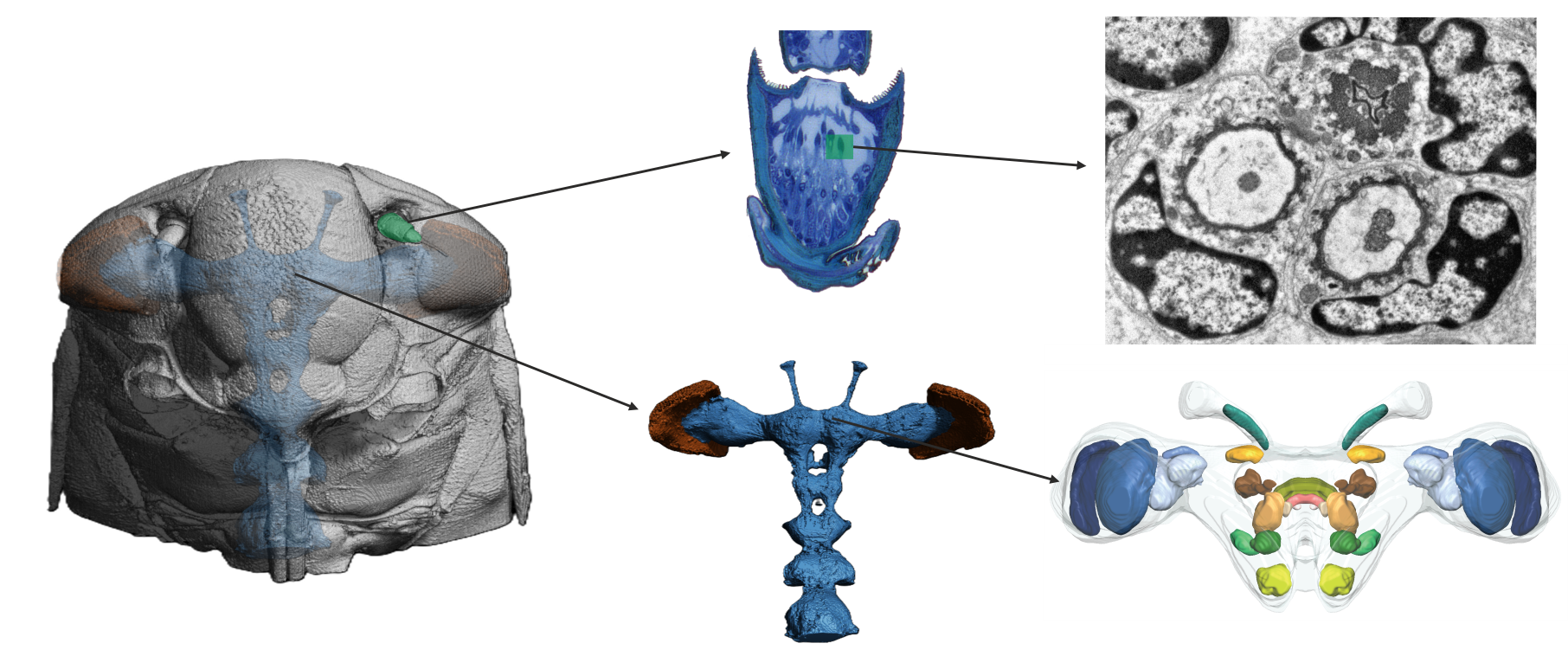
1. Introduction
Insects orient themselves in the environment using different sensory modalities (i.e., chemical, mechanical, physical) [
1,
2,
3,
4], mediated by different stimuli that are primarily detected by the antennae, considered the insect’s primary sensory organs [
5]. The functional units responsible for perceiving these stimuli are the sensilla, i.e., minute functional organs capable of detecting specific stimuli. Given the extraordinarily high diversity of insects and the variety of stimuli on which they rely throughout their lives, sensilla are adapted to specific functions. In chemoreceptors, pores are usually located at the sensillum tip or scattered on the sensillum cuticular wall [
6,
7]. The sensilla sensory neurons axons project to the central nervous system and terminate at specific neuropiles [
8,
9,
10,
11].
In addition to externally visible sensilla, insects possess sensilla present in the internal lumen of various organs/body parts, including legs, wings, antennae, abdomen, and tympanal organ. These internal sensilla are known as scolopidia and are particularly prominent in the insects’ antennae [
12]. In the second antennal segment, the pedicel, scolopidia are arranged in a circular pattern, forming a specialized structure called Johnston’s organ (JO) [
12,
13,
14]. Scolopidia are extremely sensitive organs, capable of responding to the slightest antennal movement. The degree of their sensitivity was recorded in
Toxyrhynchites brevipalpis females, which responded to antennal deflections of ± 0.0005° induced by ± 11 nm air particles [
15]. To date, Johnston’s organ has been associated with various functions, from gravireception to sound perception, flight control, airflow detection, and vibrational sensing [
16,
17,
18].
The axons of all antennal sensory organs are merged into the antennal nerve and project to the primary neuropiles in the brain. Different neuropiles are specialized for processing specific sensory signals. For instance, visual signals from eyes are processed in the optic lobes (Ols), mechanical signals are analyzed in the antennal mechanosensory and motor center (AMMC), whereas the olfactory signals are primarily analyzed in the antennal lobes (Als). The Als, considered as the primary olfactory centers of insect’s brain, are organized into small spherical functional units, called olfactory glomeruli. The number of glomeruli ranges from fewer than 10 up to several hundred, and each glomerulus receives inputs from the olfactory sensory neurons that express the same type of olfactory receptors [
9,
11,
19]. Olfactory information is subsequently modulated by local interneurons, and relayed to higher brain regions via projection neurons. Projection neurons convey information to the higher olfactory centers, including lateral horn and the mushroom bodies [
10,
11,
20,
21].
Sensory organs are adapted to specific ecological needs, resulting in their better performance (i.e., finding a food source, mate etc.). This is especially evident in insects which have developed macroglomeruili in the antennal lobe which are common in insects that use pheromones for long-range communication. However, it has been shown that macroglomeruli are also associated with food odours [
22,
23]. The changes in glomeruli size are primarily associated with the number of OSNs they receive [
20,
24] and the number of synapses [
25]. In addition to differences in the size of individual glomeruli, significant variations have been observed in the number of glomeruli between various species. In most species, the AL is composed of approximately 50-200 glomeruli [
26,
27,
28,
29,
30]. However, extreme values were found in some species, as in the case of locust, which shows between 1000 and 3000 glomeruli [
31,
32]. In contrast, species like aphids [
33,
34], dragonflies [
35], planthoppers and leafhoppers [
36] either lack glomeruli or only have a very low number. Moreover, variations were observed in the mushroom body development, being larger in social insects that display non-stereotyped behavioural responses [
37].
Philaenus spumarius L. (Hemiptera: Aphrophoridae), commonly known as the meadow spittlebug, is a species that has received increasing attention in recent years due to its role as the primary vector of
Xylella fastidiosa, a destructive xylem-limited bacterium currently spreading across Italy and Europe [
38]. Beyond its characteristic xylem-feeding behaviour and spittle mass production,
P. spumarius is characterized by having a significantly reduced number of antennal sensilla [
39]. A recent investigation of
P. spumarius antennae revealed the presence of only three olfactory sensilla (sensilla basiconica), each one housing 21 olfactory sensory neurons (OSN) [
39]. Despite this limited number of olfactory sensilla,
P. spumarius has shown positive responses to a variety of chemical compounds from plants and conspecifics in laboratory behavioural bioassays [
40,
41,
42,
43,
44]. In addition to olfactory cues,
P. spumarius utilizes vibrational signals for communication. The vibrational repertoire of
P. spumarius has been characterized, revealing the use of vibrational signals in intraspecific sexual communication [
45]. Thus,
P. spumarius relies on a multimodal communication system.
This study aimed to investigate how the simplified sensory system of P. spumarius antennae correlates with brain development. To do this, we first investigated the ultrastructural organization of Johnston’s organ by scanning and transmission electron microscopy. By Micro-CT and confocal laser scanning microscopy, we studied the brain organization and identified the primary neuropiles. Moreover, we conducted antennal and single sensilla backfills, enabling us to investigate the target regions within the P. spumarius brain.
2. Materials and Methods
Adults of P. spumarius were collected during 2020 and 2021 on meadows near University of Konstanz (Germany) and in Umbria region (Italy). Once captured, insects were transferred to the rearing facility. Insects were reared in mesh cages (Kweekkooi 40x40x60 cm, Vermandel, Hulst, The Netherlands) under controlled conditions (25±2°C, L16:D8, RH 60±5%). Fresh Vicia faba L. plants were placed inside the cage as a food source and replaced every 10 days.
