1. Introduction
The hydrophilicity and hydrophobicity of substrates are determined by the Young’s contact angle
defined by [
1]
where
,
and
are the liquid-vapor, solid-vapor, and solid-liquid surface tensions, respectively. If
is less than 90°, the substrate is classified as hydrophilic; if
is greater than 90°, the substrate is classified as hydrophobic.
Experimentally, the most common method of measuring contact angle
is to observe sessile droplets: A small amount of liquid is deposited on a smooth and flat substrate, forming a droplet whose geometrical contact angle
is measured with a telescope or microscope [
2]. The morphology of the droplet changes from a shallow cap shape with a contact angle
smaller than
as shown in
Figure 1a to a nearly spherical deep cap shape with a contact angle larger than
as shown in
Figure 1b. The measured droplet’s contact angle
is then identified as the Young’s contact angle
.
Theoretically, when considering a macroscopic equilibrium wetting film of thickness
, Young’s equation (
1) is transformed into the form [
3,
4,
5]
where
represents the surface potential
of the wetting film thickness
h at the equilibrium thickness
of the wetting (adsorbed) film. This surface potential describes the molecular interaction between the liquid molecules and the substrate. The subscript “m” is used instead of “Y” to emphasize that this contact angle corresponds to that of a macroscopic droplet.
Equation (
2) is equivalent to the formula know as the Derjaguin-Frumkin formula [
6,
7,
8,
9]
where the function
is know as the disjoining pressure, which is related to the surface potential
in Eq. (
2) through:
Therefore,
. Since the formulas in Eqs. (
2)-(
4) are derived by considering the uniform thin wetting film adsorbed on to the substrate, the contact angle
calculated from them does not necessarily guarantee that they represent the actual geometrical contact angle
of the finite size droplet. Furthermore, since these formulas assume a wetting film, their utility to the hydrophobic substrate is not clear.
A connection between the surface potential
or the disjoining pressure
and the droplet’s geometrical contact angle
rather than the thermodynamic contact angle
is only establihsed through a theoretical free-energy functional model of varying degrees of sophistication. [
10,
11,
12,
13,
14,
15,
16,
17,
18]. Among these models, Starov and Velarde [
14] provided a clear understanding of the geometrical implications of the Derjaguin-Frumkin formula (
3) for
hydrophilic substrates based on the droplet’s morphology derived from the free-energy functional model.
In this paper, we first extend Starov and Velarde’s approach to include both hydrophilic and
hydrophobic substrates. We theoretically analyze the morphology and contact angle of droplets using a simple free-energy functional model. Droplets on hydrophilic substrates are referred to as hydrophilic droplets, with their morphologies known as hydrophilic morphologies. Similarly, droplets on hydrophobic substrates are referred to as hydrophobic droplets, with their morphologies known as hydrophobic morphologies. Next, we calculate the geometrical contact angle of droplets down to the nanoscale using the developed free-energy functional model to determine if the wettability (hydrophilicity/hydrophobicity) from the geometrical contact angle is consistent with that predicted from the macroscopic contact angles calculated from Eqs. (
2) to (
3).
The organization of the present paper is as follows: We consider a strictly two dimensional system throughout to maintain the connection to the thermodynamic formulation in Eqs. (
2) to (
3). We closely follow Starov and Velarde’s work [
14] and formulate the Euler-Lagrange equation to determine the droplet morphology of not only the hydrophilic droplet but also the hydrophobic droplet from the free energy functional model that includes the effect of disjoining pressure in
Section 2. There, we also re-derive the Derjaguin-Frumkin formula [
6,
7,
14] for macroscopic contact angles for
hydrophobic droplets. In
Section 3 we use the simplest one-parameter disjoining pressure model [
19,
20,
21] and study the effect of the surface force on the morphology of droplets. In particular, we pay attention to the transition of morphology from hydrophilic to hydrophobic, as shown in
Figure 1, by changing the parameters of the surface force and the droplet’s size. In
Section 4 we will discuss the implications of our theoretical results for the concept of line tension and droplet size dependence of contact angle. Finally in
Section 5 we conclude with a short comment on our theoretical results on the wettability (hydrophilicity/hydrophobicity) at the nanoscale.
3. Geometrical Contact Angle Using a Simplified Disjoining Pressure
To study the morphology and contact angle of both hydrophilic and hydrophobic droplets, we will revisit the simplest model studied by Pekker et al. [
21]. This model employed the simplest one-parameter disjoining pressure [
19,
20,
21]
where
is the strength of the disjoining pressure,
characterizes the wetting film thickness, and
m and
n are the power-law exponents of the repulsive and attractive parts of the disjoining pressure. Then, the pressure
P in Eq. (
10) can be expressed as:
We closely follow Pekker et al. [
21] and introduce non-dimensionalization, as follows:
Equation (
14) is transformed into [
21]
where
instead of
in Eq. (
14) defined as
and
. Furthermore, a small parameter
is defined [
21] as
to characterize the wetting film thickness
and the excess pressure
P. Now the problem of morphology (hydrophilic/hydrophobic) is completely characterized by two parameters:
and
. The former represents the surrounding vapor phase through the pressure
P in Eq. (
35), and the latter represents the liquid-substrate interaction
through Eq. (
36).
