Submitted:
13 November 2025
Posted:
17 November 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Methods
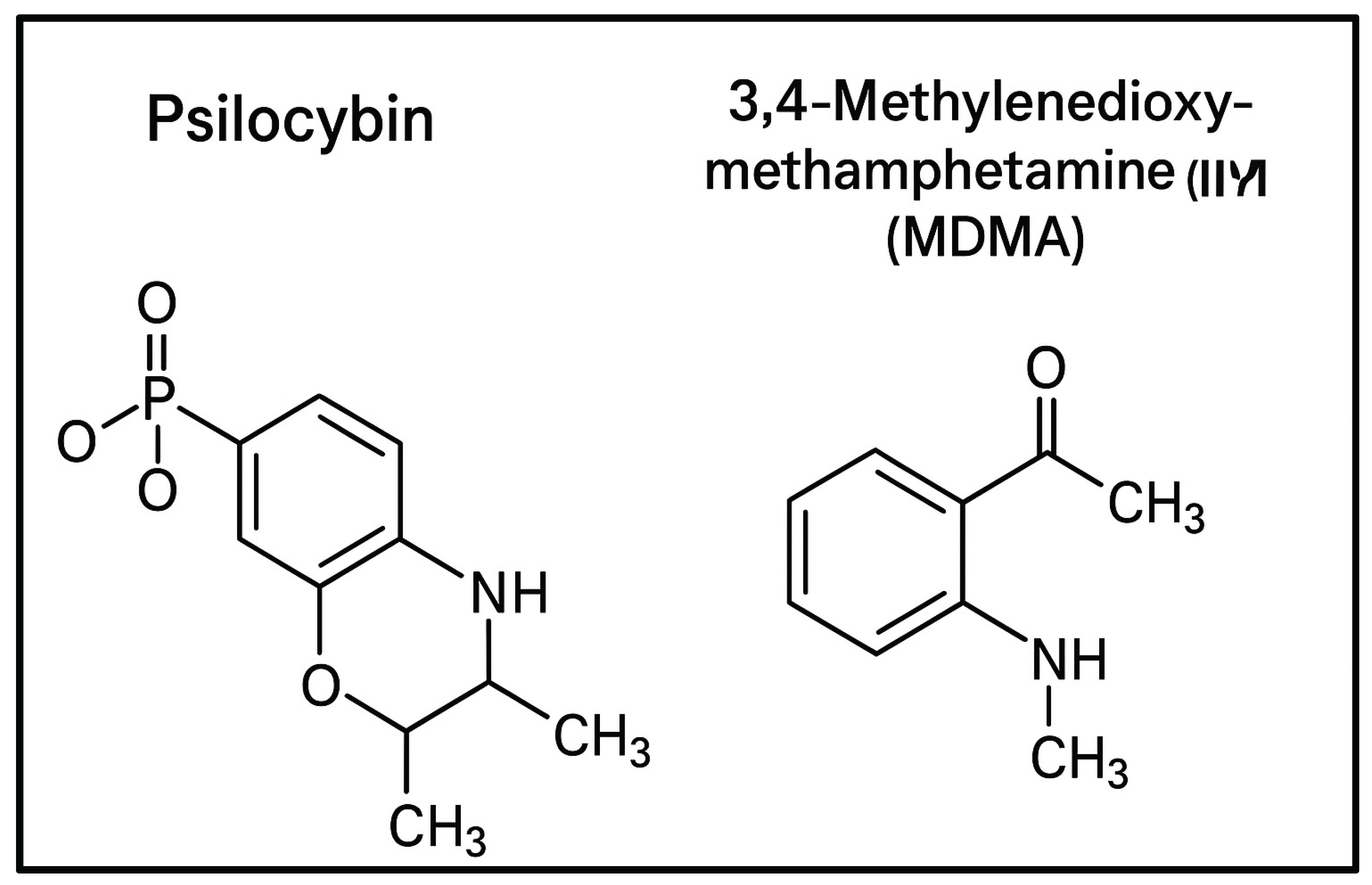
2.1. Mechanisms: Molecule, “Trip,” or Both?
2.2. What We Actually Know in Adult Trials
2.3. Why Adolescents Might Benefit and Why Caution Is Required
2.4. Potential Role of Psychedelic-Assisted Therapy for Neurodevelopmental Disorders
3. Discussion
- 1.
