Submitted:
15 November 2025
Posted:
18 November 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
- Identified trends and gaps in completed trials, including patterns in evidence dissemination and translation into clinical practice.
- Assessed the contributions of 3CTN-supported trials to peer-reviewed publications and clinical treatment guidelines.
2. Materials and Methods
3. Results
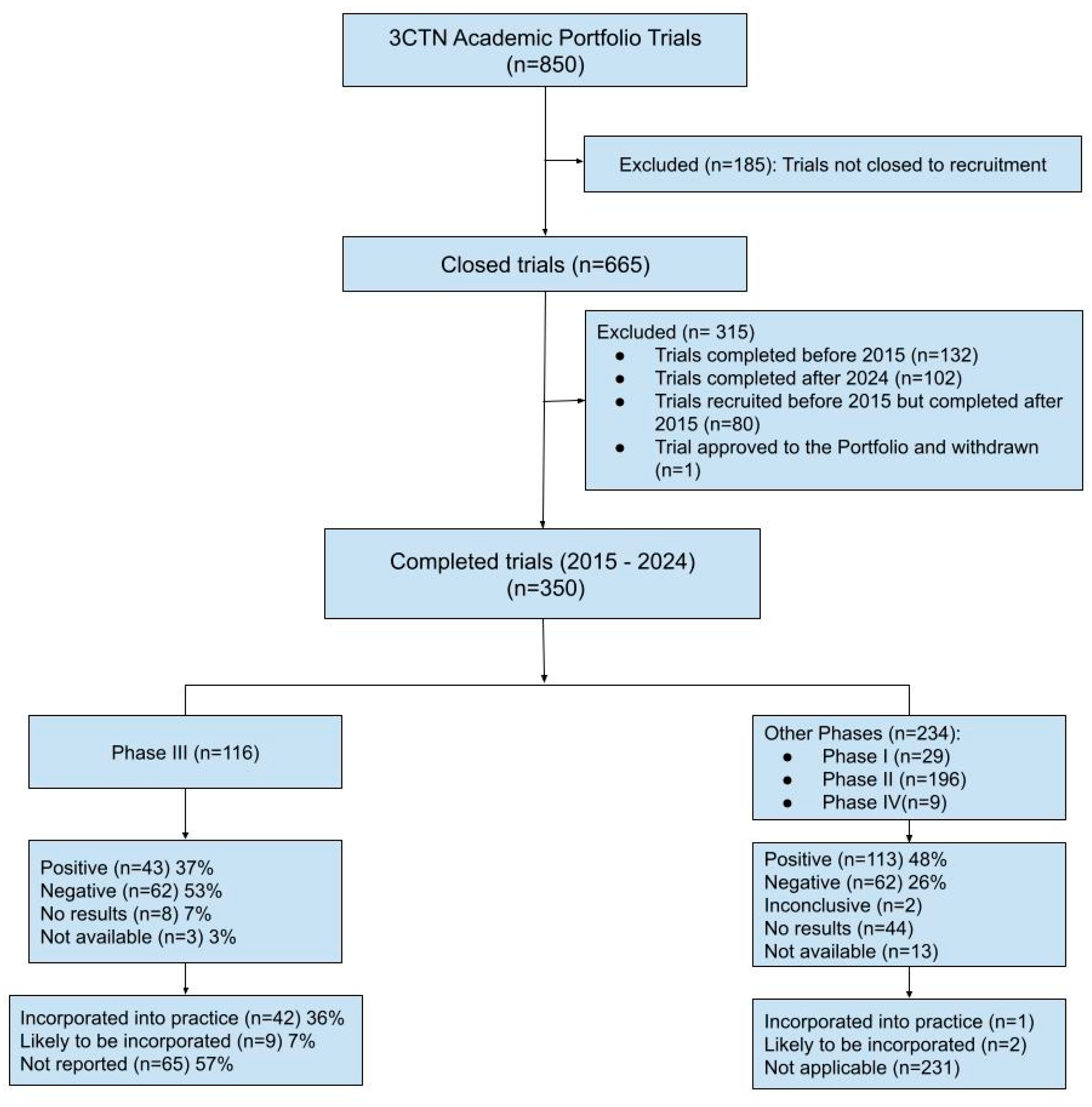
3.1. Trial Characteristics
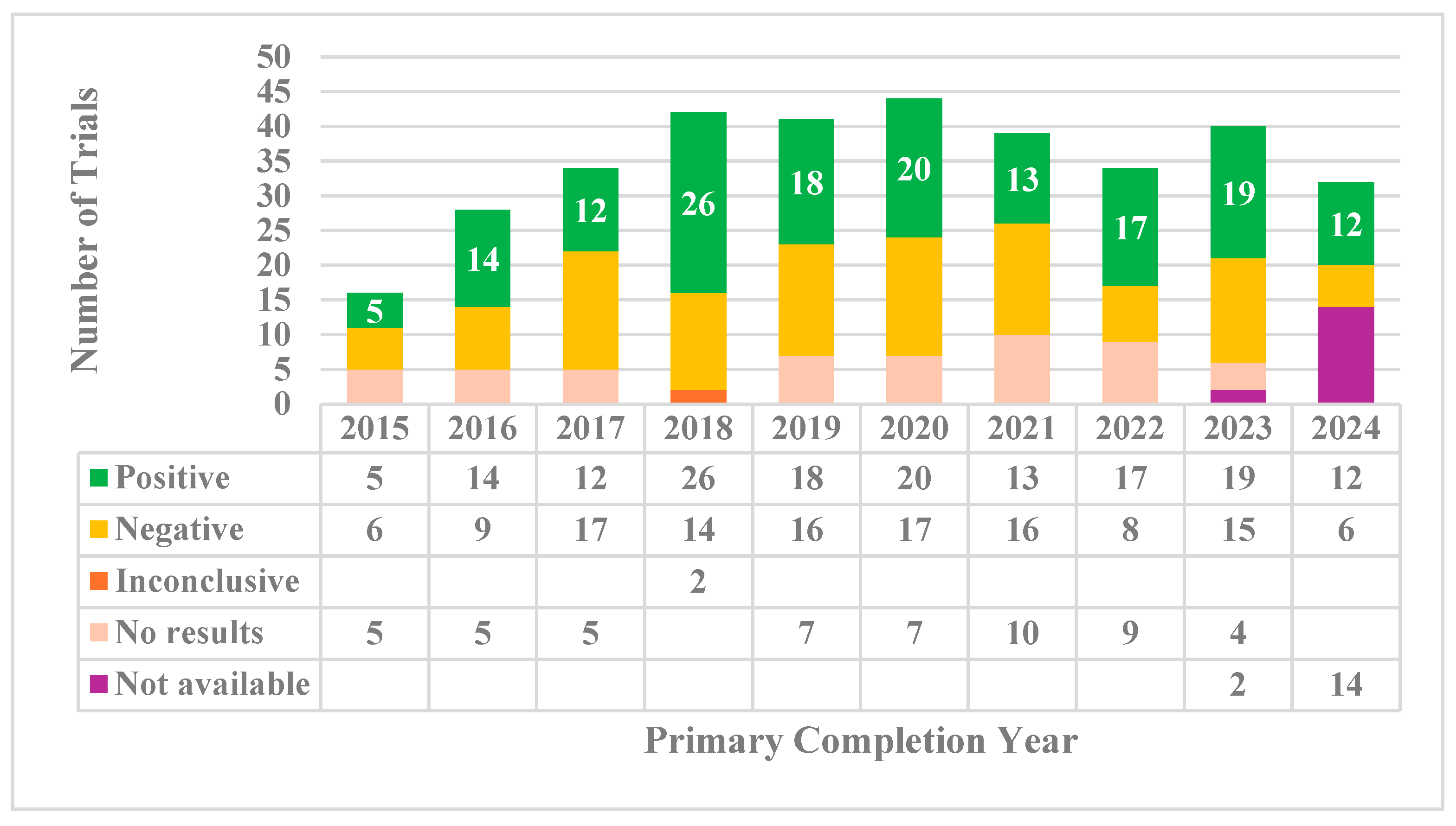
3.2. Trial Outcomes
3.2.1. Reporting and Publication Rates
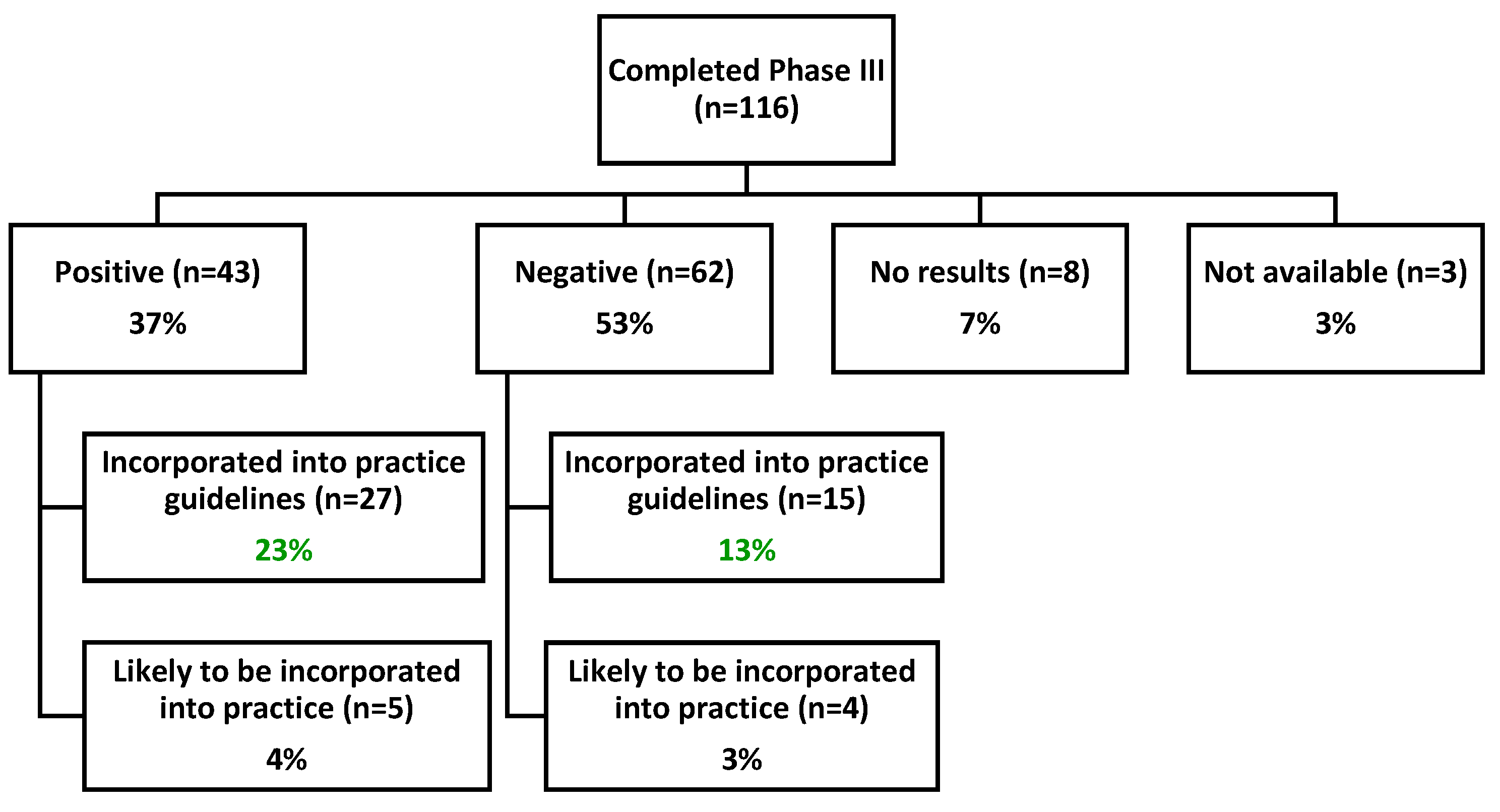
3.2.2. Practice Guideline Incorporation
3.2.3. Sponsorship and Practice-Changing Impact
3.3. Recruitment Contributions from Network Sites
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 3CTN | Canadian Cancer Clinical Trials Network |
| ACCT | Academic Cancer Clinical Trials |
| ALL | Acute Lymphoblastic Leukemia |
| ASCO | American Society of Clinical Oncology |
| ASTRO | American Society for Radiation Oncology |
| CCO | Cancer Care Ontario |