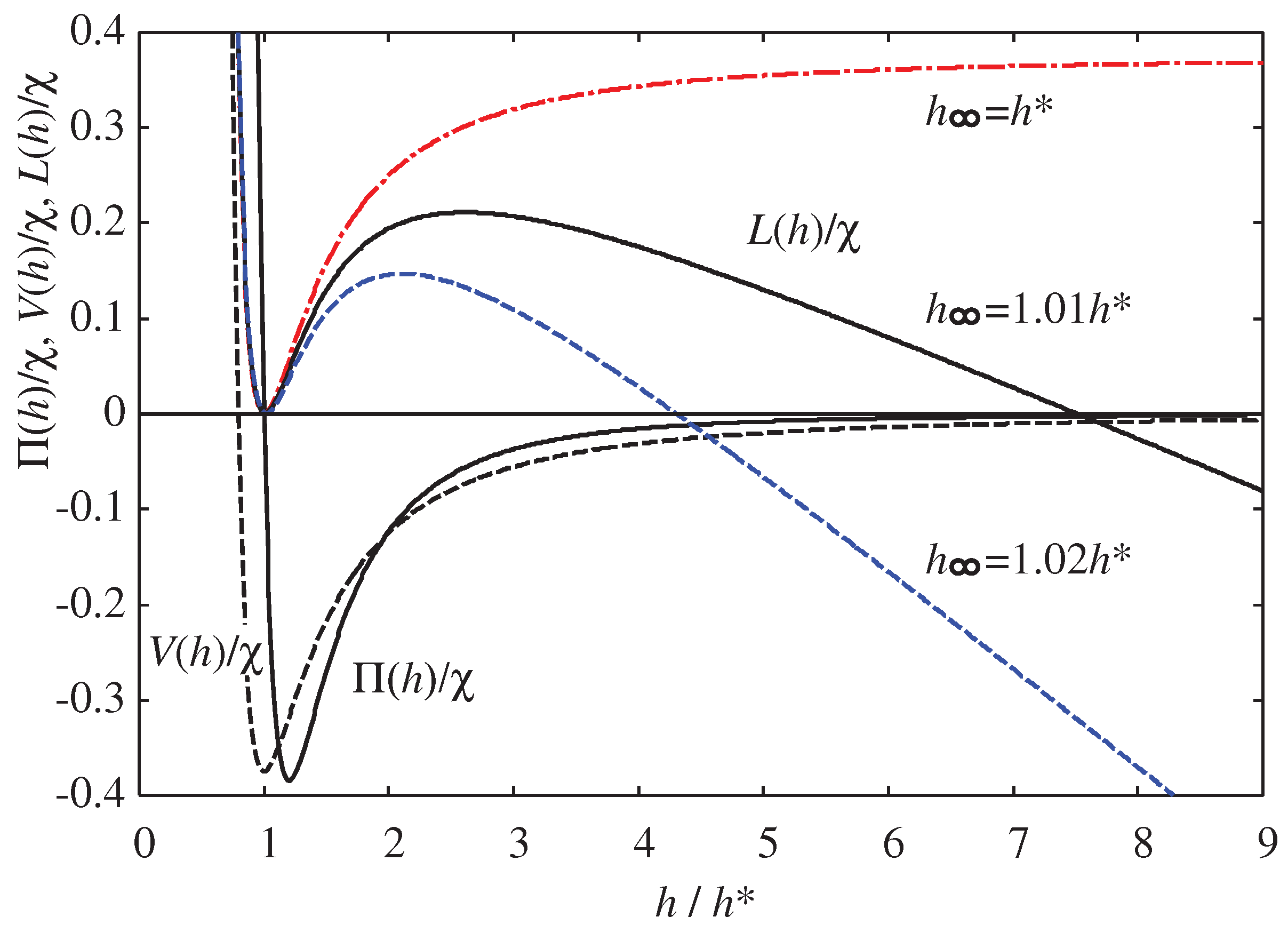
Figure 2 shows the disjoining pressure
in Eq. (
34), along with the corresponding
calculated from Eq. (
4) and
defined in Eq. (
13) for various values of
when
and
. We will utilize
and
, which resembles the Lennard-Jones potential, for numerical calculation. It is evident that
reaches a maximum near
, which is crucial for understanding the hydrophilic-hydrophobic morphological changes.
The excess pressure
P in Eq. (
35) is determined by
.
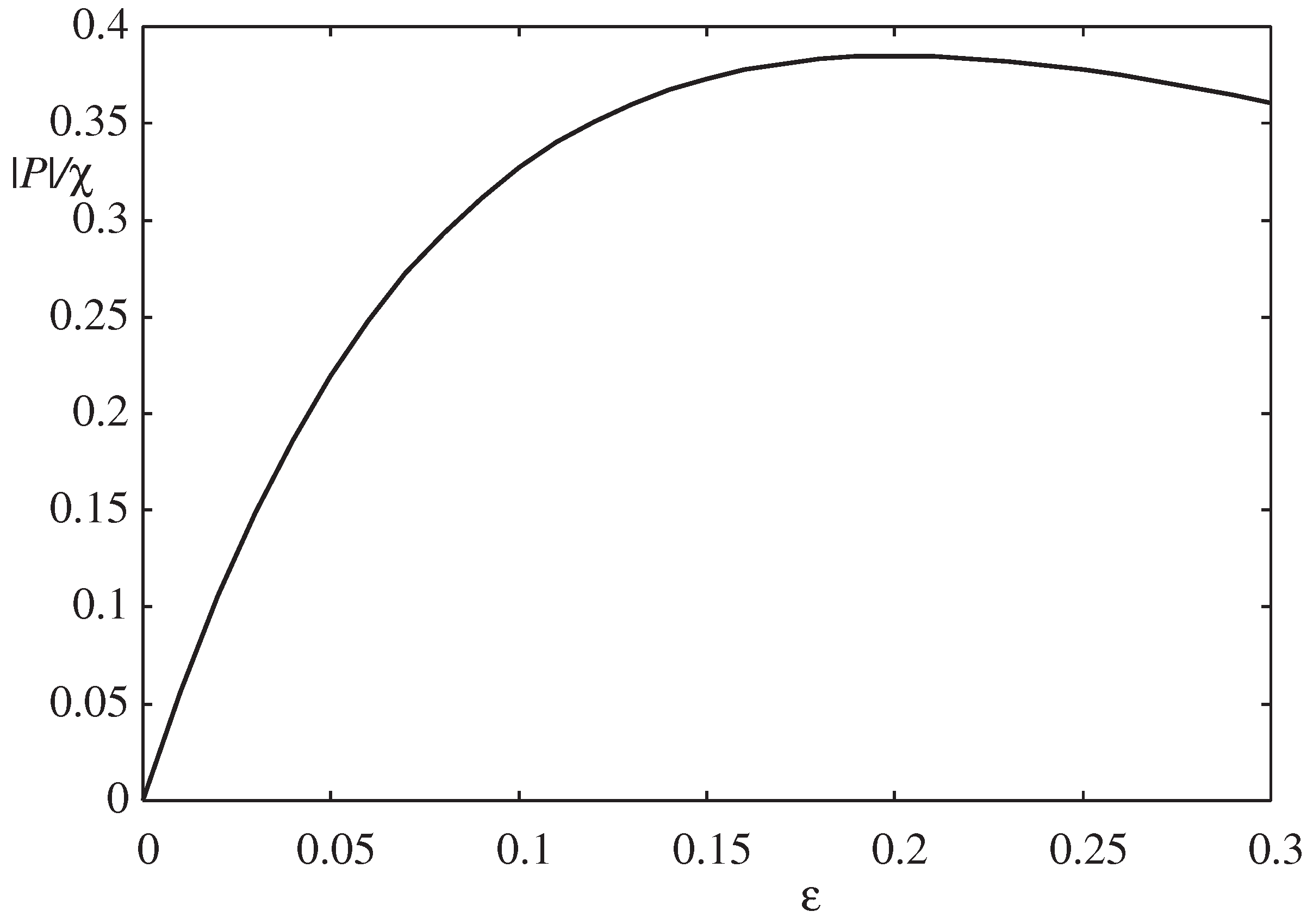
Figure 3 displays the absolute value of the pressure
as a function of the thickness parameter
instead of
when
and
. The wetting film is thinnest (
) when
and thickest (
) when [
21]
where the pressure
is at its minimum (
). For
and
,
. As the oversaturation of the surrounding vapor increases, the absolute value of the excess pressure
also increases, causing the wetting film to become thicker and the parameter
to become greater.
The morphology of a droplet is determined by Eq. (
37), and
Figure 4 shows the functional form of
for various values of
(
). The function
in
Figure 4 demonstrates the maximum
when
at
, which corresponds to the peak of
shown in
Figure 2. As the oversaturation of the surrounding vapor increases, the parameter
increases from 0, and
starts to show the maximum, with its position
becoming smaller.
