Submitted:
08 December 2025
Posted:
09 December 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Experimental Program
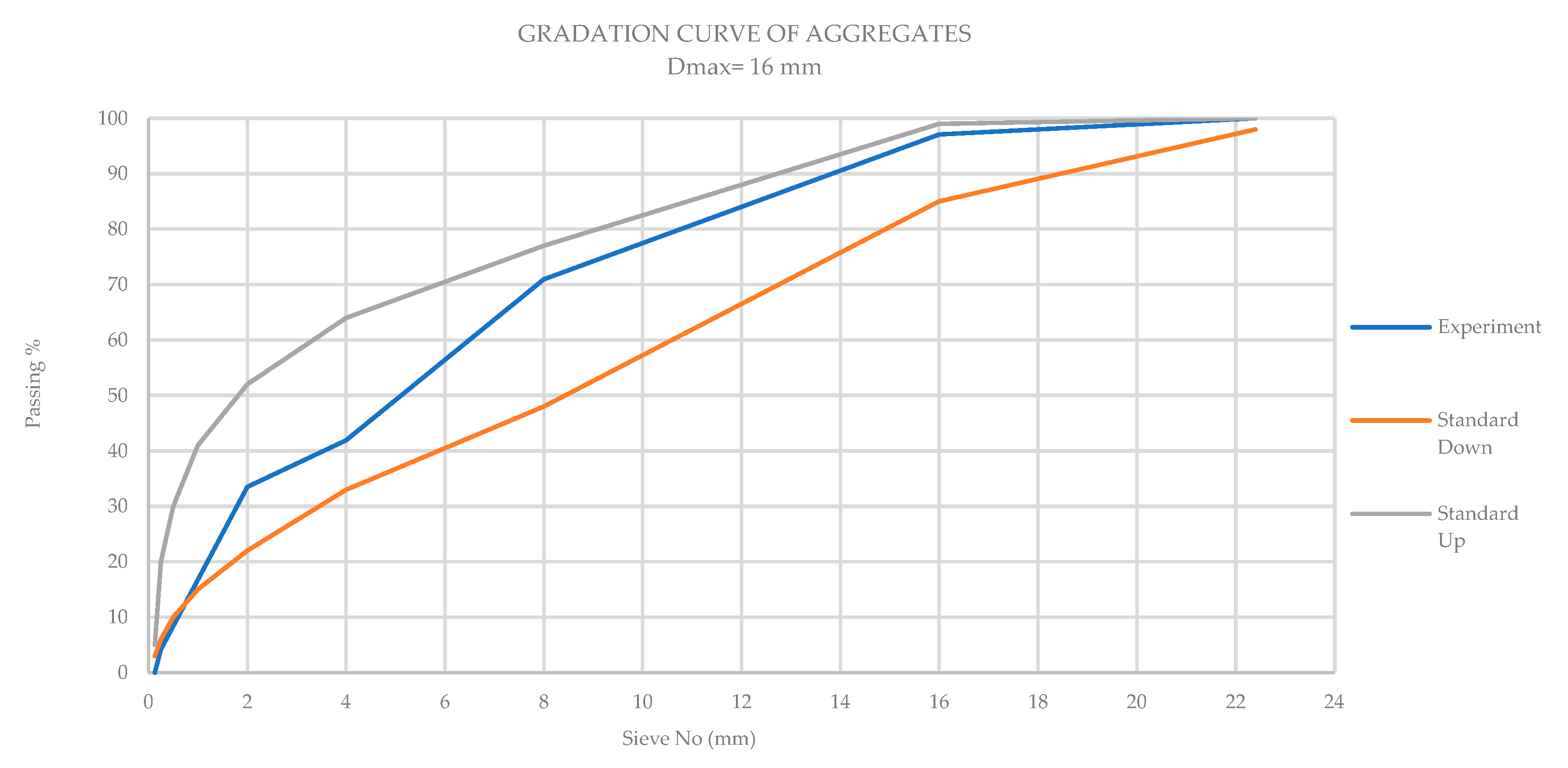
2.1. Materials and Mix Design
2.2. Preparation of Specimens and Curing Conditions
