Background
For the past decade, ketamine and its S-enantiomer, esketamine, have offered something close to a miracle for people whose depression laughs at ordinary medicines. A single infusion or nasal spray can melt suicidal despair in the space of an afternoon, with dozens of trials now documenting these overnight turnarounds [
1]. The same biology—an abrupt jolt to glutamatergic signalling—has begun to show promise in stubborn obsessive–compulsive disorder as well, giving respite to patients who have already maxed out serotonin reuptake inhibitors and exposure therapy [
2].
Yet the everyday reality is less inspiring. Intravenous ketamine demands specialised monitoring; intranasal esketamine requires two hours of supervised recovery after each dose; and both carry price tags that put them beyond reach for most clinics, let alone patients, around the world. What good is a wonder drug if you can’t afford the wonder?
That dilemma inspired a different tack. In 2025, Cheung outlined a purely oral, shoestring alternative built from familiar components: dextromethorphan to block NMDA receptors, a strong CYP2D6-inhibiting antidepressant to keep dextromethorphan in circulation, piracetam to push the downstream AMPA receptors, and optional L-glutamine to top up the glutamate pool [
3]. Early case reports hint that this stack can ignite the same NMDA-to-AMPA-to-BDNF cascade credited for ketamine’s speed and durability—only at supermarket prices [
4,
5].
The present report adds a real-world test. We describe a 48-year-old man whose life-threatening depression and later violent-imageried OCD melted after one dose of esketamine, then roared back when he had to stop for financial reasons. Conventional drug combinations failed. Switching him to the low-cost oral stack not only rekindled the initial response but has kept him well for months. His story suggests that the door ketamine opens doesn’t have to slam shut when the money runs out; with the right pharmacological workaround, it may stay open for good.
Methods
We pieced this report together by looking back through the ordinary clinical records kept in a private practice in Hong Kong (Cheung Ngo Medical Limited). Everything the psychiatrist had entered between September 2023 and December 2025—progress notes, medication lists, and the scores from the office’s routine questionnaires—was reviewed after the fact. Depression and anxiety were tracked with the Patient Health Questionnaire-9 (PHQ-9) and the Generalized Anxiety Disorder-7 (GAD-7); suicide- and anxiety-risk flags generated by the electronic chart were noted as well. Each diagnosis reflected the treating clinician’s judgement at the time of the visit, using DSM-5-TR criteria.
The glutamatergic regimen was introduced in March 2025 and inched upward over roughly three months. Dose changes and side-effect checks were folded into standard 20- to 30-minute appointments every two to six weeks. Besides the PHQ-9 and GAD-7, the psychiatrist asked open questions about the frequency and emotional punch of intrusive images, compulsions, and suicidal thoughts. Formal OCD scales such as the Y-BOCS were not part of routine practice, so none were collected.
Because this is a single-patient account, no formal ethics-committee approval was required under local guidelines. The patient reviewed a draft of the manuscript, confirmed its accuracy, and signed a consent form allowing his anonymised story to be published.
Case Presentation
In September 2023, a 48-year-old office worker came to clinic burdened by a lifelong tendency toward anxiety and, more recently, a full-blown major depressive episode marked by intense worry and thoughts of suicide. His mood had been sliding ever since shoulder surgery left him with chronic pain; he was also preoccupied with fears of "old-age diseases," under continual pressure at work, and increasingly estranged from his family. Because of the acuity of his suicidal thinking, we administered a single intranasal dose of esketamine (Spravato 56 mg). The response was dramatic—within hours his mood brightened and the suicidal ideation receded—but he could not afford additional esketamine sessions, and over the next few weeks his depression and anxiety crept back.
By November, the combination tablet flupentixol/melitracen (Deanxit) that he had been taking for years was plainly insufficient. We tried to replicate the glutamatergic effect of ketamine with an oral mixture of dextromethorphan 90 mg per day and bupropion XL 150 mg. The results were better but still fell short of remission.
The Patient defaulted his appointment, and presented to us again in February 2025. His PHQ-9 score had climbed to 17, the GAD-7 registered 21, and he described fragmented sleep along with a torrent of intrusive violent and self-destructive images, now occurring many times a day. The picture met DSM-5 criteria for obsessive-compulsive disorder with poor insight layered on top of recurrent major depression. He had stopped all medication two months earlier, after years of partial benefit from polypharmacy in mainland China, and was desperate for relief.