At the apex of a droplet,
, so the maximum height
of the droplet is determined by solving
from Eq. (
37). The analytical solution of Eq. (
42) in the limit
is [
21]
It is important to note that this result is independent of the strength of the disjoining pressure
. Therefore, once the pressure
P or the film thickness
is fixed, the maximum height is determined from Eq. (
42) regardless of the droplet’s hydrophilic or hydrophobic nature. In
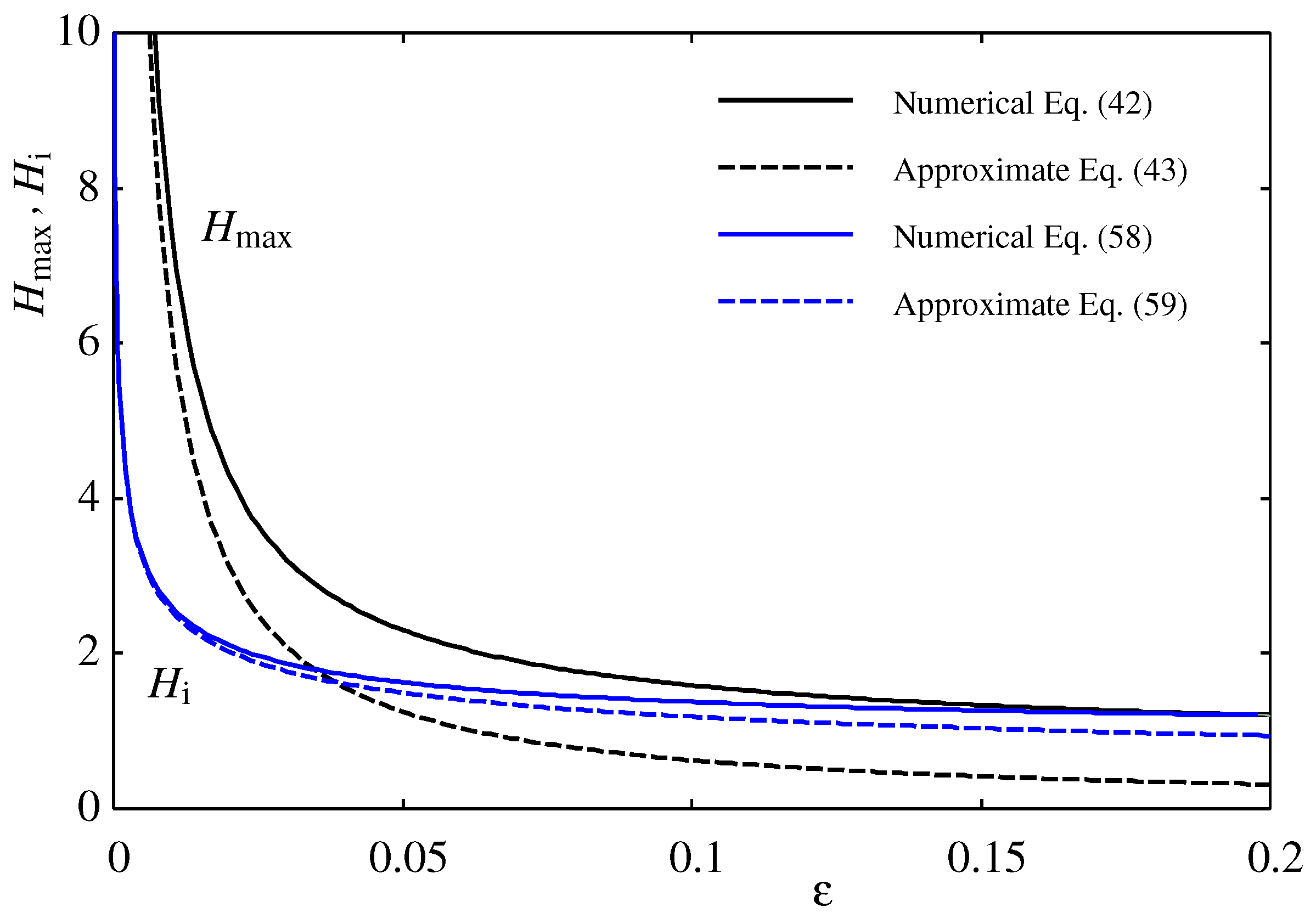
Figure 5, we compare the maximum height
calculated numerically from Eq. (
42) and from the approximate analytical formula [
21] in Eq. (
43). The droplet’s maximum height
decreases as the oversaturation and
increase. The analytical formula is most accurate near
for large droplets (large
).
A droplet solution can only exist when
in Eq. (
37) or
where
is the maximum of
. Equation (
37) can be written as
The sign (±) should be chosen according to the droplet’s hydrophilic or hydrophobic nature and the position
H of the meniscus. For
, the sign should always be negative (
) for hydrophilic droplets (see
Figure 1a). However, for hydrophobic droplets, it changes sign: it is negative (
) near the apex
and changes sign to
at the middle, and changes again to
near the wetting film at
(see
Figure 1b)). Equation (
45) can be easily integrated by choosing the appropriate sign to give the morphology of the droplet, which is controlled by
and
. The former,
, determines the functional form of
and the latter
determines the morphology (a hydrophilic or a hydrophobic droplet).
The morphology of a droplet can be controlled by the strength
of the surface potential or the disjoining pressure. In a hydrophobic droplet in
Figure 1b, the lateral size reaches a maximum and minimum at two critical height,
and
(
and
in
Figure 1), where
and
, respectively (see
Figure 1b). These conditions are met when
from Eq. (
45).
Figure 4 also displays the graphical determination of the intersections
and
. It is evident from this figure that the condition for the appearance of a hydrophobic droplet is when
which combined with Eq. (
44), results in
or
using the relation between
and
in Eq. (
39), where
is the maximum of
(see
Figure 2 and
Figure 4).
Therefore, a scenario different from Eq. (
33) emerges in Eq. (
49) to distinguish between the hydrophilicity and hydrophobicity of finite size droplets. A macroscopic droplet with
is realized when
(See
Figure 5), resulting in the vanishing of excess pressure
(
Figure 3) and the increase of the maximum position
of
to
(
Figure 4) and
(
Figure 2). Consequently,
from Eq. (
13), and Eq. (
49) simplifies to Eq. (
33). However, microscopic and nanoscopic droplets whose wettability is predicted from Eqs. (
48) and (
49) and macroscopic droplets whose wettability is predicted from Eq. (
33) may exhibit contradicting wettability (hydrophilic/hydrophobic) depending on the droplet size.
