1. Introduction
During the course of the previous several decades, there has been a dramatic revolution in the surgical approach to treating melanoma. Historically, the therapy consisted of vigorous, extensive resections that were accompanied with elective lymph node dissection. These were procedures that were intended to prevent the cancer from spreading to other regions, but they were associated with a significant amount of morbidity. [
1,
2] Over time, the accumulation of information from large-scale randomised research, the improvement of pathologic staging, and the expansion of understanding regarding the biology of tumors have all contributed to the development of treatments that are more conservative but nonetheless as effective.
Since the introduction of sentinel lymph node biopsy (SLNB), staging and risk stratification have been completely transformed. This has made it possible for surgeons to identify patients who might benefit from adjuvant therapy with a much higher degree of accuracy. [
1,
3,
4]" During the same time period, the introduction of immune checkpoint inhibitors and molecular-targeted therapy has brought about a significant shift in the expectations for survival in advanced illness. This shift has resulted in modifications to the timing and scope of surgery, as well as the promotion of multidisciplinary decision-making.
Contemporary melanoma surgery has an emphasis on precision rather than radicality, integrating anatomical, molecular, and immunologic aspects into the treatment process. Within the scope of this study, core ideas are expanded upon, changing practices are critically examined, and the ways in which forthcoming technology and research orientations may transform the area are investigated.
2. Framework
2.1. Excision Margins and Local Management
For patients with localized melanoma, the primary curative therapy that is still used is wide local excision. Margin selection, which was historically defined by Breslow depth, has been modified over several decades of clinical studies, which have shown that larger margins do not offer significant survival advantage, but do increase morbidity such as the requirement for grafts or flaps, wound problems, and functional limits.[
1,
2]