- Preclinical studies targeting adolescence-equivalent developmental stages in animal models to examine long-term neurodevelopmental and behavioral outcomes of classic psychedelics.
- 2.
- Phase I/II safety and feasibility trials in older adolescents with closely defined enrollment criteria, rigorous assent and consent procedures, independent monitoring of adverse events, structured psychotherapy protocols, and long-term follow-up.
- 3.
- Large-scale, multisite RCTs that include neurodevelopmental phenotypes, diverse demographic sampling, and standardized psychotherapeutic adjuncts.
- 4.
- Mechanistic investigations, including functional neuroimaging, network connectivity, and biomarkers such as brain-derived neurotrophic factor (BDNF), cortical thickness, white-matter integrity, and 5-HT2A receptor imaging, to elucidate mediators and developmental moderators of response.
- 5.
- Ethical, regulatory, and access considerations, including frameworks that guard against commercial exploitation, overmedicalization of adolescent populations, and undue hype while ensuring equitable access. The adult research landscape already signals potential conflicts of interest and issues of transparency in for-profit sponsorship.
- 6.
- Integration into clinical services: If adolescent-appropriate safety and efficacy are established, the next challenge will be therapist training, standardization of psychotherapy models, cost-effectiveness analyses, and system-level implementation that includes informed assent, family involvement, and safeguarding.
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- D. E. Nichols, “Psychedelics,” Pharmacol. Rev., vol. 68, no. 2, pp. 264–355, Apr. 2016. [CrossRef]
- Psychedelic Drugs Reconsidered | Office of Justice Programs.” Accessed: Nov. 10, 2025. [Online]. Available: https://www.ojp.gov/ncjrs/virtual-library/abstracts/psychedelic-drugs-reconsidered.
- R. L. Carhart-Harris et al., “Psilocybin for treatment-resistant depression: fMRI-measured brain mechanisms,” Sci. Rep., vol. 7, no. 1, p. 13187, Oct. 2017. [CrossRef]
- A. K. Davis et al., “Effects of Psilocybin-Assisted Therapy on Major Depressive Disorder: A Randomized Clinical Trial,” JAMA Psychiatry, vol. 78, no. 5, pp. 481–489, May 2021. [CrossRef]
- Psychedelics Promote Structural and Functional Neural Plasticity—PubMed. Accessed: Nov. 10, 2025. [Online]. Available: https://pubmed.ncbi.nlm.nih.gov/29898390/.
- A Multi-Site Phase 3 Study of MDMA-Assisted Therapy for PTSD (MAPP1)—Multidisciplinary Association for Psychedelic Studies—MAPS.” Accessed: Nov. 10, 2025. [Online]. Available: https://maps.org/mdma/ptsd/mapp1/.
- Psilocybin-assisted psychotherapy for treatment resistant depression: A randomized clinical trial evaluating repeated doses of psilocybin—PubMed. Accessed: Nov. 10, 2025. [Online]. Available: https://pubmed.ncbi.nlm.nih.gov/38359838/.
- Psilocybin Therapy for Clinicians With Symptoms of Depression From Frontline Care During the COVID-19 Pandemic: A Randomized Clinical Trial | Depressive Disorders | JAMA Network Open | JAMA Network. Accessed: Nov. 10, 2025. [Online]. Available: https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2827553.