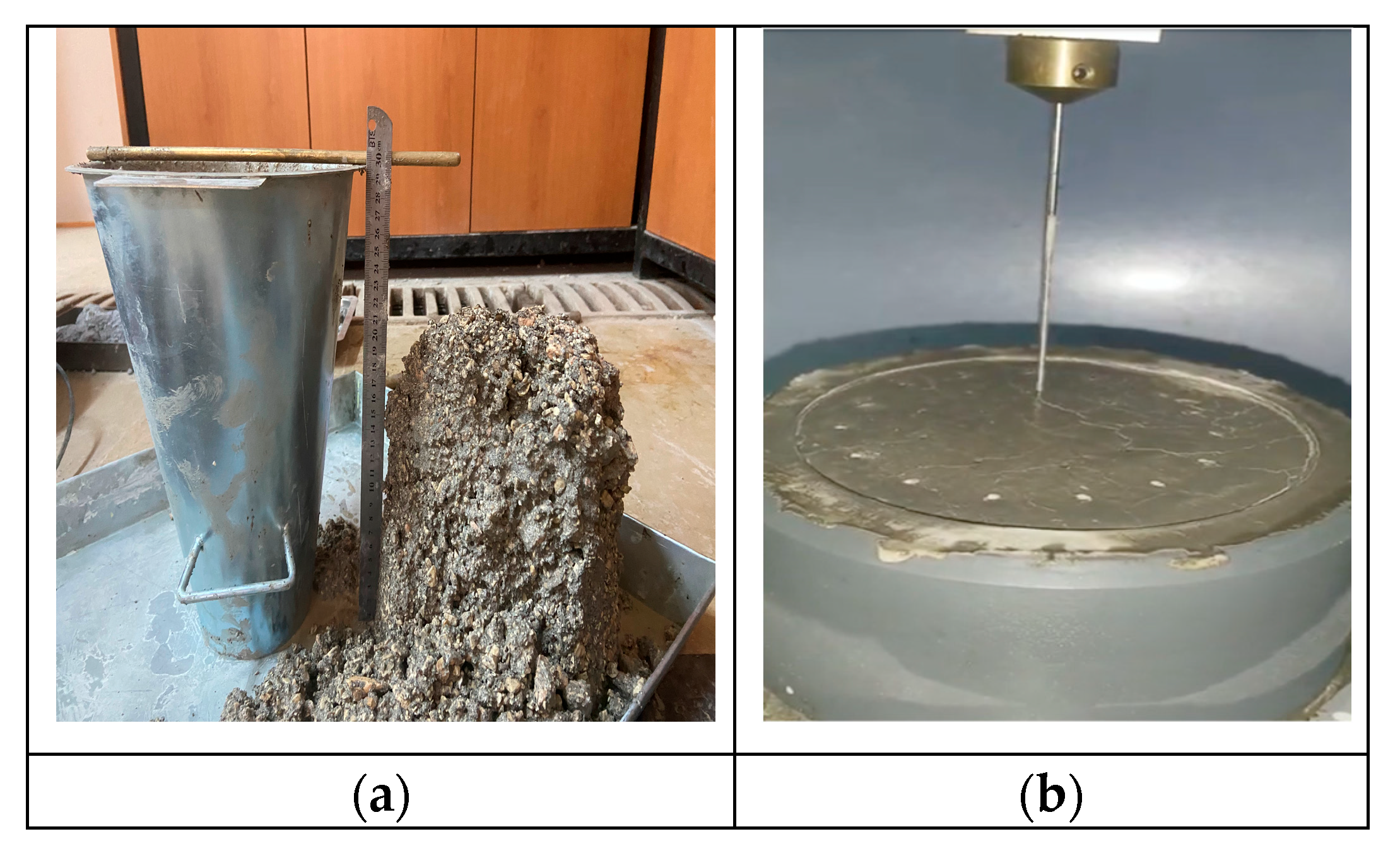
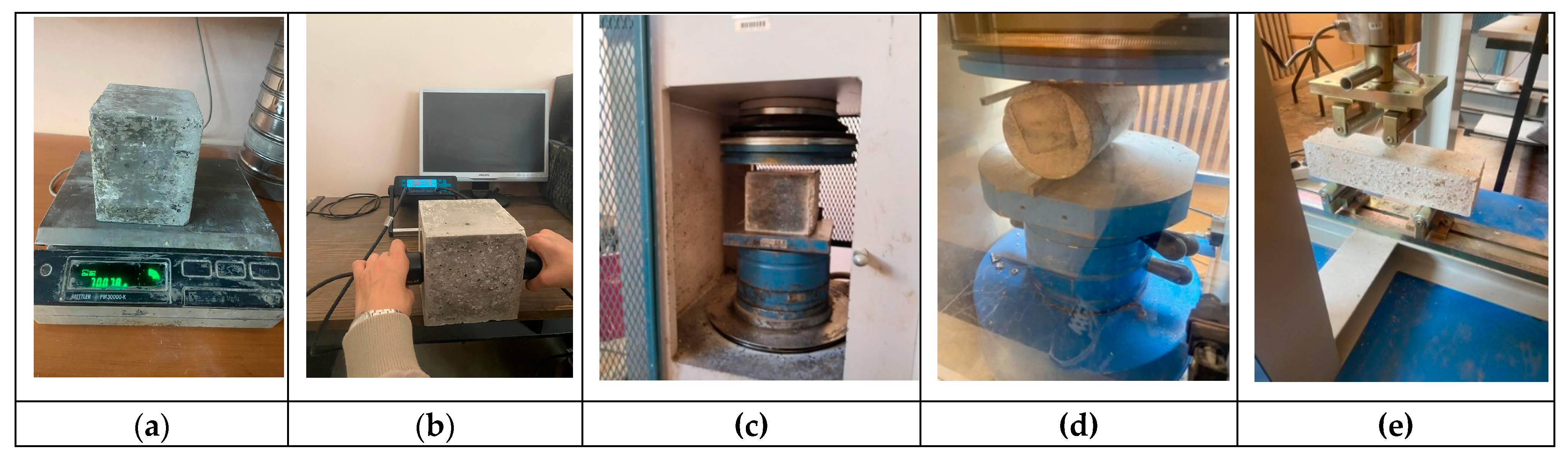
2.3. Testing Method
3. Results and Discussion
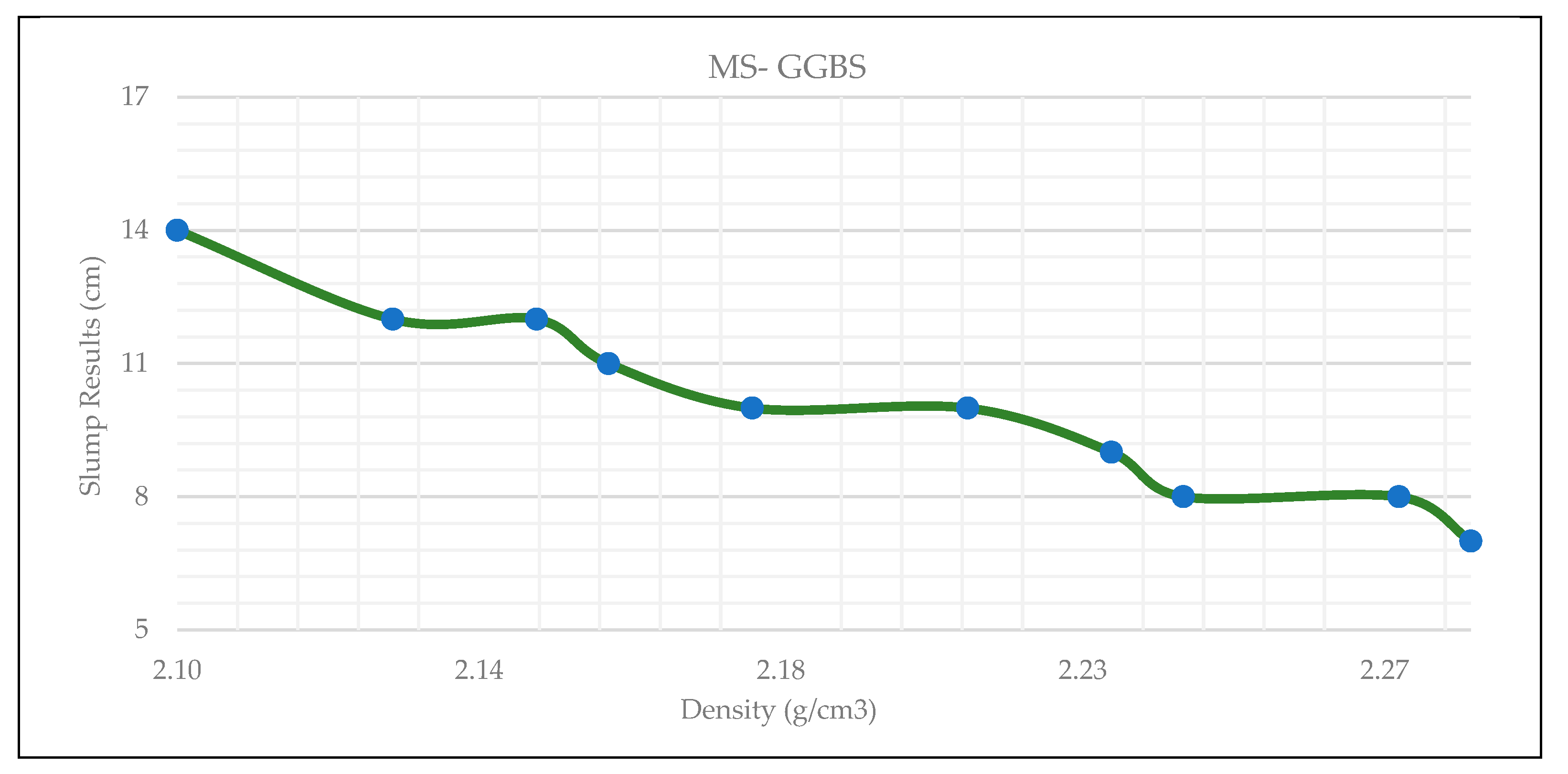
3.1. Workability
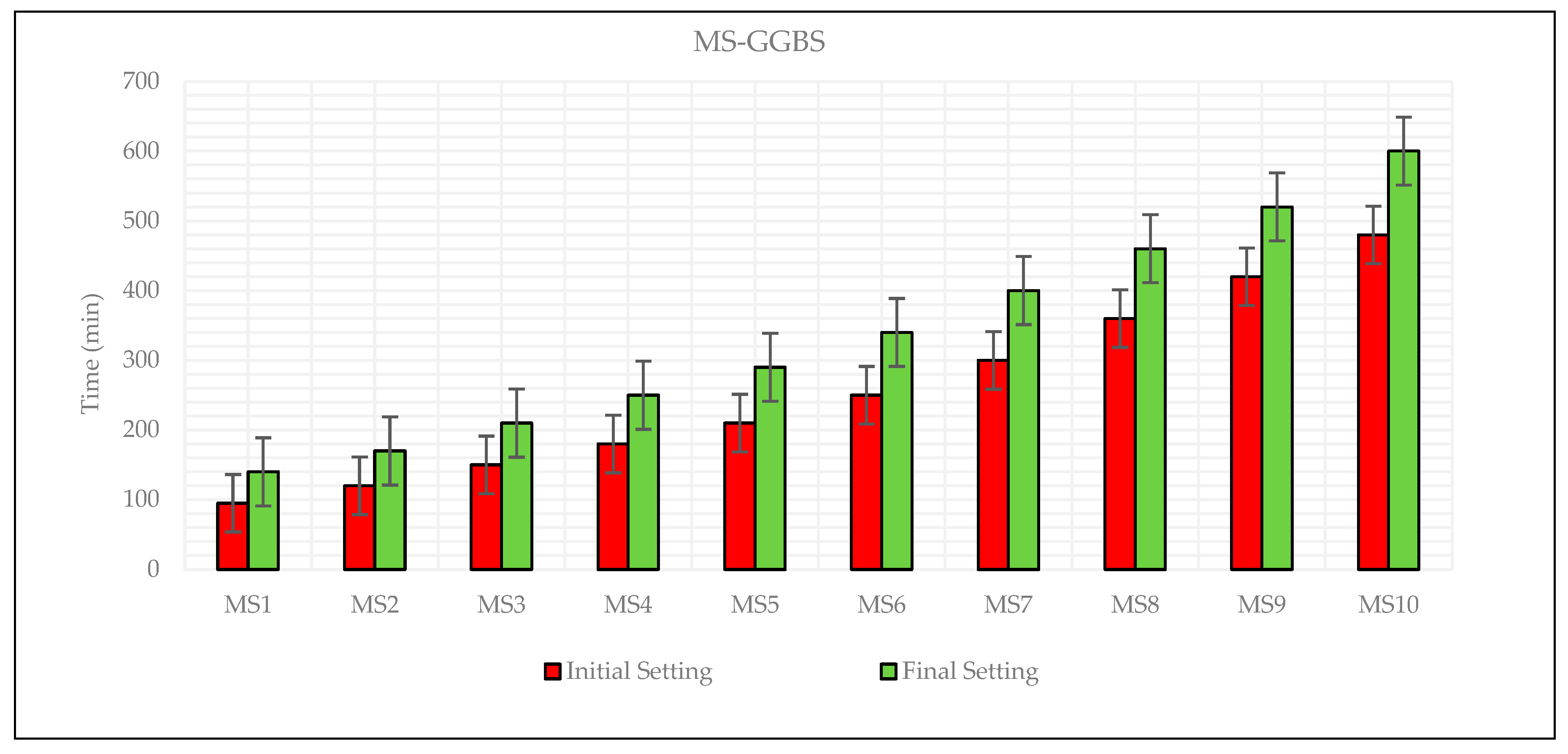