| CCTG | Canadian Cancer Trials Group |
| CNS | Central Nervous System |
| COG | Children’s Oncology Group |
| COVID-19 | Coronavirus Disease 2019 |
| CMA | Canadian Medical Association |
| DSMB | Data Safety Monitoring Board |
| EANM | European Association of Nuclear Medicine |
| ESMO | European Society for Medical Oncology |
| GU | Genitourinary |
| ML-DS | Myeloid Leukemia of Down syndrome |
| NCCN | National Comprehensive Cancer Network |
| OICR | Ontario Institute for Cancer Research |
| SIOPE | European Society for Paediatric Oncology |
| SNMMI | Society of Nuclear Medicine and Molecular Imaging |
Appendix A
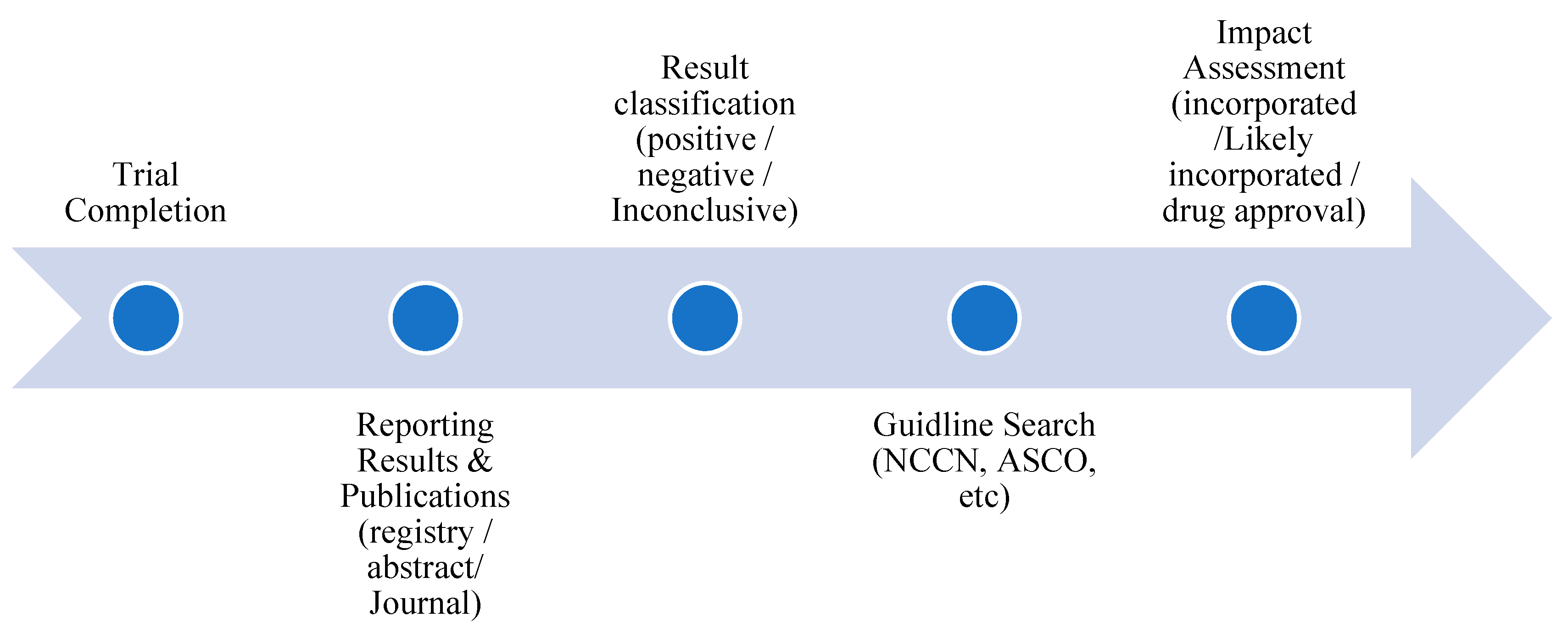
Appendix A.1 Definitions and Flow Diagram of Impact Tracking Process for Practice Change Assessment
- For phase III trials, a positive primary outcome was defined as a statistically significant (p < 0.05, or per the study’s pre specified threshold) and favourable effect for the experimental treatment compared with a placebo or active comparator.
