Submitted:
01 December 2025
Posted:
03 December 2025
Read the latest preprint version here
Abstract
Keywords:
1. Framing the Journey—Prompts
Box A—Key Terms
2. Early Paradigms & Assumptions (1960s–1990s)
3. Pivot Suite (10 Mini-Cards)
3.1. Plasticity & Circuit Control of Depressive States
3.1.1. Synaptic Plasticity & Intrinsic Excitability
- Long-term potentiation and depression, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor throughput, and evoked excitatory postsynaptic potentials (eEPSPs) index rapid remodeling, while neurogenesis and extracellular signal-regulated kinase (ERK)-sensitive priming by ketamine extend effects [42,43,44,45].
3.1.2. Glutamate/γ-Aminobutyric Acid (GABA) Microcircuit Control
3.1.3. Circuit-Level Nodes (Drugs & Devices)
- Circuit-level nodes highlight how drugs and devices now target networks connecting habenula, subcallosal cingulate, and ventromedial prefrontal cortex.
- Deep brain, vagus nerve, and transcranial magnetic stimulation, including accelerated theta-burst protocols, are steered by connectivity-guided targeting and TMS-EEG physiology.
- Proof of target engagement, intensified dosing schedules, and responder enrichment by baseline network topology redefine how device parameters map onto durable outcomes [57].
3.2. Reward, Motivation & Stress Systems
3.2.1. Reward, motivation, and stress–opioid tone
3.2.2. HPA–Circadian–Stress Axis
3.3. Immune–Metabolic–Genomic Modifiers of Risk and Treatment Response
3.3.1. Tryptophan (Trp)–kynurenine (KYN) steering
3.3.2. Neuroimmune and glia
- Trials should treat sex, inflammatory state, and electroconvulsive or stimulation induced immune shifts as designable dimensions for precision psychiatry [95].
3.3.3. Metabolic–endocrine crosstalk
- Metabolic endocrine crosstalk highlights insulin resistance, adiposity, and glucagon-like peptide-1 (GLP-1) signaling as levers that couple energy allocation to mood, cognition, and treatment response.
- Metabolic syndrome phenotypes and type 2 diabetes (T2D) comorbidity flag patients in whom antidepressant efficacy, tolerability, and neuromodulation outcomes hinge on brain insulin signaling.
- Pragmatic trials should embed metabolic stratification and functional endpoints.
3.3.4. Epigenetic/transcriptional gating
3.4. Multi-Point Precision Strategies & Emerging Targets
3.4.1. Multi-point strategies and next-wave targets
4. Divergence → Reconnection
| Human construct | Preclinical assay | Readout | Clinical analog | Status | Design tip |
| Anhedonia/ motivational deficit | Effort-based decision tasks (progressive ratio, T-maze barrier, operant sucrose) | Breakpoint, lever presses, willingness to work under stress or inflammation | Probabilistic reward tasks, EEfRT, ventral striatal BOLD, anhedonia scales | Emerging trial biomarker | Separate hedonic “liking” from motivational “wanting”; include stress/inflammation challenge blocks. |
| Negative affect/threat bias | Fear conditioning and extinction; chronic social defeat | Freezing/avoidance, extinction curves, startle, social withdrawal | Fear-learning and extinction tasks, startle paradigms, threat-bias tasks in anxious/MDD subgroups | Robust basic science; limited clinical use | Use as domain-specific endpoint in anxious and trauma-loaded depression; pair behavior with EEG/fMRI. |
| Cognitive control/executive dysfunction | Attentional set-shifting, 5-CSRTT, reversal learning | Errors, omissions, reaction times, perseveration indexes | Set-shifting (e.g., CANTAB), n-back, Stroop, Trail Making, DLPFC activation | Secondary endpoint in several trials | Pre-stratify “cognitively loaded” depression; link change to functioning and return-to-work outcomes. |
| Sleep and circadian disruption | Rodent EEG/EMG with chronic stress or light-cycle shift; REM-deprivation models | REM latency/density, NREM slow-wave power, activity rhythms, phase shifts | Polysomnography, actigraphy, DLMO, sleep/circadian questionnaires | Strong observational; emerging endpoints | Align dosing and assessments with chronotype; treat sleep/circadian metrics as primary modifiable targets. |
| HPA axis and stress reactivity | Chronic mild stress, restraint, social defeat; Dex/CRH challenges | Corticosterone profiles, GR sensitivity, coping style, stress-induced behavioral shift | Cortisol awakening response, DST, lab stress tests, hair cortisol | Mixed but promising for subtyping | Sample across diurnal cycle; co-model stress markers with symptom domains (anergy, anxiety, cognitive fog). |
| Inflammation–KYN steering | LPS/IFN-α or stress-sensitized immune activation; Trp–KYN pathway assays | KYN/Trp ratio, QA/KYNA balance, microglial activation, cytokine panels | CRP, IL-6/TNF panels, plasma KYN/Trp, symptom clusters (anergia, anhedonia, psychomotor slowing) | High translational interest | Pre-specify “inflammation-high” strata; collect longitudinal KYN panels and align with treatment response. |
| Metabolic–endocrine load | High-fat diet, genetic obesity, insulin-resistance models | Glucose tolerance, insulin signaling, adiposity, spontaneous activity | BMI, waist-to-hip ratio, HOMA-IR, HbA1c, metabolic-syndrome indices | Growing but underused in trials | Embed metabolic panels into TRD studies; design dedicated obesity/T2D depression trials with functional endpoints. |
| Synaptic plasticity / rapid-acting response | Ketamine/psychedelic paradigms; LTP/LTD, in vivo spine imaging, AMPA-forward assays | Spine density, AMPA/NMDA ratio, LTP/LTD magnitude, early oscillatory changes | Early EEG/MEG plasticity markers, TMS-LTP readouts, 24–72 h symptom and cognition shifts | Strong mechanistic, clinical for ketamine | Build in early (24–72 h) windows and plasticity markers as key secondary endpoints in rapid-acting trials. |
