, 845–855. [CrossRef]
- Pretorius, E.; Vlok, M.; Venter, C.; Bezuidenhout, J.A.; Laubscher, G.J.; Steenkamp, J.; Kell, D.B. Persistent clotting protein pathology in Long COVID/Post-Acute Sequelae of COVID-19 (PASC) is accompanied by increased levels of antiplasmin. Cardiovascular Diabetology 2021, 20, 172. [CrossRef]
- Haffke, M.; Freitag, H.; Rudolf, G.; Seifert, M.; Doehner, W.; Scherbakov, N.; Hanitsch, L.; Wittke, K.; Bauer, S.; Konietschke, F.; et al. Endothelial dysfunction and altered endothelial biomarkers in patients with post-COVID-19 syndrome and chronic fatigue syndrome (ME/CFS). Journal of Translational Medicine 2022, 20, 138. [CrossRef]
- Fingleton, B. Matrix metalloproteinases as regulators of inflammatory processes. Biochimica et Biophysica Acta (BBA) – Molecular Cell Research 2017, 1684, 2036–2042. [CrossRef]
- Smigiel, K.S.; Parks, W.C. Matrix metalloproteinases and leukocyte activation. Progress in Molecular Biology and Translational Science 2017, 147, 167–195. [CrossRef]
- Jacob, M.P.; Badier-Commander, C.; Fontaine, V.; Benazzoug, Y.; Feldman, L.; Michel, J.B. Extracellular matrix remodeling in the vascular wall. Pathologie Biologie 2001, 49, 326–332. [CrossRef]
- Davey, A.; McAuley, D.F.; O'Kane, C.M. Matrix metalloproteinases in acute lung injury: mediators of injury and drivers of repair. European Respiratory Journal 2011, 38, 959–970. [CrossRef]
- Tsarouhas, K.; Tsitsimpikou, C.; Apostolakis, S.; Haliassos, A.; Tzardi, M.; Panagiotou, M.; Tsatsakis, A.; Spandidos, D.A. Homocysteine and metalloprotease-3 and -9 in patients with ascending aorta aneurysms. Thrombosis Research 2011, 128, e95–e99. [CrossRef]
- de Jager, S.C.A.; Hoefer, I.E. Beyond the matrix: MMP2 as critical regulator of inflammation-mediated vascular dysfunction. Cardiovascular Research 2017, 113, 1705–1707. [CrossRef]
- Iyer, R.P.; de Castro Brás, L.E.; Patterson, N.L.; Bhowmick, M.; Flynn, E.R.; Asher, M.; Cannon, P.L.; Deleon-Pennell, K.Y.; Fields, G.B.; Lindsey, M.L. Early matrix metalloproteinase-9 inhibition post-myocardial infarction worsens cardiac dysfunction by delaying inflammation resolution. Journal of Molecular and Cellular Cardiology 2016, 100, 109–117. [CrossRef]
- Berry, E.; Hernandez-Anzaldo, S.; Ghomashchi, F.; Lehner, R.; Murakami, M.; Gelb, M.H.; Kassiri, Z.; Wang, X.; Fernandez-Patron, C. Matrix metalloproteinase-2 negatively regulates cardiac secreted phospholipase A2 to modulate inflammation and fever. Journal of the American Heart Association 2015, 4, e001868. [CrossRef]
- Fernandez-Patron, C.; Kassiri, Z.; Leung, D. Modulation of systemic metabolism by MMP-2: from MMP-2 deficiency in mice to MMP-2 deficiency in patients. Comprehensive Physiology 2016, 6, 1935–1949. [CrossRef]
- Lafrenie, R.M.; Wahl, L.M.; Epstein, J.S.; Hewlett, I.K.; Yamada, K.M.; Dhawan, S. HIV-1-Tat modulates the function of monocytes and alters their interactions with microvessel endothelial cells. A mechanism of HIV pathogenesis. The Journal of Immunology 1996, 156, 1638–1645.
