1. Historical Foundation and Evolution of Lipid-Based Drug Delivery
The theoretical framework of lipid-based drug delivery was built on the early revelations that phosphatidic acid vesicles led to the trapping of the therapeutic agents and thus resulting in their stability and localization increase. One of the first original steps of this process was the invention of long-flowing liposomes that were incrementally prolonged in their blood residence and the tumor accumulation increase due to passive targeting through the enhanced permeability and retention (EPR) effect [
1]. These early formulations illuminated the benefit of changing lipid composition to boost pharmacokinetic ability.
The liposome has taken a long and winding but victorious path from a mere vesicle to a sophisticated pharmaceutical carrier with the qualities of greater biocompatibility, drug-loading capacity, and targeting. Torchilin insisted that liposomes were of vital importance in getting both poorly soluble and soluble drugs into the body. This was a huge step toward the making of multifunctional nanocarrier systems, which, together with the intracellular delivery, stimulus-responsive release, and ligand-mediated targeting, could Sproule [
2]. It was also during this time that liposomes were recognized as the link between conventional dosage forms and modern nanomedicine.
The advent of stealth liposomes marked a turning point in the history of lipid-based drug delivery. The PE (polyethylene glycol) coating of the liposome rendered the drug less visible to the mononuclear phagocyte system. This change resulted in the drug’s strikingly longer circulation time and, consequently, better bioavailability and therapeutic effect which was even more pronounced in oncology applications [
3]. The development of the clinically approved formulations, Doxil
®, among others, having lesser systemic toxicity than the conventional chemotherapy was made possible by these advances.
Slowly but surely, liposomal systems were gradually transformed from laboratory concepts to clinically accepted technologies over the year. Allen and Cullis pointed out that rational design, lipid composition optimization, and surface engineering conducted to better stability, drug release control, and targeted delivery, making liposomes a vital part of nanocarrier-based therapeutics in modern medicine [
4]. These innovations created the scientific basis for the development of the new lipid-based drug delivery platforms.
2. Classical Liposomes and Early Pharmaceutical Developments
Classical liposomes indicated a landmark moment in the history of lipid-based drug delivery, a phalanx of their property being the phospholipid bilayer structure, which was capable of fooling biological membranes. The features of these systems were biocompatibility, the capability to enclose both water-soluble and fat-soluble medicines, and the possibility of controlled release, which made them the most advanced and the first to be adopted pharmaceutical carriers among others in the development of new drug delivery systems [
5]. They also had better cellular uptake and less toxicity than standard drug delivery systems because of their close structural resemblance to cell membranes.
The early developments in drugs aimed at the pharmaceutical industry were meant to optimize the components like lipid composition, size, lamellarity, and drug entrapment efficiency, in order to get better therapeutic performance. Scientists showed that changing the lipid ratios in liposomes remarkably affected drug retention, stability, and release kinetics. The invention of multilamellar vesicles (MLVs), large unilamellar vesicles (LUVs), and small unilamellar vesicles (SUVs) opened up the applications in oncology, infectious diseases, and vaccine delivery to a wider extent [
6]. The developments of those days became the basis of the design of tunable liposomal systems that are now appropriate for therapeutics.
The use of liposomes in pharmaceuticals was not only tied up with their identification as drug carriers that increase bioavailability and lower dosing frequency by the mere fact of being water-soluble and non-toxic, but also with the development of their case-turning practices such as thin-film hydration, reverse-phase evaporation, and microfluidic techniques which improved reproducibility, particle uniformity, and scalability—important for commercial applications [
7]. In addition, liposomes were recognized for their ability to protect the encapsulated drugs from bioavailability degradation, thus increasing one-off intake and reducing dosing frequency.
On the other hand, the evolution of classical liposomes included not only structural and formulation advancements but also drug targeting and release behavior. Studies pointed out the role of lipid choice, charge modification, and bilayer fluidity in controlling drug release dynamics and cellular interaction profiles [
8]. These advances were one of the key factors that led to the approval of clinical liposomal formulations and opened the door for next-generation nanocarriers with even more features.
