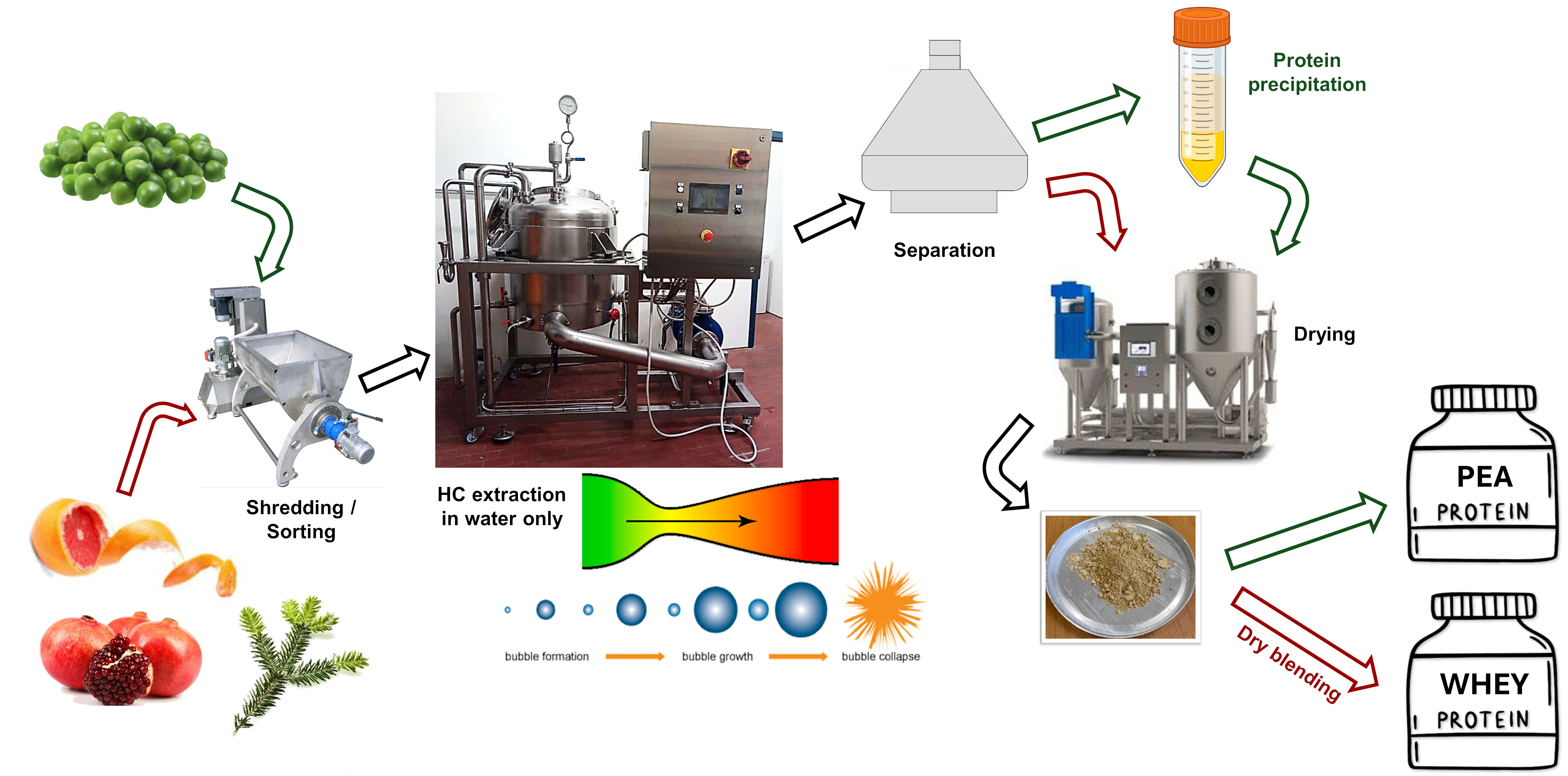
1. Introduction
The valorization of agri-food and forestry byproducts represents a cornerstone of the circular bioeconomy, with global waste streams exceeding 1.3 billion tons annually [
1]. The fruit processing industry contributes substantially, with citrus processing alone generating about 15 million tons of peel waste (CPW) [
2], and pomegranate processing more than 1.5 million tons [
3]. On the other hand, forestry residues account for 60% of harvested biomass [
4]. These underutilized resources harbor valuable bioactive compounds—flavonoids, pectin, terpenes—with proven health benefits ranging from cardioprotection [
5,
6] to hepatoprotection and neuroprotection [
7,
8,
9].
