1. Introduction
Sudden unexplained cardiac death in the young represents a devastating, up to
40% unexplained and poorly understood phenomenon that continues to challenge clinicians and researchers worldwide. Although its overall incidence is relatively low compared to adult sudden cardiac death, the societal and emotional impact of losing apparently healthy adolescents and young adults to unexpected fatal arrhythmias is profound. Despite advances in cardiac imaging, electrophysiological testing, and molecular genetics, many cases
remain unexplained even after autopsy and extensive diagnostic workup. This diagnostic gap highlights the presence of subtle, preclinical molecular alterations that escape conventional screening modalities [
16,
17].
Growing evidence implicates oxidative stress and redox imbalance as central contributors to cardiac electrical instability. Reactive oxygen and nitrogen species influence multiple targets involved in cardiac excitability, including ion channels, calcium-handling proteins, and mitochondrial pathways. Under physiological conditions, the heart maintains a delicate balance between pro-oxidant and antioxidant mechanisms. Disruption of this equilibrium can induce electrophysiological heterogeneity, prolong repolarization, and trigger arrhythmogenic afterdepolarizations, even in the absence of an overt structural pathology. Detecting such early redox alterations could provide a molecular tool for early risk prediction in individuals who are appearing healthy [
1,
3,
13].
Given its accessibility, saliva has recently emerged as a promising diagnostic fluid for non-invasive biomarker discovery [
4,
6,
8]. Salivary components reflect systemic biochemical status through capillary filtration and glandular secretion, offering a practical and ethically acceptable method for repeated sampling. This review explores the scientific and mechanistic basis for using salivary redox biomarkers as potential early indicators of SUCD risk and outlines the pathway toward clinical validation and translational implementation.
2. Aim of the Literature Review
The aim of this literature review is to explore and critically synthesize current scientific evidence linking oxidative stress and redox imbalance to the pathophysiology of sudden unexplained cardiac death and sudden cardiac death (SCD) in young healthy individuals and athletes. This work is designed as a provisional and mechanistic study to support important future clinical research on the proposed concept of salivary redox biomarkers (“Salivary Redoxome”) as a non-invasive screening tool for early cardiac risk detection.
The specific objectives of this review are to:
- -
Clarify the molecular and electrophysiological mechanisms through which oxidative stress contributes to cardiac electrical instability and arrhythmogenesis;
- -
Assess the current state of knowledge regarding oxidative and antioxidative biomarkers—particularly those measurable in saliva, such as SOD, CAT, GPx, MDA, and total antioxidant capacity (TAC);
- -
Identify the limitations, inconsistencies, and research gaps in existing studies that hinder the translation of salivary redox markers into predictive clinical tools;
- -
Describe the steps for future clinical validation studies, depending on future funding, to assess whether the Salivary redoxome can help diagnose and predict risk in young people at risk.
Ultimately, this literature review aims to provide a scientific and conceptual basis for developing a novel, non-invasive, and cost-effective biomarker strategy capable of improving early detection and prevention of SUCD in young individuals and athletes.
Research Question
Can we rely on oxidative stress biomarkers to become the first molecular screening tool for early detection of Sudden Unexplained Cardiac Death risk?
3. Methodology
This work was conducted as a narrative and mechanistic literature review to identify and synthesize current evidence linking oxidative stress and redox imbalance with sudden unexplained cardiac death and sudden cardiac death in young healthy individuals and athletes. A structured literature search was performed in PubMed, Scopus, Web of Science, and Google Scholar for articles published between 2000 and 2025, using combinations of the keywords oxidative stress, redox imbalance, cardiac ion channels, arrhythmia, sudden cardiac death, salivary biomarkers, superoxide dismutase, catalase, glutathione peroxidase, malondialdehyde, and total antioxidant capacity. Priority was given to original research papers, systematic reviews, and meta-analyses addressing oxidative mechanisms in cardiac electrophysiology and salivary redox biomarkers. Studies outside the oxidative or cardiac context were excluded. Extracted data were analyzed qualitatively to identify molecular mechanisms, methodological limitations, and research gaps that could inform future clinical validation studies. As the work relies exclusively on published data, ethical approval was not required.