- For earlier phase trials, a positive primary outcome was defined as sufficient efficacy/safety evidence to justify progression to a later phase trial, as stated by the authors.
- For phase III trials, a negative primary outcome was defined as an effect that is not statistically significant or is statistically significant but favours the control arm.
- For earlier phase trials, a negative primary outcome was defined as insufficient evidence to justify progression to a later phase trial.
- Not available: No peer reviewed results found, and the primary study completion date is < 24 months ago.
- No results: No peer reviewed results found, and the primary study completion date is ≥ 24 months ago.
- Pending final publication: No final, peer reviewed results for the entire study population, but interim or subgroup results are available, or a peer reviewed source explicitly states that results are pending or expected.
Appendix A2. Oncology Guidelines Reviewed for Practice Change Assessment
-
National Comprehensive Cancer Network (NCCN)
- ⚬
- via both the JNCCN journal (https://jnccn.org )
- ⚬
- and the official NCCN guideline PDFs (https://www.nccn.org/professionals/physician_gls ).
-
American Society of Clinical Oncology (ASCO)
- ⚬
- ⚬
- and ASCO Publications (https://ascopubs.org ) for joint or collaborative guidelines updates.
- American Society for Radiation Oncology (ASTRO) Guidelines
- European Society for Paediatric Oncology (SIOP Europe or SIOPE)
- International Childhood Liver Tumors Strategy Group – for pediatric liver cancer
- Cancer Care Ontario (CCO)
- American Urological Association Guidelines
- European Association of Urology – for prostate and bladder cancer.
- European Society for Radiotherapy and Oncology
- European Society for Medical Oncology (ESMO) – for European oncology practice
- European Association of Neuro-Oncology – for neuro-oncology
- Canadian Medical Association (CMA)
- Trip Database- https://www.tripdatabase.com – aggregates global guidelines.
- EANM/SNMMI Joint Guidelines for trials involving diagnostic imaging. These guidelines are published by the European Association of Nuclear Medicine and the Society of Nuclear Medicine and Molecular Imaging.