Conventional extraction methods (e.g., Soxhlet, maceration) face persistent barriers categorized as technological, economic, and regulatory [
10]. Technologically, these methods suffer from low yields (typically <30% for polyphenols) [
11], extended processing times (up to 24 hours) [
12], and poor scalability to industrial volumes [
13]. Economically, solvent costs and purification steps consume 40-60% of operational expenses [
14], while regulatory hurdles like the EU Novel Food Regulation (2015/2283) impose lengthy approval processes for extracts obtained via novel technologies [
15].
Emerging green techniques—ultrasound (UAE), microwave (MAE), pulsed electric field (PEF)—have improved sustainability metrics but encounter scalability limitations. UAE, while efficient at lab scale, shows inconsistent cavitation patterns in reactors >50 L due to acoustic field dissipation [
16]. MAE suffers from thermal degradation at industrial power levels (>5 kW) [
17], and PEF primarily serves as a pretreatment requiring downstream extraction [
18].
Hydrodynamic cavitation (HC) stands out among green extraction methods for a high energy density delivered to particles in circulating liquid or mixed liquid-solid media and for the excess generation of hydroxyl radicals, in turn leading to the intensification of physical, chemical and biochemical processes [
14]. The drastic intensification of the temperature-dependent conversion reaction of S-methyl-methionine to free dimethyl sulfide in brewer’s wort and the rapid degassing of the latter, which have long been bottlenecks constraining conventional brewer’s wort processing, was a striking example of HC capabilities [
19].
In the field of the extraction of natural products, HC has emerged not only as the most efficient technique in terms of extraction yield and especially process yield (amount of bioactives recovered per unit energy consumed), but also—at least in the technical variant including linear static reactors such as Venturi tubes—as a straightforwardly scalable extraction technique [
14]. Beyond effectiveness and efficiency, HC showed the ability to stably conjugate low methoxy pectin, such as found in most citrus peel, with flavonoids and volatile terpenoids [
20], likely (at least with flavonoids) through both non-covalent and stronger covalent bonds, the latter allowed by the excess generation of hydroxyl radicals [
8]. This unique property, obtained without further processing, afforded the controlled release of flavonoids and terpenoids and the sustained biological activities such as antioxidant, antimicrobic and anti-inflammatory [
20], as well as the in vivo biological effectivity at far lower doses compared to pure compounds [
6,
8]. The observation of comparatively lower effective doses for a HC-based extract of pomegranate byproducts suggests the possibility of the stable conjugation also in the case of high-methoxy pectin [
21].
HC showed more than sevenfold higher efficiency compared to hot water technique with the extraction of spruce bark as a byproduct of conifer wood supply chains [
4], while retaining volatiles which were deemed responsible for the higher antibacterial activity.
With the potential for HC to become an effective enabler of the sustainable exploitation of vegetable byproducts, either from agri-food or forestry supply chains, the food supplement market appears as the natural outlet, given its astonishing revenues surpassing USD 180 billion, with projections for further sustained growth [
22]. However, the market has been flooded with food supplements and any new single product not only has to compete with all the others, but also faces ever stricter regulations regarding safety and claims [
22,
23].
This study explores a likely smoother approach to the marketing of HC-derived bioactive extracts compared with dedicated food supplements, namely their integration with dry protein isolate (DPI) products, which are widespread and growing in consumption. For example, the market for whey protein isolate (WPI), a highly valuable and easily digestible source of amino acids, amounted to nearly USD 9 billion in 2023 and is projected to more than double in 2030 [
24], with plant-based DPI gaining market shares also due to personal attitudes and environmental concerns [
25].