- Giraudon, P.; Szymocha, R.; Buart, S.; Bernard, A.; Cartier, L.; Belin, M.F.; Akaoka, H. T lymphocytes activated by persistent viral infection differentially modify the expression of metalloproteinases and their endogenous inhibitors, TIMPs, in human astrocytes: relevance to HTLV-I-induced neurological disease. The Journal of Immunology 2000, 164, 2718–2727. [CrossRef]
- Missé, D.; Esteve, P.O.; Renneboog, B.; Vidal, M.; Cerutti, M.; St Pierre, Y.; Yssel, H.; Parmentier, M.; Veas, F. HIV-1 glycoprotein 120 induces the MMP-9 cytopathogenic factor production that is abolished by inhibition of the p38 mitogen-activated protein kinase signaling pathway. Blood 2001, 98, 541–547. [CrossRef]
- Chung, T.W.; Lee, Y.C.; Kim, C.H. Hepatitis B viral HBx induces matrix metalloproteinase-9 gene expression through activation of ERK and PI-3K/AKT pathways: involvement of invasive potential. The FASEB Journal 2004, 18, 1123–1125. [CrossRef]
- Hazra, S.; Chaudhuri, A.G.; Tiwary, B.K.; Chakrabarti, N. Matrix metallopeptidase 9 as a host protein target of chloroquine and melatonin for immunoregulation in COVID-19: A network-based meta-analysis. Life Sciences 2020, 257, 118096. [CrossRef]
- Lerum, T.V.; Maltzahn, N.N.; Aukrust, P.; Trøseid, M.; Henriksen, K.N.; Kåsine, T.; Dyrhol-Riise, A.M.; Stiksrud, B.; Haugli, M.; Blomberg, B.; et al. Persistent pulmonary pathology after COVID-19 is associated with high viral load, weak antibody response, and high levels of matrix metalloproteinase-9. Scientific Reports 2021, 11, 23205. [CrossRef]
- Martinez Mesa, A.; Cabrera César, E.; Martín-Montañez, E.; Sanchez Alvarez, E.; Lopez, P.M.; Romero-Zerbo, Y.; Garcia-Fernandez, M.; Velasco Garrido, J.L. Acute lung injury biomarkers in the prediction of COVID-19 severity: total thiol, ferritin and lactate dehydrogenase. Antioxidants 2021, 10, 1221. [CrossRef]
- Bonetto, V.; Pasetto, L.; Lisi, I.; Carbonara, M.; Zangari, R.; Ferrari, E.; Punzi, V.; Luotti, S.; Bottino, N.; Biagianti, B.; et al. Markers of blood-brain barrier disruption increase early and persistently in COVID-19 patients with neurological manifestations. Frontiers in Immunology 2022, 13, 1070379. [CrossRef]
- Carolina, D.A.-M.; Couto, A.E.S.; Campos, L.C.B.; Vasconcelos, T.F.; Michelon-Barbosa, J.; Corsi, C.A.C.; Mestriner, F.; Petroski-Moraes, B.C.; Garbellini-Diab, M.J.; Couto, D.M.S.; et al. MMP-2 and MMP-9 levels in plasma are altered and associated with mortality in COVID-19 patients. Biomedicine & Pharmacotherapy 2021, 142, 112067. [CrossRef]
- Fernandez-Patron, C.; Hardy, E. Matrix metalloproteinases in health and disease in the times of COVID-19. Biomolecules 2022, 12, 692. [CrossRef]
- da Silva-Neto, P.V.; do Valle, V.B.; Fuzo, C.A.; Fernandes, T.M.; Toro, D.M.; Fraga-Silva, T.F.C.; Basile, P.A.; de Carvalho, J.C.S.; Pimentel, V.E.; Pérez, M.M.; et al. Matrix metalloproteinases on severe COVID-19 lung disease pathogenesis: cooperative actions of MMP-8/MMP-2 axis on immune response through HLA-G shedding and oxidative stress. Biomolecules 2022, 12, 604. [CrossRef]
- Patrucco, F.; Solidoro, P.; Gavelli, F.; Apostolo, D.; Bellan, M. Idiopathic pulmonary fibrosis and post-COVID-19 lung fibrosis: links and risks. Microorganisms 2023, 11, 895. [CrossRef]
- Mara, G.; Nini, G.; Frent, S.M.; Cotoraci, C. Hematologic and immunologic overlap between COVID-19 and idiopathic pulmonary fibrosis. Journal of Clinical Medicine 2025, 14, 5229. [CrossRef]
- Robu Popa, D.; Melinte, O.E.; Dobrin, M.-E.; Cernomaz, A.T.; Grigorescu, C.; Nemes, A.F.; Todea, D.A.; Vulturar, D.M.; Grosu-Creangă, I.A.; Lunguleac, T.; Trofor, A.C. Laboratory diagnostics accuracy for COVID-19 versus post-COVID-19 syndrome in lung disease patients with multimorbidity. Journal of Personalized Medicine 2024, 14, 171. [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [CrossRef]
- Kurbatova, I.V.; Topchieva, L.V.; Dudanova, O.P.; Shipovskaya, A.A. Role of MMP-2 and MMP-9 in the relationship between inflammation, fibrosis, and apoptosis during progression of non-alcoholic fatty liver disease and diagnostic significance of plasma levels of their active forms. Biochemistry (Moscow) 2024, 89, 1998-2022. [CrossRef]
- Gelzo, M.; Cacciapuoti, S.; Pinchera, B.; De Rosa, A.; Cernera, G.; Scialò, F.; Comegna, M.; Mormile, M.; Fabbrocini, G.; Parrella, R.; et al. Matrix metalloproteinases (MMP) 3 and 9 as biomarkers of severity in COVID-19 patients. Scientific Reports 2022, 12, 1212. [CrossRef]
- Wynn, T.A.; Ramalingam, T.R. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nature Medicine 2013, 18, 1028–1040. [CrossRef]
- Swigris, J.J.; Brown, K.K. The role of endothelin-1 in the pathogenesis of idiopathic pulmonary fibrosis. BioDrugs 2010, 24, 49–54. [CrossRef]
- Sultan S.; Gosling, M.; Nagase, H.; Powell J.T. Shear stress-induced shedding of soluble intercellular adhesion molecule-1 from saphenous vein endothelium. FEBS Letters 2004, 564, 161-165. [CrossRef]
- Fernandez-Patron, C.; Radomski, M.W.; Davidge, S.T. Vascular matrix metalloproteinase-2 cleaves big endothelin-1 yielding a novel vasoconstrictor. Circulation Research 1999, 85, 906-911. [CrossRef]
- Lin, C.C.; Lin, W.N.; Hou, W.C.; Hsiao, L.D.; Yang, C.M. Endothelin-1 induces VCAM-1 expression mediated inflammation via receptor tyrosine kinases and Elk/p300 in human tracheal smooth muscle cells. American Journal of Physiology-Lung Cellular and Molecular Physiology 2015, 309, L211–L225. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).