- The American Society for Transplantation and Cellular Therapy Practice Guidelines
- Society of American Gastrointestinal and Endoscopic Surgeons - GI Surgery for all GI Surgeons
| Reasons Cited for Termination | Phase I | Phase II | Phase III | Phase IV |
Grand Total |
| Drug company decision | 2 | 1 | 3 | ||
| DSMB review | 1 | 1 | |||
| Lack of funding | 1 | 1 | |||
| Negative study | 2 | 2 | |||
| Poor accrual | 3 | 14 | 1 | 1 | 19 |
| Staffing issues | 1 | 1 | |||
| Unacceptable Toxicity | 1 | 1 | |||
| Unknown | 2 | 2 | |||
| COVID-19 pandemic | 1 | 1 | |||
| Total | 3 | 22 | 4 | 2 | 31 |
| Trial Characteristics | Analysis | Phase III | Overall |
| Number of trials | N | 116 | 350 |
| Study Phase | I II III IV |
- - 116 - |
29(8%) 196(56%) 116(33%) 9(3%) |
| Disease Site | Bone Brain/CNS Breast Gastrointestinal Genito-Urinary Gynecological Head and neck Hematology Lung Neuroblastoma Other Sarcoma Skin / Melanoma |
1(1%) 8(7%) 16(14%) 11(9%) 17(15%) 12(10%) 3(3%) 23(20%) 10(9%) 1(1%) 9(8%) 4(3%) 1(1%) |
5(1%) 19(5%) 49(14%) 32(9%) 56(16%) 25(7%) 11(3%) 55(16%) 32(9%) 7(2%) 43(12%) 9(3%) 7(2%) |
| Country of Sponsor | Canada United States Other |
33(28%) 71(61%) 13(11%) |
192(55%) 142(41%) 16(5%) |
| Sponsor | NCI(USA) CCTG COG |
67(58%) 39 (33%) 21 (6%) |
123 (35%) 87 (25%) 41(12%) |
| Special Interest | Lifestyle Interventions Novel therapy Rare cancer setting Vulnerable populations Precision medicine |
4(3%) 9(8%) 39(33%) 26(22%) 80(68%) |
10(3%) 69(20%) 109(31%) 71(20%) 80(23%) |
| Interventions | Behavioral Drug Device Radiation Procedure Biological |
3(3%) 75(64%) 3(3%) 39(33%) 0(0%) 24(21%) |
13(4%) 232(66%) 8(2%) 82(23%) 58(17%) 51(15%) |
| Type of Design | Basket Trial Platform Trial Umbrella Trial Low complexity method Multiple steps |
0101 13 |
2 5 1 5 22 |
| Completion status | Closed to recruitment Completed Prematurely completed (terminated or withdrawn) |
60(52%) 52(45%) 4(3%) |
129(37%) 190(54%) 31(9%) |
| Trial | Practice-defining trial outcome | Disease Site | Active recruitment | NCT Number | Recruitment Contribution (%) | Publication | Practice Guidelines Changed |
| NRG-CC001 |
Recommended HA-WBRT plus memantine to reduce neurocognitive decline in patients with brain metastases. | Brain Metastases | 2016 - 2018 | NCT02360215 |
8.9% (46/518) |
[18] | NCCN [19] |
| (CCTG) MA.36 / Olympia |
Adjuvant Olaparib recommended for HER2-negative, BRCA-mutated early breast cancer with residual disease after neoadjuvant chemotherapy, based on Olympia trial results. FDA approves Olaparib for adjuvant treatment of high-risk early breast cancer. |
Breast | 2015 - 2019 | NCT02032823 | 1.9% (35/1837) |
[20] |
NCCN [21] |
| OCOG-2016-PETABC |
PETABC trial supported guideline recommendation for using 18F-FDG PET/CT in staging stage IIB–III breast cancer, showing improved detection of stage IV disease and influencing treatment decisions | Breast | 2016 -2022 | NCT02751710 | 100% (369/369) |
[22] |
EJNMMI [23] |
| (CCTG) MA.37 / PALLAS |
PALLAS trial showed no benefit of adjuvant palbociclib, leading to guideline recommendations against its use in early breast cancer. | Breast | 2017-2025 | NCT02513394 | 2.6% (152/5796) |
[24] | NCCN [25] |
| (EORTC) 1333-GUCG/PEACE III |
Combining radium-223 with enzalutamide for mCRPC showed improved progression-free survival and potential overall survival benefit. | GU/Prostate | 2018 - 2023 | NCT02194842 | 4.5% (20/446) |
[26] | NCCN [27] |
| GOG – 0275 |
For low-risk gestational trophoblastic neoplasia, supporting methotrexate and actinomycin-D are effective first-line single-agent therapies. |
Gyne/Gestational Trophoblastic | 2015 - 2017 | NCT01535053 | 3.5% (2/57) | [28] | NCCN [29] |
| (CCTG) ENC.1 / NRG-GY018 / MK-3475-868 | Pembrolizumab plus chemotherapy as a new standard for advanced or recurrent endometrial cancer, regardless of mismatch repair status; led to FDA approval and guideline inclusion. | Gyne/Endometrial | 2021 - 2022 | NCT03914612 | 3.7% (30/813) |
[30] | NCCN [31] |
| (CCTG) CLC.2 / Alliance A041202 |
Ibrutinib was superior to Bendamustine–rituximab for older patients with untreated CLL, supporting guideline recommendations and FDA-approved frontline use. | Chronic Lymphocytic Leukemia (CLL) | 2015 – 2016 | NCT01886872 |
7.9% (43/547) |
[32] | NCCN [33] |
| (COG) AHOD1331 |
Use of brentuximab Vedotin with AVE-PC for high-risk pediatric Hodgkin lymphoma showed superior efficacy and reduced need for radiation. | Hodgkin lymphoma | 2015 - 2019 | NCT02166463 |
6.5% (39/600) |
[34] | NCCN [35] |
| (COG) AALL1331 |