| Network-level connectivity biotypes | Chemogenetic/optogenetic PFC–striatal/limbic manipulation; rodent rsfMRI/EEG | Resting-state connectivity, oscillatory coupling, causal node influence, behavior under circuit control | rsfMRI biotypes, TMS-EEG connectivity, SCC/vmPFC network markers for neuromodulation targeting | Emerging targeting tool | Require “target engagement” thresholds for drugs/devices; enrich samples by baseline network topology. |
| Digital behavior and passive monitoring | Home-cage automated monitoring of movement, sleep, and social interaction | Continuous activity, sleep–wake structure, social proximity, exploration patterns | Smartphone-based mobility, call/text patterns, speech and behavior passively captured by sensors | Early exploratory | Predefine digital endpoints (e.g., mobility, social withdrawal) and link them to functional and relapse outcomes. |
5. Clinical Applications Today
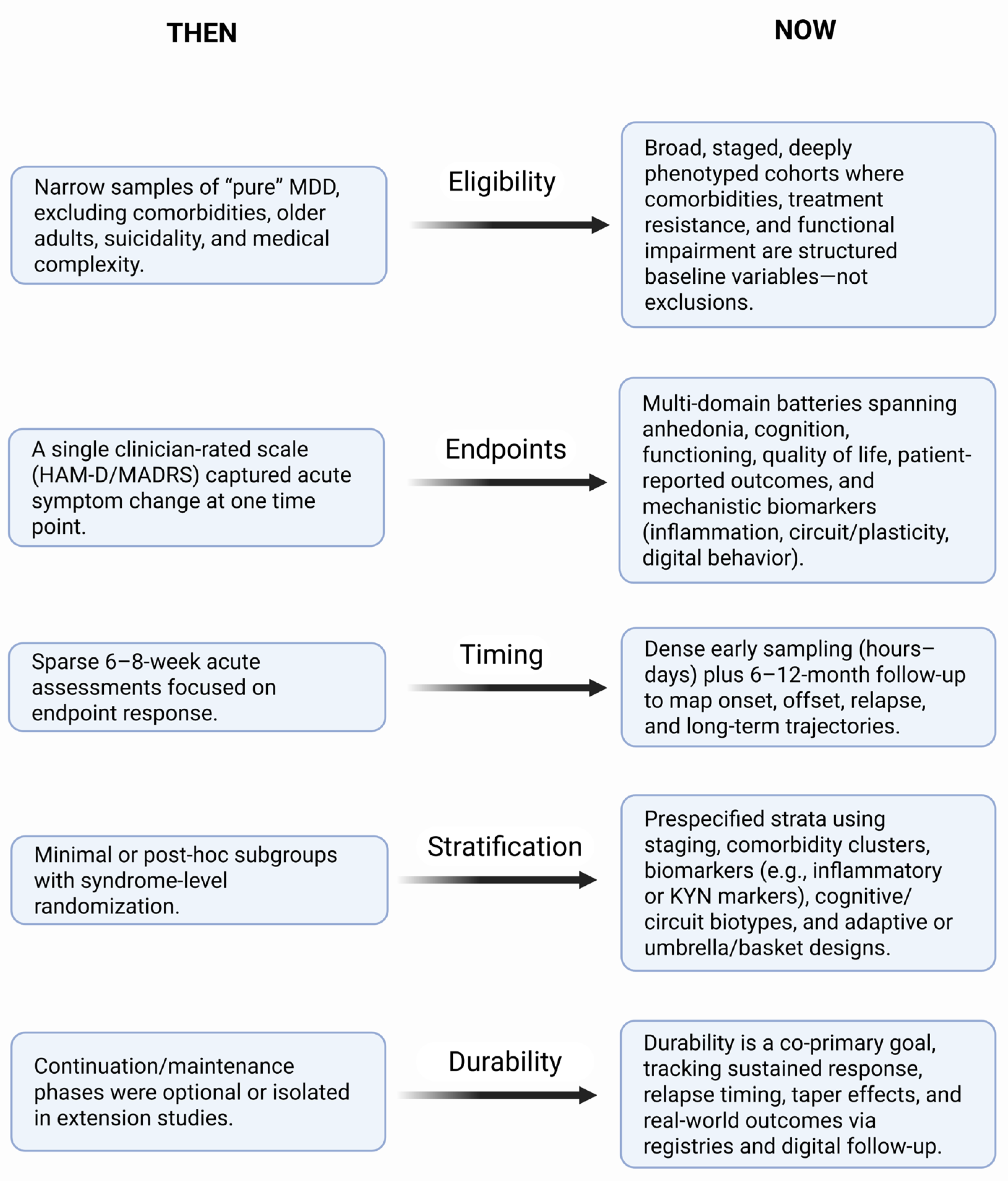
6. What We Got Wrong/Right
7. Outlook
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-CSRTT | five-choice serial reaction time task |
| AhR | aryl hydrocarbon receptor |
| AMPA | α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid |
| ATAC | assay for transposase-accessible chromatin |
| BMI | body mass index |
| BOLD | blood-oxygen-level–dependent |
| CANTAB | Cambridge Neuropsychological Test Automated Battery |
| CRP | C-reactive protein |
| CSF1R | colony-stimulating factor 1 receptor |
| Dex/CRH | dexamethasone/corticotropin-releasing hormone |
| DLMO | dim light melatonin onset |
| DLPFC | dorsolateral prefrontal cortex |
| DNMTs | DNA methyltransferases |
| DSM | Diagnostic and Statistical Manual of Mental Disorders |
| EEG | electroencephalography |
| EefRT | Effort Expenditure for Rewards Task |
| eEPSPs | evoked excitatory postsynaptic potentials |
| ERK | extracellular signal-regulated kinase |
| EMG | electromyography |
| fMRI | functional magnetic resonance imaging |
| GABA | γ-aminobutyric acid |
| GLP-1 | glucagon-like peptide-1 |
| GR | glucocorticoid receptor |
| HAM-D | Hamilton Depression Rating Scale |
| HbA1c | glycated hemoglobin |
| HDACs | histone deacetylases |
| HOMA-IR | homeostatic model assessment of insulin resistance |
| HPA | hypothalamic–pituitary–adrenal axis |
| IDO | indoleamine 2,3-dioxygenase |
| IFN-α | interferon-alpha |
| IL-6 | interleukin-6 |
| IPSPs | inhibitory postsynaptic potentials |
| κ | kappa |
| KYN | kynurenine |
| KYNA | kynurenic acid |
| LFP | local field potential |
| LPS | lipopolysaccharide |
| LSD1 | lysine-specific demethylase 1 |
| LTD | long-term depression |
| LTP | long-term potentiation |
| MADRS | Montgomery–Åsberg Depression Rating Scale |
| MDD | major depressive disorder |
| MEG | magnetoencephalography |
| NMDA | N-methyl-D-aspartate |
| PET | positron emission tomography |
| PFC | prefrontal cortex |
| QA | quinolinic acid |
| RDoC | Research Domain Criteria |
| REM | rapid eye movement |
| RNA | ribonucleic acid |
| rsfMRI | resting-state functional magnetic resonance imaging |
| SCC | subcallosal cingulate cortex |
| T2D | type 2 diabetes |
| TDO | tryptophan 2,3-dioxygenase |
| TMS | transcranial magnetic stimulation |
| TMS-EEG | transcranial magnetic stimulation–electroencephalography |
| TNF | tumor necrosis factor |
| TRD | treatment-resistant depression |
| Trp | tryptophan |
| vmPFC | ventromedial prefrontal cortex |
| VNS | vagus nerve stimulation |
References
- Hirschfeld, R.M. History and evolution of the monoamine hypothesis of depression. J Clin Psychiatry 2000, 61 Suppl 6, 4-6.
- Boku, S.; Nakagawa, S.; Toda, H.; Hishimoto, A. Neural basis of major depressive disorder: Beyond monoamine hypothesis. Psychiatry Clin Neurosci 2018, 72, 3-12. [CrossRef]
- Liu, B.; Liu, J.; Wang, M.; Zhang, Y.; Li, L. From Serotonin to Neuroplasticity: Evolvement of Theories for Major Depressive Disorder. Front Cell Neurosci 2017, 11, 305. [CrossRef]
- Spellman, T.; Liston, C. Toward Circuit Mechanisms of Pathophysiology in Depression. Am J Psychiatry 2020, 177, 381-390. [CrossRef]
- Fries, G.R.; Saldana, V.A.; Finnstein, J.; Rein, T. Molecular pathways of major depressive disorder converge on the synapse. Mol Psychiatry 2023, 28, 284-297. [CrossRef]
- Brandl, F.; Weise, B.; Mulej Bratec, S.; Jassim, N.; Hoffmann Ayala, D.; Bertram, T.; Ploner, M.; Sorg, C. Common and specific large-scale brain changes in major depressive disorder, anxiety disorders, and chronic pain: a transdiagnostic multimodal meta-analysis of structural and functional MRI studies. Neuropsychopharmacology 2022, 47, 1071-1080. [CrossRef]
- Sindermann, L.; Redlich, R.; Opel, N.; Böhnlein, J.; Dannlowski, U.; Leehr, E.J. Systematic transdiagnostic review of magnetic-resonance imaging results: Depression, anxiety disorders and their co-occurrence. J Psychiatr Res 2021, 142, 226-239. [CrossRef]
- Arnaud, A.M.; Brister, T.S.; Duckworth, K.; Foxworth, P.; Fulwider, T.; Suthoff, E.D.; Werneburg, B.; Aleksanderek, I.; Reinhart, M.L. Impact of Major Depressive Disorder on Comorbidities: A Systematic Literature Review. J Clin Psychiatry 2022, 83. [CrossRef]
- Tanaka, M.; Battaglia, S.; Liloia, D. Navigating Neurodegeneration: Integrating Biomarkers, Neuroinflammation, and Imaging in Parkinson's, Alzheimer's, and Motor Neuron Disorders. Biomedicines 2025, 13. [CrossRef]
- Berk, M.; Köhler-Forsberg, O.; Turner, M.; Penninx, B.; Wrobel, A.; Firth, J.; Loughman, A.; Reavley, N.J.; McGrath, J.J.; Momen, N.C.; et al. Comorbidity between major depressive disorder and physical diseases: a comprehensive review of epidemiology, mechanisms and management. World Psychiatry 2023, 22, 366-387. [CrossRef]
- Tanaka, M.; Battaglia, S. From Biomarkers to Behavior: Mapping the Neuroimmune Web of Pain, Mood, and Memory. Biomedicines 2025, 13. [CrossRef]