3.2. Setting Time
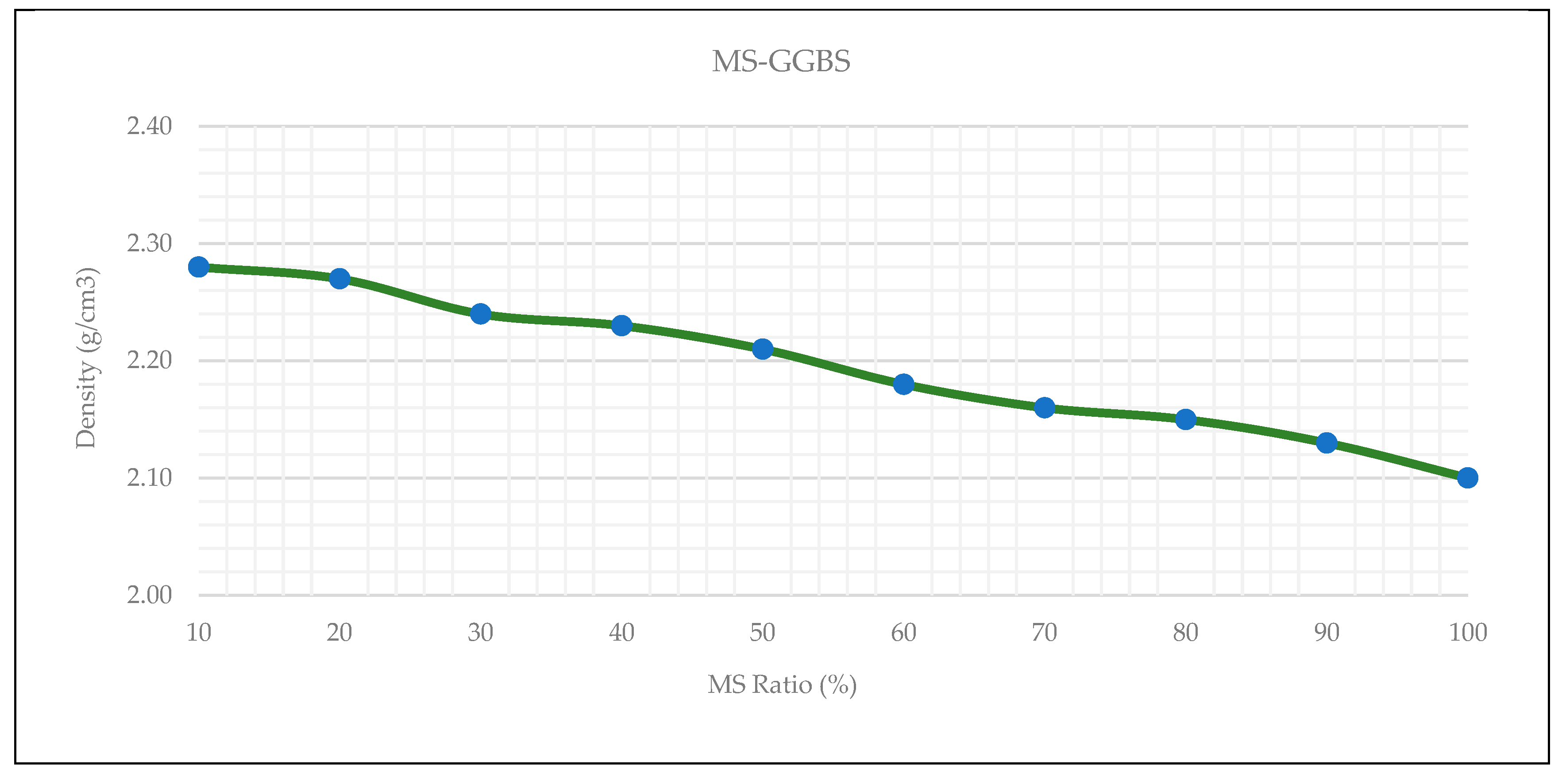
3.3. Wet Apparent Density
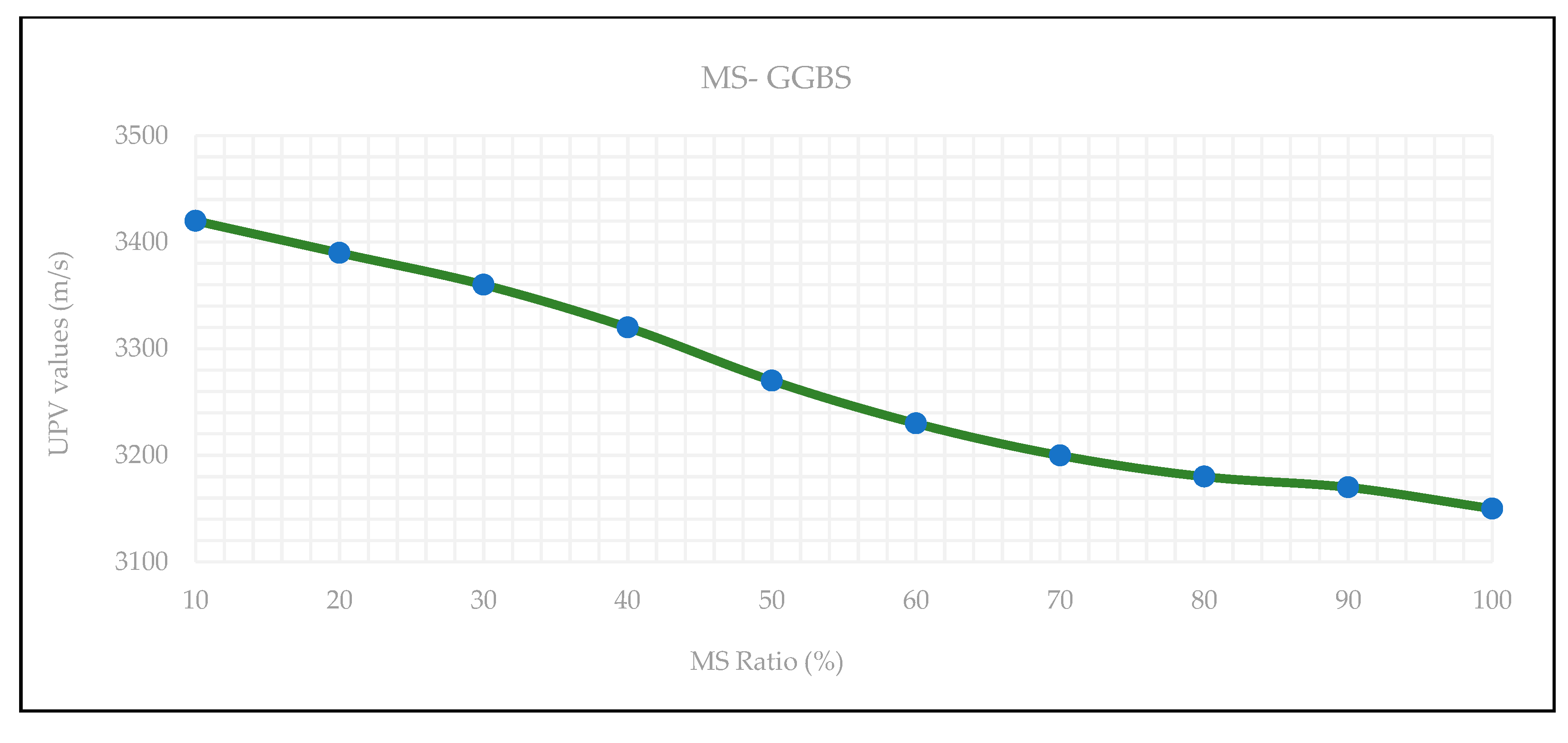
3.4. Ultrasonic Pulse Velocity (UPV)
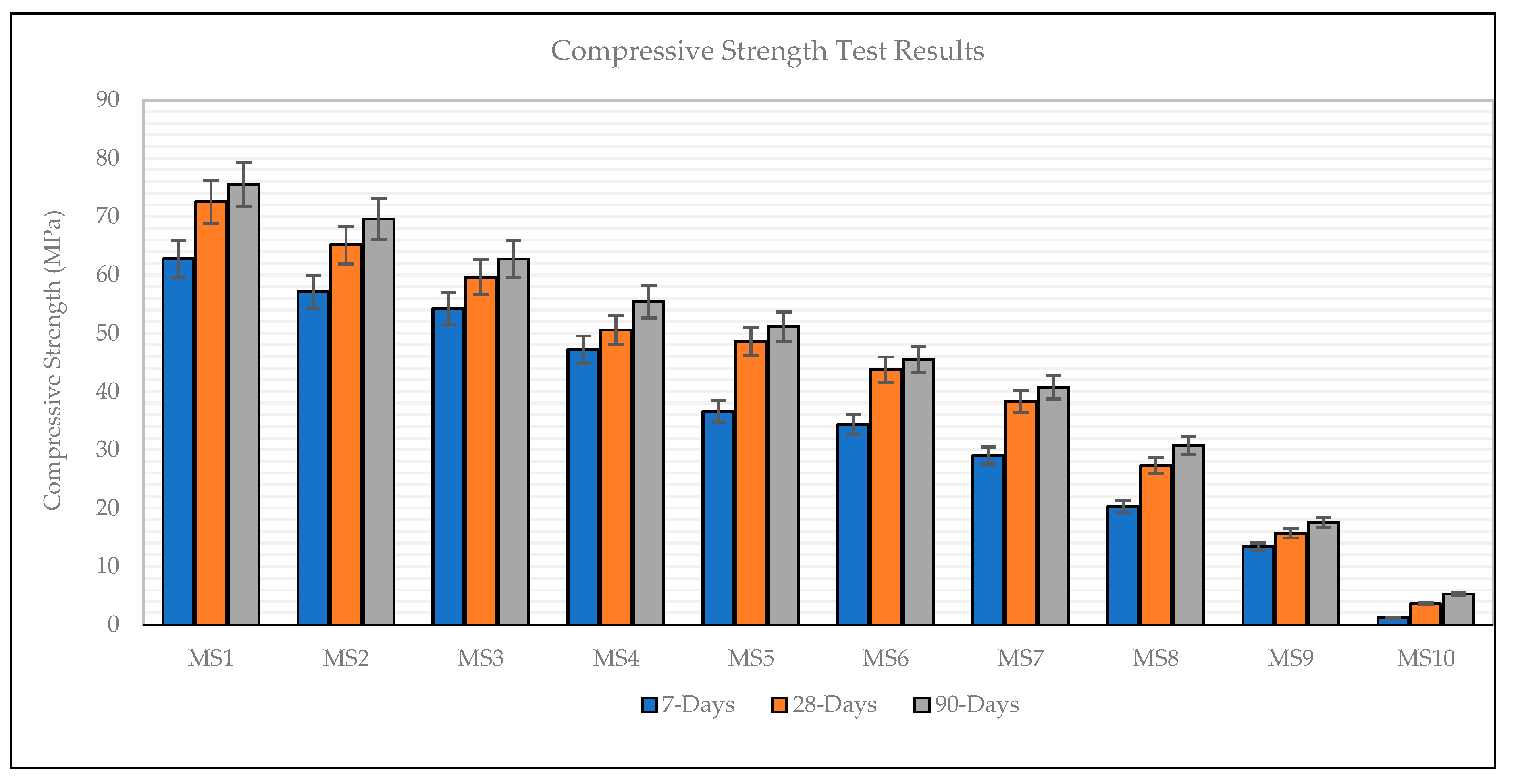
3.5. Compressive Strength