Beginning in March 2025 we shifted to a fully oral, glutamatergic-focused strategy. Dextromethorphan was increased to 60 mg BD. Fluoxetine 20 mg BD was added, chosen as a strong CYP2D6 inhibitor to prolong dextromethorphan’s effect. We complemented these with piracetam 600mg BD, seeking AMPA modulation. Lower doses of risperidone, valproate, pregabalin, and the old flupentixol/melitracen tablet were kept on board mainly for sleep and residual anxiety.
Within a month the change was striking. By June—and persisting through the autumn—episodes of violent or suicidal imagery had dwindled from dozens each day to once every four or five days, and when they did surface he could shrug them off. Depression scores settled around 5–6 on the PHQ-9; anxiety dropped to the mild range with a GAD-7 of 7; sleep consolidated. He told us in October that he felt "much better overall" and was working effectively, no longer derailed by mental intrusions.
At his last visit on 1 December 2025 he remained on the same inexpensive regimen, was free of suicidal thoughts, and experienced only occasional, easily dismissed intrusive images. The case suggests that a carefully constructed oral combination—dextromethorphan boosted by fluoxetine’s CYP2D6 inhibition and partnered with piracetam’s AMPA activity—can, in some patients, reproduce the durable benefits first glimpsed with ketamine-class therapy, without the cost or logistical hurdles of ongoing esketamine infusions.
Conclusion
The journey described here started with a single 56-mg spray of esketamine that lifted mood and shut down suicidal thinking within hours. That lightning-fast turnaround told us something important about the biology driving this man’s illness: his circuits were unusually responsive to a glutamatergic push, especially the AMPA "throughput" that follows NMDA blockade [
6,
7]. Unfortunately, the price of continued intranasal treatment—well over HK
$6,000 per dose—put that option out of reach.
The question, then, was whether we could bottle the same biology in pills the patient could actually afford. The answer turned out to be a three-drug stack already sitting on most pharmacy shelves: dextromethorphan, fluoxetine, and piracetam. Fluoxetine slows CYP2D6, stretching dextromethorphan’s half-life; piracetam leans on AMPA receptors to amplify the downstream signal. In effect, the trio recreates the whole NMDA-to-AMPA-to-BDNF cascade that makes ketamine unique—an idea first sketched out in the proposal for a "ketamine-class oral stack" [
3].
Clinical reality matched the theory. Once the patient hit dextromethorphan 120 mg/day, fluoxetine 20–40 mg/day, and piracetam 1.2 g/day, the violent intrusive images that had dominated his days shrank to brief, ignorable flickers. PHQ-9 scores slid into the single digits and stayed there; GAD-7 scores followed. The pattern mirrors other real-world reports in depression, OCD, PTSD, binge-eating, and functional somatic syndromes, all pointing to the same low-cost glutamatergic recipe [
4,
5,
8,
9,
10,
11].
Cost is hard to ignore. The daily price of this regimen in Hong Kong—about HK $15–20—represents a 95 percent saving compared with maintenance esketamine, yet it preserved the rapid relief that matters most when suicide is on the table. For health systems as well as patients, such economics deserve attention.
Of course, a single case cannot settle questions of efficacy or safety. Ratings were unblinded, and low-dose risperidone, valproate, and pregabalin were kept in place. Still, the tight temporal link between titrating the stack and watching years-long symptoms fade, after prior confirmation of ketamine sensitivity, makes a spontaneous remission unlikely.
Taken together, the findings add one more piece to a growing puzzle: people who blossom on ketamine may not need ketamine forever. With careful pharmacokinetics—and a little help from an AMPA booster—they may do just as well on familiar, inexpensive drugs.