- J. M. Mitchell et al., “MDMA-assisted therapy for moderate to severe PTSD: a randomized, placebo-controlled phase 3 trial, Nat. Med., vol. 29, no. 10, pp. 2473–2480, Oct. 2023. [CrossRef]
- Single-Dose Psilocybin Treatment for Major Depressive Disorder: A Randomized Clinical Trial—PubMed. Accessed: Nov. 10, 2025. [Online]. Available: https://pubmed.ncbi.nlm.nih.gov/37651119/.
- G. M. Goodwin et al., “Single-Dose Psilocybin for a Treatment-Resistant Episode of Major Depression, N. Engl. J. Med., vol. 387, no. 18, pp. 1637–1648, Nov. 2022. [CrossRef]
- R. Carhart-Harris et al., “Trial of Psilocybin versus Escitalopram for Depression,” N. Engl. J. Med., vol. 384, no. 15, pp. 1402–1411, Apr. 2021. [CrossRef]
- K. Rajwani et al., “Clinical psychedelic research in adolescents: a scoping review and overview of ethical considerations,” Lancet Child Adolesc. Health, vol. 9, no. 10, pp. 744–752, Oct. 2025. [CrossRef]
- Psychedelic microdosing benefits and challenges: an empirical codebook—PubMed. Accessed: Nov. 10, 2025. [Online]. Available: https://pubmed.ncbi.nlm.nih.gov/31288862/.
- Brain development during childhood and adolescence: a longitudinal MRI study—PubMed.” Accessed: Nov. 10, 2025. [Online]. Available: https://pubmed.ncbi.nlm.nih.gov/10491603/.
- Exploring Novel Antidepressants Targeting G Protein-Coupled Receptors and Key Membrane Receptors Based on Molecular Structures. Accessed: Nov. 10, 2025. [Online]. Available: https://www.mdpi.com/1420-3049/29/5/964.
- F. X. Vollenweider and K. H. Preller, “Psychedelic drugs: neurobiology and potential for treatment of psychiatric disorders, Nat. Rev. Neurosci., vol. 21, no. 11, pp. 611–624, Nov. 2020. [CrossRef]
- R. L. Carhart-Harris and K. J. Friston, “REBUS and the Anarchic Brain: Toward a Unified Model of the Brain Action of Psychedelics, Pharmacol. Rev., vol. 71, no. 3, pp. 316–344, July 2019. [CrossRef]
- R. R. Griffiths et al., “Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: A randomized double-blind trial, J. Psychopharmacol. Oxf. Engl., vol. 30, no. 12, pp. 1181–1197, Dec. 2016. [CrossRef]
- K. Rajwani et al., “Clinical psychedelic research in adolescents: a scoping review and overview of ethical considerations, Lancet Child Adolesc. Health, vol. 9, no. 10, pp. 744–752, Oct. 2025. [CrossRef]
- B. J. Casey, R. M. Jones, and T. A. Hare, “The adolescent brain,” Ann. N. Y. Acad. Sci., vol. 1124, pp. 111–126, Mar. 2008. [CrossRef]
- N. Izmi, R. L. Carhart-Harris, and H. Kettner, “Psychological effects of psychedelics in adolescents, Front. Child Adolesc. Psychiatry, vol. 3, p. 1364617, 2024. [CrossRef]
- Adolescent Psychedelic Use and Psychotic or Manic Symptoms | Bipolar and Related Disorders | JAMA Psychiatry | JAMA Network. Accessed: Nov. 12, 2025. [Online]. Available: https://jamanetwork.com/journals/jamapsychiatry/fullarticle/2816354.
- C. Agnorelli et al., “Neuroplasticity and psychedelics: A comprehensive examination of classic and non-classic compounds in pre and clinical models, Neurosci. Biobehav. Rev., vol. 172, p. 106132, May 2025. [CrossRef]
- A Systematic Review of the Neurocognitive Effects of Psychedelics in Healthy Populations: Implications for Depressive Disorders and Post-Traumatic Stress Disorder—PubMed. Accessed: Nov. 12, 2025. [Online]. Available: https://pubmed.ncbi.nlm.nih.gov/38539636/.