The position
of the maximum of
is determined by solving
or
from Eq. (
38), and the maximum is given by
In the limit
(
), the approximate solution of Eq. (
50) becomes
and Eq. (
51) becomes
In
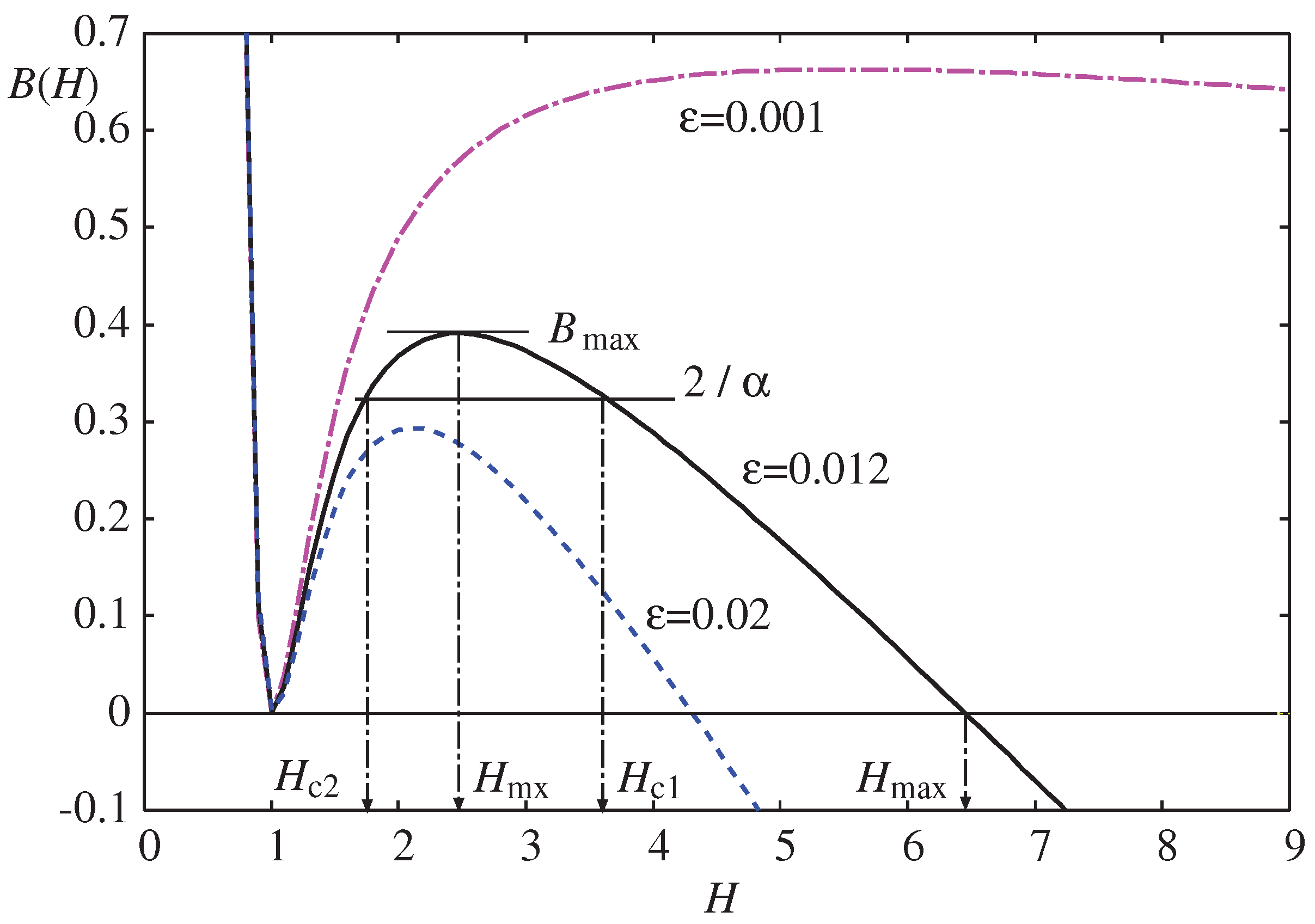
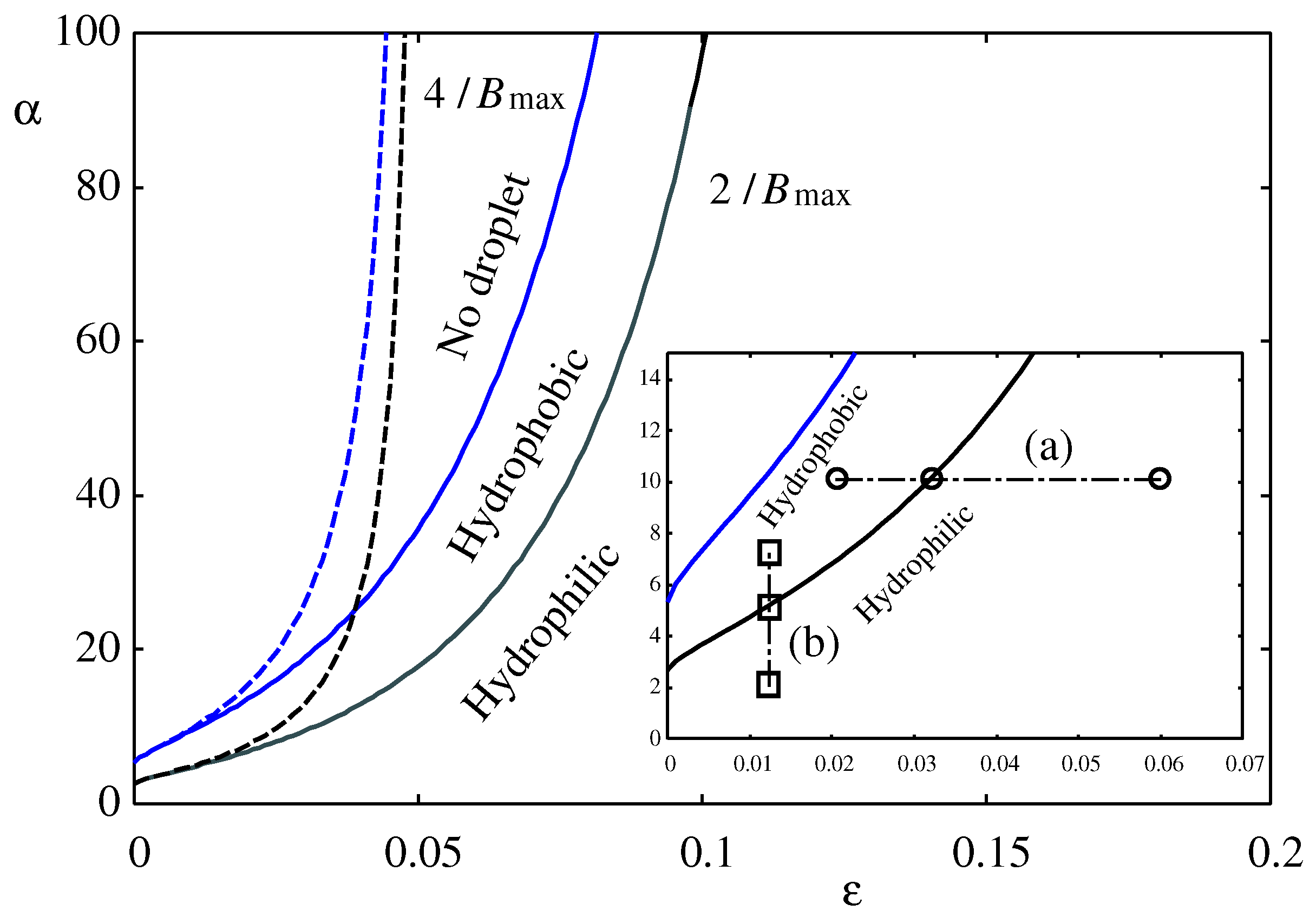
Figure 6, the boundary values for no-droplet,
, and hydrophilic-hydrophobic,
are plotted as a function of
. The dashed lines represent the approximate formula in Eq. (
53), which is only valid for
. As
, these boundaries converge to
and
as predicted by Eq. (
53). The inset of
Figure 6 shows an expanded view around
with two paths (a) and (b) along which the morphologies in
Figure 7a and
Figure 7b are calculated.