4. Scientific Background and Clinical Perspectives
4.1. Epidemiology
In the European Union, SCD approximately a quarter of a million deaths are reported annually and 4 million worldwide, which is representing a major public health problem [
18].
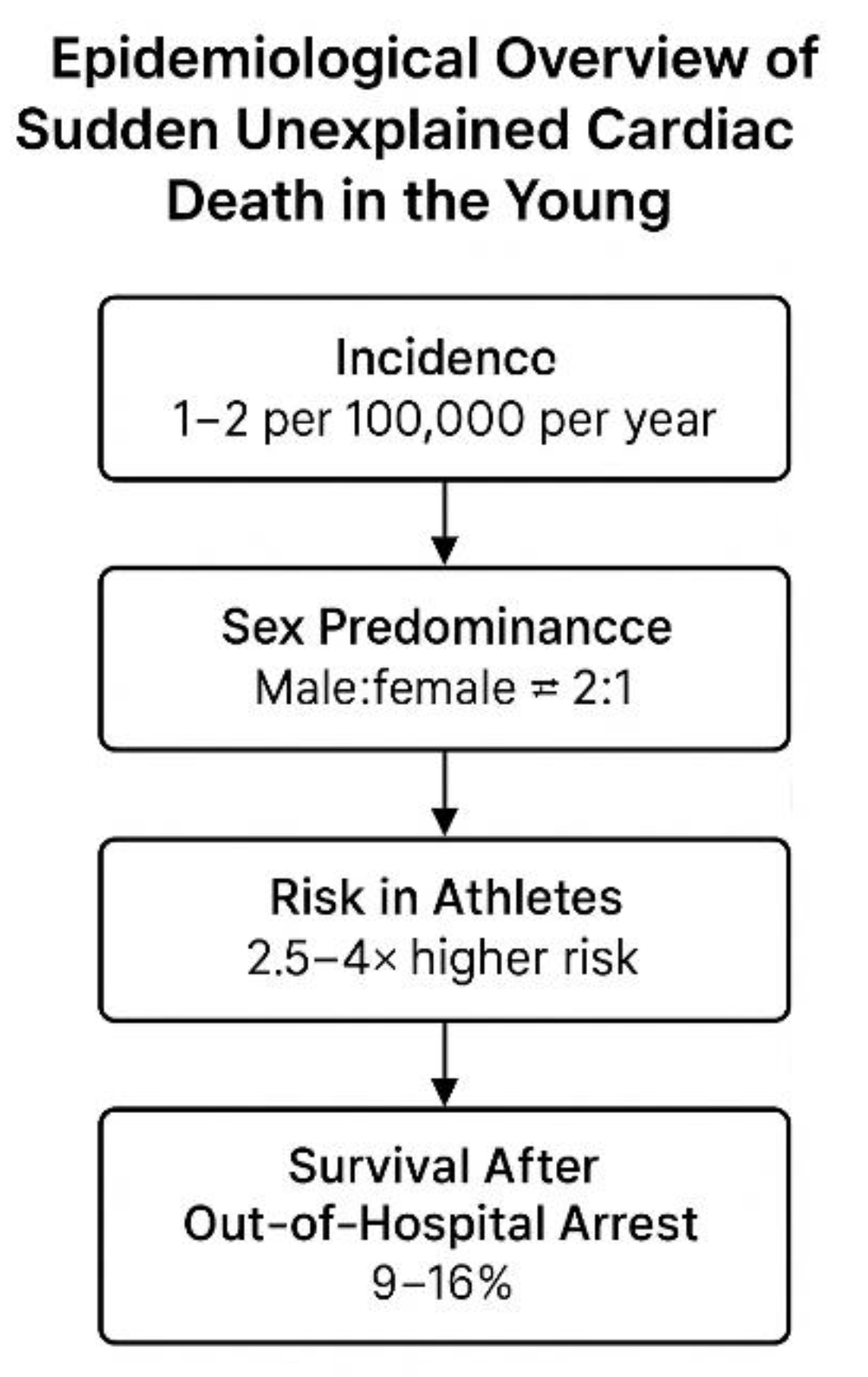
Figure 1.