- M. W. Johnson, P. S. Hendricks, F. S. Barrett, and R. R. Griffiths, “Classic psychedelics: An integrative review of epidemiology, therapeutics, mystical experience, and brain network function, Pharmacol. Ther., vol. 197, pp. 83–102, May 2019. [CrossRef]
- Khaleel Rajwani, Should Adolescents be Included in Emerging Psychedelic Research?—PhilArchive. Accessed: Nov. 12, 2025. [Online]. Available: https://philarchive.org/rec/RAJSAB-3.
- R. Nardou et al., “Psychedelics reopen the social reward learning critical period, Nature, vol. 618, no. 7966, pp. 790–798, June 2023. [CrossRef]
| Feature | 5-HT2A Receptor | 5-HT1A Receptor | Clinical Implications |
| Receptor Type/Coupling | GPCR (Gq/11-coupled) → ↑ IP3, Ca2+, PKC activation | GPCR (Gi/o-coupled) → ↓ cAMP, neuronal inhibition | 5-HT2A activation increases excitatory signaling; 5-HT1A activation exerts inhibitory, anxiolytic effects. |
| Brain Locations | Prefrontal, somatosensory, and visual cortices; claustrum | Raphe nuclei, hippocampus, amygdala, prefrontal cortex | Cortical 5-HT2A influences perception and cognition; 5-HT1A regulates mood and anxiety circuits. |
| Typical Agonists | Psilocin, LSD, DOI, mescaline | Serotonin, 8-OH-DPAT, buspirone (partial) | 5-HT2A agonism produces perceptual and cognitive changes; 5-HT1A agonism reduces anxiety and enhances mood. |
| Typical Antagonists | Mirtazapine, clozapine, risperidone, ketanserin | WAY-100635, pindolol (partial) | 5-HT2A blockade reduces hallucinations and contributes to antidepressant and antipsychotic effects; 5-HT1A antagonism is mainly experimental. |
| Partial Agonists | Aripiprazole (functional), brexpiprazole | Buspirone, vilazodone, vortioxetine | Partial agonism stabilizes serotonergic tone and can enhance antidepressant efficacy. |
| Functional Role | Modulates perception, sensory integration, and neuroplasticity | Mediates serotonergic feedback, mood, and anxiety regulation | 5-HT2A activation promotes cortical excitation and plasticity; 5-HT1A activation dampens stress responses and improves emotional regulation. |
| Citation (first author, journal) | Design/Phase | Sample (N, population) | Intervention (dose/sessions) | Primary outcome (timepoint) | Key result |
| Rosenblat et al., 2024 [7] | Randomized clinical trial (phase 2 style) | N ≈ (reported) adults with treatment resistant depression; multisite | Repeated doses of psilocybin with psychotherapy (protocolized) | Depression severity (weeks 6–12) | Repeated-dose psilocybin demonstrated clinically meaningful reductions in depressive symptoms versus control; supports feasibility of repeated dosing in TRD. |
| Back et al., 2024[8] | Randomized clinical trial | N = 30 clinicians with depression, burnout, PTSD symptoms | Single or limited session psilocybin therapy with psychotherapeutic support | Depression symptom change (day 28) | Significant reduction in depressive symptoms by day 28 after psilocybin administration in this small RCT |
| Mitchell et al., [9] | Multisite, randomized, double blind, confirmatory phase 3 | N large multisite sample; adults with moderate to severe PTSD | MDMA assisted therapy (manualized psychotherapy + MDMA) vs. placebo + therapy (multiple sessions) | PTSD symptom severity and functional impairment (primary endpoint timepoint per protocol) | MDMA assisted therapy significantly reduced PTSD symptoms and functional impairment with an acceptable safety profile in the trial population |
| Raison et al., 2023[10] | Randomized, multiblinded clinical trial | Adults with major depressive disorder, randomized | Single-dose psilocybin vs. active placebo comparator (niacin) with blinding procedures | Depression severity over six weeks | Demonstrated onset of antidepressant effect and durability over six weeks; used centralized blinded raters to evaluate timing and safety |