Therefore, within this disjoining pressure model in Eq. (
34) the condition for macroscopic droplets in Eq. (
33) is now given as
derived from Eq. (
48) when
.
For a fixed
, the height of the droplet
(Eq. (
42)) as well as the excess pressure
P (Eq. (
35)) and, therefore, the Laplace radius
(Eq. (
17)) are fixed. As the strength of the liquid-substrate interaction
(
) is increased, making the disjoining pressure
stronger and the surface potential
more attractive (see
Figure 2),
Figure 6 indicate that the droplet morphology changes from hydrophilic to hydrophobic as shown in
Figure 1. Finally, the droplet is detached from the substrate and disappears, leaving a wetting film behind. This scenario is consistent with the Frumkin-Derjyaguin formula in Eqs. (
2) and (
3).
When the liquid is more strongly attracted to the substrate, the droplet will spread and the substrate will be more hydrophilic. In fact, many numerical simulations mostly for the Lennard-Jones model fluid using molecular dynamics [
23,
24,
25,
26] or density functional theory [
16,
27] indicate that when the liquid-substrate molecular interaction is more attractive, the contact angle becomes smaller making the droplet more hydrophilic. Superficially, these results seem to contradict the prediction of
Figure 6 based on disjoining pressure.
The statistical mechanical definition of the disjoining pressure [
9,
28,
29] that links fluid-wall molecular interactions to the disjoining pressure is established. Several attempts have been made to determine the disjoining pressure and surface potential using Monte Carlo simulation [
28] and density functional theory [
16,
30,
31,
32], primarily for the Lennard-Jones model fluid. These results suggest that as the fluid-wall interaction becomes stronger and more attractive, the surface potential
or the disjoining pressure
becomes less attractive. The potential minimum (
Figure 2) also becomes shallower, resulting in a lower contact angle from Eq. (
2). Therefore, the hydrophilic-hydrophobic boundary in
Figure 6 and the Derjaguin-Frumkin formula in Eqs. (
2)-(
4) do not contradict the prediction of simulations [
16,
23,
24,
25,
26,
27].
Knowing the conditions in Eqs. (
48) and (
49), we consider the morphology of the droplet in more detail. First, we will examine the simple hydrophilic morphology. In this case, the function
does not intersect
because
(see
Figure 4) and
for
from the apex at
down to the wetting film at
(
Figure 1a). The meniscus is obtained simply by integrating
from Eq. (
45) because
, and the meniscus is given by
which can be evaluated numerically.
Next we will consider the hydrophobic morphology. In this case, Eq. (
46) has two solutions
and
(
, see
Figure 4). Within the interval
,
(see
Figure 1b) and
once again (
Figure 4). Therefore, the meniscus is determined by Eq. (
55). However, in the interval