The standard recommendations continue to maintain a margin of 1 centimeter for tumors that are less than 2 millimeters in size.
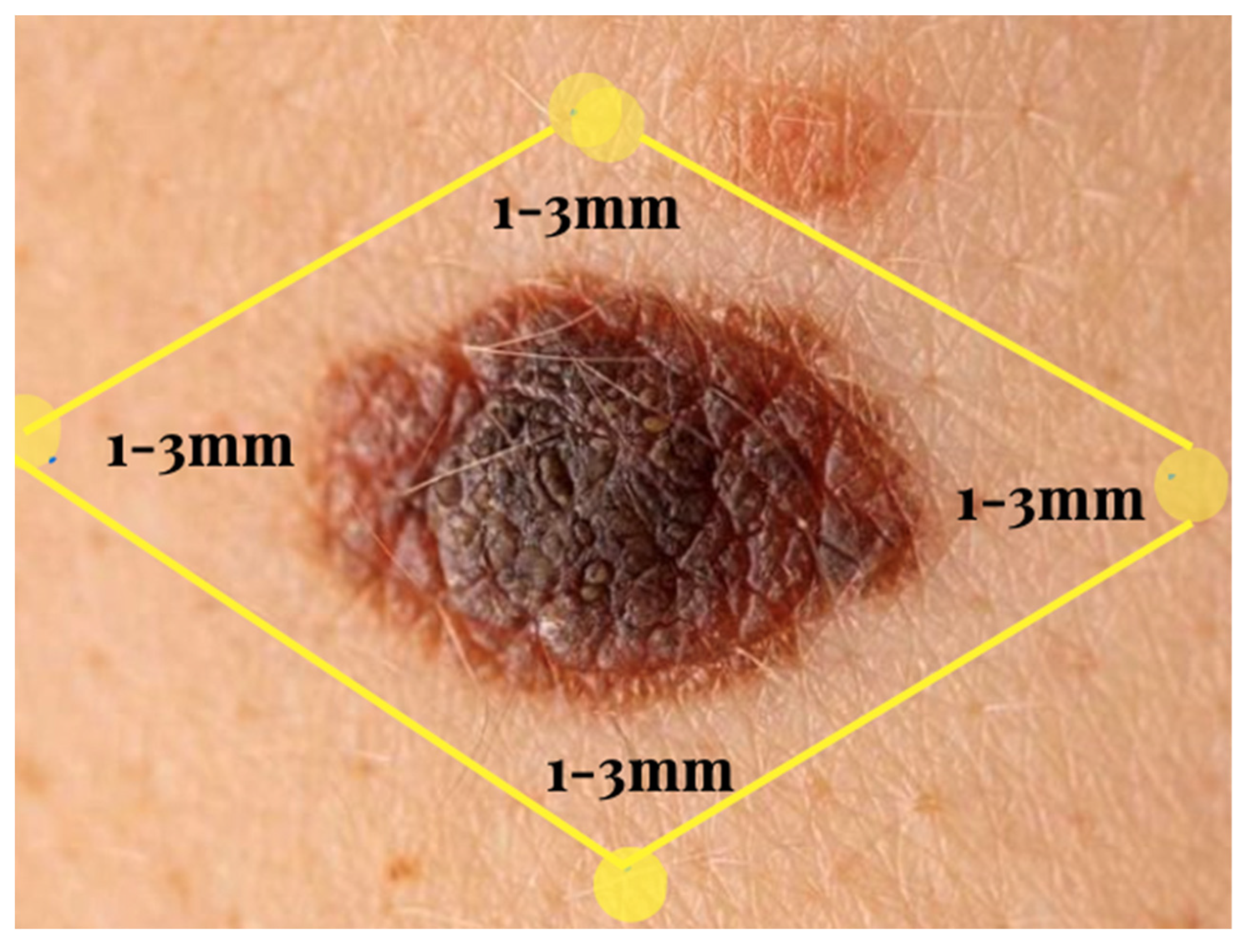
Figure 1.
Excision margins. There is a 2-cm margin for lesions that are at least 2 mm in size.
Figure 1.
Excision margins. There is a 2-cm margin for lesions that are at least 2 mm in size.
In the ongoing MelMarT-II experiment, researchers are investigating whether or not thinner margins of one centimeter might be similarly safe for bigger melanomas. If this is the case, it could possibly provide less morbidity, shorter operational durations, and superior esthetic results..[
2,
3]
Narrower surgical margins (five to ten millimeters) are often sufficient for melanoma in situ, particularly lentigo maligna on cosmetically delicate areas (facial, scalp, ears, and fingers), but the risk of local recurrence varies. Techniques such as Mohs micrographic surgery, phased excision, or margin-controlled procedures continue to be preferred in areas that are either high-risk or anatomically complicated. [
2,
4]
Although Mohs surgery has shown great cure rates in melanoma in situ and lentigo maligna, its value in invasive melanoma is still confined to chosen locations that have specialist immunostaining skills. Mohs surgery enables real-time histologic control, maximizes tissue preservation, and has showed excellent cure rates.
2.2. Sentinel Lymph Node Biopsy and Regional Management:
When it comes to staging intermediate- and high-risk melanomas, the standard of treatment has shifted away from elective lymph node dissection and toward SLNB alone. Not only does it provide essential prognostic information, but it also assists in the identification of candidates for adjuvant immunotherapy or targeted treatment, which has a significant impact on survival outcomes in contemporary clinical practice. [
1,
5] Based on the findings of MSLT-II and DeCOG-SLT, it was determined that doing regular complete lymph node dissection following a positive SLNB does not improve melanoma-specific survival, but it did increase the frequency of lymphedema and sensory impairments. [
1,
2,
3,
4,
5,
6] The use of regular nodal ultrasonography for observation is now considered conventional.