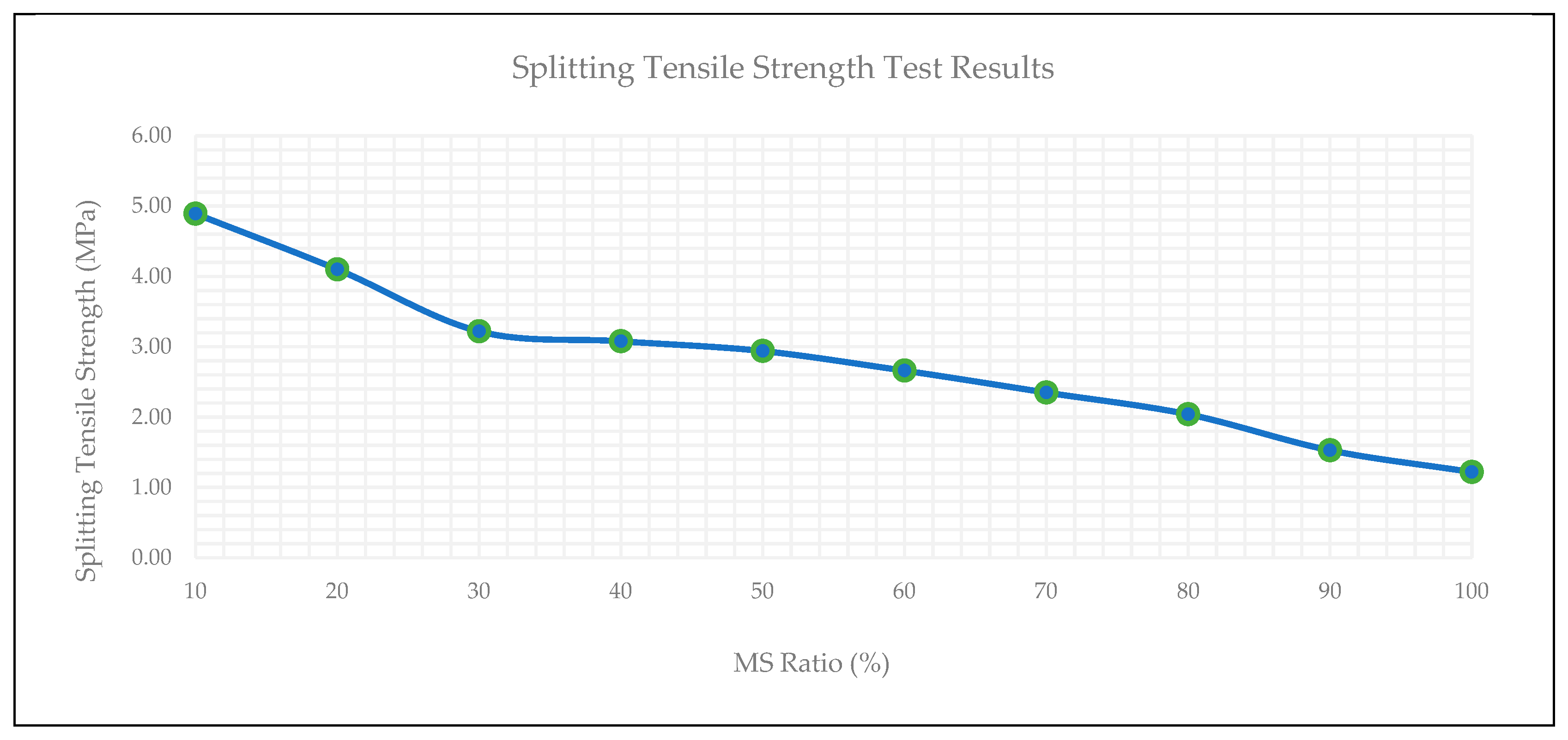
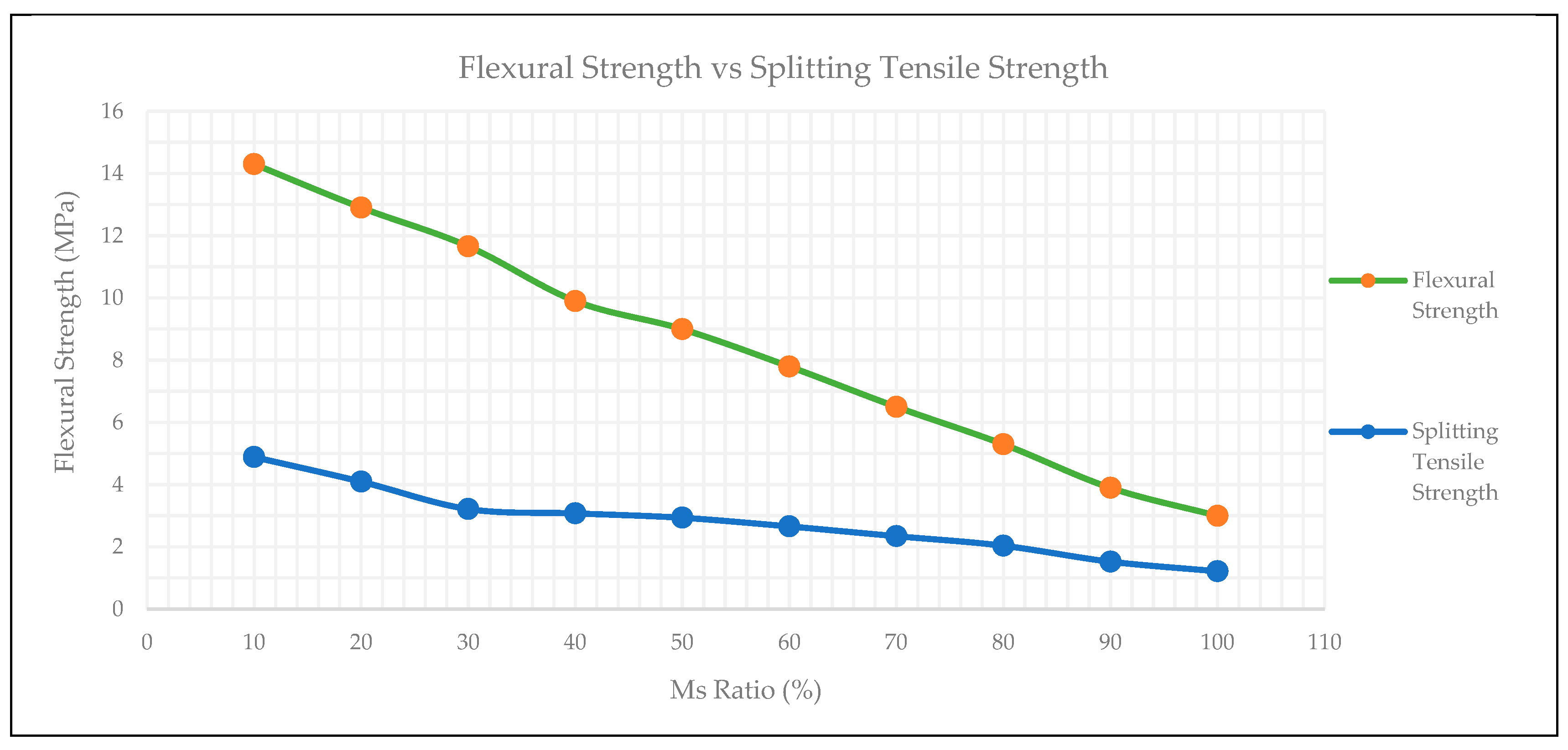
3.6. Splitting Tensile Strength
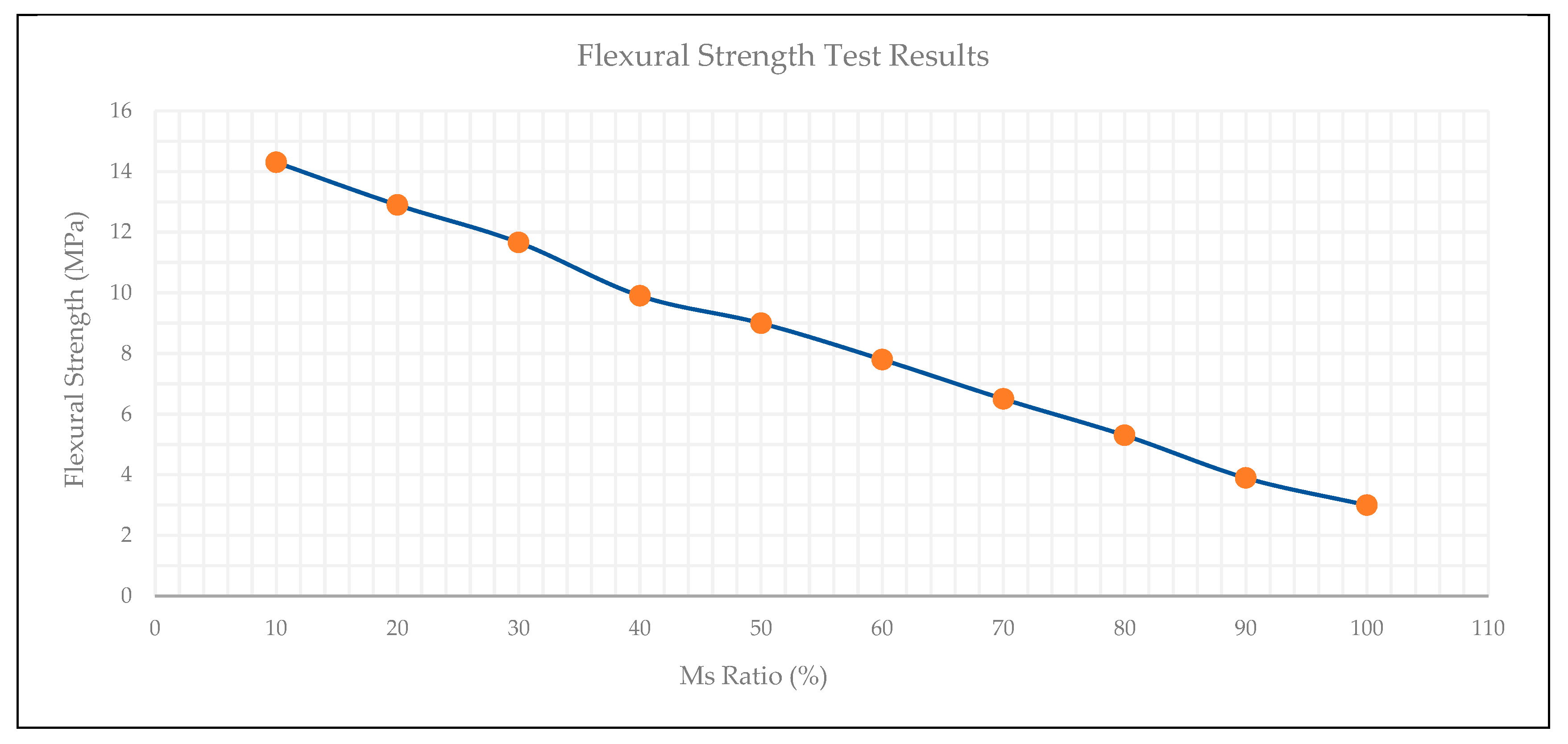
3.7. Flexural Strength
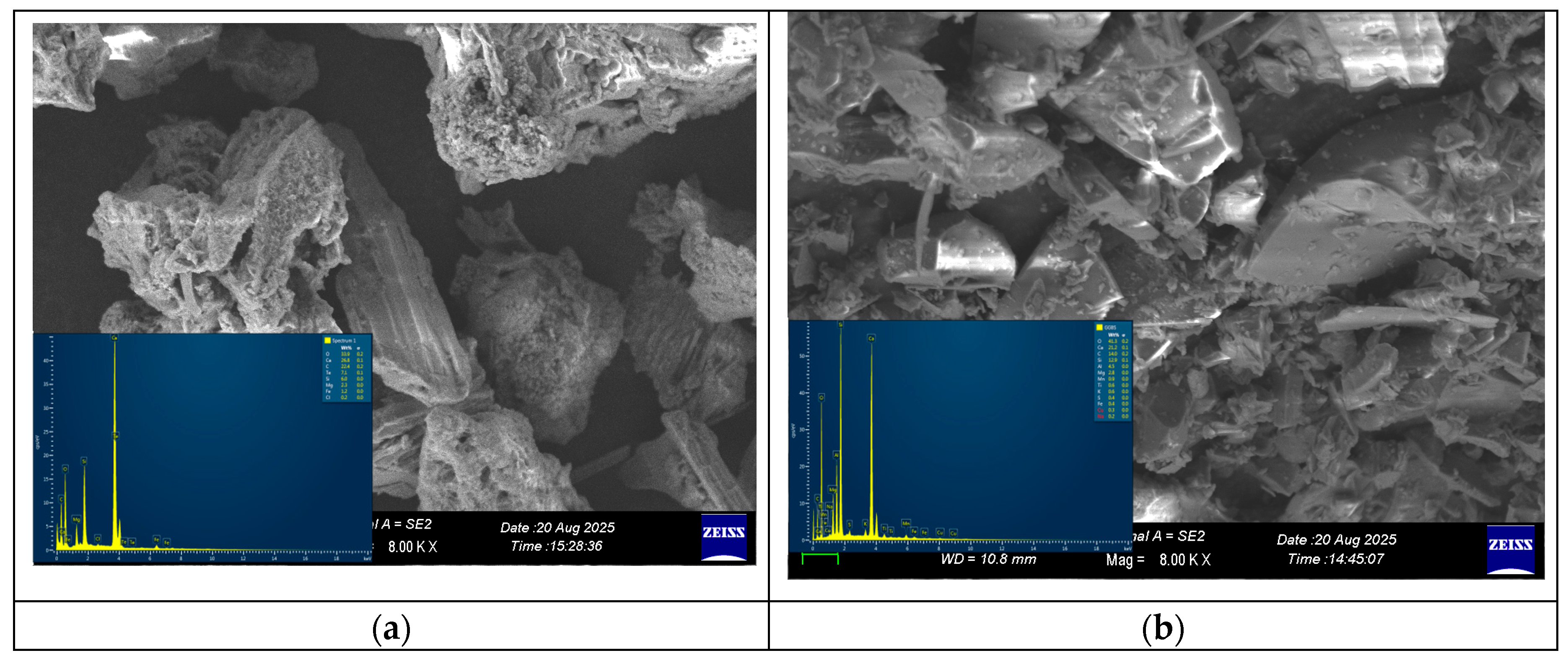