Epidemiological overview of sudden unexplained cardiac death (SUCD) in young individuals. The figure summarizes key epidemiological indicators: an estimated incidence of 1–2 cases per 100,000 persons per year, a male-to-female predominance of approximately 2:1, a 2.5–4-fold higher risk among athletes compared with non-athletes, and a 9–16 % survival rate following out-of-hospital cardiac arrest. Figure created by the author.
Figure 1.
Epidemiological overview of sudden unexplained cardiac death (SUCD) in young individuals. The figure summarizes key epidemiological indicators: an estimated incidence of 1–2 cases per 100,000 persons per year, a male-to-female predominance of approximately 2:1, a 2.5–4-fold higher risk among athletes compared with non-athletes, and a 9–16 % survival rate following out-of-hospital cardiac arrest. Figure created by the author.
Sudden
unexplained cardiac death in young, apparently healthy individuals remains a rare yet deeply impactful phenomenon within global cardiovascular mortality. Contemporary data indicate that the incidence of sudden cardiac death among individuals under 35–40 years of age in “Western/European populations” ranges between 1 and 2 cases per 100,000 persons per year [
16,
17]
, with considerable regional and demographic variability. Although recent advances in imaging, genetics, and emergency care have improved the diagnosis and survival of some
explained cases of SCD in Europe, these tools have had almost no impact on sudden
unexplained cardiac death. Young individuals who experience SUCD often show completely normal findings before the event, and the cause remains
unknown even after a full investigation. Recent North American trends demonstrate a gradual increase in SCD incidence among younger adults, particularly those aged 25–44 years. This rise has been attributed to multifactorial influences, including lifestyle factors, metabolic stress, subclinical cardiomyopathies, and environmental contributors such as air pollution and psychosocial stress [
9,
16].
Despite improved autopsy techniques and the incorporation of molecular autopsy into forensic protocols, a substantial proportion—estimated at 30% to 40%—of sudden deaths in the young remain unexplained, lacking identifiable structural, toxicological, or genetic causes [
17,
19].
This unexplained subset represents the critical target population for novel biomarker-based screening strategies.
Epidemiological observations consistently reveal a male predominance, with male-to-female ratios approximating 2:1 across most cohorts, likely reflecting sex-related differences in myocardial repolarization, hormonal modulation of ion channels, and exposure to physical stressors. Geographic and ethnic disparities are also evident, with higher incidence rates observed in specific minority and low-resource populations.
In athletes and individuals engaged in high-intensity physical activity, the risk of sudden cardiac arrest is elevated—approximately 2.5- to 4-fold greater than in age-matched non-athletic counterparts [
16]—underscoring the limitations of conventional pre-participation screening. Survival after out-of-hospital cardiac arrest in young adults remains disappointingly low, typically ranging from 9% to 16% worldwide. Collectively, these epidemiological patterns highlight the persistent
diagnostic blind spot preceding SUCD and reinforce the necessity for early, molecular-level screening approaches capable of detecting preclinical electrical and redox instability.