Ongoing research is aimed at refining SLNB indications by the utilization of predictive methods like as nomograms, molecular classifiers, and gene expression profiling. This might ultimately lead to a reduction in the number of needless operations performed on patients with little risk while still preserving accuracy. [
6] When it comes to patients who require therapeutic lymphadenectomy, advances such as rapid lymphatic reconstruction, which includes lymphovenous anastomosis, are demonstrating encouraging outcomes in terms of minimizing postoperative lymphedema and enhancing quality of life. [
4]
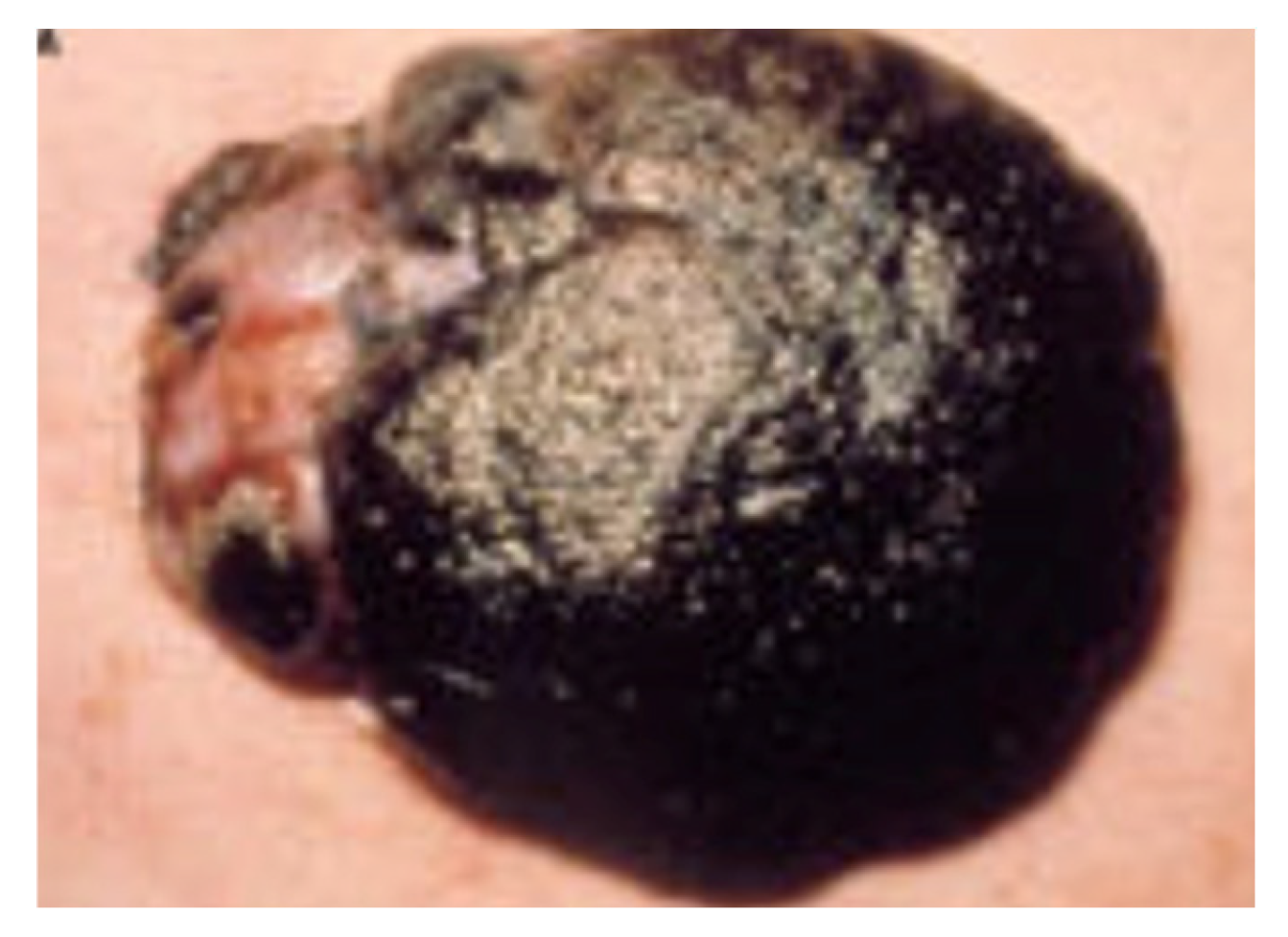
Figure 2.
Macroscopic image of melanoma.
Figure 2.
Macroscopic image of melanoma.