Scanning Electron Microscopy
Scanning electron observations were conducted on 10 individuals, which were anesthetized by low-temperature exposure (-18°C for 2 min) and placed in 50% ethanol. To observe antennae in natural position, the whole head with the antennae was detached from the rest of the body. Moreover, to observe the antennae from all sides, they were removed from the head by a fine scalpel blade under a stereomicroscope. The prepared specimens were dehydrated in a series of graded ethanol (60, 70, 80, 90, 95, and 99%), each step for 15 min. After dehydration, specimens were submerged in pure HMDS (Hexamethyldisilazane, Sigma-Aldrich, Dorset, UK), and allowed to dry under the hood, at room temperature. Samples were mounted on aluminium stubs, and to obtain a clear view of the different sides, the specimens were positioned with different orientations. Mounted specimens were gold-sputtered using a “Balzers Union® SCD 040” unit (Balzers, Vaduz, Liechtenstein). The observations were carried out using a FE-SEM Zeiss® SUPRA 40 (Carl Zeiss NTS GmbH, Oberkochen, Germany) and a Philips® XL 30 (Eindhoven, The Netherlands) operating at 7-10 KV, WD 9-10 mm, and analyzed by a SMART-SEM® software.
Light and Transmission Electron Microscopy
Ten individuals were anesthetized by exposure to low temperature (-18°C for 1 min). Immediately after, they were immersed in a solution of 2% glutaraldehyde and 2.5% paraformaldehyde in 0.1 M cacodylate buffer+5% sucrose, pH 7.2–7.3. Complete antennae were detached from the head and left at 4°C for 2h. The specimens were kept at 4 °C overnight in the same buffer, then the specimens were post-fixed in 1% OsO4 (osmium tetroxide) for 1 h at 4 °C and rinsed in the same buffer. Later, the specimens were dehydrated in a series of graded ethanol from 60 to 99% and embedded in Epon-Araldite with propylene oxide as bridging solvent. Thin sections were taken with a diamond knife on a LEICA ULTRACUT R ultramicrotome (Leica®) and mounted on formvar-coated 50 mesh grids. Then, sections on grids were stained with uranyl acetate (20 min, room temperature) and lead citrate (5 min, room temperature). Finally, the sections were investigated with a Philips® EM 208. Digital pictures (1376 x 1032 pixels, 8b, uncompressed greyscale TIFF files) were obtained using a high-resolution digital camera MegaViewIII (SIS®) connected to the TEM.
Histology and Immunocytochemistry
Live P. spumarius individuals were anesthetized by placing them in a freezer for 2 minutes. Using fine tweezers, brains were carefully dissected and immediately placed in freshly prepared 4% paraformaldehyde (PFA, Electron Microscopy Science, USA) dissolved in 0.01 M phosphate-buffered saline solution (PBS) for fixation. The samples were kept at room temperature on a shaker for 1 hour. After fixation, the brains were washed 7 times for 15 minutes with PBS containing 1% Triton X-100 (PBS-Tx, Sigma Aldrich, USA). The brains were preincubated overnight in an antibody blocking solution. Subsequently, the brains were incubated with primary antibody, monoclonal anti-mouse Synapsin 1 (Hybridoma Bank, USA, SYNORF1) at a 1:30 dilution for 3 days at room temperature on a shaker. After primary antibody incubation, the brains were rinsed 8 times for 30 minutes with PBS-Tx and incubated for 3 days with the secondary goat anti-mouse conjugated to Alexa Fluor 546 antibody (Thermo Fisher Scientific, USA) diluted 1:500. To visualize cell nuclei, DAPI (Sigma-Aldrich, Germany) at a dilution of 1:500 was added simultaneously with the secondary antibody. After antibody incubations, the brains were washed 11 times for 20 minutes with PBS-Tx and dehydrated in an ethanol series of increasing concentrations (50, 75, 95, and twice in 100%) for 30 minutes each step. Following dehydration, the brains were treated with xylene for 2 minutes and embedded in DPX mounting medium (Sigma-Aldrich, USA) between two cover slips separated by a custom-made metal spacer.
Antennal Backfills
Insect were anesthetized for 2 minutes in a freezer and immobilized on a cover glass. The antennae were positioned vertically and secured using Patafix, which was shaped into a wall around the scape. Within this Patafix wall, one µl of 4% Neurobiotin (Neurobiotin Plus, Vector Laboratories) was applied, after which the flagellum was removed by a razor. The insects were then placed in a dark chamber for 2 hours with a piece of wet paper to maintain high humidity. Brains dissection and fixation were carried out as described above. Synapsin-rich neuropiles were stained with a monoclonal anti-mouse Synapsin 1 was applied at 1:30 dilution followed by 8 washes PBS-Tx for 30 min each. The brains were subsequently incubated with Cy3 conjugated Streptavidin (Jackson ImmunoResearch) at a 1:400 dilution, goat anti-mouse conjugated to Alexa Fluor 488 (Thermo Fisher Scientific, USA, 1:500) and DAPI (Sigma-Aldrich, Germany, 1:500) for 3 days. Subsequently, the brains were washed 11 times in PBS-Tx for 20 min each, dehydrated in graded series of ethanol, cleared in xylene and mounted in DPX as reported above.
Single Sensillum Backfills
Insects were immobilized on a cover glass with ventral side facing upward using dental wax. A thin layer of the dental wax was applied on the antennal ledge, on which pedicel was attached. To facilitate the approach of the glass electrode to the sensilla basiconica, the arista was halved using micro scissors. The prepared specimens were positioned under light microscope (Examiner A1, Zeiss, Germany) equipped with a 250x magnification objective lens. A glass electrode, prepared using a micropipette puller (Sutter instrument, P-2000, CA, USA), was connected to a frequency generator via piezo element and mounted on a micromanipulator. When the electrode was positioned near the sensillum basiconica, the frequency was adjusted such that the glass electrode started to vibrate, causing the sensillum to break. Immediately afterward, 1 µl of 4% Neurobiotin (Neurobiotin Plus, SP-1150; Vector) was applied over the broken sensillum. The specimens were then placed in a dark box for 2 hours. Subsequently, brains were dissected and fixed as previously described. To visualize neurobiotin, Alexa Fluor 488 conjugated streptavidin was applied (Thermo Fisher Scientific, 1:400), whereas for neuropile identification monoclonal anti-mouse synapsin 1 followed with secondary goat anti-mouse conjugated to Alexa Fluor 546 antibody.
Confocal Laser Scanning Microscopy
Whole-mount preparations were scanned with a Zeiss LSM 510 or a Zeis LSM 880 confocal laser scanning microscope, equipped with a 10x and 20x objective lenses. Images were acquired in sequential mode, utilizing three different excitation wavelengths simultaneously (405 nm for DAPI, 488 nm for Alexa Fluor 488 and Alexa Fluor 488 streptavidin, 540 nm for Cy3 and 560 nm for Alexa Fluor 546). These wavelengths allowed for the visualization of antisynapsin staining, antennal nerve fill and cell body staining. Images were taken at intervals of 0.5 or 1 µm.
The 3D reconstruction of identified neuropiles was performed using Amira software (Amira 5.3, Visage Imaging, Fürth, Germany). Neuropiles were labelled using the segmentation editor with the interpolation option and were subsequently manually verified for accuracy. The final labels were rendered using the SurfaceGen tool.
Micro Computed Tomography (Micro-CT)
Individuals of P. spumarius were anesthetized by exposure to low temperatures (-20°C) for two minutes and immersed in a solution of 2% glutaraldehyde and 2.5% paraformaldehyde in 0.1 M cacodylate buffer +5% sucrose, pH 7.2–7.3 for 24 h at 4°C. Following fixation, specimens were washed with the same buffer two times for 15 minutes and stored in 99% ethanol. Specimens were subsequently stained with Lugol’s iodine solution for one week at 4°C, then washed two times for 15 minutes with 99% ethanol, mounted in a pipette tip filed with 99% ethanol and sealed with the parafilm. Micro-CT scanning was performed using a Bruker-SkyScan 1172 (Bruker, MA, USA) with a voltage of 60 kV and an amperage of 167 uA, a 360° rotation and step size of 0.15°. The resulting image projections were processed with NRecon (Version 1.7.4.6) and exported as an 8-bit BMP image series. The image series were subsequently transformed into a single 8-bit TIFF file. Segmentation was carried out in Dragonfly 2022.2 (Comet Technologies, Canada). To ensure uniformity in the segmentation an OTSU thresholding was applied. The cuticle, brain, and retina were pre-segmented by manually annotating every 25th slice. Regions of interest (ROIs) were exported as a single grayscale TIFF image, where each ROI was assigned a unique label (1-3) and everything else was labelled as background (0). Both the greyscale image and Micro-CT dataset were uploaded to Biomedisa, an online platform for semi-automatic segmentation. The resulting segmentations were imported back to Dragonfly, where ROIs were extracted and compared to assess the segmentation accuracy. Lastly, areas with spilling into the wrong ROI were manually corrected, smoothed, and rendered in 3D using Dragonfly.