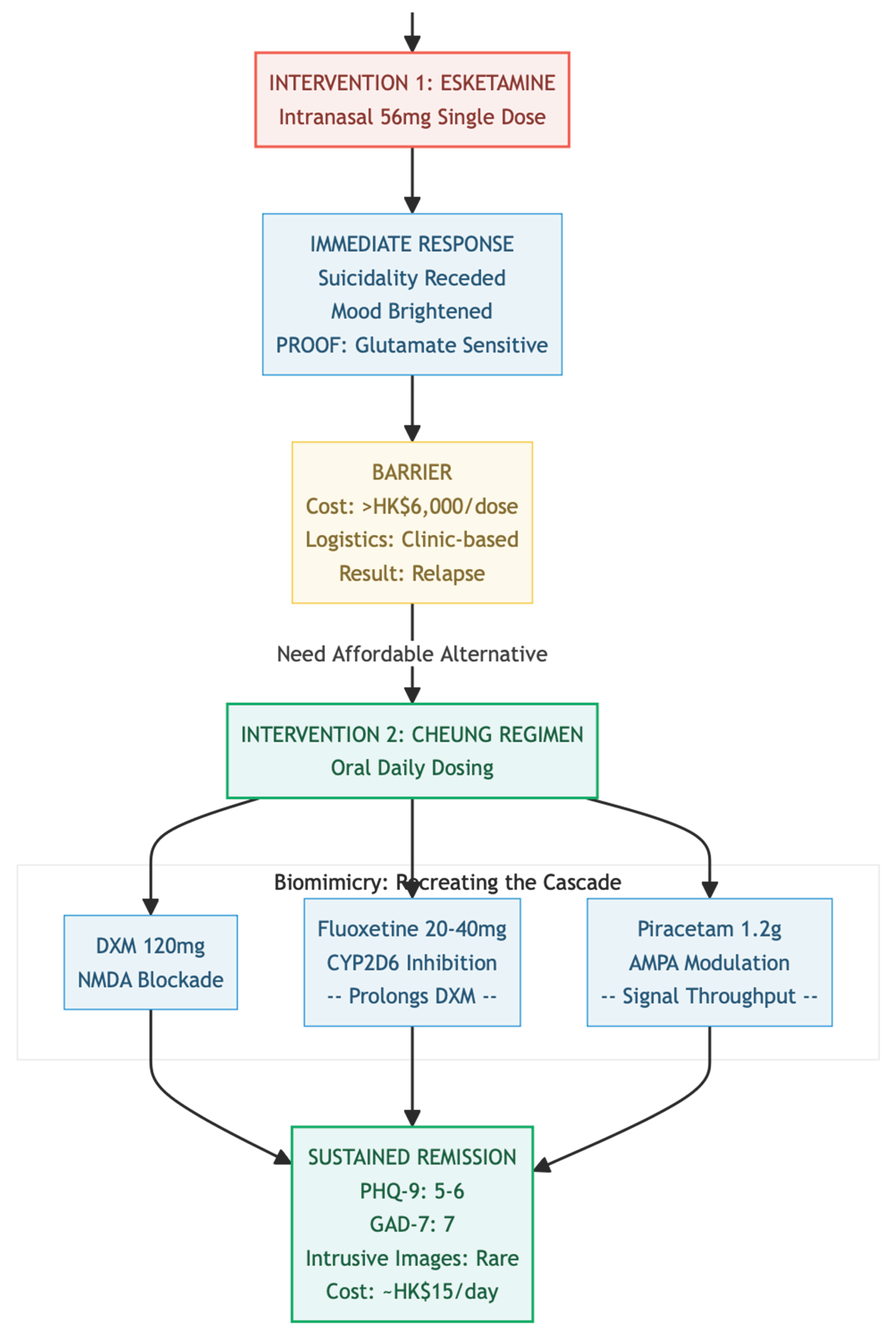
Figure 1.
The Biological Signal emerged when a single intranasal dose of Esketamine produced a rapid anti-suicidal effect, serving not only as an intervention but also as a diagnostic indicator that the patient’s neural circuits were highly responsive to glutamatergic modulation—specifically the NMDA-to-AMPA throughput. This dramatic response provided "biological proof" that justified transitioning to a targeted oral regimen. However, The Pharmacokinetic Hack was necessary due to Dextromethorphan’s (DXM) short half-life, which limits its efficacy as an NMDA antagonist. To address this, Fluoxetine—a potent CYP2D6 inhibitor—was introduced to slow DXM metabolism, sustaining its presence in the system long enough to deliver the "priming" signal required for neuroplasticity. Finally, The Economic Reality underscores the treatment’s accessibility: while Esketamine costs over HK$6,000 per dose, the oral stack (DXM, Fluoxetine, Piracetam) costs approximately HK$15 per day, achieving over 95% in cost savings and offering a sustainable, off-patent alternative that brings "Ketamine-class" efficacy within reach for patients unable to afford ongoing clinic-based infusions.