2.3. Integration of Systemic Therapies and Neoadjuvant Approache:
Increasing data suggests that systemic therapy should be administered prior to surgical treatment in cases of locoregionally advanced melanoma. A reduction in the burden of the tumor, the generation of powerful systemic immune responses, and an improvement in long-term outcomes are the goals of neoadjuvant immunotherapy. The SWOG1801 study indicated that patients who received neoadjuvant anti–PD-1 treatment had significantly better event-free survival when compared to individuals who received adjuvant therapy alone. In addition, the PRADO and NADINA studies provide evidence for a response-adapted surgical strategy. This method involves tailoring the scope of surgery to the patient's pathologic or radiologic response to neoadjuvant treatment. [
7,
8]
A reduction in surgical morbidity, the avoidance of needless node dissections, and the personalization of treatment intensity are all potential outcomes of this paradigm change. However, optimizing neoadjuvant regimens, determining the appropriate scheduling of surgery, and identifying biomarkers that predict response are still active areas of research.
2.4. Reconstructive and Functional Considerations
In the case of large excisions, particularly those performed on the extremities or the head and neck, sophisticated reconstruction may be required. As reconstructive techniques become more prevalent, function, cosmesis, and lymphatic preservation are becoming increasingly important. There are a variety of trunk and limb abnormalities that may be treated using keystone, V-Y, and perforator-based flaps. These flaps provide long-lasting covering with little morbidity. [
4] Digit-sparing surgery has gained favor in the treatment of sub-ungual melanoma in certain individuals who are in the early stages of the illness and have minimal bone involvement. This presents a challenge to the conventional procedures of regular amputation. [
2,
3,
4] Obtaining simultaneous on-cologic clearance and achieving optimal cosmetic results is made possible by collaboration with reconstructive specialists. The move toward functional rehabilitation and long-term survivability is reflected in the development of new procedures such as lymphatic mapping and preservation, supermicrosurgery, and physiologic reconstruction schemes.
2.5. Management of In-Transit and Locoregional Metastases
Metastases that are in transit create a barrier for the treatment process. Isolated limb infusion, intralesional treatments (such as T-VEC), electrochemotherapy, and ablative laser procedures are examples of minimally invasive modalities that provide localized control while avoiding significant surgical procedures..[
4,
6]
These methods are in accordance with contemporary ideas of de-escalation, which suggest that substantial resections should be reserved exclusively for instances that are either symptomatic or treatment-resistant.
3. Discussion
3.1. Evolving Role of Surgery in the Immunotherapy Era
The natural history of metastatic melanoma has been significantly altered as a result of the introduction of immune checkpoint inhibitors (ICIs), which include anti-PD-1 and anti-CTLA-4 treatments, as well as BRAF/MEK inhibitors for molecularly targeted therapy. [
5,
10] They have brought forth novel intersections between surgical and systemic treatment as a result of their debut. In recent years, surgical procedures are increasingly being seen not as a standalone curative modality but rather as a component of an integrated therapeutic continuum. individuals who experience substantial systemic responses with immunotherapy may be able to avoid the necessity for major surgery. On the other hand, surgery may be deliberately utilized for the purpose of oligoprogression, local control, or consolidation in certain individuals.
For the purpose of maximizing future surgical decision-making, it will be essential to have a solid understanding of immune response patterns, resistance mechanisms, and the behavior of residual illness.
3.2. Precision Surgery and Technological Advances
The accuracy of intraoperative procedures has been significantly improved by technological advancements. The delineation of lymphatic drainage patterns, the improvement of sentinel node identification, and the enhancement of margin visualization are all made possible with the use of tools such as near-infrared fluorescence imaging, targeted radiotracers, and molecular probes. Because of their enhanced dexterity and visualization capabilities, robotic-assisted procedures are becoming increasingly popular for the purpose of performing lymphadenectomy in anatomically problematic locations.[
8,
9]
Furthermore, the integration of digital pathology, artificial intelligence-driven margin evaluation, and high-resolution imaging gives the possibility of real-time intraoperative decision assistance, which has the potential to reduce the number of re-excisions being performed and improve long-term results.
3.3. Reconstruction and Quality of Life
Outcomes that are centered on the patient are receiving a larger amount of attention as surgical procedures grow more conservative. There has been a movement toward survivor-ship-oriented treatment, which is seen in the inclusion of oncoplastic concepts, scar optimization, psychological support, and patient education.
Increasingly, quality-of-life indicators are being integrated into clinical studies, and it is anticipated that they will play a role in the development of future guidelines.