Table.
Classical Liposomes and Early Pharmaceutical Developments
Table.
Classical Liposomes and Early Pharmaceutical Developments
| Subtopic |
Description |
Key Contributions / Advantages |
References |
| Phospholipid Bilayer-Based Liposomes |
Early liposomes featured a phospholipid bilayer structure capable of mimicking biological membranes. They could encapsulate both hydrophilic and lipophilic drugs. |
High biocompatibility; reduced toxicity; controlled release; enhanced cellular uptake. |
[5] |
| Early Pharmaceutical Optimization |
Formulation studies focused on optimizing size, lipid composition, lamellarity, and drug entrapment efficiency to improve therapeutic performance. |
Improved drug retention, stability, and release kinetics. |
[6] |
| Liposome Types: MLVs, SUVs, LUVs |
Development of multilamellar vesicles (MLVs), small unilamellar vesicles (SUVs), and large unilamellar vesicles (LUVs) expanded clinical applications. |
Extended uses in oncology, vaccine delivery, and infectious diseases. |
[6] |
| Manufacturing Techniques |
Introduction of thin-film hydration, reverse-phase evaporation, and microfluidic techniques enhanced commercial scalability and reproducibility. |
Higher particle uniformity; improved scalability; better drug protection and dosing frequency reduction. |
[7] |
| Drug Stability and Improved Bioavailability |
Liposomes increased drug protection against degradation and extended circulation, enhancing one-time dosing and bioavailability. |
Increased bioavailability; reduced dosing frequency; enhanced therapeutic outcomes. |
[7] |
| Structural and Surface Modification |
Advances included tailoring lipid composition, surface charge, and membrane fluidity to control release and cellular interaction. |
Enabled targeted delivery; optimized release behavior; led to clinical approvals. |
[8] |
3. Fundamentals of Liposomal Structure Composition and Classification
The liposomal structure is mainly a double layer of phospholipids that surrounds a water core and thus can encapsulate both types of drugs—hydrophilic and hydrophobic. The choice of lipids like cholesterol and phosphatidylcholine determines the properties like rigidity, permeability, and drug retention of the vesicles. The most flexible liposomes, i.e., transfersomes and ethosomes, employ edge activators like surfactants, which make the membranes more elastic and thus allow for both dermal and transdermal drug delivery [
9]. With this modification, liposomes can access and penetrate biological barriers, an operation that is performed poorly by traditional vesicles.
Liposomes are classified by their size, number of layers, type of materials used, and their surface properties. MLVs (Multilamellar vesicles) consist of many layers of lipids and are most useful in the production of sustained-release formulations, while SUVs (Small Unilamellar Vesicles) and LUVs (Large Unilamellar Vesicles) are the preferred forms of target-release due to their small size and high encapsulation efficiency. Nanovesicular liposomes, such as virosomes, archaeosomes, and niosomes, have very higher stability, immunogenicity modulation, and even more cellular uptake that extends their use in the biomedical field [
10]. These advanced types of vesicles are said to have both enhanced drug protection and targeted delivery capabilities.
The composition of liposomes has a significant impact on their stability, encapsulation efficiency, and efficacy as a drug. The use of the Quality by Design (QbD) technique is essential in determining proper lipid ratio, and particle size, adjusting surface charge and selecting the right manufacturing conditions. It is evident from the research that by designing and controlling the critical formulation attributes systematically, reproducibility is improved, performance is enhanced, and quicker regulatory approval paths are created [
11]. This method has been a major factor in the commercial success of liposomal products.
Today, the focus in liposomal technology is directed towards the development of vesicles with optimal surface modifications such as PEGylation, ligand conjugation, and charge alteration. These modifications lead to enhanced blood circulation, more effective active targeting, and a higher likelihood of stimuli-responsive drug release. Cationic, pH-sensitive, and immunoliposomes among functionalized liposomes are considered to be the best choice for the application in vaccines, cancer treatment, and gene therapy [
12]. The technology has led to the classification of liposomes as all-purpose nano-carriers capable of solving different therapeutic problems.