- Bottaccioli, A.G.; Bologna, M.; Bottaccioli, F. Rethinking Depression-Beyond Neurotransmitters: An Integrated Psychoneuroendocrineimmunology Framework for Depression's Pathophysiology and Tailored Treatment. Int J Mol Sci 2025, 26. [CrossRef]
- Tozzi, L.; Zhang, X.; Pines, A.; Olmsted, A.M.; Zhai, E.S.; Anene, E.T.; Chesnut, M.; Holt-Gosselin, B.; Chang, S.; Stetz, P.C.; et al. Personalized brain circuit scores identify clinically distinct biotypes in depression and anxiety. Nat Med 2024, 30, 2076-2087. [CrossRef]
- Jiao, W.; Lin, J.; Deng, Y.; Ji, Y.; Liang, C.; Wei, S.; Jing, X.; Yan, F. The immunological perspective of major depressive disorder: unveiling the interactions between central and peripheral immune mechanisms. J Neuroinflammation 2025, 22, 10. [CrossRef]
- Tanaka, M.; Battaglia, S. Dualistic Dynamics in Neuropsychiatry: From Monoaminergic Modulators to Multiscale Biomarker Maps. Biomedicines 2025, 13. [CrossRef]
- Drevets, W.C.; Wittenberg, G.M.; Bullmore, E.T.; Manji, H.K. Immune targets for therapeutic development in depression: towards precision medicine. Nat Rev Drug Discov 2022, 21, 224-244. [CrossRef]
- Tafet, G.E.; Nemeroff, C.B. The Links Between Stress and Depression: Psychoneuroendocrinological, Genetic, and Environmental Interactions. J Neuropsychiatry Clin Neurosci 2016, 28, 77-88. [CrossRef]
- Yankelevitch-Yahav, R.; Franko, M.; Huly, A.; Doron, R. The forced swim test as a model of depressive-like behavior. J Vis Exp 2015. [CrossRef]
- Yan, H.C.; Cao, X.; Das, M.; Zhu, X.H.; Gao, T.M. Behavioral animal models of depression. Neurosci Bull 2010, 26, 327-337. [CrossRef]
- Dale, E.; Bang-Andersen, B.; Sánchez, C. Emerging mechanisms and treatments for depression beyond SSRIs and SNRIs. Biochem Pharmacol 2015, 95, 81-97. [CrossRef]
- Abelaira, H.M.; Réus, G.Z.; Quevedo, J. Animal models as tools to study the pathophysiology of depression. Braz J Psychiatry 2013, 35 Suppl 2, S112-120. [CrossRef]
- Sharma, S.; Chawla, S.; Kumar, P.; Ahmad, R.; Kumar Verma, P. The chronic unpredictable mild stress (CUMS) Paradigm: Bridging the gap in depression research from bench to bedside. Brain Res 2024, 1843, 149123. [CrossRef]
- Ji, N.; Lei, M.; Chen, Y.; Tian, S.; Li, C.; Zhang, B. How Oxidative Stress Induces Depression? ASN Neuro 2023, 15, 17590914231181037. [CrossRef]
- Bech, P. Modern psychometrics in clinimetrics: impact on clinical trials of antidepressants. Psychother Psychosom 2004, 73, 134-138. [CrossRef]
- Regier, D.A.; Narrow, W.E.; Kuhl, E.A.; Kupfer, D.J. The conceptual development of DSM-V. Am J Psychiatry 2009, 166, 645-650. [CrossRef]
- Hengartner, M.P.; Jakobsen, J.C.; Sørensen, A.; Plöderl, M. Efficacy of new-generation antidepressants assessed with the Montgomery-Asberg Depression Rating Scale, the gold standard clinician rating scale: A meta-analysis of randomised placebo-controlled trials. PLoS One 2020, 15, e0229381. [CrossRef]
- Khan, A.; Khan, S.R.; Shankles, E.B.; Polissar, N.L. Relative sensitivity of the Montgomery-Asberg Depression Rating Scale, the Hamilton Depression rating scale and the Clinical Global Impressions rating scale in antidepressant clinical trials. Int Clin Psychopharmacol 2002, 17, 281-285. [CrossRef]
- Guizzaro, L.; Morgan, D.D.V.; Falco, A.; Gallo, C. Hamilton scale and MADRS are interchangeable in meta-analyses but can disagree at trial level. J Clin Epidemiol 2020, 124, 106-117. [CrossRef]
- Leucht, S.; Fennema, H.; Engel, R.R.; Kaspers-Janssen, M.; Szegedi, A. Translating the HAM-D into the MADRS and vice versa with equipercentile linking. J Affect Disord 2018, 226, 326-331. [CrossRef]
- Santi, N.S.; Biswal, S.B.; Naik, B.N.; Sahoo, J.P.; Rath, B. Comparison of Hamilton Depression Rating Scale and Montgomery-Åsberg Depression Rating Scale: Baked Straight From a Randomized Study. Cureus 2023, 15, e45098. [CrossRef]
- Ratheesh, A.; Berk, M.; Schmaal, L. Can we overcome the heterogeneity of mood disorders in clinical trials? Aust N Z J Psychiatry 2023, 57, 309-311. [CrossRef]
- Su, Z.; Yang, X.; Hou, J.; Liu, S.; Wang, Y.; Chen, Z. Gender differences in the co-occurrence of anxiety and depressive symptoms among early adolescents: A network approach. J Psychiatr Res 2024, 179, 300-305. [CrossRef]
- Vasiliadis, H.M.; Desjardins, F.; Roberge, P.; Grenier, S. Sex Differences in Anxiety Disorders in Older Adults. Curr Psychiatry Rep 2020, 22, 75. [CrossRef]
- Thompson, K.N.; Hübel, C.; Cheesman, R.; Adey, B.N.; Armour, C.; Davies, M.R.; Hotopf, M.; Jones, I.R.; Kalsi, G.; McIntosh, A.M.; et al. Age and sex-related variability in the presentation of generalized anxiety and depression symptoms. Depress Anxiety 2021, 38, 1054-1065. [CrossRef]
- Pavlidi, P.; Kokras, N.; Dalla, C. Sex Differences in Depression and Anxiety. Curr Top Behav Neurosci 2023, 62, 103-132. [CrossRef]
- Kuper, P.; Miguel, C.; Cuijpers, P.; Apfelbacher, C.; Buntrock, C.; Karyotaki, E.; Sprenger, A.A.; Harrer, M. Sample size and geographical region predict effect heterogeneity in psychotherapy research for depression: a meta-epidemiological study. J Clin Epidemiol 2025, 183, 111779. [CrossRef]
- Cai, N.; Choi, K.W.; Fried, E.I. Reviewing the genetics of heterogeneity in depression: operationalizations, manifestations and etiologies. Hum Mol Genet 2020, 29, R10-r18. [CrossRef]
- Hickie, I.B.; Berk, M.; Scott, J.; Crouse, J.; Scott, E.; Wray, N.; Iorfino, F. What are the best strategies for stratification of clinical cohorts with depression and other mood disorders? Research Directions: Depression 2024, 1, e18.
- Dunlop, K.; Grosenick, L.; Downar, J.; Vila-Rodriguez, F.; Gunning, F.M.; Daskalakis, Z.J.; Blumberger, D.M.; Liston, C. Dimensional and categorical solutions to parsing depression heterogeneity in a large single-site sample. Biological Psychiatry 2024, 96, 422-434.