For relapsed pediatric B-ALL, Blinatumomab was as a treatment option despite early trial termination and no significant difference in disease-free survival. | B-ALL |
2015 - 2019 | NCT02101853 | 7.9% (53/669) | [36] | NCCN [37] |
| (COG) AAML1531 |
For ML-DS, supporting risk-based treatment and use of HD-AraC to improve outcomes in standard-risk patients. | ML-DS | 2016 - 2022 | NCT02521493 |
5.4% (15/280) | [38] | SIOP Europe [39] |
| (CCTG) ALC.4 (ECOG E1910) |
Adding Blinatumomab to consolidation chemotherapy for newly diagnosed B-lineage ALL, improving overall survival and establishing a new standard for BCR::ABL1-negative patients; | ALL | 2017 - 2019 | NCT02003222 | 1.8% (9/488) |
[40] | NCCN [41] |
| (CCTG) HDC.1/SWOG S1826 |
Nivolumab + AVD as first-line treatment for advanced-stage Hodgkin lymphoma, showing better progression-free survival than BV + AVD; now a Category 1 recommendation in NCCN guidelines. | Hodgkin Lymphoma | 2021 - 2022 | NCT03907488 | 1.8% (18/994) | [42] | NCCN [43] |
| (EORTC) STRASS |
No overall benefit of preoperative radiotherapy for retroperitoneal sarcoma, but supported selective use in Liposarcoma. | Sarcoma | 2015 – 2017 | NCT01344018 |
4.5% (12/266) |
[44] | NCCN [45] |
| (CCTG) SRC.7 / Alliance A091105 |
Sorafenib significantly improved progression-free survival in desmoid tumors; now recommended in NCCN guidelines as a systemic therapy option. | Sarcoma | 2015 – 2016 | NCT02066181 | 5.7% (5/87) |
[46] | NCCN [45] |
| (CCTG) SC.24 |
SC.24 trial showed stereotactic body radiotherapy improved pain control over conventional radiotherapy for spinal metastases; cited in Ontario guidelines for spine SBRT planning and delivery. | Spinal Metastases | 2015 – 2019 | NCT02512965 | 76.4% (175/229) | [47] | CCO [48] |
References
- Medicine, I.o., A National Cancer Clinical Trials System for the 21st Century: Reinvigorating the NCI Cooperative Group Program, ed. S.J. Nass, H.L. Moses, and J. Mendelsohn. 2010, Washington, DC: The National Academies Press. 316.
- Canadian Cancer Research Alliance, Report on the State of Cancer Clinical Trials in Canada. 2011: Toronto.
- Schilsky, R.L., Publicly Funded Clinical Trials and the Future of Cancer Care. The Oncologist, 2013. 18(2): p. 232-238.
- Unger, J.M., et al., Association of National Cancer Institute–Sponsored Clinical Trial Network Group Studies With Guideline Care and New Drug Indications. JAMA Network Open, 2019. 2(9): p. e1910593-e1910593.
- Tang, P.A.P., Joe; Thiessen, Maclean Harvey; Lee-Ying, Richard M; Monzon, Jose Gerard; Cheung, Winson Y., Impact of Canadian Cancer Trials Group (CCTG) phase III trials (P3Ts), in 2018 ASCO Annual Meeting. 2018.
- Bennette, C.S., et al., Predicting Low Accrual in the National Cancer Institute’s Cooperative Group Clinical Trials. JNCI: Journal of the National Cancer Institute, 2015. 108(2).
- Hauck, C.L., et al., Trial-level factors affecting accrual and completion of oncology clinical trials: A systematic review. Contemp Clin Trials Commun, 2021. 24: p. 100843.
- Seruga, B., et al., Barriers and challenges to global clinical cancer research. Oncologist, 2014. 19(1): p. 61-7.
- Bentley, C., et al., Barriers to conducting cancer trials in Canada: an analysis of key informant interviews. Curr Oncol, 2020. 27(3): p. e307-e312.
- Smalheiser, N.R. and A.W. Holt, A web-based tool for automatically linking clinical trials to their publications. J Am Med Inform Assoc, 2022. 29(5): p. 822-830.
- Schoales, J., et al., A novel and comprehensive framework for categorizing and evaluating the potential impact of academic cancer clinical trials [Poster Presentation], in 2019 Canadian Cancer Research Conference. 2019: Ottawa, Ontario, Canada.
- Elimova, E., et al., Updating Reports of Phase 3 Clinical Trials for Cancer. JAMA Oncology, 2021. 7(4): p. 593-596.
- Canadian Cancer Clinical Trials Network. Outcomes and Publication Search. 2025 October 27, 2025]; Available from: https://3ctn.ca/outcomes-and-publication-search/.
- Dancey, J., Canada needs a national system for cancer clinical trials, in Toronto Star. 2024.
- Joober, R., et al., Publication bias: what are the challenges and can they be overcome? J Psychiatry Neurosci, 2012. 37(3): p. 149-52.
- Dickersin, K. and I. Chalmers, Recognizing, investigating and dealing with incomplete and biased reporting of clinical research: from Francis Bacon to the WHO. J R Soc Med, 2011. 104(12): p. 532-8.
- Allegra, C.J., P.J. Goodwin, and P.A. Ganz, Can We Find the Positive in Negative Clinical Trials? JNCI: Journal of the National Cancer Institute, 2019. 111(7): p. 637-638.