4. Quality by Design Formulation Techniques and Characterization
Quality by Design (QbD) has become a powerful tool in the production of liposomal formulations, wherein the entire process systematically looks for critical quality attributes (CQAs) that define and control the products, among them particle size, polydispersity, zeta potential, and encapsulation efficiency. With the help of QbD, these parameters are fully understood and controlled thus the safety, targeting capability, stability, and therapeutic efficiency of the nanocarriers are guaranteed. The use of artificial intelligence in the QbD process supports predictive modeling which reduces the number of experiments and increases the accuracy of formulations thus speeding up the process of drug development [
13].
The use of rational formulation strategies is essential to not only improve the effectiveness of liposomal drug delivery systems but also to reduce the toxicity of these systems. Commonly, the lipid ratios are varied, cholesterol is added, and the vesicle surface properties are altered to increase stability and control release. Several techniques like thin film hydration, freeze-thaw cycles, and solvent injection have been improved to facilitate the reproducibility and upscaling of the liposomal preparations [
14]. Such improvements have made it easier to transfer laboratory prototypes to clinically acceptable liposomal products.
Choosing the right types of nanocarriers is the key to formulating, and the understanding of tocosomes, liposomes, and nanoliposomes in comparison to each other has contributed to it. Tocosomes are made from tocopherol derivatives, which provide them with very strong antioxidant capacities, high biocompatibility, and thus, the ability to be applied in the nutrition and medicine sectors. Nanoliposomes are now more placebo-reactive than before due to their size and permeability which makes them useful in drug delivery and targeted therapies [
15]. The difference in structures allows the formulation scientists to personalize their carriers according to the needs of the therapy.
Characterization is a very important factor in the assessing the structural integrity and internal arrangement of lipid nanoparticles. Small-Angle X-ray Scattering (SAXS) is one of the techniques that provide the researchers with a lot of information about the lipid bilayer thickness, the core-shell architecture, vesicle organization, and internal packing behavior. The structural analysis is, therefore, a way of knowing how the ADA works and stability which the nanocarrier systems will always deliver [
16]. Characterization techniques are, thus, the backbone of the development of liposomal formulations that are robust, stable, and compliant with the regulations.
5. Lipid Nanocarriers Tocosomes Nanoliposomes and Flexible Nanovesicles
Lipid nanocarriers are considered the most sophisticated delivery systems that have been developed to improve drug stability, targeting efficiency, and therapeutic performance. The recent studies revealed that through sequential polyelectrolyte adsorption, strong vesicular capsules are made of charged liposomes. This agent reinforcing structure significantly enhances the - mechanical and prevents premature leakage while boosting the circulation times, hence they are often used for fragile biopharmaceuticals and hydrophilic drugs [
17]. These modified liposomes can find their way in both passive and active targeting applications depending on the need.
Lipid nanoparticles (LNPs) have a vast range of applications, including solid lipid nanoparticles and nanostructured lipid carriers, that have evolved to serve as delivery vehicles only. They also act as bioactive platforms to modulate immune responses, offer tissue-specific delivery, and support gene therapy. Due to their physicochemical adaptability, scalability, and compatibility with diverse therapeutic agents, LNPs have been regarded as one of the most—if not the most—versatile nanocarrier systems in modern pharmaceutical applications [
18]. Their properties have led to the successful integration of LNPs aiming for mRNA vaccine technology and targeted oncology.
The highly adaptable pLNPs are indeed a revolutionary step forward in drug delivery systems, created using the Four-Domain Model, which that combines targeting, release, protection, and sensing into one single carrier. The intelligent nanoparticles are made to react to certain pH levels, enzymes, or heat, thereby discharging their contents right at the disease area. By the way, these particles are not only made to behave in a certain way; they have also been created in such a way that they could be used in many areas of biomedical research such as gene silencing and precision therapy, thanks to the use of lipids, polymers, and ligand conjugates [
19].