- Marsden, W.N. Synaptic plasticity in depression: molecular, cellular and functional correlates. Prog Neuropsychopharmacol Biol Psychiatry 2013, 43, 168-184. [CrossRef]
- Shenoy, S.; Ibrahim, S. Perinatal Depression and the Role of Synaptic Plasticity in Its Pathogenesis and Treatment. Behav Sci (Basel) 2023, 13. [CrossRef]
- Brown, J.C.; Higgins, E.S.; George, M.S. Synaptic Plasticity 101: The Story of the AMPA Receptor for the Brain Stimulation Practitioner. Neuromodulation 2022, 25, 1289-1298. [CrossRef]
- He, J.G.; Zhou, H.Y.; Wang, F.; Chen, J.G. Dysfunction of Glutamatergic Synaptic Transmission in Depression: Focus on AMPA Receptor Trafficking. Biol Psychiatry Glob Open Sci 2023, 3, 187-196. [CrossRef]
- Brager, D.H.; Johnston, D. Plasticity of intrinsic excitability during long-term depression is mediated through mGluR-dependent changes in I(h) in hippocampal CA1 pyramidal neurons. J Neurosci 2007, 27, 13926-13937. [CrossRef]
- Cavalleri, L.; Merlo Pich, E.; Millan, M.J.; Chiamulera, C.; Kunath, T.; Spano, P.F.; Collo, G. Ketamine enhances structural plasticity in mouse mesencephalic and human iPSC-derived dopaminergic neurons via AMPAR-driven BDNF and mTOR signaling. Mol Psychiatry 2018, 23, 812-823. [CrossRef]
- Appelbaum, L.G.; Shenasa, M.A.; Stolz, L.; Daskalakis, Z. Synaptic plasticity and mental health: methods, challenges and opportunities. Neuropsychopharmacology 2023, 48, 113-120. [CrossRef]
- Cantone, M.; Bramanti, A.; Lanza, G.; Pennisi, M.; Bramanti, P.; Pennisi, G.; Bella, R. Cortical Plasticity in Depression. ASN Neuro 2017, 9, 1759091417711512. [CrossRef]
- Hu, Y.T.; Tan, Z.L.; Hirjak, D.; Northoff, G. Brain-wide changes in excitation-inhibition balance of major depressive disorder: a systematic review of topographic patterns of GABA- and glutamatergic alterations. Mol Psychiatry 2023, 28, 3257-3266. [CrossRef]
- Fee, C.; Banasr, M.; Sibille, E. Somatostatin-Positive Gamma-Aminobutyric Acid Interneuron Deficits in Depression: Cortical Microcircuit and Therapeutic Perspectives. Biol Psychiatry 2017, 82, 549-559. [CrossRef]
- Kuki, T.; Fujihara, K.; Miwa, H.; Tamamaki, N.; Yanagawa, Y.; Mushiake, H. Contribution of parvalbumin and somatostatin-expressing GABAergic neurons to slow oscillations and the balance in beta-gamma oscillations across cortical layers. Front Neural Circuits 2015, 9, 6. [CrossRef]
- Chen, G.; Zhang, Y.; Li, X.; Zhao, X.; Ye, Q.; Lin, Y.; Tao, H.W.; Rasch, M.J.; Zhang, X. Distinct Inhibitory Circuits Orchestrate Cortical beta and gamma Band Oscillations. Neuron 2017, 96, 1403-1418.e1406. [CrossRef]
- Tsuboi, D.; Nagai, T.; Yoshimoto, J.; Kaibuchi, K. Neuromodulator regulation and emotions: insights from the crosstalk of cell signaling. Front Mol Neurosci 2024, 17, 1376762. [CrossRef]
- Wagatsuma, N.; Nobukawa, S.; Fukai, T. A microcircuit model involving parvalbumin, somatostatin, and vasoactive intestinal polypeptide inhibitory interneurons for the modulation of neuronal oscillation during visual processing. Cereb Cortex 2023, 33, 4459-4477. [CrossRef]
- Tahvili, F.; Vinck, M.; di Volo, M. PV and SOM cells play distinct causal roles in controlling network oscillations and stability. Cell Rep 2025, 44, 116131. [CrossRef]
- Mazza, F.; Guet-McCreight, A.; Valiante, T.A.; Griffiths, J.D.; Hay, E. In-silico EEG biomarkers of reduced inhibition in human cortical microcircuits in depression. PLoS Comput Biol 2023, 19, e1010986. [CrossRef]
- Rademacher, J.; Grent-'t-Jong, T.; Rivolta, D.; Sauer, A.; Scheller, B.; Gonzalez-Burgos, G.; Metzner, C.; Uhlhaas, P.J. Computational modeling of ketamine-induced changes in gamma-band oscillations: The contribution of parvalbumin and somatostatin interneurons. PLoS Comput Biol 2025, 21, e1013118. [CrossRef]
- Tanaka, M.; He, Z.; Han, S.; Battaglia, S. Editorial: Noninvasive brain stimulation: a promising approach to study and improve emotion regulation. Front Behav Neurosci 2025, 19, 1633936. [CrossRef]
- Bruijnzeel, A.W. kappa-Opioid receptor signaling and brain reward function. Brain Res Rev 2009, 62, 127-146. [CrossRef]
- Wallace, C.W.; Holleran, K.M.; Slinkard, C.Y.; Centanni, S.W.; Lapish, C.C.; Jones, S.R. Kappa opioid receptors diminish spontaneous dopamine signals in awake mice through multiple mechanisms. Neuropharmacology 2025, 273, 110458. [CrossRef]
- Bekhbat, M.; Li, Z.; Dunlop, B.W.; Treadway, M.T.; Mehta, N.D.; Revill, K.P.; Lucido, M.J.; Hong, C.; Ashchi, A.; Wommack, E.C.; et al. Sustained effects of repeated levodopa (L-DOPA) administration on reward circuitry, effort-based motivation, and anhedonia in depressed patients with higher inflammation. Brain Behav Immun 2025, 125, 240-248. [CrossRef]
- Treadway, M.; Etuk, S.; Cooper, J.; Hossein, S.; Hahn, E.; Betters, S.; Liu, S.; Arulpragasam, A.; DeVries, B.; Irfan, N.; et al. A randomized proof-of-mechanism trial of TNF antagonism for motivational anhedonia and related corticostriatal circuitry in depressed patients with high inflammation. Res Sq 2024. [CrossRef]
- Felger, J.C.; Treadway, M.T. Inflammation Effects on Motivation and Motor Activity: Role of Dopamine. Neuropsychopharmacology 2017, 42, 216-241. [CrossRef]
- Lucido, M.J.; Bekhbat, M.; Goldsmith, D.R.; Treadway, M.T.; Haroon, E.; Felger, J.C.; Miller, A.H. Aiding and Abetting Anhedonia: Impact of Inflammation on the Brain and Pharmacological Implications. Pharmacol Rev 2021, 73, 1084-1117. [CrossRef]
- Hasbi, A.; Madras, B.K.; George, S.R. Daily Δ(9)-Tetrahydrocannabinol and Withdrawal Increase Dopamine D(1)-D(2) Receptor Heteromer to Mediate Anhedonia- and Anxiogenic-like Behavior Through a Dynorphin and Kappa Opioid Receptor Mechanism. Biol Psychiatry Glob Open Sci 2023, 3, 550-566. [CrossRef]
- Thomas, C.S.; Mohammadkhani, A.; Rana, M.; Qiao, M.; Baimel, C.; Borgland, S.L. Optogenetic stimulation of lateral hypothalamic orexin/dynorphin inputs in the ventral tegmental area potentiates mesolimbic dopamine neurotransmission and promotes reward-seeking behaviours. Neuropsychopharmacology 2022, 47, 728-740. [CrossRef]
- Albrecht, U. Molecular Mechanisms in Mood Regulation Involving the Circadian Clock. Front Neurol 2017, 8, 30. [CrossRef]
- Focke, C.M.B.; Iremonger, K.J. Rhythmicity matters: Circadian and ultradian patterns of HPA axis activity. Mol Cell Endocrinol 2020, 501, 110652. [CrossRef]
- Nader, N.; Chrousos, G.P.; Kino, T. Interactions of the circadian CLOCK system and the HPA axis. Trends Endocrinol Metab 2010, 21, 277-286. [CrossRef]
- Lightman, S.L.; Birnie, M.T.; Conway-Campbell, B.L. Dynamics of ACTH and Cortisol Secretion and Implications for Disease. Endocr Rev 2020, 41. [CrossRef]
- Carpenter, J.S.; Crouse, J.J.; Shin, M.; Tonini, E.; Hindmarsh, G.; de Haan, Z.; Iorfino, F.; Robillard, R.; Naismith, S.; Scott, E.M.; et al. Evidence for Internal Misalignment of Circadian Rhythms in Youth With Emerging Mood Disorders. J Biol Rhythms 2025, 40, 424-440. [CrossRef]
- Skubic, C.; Zevnik, U.; Nahtigal, K.; Dolenc Grošelj, L.; Rozman, D. Circadian Biomarkers in Humans: Methodological Insights into the Detection of Melatonin and Cortisol. Biomolecules 2025, 15. [CrossRef]
- Schrire, Z.M.; Naismith, S.L.; Pye, J.; Duffy, S.L.; Gordon, C.J.; Lewis, S.J.; Hoyos, C.M. Circadian rhythms and misalignment in older adults and those ‘at risk’for dementia: A study examining correlates of dim-light melatonin onset: Biomarkers (non-neuroimaging)/Prognostic utility. Alzheimer's & Dementia 2020, 16, e045525.