4. Discussion
The present study revealed the ultrastructural organization of the Johnston’s organ in
P. spumarius and provided an overview of its general brain organization. In many insects, intra- and interspecific communication signals are generated as mechanical stimuli resulting from abdomen trembling, wing spreading, stridulation, or drumming [
13]. These signals are detected by chordotonal organs (such as tympanal organs and the Johnston’s organ) located in key positions, including the wing base, femur, ventral abdomen, larval abdominal wall, or antennae. The auditory organ consists of a series of scolopidia grouped into a larger structural unit—the Johnston’s organ [
13,
46,
47].
The number of scolopidia that make up the Johnston’s organ varies across taxa. The most advanced Johnston’s organ, found in mosquitoes, consists of over 7,000 scolopidia [
48,
49]. In contrast,
Periplaneta americana has a Johnston’s organ with approximately 150 scolopidia, while in
P. spumarius, we recorded 110 scolopidia. This number is comparable to those observed in related groups, such as Heteroptera and Sternorrhyncha (Aphididae), where the number of scolopidia typically does not exceed 50 [
50,
51]. However, among other closely related species, scolopidia counts vary, ranging from 25 in
Scaphoideus titanus Ball, to 66 in
Hyalesthes obsoletus Signoret, and 72 in
Metcalfa pruinosa Say [
52].
The complexity of the Johnston’s organ reflects the selective pressure for intra- and interspecific communication. In honeybees, the Johnston’s organ detects the vibrations generated during the waggle dance [
3], while in
Drosophila melanogaster, it responds to courtship signals produced by female wing vibrations. In planthoppers, the Johnston’s organ is involved in vibrational communication via substrate-borne signals.
In
P. spumarius, we identified two types of scolopidia, distinguished by the number of sensory neurons they contain. This variation may suggest a potential multifunctional role for each type. The most common type contained two sensory neurons, while the scolopidia with one sensory neuron was less prevalent. Scolopidia with two sensory neurons are commonly observed in other insects, including aphids,
Periplaneta americana,
Drosophila, and mosquitoes [
48,
49,
53,
54]. In
Aedes aegypti, this type of scolopidium is the most abundant and plays a key role in sound perception, accounting for 97% of the total scolopidia [
48,
49]. Recent physiological studies in
D. melanogaster have shown that within these two-neuron scolopidia, one sensory cell is sensitive to vibrations, while the other responds to static deflections [
55]. These findings support the hypothesis that one sensory neuron in the Johnston’s organ is specialized for hearing, while the other is involved in detecting gravity and wind.
In addition to the similarities in the ultrastructural organization of scolopidia, we aim to draw a parallel between the antennal organization and signal transmission mechanisms in
D. melanogaster and
P. spumarius. The antennae of
D. melanogaster consist of three segments, with a feather-like arista located on the final segment, the funiculus [
56]. In
Drosophila, the arista and funiculus are the primary mechanotransducers, closely linked to one another. When sound particles or wind cause the displacement of the arista, it induces the rotation of the funiculus. This rotation generates a force on the scolopidia at the attachment point, stretching them and triggering mechanosensory cilia in the nerve cells. This process opens stretch-gated mechanotransduction channels, resulting in action potentials being transmitted along the antennal nerve [
57,
58,
59].
A similar signal reception pathway likely occurs in P. spumarius, although with a key difference in antennal structure. In Drosophila, the arista is feather-like, while in P. spumarius, it is replaced by a long, thread-like filament. In P. spumarius, displacement of the flagellum by sound waves may transmit mechanical forces to the scolopidia of the Johnston’s organ, which are attached at the base of the flagellum, within the pedicel.
Apart the well-developed Johnston’s organ, a single sensillum campaniform and a single nerve at the base of the thread flagellum were observed. Taking into consideration the campaniform sensillum position (at the cuticular base of the flagellum), this sensillum could easily perceive slight flagellar movement, which could induce pressure on the sensillum. The direct pressure on the tip of the sensilla would result in its depolarization or, in contrast, hyperpolarization, similarly to the response of scolopidia in
Drosophila [
60]. Besides, the presence of the sensory neuron at the flagellum base indicates a potential proprioceptive role of this structure.
The brain of
P. spumarius followed the general organization found in the clade. However, when compared with the brain of true bugs (Hemiptera), it had less prominent MB and low delimitation of the AL glomeruli [
30]. Moreover, differences were found at the ganglia level, which appeared to be highly fused in aphids, whereas in
P.
spumarius they were separated by the short connectives [
33].
Previous studies on the antennal lobe organization in Homoptera and Auchenorrhyncha have reported the presence of small, aglomerular or glomerular ALs [
33,
34,
36]. Our observations are consistent with these findings, as the AL in
P. spumarius occupies approximately 0.4% of the total brain volume. A similar size has been reported for
Hyalesthes obsoletus, where the AL constitutes about 0.6% of the brain [
36]. This relatively small size stands in sharp contrast to the well-developed ALs of commonly studied model organisms. For instance, in
Nasonia vitripennis (Walker) (Pteromalidae), the AL accounts for 12% of the total brain volume [
27]; in
Apis mellifera, it represents approximately 5.7% [
37]; in
Bombus terrestris L., 5.2% [
61]; and in
D. melanogaster, around 5% [
62,
63]. Moreover, in
Apolygus lucorum Meyer-Dür (Miridae), the AL occupies nearly 15% of the brain volume, making it the species with the most prominent AL reported to date [
30].
The relatively small volume of the antennal lobe (AL) in the brain of
P. spumarius likely reflects its sensory ecology. Indeed, the antennae of
P. spumarius exhibit a significantly reduced sensory system, consisting of only 15 sensilla, of which just three are olfactory [
39]. This low number of olfactory sensilla likely contributes to the small size of the glomeruli and the AL overall. A similarly reduced number of olfactory sensory neurons (OSNs) has been reported in other species, such as psyllids and larvae of
D. melanogaster [
64,
65].
No sexual dimorphism in AL size was observed. This is not surprising, as both sexes share a similar sensory system organization and ecological function. Differences in AL size between sexes have been reported in species where males and females exhibit distinct ecological behaviors, such as in the ant
Camponotus japonicus. In this species, significant differences in AL size were found between males and unmated queens; however, no difference was observed between queens and workers [
66].
Our staining of synapsin-rich neuropiles was largely successful. However, the glomeruli within the antennal lobes (ALs) appeared poorly delimited, which prevented us from performing a 3D reconstruction. This low glomerular resolution may be associated with the presence of a thin glial sheath, which plays a crucial role in glomerular organization, as previously reported in Diptera [
67].
Antennal backfill analysis revealed a prominent antennal nerve projecting into both the antennal mechanosensory and motor center (AMMC) and the AL. Most of the fibers were associated with the AMMC, while only a few entered the AL. To further characterize the spatial organization of the AL, we performed single sensillum backfills. However, in addition to labelling sensory neurons from the sensilla basiconica, we also stained the nerve from the Johnston’s organ. To better visualize the sensilla and isolate individual sensilla, we extended the antennae up to the antennal ledge. This manipulation, however, led to the rupture of Johnston’s organ nerves. As a result, when neurobiotin was applied, it labelled both the targeted sensillum neurons and residual nerves from the Johnston’s organ. Despite this overlap, the staining clearly showed that the fibers entering the AL originated from the sensilla basiconica, and not from other sensillum types.
Our single sensillum backfills performed on sensilla basiconica revealed sensory neuron terminals arborizing across a large portion of the AL, without a clear functional subdivision in their arborization pattern. This diffuse localization may reflect the number of OSNs housed within each sensillum basiconicum and the total number of glomeruli. A previous study showed that each sensillum basiconicum contains 21 OSNs [
39]. It is therefore conceivable that multiple OSNs from a single sensillum converge onto the same glomerulus. Similar findings have been reported in other species. In the mite
Phytoseiulus persimilis, the peripheral olfactory system comprises five putative olfactory sensilla, with each glomerulus receiving input from a single sensory neuron [
68]. A similar arrangement is also observed in the larvae of
D. melanogaster, where 21 OSNs project to a single glomerulus [
65]. However, in other insects, sensory neurons can be functionally arborized within the AL, receiving input from particular types of sensory neurons, such as olfactory or thermo-/hygroreceptive neurons [
69,
70,
71,
72] or receive inputs from multiple OSN [
73].
The overall neural pattern of the Johnston’s organ afferents in
P. spumarius is comparable with other species. In
Drosophila, ants, and
Mythimna separata a major part of the Johnston’s organ projects to the AMMC [
74,
75,
76]. In the earlier study on Drosophila, the AMMC was divided into five zones, depending on the spatial organization of the Johnston’s organ afferents [
53,
76,
77,
78,
79]. Furthermore, each zone was associated with the specific function. Zones A and B receive inputs from the nerve cells responsible for detection of near-sound and high-frequency vibrations. While zones C and E are associated with the gravitational forces and wind-induced deflections. Contrary to
Drosophila and
P. spumarius, in honeybees, the afferents from Johnston’s organ are slightly different diverged in the brain [
80]. Most of the afferents projects to the posterior protocerebrum and subesophageal ganglion, while just a small number of afferents terminates in the AMMC.