The use of lipid-based carriers has had a remarkable impact on drug delivery to the cells and tissues due to their capacity to encapsulate and at the same time keep the activity of the proteins, nucleic acids, or peptides. They are biodegradable and less immunogenic; therefore, they can be considered as proper substitutes for polymeric carriers. Furthermore, their capability to be modified to suit the surface of the drug provides an easy way to give the drug directly to patients with chronic diseases, autoimmune conditions, and even cancer [
20]. These innovations serve as a signal of the transition to multifunctional nanocarriers that can cope with a wide range of therapeutic hurdles.
Table.
Lipid Nanocarriers – Tocosomes, Nanoliposomes, and Flexible Nanovesicles.
Table.
Lipid Nanocarriers – Tocosomes, Nanoliposomes, and Flexible Nanovesicles.
| Subtopic / Carrier Type |
Description & Structural Features |
Key Advantages / Functions |
Applications |
References |
| Polyelectrolyte-Reinforced Liposomes |
Charged liposomes reinforced through sequential polyelectrolyte adsorption, creating strong vesicular capsules. |
Enhanced mechanical strength, reduced premature leakage, improved circulation time. |
Delivery of fragile biopharmaceuticals, hydrophilic drugs; passive and active targeting. |
[17] |
| Lipid Nanoparticles (LNPs) |
Includes solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs); high physicochemical adaptability. |
High drug loading, immune modulation, gene therapy compatibility, scalable production. |
mRNA vaccine technology, targeted oncology, gene delivery. |
[18] |
| Programmable Lipid Nanoparticles (pLNPs) |
Developed using the Four-Domain Model (targeting, release, protection, sensing); stimuli-responsive. |
Respond to pH, enzymes, or heat; smart release at disease site; multifunctional precision carriers. |
Gene silencing, precision medicine, controlled release therapy. |
[19] |
| Flexible Nanovesicles (e.g., Tocosomes, Nanoliposomes) |
Lipid-based vesicles engineered for high deformability, biocompatibility, and biomolecule delivery. |
Encapsulate and preserve proteins, peptides, and nucleic acids; high tissue penetration; non-immunogenic. |
Chronic diseases, autoimmune conditions, cancer therapy, biologics delivery. |
[20] |
6. Comparative Insights into Surfactant-Based, Liposomal, and Lipid Nanoparticle Systems
The highly adaptable pLNPs are indeed a revolutionary step forward in drug delivery systems, created using the Four-Domain Model, which that combines targeting, release, protection, and sensing into one single carrier. The intelligent nanoparticles are made to react to certain pH levels, enzymes, or heat, thereby discharging their contents right at the disease area. By the way, these particles are not only made to behave in a certain way; they have also been created in such a way that they could be used in many areas of biomedical research such as gene silencing and precision therapy, thanks to the use of lipids, polymers, and ligand conjugates [
19].
The use of lipid-based carriers has had a remarkable impact on drug delivery to the cells and tissues due to their capacity to encapsulate and at the same time keep the activity of the proteins, nucleic acids, or peptides. They are biodegradable and less immunogenic; therefore, they can be considered as proper substitutes for polymeric carriers. Furthermore, their capability to be modified to suit the surface of the drug provides an easy way to give the drug directly to patients with chronic diseases, autoimmune conditions, and even cancer [
20]. These innovations serve as a signal of the transition to multifunctional nanocarriers that can cope with a wide range of therapeutic hurdles.