- Scott, M.R.; McClung, C.A. Circadian Rhythms in Mood Disorders. Adv Exp Med Biol 2021, 1344, 153-168. [CrossRef]
- Johnson, C.; Brzezynski, J.; Bair, A.; Smieszek, S.; Bai, H.; Polymeropoulos, C.; Birznieks, G.; Polymeropoulos, M. 0879 Dim Light Melatonin Onset in a Delayed Sleep-Wake Phase Disorder Cohort: Ongoing Clinical Trial Update. Sleep 2025, 48, A382-A382.
- Tanaka, M.; Tóth, F.; Polyák, H.; Szabó, Á.; Mándi, Y.; Vécsei, L. Immune Influencers in Action: Metabolites and Enzymes of the Tryptophan-Kynurenine Metabolic Pathway. Biomedicines 2021, 9. [CrossRef]
- Badawy, A.A. Kynurenine Pathway of Tryptophan Metabolism: Regulatory and Functional Aspects. Int J Tryptophan Res 2017, 10, 1178646917691938. [CrossRef]
- Salminen, A. Role of indoleamine 2,3-dioxygenase 1 (IDO1) and kynurenine pathway in the regulation of the aging process. Ageing Res Rev 2022, 75, 101573. [CrossRef]
- Stone, T.W.; Williams, R.O. Tryptophan metabolism as a 'reflex' feature of neuroimmune communication: Sensor and effector functions for the indoleamine-2, 3-dioxygenase kynurenine pathway. J Neurochem 2024, 168, 3333-3357. [CrossRef]
- MacDowell, K.S.; Munarriz-Cuezva, E.; Meana, J.J.; Leza, J.C.; Ortega, J.E. Paliperidone Reversion of Maternal Immune Activation-Induced Changes on Brain Serotonin and Kynurenine Pathways. Front Pharmacol 2021, 12, 682602. [CrossRef]
- Pathak, S.; Nadar, R.; Kim, S.; Liu, K.; Govindarajulu, M.; Cook, P.; Watts Alexander, C.S.; Dhanasekaran, M.; Moore, T. The Influence of Kynurenine Metabolites on Neurodegenerative Pathologies. Int J Mol Sci 2024, 25. [CrossRef]
- Schröcksnadel, K.; Wirleitner, B.; Winkler, C.; Fuchs, D. Monitoring tryptophan metabolism in chronic immune activation. Clin Chim Acta 2006, 364, 82-90. [CrossRef]
- Alfaro-Rodríguez, A.; Reyes-Long, S.; Roldan-Valadez, E.; González-Torres, M.; Bonilla-Jaime, H.; Bandala, C.; Avila-Luna, A.; Bueno-Nava, A.; Cabrera-Ruiz, E.; Sanchez-Aparicio, P.; et al. Association of the Serotonin and Kynurenine Pathways as Possible Therapeutic Targets to Modulate Pain in Patients with Fibromyalgia. Pharmaceuticals (Basel) 2024, 17. [CrossRef]
- Szabó, Á.; Galla, Z.; Spekker, E.; Martos, D.; Szűcs, M.; Fejes-Szabó, A.; Fehér, Á.; Takeda, K.; Ozaki, K.; Inoue, H.; et al. Behavioral Balance in Tryptophan Turmoil: Regional Metabolic Rewiring in Kynurenine Aminotransferase II Knockout Mice. Cells 2025, 14. [CrossRef]
- Tanaka, M.; Vécsei, L. From Microbial Switches to Metabolic Sensors: Rewiring the Gut-Brain Kynurenine Circuit. Biomedicines 2025, 13. [CrossRef]
- Martos, D.; Lőrinczi, B.; Szatmári, I.; Vécsei, L.; Tanaka, M. Decoupling Behavioral Domains via Kynurenic Acid Analog Optimization: Implications for Schizophrenia and Parkinson's Disease Therapeutics. Cells 2025, 14. [CrossRef]
- Juhász, L.; Spisák, K.; Szolnoki, B.Z.; Nászai, A.; Szabó, Á.; Rutai, A.; Tallósy, S.P.; Szabó, A.; Toldi, J.; Tanaka, M.; et al. The Power Struggle: Kynurenine Pathway Enzyme Knockouts and Brain Mitochondrial Respiration. J Neurochem 2025, 169, e70075. [CrossRef]
- Tanaka, M.; Szatmári, I.; Vécsei, L. Quinoline Quest: Kynurenic Acid Strategies for Next-Generation Therapeutics via Rational Drug Design. Pharmaceuticals (Basel) 2025, 18. [CrossRef]
- Naffaa, M.M. Mechanisms of astrocytic and microglial purinergic signaling in homeostatic regulation and implications for neurological disease. Exploration of Neuroscience 2025, 4, 100676.