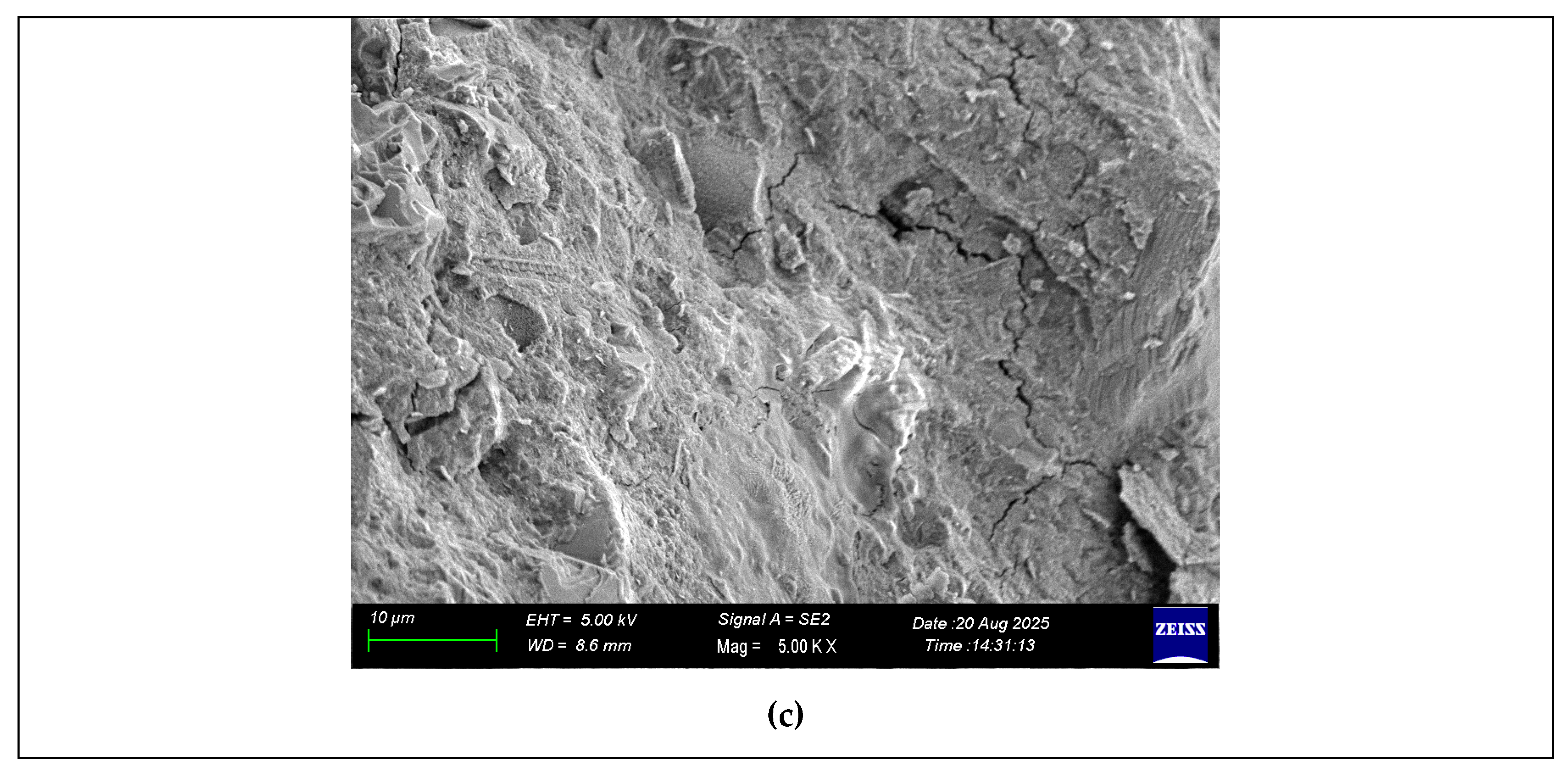
3.8. Scanning Electron Microscopy (SEM)
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MS | Magnesium Slag |
| OPGC | One Part Geopolymer Concrete |
| GGBS | Ground Granulated Blast Furnace Slag |
| UPV | Ultrasonic Pulse Velocity |
References
- El-Mir, A., Hwalla, J., El-Hassan, H., Assaad, J. J., El-Dieb, A., Shehab, E. Valorization of waste perlite powder in geopolymer composites. Construction and Building Materials, 2023, 368, 130491.
