Submitted:
21 November 2025
Posted:
25 November 2025
You are already at the latest version
Abstract
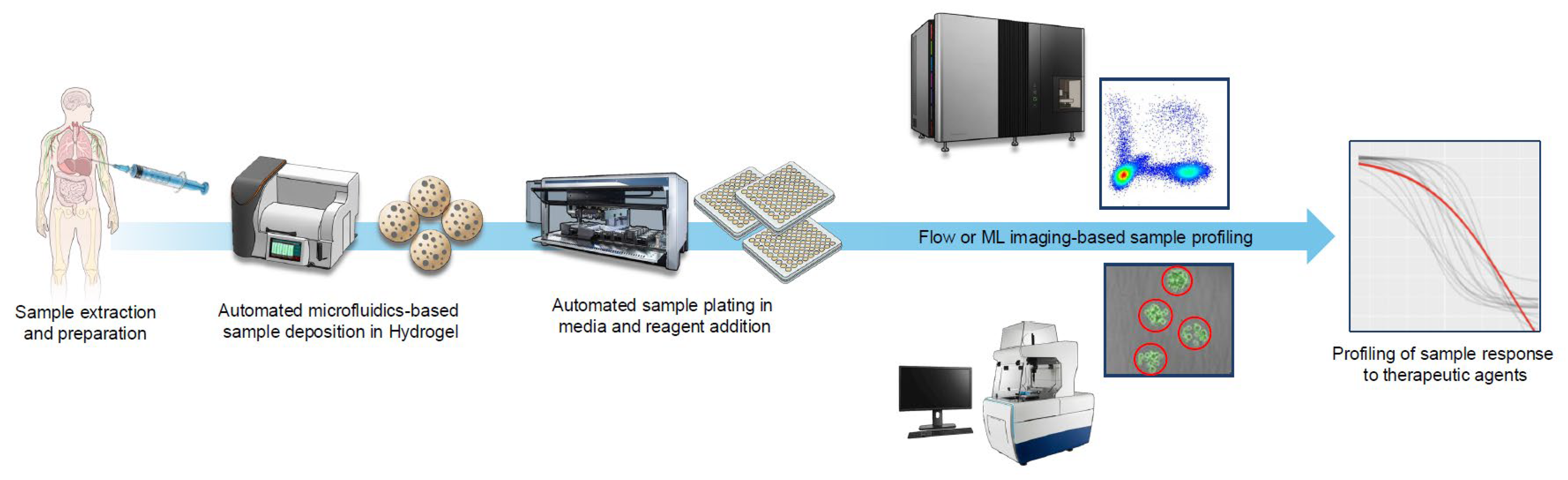
Breast cancer–associated malignant pleural effusion (MPE) is a common and debilitating manifestation of advanced disease, yet current management is largely limited to indwelling pleural catheters and chemical pleurodesis and offers only transient palliation without addressing the underlying tumor biology. We propose that integrating patient-derived organoid modeling of pleural tumor cells with characterization via technologies like next-generation sequencing could shift MPE care from symptom management toward precision intervention. Organoid-based drug testing enables ex vivo evaluation of local therapeutic agents, including intrapleural chemotherapy, immune modulators, and bispecific antibodies, while paired genomic profiling may reveal actionable resistance pathways unique to pleural metastases. Together, these approaches could identify rational, localized combination therapies that improve local control, reduce effusion recurrence, and ultimately extend survival. By coupling functional and molecular analyses directly to the pleural compartment, we envision a translational framework that redefines breast MPE from a purely palliative condition to one amenable to mechanism-driven, patient-tailored therapy.
Keywords:
1. Introduction
1.1. Standard-of-Care
1.1. Alternatives to Standard-of-Care
1.2. New Models and Approaches
1.3. Discussion
Author Contributions
Acknowledgments
Conflict of Interest
References
- Filho AM, Laversanne M, Ferlay J, Colombet M, Piñeros M, Znaor A, et al. The GLOBOCAN 2022 cancer estimates: Data sources, methods, and a snapshot of the cancer burden worldwide. Int J Cancer. 2025 Apr 1;156(7):1336–46. [CrossRef]
- Winters S, Martin C, Murphy D, Shokar NK. Breast cancer epidemiology, prevention, and screening. Prog Mol Biol Transl Sci. 2017 Oct 10;151:1–32.