- Bedetta, M.; Pizzo, P.; Lia, A. The Multifaceted Role of P2X7R in Microglia and Astrocytes. Neurochem Res 2025, 50, 239. [CrossRef]
- Batista, A.F.; Khan, K.A.; Papavergi, M.T.; Lemere, C.A. The Importance of Complement-Mediated Immune Signaling in Alzheimer's Disease Pathogenesis. Int J Mol Sci 2024, 25. [CrossRef]
- Liu, Y.D.; Chang, Y.H.; Xie, X.T.; Wang, X.Y.; Ma, H.Y.; Liu, M.C.; Zhang, H.M. PET Imaging Unveils Neuroinflammatory Mechanisms in Psychiatric Disorders: From Microglial Activation to Therapeutic Innovation. Mol Neurobiol 2025, 62, 15318-15335. [CrossRef]
- Werry, E.L.; Bright, F.M.; Piguet, O.; Ittner, L.M.; Halliday, G.M.; Hodges, J.R.; Kiernan, M.C.; Loy, C.T.; Kril, J.J.; Kassiou, M. Recent Developments in TSPO PET Imaging as A Biomarker of Neuroinflammation in Neurodegenerative Disorders. Int J Mol Sci 2019, 20. [CrossRef]
- Enache, D.; Pariante, C.M.; Mondelli, V. Markers of central inflammation in major depressive disorder: A systematic review and meta-analysis of studies examining cerebrospinal fluid, positron emission tomography and post-mortem brain tissue. Brain Behav Immun 2019, 81, 24-40. [CrossRef]
- Dupont, A.C.; Largeau, B.; Santiago Ribeiro, M.J.; Guilloteau, D.; Tronel, C.; Arlicot, N. Translocator Protein-18 kDa (TSPO) Positron Emission Tomography (PET) Imaging and Its Clinical Impact in Neurodegenerative Diseases. Int J Mol Sci 2017, 18. [CrossRef]
- Meyer, J.H.; Cervenka, S.; Kim, M.J.; Kreisl, W.C.; Henter, I.D.; Innis, R.B. Neuroinflammation in psychiatric disorders: PET imaging and promising new targets. Lancet Psychiatry 2020, 7, 1064-1074. [CrossRef]
- Baek, S.Y.; Lee, J.; Kim, T.; Lee, H.; Choi, H.S.; Park, H.; Koh, M.; Kim, E.; Jung, M.E.; Iliopoulos, D.; et al. Development of a novel histone deacetylase inhibitor unveils the role of HDAC11 in alleviating depression by inhibition of microglial activation. Biomed Pharmacother 2023, 166, 115312. [CrossRef]
- Du, J.; Liu, R.; Ma, L.; Liu, Y.; Wei, W.; Liu, N.; Cao, Q.; Yu, J. Novel histone deacetylase-5 inhibitor T2943 exerts an anti-depressive effect in mice by enhancing GRID1 expression. Sci Rep 2025, 15, 4522. [CrossRef]
- Sun, Z.; Zhang, B.; Zhou, J.; Luo, Y.; Zhu, X.; Wang, Y.; He, Y.; Zheng, P.; Zhang, L.; Yang, J.; et al. Integrated Single-Cell RNA-seq and ATAC-seq Reveals Heterogeneous Differentiation of CD4(+) Naive T Cell Subsets is Associated with Response to Antidepressant Treatment in Major Depressive Disorder. Adv Sci (Weinh) 2024, 11, e2308393. [CrossRef]
- Taavitsainen, S.; Engedal, N.; Cao, S.; Handle, F.; Erickson, A.; Prekovic, S.; Wetterskog, D.; Tolonen, T.; Vuorinen, E.M.; Kiviaho, A.; et al. Single-cell ATAC and RNA sequencing reveal pre-existing and persistent cells associated with prostate cancer relapse. Nat Commun 2021, 12, 5307. [CrossRef]
- Grandi, F.C.; Modi, H.; Kampman, L.; Corces, M.R. Chromatin accessibility profiling by ATAC-seq. Nat Protoc 2022, 17, 1518-1552. [CrossRef]
- Kajumba, M.M.; Kakooza-Mwesige, A.; Nakasujja, N.; Koltai, D.; Canli, T. Treatment-resistant depression: molecular mechanisms and management. Mol Biomed 2024, 5, 43. [CrossRef]
- Daeli, J.I.D.; Soemara, A. How Do Combined Pharmacological And Psychotherapeutic Interventions Impact Treatment Outcomes For Patients With Treatment-Resistant Major Depressive Disorder?: A Systematic Review. The International Journal of Medical Science and Health Research 2025, 12, 49-99.
- Ruberto, V.L.; Jha, M.K.; Murrough, J.W. Pharmacological Treatments for Patients with Treatment-Resistant Depression. Pharmaceuticals (Basel) 2020, 13. [CrossRef]
- McIntyre, R.S.; Alsuwaidan, M.; Baune, B.T.; Berk, M.; Demyttenaere, K.; Goldberg, J.F.; Gorwood, P.; Ho, R.; Kasper, S.; Kennedy, S.H.; et al. Treatment-resistant depression: definition, prevalence, detection, management, and investigational interventions. World Psychiatry 2023, 22, 394-412. [CrossRef]
- Amasi-Hartoonian, N.; Pariante, C.M.; Cattaneo, A.; Sforzini, L. Understanding treatment-resistant depression using "omics" techniques: A systematic review. J Affect Disord 2022, 318, 423-455. [CrossRef]
- Garcia, G.; Cotner, C.; Spano, R. Precision medicine in psychiatry: Case series on pharmacogenomic solutions for treatment-resistant depression. J Am Assoc Nurse Pract 2025, 37, 471-476. [CrossRef]
- Baum, M.L.; Widge, A.S.; Carpenter, L.L.; McDonald, W.M.; Cohen, B.M.; Nemeroff, C.B. Pharmacogenomic Clinical Support Tools for the Treatment of Depression. Am J Psychiatry 2024, 181, 591-607. [CrossRef]
- Young, J.W. Development of cross-species translational paradigms for psychiatric research in the Research Domain Criteria era. Neurosci Biobehav Rev 2023, 148, 105119. [CrossRef]
- Iturra-Mena, A.M.; Kangas, B.D.; Luc, O.T.; Potter, D.; Pizzagalli, D.A. Electrophysiological signatures of reward learning in the rodent touchscreen-based Probabilistic Reward Task. Neuropsychopharmacology 2023, 48, 700-709. [CrossRef]
- Cavanagh, J.F.; Olguin, S.L.; Talledo, J.A.; Kotz, J.E.; Roberts, B.Z.; Nungaray, J.A.; Sprock, J.; Gregg, D.; Bhakta, S.G.; Light, G.A.; et al. Amphetamine alters an EEG marker of reward processing in humans and mice. Psychopharmacology (Berl) 2022, 239, 923-933. [CrossRef]
- Pizzagalli, D.A.; Sherwood, R.J.; Henriques, J.B.; Davidson, R.J. Frontal brain asymmetry and reward responsiveness: a source-localization study. Psychol Sci 2005, 16, 805-813. [CrossRef]
- Kheirkhah, M.; Duncan, W.C., Jr.; Yuan, Q.; Wang, P.R.; Jamalabadi, H.; Leistritz, L.; Walter, M.; Goldman, D.; Zarate, C.A., Jr.; Hejazi, N.S. REM density predicts rapid antidepressant response to ketamine in individuals with treatment-resistant depression. Neuropsychopharmacology 2025, 50, 941-946. [CrossRef]
- Pesonen, A.K.; Gradisar, M.; Kuula, L.; Short, M.; Merikanto, I.; Tark, R.; Räikkönen, K.; Lahti, J. REM sleep fragmentation associated with depressive symptoms and genetic risk for depression in a community-based sample of adolescents. J Affect Disord 2019, 245, 757-763. [CrossRef]
- Lechinger, J.; Koch, J.; Weinhold, S.L.; Seeck-Hirschner, M.; Stingele, K.; Kropp-Näf, C.; Braun, M.; Drews, H.J.; Aldenhoff, J.; Huchzermeier, C.; et al. REM density is associated with treatment response in major depression: Antidepressant pharmacotherapy vs. psychotherapy. J Psychiatr Res 2021, 133, 67-72. [CrossRef]
- Luc, O.T.; Kangas, B.D. Validation of a touchscreen probabilistic reward task for mice: A reverse-translated assay with cross-species continuity. Cogn Affect Behav Neurosci 2024, 24, 281-288. [CrossRef]
- Palmer, D.; Dumont, J.R.; Dexter, T.D.; Prado, M.A.M.; Finger, E.; Bussey, T.J.; Saksida, L.M. Touchscreen cognitive testing: Cross-species translation and co-clinical trials in neurodegenerative and neuropsychiatric disease. Neurobiol Learn Mem 2021, 182, 107443. [CrossRef]
- Molendijk, M.L.; de Kloet, E.R. Coping with the forced swim stressor: Current state-of-the-art. Behav Brain Res 2019, 364, 1-10. [CrossRef]
- Commons, K.G.; Cholanians, A.B.; Babb, J.A.; Ehlinger, D.G. The Rodent Forced Swim Test Measures Stress-Coping Strategy, Not Depression-like Behavior. ACS Chem Neurosci 2017, 8, 955-960. [CrossRef]
- Molendijk, M.L.; de Kloet, E.R. Immobility in the forced swim test is adaptive and does not reflect depression. Psychoneuroendocrinology 2015, 62, 389-391. [CrossRef]
- Parekh, P.K.; Johnson, S.B.; Liston, C. Synaptic Mechanisms Regulating Mood State Transitions in Depression. Annu Rev Neurosci 2022, 45, 581-601. [CrossRef]
- Aleksandrova, L.R.; Wang, Y.T.; Phillips, A.G. Evaluation of the Wistar-Kyoto rat model of depression and the role of synaptic plasticity in depression and antidepressant response. Neurosci Biobehav Rev 2019, 105, 1-23. [CrossRef]
- Petković, A.; Chaudhury, D. Encore: Behavioural animal models of stress, depression and mood disorders. Front Behav Neurosci 2022, 16, 931964. [CrossRef]
- Della Valle, A.; De Carlo, S.; Sonsini, G.; Pilati, S.; Perali, A.; Ubaldi, M.; Ciccocioppo, R. Machine learning-based model for behavioural analysis in rodents applied to the forced swim test. Sci Rep 2025, 15, 22314. [CrossRef]