- Brown, P.D., et al., Hippocampal Avoidance During Whole-Brain Radiotherapy Plus Memantine for Patients With Brain Metastases: Phase III Trial NRG Oncology CC001. J Clin Oncol, 2020. 38(10): p. 1019-1029.
- Nabors, L.B., et al., Central Nervous System Cancers, Version 3.2020, NCCN Clinical Practice Guidelines in Oncology. Journal of the National Comprehensive Cancer Network J Natl Compr Canc Netw, 2020. 18(11): p. 1537-1570.
- Tutt, A.N.J., et al., Adjuvant Olaparib for Patients with BRCA1- or BRCA2-Mutated Breast Cancer. N Engl J Med, 2021. 384(25): p. 2394-2405.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) Breast Cancer. 2025 [cited 2025 July 31]; Version 1.2024:[Available from: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf.
- Dayes, I.S., et al., Impact of 18F-Labeled Fluorodeoxyglucose Positron Emission Tomography-Computed Tomography Versus Conventional Staging in Patients With Locally Advanced Breast Cancer. Journal of Clinical Oncology, 2023. 41(23): p. 3909-3916.
- Vaz, S.C., et al., Joint EANM-SNMMI guideline on the role of 2-[18F]FDG PET/CT in no special type breast cancer. European Journal of Nuclear Medicine and Molecular Imaging, 2024. 51(9): p. 2706-2732.
- Gnant, M., et al., Adjuvant Palbociclib for Early Breast Cancer: The PALLAS Trial Results (ABCSG-42/AFT-05/BIG-14-03). Journal of Clinical Oncology, 2022. 40(3): p. 282-293.
- Giordano, S.H., A.D. Elias, and W.J. Gradishar, NCCN Guidelines Updates: Breast Cancer. Journal of the National Comprehensive Cancer Network J Natl Compr Canc Netw, 2018. 16(5S): p. 605-610.
- Gillessen, S., et al., LBA1 A randomized multicenter open label phase III trial comparing enzalutamide vs a combination of Radium-223 (Ra223) and enzalutamide in asymptomatic or mildly symptomatic patients with bone metastatic castration-resistant prostate cancer (mCRPC): First results of EORTC-GUCG 1333/PEACE-3. Annals of Oncology, 2024. 35: p. S1254.
- Schaeffer, E.M., et al., Prostate Cancer, Version 4.2023, NCCN Clinical Practice Guidelines in Oncology. Journal of the National Comprehensive Cancer Network, 2023. 21(10): p. 1067-1096.
- Schink, J.C., et al., An international randomized phase III trial of pulse actinomycin-D versus multi-day methotrexate for the treatment of low risk gestational trophoblastic neoplasia; NRG/GOG 275. Gynecol Oncol, 2020. 158(2): p. 354-360.
- Abu-Rustum, N.R., et al., Gestational Trophoblastic Neoplasia, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. Journal of the National Comprehensive Cancer Network J Natl Compr Canc Netw, 2019. 17(11): p. 1374-1391.
- Eskander, R.N., et al., Pembrolizumab plus Chemotherapy in Advanced Endometrial Cancer. New England Journal of Medicine, 2023. 388(23): p. 2159-2170.
- Abu-Rustum, N.R., et al., NCCN Guidelines® Insights: Uterine Neoplasms, Version 3.2025: Featured Updates to the NCCN Guidelines®. Journal of the National Comprehensive Cancer Network, 2025. 23(8): p. 284-291.
- Woyach, J.A., et al., Ibrutinib Regimens versus Chemoimmunotherapy in Older Patients with Untreated CLL. New England Journal of Medicine, 2018. 379(26): p. 2517-2528.
- Wierda, W.G., et al., Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma, Version 2.2024, NCCN Clinical Practice Guidelines in Oncology. Journal of the National Comprehensive Cancer Network, 2024. 22(3): p. 175-204.
- Castellino, S.M., et al., Brentuximab Vedotin with Chemotherapy in Pediatric High-Risk Hodgkin’s Lymphoma. New England Journal of Medicine, 2022. 387(18): p. 1649-1660.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) Pediatric Hodgkin Lymphoma. 2025 June 9, 2025 [cited 2025 July 31]; Version 2.2025:[Available from: https://www.nccn.org/professionals/physician_gls/pdf/ped_hodgkin.pdf.
- Brown, P.A., et al., Effect of Postreinduction Therapy Consolidation With Blinatumomab vs Chemotherapy on Disease-Free Survival in Children, Adolescents, and Young Adults With First Relapse of B-Cell Acute Lymphoblastic Leukemia: A Randomized Clinical Trial. JAMA, 2021. 325(9): p. 833-842.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) Pediatric Acute Lymphoblastic Leukemia. 2025 August 11, 2025 [cited 2025 August 15]; Version 1.2026:[Available from: https://www.nccn.org/professionals/physician_gls/pdf/ped_all.pdf.
- Hitzler, J., et al., High-dose AraC is essential for the treatment of ML-DS independent of postinduction MRD: results of the COG AAML1531 trial. Blood, 2021. 138(23): p. 2337-2346.