- Vrancken Peeters NJMC, Kerklaan R, Vlooswijk C, Bijlsma RM, Kaal SEJ, Tromp JM, et al. Long-term health-related quality of life among adolescent and young adult breast cancer survivors. Qual Life Res. 2025 May;34(5):1483–500. [CrossRef]
- Cohn JG, Locke SC, Herring KW, Dent SF, LeBlanc TW. Palliative care use and end-of-life care quality in HR+/HER2- metastatic breast cancer. Breast Cancer Res Treat [Internet]. 2025 Aug 16; Available from: . [CrossRef]
- Han Y-M, Yan-Dong, Wang H-L, Li X-M, Zhang X-, Wei X-Y, et al. Prognostic significance of malignant pleural effusions in patients with advanced luminal B breast cancer. BMC Womens Health. 2024 Oct 14;24(1):562.
- Soni A, Ren Z, Hameed O, Chanda D, Morgan CJ, Siegal GP, et al. Breast cancer subtypes predispose the site of distant metastases. Am J Clin Pathol. 2015 Apr;143(4):471–8.
- Piggott LM, Hayes C, Greene J, Fitzgerald D. Malignant pleural disease. Breathe (Sheff) [Internet]. 2023 Dec 1;19. [CrossRef]
- Zamboni MM, da Silva CT Jr, Baretta R, Cunha ET, Cardoso GP. Important prognostic factors for survival in patients with malignant pleural effusion. BMC Pulm Med. 2015 Mar 28;15(1):29.
- Fentiman IS, Millis R, Sexton S, Hayward JL. Pleural effusion in breast cancer: a review of 105 cases. Cancer. 1981 Apr 15;47(8):2087–92.
- Wang Y, Zhou T, Zhao S, Li N, Sun S, Li M. A novel clinical prognostic model for breast cancer patients with malignant pleural effusion: Avoiding chemotherapy in low-risk groups? Cancer Manag Res. 2023 May 11;15:409–22. [CrossRef]
- Munavvar M, Bodtger U, Carus A, Cordovilla R, Naik S, Salud A, et al. Current trends in treating malignant pleural effusion: Evidence, guidelines, and best practice recommendations. JCO Oncol Pract. 2025 June;21(6):759–65. [CrossRef]
- Zaza C, Baine N. Cancer pain and psychosocial factors: a critical review of the literature. J Pain Symptom Manage. 2002 Nov;24(5):526–42.
- Guo Y-Q, Ju Q-M, You M, Liu Y, Yusuf A, Soon LK. Depression, anxiety and stress among metastatic breast cancer patients on chemotherapy in China. BMC Nurs. 2023 Feb 6;22(1):33. [CrossRef]
- Economidou F, Margaritopoulos G, Antoniou KM, Siafakas NM. The angiogenetic pathway in malignant pleural effusions: Pathogenetic and therapeutic implications. Exp Ther Med. 2010 Jan;1(1):3–7.
- Jovanovic D. Etiopathogenesis of malignant pleural effusion. AME Med J. 2021 Sept;6(0):28–28.
- Donnenberg VS, Luketich JD, Sultan I, Lister J, Bartlett DL, Ghosh S, et al. A maladaptive pleural environment suppresses preexisting anti-tumor activity of pleural infiltrating T cells. Front Immunol. 2023 Mar 30;14:1157697. [CrossRef]
- Sivabalah K, Balata H, Craig C, Alsaaty A, Conroy K, Ong WH, et al. The 2023 British Thoracic Society guideline for pleural disease update on malignant pleural effusion. JoR. 2024 Nov 26;4(4):210–22. [CrossRef]
- Shafiq M, Frick KD, Lee H, Yarmus L, Feller-Kopman DJ. Management of malignant pleural effusion. J Bronchology Interv Pulmonol. 2015 July;22(3):215–25.