- Cuthbert, B.N. Research Domain Criteria (RDoC): Progress and Potential. Curr Dir Psychol Sci 2022, 31, 107-114. [CrossRef]
- Anderzhanova, E.; Kirmeier, T.; Wotjak, C.T. Animal models in psychiatric research: The RDoC system as a new framework for endophenotype-oriented translational neuroscience. Neurobiol Stress 2017, 7, 47-56. [CrossRef]
- Nusslock, R.; Alloy, L.B. Reward processing and mood-related symptoms: An RDoC and translational neuroscience perspective. J Affect Disord 2017, 216, 3-16. [CrossRef]
- Cavanagh, J.F.; Gregg, D.; Light, G.A.; Olguin, S.L.; Sharp, R.F.; Bismark, A.W.; Bhakta, S.G.; Swerdlow, N.R.; Brigman, J.L.; Young, J.W. Electrophysiological biomarkers of behavioral dimensions from cross-species paradigms. Transl Psychiatry 2021, 11, 482. [CrossRef]
- Fernandes, B.S.; Karmakar, C.; Tamouza, R.; Tran, T.; Yearwood, J.; Hamdani, N.; Laouamri, H.; Richard, J.R.; Yolken, R.; Berk, M.; et al. Precision psychiatry with immunological and cognitive biomarkers: a multi-domain prediction for the diagnosis of bipolar disorder or schizophrenia using machine learning. Transl Psychiatry 2020, 10, 162. [CrossRef]
- Sanislow, C.A.; Ferrante, M.; Pacheco, J.; Rudorfer, M.V.; Morris, S.E. Advancing Translational Research Using NIMH Research Domain Criteria and Computational Methods. Neuron 2019, 101, 779-782. [CrossRef]
- Morris, S.E.; Sanislow, C.A.; Pacheco, J.; Vaidyanathan, U.; Gordon, J.A.; Cuthbert, B.N. Revisiting the seven pillars of RDoC. BMC Med 2022, 20, 220. [CrossRef]
- Arns, M.; van Dijk, H.; Luykx, J.J.; van Wingen, G.; Olbrich, S. Stratified psychiatry: Tomorrow's precision psychiatry? Eur Neuropsychopharmacol 2022, 55, 14-19. [CrossRef]
- Milaneschi, Y.; Kappelmann, N.; Ye, Z.; Lamers, F.; Moser, S.; Jones, P.B.; Burgess, S.; Penninx, B.; Khandaker, G.M. Association of inflammation with depression and anxiety: evidence for symptom-specificity and potential causality from UK Biobank and NESDA cohorts. Mol Psychiatry 2021, 26, 7393-7402. [CrossRef]
- Tanaka, M. Special Issue "Translating Molecular Psychiatry: From Biomarkers to Personalized Therapies". Int J Mol Sci 2025, 26. [CrossRef]
- Bekhbat, M.; Treadway, M.T.; Goldsmith, D.R.; Woolwine, B.J.; Haroon, E.; Miller, A.H.; Felger, J.C. Gene signatures in peripheral blood immune cells related to insulin resistance and low tyrosine metabolism define a sub-type of depression with high CRP and anhedonia. Brain Behav Immun 2020, 88, 161-165. [CrossRef]
- Baune, B.T.; Fromme, S.E.; Aberg, M.; Adli, M.; Afantitis, A.; Akkouh, I.; Andreassen, O.A.; Angulo, C.; Barlati, S.; Brasso, C.; et al. A stratified treatment algorithm in psychiatry: a program on stratified pharmacogenomics in severe mental illness (Psych-STRATA): concept, objectives and methodologies of a multidisciplinary project funded by Horizon Europe. Eur Arch Psychiatry Clin Neurosci 2025, 275, 1453-1464. [CrossRef]
- Abi-Dargham, A.; Horga, G. The search for imaging biomarkers in psychiatric disorders. Nat Med 2016, 22, 1248-1255. [CrossRef]
- Guo, Q.; Guo, L.; Wang, Y.; Shang, S. Efficacy and safety of eight enhanced therapies for treatment-resistant depression: A systematic review and network meta-analysis of RCTs. Psychiatry Res 2024, 339, 116018. [CrossRef]
- Łysik, A.; Logoń, K.; Szczygieł, A.; Wołoszczak, J.; Wrześniewska, M.; Leszek, J. Innovative approaches in the treatment-resistant depression: exploring different therapeutic pathways. Geroscience 2025, 47, 5543-5558. [CrossRef]
- Salahudeen, M.S.; Wright, C.M.; Peterson, G.M. Esketamine: new hope for the treatment of treatment-resistant depression? A narrative review. Ther Adv Drug Saf 2020, 11, 2042098620937899. [CrossRef]
- Brietzke, E.; Hawken, E.R.; Idzikowski, M.; Pong, J.; Kennedy, S.H.; Soares, C.N. Integrating digital phenotyping in clinical characterization of individuals with mood disorders. Neurosci Biobehav Rev 2019, 104, 223-230. [CrossRef]
- Baldwin, H.; Loebel-Davidsohn, L.; Oliver, D.; Salazar de Pablo, G.; Stahl, D.; Riper, H.; Fusar-Poli, P. Real-World Implementation of Precision Psychiatry: A Systematic Review of Barriers and Facilitators. Brain Sci 2022, 12. [CrossRef]
- Dhieb, D.; Bastaki, K. Pharmaco-Multiomics: A New Frontier in Precision Psychiatry. Int J Mol Sci 2025, 26. [CrossRef]
- Zanardi, R.; Prestifilippo, D.; Fabbri, C.; Colombo, C.; Maron, E.; Serretti, A. Precision psychiatry in clinical practice. Int J Psychiatry Clin Pract 2021, 25, 19-27. [CrossRef]
- Fusar-Poli, P.; Manchia, M.; Koutsouleris, N.; Leslie, D.; Woopen, C.; Calkins, M.E.; Dunn, M.; Tourneau, C.L.; Mannikko, M.; Mollema, T.; et al. Ethical considerations for precision psychiatry: A roadmap for research and clinical practice. Eur Neuropsychopharmacol 2022, 63, 17-34. [CrossRef]
- Brown, J.E.H.; Young, J.L.; Martinez-Martin, N. Psychiatric genomics, mental health equity, and intersectionality: A framework for research and practice. Front Psychiatry 2022, 13, 1061705. [CrossRef]
- Giusti-Rodríguez, P.; Okewole, N.; Jain, S.; Montalvo-Ortiz, J.L.; Peterson, R.E. Diversifying Psychiatric Genomics: Globally Inclusive Strategies Toward Health Equity. Psychiatr Clin North Am 2025, 48, 241-256. [CrossRef]
- Blackburn, T.P. Depressive disorders: Treatment failures and poor prognosis over the last 50 years. Pharmacol Res Perspect 2019, 7, e00472. [CrossRef]
- Oasi, O.; Critchfield, K.L.; Werbart, A. Editorial: Rethinking unsuccessful psychotherapies: when and how do treatments fail? Front Psychol 2024, 15, 1514654. [CrossRef]
- Petter, J.; Schumacher, L.; Echterhoff, J.; Klein, J.P.; Schramm, E.; Härter, M.; Hautzinger, M.; Kriston, L. Heterogeneity of Treatment Outcomes Across Therapists and Sites in a Randomized Multicentre Psychotherapy Trial. Clin Psychol Psychother 2025, 32, e70087. [CrossRef]
- Huneke, N.T.M.; Fusetto Veronesi, G.; Garner, M.; Baldwin, D.S.; Cortese, S. Expectancy Effects, Failure of Blinding Integrity, and Placebo Response in Trials of Treatments for Psychiatric Disorders: A Narrative Review. JAMA Psychiatry 2025, 82, 531-538. [CrossRef]
- Bjurner, P.; Isacsson, N.H.; Abdesslem, F.B.; Boman, M.; Forsell, E.; Kaldo, V. Study protocol for a triple-blind randomised controlled trial evaluating a machine learning-based predictive clinical decision support tool for internet-delivered cognitive behaviour therapy (ICBT) for depression and anxiety. Internet Interv 2025, 40, 100816. [CrossRef]
- Lutz, W.; Deisenhofer, A.K.; Rubel, J.; Bennemann, B.; Giesemann, J.; Poster, K.; Schwartz, B. Prospective evaluation of a clinical decision support system in psychological therapy. J Consult Clin Psychol 2022, 90, 90-106. [CrossRef]
- Kwan, J.L.; Lo, L.; Ferguson, J.; Goldberg, H.; Diaz-Martinez, J.P.; Tomlinson, G.; Grimshaw, J.M.; Shojania, K.G. Computerised clinical decision support systems and absolute improvements in care: meta-analysis of controlled clinical trials. Bmj 2020, 370, m3216. [CrossRef]
- Rybak, Y.E.; Lai, K.S.P.; Ramasubbu, R.; Vila-Rodriguez, F.; Blumberger, D.M.; Chan, P.; Delva, N.; Giacobbe, P.; Gosselin, C.; Kennedy, S.H.; et al. Treatment-resistant major depressive disorder: Canadian expert consensus on definition and assessment. Depress Anxiety 2021, 38, 456-467. [CrossRef]
- Mora, F.; Ramos-Quiroga, J.A.; Baca-García, E.; Crespo, J.M.; Gutiérrez-Rojas, L.; Madrazo, A.; Pérez Costillas, L.; Saiz, P.A.; Tordera, V.; Vieta, E. Treatment-resistant depression and intranasal esketamine: Spanish consensus on theoretical aspects. Front Psychiatry 2025, 16, 1623659. [CrossRef]
- Ivarsson, M.; Danielsson, H.; Imms, C. Measurement issues in longitudinal studies of mental health problems in children with neurodevelopmental disorders. BMC Psychol 2025, 13, 267. [CrossRef]
- Lee, W.; Bindman, J.; Ford, T.; Glozier, N.; Moran, P.; Stewart, R.; Hotopf, M. Bias in psychiatric case-control studies: literature survey. Br J Psychiatry 2007, 190, 204-209. [CrossRef]
- Abbaz Yazdian, F.; Khodabakhshi-Koolaee, A. Exploring the Counselors and Psychotherapists Perceptions of Therapeutic Errors in the Treatment Room. SAGE Open 2024, 14, 21582440241257320.