Across all potential sources of bioactive extracts suitable for the functionalization of DPI products, this study focuses on the byproducts of the supply and processing chains of two classes of natural products, citrus fruits and conifer plants. Such sources are quite familiar to the authors, sufficiently different in nature and composition and increasingly considered for valorization through extraction due to their recognized biological activities and widespread availability of the raw materials. Citrus byproducts are an agri-food resource dominated by polysaccharide compounds, while conifer plants byproducts are a forest resource dominated by lignocellulosic material.
Beyond direct blending of DPI products with dry polyphenol-rich extracts, this study briefly reviews the emerging distinct features of HC-based extraction of proteins from plant sources, and the prospective opportunity and potential advantages of coprocessing DPI products with HC-derived isolated polyphenols of polyphenol-rich phytocomplexes.
This is a narrative, expert-judgement review rather than a systematic review. Study selection was purposive: papers were included when, in the authors’ assessment, they materially informed process understanding, energy/use-of-water implications, product functionality, or scale-up of HC methods within agri-food/forestry streams. Therefore, the coverage is illustrative and may omit relevant studies. The intent was to synthesize practice-relevant evidence, surface consistent patterns and constraints, and delineate priorities for future systematic evaluations.
2. Comparative Analysis of Green Extraction Techniques of Bioactive Compounds
Among the resources focused on in this study, CPW has been the most studied for extraction methods, with sufficient literature about both conventional and novel green methods, and will be the focus of this Section. As the widely adopted green extraction principles (GEPs), lately formulated within a rigorous sustainability benchmark by de Souza Mesquita and coworkers [
26], foresee the use of water as the only solvent or at least safe solvents (principle 1), non-denaturing conditions, implying mild extraction environments (principle 2) and the possibility to use renewable energy sources (principle 5), only novel green methods are discussed in the following. Among these methods, to the best knowledge of the authors, only subcritical water extraction (SWE) and HC have also been tested on conifer byproducts.
2.1. Ultrasound-Assisted Extraction
UAE methods are the most common across non-conventional green methods to obtain phenolic and volatile compounds from citrus byproducts. UAE is a solid-liquid extraction method, based on the phenomenon of acoustic cavitation, i.e., generating vapor bubbles within a liquid, the implosion of which produces the fragmentation of molecules cell walls, increases the exchange surface and promotes the release of bioactive compounds [
27].
Cavitation in liquid media is a multiphase phenomenon consisting of the generation, growth and quasi-adiabatic collapse of vapor-filled bubbles under an oscillating pressure field, resulting in pressure shockwaves (up to 1000 bar), hydraulic jets, extreme local temperatures (up to thousands of K), and the generation of free radicals [
28,
29].
The main parameters affecting the recovery efficiency are particle size, temperature, time, solvent type, and ultrasonic power and frequency [
30]. Using water as the only solvent, the extraction rate of phenolic acids (
p-coumaric, caffeic and chlorogenic) and hesperidin from
Citrus limon L. pomace (peel, membranes and seeds) significantly increased with increasing temperature and decreasing particle size [
31]. In the case of pectin recovery, the extraction rate negatively correlated with the liquid-to-solid ratio, with a significant decrease in pectin yield with increasing solvent volume [
32]. Several studies were performed to validate and optimize UAE for the extraction of polyphenols, pectin, essential oils and carotenoids [
33], although transition to full scale appears intrinsically problematic [
13].
2.2. Pulsed Electric Field
PEF is an emerging non-thermal method for the extraction of bioactive compounds, based on the application of microsecond pulses of high-intensity electric fields that induce a non-reversible electroporation in cell membranes, with the cell disintegration index used to evaluate the appropriate PEF treatment conditions [
34]. The intensity of the electric field, affecting the structural characteristics of the target material, is the most important determinant of the extraction yield, followed by the frequency and amplitude of the pulses, the wave shape, and the exposure time [
35]. The temperature of the solvent in the treatment chamber is a decisive parameter for the diffusion of electrical pulses, with the process temperatures of extractive processes usually below 90°C, both to ensure the preservation of functional compounds and to avoid the reduction in water viscosity that occurs at high temperatures.