7. Emerging Oral, Dermal, and Rapidly Dissolving Drug Delivery Platforms
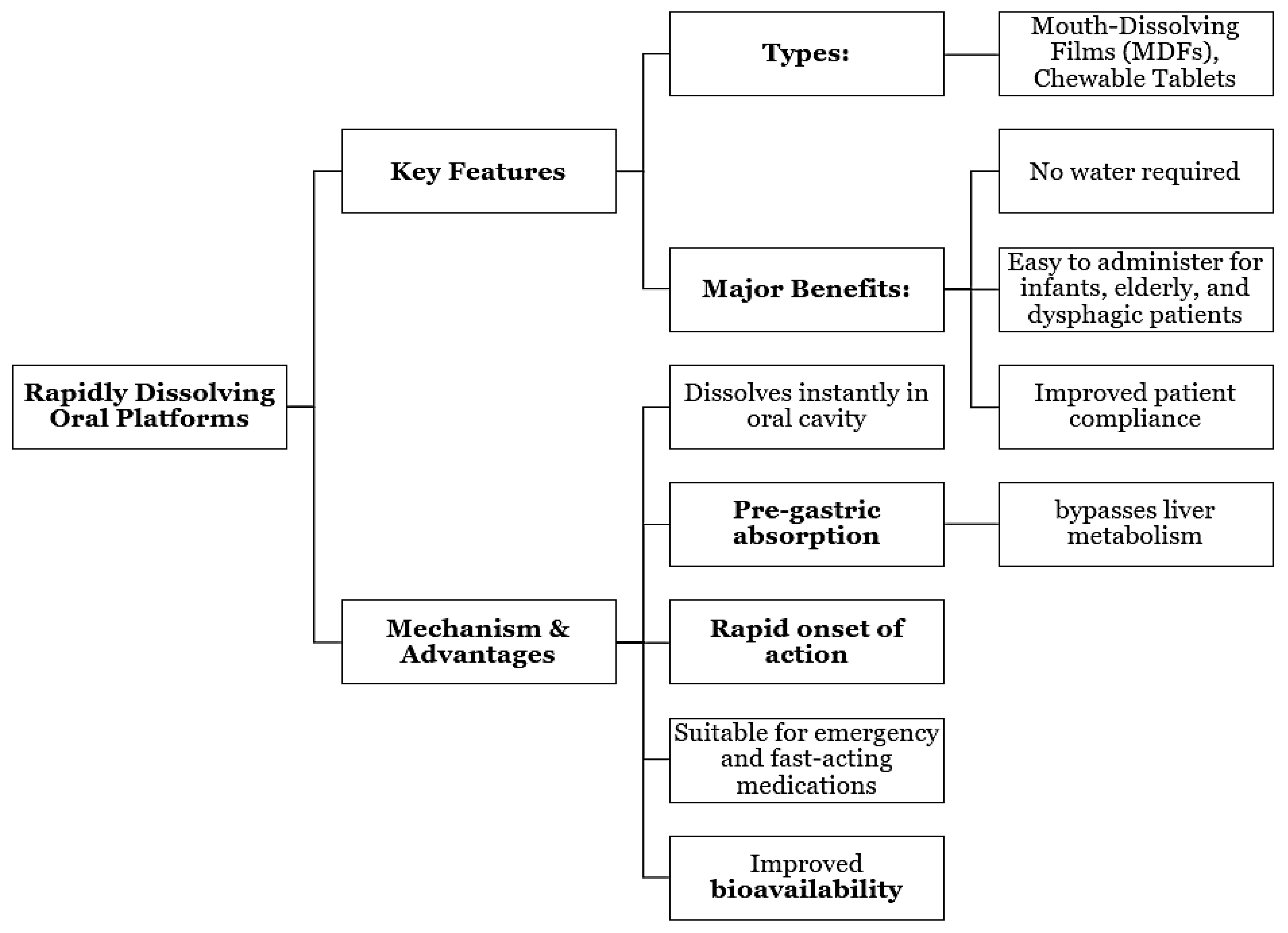
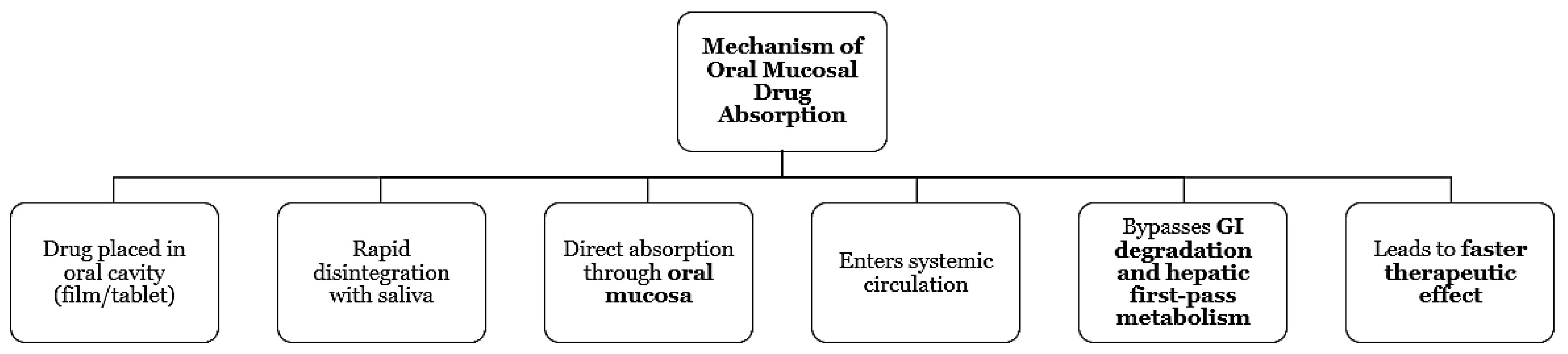
Mouth-dissolving films and chewable tablets are rapidly dissolving formulations that have been receiving a lot of attention due to their benefits over the traditional methods of drug administration, patient compliance and ease of use in the case of infants and elderly people. These oral delivery systems allowing fast absorption through the mouth, thereby making the drug suitable for painless, quick and emergency [
24] medications, etc., because they also eliminate liver metabolism, cause rapid onset of action, and improve bioavailability via the above-mentioned route. At the same time, lipid nanocarriers and liposomes are developed for controlled oral and dermal delivery, by enhancing drug solubility, mucosal retention, and sustaining therapeutic effect. Their ability to encase peptide, protein, and unstable compound going for targeting delivery while reducing degradation in GI or dermal environments [
25]. Such technological advances point out the turning point in medical deliveries that are patient-oriented, fast-acting, and biocompatible-related that are particularly suitable for chronic and acute therapies.
Figure 1.
Rapidly Dissolving Oral Platforms.
Figure 1.
Rapidly Dissolving Oral Platforms.
Figure 2.
Mechanism of Oral Mucosal Drug Absorption.
Figure 2.
Mechanism of Oral Mucosal Drug Absorption.
8. Targeted Delivery Strategies for Neurological, Oncological, and Systemic Diseases
Lipid nanoparticles have become very popular in the therapy of neurological disorders, particularly in Alzheimer’s disease, mainly due to their capability of passing through the blood-brain barrier, thus, protecting the therapeutic agents from degradation by enzymes and eventually giving a controlled release of the drug over a long period of time. The combination of targeting ligands and surface-modifying polymers in LNPs leads to better brain uptake and increased neuronal delivery efficiency [
26]. In cancer treatment, lipid nanoparticles can deliver the drugs straight to the tumor site due to the EPR effect, which is the result of the increased permeability and retention of the tumor, thus allowing the use of less toxic chemotherapy and increasing the overall therapeutic effect [
18]. Furthermore, for systemic diseases like colorectal cancer, nano-formulations are made with the help of ligand-mediated targeting, which results in better localization, internalization, and cellular uptake, and thus, it can be aimed at personalized treatment [
22].