- Hayes, S.C.; Hofmann, S.G. "Third-wave" cognitive and behavioral therapies and the emergence of a process-based approach to intervention in psychiatry. World Psychiatry 2021, 20, 363-375. [CrossRef]
- Tanaka, M. Parkinson's Disease: Bridging Gaps, Building Biomarkers, and Reimagining Clinical Translation. Cells 2025, 14. [CrossRef]
- Meehan, A.J.; Lewis, S.J.; Fazel, S.; Fusar-Poli, P.; Steyerberg, E.W.; Stahl, D.; Danese, A. Clinical prediction models in psychiatry: a systematic review of two decades of progress and challenges. Mol Psychiatry 2022, 27, 2700-2708. [CrossRef]
- Figueiredo Godoy, A.C.; Frota, F.F.; Araújo, L.P.; Valenti, V.E.; Pereira, E.; Detregiachi, C.R.P.; Galhardi, C.M.; Caracio, F.C.; Haber, R.S.A.; Fornari Laurindo, L.; et al. Neuroinflammation and Natural Antidepressants: Balancing Fire with Flora. Biomedicines 2025, 13. [CrossRef]
- Haslbeck, J.; Ryan, O.; Robinaugh, D.J.; Waldorp, L.J.; Borsboom, D. Modeling psychopathology: From data models to formal theories. Psychological Methods 2022, 27, 930.
- Halassa, M.M.; Frank, M.J.; Garety, P.; Ongur, D.; Airan, R.D.; Sanacora, G.; Dzirasa, K.; Suresh, S.; Fitzpatrick, S.M.; Rothman, D.L. Developing algorithmic psychiatry via multi-level spanning computational models. Cell Rep Med 2025, 6, 102094. [CrossRef]
- Tan, T.; Wang, W.; Liu, T.; Zhong, P.; Conrow-Graham, M.; Tian, X.; Yan, Z. Neural circuits and activity dynamics underlying sex-specific effects of chronic social isolation stress. Cell Rep 2021, 34, 108874. [CrossRef]
- Bowman, R.; Frankfurt, M.; Luine, V. Sex differences in anxiety and depression: insights from adult rodent models of chronic stress and neural plasticity. Front Behav Neurosci 2025, 19, 1591973. [CrossRef]
- Mingardi, J.; Giovenzana, M.; Nicosia, N.; Misztak, P.; Ieraci, A.; Musazzi, L. Sex and Circadian Rhythm Dependent Behavioral Effects of Chronic Stress in Mice and Modulation of Clock Genes in the Prefrontal Cortex. Int J Mol Sci 2025, 26. [CrossRef]
- Bekhbat, M.; Ulukaya, G.B.; Bhasin, M.K.; Felger, J.C.; Miller, A.H. Cellular and immunometabolic mechanisms of inflammation in depression: Preliminary findings from single cell RNA sequencing and a tribute to Bruce McEwen. Neurobiol Stress 2022, 19, 100462. [CrossRef]
- Koppe, G.; Meyer-Lindenberg, A.; Durstewitz, D. Deep learning for small and big data in psychiatry. Neuropsychopharmacology 2021, 46, 176-190. [CrossRef]
- Driessen, E.; Efthimiou, O.; Wienicke, F.J.; Breunese, J.; Cuijpers, P.; Debray, T.P.A.; Fisher, D.J.; Fokkema, M.; Furukawa, T.A.; Hollon, S.D.; et al. Developing a multivariable prediction model to support personalized selection among five major empirically-supported treatments for adult depression. Study protocol of a systematic review and individual participant data network meta-analysis. PLoS One 2025, 20, e0322124. [CrossRef]
- Luedtke, A.; Sadikova, E.; Kessler, R.C. Sample size requirements for multivariate models to predict between-patient differences in best treatments of major depressive disorder. Clinical Psychological Science 2019, 7, 445-461.
- Watkins, E.R.; Newbold, A. Factorial Designs Help to Understand How Psychological Therapy Works. Front Psychiatry 2020, 11, 429. [CrossRef]
- Ciolino, J.D.; Scholtens, D.M.; Bonner, L.B. Factorial Clinical Trial Designs. Jama 2025, 333, 532-533. [CrossRef]
- Watkins, E.; Newbold, A.; Tester-Jones, M.; Javaid, M.; Cadman, J.; Collins, L.M.; Graham, J.; Mostazir, M. Implementing multifactorial psychotherapy research in online virtual environments (IMPROVE-2): study protocol for a phase III trial of the MOST randomized component selection method for internet cognitive-behavioural therapy for depression. BMC Psychiatry 2016, 16, 345. [CrossRef]
- Kotecha, G.; Ventz, S.; Fortini, S.; Trippa, L. Uncertainty directed factorial clinical trials. Biostatistics 2024, 25, 833-851. [CrossRef]
- Victor, T.A.; Khalsa, S.S.; Simmons, W.K.; Feinstein, J.S.; Savitz, J.; Aupperle, R.L.; Yeh, H.W.; Bodurka, J.; Paulus, M.P. Tulsa 1000: a naturalistic study protocol for multilevel assessment and outcome prediction in a large psychiatric sample. BMJ Open 2018, 8, e016620. [CrossRef]
- Nelson, B.; McGorry, P.D.; Wichers, M.; Wigman, J.T.W.; Hartmann, J.A. Moving From Static to Dynamic Models of the Onset of Mental Disorder: A Review. JAMA Psychiatry 2017, 74, 528-534. [CrossRef]
- Harkness, A.R.; Reynolds, S.M.; Lilienfeld, S.O. A review of systems for psychology and psychiatry: adaptive systems, personality psychopathology five (PSY-5), and the DSM-5. J Pers Assess 2014, 96, 121-139. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