- Siefen A-C, Eilers L, Baltin CT, Kron F. Cost comparison of treatment alternatives for pleural effusion and ascites from a payer perspective: Are there cost savings from indwelling catheters? J Palliat Med. 2023 Nov;26(11):1510–20. [CrossRef]
- Duong V, Hargreaves B, Muruganandan S. Management of malignant pleural effusion in 2024: A definitive and unified global approach. JCO Oncol Pract. 2025 June;21(6):739–41. [CrossRef]
- Peel AM, Mishra EK. The psychosocial impact of Indwelling Pleural Catheters: A scoping review. Cureus. 2023 July;15(7):e41689. [CrossRef]
- Iqbal B, Bedawi E, Rahman NM. Pro: indwelling pleural catheters cause harm to patients. Breathe (Sheff). 2024 Oct;20(3):240034. [CrossRef]
- Sidhu C, Wright G, Peddle-McIntyre CJ, Tan AL, Lee YCG. Management of malignant pleural effusion and trapped lung: a survey of respiratory physicians and thoracic surgeons in Australasia. Intern Med J. 2024 July;54(7):1119–25. [CrossRef]
- Mei F, Tamburrini M, Gonnelli F, Morandi L, Bonifazi M, Sediari M, et al. Management of malignant pleural effusion in Italian clinical practice: a nationwide survey. BMC Pulm Med. 2023 July 10;23(1):252. [CrossRef]
- Sarkar RR, Courtney PT, Bachand K, Sheridan PE, Riviere PJ, Guss ZD, et al. Quality of care at safety-net hospitals and the impact on pay-for-performance reimbursement. Cancer. 2020 Oct 15;126(20):4584–92.
- Thomas R, Francis R, Davies HE, Lee YCG. Interventional therapies for malignant pleural effusions: the present and the future: Interventions for MPE. Respirology. 2014 Aug;19(6):809–22.
- Gonnelli F, Hassan W, Bonifazi M, Pinelli V, Bedawi EO, Porcel JM, et al. Malignant pleural effusion: current understanding and therapeutic approach. Respir Res. 2024 Jan 19;25(1):47. [CrossRef]
- Orlandi R, Cara A, Cassina EM, Degiovanni S, Libretti L, Pirondini E, et al. Malignant pleural effusion: Diagnosis and treatment-up-to-date perspective. Curr Oncol. 2024 Nov 2;31(11):6867–78. [CrossRef]
- Boshuizen RC, Thomas R, Lee YCG. Advantages of indwelling pleural catheters for management of malignant pleural effusions. Curr Respir Care Rep. 2013 June;2(2):93–9. [CrossRef]
- Bhatnagar R, Kahan BC, Morley AJ, Keenan EK, Miller RF, Rahman NM, et al. The efficacy of indwelling pleural catheter placement versus placement plus talc sclerosant in patients with malignant pleural effusions managed exclusively as outpatients (IPC-PLUS): study protocol for a randomised controlled trial. Trials. 2015 Feb 12;16:48. [CrossRef]
- Semenova Y, Burkitbayev Z, Kalibekov N, Digay A, Zhaxybayev B, Shatkovskaya O, et al. The evolving role of chemotherapy in the management of pleural malignancies: Current evidence and future directions. Cancers (Basel) [Internet]. 2025 June 25. [CrossRef]
- Mitchell MA, Deschner E, Dhaliwal I, Robinson M, Li P, Kwok C, et al. Patient perspectives on the use of indwelling pleural catheters in malignant pleural effusions. Thorax. 2023 Nov;78(11):1111–7. [CrossRef]
- Wang S, Zhang R, Wan C, Qin J, Hu X, Shen Y, et al. Incidence of complications from indwelling pleural catheter for pleural effusion: A meta-analysis. Clin Transl Sci. 2023 Jan;16(1):104–17. [CrossRef]
- Zhang J, Liang J, Kadwani O, Agoramoorthy L, Montalvo S, Radcliffe G, et al. S138 Malignant pleural effusions: evaluating the psychosocial impact of indwelling pleural catheters on patients (MY-IPC) – an interim analysis. In: ‘Bridge over troubled waters’ – Managing the exudative effusion [Internet]. BMJ Publishing Group Ltd and British Thoracic Society; 2023. Available from: http://dx.doi.org/10.1136/thorax-2023-btsabstracts.144.