- Guo, S., Ma, C., Long, G., Xie, Y., Cleaner one-part geopolymer prepared by introducing fly ash sinking spherical beads: properties and geopolymerization mechanism, Journal of Cleaner Production, 2019, 219, 686–697.
- Sumesh, M., Alengaram, U. J., Jumaat, M. Z., Mo, K. H., Alnahhal, M. F., Incorporation of nano-materials in cement composite and geopolymer based paste and mortar–A review, Construction and Building Materials, 2017, 148, 62–84.
- Meng, Y., Ling, T. C., Mo, K. H., Recycling of wastes for value-added applications in concrete blocks: An overview, Resources, Conservation and Recycling, 2018, 138, 298–312.
- Oderji, S. Y., Chen, B., Ahmad, M. R., Shah, S. F. A., Fresh and hardened properties of one-part fly ash-based geopolymer binders cured at room temperature: Effect of slag and alkali activators, Journal of Cleaner Production, 2019, 225, 1–10.
- Pacheco-Torgal, F., Introduction to handbook of alkali-activated cements, mortars and concretes, In: Handbook of alkali-activated cements, mortars and concretes, 2015, pp. 1–16, Woodhead Publishing.
- Zhang, Y. J., Wang, Y. C., Li, S., Mechanical performance and hydration mechanism of geopolymer composite reinforced by resin, Materials Science and Engineering, 2010, 527(24-25), 6574–6580.
- Aiken, T. A., Kwasny, J., Sha, W., Soutsos, M. N., Effect of slag content and activator dosage on the resistance of fly ash geopolymer binders to sulfuric acid attack, Cement and Concrete Research, 2018, 111, 23–40.
- Yao, X., Zhang, Z., Zhu, H., Chen, Y., Geopolymerization process of alkali–metakaolinite characterized by isothermal calorimetry, Thermochimica Acta, 2009, 493(1-2), 49–54.
- Sagoe-Crentsil, K., Brown, T., Taylor, A., Drying shrinkage and creep performance of geopolymer concrete, Journal of Sustainable Cement-Based Materials, 2013, 2(1), 35–42.
- Kong, D. L., Sanjayan, J. G., Effect of elevated temperatures on geopolymer paste, mortar and concrete, Cement and Concrete Research, 2010, 40(2), 334–339.
- Luukkonen, T., Abdollahnejad, Z., Yliniemi, J., Kinnunen, P., Illikainen, M., Comparison of alkali and silica sources in one-part alkali-activated blast furnace slag mortar, Journal of Cleaner Production, 2018, 187, 171–179.
- Abdel-Gawwad, H. A., Rashad, A. M., Heikal, M., Sustainable utilization of pre-treated concrete waste in the production of one-part alkali-activated cement, Journal of Cleaner Production, 2019, 232, 318–328.
- Koloušek, D., Brus, J., Urbanova, M., Andertova, J., Hulinsky, V., Vorel, J., Preparation, structure and hydrothermal stability of alternative (sodium silicate-free) geopolymers, Journal of Materials Science, 2007, 42, 9267–9275.
- Peng, M. X., Wang, Z. H., Shen, S. H., Xiao, Q. G., 2015. Synthesis, characterization and mechanisms of one-part geopolymeric cement by calcining low-quality kaolin with alkali, Materials and Structures, 48, 699–708.
- Ma, C., Long, G., Shi, Y., Xie, Y., 2018. Preparation of cleaner one-part geopolymer by investigating different types of commercial sodium metasilicate in China, Journal of Cleaner Production, 201, 636–647.
- Zhang, T., Zhang, Y., He, Y., Zhao, Y. and Shi, C., 2022. Alkali-activated materials from metakaolin and MSW incineration fly ash: Reaction kinetics and microstructure. Cement and Concrete Composites, 130, p.104483.
- Lao, J. C., Xu, L. Y., Huang, B. T., Zhu, J. X., Khan, M., Dai, J. G., 2023. Utilization of sodium carbonate activator in strain-hardening ultra-high-performance geopolymer concrete, Frontiers in Materials, 10, 1142237.
- Alhamoud, A., Tajmir Riahi, H., Ataei, A., 2024. A Practical Mix Design Method of Ground Granulated Blast-Furnace Slag-Based One-Part Geopolymer Concrete, Arab Journal Science and Engineering, 49, 5447–5466.
- Lu, P., Zhao, Y., Zhang, N., Wang, Y., Zhang, J., Zhang, Y., and Liu, X., Structural characteristics and cementitious behavior of magnesium slag in comparison with granulated blast furnace slag. Materials, 2024, 17(2), 360.
- Kumar, S., Kumar, R. and Mehrotra, S.P., Influence of granulated blast furnace slag on the reaction, structure and properties of fly ash-based geopolymer. Journal of Materials Science, 2017, 52(5), pp.2618–2630.
- Frías, M., Moreno-Reyes, A. M., Vigil, R., García, R., Villar, E., Oleaga, A., and Vegas, I. Scientific advances regarding the effect of carbonated alkaline waste materials on pozzolanic reactivity. Journal of Building Engineering, 2024, 98, 111423.
- Zhu, M., Zhai, R., Zhu, M., and He, J. Evaluating Alkali Activation in Magnesium Slag Carbonization and Its Mechanism, Crystals, 2024, 14(10), 847.
- Zhang, L., Zhang, Y., Zhang, F., Liang, H., Niu, D., and Li, H., Mechanical and Ecological Properties of CO2 Curing Magnesium Slag Concrete. Materials, 18(1), 2024, 109.
- Nawaz, M., Heitor, A., Sivakumar, M., Geopolymers in construction-recent developments, Construction and Building Materials, 2020, 260, 120472.
- Sharma, A., Basumatary, N., Singh, P., Kapoor, K., and Singh, S. P., Potential of geopolymer concrete as substitution for conventional concrete: A review, Materials Today: Proceedings, 2022, 57, 1539–1545.
- Neupane, K., 2016. Fly ash and GGBFS based powder-activated geopolymer binders: A viable sustainable alternative of portland cement in concrete industry, Mechanics of Materials, 103, 110–122.
- Deb, P. S., Nath, P., and Sarker, P. K., The effects of ground granulated blast-furnace slag blending with fly ash and activator content on the workability and strength properties of geopolymer concrete cured at ambient temperature, Materials & Design, 2014, 62, 32–39.
- Yazıcı, N., Karagöl, F., 2022. Investigation of mechanical and durability properties of fly ash-based and slag-incorporated geopolymer concretes, Journal of the Institute of Science and Technology, 12(3), 1592–1606.
- Fouad, H. E. E., Serag, M. I., Ragab, A., & Hussein, A. (2025). Mechanical Properties and Temperature Resistance of Fly Ash-Based Geopolymer Concrete. Mansoura Engineering Journal, 50(3), 13.
- Altundal, M. B., 2019. Mechanical behavior of geopolymer concretes containing ground granulated blast furnace slag and fly ash under 5% sulfuric acid exposure, Master’s Thesis.
- Erdoğdu, Ş., Kurbetci, Ş., Kandil, U., Nayır, S., Nas, M., 2021. Some mechanical and durability properties of blast furnace slag concrete, Hazır Beton, 167.
- Çakır, Ö., 2006. The effect of ground granulated blast furnace slag on the durability of concrete and reinforced concrete, Master’s Thesis.
- Justnes, H., Martius-Hammer, T. A., 2016. Sustainability – The pioneering role in concrete innovation, Hazır Beton, 23, 77–82.
- Lu, P., Zhao, Y., Zhang, N., Wang, Y., Zhang, J., Zhang, Y., and Liu, X., Structural characteristics and cementitious behavior of magnesium slag in comparison with granulated blast furnace slag. Materials, (2024)., 17(2), 360.