Apart from the AMMC, the afferent neurons often project to other neuropiles. In
Drosophila they are gnathal ganglia, wedge, thoracic-abdominal ganglia, anterior ventrolateral protocerebrum, saddle, ventrolateral protocerebrum [
81]. The former neuropile (ventrolateral protocerebrum) receives intensive inputs from the visual interneurons, thus is also known as a visual centre in the central brain. Moreover, it receives inputs from the lateral horn. Therefore, the extension of the Johnston’s organ afferent to the ventrolateral protocerebrum may allow integration of both visual and mechanosensory signals, possibly improving continuous coordination during the flight by detecting wind currents [
53,
80].
Although the subesophageal ganglion is the primary centre of gustatory neurons of the mouth parts, in
Drosophila brain the gustatory and Johnston’s organ neurons do not intersect. Thus, it is unlikely that these neurons have a direct contact. Moreover, as the subesophageal ganglion houses terminals of the neurons derived from the thoracic and abdominal ganglia, it was proposed that this neuropile acts as an integration centre for mechanosensory inputs from the Johnston’s organ and other parts of the brain [
53].
In
P. spumarius brain, we recorded an additional output centre of the Johnston’s organ centre, the prothoracic ganglia. This neuronal cell could simply be involved in transmitting information of different mechanosensory modalities to the prothoracic ganglia [
78].
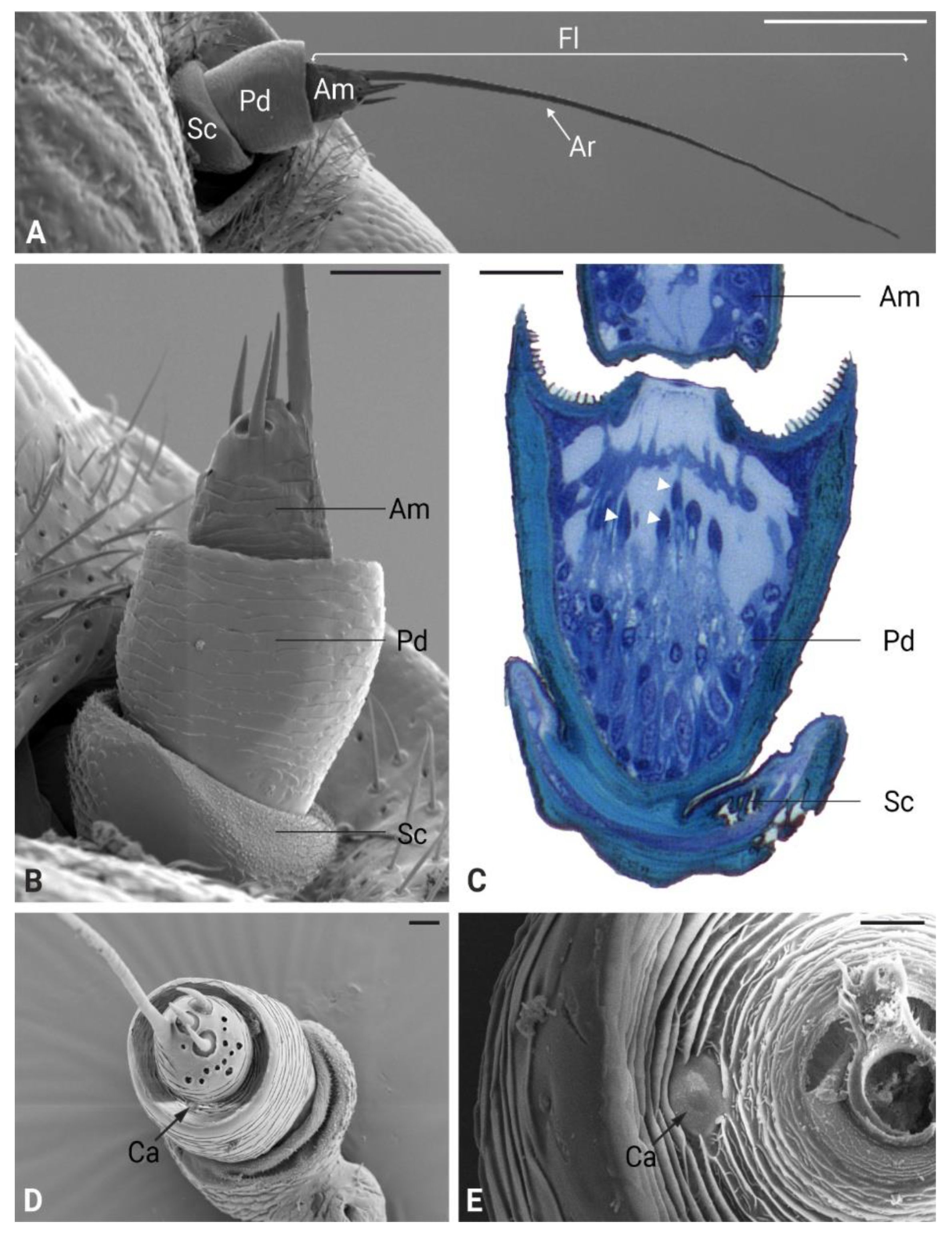
Figure 1.
Scanning and light microscopy micrograph of Philaenus spumarius antennae. A) Ventral view of the antenna showing a short scape (Sc), a large pedicel (Pd) and the flagellum (Fl) which is composed of a basal ampulla (Am) and a long arista (Ar). B) Close-up view of the basal part of the antenna. C) Longitudinal LM section of the pedicel, showing several scolopidia (white arrowheads) belonging to the Johnston’s organ. D) Frontal view of the antennae showing sensory structures present on the ampulla and the area between the ampulla and the apical part of the pedicel. A campaniform sensillum (Ca) can be observed at the apical part of the pedicel. E) Higher magnification image of the Ca sensillum. Scale bars: A = 200 μm, B = 50 μm, C = 25 μm, D = 20 μm, E = 10 μm.
Figure 1.
Scanning and light microscopy micrograph of Philaenus spumarius antennae. A) Ventral view of the antenna showing a short scape (Sc), a large pedicel (Pd) and the flagellum (Fl) which is composed of a basal ampulla (Am) and a long arista (Ar). B) Close-up view of the basal part of the antenna. C) Longitudinal LM section of the pedicel, showing several scolopidia (white arrowheads) belonging to the Johnston’s organ. D) Frontal view of the antennae showing sensory structures present on the ampulla and the area between the ampulla and the apical part of the pedicel. A campaniform sensillum (Ca) can be observed at the apical part of the pedicel. E) Higher magnification image of the Ca sensillum. Scale bars: A = 200 μm, B = 50 μm, C = 25 μm, D = 20 μm, E = 10 μm.
Figure 2.
Ultrastructural organization of Philaenus spumarius antennae. A) Cross-section of the middle portion of the arista showing an electron-dense cuticular wall (CW): on one side, a single sensory neuron surrounded by a dendrite sheath is present. Inset: higher magnification image of the sensory neuron. B) Longitudinal section of sensilla campaniformia showing the cuticular cap (CC) inserted on the thicker antennal wall (CW). A single sensory neuron surrounded by the dendrite sheath (DS) enters the Ca lumen and ends just below the CC. The apical tubular body (TB) is clearly visible. The sensory neuron is positioned below the cuticular cap and surrounded by a socket septum (SS). C) Longitudinal section at the joint level between the pedicel and the ampulla of the flagellum. The joint membrane (JM) extends over the apical part of the pedicel and attaches to the flagellum. The flagellum is inserted on the suspension fibers (arrowheads) which connect to the apodeme (AP). At the central lumen of the pedicel a series of scolopidia (SC) are visible. D). Higher magnification image of the suspension fibers. E) Cross section of the apical part of the pedicel with the basal flagellum at its center. The antennal nerve occupies a large portion of the flagellum’s lumen. The scolopidia of Johnston’s organ are distributed in a circular pattern around the flagellum. Individual scolopidia are embedded in the suspension fibers between pedicel and flagellum. E) High magnification image of the scolopidia attachment to the flagellum. Scale bars: A = 5 μm, B = 2 μm, C = 10 μm, D = 2 μm, E = 5 μm, F = 0.5 μm.
Figure 2.
Ultrastructural organization of Philaenus spumarius antennae. A) Cross-section of the middle portion of the arista showing an electron-dense cuticular wall (CW): on one side, a single sensory neuron surrounded by a dendrite sheath is present. Inset: higher magnification image of the sensory neuron. B) Longitudinal section of sensilla campaniformia showing the cuticular cap (CC) inserted on the thicker antennal wall (CW). A single sensory neuron surrounded by the dendrite sheath (DS) enters the Ca lumen and ends just below the CC. The apical tubular body (TB) is clearly visible. The sensory neuron is positioned below the cuticular cap and surrounded by a socket septum (SS). C) Longitudinal section at the joint level between the pedicel and the ampulla of the flagellum. The joint membrane (JM) extends over the apical part of the pedicel and attaches to the flagellum. The flagellum is inserted on the suspension fibers (arrowheads) which connect to the apodeme (AP). At the central lumen of the pedicel a series of scolopidia (SC) are visible. D). Higher magnification image of the suspension fibers. E) Cross section of the apical part of the pedicel with the basal flagellum at its center. The antennal nerve occupies a large portion of the flagellum’s lumen. The scolopidia of Johnston’s organ are distributed in a circular pattern around the flagellum. Individual scolopidia are embedded in the suspension fibers between pedicel and flagellum. E) High magnification image of the scolopidia attachment to the flagellum. Scale bars: A = 5 μm, B = 2 μm, C = 10 μm, D = 2 μm, E = 5 μm, F = 0.5 μm.