- Kathamuthu V, Balakrishnan R, Rajendran S, Rathinam P. The safety and efficacy of chemical pleurodesis agents in patients with malignant pleural effusion admitted in tertiary care hospital. Journal of Association of Pulmonologist of Tamil Nadu. 2025 Jan;8(1):17–22. [CrossRef]
- Kwok C, Thavorn K, Amjadi K, Aaron SD, Kendzerska T. Mortality after treatment of malignant pleural effusions with indwelling pleural catheters versus chemical pleurodesis: a population-based study. Respir Res. 2024 Nov 13;25(1):409. [CrossRef]
- Baiu I, Yevudza E, Shrager JB. Talc pleurodesis: A medical, medicolegal, and socioeconomic review. Ann Thorac Surg. 2020 Apr;109(4):1294–301. [CrossRef]
- Zhang W, Zhao Y-L, Li S-J, Zhao Y-N, Guo N-N, Liu B. Complications of thoracoscopic talc insufflation for the treatment of malignant pleural effusions: a meta-analysis. J Cardiothorac Surg. 2021 May 4;16(1):125. [CrossRef]
- Chinese Thoracic Society, Chinese Medical Association. Chinese expert consensus on treatment of malignant pleural effusion (2023 Edition). Zhonghua Jie He He Hu Xi Za Zhi. 2023 Dec 12;46(12):1189–203.
- Xu Y, Cui Y, Jiang L, Yu Y, Si W, Zhu X. Thoracic perfusion of antiangiogenic agents combined with chemotherapy for treating malignant pleural effusion in non-small cell lung cancer: a network meta-analysis. BMJ Open. 2024 Dec 20;14(12):e080703. [CrossRef]
- Wang C-Q, Huang X-R, He M, Zheng X-T, Jiang H, Chen Q, et al. Intrapleural administration with Rh-endostatin and chemical irritants in the control of malignant pleural effusion: A systematic review and meta-analysis. Front Oncol. 2021 Aug 3;11:649999. [CrossRef]
- Fan Y, Zou Q, Li X, Qi X, Dong J, Liu J, et al. Analysis of the Efficacy of Endostar Thoracic Perfusion and DDP Intravenous Chemotherapy for Malignant Pleural Effusion of Breast Cancer. Pract J Cancer. 2018;33(7):1175–7. [CrossRef]
- Biaoxue R, Xiguang C, Hua L, Wenlong G, Shuanying Y. Thoracic perfusion of recombinant human endostatin (Endostar) combined with chemotherapeutic agents versus chemotherapeutic agents alone for treating malignant pleural effusions: a systematic evaluation and meta-analysis. BMC Cancer. 2016 Nov 14;16(1):888.
- Hao Y, Gkasti A, Managh AJ, Dagher J, Sifis A, Tiron L, et al. Hyperthermic intrathoracic chemotherapy modulates the immune microenvironment of pleural mesothelioma and improves the impact of dual immune checkpoint inhibition. Cancer Immunol Res. 2025 Feb 3;13(2):185–99.
- Khosrawipour C, Nicpoń J, Kiełbowicz Z, Prządka P, Liszka B, Zielinski K, et al. First in vivo applicational data of foam-based intrathoracic chemotherapy (FBiTC) in a swine model. Pharmaceuticals (Basel) [Internet]. 2023 Dec 27;17(1). [CrossRef]
- Sebastian M, Kiewe P, Schuette W, Brust D, Peschel C, Schneller F, et al. Treatment of malignant pleural effusion with the trifunctional antibody catumaxomab (Removab) (anti-EpCAM x Anti-CD3): results of a phase 1/2 study. J Immunother. 2009 Feb;32(2):195–202.