,
(
Figure 1b) and
(
Figure 4). Despite this, the meniscus is still determined by Eq. (
55) because the denominator is negative (
). Lastly, within the interval
,
(
Figure 1b) and
(
Figure 4) resulting in the meniscus being determined by Eqs. (
55) since
.
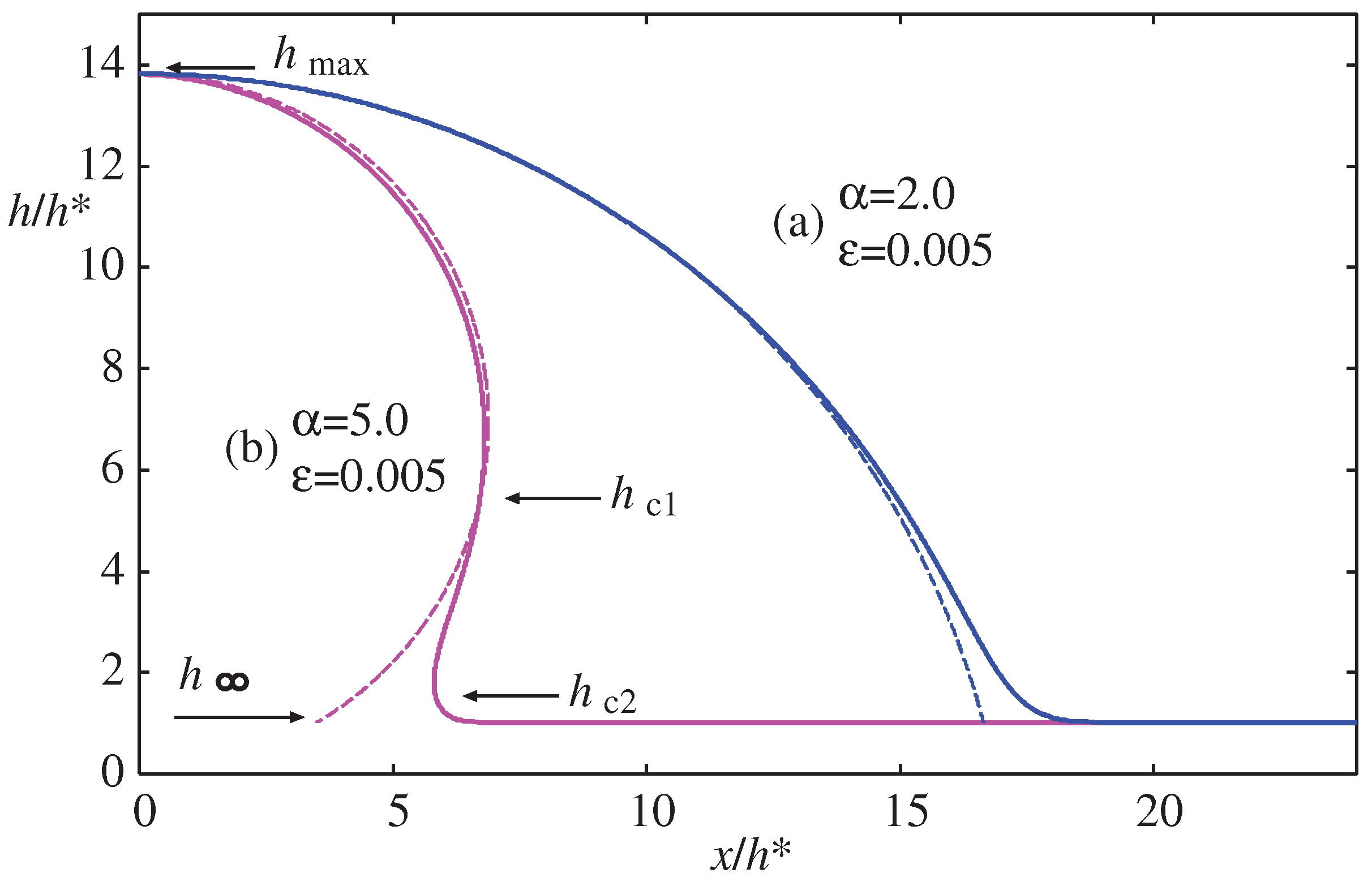
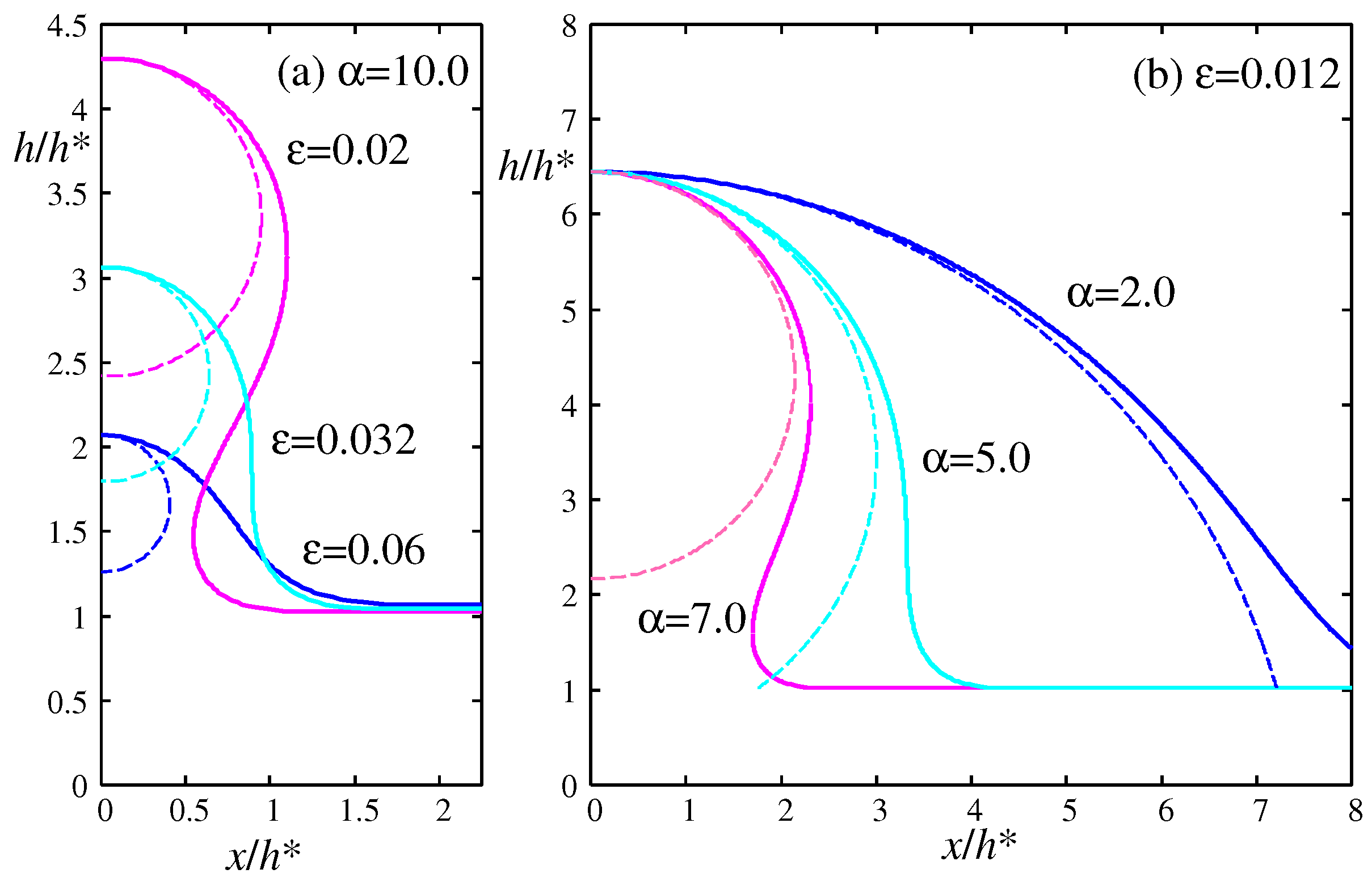
Figure 7a illustrates the numerically calculated morphology of the meniscus for the same value of
but different values of
along the path (a) indicated in the inset of
Figure 6. The morphology transitions from hydrophobic (
) to neutral (
) with a contact angle of nearly
, to hydrophilic (
). The dashed lines represent the cylindrical meniscus with the Laplace radius calculated from Eq. (
17). This approximation is not suitable for smaller droplets because the cylindrical meniscus cannot reach the wetting film, and the contact angle cannot be determined.
Figure 7b shows the morphology of the meniscus for the same value of
but different values of
along the path (b) indicated in the inset of
Figure 6. The morphology changes from hydrophobic (
) to neutral (
) to hydrophilic (
). In this case (b) the height
is fixed as it does not depend on
from Eq. (
42). The parameter sets
used to calculate the morphologies in
Figure 7 are indicated by circular and square symbols in the inset of
Figure 6 and
Figure 8.
Finally, we consider the contact angle which depends on the definition. Pekker et al. [
21] derived an approximate analytical formula for the contact angle of this simplified droplet model. They defined the contact angle
as the slope of the meniscus:
at the inflection point (height)
(
) defined by
, which leads to
from Eqs. (
9) and (
10). The approximate solution of Eq. (
58) in the limit
is obtained as [
21]
and
Then, the effective contact angle
is given by [
21]
from Eqs. (
55) and (
57) for both a hydrophilic morphology and a hydrophobic morphology. In
Figure 5 we also compared the height of the inflection point
calculated from the approximate formula in Eq. (
52) with the exact numerical results from
in Eq. (
58). Apparently, the approximate formula in Eq. (
59) and therefore that in Eq. (
60) are valid only near
. In particular, the formula in Eq. (
60) does not depend on
, so Eq. (
61) must be valid only near
.
Similarly, the integration in Eq. (
3) can be obtained analytically, providing us with an explicit expression for the macroscopic contact angle
:
from the Derjaguin-Frumkin formula [
6,
7,
14] in Eq. (
3), which simplifies to
in the limit
. It is evident from Eqs. (
61) and (
63) that the contact angle
from the formula of Pekker et al. [
21] in Eq. (
61) and
from the Derjaguin-Frumkin formula in Eq. (
63) are identical as
. Equation (
63) and, thererfore, Eq. (
61) have a solution only when
and the hydrophilic-hydrophobic boundary is at
as summarized in Eq. (
54).