PEF is also a technique applied in the pre-treatment of biomass to increase the extraction rate in subsequent processes. Hwang et al. compared conventional flavonoids extraction of Citrus
unshiu with PEF followed by SWE, with an increase of hesperidin concentration by about 22.4, 2.1 and 1.2 times in comparison with hot water, methanol and SWE, respectively [
36]. Moreover, Luengo et al. found an increase by 20 to 159% of the extraction yield of the most abundant polyphenols from orange peels, like naringin and hesperidin, applying PEF before conventional pressing [
37].
As a pre-treatment of orange peels, PEF also showed advantages in increasing the EOs extraction yield, with a 33% increase in limonene extraction when combined with ethanol [
38]. Notably, the selectivity of the extracted aromatic compounds depended on the type of solvent used and the relative affinity to intracellular compounds that solubilize easily following treatment with PEF.
2.3. Microwave-Assisted Extraction
MAE takes advantage of the dielectric properties of the plant matrix, sustaining the interaction of the polar molecules of the sample with the solvent, which in turn causes rapid heating and rupture of plant cell walls. MAE operates trough two mechanisms of heat generation: dipolar rotation, with water evaporation; ionic conduction trough the resistance of the solution to ion flow, producing friction and heat [
27].
Combined MAE with solvent (ethanol) extraction showed superior flavonoid recovery from orange peels compared with conventional methods, operating at low temperatures and in a short time [
39]. However, optimization of the microwave power and the exposure time to microwaves is critical to avoid degradation of phenolic and thermolabile compounds [
40]. MAE, combined with steam diffusion, was used to increase the extraction yield of EOs from citrus byproducts, such as D-limonene, reducing the processing time at about 3 to 20 times and achieving a better score of aroma sensory profile [
41].
2.4. Enzyme-Assisted Extraction
Enzyme-assisted extraction (EAE) can be useful in the case of particularly tight molecular bonds that are difficult to cleave. The enzyme used hydrolyzes the cell walls, releasing the target compounds contained in the plant biomass [
42]. The main parameters that affected the extraction yield of phenolics, while preserving their biological properties, are the condition of the peels, the temperature of the extraction, pH, the types of enzymes, the enzyme concentration and the citrus species [
43]. EAE offers several advantages in pectin extraction from plant resources such as CPW, because this technique can increase the pectin extraction yield compared to traditional processes and can be used under low process temperatures, thereby reducing the energy consumption [
44]. EAE also acts as an effective CPW pretreatment method to improve the distillation of EOs, with an increase in extraction yields by up to 6-fold compared to traditional steam distillation, as well as presents an opportunity for the complete reuse of plant biomass as it generates high amounts of sugars following enzymatic hydrolysis [
45]. Finally, EAE can be coupled with UAE, favoring the extraction of compounds most closely bound to the raw material, replacing heat pre-treatment, reducing the ultrasonic extraction time and consequently increasing the yields by as much as 2 times [
46].
2.5. Subcritical Water Extraction
The SWE method involves the use of water at temperature and pressure conditions below the critical point of 374.15°C and 22.1 MPa. The change in chemical and physical properties allows the selective extraction of polar and non-polar compounds [
47], and the extraction of hydrophilic and lipophilic products [
48]. SWE was used for the enhancement of the extraction yield of mandarin (
Citrus unshiu Markovich) peels in at the laboratory (1 g of sample) and the pilot scale (100 g of sample), with processing time between 10 and 15 minutes, finding similar yields of total phenolic compounds, an increase in the extraction yield of hesperidin and narirutin at 130°C and a subsequent decrease at 150°C, while naringenin showed higher yields at 170°C [
49].