- Ammouri L, Prommer EE. Palliative treatment of malignant ascites: profile of catumaxomab. Biologics. 2010 May 25;4:103–10. [CrossRef]
- Aggarwal C, Haas AR, Metzger S, Aguilar LK, Aguilar-Cordova E, Manzanera AG, et al. Phase I study of intrapleural gene-mediated cytotoxic immunotherapy in patients with malignant pleural effusion. Mol Ther. 2018 May 2;26(5):1198–205. [CrossRef]
- Park H, Lewis C, Dadgar N, Sherry C, Evans S, Ziobert S, et al. Intra-pleural and intra-peritoneal tocilizumab therapy for managing malignant pleural effusions and ascites: The Regional Immuno-Oncology Trial (RIOT)−2 study protocol. Surg Oncol Insight. 2024 June;1(2):100045. [CrossRef]
- Donnenberg AD, Luketich JD, Dhupar R, Donnenberg VS. Treatment of malignant pleural effusions: the case for localized immunotherapy. J Immunother Cancer. 2019 Apr 18;7(1):110. [CrossRef]
- Li X, Wu G, Chen C, Zhao Y, Zhu S, Song X, et al. Intrapleural injection of anti-PD1 antibody: A novel management of malignant pleural effusion. Front Immunol. 2021 Dec 13;12:760683. [CrossRef]
- Wang P, Zhang C, Hao P, Wang S, Zhu R, Li J, et al. The Observation of Clinical Efficacy and Safety of De-Platinum-Based Pleural Perfusion in the Treatment of Malignant Pleural Effusion and Its Correlation with the Expression of VEGF in Pleural Fluid. Journal of Cancer Therapy. 2024;15(12):432–45. [CrossRef]
- Kroesen BJ, Nieken J, Sleijfer DT, Molema G, de Vries EG, Groen HJ, et al. Approaches to lung cancer treatment using the CD3 x EGP-2-directed bispecific monoclonal antibody BIS-1. Cancer Immunol Immunother. 1997 Nov;45(3–4):203–6. [CrossRef]
- Cai J, Zhang F, Song Z, Jin J, Lv D, Pang W, et al. 1371P An anti-EpCAM x CD3 bispecific antibody, M701, for the treatment of malignant pleural effusion in NSCLC patients: Intermediate results of a prospective multicenter phase Ib trial. Ann Oncol. 2024 Sept;35:S862. [CrossRef]
- He D, Ding R, Wen Q, Chen L. Novel therapies for malignant pleural effusion: Anti-angiogenic therapy and immunotherapy (Review). Int J Oncol. 2021 Mar;58(3):359–70. [CrossRef]
- Murthy V, Katzman D, Sterman DH. Intrapleural immunotherapy: An update on emerging treatment strategies for pleural malignancy. Clin Respir J. 2019 May;13(5):272–9. [CrossRef]
- Wong T, Fuld AD, Feller-Kopman DJ. Malignant pleural effusions in the era of immunotherapy and antiangiogenic therapy. Semin Respir Crit Care Med. 2023 Aug;44(4):447–53. [CrossRef]
- Wang D-X, Zhu M, Guo D-H, Gu J, Xia L, Huang X-W, et al. Safety of Endostar in combination with chemotherapy in patients with cancer. Indian J Cancer. 2024 Oct 1;61(4):694–702. [CrossRef]
- Penz E, Watt KN, Hergott CA, Rahman NM, Psallidas I. Management of malignant pleural effusion: challenges and solutions. Cancer Manag Res. 2017 June 23;9:229–41. [CrossRef]
- Honkala A, Malhotra SV, Kummar S, Junttila MR. Harnessing the predictive power of preclinical models for oncology drug development. Nat Rev Drug Discov. 2022 Feb;21(2):99–114. [CrossRef]
- Mak IW, Evaniew N, Ghert M. Lost in translation: animal models and clinical trials in cancer treatment. Am J Transl Res. 2014 Jan 15;6(2):114–8.
- NIH animal model funding. https://grants.nih.gov/news-events/nih-extramural-nexus-news/2025/07/nih-funding-announcements-to-align-with-nih-initiative-to-prioritize-human-based-research.
- Brugge J, Chang K-C, Silvestri F, Olipant M, Martinez-Gakidis MA, Orgill D, et al. Breast organoid suspension cultures maintain long-term estrogen receptor expression and responsiveness [Internet]. Res. Sq. 2024. Available from: https://www.researchsquare.com/article/rs-4463390/v1.