4.2. Mechanisms of Sudden Cardiac Death
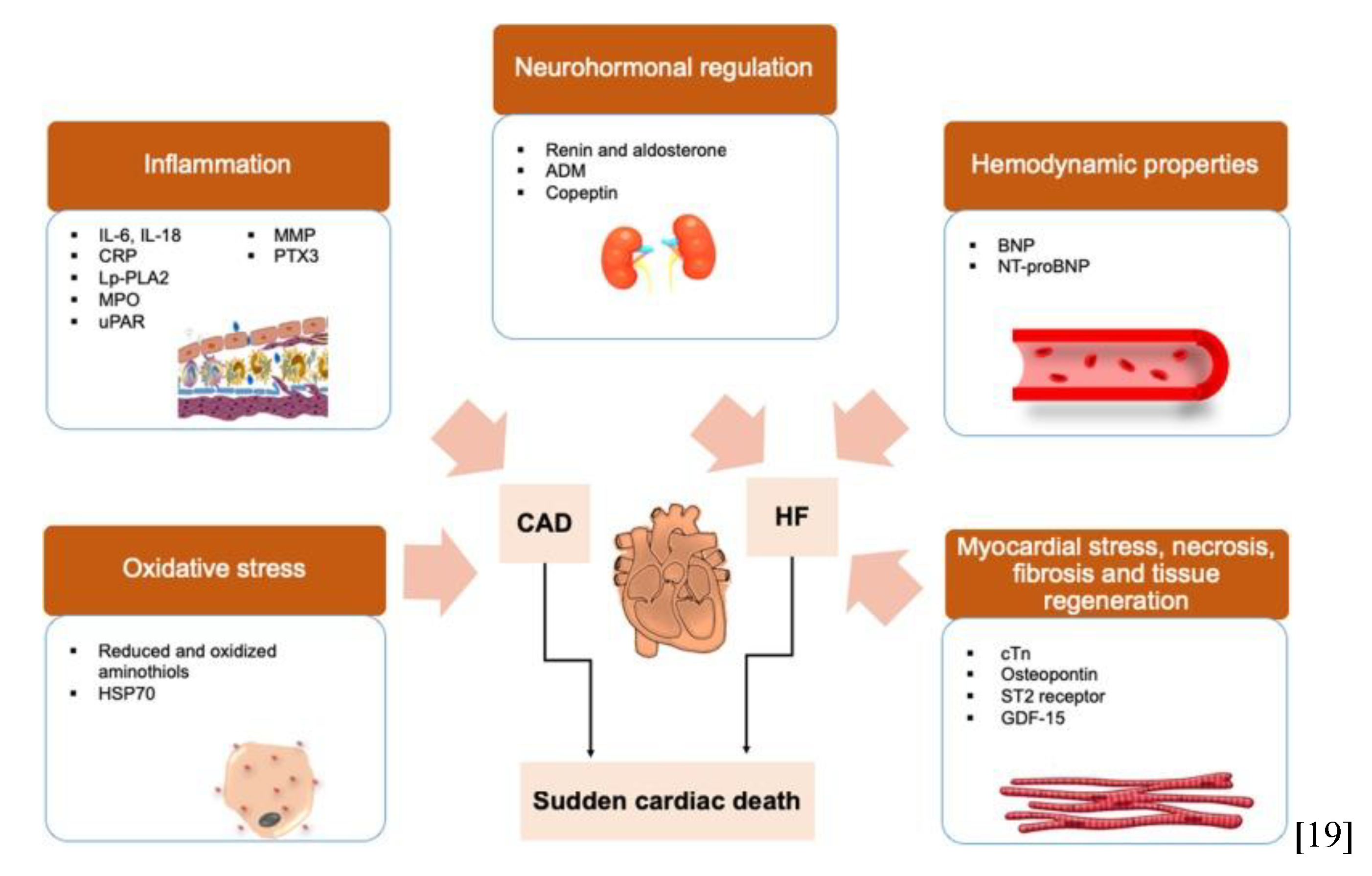
Figure 2.
Biomarker pathways involved in sudden cardiac death. Protein biomarkers for assessing risks of SCD. Surrogate biomarkers that reflect the development of oxidative stress and inflammation are associated with coronary artery diseases (CAD). While biomarkers that reflect the neurohormonal regulation process, hemodynamic properties and myocardial stress are often associated with heart failure (HF). Adapted from Osman J. et al., 2019.
Figure 2.
Biomarker pathways involved in sudden cardiac death. Protein biomarkers for assessing risks of SCD. Surrogate biomarkers that reflect the development of oxidative stress and inflammation are associated with coronary artery diseases (CAD). While biomarkers that reflect the neurohormonal regulation process, hemodynamic properties and myocardial stress are often associated with heart failure (HF). Adapted from Osman J. et al., 2019.