- Childhood Liver Tumors Strategy Group (SIOPEL). Standard Clinical Practice Recommendations for Acute Myeloid Leukemia (AML) in Children and Adolescents. 2020 [cited 2025 July 31]; Available from: https://siope.eu/media/documents/acute-myeloid-leukemia.pdf.
- Litzow, M.R., et al., Consolidation Therapy with Blinatumomab Improves Overall Survival in Newly Diagnosed Adult Patients with B-Lineage Acute Lymphoblastic Leukemia in Measurable Residual Disease Negative Remission: Results from the ECOG-ACRIN E1910 Randomized Phase III National Cooperative Clinical Trials Network Trial. Blood, 2022. 140(Supplement 2): p. LBA-1-LBA-1.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) Acute Lymphoblastic Leukemia. 2025 June 27, 2025 [cited 2025 July 31]; Version 2.2025:[Available from: https://www.nccn.org/professionals/physician_gls/pdf/all.pdf.
- Herrera, A.F., et al., Nivolumab+AVD in Advanced-Stage Classic Hodgkin’s Lymphoma. New England Journal of Medicine, 2024. 391(15): p. 1379-1389.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) Hodgkin Lymphoma. 2025 January 30, 2025 [cited 2025 July 31]; Version 2.2025:[Available from: https://www.nccn.org/professionals/physician_gls/pdf/hodgkins.pdf.
- Bonvalot, S., et al., STRASS (EORTC 62092): A phase III randomized study of preoperative radiotherapy plus surgery versus surgery alone for patients with retroperitoneal sarcoma. Journal of Clinical Oncology, 2019. 37(15_suppl): p. 11001-11001.
- von Mehren, M., et al., Soft Tissue Sarcoma, Version 2.2018, NCCN Clinical Practice Guidelines in Oncology. Journal of the National Comprehensive Cancer Network J Natl Compr Canc Netw, 2018. 16(5): p. 536-563.
- Gounder, M.M., et al., Sorafenib for Advanced and Refractory Desmoid Tumors. New England Journal of Medicine, 2018. 379(25): p. 2417-2428.
- Sahgal, A., et al., Stereotactic body radiotherapy versus conventional external beam radiotherapy in patients with painful spinal metastases: an open-label, multicentre, randomised, controlled, phase 2/3 trial. The Lancet Oncology, 2021. 22(7): p. 1023-1033.
- A. Sahgal, S.K., T. Nguyen, P. Maralani, J. Greenspoon, K. Linden, A. Pearce, F. Siddiqi, M. Ruschin, SBRT for Spine Expert Panel. Consensus-based organizational guideline for the planning and delivery of spine stereotactic body radiotherapy treatment in Ontario. 2023 April 21, 2023 [cited 2025 July 31]; 21-6:[Available from: https://www.cancercareontario.ca/en/guidelines-advice/types-of-cancer/74056.
| Phase | All trials | Reported in Registry | Journal Publication |
| Phase I | 29 | 2 (7%) | 21 (72%) |
| Phase II | 196 | 79 (40%) | 148 (76%) |
| Phase III | 116 | 76 (66%) | 105 (91%) |
| Phase IV | 9 | 2 (22%) | 8 (89%) |
| Total | 350 | 159 (45%) | 282 (81%) |
| Trial Completion Year | # of ACCT Trials | Sample Size | Global Recruitment * | 3CTN Sites Recruitment | 3CTN Sites Contribution (%) |
| 2015 | 16 | 2930 | 2158 | 181 | 8% |
| 2016 | 28 | 4406 | 2894 | 836 | 29% |
| 2017 | 34 | 8861 | 7596 | 859 | 11% |
| 2018 | 42 | 14873 | 14231 | 3134 | 22% |
| 2019 | 41 | 14536 | 16554 | 2595 | 16% |
| 2020 | 44 | 20217 | 20458 | 2962 | 14% |
| 2021 | 39 | 11886 | 11221 | 2312 | 21% |
| 2022 | 34 | 25381 | 18903 | 2894 | 15% |
| 2023 | 40 | 18777 | 17821 | 2521 | 14% |
| 2024* | 32 | 11553 | 8223 | 2681 | 33% |
| Total | 350 | 133420 | 120059 | 20975 | 17% |
| Median | 36.5 | 13211 | 12726 | 2558 | 15% |
| IQR | 8.25 | 8267 | 9751.5 | 1618.5 | 7% |
| Study Results | Number of Trials | Total Recruitment | 3CTN Member Sites Recruitment |
| Negative | 62 (53%) | 52999 | 3763 (7%) |
| No results | 8 (7%) | 1500 | 355 (24%) |
| Not available | 3 (3%) | 385 | 139 (36%) |
| Positive | 43 (37%) | 37679 | 3771 (10%) |
| Total | 116 | 92563 | 8028 (9%) |
| Study Results | Number of Trials* | Total Recruitment | 3CTN Sites Recruitment |
| Negative | 15 | 211518 | 958 (4.5%) |
| Positive | 28 | 32826 | 2593 (7.1%) |
| Total | 43 | 54007 | 3551 (6.6%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