9. Programmable Lipid-Based Nanoparticles and Artificial Intelligence Integration
The programmable lipid nanoparticles (pLNPs) made using the Four-Domain Model allow for modular customization for protection, targeting, sensing, and release, thus making them fit for the applications of gene therapy, mRNA delivery, and precision medicine. These carriers are able to react to either internal or external biological stimuli, so they are able to deliver the drug precisely and with better accuracy [
19]. The use of small-angle X-ray scattering (SAXS) and other techniques for structural characterization provides more knowledge about nanoparticle morphology, stability, and drug-loading behavior, thereby helping in the development of optimized formulation [
16]. Lipid nanoparticles have already taken on the role of passive carriers; they are now being designed for immune modulation, disease detection, and smart drug delivery roles, particularly in the areas of mRNA vaccine technology and regenerative medicine [
18]. AI is also an important factor in these advances because it helps to predict the composition of formulations, create the optimal particle size and stability, and speed up the nanocarrier design process by using machine learning-based predictive modeling [
13].
10. Future Perspectives, Translational Challenges, and Clinical Adaptability
Even though there is a lot of clinical translation, at the same time, scale-up, stability, immunogenicity, and regulatory approval of liposomal and lipid nanoparticle formulations will be the major issues to be solved in future studies. The reproducibility and safety requirements are still the factors that have to be tackled before the laboratory product gets to the market [
3]. The modern liposomal formulations are focused on reducing toxicity and increasing therapeutic efficacy at the same time, with attention given to the selection of excipients and pharmacokinetic performance complying with regulatory requirements [
14]. Lipid nanoparticles with their customizable properties have been recognized as clinically adaptable platforms for vaccines, gene therapies, and targeted therapies; however, the widespread adoption of this technology requires standardized formulation protocols and long-term safety data [
18]. The future of drug delivery is going to be technology that combines the use of nanotechnology with personalized medicine, real-time monitoring, and the use of hybrid systems that mix biological and synthetic components for advanced therapeutic applications [
23].
Conclusion
This document has delved into the detailed metamorphosis, progression, and the future of lipid-based drug delivery systems and nanocarrier therapeutics, stressing their great influence on the present-day medical practice. After outlining the historical background of the classical liposomes, we sequenced the progress from basic phospholipid bilayers to the highly advanced nanocarrier systems like nanoliposomes, tocosomes, flexible vesicles, and programmable lipid nanoparticles. The main factors of liposomal composition and classification were demonstrated, indicating how the structure has a direct impact on efficacy, targeting capability, and drug release mechanisms.
The principles of Quality by Design and state-of-the-art analysis methods were discussed as important factors in enhancing the performance, consistency, and acceptance of the formulation through the regulatory process.
Through comparative studies, the properties that surfactant-based systems, phospholipid liposomes, and modern lipid nanoparticles have were uncovered, together with that which each of them lacks, this way, their potential for disease-specific application was highlighted. A special emphasis was laid on the coming oral, dermal, and rapidly dissolving platforms which not only attract patients but also increase the drug’s bioavailability. Applications in oncology, neurology, and systemic diseases showcased the importance of such factors as ligand-based targeting, EPR effects, immune modulation, and BBB penetration. The use of artificial intelligence has also played a major role in the development of the formulation, real-time prediction of nanoparticle behavior, and personalized therapy design.
Although there have been remarkable developments, still there are challenges such as large-scale production, immunogenicity, long-term safety assessment, and regulatory standardization that weigh heavily. If lipid nanocarriers were to metamorphose from being merely passive carriers to becoming intelligent therapeutic platforms, their clinical adaptability would in turn grow and thus, the future of precision and translational medicine would be shaped accordingly. In unison, lipid-based delivery systems are the very foundation of a change-over in the drug production process that is going to be of huge impact giving a new lease of life to the global therapeutic outcomes.
References
- Gabizon, A., & Papahadjopoulos, D. (1988). Liposome formulations with prolonged circulation time in blood and enhanced uptake by tumours. Proceedings of the National Academy of Sciences, 85(18), 6949–6953. [CrossRef]
- Torchilin, V. (2005). Recent advances with liposomes as pharmaceutical carriers. Nature Reviews Drug Discovery, 4(2), 145–160. [CrossRef]
- Immordino, M. L., Dosio, F., & Cattel, L. (2006). Stealth liposomes: review of the basic science, rationale, and clinical applications. International Journal of Nanomedicine, 1(3), 297–315.