SCD results from the abrupt loss of effective cardiac output, most often due to malignant ventricular arrhythmias such as ventricular tachycardia or ventricular fibrillation. These arrhythmias arise from disturbances in myocardial excitability, conduction, and repolarization that promote re-entry circuits, triggered activity, or abnormal automaticity [
1,
3].
In younger individuals and athletes, SCD typically occurs in the absence of coronary artery disease and is associated with inherited or acquired electrical and structural disorders, including ion-channelopathies—such as long QT syndrome, Brugada syndrome, and catecholaminergic polymorphic ventricular tachycardia—and cardiomyopathies, particularly hypertrophic and arrhythmogenic forms [
3,
13]. Despite these recognized entities, a considerable proportion of cases remain unexplained at autopsy or molecular analysis, classified as SUCD [
19].
At the cellular level, oxidative stress and redox imbalance have emerged as key contributors to arrhythmogenic remodeling. Reactive oxygen species (ROS) modify ion-channel function, disrupt calcium handling, and impair mitochondrial energy metabolism, leading to electrical heterogeneity and instability even in structurally normal hearts [
1,
2,
15]. Oxidative activation of Ca²⁺/calmodulin-dependent protein kinase II (CaMKII) further amplifies calcium leakage and afterdepolarizations, establishing a substrate conducive to
lethal ventricular arrhythmias.
SCD therefore represents the final common pathway of diverse insults that converge on oxidative and electrophysiological dysregulation of the myocardium, underscoring the rationale for investigating redox biomarkers as early indicators of electrical vulnerability [
2,
14,
19].
Figure 3.
A simple mechanistic framework linking oxidative stress, redox imbalance, and cardiac electrical instability leading to sudden unexplained cardiac death (SUCD). The diagram illustrates the conceptual sequence from oxidative stress to SUCD risk. Figure created by the author.
Figure 3.
A simple mechanistic framework linking oxidative stress, redox imbalance, and cardiac electrical instability leading to sudden unexplained cardiac death (SUCD). The diagram illustrates the conceptual sequence from oxidative stress to SUCD risk. Figure created by the author.
4.3. Oxidative Stress and Cardiac Electrophysiology
Oxidative stress represents an imbalance between the production of ROS and the capacity of antioxidant defenses to neutralize them. In cardiac tissue, ROS generation arises from mitochondrial respiration, NADPH oxidases, xanthine oxidase, and uncoupled nitric oxide synthase [
1,
2]. Excessive ROS modifies key electrophysiological components, including sodium (Na⁺), calcium (Ca²⁺), and potassium (K⁺) channels, thereby disturbing action potential formation and propagation [
3,
13]. Oxidative modifications such as S-nitrosylation and S-glutathionylation alter ion channel gating, decrease sodium current amplitude, and prolong repolarization, increasing susceptibility to arrhythmia [
1,
3].
4.4. Molecular Pathways Linking Redox Imbalance to Arrhythmogenesis
Beyond direct channel modulation, oxidative stress affects calcium handling by altering the function of ryanodine receptors, sarcoplasmic reticulum Ca²⁺-ATPase, and CaMKII [
1,
3,
5]. Oxidized CaMKII triggers abnormal calcium leakage and afterdepolarizations that can initiate lethal arrhythmias [
1,
13]. Mitochondrial dysfunction further amplifies ROS production, disrupting ATP generation and promoting electrical instability [
2,
3]. Together, these mechanisms form a self-sustaining cycle of redox dysregulation, energetic failure, and arrhythmogenic remodeling [
1,
5].
4.5. Salivary Redox Biomarkers: Composition and Systemic Reflection
Saliva contains numerous oxidative stress markers that mirror systemic redox status. Antioxidant enzymes such as SOD, CAT, and GPx constitute the enzymatic defense line against ROS. SOD catalyzes the conversion of superoxide anion into hydrogen peroxide, which is then decomposed by CAT and GPx. Non-enzymatic antioxidants, including uric acid, glutathione, and ascorbic acid, contribute to TAC. MDA, a lipid peroxidation product, serves as an indicator of oxidative damage [
2,
4,
10,
12]. Evidence suggests that these salivary parameters correlate with plasma or serum equivalents, supporting saliva as a valid diagnostic matrix [
6,
12,
15].