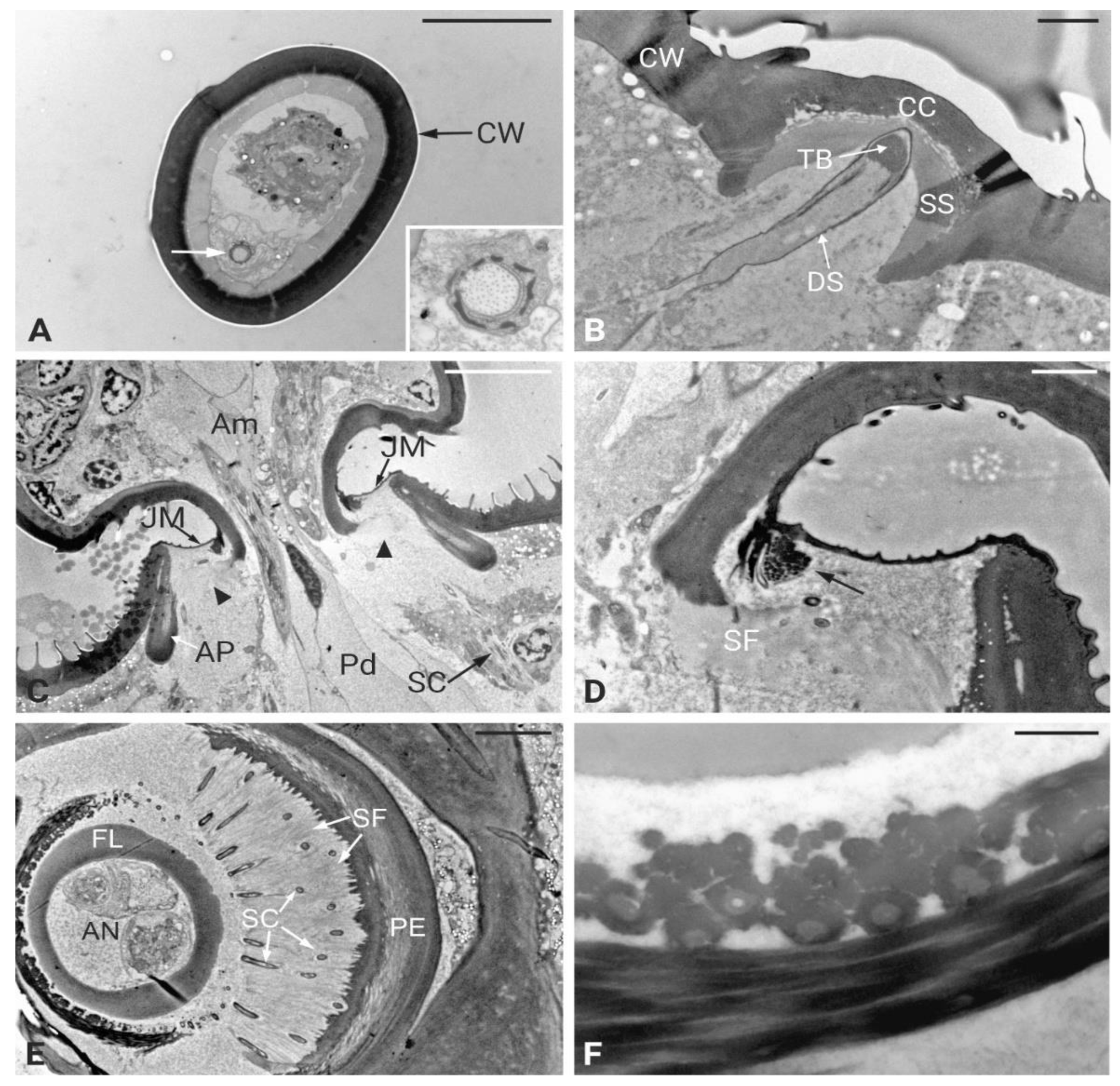
Figure 3.
Ultrastructural organization of the scolopidia of Johnston’s organ in Philaenus spumarius. A) Cross-section of three scolopidia, each containing a different number of cilia and a large nucleus. B) Distal part of a scolopidium showing two cilia (C) surrounded by an extracellular cap (EC). The scolopale rods (SR) are almost fused at this stage and the scolopale cell is significantly reduced. C) More proximal section of the scolopidium showing two almost fused cilia. D) Sections taken at the basal part of the scolopidium showing the ciliary roots (CR) and desmosome (DE). Within the scolopale cell, six segments of scolopale rods (SR) are visible. E) Longitudinal section taken at the basal part of the pedicel showing several scolopidia with large nuclei. F) Longitudinal section of scolopidia surrounded by scolopale rods (SR). Within the rods, a cilium (C) is visible at its proximal part, while at the distal part, the basal bodies (BB) and granular material (GM) are present. Inset: high magnification image of the proximal and distal basal bodies. Scale bars: , A, B = 1 μm; E = 5 μm; F, C = 2 μm; D = 0.5 μm.
Figure 3.
Ultrastructural organization of the scolopidia of Johnston’s organ in Philaenus spumarius. A) Cross-section of three scolopidia, each containing a different number of cilia and a large nucleus. B) Distal part of a scolopidium showing two cilia (C) surrounded by an extracellular cap (EC). The scolopale rods (SR) are almost fused at this stage and the scolopale cell is significantly reduced. C) More proximal section of the scolopidium showing two almost fused cilia. D) Sections taken at the basal part of the scolopidium showing the ciliary roots (CR) and desmosome (DE). Within the scolopale cell, six segments of scolopale rods (SR) are visible. E) Longitudinal section taken at the basal part of the pedicel showing several scolopidia with large nuclei. F) Longitudinal section of scolopidia surrounded by scolopale rods (SR). Within the rods, a cilium (C) is visible at its proximal part, while at the distal part, the basal bodies (BB) and granular material (GM) are present. Inset: high magnification image of the proximal and distal basal bodies. Scale bars: , A, B = 1 μm; E = 5 μm; F, C = 2 μm; D = 0.5 μm.