Figure 1.
The Biological Signal emerged when a single intranasal dose of Esketamine produced a rapid anti-suicidal effect, serving not only as an intervention but also as a diagnostic indicator that the patient’s neural circuits were highly responsive to glutamatergic modulation—specifically the NMDA-to-AMPA throughput. This dramatic response provided "biological proof" that justified transitioning to a targeted oral regimen. However, The Pharmacokinetic Hack was necessary due to Dextromethorphan’s (DXM) short half-life, which limits its efficacy as an NMDA antagonist. To address this, Fluoxetine—a potent CYP2D6 inhibitor—was introduced to slow DXM metabolism, sustaining its presence in the system long enough to deliver the "priming" signal required for neuroplasticity. Finally, The Economic Reality underscores the treatment’s accessibility: while Esketamine costs over HK$6,000 per dose, the oral stack (DXM, Fluoxetine, Piracetam) costs approximately HK$15 per day, achieving over 95% in cost savings and offering a sustainable, off-patent alternative that brings "Ketamine-class" efficacy within reach for patients unable to afford ongoing clinic-based infusions.
References
- Berman, R.M.; Cappiello, A.; Anand, A.; Oren, D.A.; Heninger, G.R.; Charney, D.S.; Krystal, J.H. Antidepressant effects of ketamine in depressed patients. Biol. Psychiatry 2000, 47, 351–354. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, C.I.; Kegeles, L.S.; Levinson, A.; Feng, T.; Marcus, S.M.; Vermes, D.; Flood, P.; Simpson, H.B. Randomized Controlled Crossover Trial of Ketamine in Obsessive-Compulsive Disorder: Proof-of-Concept. Neuropsychopharmacology 2013, 38, 2475–2483. [Google Scholar] [CrossRef] [PubMed]
- Cheung, N. DXM, CYP2D6-inhibiting antidepressants, piracetam, and glutamine: Proposing a ketamine-class antidepressant regimen with existing drugs. Preprints 2025. [Google Scholar] [CrossRef]
- Cheung, N. Clinical experience and optimisation of the Cheung Glutamatergic Regimen for refractory psychiatric diseases. Preprints 2025. [Google Scholar] [CrossRef]
- Cheung, N. Case series: Marked improvement in treatment-resistant obsessive–compulsive symptoms with over-the-counter glutamatergic augmentation in routine clinical practice. Preprints 2025. [Google Scholar] [CrossRef]
- Maeng, S.; Zarate, C.A.; Du, J.; Schloesser, R.J.; McCammon, J.; Chen, G.; Manji, H.K. Cellular Mechanisms Underlying the Antidepressant Effects of Ketamine: Role of α-Amino-3-Hydroxy-5-Methylisoxazole-4-Propionic Acid Receptors. Biol. Psychiatry 2008, 63, 349–352. [Google Scholar] [CrossRef] [PubMed]
- Zanos, P.; Moaddel, R.; Morris, P.J.; Georgiou, P.; Fischell, J.; Elmer, G.I.; Alkondon, M.; Yuan, P.; Pribut, H.J.; Singh, N.S.; et al. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature 2016, 533, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Cheung, N. An oral "ketamine-like" NMDA/AMPA modulation stack restores cognitive capacity in a young man with schizoaffective disorder—Case report. Preprints 2025. [Google Scholar] [CrossRef]
- Cheung, N. Case report: Rapid remission of adolescent binge-eating disorder after over-the-counter glutamatergic augmentations to bupropion. Preprints 2025. [Google Scholar] [CrossRef]
- Cheung, N. OTC glutamatergic augmentation resolves adolescent refractory somatic symptoms. Preprints 2025. [Google Scholar] [CrossRef]
- Cheung, N. Oral glutamatergic augmentation for trauma-related disorders with fluoxetine- / bupropion-potentiated dextromethorphan ± piracetam: A four-patient case series. Preprints 2025. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