4.6. Analytical and Clinical Validity of Salivary Oxidative Markers
Quantifying salivary redox biomarkers requires standardized collection and storage procedures to avoid pre-analytical variability. Both spectrophotometric and ELISA-based assays have been used for measuring SOD, CAT, GPx, MDA, and TAC, demonstrating reasonable reproducibility [
4,
11,
12]. Clinically, altered salivary oxidative profiles have been reported in cardiovascular, metabolic, and neurodegenerative diseases, indicating their broader systemic relevance [
2,
6,
7,
14]. Translating these observations into cardiac risk screening necessitates validation in young healthy cohorts, correlation with electrophysiological indices such as QT dispersion or heart rate variability, and longitudinal follow-up to link biomarker shifts with clinical outcomes [
19].
4.7. Pre-Analytical and Physiological Confounders
Several factors influence salivary oxidative parameters, including circadian rhythm, diet, exercise, oral hygiene, and inflammatory conditions of the oral cavity. Controlling these variables is critical for biomarker reliability. Standardized sampling protocols—such as morning fasting collection, avoidance of physical exertion and smoking before sampling, and exclusion of participants with periodontal disease—are recommended for reproducibility and comparability across studies [
4,
11,
12].
4.8. Emerging Technologies and Biosensor Innovations
Advances in microfluidics, nanomaterials, and electrochemical sensing have accelerated the development of portable salivary biosensors capable of real-time redox measurement. Integration of these devices with smartphone-based data acquisition could enable decentralized screening of at-risk populations [
8,
11]. The envisioned “Salivary Redoxome” platform would combine multiplex detection of enzymatic and non-enzymatic markers, applying machine-learning algorithms to classify redox risk profiles and predict susceptibility to SUCD [
8,
14]. Such innovations align with the European Union’s strategic goals for preventive, personalized, and digital health technologies.
5. Discussion
Integrating the reviewed evidence, it becomes clear that oxidative stress serves as a mechanistic bridge between molecular changes and electrical vulnerability in SUCD. While serum-based oxidative biomarkers have been extensively studied, saliva offers a more accessible medium for community-level screening, particularly in young individuals without overt disease.
The correlation between salivary and systemic antioxidant status indicates that salivary biomarkers could serve as early reporters of subclinical redox imbalance preceding electrical instability. For translational application, a structured validation pathway is essential. Initial pilot studies should establish normative salivary redox reference ranges in healthy young populations. Subsequent cross-sectional studies can compare individuals with benign ECG abnormalities or genetic variants predisposing to arrhythmia, while longitudinal designs can explore predictive value for future cardiac events. A saliva-based redox test costing only €3–€5 could help prevent sudden cardiac events that typically associated with medical and societal costs exceeding €100,000, making this approach a highly cost-efficient public health strategy. Also, saliva sampling poses minimal to no harm, making it an ethically robust approach suitable for young individuals, athletes, and community-wide screening. Such a screening tool could help prevent unexpected cardiac deaths in high-risk community settings, including schools, universities, athletic programs, sport clubs, and military medical programs. Medical staff, particularly young residents working long hours under high stress, also represent an overlooked group in whom unexpected sudden cardiac events have been increasingly reported. Collaboration between clinical and laboratory teams will facilitate integration of salivary redox profiling into existing screening programs.
Importantly, Because SUCD incidence varies across Europe, research funding should prioritize multicenter European initiatives to harmonize biomarker protocols, build biobanks of salivary samples linked with ECG and genetic data, and test cost-effectiveness across diverse populations. Collaboration among centers in Denmark, the Netherlands, Italy, the UK, Romania, and Greece would ensure reliable EU-wide applicability, which fits well with current European efforts to improve early detection and preventive strategies in cardiovascular health.