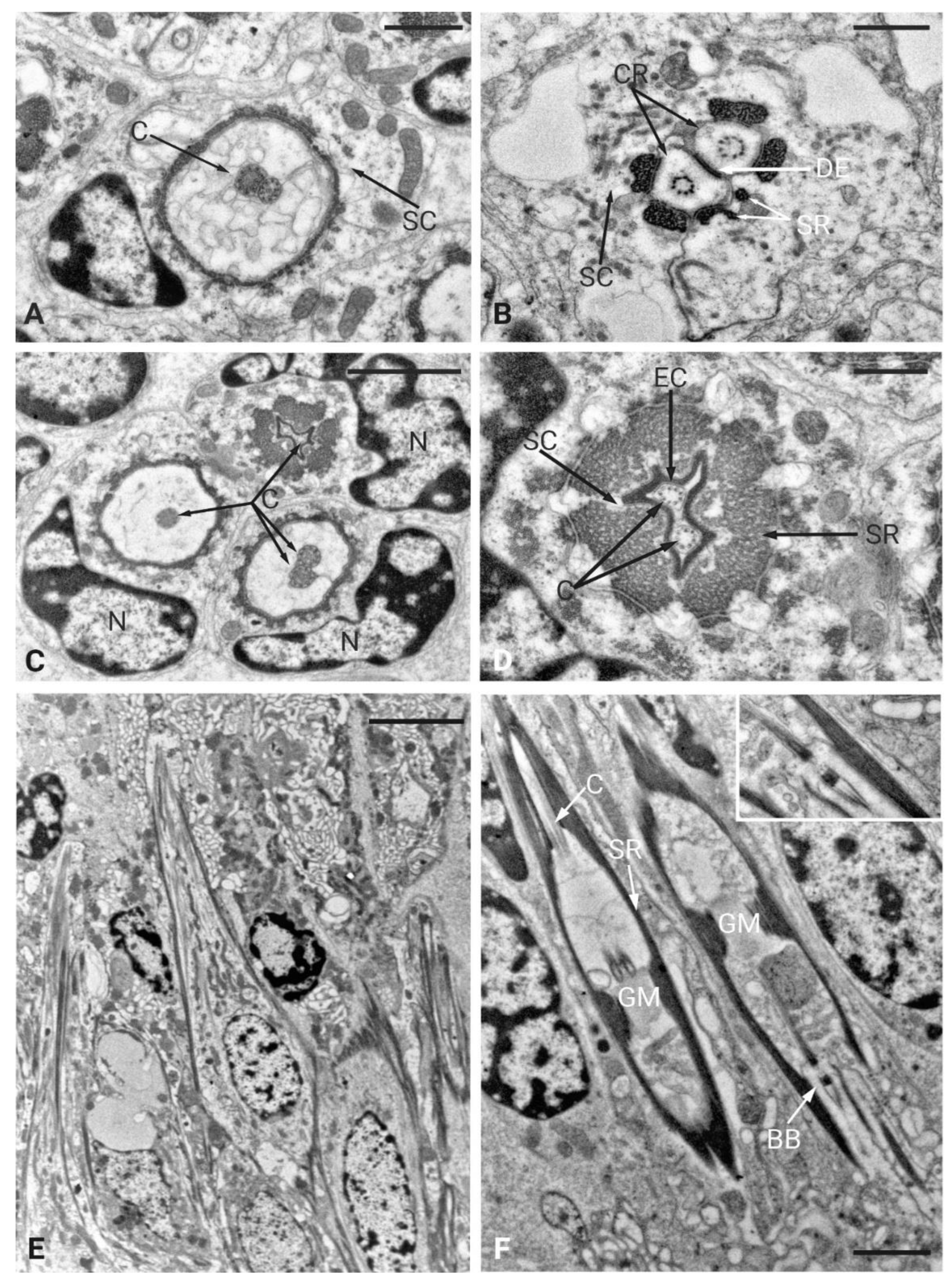
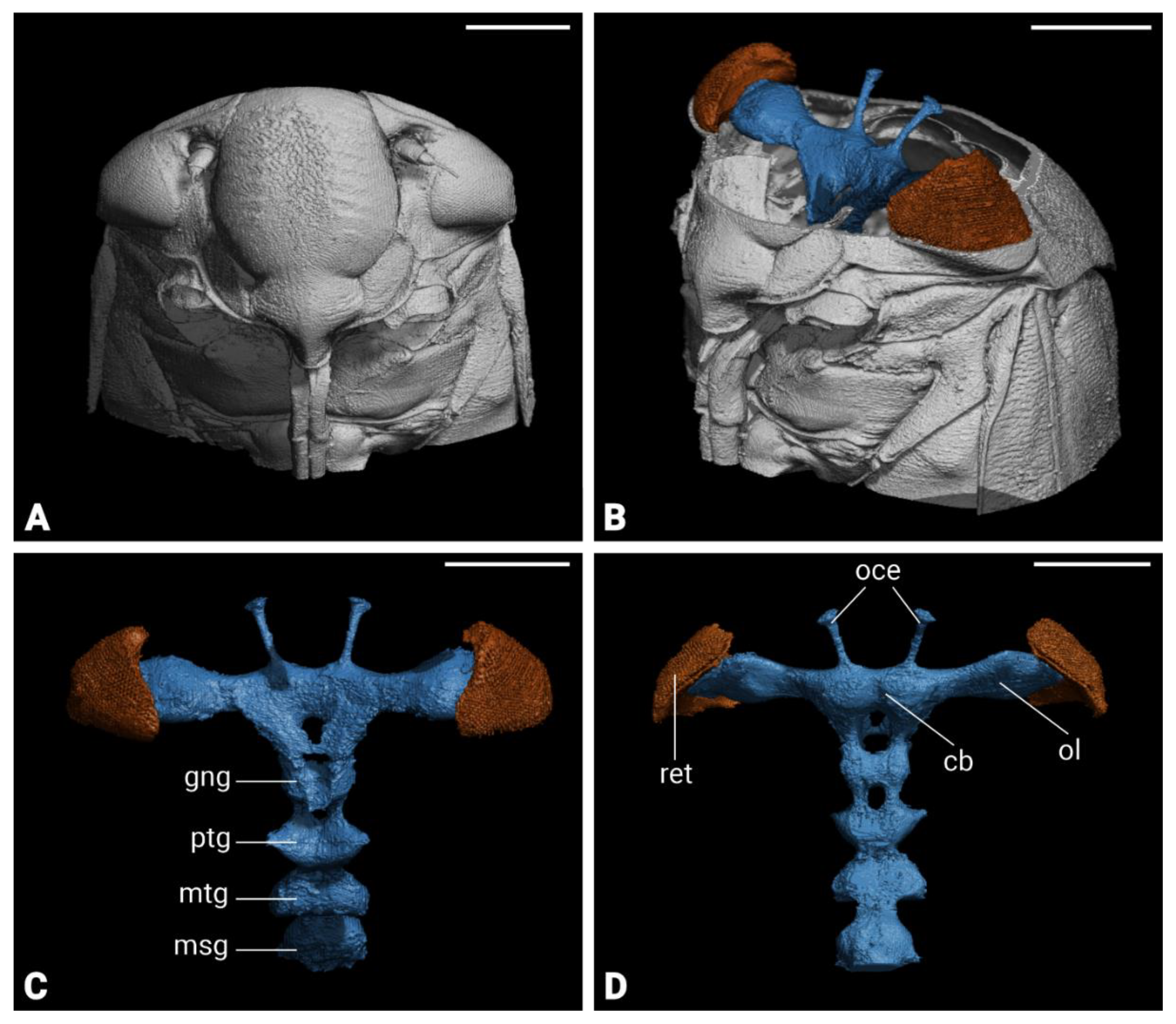
Figure 4.
Micro-CT based 3D reconstruction of the head and brain in Philaenus spumarius. A) 3D Reconstruction of the head of P. spumarius. B) Cross section of the head showing the brain within the internal lumen and retina attached to the cuticle. C) Ventral view of P. spumarius brain with the gnathal (gng) and three thoracic ganglia (ptg, mtg, msg). D) Dorsal side of P. spumarius brain. retina (ret), ocelli (oce), central brain (cb), optic lobes (ol). Scale bars: A, B, C, D = 500 μm.
Figure 4.
Micro-CT based 3D reconstruction of the head and brain in Philaenus spumarius. A) 3D Reconstruction of the head of P. spumarius. B) Cross section of the head showing the brain within the internal lumen and retina attached to the cuticle. C) Ventral view of P. spumarius brain with the gnathal (gng) and three thoracic ganglia (ptg, mtg, msg). D) Dorsal side of P. spumarius brain. retina (ret), ocelli (oce), central brain (cb), optic lobes (ol). Scale bars: A, B, C, D = 500 μm.
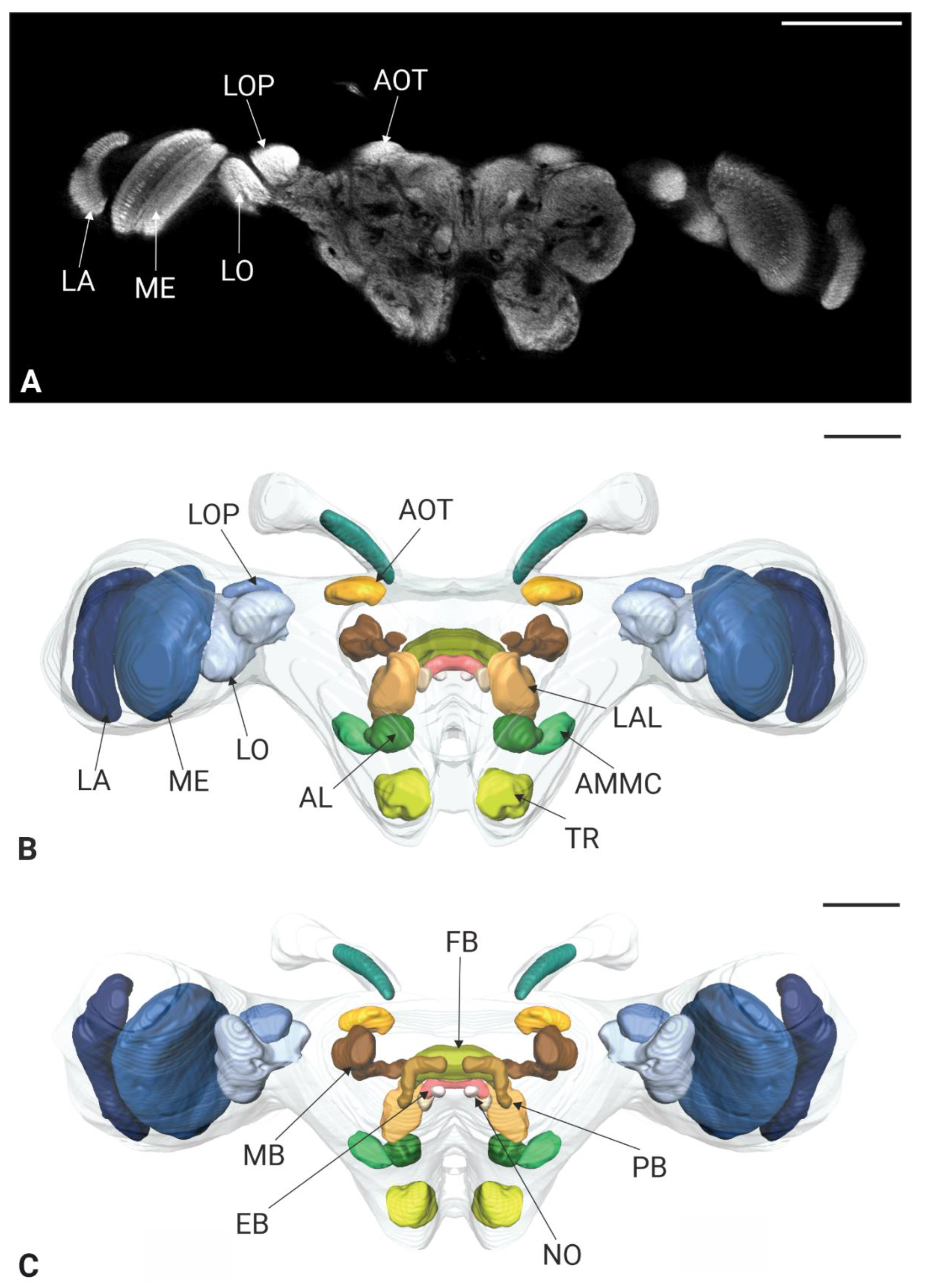
Figure 5.