| Goodwin et al., 2022[11] | Phase 2, double blind randomized trial | Adults with treatment resistant depression | Single dose proprietary psilocybin formulation with psychotherapy vs. control | Depression severity (primary endpoint weeks 3–6) | Single dose psilocybin produced clinically meaningful improvement in depressive symptoms compared with control in TRD cohort |
| Carhart-Harris et al., 2021 [12] | Double blind randomized controlled trial | N = 59 adults with major depression | Two doses of psilocybin (with psychological support) vs. daily escitalopram (active SSRI) for six weeks | Change in depression rating scales at week 6 | Psilocybin showed clinically important improvements but the trial did not demonstrate a statistically significant difference from escitalopram on the primary endpoint; issues of expectancy and blinding were noted |
| Davis et al., 2021[4] | Randomized clinical trial | Adults with major depressive disorder | Psilocybin assisted therapy (two dosing sessions) vs. delayed treatment/waitlist | Depression outcomes up to 4–6 weeks and longer follow up | Psilocybin with psychotherapy produced rapid, large and sustained antidepressant effects compared with control conditions in this trial |
| Domain | Current Evidence and Insights | Limitations/Risks | Research and Translational Priorities |
| Mechanistic Basis | Classic psychedelics (psilocybin, LSD, MDMA) act primarily through 5-HT2A receptor agonism, promoting cortical excitation, altered network dynamics, and potential neuroplasticity [24,25] | Mechanistic data largely derived from adult and animal models; age-specific receptor density and synaptic pruning effects remain poorly characterized. | Conduct developmentally calibrated preclinical studies examining 5-HT2A signaling, cortical maturation, and dopaminergic modulation during adolescence. |
| Clinical Efficacy | Multiple adult RCTs demonstrate rapid antidepressant and anxiolytic effects of psilocybin and MDMA[4,12]. | Limited generalizability: adult samples are predominantly middle-aged, White, and medically healthy; no completed adolescent RCTs to date[20]. | Initiate Phase I/II safety and feasibility trials in older adolescents with treatment-resistant depression or PTSD using rigorous consent and safety monitoring. |
| Safety and Tolerability | Adult studies report transient physiological effects (e.g., ↑ systolic BP = 13–24 mmHg) and low incidence of severe adverse events [26]. | Adolescents show higher rates of hallucinogen-persisting perceptual phenomena [22], possible manic risk in genetically vulnerable youth [23]. | Implement age-specific risk stratification, independent safety boards, and long-term neurodevelopmental follow-up. |
| Ethical and Developmental Context | Growing ethical discourse supports inclusion of capable adolescents in research with enhanced consent frameworks[27]. | Uncertainty regarding capacity, assent, and informed decision-making; lack of trauma-informed and family-integrated models. | Develop standardized assent templates, family engagement protocols, and trauma-sensitive psychotherapy adjuncts for minors. |
| Regulatory and Implementation Issues | Psychedelics are undergoing regulatory reevaluation (e.g., FDA breakthrough status for psilocybin and MDMA) in adults. | Youth inclusion remains legally restricted, with undefined pathways for therapeutic exemption or compassionate use. | Advocate for regulatory guidance on adolescent research, conflict-of-interest oversight, and equity in access once safety is established. |
| Future Research Directions | Emerging neuroscience suggests psychedelics may reopen critical periods of social learning and plasticity[28] | Mechanistic plausibility does not equal therapeutic safety; dose–developmental interaction data absent. | Integrate multimodal neuroimaging, biomarker studies (e.g., BDNF, cortical thickness), and network-level analyses in early trials. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