6. Conclusion
Salivary redox biomarkers represent promising non-invasive indicators of early cardiac electrical vulnerability in apparently healthy young individuals. By reflecting systemic oxidative balance, these biomarkers could cover a huge research and diagnostic gap left by conventional screening tools. The integration of salivary redox profiling with clinical, electrocardiographic, genetic, and lifestyle data represents an innovative direction in cardiology and in preventive medicine. Further collaborative, interdisciplinary research and advances in biomarkers technology could transform the concept of the “Salivary Redoxome” from a theoretical framework into an easy and practical screening tool, for the prevention of sudden unexplained cardiac death in the young.
7. Statement of Novelty
Based on the current available scientific knowledge, no published studies have evaluated the use of salivary redox biomarkers as non-invasive screening approach for assessing the risk of sudden unexplained cardiac death (SUCD) or sudden cardiac death (SCD) in young healthy individuals. Existing research on salivary oxidative stress has primarily focused on metabolic, neurodegenerative, and oral diseases, with limited exploration in cardiovascular contexts such as myocardial infarction and heart failure.
This study presents, for the first time, the concept of a combined biomarkers profile, “Salivary Redoxome”, which includes antioxidant enzymes and markers of oxidative damage as a potential early indicator of cardiac electrical vulnerability.
Thus, this literature-based mechanistic framework establishes a novel translational direction for future clinical validation studies, positioning saliva as a practical, non-invasive biospecimen for early cardiac risk detection and precision prevention.
8. Future Perspectives
Future research should focus on translating this concept into well-designed clinical studies to assess and to validate the diagnostic and predictive value of salivary redox biomarkers in young healthy populations. The next phase should aim to establish reference ranges and analytical standardization for key oxidative and antioxidative markers, followed by correlation with electrophysiological, genetic, and lifestyle risk factors associated with cardiac electrical instability. Integration of these findings with emerging biosensor and digital health technologies could enable the development of a portable, real-time saliva-based screening platform suitable for large-scale population use.
To Move this project forward will require collaboration between several research centers, supported by European and international funding programs. Such coordinated efforts are important for evaluating the ‘Salivary Redoxome’ concept across diverse populations and for determining how it may complement OMICS-based biomarker discovery, and how it could be applied in cardiology and in preventive medicine. Also, this work aligns directly with the EU Mission on Cardiovascular Disease (2021–2030), which prioritizes early detection, prevention, and innovative screening tools for reducing sudden cardiac events across Europe and worldwide.
This work also aligns with the WHO global strategy to reduce premature cardiovascular mortality through early detection and low-cost preventive tools.
Author Contributions
The conceptual framework of the “Salivary Redoxome” and its proposed application as a non-invasive screening approach for early detection of sudden unexplained cardiac death (SUCD) in young healthy individuals were originally conceived and registered by
Ahmed Adel Mansour Kamar. This concept was publicly disclosed on the Zenodo platform to establish authorship and to invite scientific collaboration for validation and translational development. Zenodo DOI:
https://doi.org/10.5281/zenodo.17450451
Abbreviations
The following abbreviations are used in this manuscript:
| ATP |
Adenosine Triphosphate |
| CAD |
Coronary Artery Disease |
CAT
CaMKII
Ca²⁺
ECG
ELISA
GPx / GPX
HF
K⁺
MDA
Na⁺
NADPH
OMICS
RNS
ROS
SCD
SOD
SUCD
TAC
VF
VT |
Catalase
Calcium/Calmodulin-Dependent Protein Kinase II
Calcium ion
Electrocardiogram / Electrocardiography
Enzyme-Linked Immunosorbent Assay
Glutathione Peroxidase
Heart Failure
Potassium ion
Malondialdehyde
Sodium ion
Nicotinamide Adenine Dinucleotide Phosphate
Comprehensive molecular profiling methods
Reactive Nitrogen Species
Reactive Oxygen Species
Sudden Cardiac Death
Superoxide Dismutase
Sudden Unexplained Cardiac Death
Total Antioxidant Capacity
Ventricular Fibrillation
Ventricular Tachycardia |
References
- Pfenniger A, et al. Oxidative stress and atrial fibrillation. J Mol Cell Cardiol. 2024;195:107–118.