Immunocytochemistry-based 3D reconstruction of the brain of Philaenus. spumarius. A) Single optical section through medial pat of the brain showing anti-synapsin staining. B) Frontal view of P. spumarius brain with major neuropiles labeled. C) Posterior view of the brain. Abbreviations: AL, antennal lobe; AMMC, antennal mechanosensory and motor centre; AOTB, anterior optic tubercle; EB, ellipsoid body; FB, fan-shaped body; LA, lamina; LAL, lateral accessory lobe; LO, lobula; LOP, lobula plate; MB, mushroom body; ME, medulla; NO, Noduli, PB, protocerebral bridge; TR, tritocerebrum. Scale bars: A = 150 μm, B, C = 100 μm.
Figure 5.
Immunocytochemistry-based 3D reconstruction of the brain of Philaenus. spumarius. A) Single optical section through medial pat of the brain showing anti-synapsin staining. B) Frontal view of P. spumarius brain with major neuropiles labeled. C) Posterior view of the brain. Abbreviations: AL, antennal lobe; AMMC, antennal mechanosensory and motor centre; AOTB, anterior optic tubercle; EB, ellipsoid body; FB, fan-shaped body; LA, lamina; LAL, lateral accessory lobe; LO, lobula; LOP, lobula plate; MB, mushroom body; ME, medulla; NO, Noduli, PB, protocerebral bridge; TR, tritocerebrum. Scale bars: A = 150 μm, B, C = 100 μm.
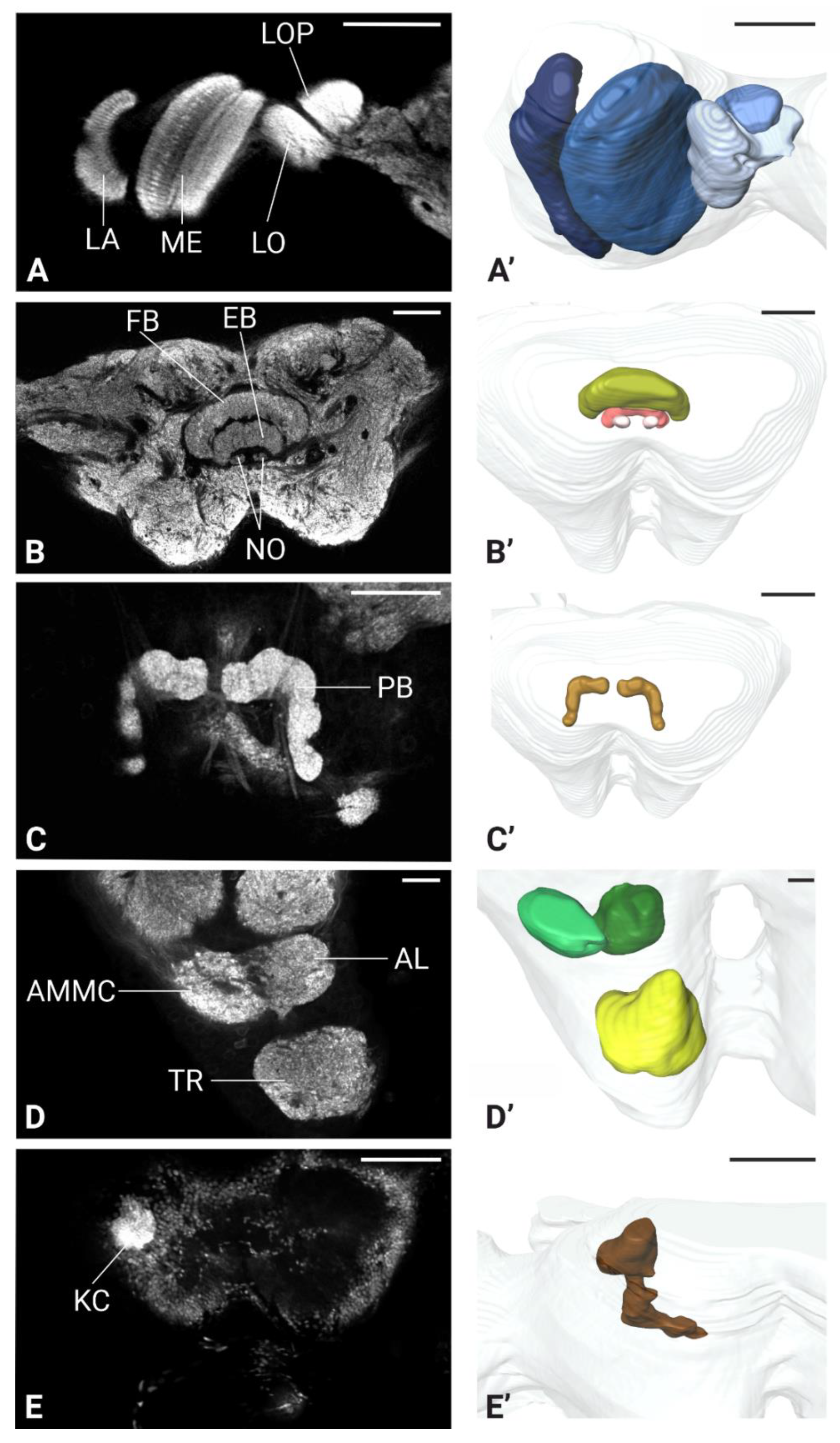
Figure 6.
Optical sections and 3D renderings of neuropules in the brain of Philaenus spumarius. A, A’) Optic lobe showing Lamina (LA), Medula (ME), Lobula (LO) and Lobula plate (LOP), B, B’) Central body and its 3D reconstruction, including the fan shaped body (FB), ellipsoid body (ED) and noduli (NO). C, C’) Protocerebral bridge (PB) and its 3D reconstruction, D, D’) Single section showing antennal lobe (AL), antennal mechanosensory and motor centre (AMMC), and tritocerebrum (TR) together with 3D reconstruction, E, E’) Section showing dense nuclei of Kenyon cells (KC) used as a landmark for the mushroom body. Scale bars: A, A’ = 100 μm , B, B’, C, C’ = 50 μm, D, D’ = 20 μm, E,E’ = 100 μm.
Figure 6.
Optical sections and 3D renderings of neuropules in the brain of Philaenus spumarius. A, A’) Optic lobe showing Lamina (LA), Medula (ME), Lobula (LO) and Lobula plate (LOP), B, B’) Central body and its 3D reconstruction, including the fan shaped body (FB), ellipsoid body (ED) and noduli (NO). C, C’) Protocerebral bridge (PB) and its 3D reconstruction, D, D’) Single section showing antennal lobe (AL), antennal mechanosensory and motor centre (AMMC), and tritocerebrum (TR) together with 3D reconstruction, E, E’) Section showing dense nuclei of Kenyon cells (KC) used as a landmark for the mushroom body. Scale bars: A, A’ = 100 μm , B, B’, C, C’ = 50 μm, D, D’ = 20 μm, E,E’ = 100 μm.