3.4. Global Disparities and Access to Care
Despite progress, discrepancies continue to exist on a worldwide scale. Countries with low and intermediate incomes have a number of obstacles, some of which include delayed diagnosis, restricted access to SLNB or immunotherapy, and an inadequate infrastructure for multidisciplinary care.
Efforts such as teledermatology, telepathology, regional training programs, and international partnerships continue to be essential in order to reduce disparities and improve the outcomes of melanoma on a worldwide scale.
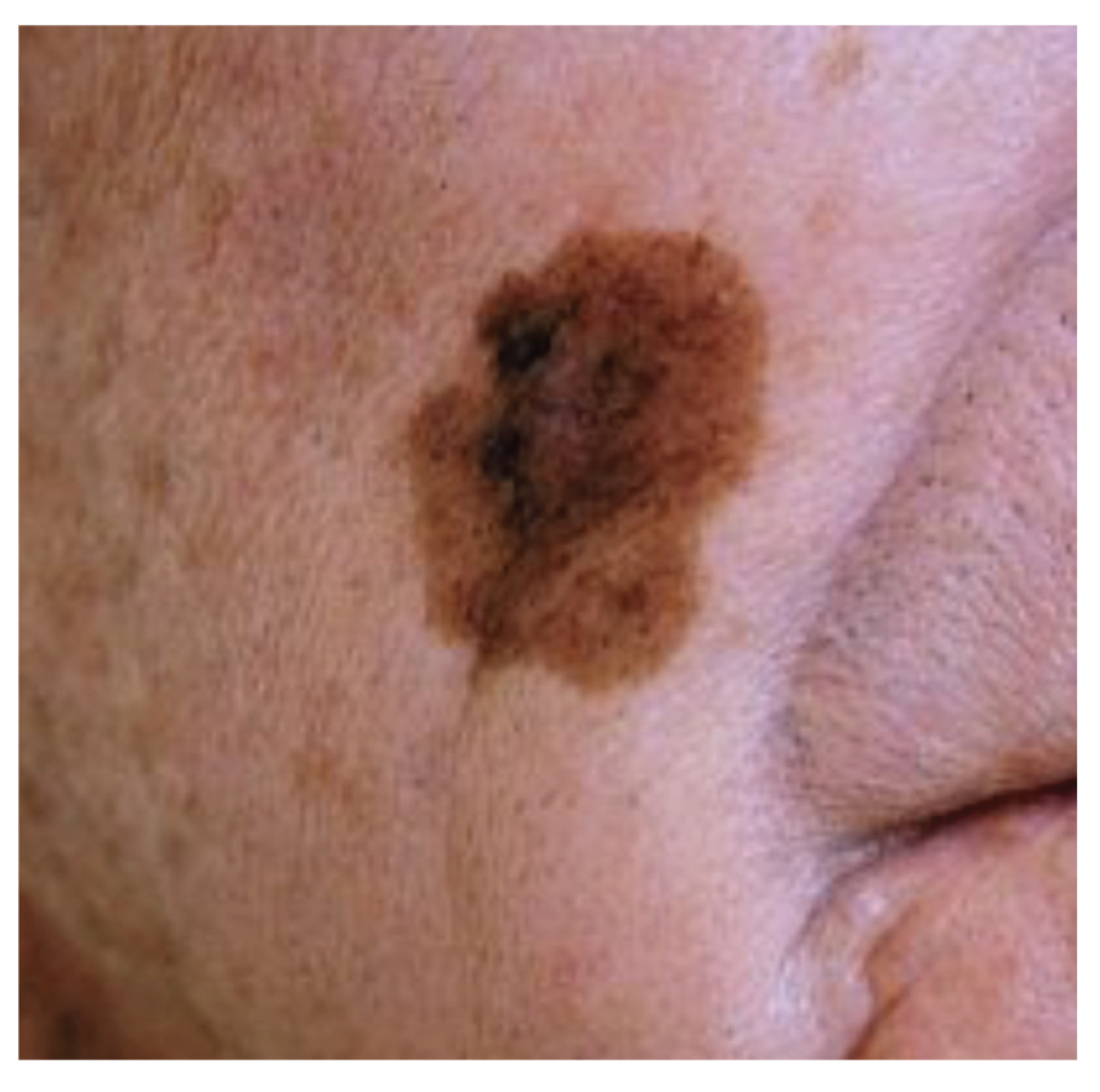
Figure 3.