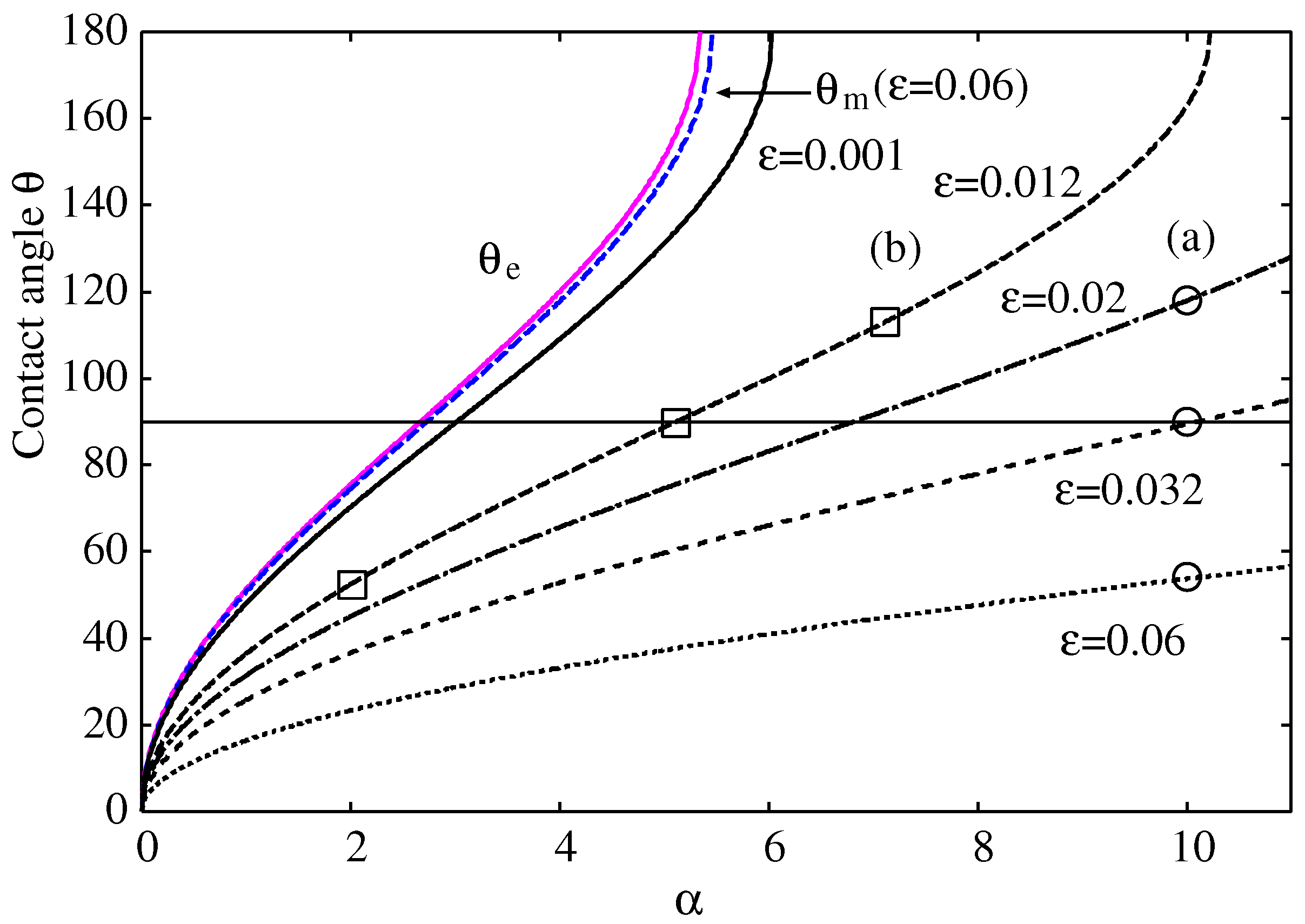
In
Figure 8, we compare the effective contact angle
and the macroscopic contact angle
calculated from the analytical formulas in Eqs. (
61) and (
62) with the exact numerical results
calculated from Eq. (
57). The formulas of Pekkers et al. [
21] and that of Derjaguin-Frumkin [
6,
7,
14] yield similar results, but they generally produce larger values than the exact numerical results, significantly overestimating the contact angles for hydrophobic droplets with larger
(stronger surface potentials) and larger
(smaller droplets, see
Figure 5). Specifically, the numerical results of the contact angle for
in
Figure 8, indicated by the square symbols, are in full agreement with the morphological change of the droplet shown in
Figure 7b. Likewise, those indicated by circular symbols with
are consistent with the morphological change in
Figure 7a.
The values of the contact angles,
, calculated numerically from the exact expression in Eq. (
57), are compared with those of
and
from the approximate formulas in Eqs. (
61) and (
62) in
Table 1 for the droplets shown in
Figure 1 and
Figure 7. The approximate formulas are reasonable for the larger droplets in
Figure 1, but they consistently overestimate the contact angles. For smaller droplets in
Figure 7, the approximate formulas significantly overestimate the contact angles, and they cannot even be used as shown in
Figure 8 because the cylindrical approximation for the meniscus completely fails as shown in
Figure 7a.
The numerical results based on the simplified disjoining pressure in
Figure 8 and
Table 1 suggest that the droplet remains hydrophilic (
) even when the two analytical formulas by Pekker et al. [
21] in Eq. (
61) and Derjaguin-Frumkin [
6,
7,
14] in Eq. (
62) predict a hydrophobic character (
) up to the limit
of Eq. (
54) for
and
. Therefore, the effect of this simplified disjoining pressure makes smaller droplets more hydrophilic and less hydrophobic, resulting in a smaller contact angle. Furthermore, a droplet can be hydrophilic or hydrophobic beyond the limit
. However, this argument does not necessarily invalidate the two analytical formulas in Eqs. (
61) and (
62) because they were originally designed for larger droplets with
, which can be regarded as cylindrical droplets. Even though we restrict ourselves to the two-dimensional cylindrical droplets throughout this paper, the effect of the surface force would be qualitatively the same for the three-dimensional spherical droplets.
4. Discussion
Finally, we will discuss the implications of our theoretical results for real experiments. Within the disjoining pressure model used in this study, the small
approximation would be reasonable only for
, as shown in
Figure 6 and
Figure 8. Specifically, the effective contact angle
in Eq. (
61) and the macroscopic contact angle
from the Derjaguin-Frumkin formula in Eq. (
3) are reasonable for both hydrophilic and hydrophobic droplets only when
, as indicated in
Figure 8. Since Eq. (
43) yields
for
,
and
, the macroscopic formulas in Eqs. (
61) and (
62) are applicable to large droplets with a height greater than approximately 63 times
from Eq. (
36). Assuming the wetting film height is
nm [
8], the minimum droplet height would be
nm. For droplets taller than, let’s say, 100 nm, the macroscopic formula in Eq. (
62) and therefore the Derjaguin-Frumkin formula in Eq. (
3) would be applicable to both hydrophilic and hydrophobic droplets. For a nanoscale droplet shorter than, let’s say, 100 nm, the total volume of the droplet is influenced by disjoining pressure, making it more hydrophilic with a lower contact angle compared to a macroscopic droplet of cm to
m size.