Compared to conventional organic solvent extraction, SWE showed higher extraction yield for hesperidin (1.4 to 5.8 times), narirutin (1.1 to 5.6 times) and polymethoxyflavones (from 1.1 to 1.6 times), with the extraction yield of citrus flavonoids directly correlating to temperature and flow rate [
50]. These results are consistent with other studies concerning the extraction yield and antioxidant activity of flavanones derived from orange defatted peels [
51]. SWE was also used in two-step processes for the extraction of citrus flavanones (hesperidin and narirutin) at 150°C and mono- and disaccharides at 200°C [
52].
2.6. Natural Deep Eutectic Solvents
Natural Deep Eutectic Solvents (NADES) are eutectic solvents that use only primary metabolites as solvents, such as sugars, organic acids and bases, and amino acids [
27]. NADES can also work in a multistep integrated process to extract different compounds (D-limonene, proteins, and polyphenols) with a unique solvent like cholinium chloride and ethylene glycol as a hydrogen bond donor [
53]. The effect of NADES can be enhanced through their combination with UAE, achieving a synergy between mass exchange and disruption of cell membranes performed by UAE, and the stabilization of bioactive compounds provided by NADES [
54]. Among the most promising NADES for application to CPW are lactic acid:glucose with a yield for the total phenolic content of 1932 ± 7.83 mgGAE/100 gdw, and L-proline:malic acid with a yield of 2164 ± 5.17 mgGAE/100 gdw, showing also a good stability of polyphenols after 30 days of storage (25°C and 4°C) and lower degradability compared to ethanol extracts [
55].
2.7. Hydrodynamic Cavitation
HC, sharing with UAE the exploitation of the cavitation phenomena in liquid media, is performed either by circulating a liquid through static constrictions of various geometries, or by special immersed rotary equipment. Contrary to UAE, HC is a straightforwardly scalable technological solution, showing outstanding effectiveness and efficiency for food processing, process intensification and extraction of natural products, besides plenty of other applications [
56,
57]. Direct pilot-scale experiments, using water as the only solvent, with 42 kg of fresh orange byproducts in 120 L water, the process afforded the extraction of a low methoxy pectin (degree of esterification of 17.05%) rich in adsorbed hesperidin, naringin, other polyphenols, and terpenes.
The application of HC to the extraction of CPW allowed obtaining a completely new class of phytocomplexes, dubbed IntegroPectin and consisting of stable conjugates of pectin, flavonoids and volatile compounds (terpenes) [
58,
59], with remarkable pharmacological activities, and much higher bioavailability compared to isolated flavonoids. The role of cavitation in the production of IntegroPectin was later confirmed by experiments using UAE [
60].
The phytocomplex effect, where conjugated molecules show greater bioactivity than isolated compounds, was further evidenced by the striking contrast of the in vitro and in vivo anti-inflammatory performances of red orange IntegroPectin [
8], which also exhibited a remarkable degree of standardization using batches of raw material collected in different years and at different times of the harvesting season.
HC-based extraction processes applied to CPW from lemon and grapefruit also revealed the stable conjugation of pectin with flavonoids and terpenes [
61,
62], spontaneously achieving a result comparable to complex manufacturing processes, for example, used to create hydroxytyrosol-pectin conjugates [
63]. An HC-based grapefruit extract was used in an in vivo study, showing anti-ischemic cardioprotective activity far exceeding that of the pure bioactive flavanone naringenin on a dose-dependent basis from cardioprotection [
6].
Finally, it is notable that insoluble residues of HC-based CPW extraction processing mainly consisted of highly micronized cellulose with high technical value [
64], thus further contributing to the citrus circular economy.
2.8. Summary of Extraction Techniques
Table 1 summarizes the main advantages and drawbacks of the considered methods for the extraction of bioactive compounds from CPW.