- Allen, T. M., & Cullis, P. R. (2013). Liposomal drug delivery systems: from concept to clinical applications. Advanced Drug Delivery Reviews, 65(1), 36–48. [CrossRef]
- Langner, M. (2024). Lipid-Based Nanotechnology: Liposome. Pharmaceutics, 16(1), 34. [CrossRef]
- Kumar, A., et al. (2024). An updated review on liposomes. International Journal of Pharmaceutics and Drug Analysis, 12(1), 8–14. [CrossRef]
- Ismail, Y., et al. (2024). Descriptive review on liposomal drug delivery system. Journal of Pharma Insights and Research, 2(4), 045–058. [CrossRef]
- Shingate, D. M., & Mulla, J. A. S. (2024). Exploring liposomes: comprehensive classification, preparation techniques, and composition insights. Indian Journal of Novel Drug Delivery, 16(2), 80–90.
- Sudhakar, K., et al. (2021). Ultraflexible liposome nanocargo as a dermal and transdermal drug delivery system. Nanomaterials, 11(10), 2557. [CrossRef]
- Chudasma, M. P., et al. (2023). Brief insight on nanovesicular liposomes as drug-delivery carriers. Journal of Exploratory Research in Pharmacology, 8(3), 222–236. [CrossRef]
- Atre, P., & Rizvi, S. A. A. (2024). Quality by design approach for pharmaceutical liposomes. RSC Pharmaceutics, 1, 675–688. [CrossRef]
- Senjab, R. M., et al. (2024). Advances in liposomal nanotechnology. RSC Pharmaceutics, 1, 928–948. [CrossRef]
- Alshawwa, S. Z., et al. (2022). Nanocarrier delivery systems: AI and future perspectives. Pharmaceutics, 14(4), Article 883. [CrossRef]
- Robson, K. (2024). Liposomal formulations: enhancing efficacy and minimizing toxicity. Research & Reviews: Journal of Pharmaceutics and Nanotechnology, 12(1), 009.
- Atrooz, O., et al. (2024). Comparative review of tocosomes, liposomes, and nanoliposomes. Biomedicines, 12(9), 2002. [CrossRef]
- Spinozzi, F. , et al. (2023). Internal structure of lipid nanoparticles by SAXS. arXiv. 2309. [Google Scholar]
- Ruano, M. , et al. (2024). Fabrication of robust capsules on charged liposomes. arXiv. 2401. [Google Scholar]
- Nanoscale (RSC Publishing). (2025). More than a delivery system: lipid nanoparticles. Nanoscale. [CrossRef]
- Liu, Z., et al. (2024). Programmable lipid nanoparticles and Four-Domain Model. arXiv.
- Kapoor, D., et al. (2024). Lipid nanoparticles for biological drug delivery. Current Pharmaceutical Biotechnology, 25(15). [CrossRef]
- Ashutosh, S. (2025). Comparative analysis of liposomal and surfactant-based delivery. Preprints. [CrossRef]
- Hasan, M. , et al. (2024). Targeted nanoparticle delivery for colorectal cancer. arXiv. 2409. [Google Scholar]
- Sengar, A., & Gupta, S. (2025). Holistic review of novel delivery systems. World Journal of Pharmaceutical Research, 14(20), 37–52.
- Sengar, A. (2025). Chewable Tablets and Mouth-Dissolving Films. International Journal of Biomedicine & Clinical Analysis, 5(1), 29–34.
- Sengar, A. (2025). Controlled Drug Delivery Through Liposomes Nanocarriers. Preprints. [CrossRef]
- Jang, Y. J., et al. (2025). Lipid nanoparticles for Alzheimer’s therapy. Journal of Nanobiotechnology, 23, 99. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).