The relationship between droplet size and contact angle has been studied experimentally for many years, with the main focus being on determining the line tension, denoted as
, defined through the phenomenological modified Young’s equation [
33,
34,
35]
where
is the radius of contact line and
is the contact angle of the macroscopic droplet. We can identify
in Eq. (
61) in the model of
Section 3. Since our model is two-dimensional and
, the contact angle should not depend on the size of the droplet as the line tension
does not play a role in modifying the contact angle. However, the contact angle does depend on the size of the droplet as shown in
Figure 8 and
Figure 9a. In
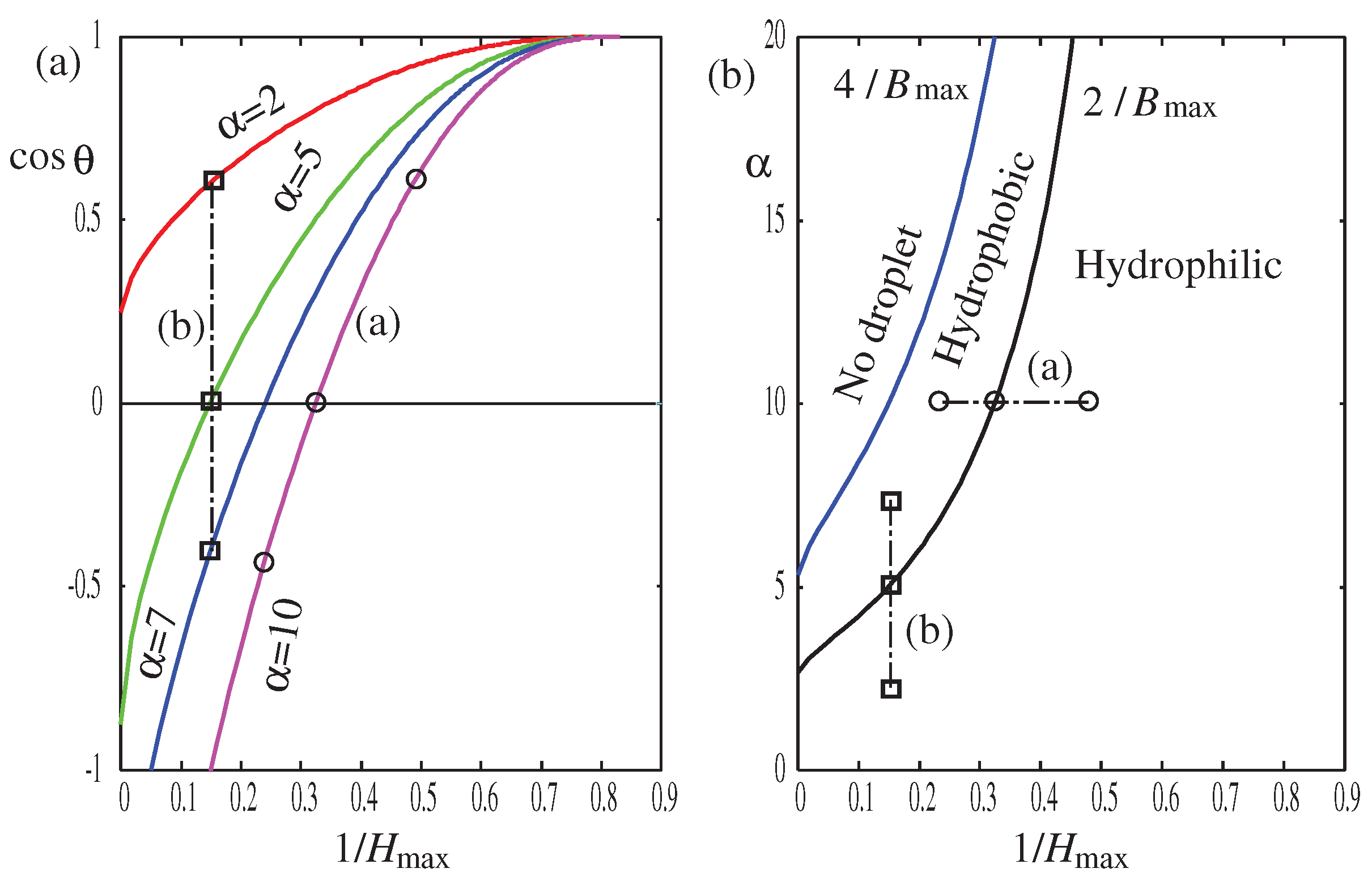
Figure 9a, we plot the cosine of the contact angle
versus the inverse of the droplet’s height
. In
Figure 9b, we redraw
Figure 6 using
instead of
, which are related through Eq. (
42) and
Figure 5.
Figure 9b shows that smaller droplets tend to be more hydrophilic.
Our calculations in
Figure 8 and
Figure 9 suggest that as the droplet size decreases (
), the droplet becomes more hydrophilic and the contact angle is smaller, indicating negative line tension
for three-dimensional droplets. Interestingly, most of the old experimental data for macroscopic droplets of cm and mm sizes show an opposite trend: as the droplet size decreases, the contact angle increases [
36,
37], meaning positive line tension
. However, experimental results for nanoscale droplets using atomic-force microscopy (AFM) [
38,
39,
40,
41] and scanning electron microscopy (SEM) [
42] are mostly consistent with our findings: the smaller the droplet, the lower the contact angle, indicating negative line tension. Furthermore, our highly nonlinear convex curve in
Figure 9a resembles some of the previous experimental results [
39,
40]. We can estimate the effective line tension by identifying
in Eq. (
64). From
Figure 9a, we can estimate the slope of the curve
as
, which can be translated into the effective line tension using
nm [
8] and
mN/m (water) [
2] as
N, which is negative and the right order of magnitude of experimental observation. The absolute magnitude
decreases as the droplet becomes smaller (
).
The size dependence of the contact angle of nanoscale droplets has also been studied by molecular dynamics simulation [
24,
25,
26] and density functional theory [
27]. Furthermore, the determination the sign and magnitude of line tension has also been attempted by molecular dynamics [
43,
44,
45,
46]. Some of them [
26,
27] indicate positive line tension (
), while some [
24,
25,
43] are consistent with our results in
Figure 9a:
or the contact angle decreases as the size of the droplet shrinks. Some [
44,
45,
46] also indicate that the sign depends on the wettability (Hydrophilic/hydrophobic) of the substrate.
Of course, our numerical results based on the one-parameter disjoining pressure model cannot resolve these contradictory results. However, our results, along with some of the previous results for cylindrical droplets [
43,
44,
45], suggest the limited utility of the line tension concept and the modified Young’s equation (
54) at the nanoscale. Although line tension includes the combined effect of various size-dependence factors such as the surface tension by Tolman’s length [
44,
47,
48], the main origin is the localized surface potential
or the disjoining pressure
acting only near the contact line [
11,
35,
38,
47,
48]. For droplets at the nanoscale, where the entire volume is under the influence of surface potential, such a line tension concept may not hold true. Instead, the volume force acting on the total volume of the droplet is responsible for the size dependence of the contact angle. This same conclusion was recently reached by Tan et al. [
49], who showed that the contact angle of mm to cm-sized droplets can be largely explained by the gravitational body force and that of nanoscale droplets can also be explained by a Lennard-Jones type inter-molecular body force between liquid molecules and the substrate. The limitation of the line tension concept for nanoscale droplet was also argued by Stocco and Möhwald [
50], who used disjoining pressure instead of intermolecular body force and studied the surface morphology of nanoscale droplets.