Lentigo maligna.
Figure 3.
Lentigo maligna.
3.5. Future Directions
It is anticipated that molecular profiling, radiogenomics, and immunological markers would be included into future cases of melanoma surgery in order to facilitate real-time therapy adaption. The development of new technologies, such as circulating tumor DNA (ctDNA), has the potential to enhance monitoring, reduce the importance of surgical interventions, and ultimately move melanoma toward a surgical modality that is guided by biological factors..[
9,
10]
As the field of molecular diagnostics continues to advance, it is possible that surgical staging procedures will become less involved or possibly eliminated entirely.
4. Conclusions
Within the realm of melanoma care, surgery continues to be an indispensable and indispensable component. On the other hand, rapidly advancing technology in immunotherapy, molecular diagnostics, and high-precision technologies have completely altered the paradigm of surgical procedures. Currently, the technique has an emphasis on individualised, less intrusive procedures that incorporate systemic treatment, improve both functional and cosmetic results, and prioritize long-term survivability.
Melanoma surgery in the future will rely more and more on tailored oncologic methods that are informed by molecular, radiologic, and immunological indicators. This will result in a change in emphasis away from drastic excision and toward biologically guided intervention. Surgeons will continue to play a major role within multidisciplinary teams, adjusting to an environment in which accuracy, integration, and results that are centered on the patient characterize optimum practice.
Author Contributions
Conceptualization, J.M.Z.T. and V.C.O.; methodology, J.M.Z.T.; software, not applicable; validation, J.M.Z.T. and V.C.O.; formal analysis, J.M.Z.T.; investigation, J.M.Z.T.; resources, V.C.O.; data curation, J.M.Z.T.; writing—original draft preparation, J.M.Z.T.; writing—review and editing, J.M.Z.T. and V.C.O.; visualization, J.M.Z.T.; supervision, V.C.O.; project administration, J.M.Z.T.; funding acquisition, not applicable. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding
Institutional Review Board Statement
Not applicable
Informed Consent Statement
Not applicable
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Curti BD, Faries MB. Recent Advances in the Treatment of Melanoma. N Engl J Med. 2021;384(23):2229–2240.
- Dixon AJ, Sladden M, Zouboulis CC, et al. Primary Cutaneous Melanoma—Management in 2024. J Clin Med. 2024;13(6):1607.
- Long GV, Swetter SM, Menzies AM, Gershenwald JE, Scolyer RA. Cutaneous Melanoma. Lancet. 2023;402(10400):485–502.
- Faries MB, Thompson JF, Cochran AJ, et al. Completion Dissection or Observation for Sentinel-Node Metastasis in Melanoma. N Engl J Med. 2017;376(23):2211–2222.
- Binks M, van Akkooi ACJ, Menzies AM, Spillane A. Cutaneous Melanoma Management—An Update and Narrative Review for the General Surgeon. ANZ J Surg. 2025.
- Santamaria-Barria JA, Mammen JMV. Surgical Management of Melanoma: Advances and Updates. Curr Oncol Rep. 2022;24(11):1425–1432.
- Siller A, DaCunha M, Coldiron BM. The Surgical Management of Cutaneous Melanoma. Dermatol Clin. 2025;43(3):461–471.
- Temple-Oberle C, Nicholas C, Rojas-Garcia P. Current Controversies in Melanoma Treatment. Plast Reconstr Surg. 2023;151(3):495e–505e.
- Sondak VK, Neves RI, Wuthrick EJ, Messina JL, Khushalani NI. Current and Future Approaches in the Surgical Management of T3b/T4 Primary and Locoregionally Advanced Melanoma. Cancer. 2022;128(21):3764–3771.
- Novis E, van Akkooi ACJ. Management of Localized Melanoma in the Anti–PD-1 Era. Curr Oncol Rep. 2024;26(8):924–933.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).