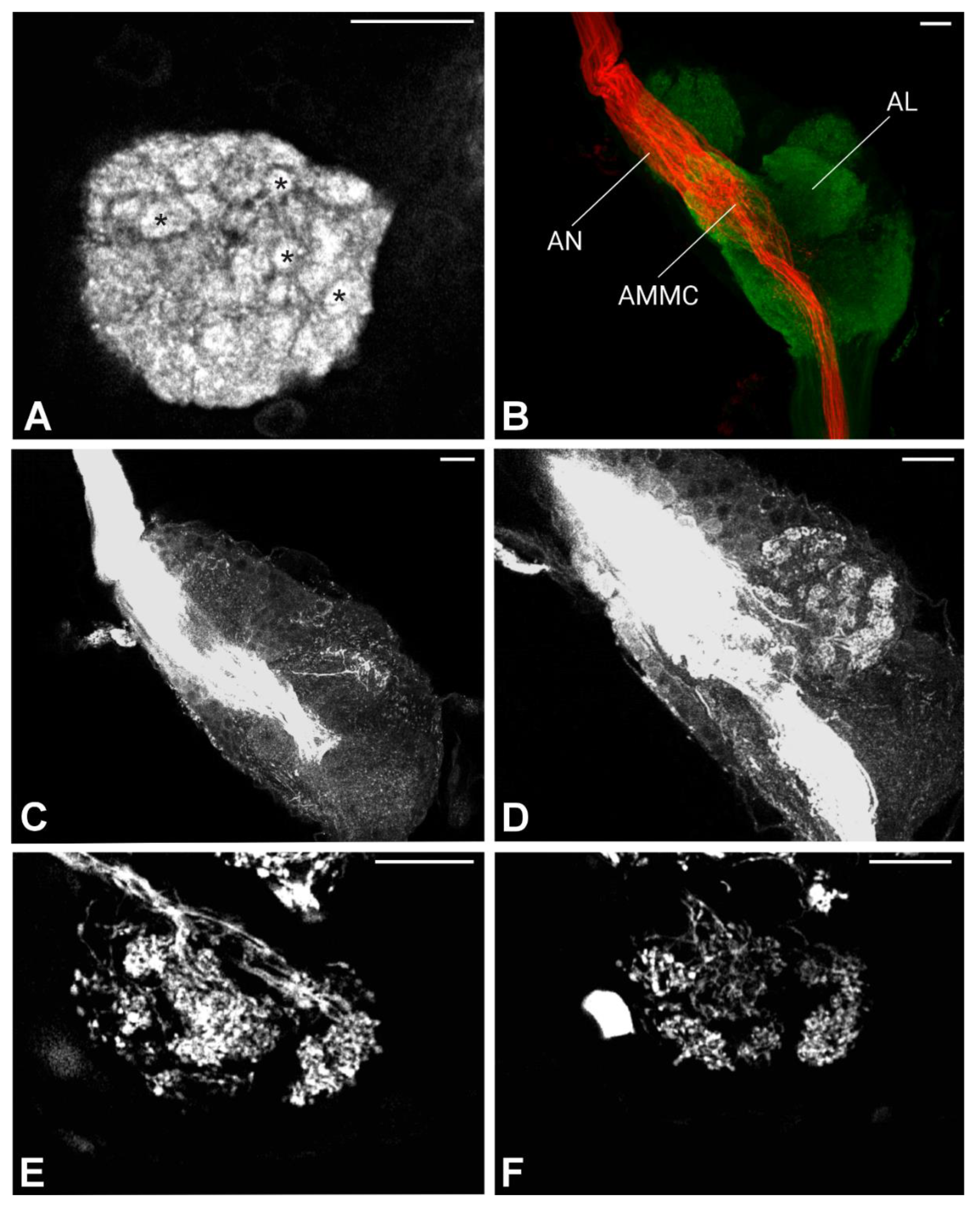
Figure 7.
Antennal lobe and antennal nerve projection in the brain of P. spumarius. A) Mid-level section of the antennal lobe showing numerous small, rounded glomeruli (*). B) Antennal backfills staining revealing the antennal nerve (AN) passing through the AMMC towards the tritocerebrum. C, D) Sensory neuros terminating in the antennal lobe (AL) shown via antennal nerve backfills. E, F), Single sensillum backfills indicating sensory neurons arborization at different levels within the antennal lobe. Scale bars: A-F = 20 μm.
Figure 7.
Antennal lobe and antennal nerve projection in the brain of P. spumarius. A) Mid-level section of the antennal lobe showing numerous small, rounded glomeruli (*). B) Antennal backfills staining revealing the antennal nerve (AN) passing through the AMMC towards the tritocerebrum. C, D) Sensory neuros terminating in the antennal lobe (AL) shown via antennal nerve backfills. E, F), Single sensillum backfills indicating sensory neurons arborization at different levels within the antennal lobe. Scale bars: A-F = 20 μm.
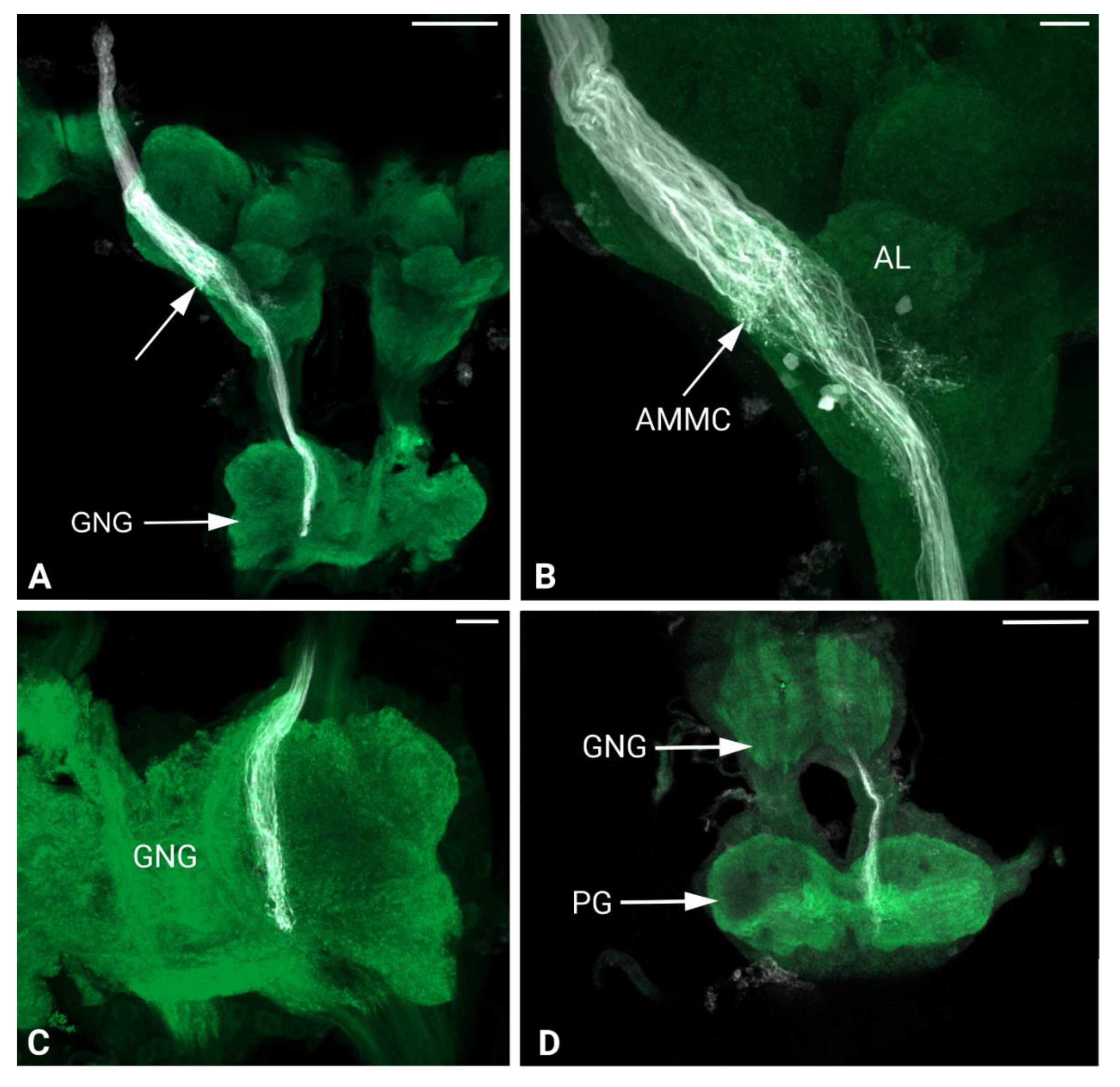
Figure 8.
Maximum intensity projections of the confocal images of Philaenus spumarius brain. A) Overview of antennal nerve projections through the brain. A massive nerve staining is visible at the apical part of the brain, which becomes reduced when arborized at the AMMC level (arrow). Here the nerve project until the gnathal ganglion. B) Johnston’s organ afferents terminating at the AMMC, before continuing downward. C) Johnston’s organ afferents at the gnathal ganglion (GNG) level. D) Johnston’s organ nerve terminating at the prothoracic ganglia (PG). Scale bars: A = 100 μm, B, C = 20 μm, D = 100 μm.
Figure 8.
Maximum intensity projections of the confocal images of Philaenus spumarius brain. A) Overview of antennal nerve projections through the brain. A massive nerve staining is visible at the apical part of the brain, which becomes reduced when arborized at the AMMC level (arrow). Here the nerve project until the gnathal ganglion. B) Johnston’s organ afferents terminating at the AMMC, before continuing downward. C) Johnston’s organ afferents at the gnathal ganglion (GNG) level. D) Johnston’s organ nerve terminating at the prothoracic ganglia (PG). Scale bars: A = 100 μm, B, C = 20 μm, D = 100 μm.