- Zhang, S. S., Wang, S., and Chen, X., 2024. Understanding the role of magnesium ions on setting of metakaolin-based geopolymer. Cement and Concrete Research, 177, 107430.
- Dung, N. T., Hooper, T. J. N., & Unluer, C. 2019, Accelerating the reaction kinetics and improving the performance of Na2CO3-activated GGBS mixes. Cement and Concrete Research, 126, 105927.
- Nath, P., and Sarker, P. K., 2014, Effect of GGBFS on setting, workability and early strength properties of fly ash geopolymer concrete cured in ambient condition. Construction and Building materials, 66, 163-171.
- Lee, N. K., and Haeng-Ki Lee. Setting and mechanical properties of alkali-activated fly ash/slag concrete manufactured at room temperature. Construction and Building Materials 47 (2013): 1201-1209.
- Hu, Y., Tang, Z., Li, W., Li, Y., and Tam, V. W., Physical-mechanical properties of fly ash/GGBFS geopolymer composites with recycled aggregates. Construction and Building Materials, 226, (2019). 139-151.
- Rakhimova, Nailia R., and Ravil Z. Rakhimov., Alkali-activated cements and mortars based on blast furnace slag and red clay brick waste, Materials & Design 85 (2015): 324-331.
- Rakhimova, Nailia R., and Ravil Z. Rakhimov., Alkali-activated cements and mortars based on blast furnace slag and red clay brick waste, Materials & Design 85 (2015): 324-331.
- Chen, C., Gong, W., Lutze, W., Pegg, I. L. and Zhai, J. Kinetics of fly ash leaching in strongly alkaline solutions. Journal of Materials Science, 46(3), (2011), 590-597.
- Espinosa, A. B., Revilla-Cuesta, V., Skaf, M., Faleschini, F., and Ortega-López, V, Utility of ultrasonic pulse velocity for estimating the overall mechanical behavior of recycled aggregate self-compacting concrete. Applied Sciences, 13(2), (2023). 874.
- Lu, P., Zhao, Y., Zhang, N., Wang, Y., Zhang, J., Zhang, Y., and Liu, X. Structural characteristics and cementitious behavior of magnesium slag in comparison with granulated blast furnace slag. Materials, (2024). 17(2), 360.
- Somna, K., Jaturapitakkul, C., Kajitvichyanukul, P., and Chindaprasirt, P., NaOH-activated ground fly ash geopolymer cured at ambient temperature, Fuel, 2011, 90(6), 2118-2124.
- Mikuni, A., Komatsu, R., and Ikeda, K., Dissolution properties of some fly ash fillers applying to geopolymeric materials in alkali solution. Journal of materials science, 42(9), 2007, 2953-2957.
- Ji, G., Peng, X., Wang, S., Hu, C., Ran, P., Sun, K., and Zeng, L. Influence of magnesium slag as a mineral admixture on the performance of concrete. Construction and Building Materials, 295, 2021, 123619.
- Lu, P., Zhao, Y., Zhang, N., Wang, Y., Zhang, J., Zhang, Y., and Liu, X., Structural characteristics and cementitious behavior of magnesium slag in comparison with granulated blast furnace slag. Materials, 17(2), 2024, 360.
- Amini, O., and Ghasemi, M. Laboratory study of the effects of using magnesium slag on the geotechnical properties of cement stabilized soil. Construction and Building Materials, 2019, 223, 409-420.
- Wei, F., Xiao, H., Zhang, J., He, Z., Cao, X., and Guan, B. Feasibility study of magnesium slag, fly ash, and metakaolin to replace part of cement as cementitious materials. Buildings, 14(12), (2024), 3874.
- Bernal, S. A., Rodríguez, E. D., Mejía de Gutiérrez, R., Gordillo, M., and Provis, J. L. Mechanical and thermal characterization of geopolymers based on silicate-activated metakaolin/slag blends. Journal of materials science, 46(16), 2011, 5477-5486.
- Provis, J. L., and Van Deventer, J. S. J. (Eds.)., Geopolymers: structures, processing, properties and industrial applications. Elsevier. 2009.
- Li, Z., Zhang, W., Wang, R., Chen, F., Jia, X., and Cong, P. (2019). Effects of reactive MgO on the reaction process of geopolymer. Materials, 12(3), 526.
- Nath, P., and Sarker, P. K. (2014). Effect of GGBFS on setting, workability and early strength properties of fly ash geopolymer concrete cured in ambient condition. Construction and Building materials, 66, 163-171.
- Provis, J. L., and Van Deventer, J. S. J. (Eds.). (2009). Geopolymers: structures, processing, properties and industrial applications. Elsevier.



| Component name | MS (wt%) | GGBS (wt%) |
| SiO2 | 29,68 | 29,40 |
| Al2O3 | 1,91 | 15,28 |
| Fe2O3 | 4,29 | 1,30 |
| CaO | 51,70 | 42,10 |
| MgO | 9,93 | 7,01 |
| SO3 | 0,52 | 2,87 |
| Na2O | 0,31 | 0,49 |
| K2O | 1,30 | 0,79 |
| Cl | 0 | 0,05 |
| Loi | 0,36 | 0,69 |
| Properties | Value |
| Molecular structure | Composed of Sodium (Na), Silicon (Si), and Oxygen (O) atoms |
| Components | Sodium Oxide (Na₂O): Provides alkaline structure Silica (SiO₂): Promotes the formation of reactive phases |
| Physical form | White granules |
| Density (solid) | 2.5 g/cm³ |
| Solubility | Easily dissolves in water; dissociates into Na⁺ and SiO₃²⁻ ions |
| Reactivity | Initiates the alkaline activation process Supports the formation of C-S-H and N-A-S-H gel phases in geopolymer concrete production |
| Moisture absorption | Rapidly absorbs moisture from the air; should be stored in a dry environment |
| Thermal stability | Stable up to 1088 °C |
| Properties | Value |
| Dry specific gravity | 2,56 |
| Saturated surface dry specific gravity | 2,66 |
| Water absorption | 1,1 |
| Materials’name | Quantities(kg/m³) |
| Coarse aggregate | 961,0 |
| Fine aggregate | 693,0 |
| Alumina-silicate | 398,8 |
| Binder | 462,6 |
| Material ID | ||||||||||
| Materials(kg/m3) | MS1 | MS2 | MS3 | MS4 | MS5 | MS6 | MS7 | MS8 | MS9 | MS10 |
| Coarse aggregate | 961,0 | 961,0 | 961,0 | 961,0 | 961,0 | 961,0 | 961,0 | 961,0 | 961,0 | 961,0 |
| Fine aggregate | 693,0 | 693,0 | 693,0 | 693,0 | 693,0 | 693,0 | 693,0 | 693,0 | 693,0 | 693,0 |
| MS | 39,9 | 79,8 | 119,6 | 159,5 | 199,4 | 239,3 | 279,2 | 319,0 | 358,9 | 398,8 |
| GGBS | 358,9 | 319,0 | 279,2 | 239,3 | 199,4 | 159,5 | 119,6 | 79,8 | 39,9 | 0,0 |
| Activator | 63,8 | 63,8 | 63,8 | 63,8 | 63,8 | 63,8 | 63,8 | 63,8 | 63,8 | 63,8 |
| Water | 217,4 | 217,4 | 217,4 | 217,4 | 217,4 | 217,4 | 217,4 | 217,4 | 217,4 | 217,4 |
| Binder | 462,6 | 462,6 | 462,6 | 462,6 | 462,6 | 462,6 | 462,6 | 462,6 | 462,6 | 462,6 |
| Material ID | Slump value (cm) | MS value (%) | GGBS value (%) |
| MS1 | 7 | 10 | 90 |
| MS2 | 8 | 20 | 80 |
| MS3 | 8 | 30 | 70 |
| MS4 | 9 | 40 | 60 |
| MS5 | 10 | 50 | 50 |
| MS6 | 10 | 60 | 40 |
| MS7 | 11 | 70 | 30 |
| MS8 | 12 | 80 | 20 |
| MS9 | 12 | 90 | 10 |
| MS10 | 14 | 100 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