[CrossRef]
- Valaitienė J, et al. Oxidative stress and its biomarkers in cardiovascular diseases. Artery Res. 2024;36:62–68.
[CrossRef]
- Lei M, et al. Cardiac arrhythmogenesis: roles of ion channels and their interaction with reactive oxygen species. Front Physiol. 2024;15:1342761. [CrossRef]
- Surdu A, Foia LG, Luchian I, et al. Saliva as a diagnostic tool for systemic diseases—a narrative review. Medicina (Kaunas). 2025;61(2):243. [CrossRef]
- Attia A, et al. Relevance of targeting oxidative stress, inflammatory, and fibrosis pathways in post-operative atrial fibrillation. Antioxidants (Basel). 2025;14(4):414. [CrossRef]
- Soltanian M, et al. Evaluation of salivary total antioxidant capacity and total oxidant status in autoimmune diseases. J Oral Biol Craniofac Res. 2025;15(1):45–52. [CrossRef]
- Karasu YÖ, Maden O, Çanakçı ÇF. Oxidative damage biomarkers and antioxidant enzymes in saliva of patients with peri-implant diseases. Int J Implant Dent. 2024;10:43. [CrossRef]
- El Bahbah AI, Negm WA, El-Gendy A, Kamel W, Hamed M. Salivary biomarkers in cardiovascular diseases: an insight into their diagnostic and prognostic potential. Diagnostics (Basel). 2021;11(1):27. [CrossRef]
- Jinarat D, et al. Particulate matter and cardiac arrhythmias: from clinical evidence to ion-channel mechanisms. Environ Res. 2025;250:118949. [CrossRef]
- Klimiuk A, Maciejczyk M, Zalewska A. The relationship between exercise and salivary oxidative stress: a review. Antioxidants (Basel). 2020;11(8):1489. [CrossRef]
- Dongiovanni P, Gaggini M, Carli F, Rametta R, Magni S, Fracanzani AL, et al. Salivary biomarkers: novel non-invasive tools to diagnose and monitor systemic diseases. Cell Mol Immunol. 2023;20:467–480. [CrossRef]
- Wang J, Schipper HM, Velly AM, Mohit S, Gornitsky M. Salivary biomarkers of oxidative stress: a critical review. Free Radic Biol Med. 2015;85:95–104. [CrossRef]
- Orfali R, Alwatban AZ, Orfali RS, et al. Oxidative stress and ion channels in neurodegenerative diseases: emerging parallels with cardiac electrophysiology. Front Physiol. 2024;15:1320086. [CrossRef]
- Song X, et al. Recent advances in biomarkers for cardiovascular disease. Biomark Med. 2025;19(3):225–240. [CrossRef]
- Chojnowska S, Baran T, Wilczyńska K, Sienicka M, Cabaj-Wiater I, Knaś M. Evaluation of salivary and serum antioxidant and oxidative stress parameters in healthy and diseased subjects. Front Physiol. 2017;8:189. [CrossRef]
- Tseng ZH, et al. Trends in sudden cardiac death among adults aged 25 to 44 years in the United States, 1999–2020. JAMA Netw Open. 2025;8(1):e2435123. [CrossRef]
- Winkel BG, et al. Nationwide decline in sudden cardiac death incidence among the young: twenty-year follow-up of the Danish Sudden Cardiac Death Registry. Circulation. 2024;149(12):1012-1023. [CrossRef]
- Radu I, Farcas AO, Nyulas V, Radu CC, Brinzaniuc K. Sudden cardiac death—etiology, risk factors and demographic characteristics: an extensive study of 1618 forensic autopsies. Diseases. 2024;12(8):168. [CrossRef]
- Osman J, Tan SC, Lee PY, Low TY, Jamal R. Sudden Cardiac Death (SCD) – risk stratification and prediction with molecular biomarkers. J Biomed Sci. 2019;26(1):39. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).