- Önder CE, Ziegler T, Becker R, Brucker S, Hartkopf A, Engler T, et al. Advancing cancer therapy predictions with patient-derived organoid models of metastatic breast cancer. Cancers (Basel) [Internet]. 2023 July 1;15. [CrossRef]
- Laberiano-Fernandez C, Gan Q, Wang SM, Tamegnon A, Wistuba I, Yoon E, et al. Exploratory pilot study to characterize the immune landscapes of malignant pleural effusions and their corresponding primary tumors from patients with breast carcinoma and lung adenocarcinoma. J Am Soc Cytopathol. 2024 May;13(3):161–73. [CrossRef]
- Pan B, Zhao D, Liu Y, Li N, Song C, Li N, et al. Breast cancer organoids from malignant pleural effusion-derived tumor cells as an individualized medicine platform. In Vitro Cell Dev Biol Anim. 2021 May;57(5):510–8. [CrossRef]
- Choi W, Kim YH, Woo SM, Yu Y, Lee MR, Lee WJ, et al. Establishment of patient-derived organoids using ascitic or pleural fluid from cancer patients. Cancer Research and Treatment: Official Journal of Korean Cancer Association. 2023;55(4):1077–86. [CrossRef]
- NIH establishes nation’s first dedicated organoid development center to reduce reliance on animal modeling [Internet]. National Institutes of Health (NIH). [cited 2025 Oct 8]. Available from: https://www.nih.gov/news-events/news-releases/nih-establishes-nations-first-dedicated-organoid-development-center-reduce-reliance-animal-modeling.
- Yang S-R, Mooney KL, Libiran P, Jones CD, Joshi R, Lau HD, et al. Targeted deep sequencing of cell-free DNA in serous body cavity fluids with malignant, suspicious, and benign cytology. Cancer Cytopathol. 2020 Jan;128(1):43–56. [CrossRef]
- Liu Y, Gan Y, AiErken N, Chen W, Zhang S, Ouyang J, et al. Combining organoid models with next-generation sequencing to reveal tumor heterogeneity and predict therapeutic response in breast cancer. J Oncol. 2022 Aug 22;2022:9390912. [CrossRef]
- Ding S, Hsu C, Wang Z, Natesh NR, Millen R, Negrete M, et al. Patient-derived micro-organospheres enable clinical precision oncology. Cell Stem Cell. 2022 June 2;29(6):905-917.e6. [CrossRef]
- Xi R, Wang X, Moseley R, Raman R, Zhang R, Jaibbar S, et al. Abstract 3412: Patient-derived MicroOrganoSpheres (MOS) enable precision clinical decision-making for multiple myeloma patients. Cancer Res. 2023 Apr 4;83(7_Supplement):3412–3412. [CrossRef]
- Hanahan D. Hallmarks of cancer: New dimensions. Cancer Discov. 2022 Jan;12(1):31–46. [CrossRef]
- Aliazis K, Christofides A, Shah R, Yeo YY, Jiang S, Charest A, et al. The tumor microenvironment’s role in the response to immune checkpoint blockade. Nat Cancer. 2025 June;6(6):924–37. [CrossRef]
- Wang Q, Shao X, Zhang Y, Zhu M, Wang FXC, Mu J, et al. Role of tumor microenvironment in cancer progression and therapeutic strategy. Cancer Med. 2023 May;12(10):11149–65. [CrossRef]
- Thorel L, Perréard M, Florent R, Divoux J, Coffy S, Vincent A, et al. Patient-derived tumor organoids: a new avenue for preclinical research and precision medicine in oncology. Exp Mol Med. 2024 July;56(7):1531–51. [CrossRef]
- Zhou C, Wu Y, Wang Z, Liu Y, Yu J, Wang W, et al. Standardization of organoid culture in cancer research. Cancer Med. 2023 July;12(13):14375–86. [CrossRef]
- Huang S, Mei Z, Wan A, Zhao M, Qi X. Application and prospect of organoid technology in breast cancer. Front Immunol. 2024 Aug 26;15:1413858. [CrossRef]
- Tzeng Y-DT, Hsiao J-H, Tseng L-M, Hou M-F, Li C-J. Breast cancer organoids derived from patients: A platform for tailored drug screening. Biochem Pharmacol. 2023 Nov;217(115803):115803. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