Across the CPW extraction methods, HC appears the most compliant with the principles of green extraction of natural products, including the use of water as the only solvent, efficiency, high-value end products, potential circularity, and scalability sustainability [
26]. However, the persistent lack of technological and process standardization represents a challenging task that needs to be urgently addressed [
65], along with the possible efficiency gains derived from coupling with pretreatment methods such as PEF or EAE.
Table 2 shows a synthetic compliance matrix of each green extraction method with GEPs, compiled by the authors based on the presented evidence, therefore to be meant as an expert assessment. Checkmarks (✓) stand for compliance and crosses (✗) for non-compliance. The compliance rate is calculated as (number of checks / 12) × 100.
HC has the potential to achieve the highest apparent compliance among surveyed methods; several items are context-dependent rate with GEPs at the state of the art, with UAE following HC but lacking the crucial criterion of scalability. Among the other green extraction techniques, PEF shows an interesting potential at least as a pre-treatment: if used with water as the only solvent, it could be coupled with HC for faster and more effective processing, at the expense of expanding the unit operations.
3. Bioactive Compounds: In Vivo, Ex Vivo and Clinical Evidence
Consumers of DPI products, either animal- or plant-based, are usually quite aware of their benefits for health and sports performances. Thus, the functionalization of DPI with bioactive compounds, either integral or purified phytocomplexes extracted from natural products, should be based on robust evidence of the additional value, confirmed by clinical trials, in vivo and ex vivo experiments, or computational predictions. Moreover, further ingredients should be as cheap as possible to avoid skyrocketing the price of functionalized DPI, which points to HC as the most efficient extraction and processing technique also leading to higher bioavailability and consequently lower effective doses.
In the following, recent evidence is presented of biological functions of dominant bioactive compounds or integral phytocomplexes extracted from resources associated with documented HC-based extraction processing: orange peel [
66], pomegranate peel [
67], and silver fir (
Abies alba) byproducts [
68].
3.1. Orange Peel Extracts
Hesperidin is by far the dominant flavanone in orange peel extracts and stands out for its many biological functions. It shows convergent evidence across clinical, in vivo, and in silico studies, with green extraction routes enabling high-yield recovery (e.g., hydrodynamic cavitation, hydroalcoholic extraction). In a randomized controlled trial, oral supplementation with a citrus-derived hesperidin formulation (with alpha-glucomannan phosphate, soy proteins and spermidine; food-grade extract, typically hydroalcoholic from peels) improved immune function, lowered biological age, and ameliorated oxidative–inflammatory status [
69]. In vivo, a hesperidin-rich red-orange byproducts phytocomplex produced via hydrodynamic cavitation in water (dubbed AL0042) countered thioacetamide-induced minimal hepatic encephalopathy in mice, showing neuroprotective, anti-inflammatory and antioxidant actions [
8]. Complementing safety and mechanism, an HC- derived citrus-peel extract showed low acute toxicity, antioxidant activity and stimulated immune responses in murine models [
70].
Computational predictions reinforce disease-modifying potential: hesperidin inhibited α-synuclein aggregation in molecular dynamics analyses, supporting anti-amyloidogenic activity relevant to neurodegeneration [
71]. Systems-level reviews further integrate ex vivo and clinical dermatology data, highlighting skin barrier support, anti-UV/anti-inflammatory effects and vascular protection from peel-derived hesperidin formulations [
72]. Finally, docking-led appraisals during the COVID-19 era consistently flagged hesperidin as a top-rank binder of viral/host targets while advocating scalable production via hydrodynamic cavitation [
66].
Collectively, orange peel-derived hesperidin—obtained by water-based hydrodynamic cavitation or conventional hydroalcoholic extraction—exhibits immunomodulatory, antioxidant, anti-inflammatory, neuroprotective, and anti-amyloidogenic functions across clinical, in vivo, and computational lines, with ex vivo/clinical dermatology evidence emerging for skin applications.
3.2. Pomegranate Peel Extracts
Punicalagin—the signature ellagitannin of pomegranate peel—anchors a growing body of translational evidence, with scalable recovery by hydroalcoholic or water-based methods, including HC. An HC pomegranate byproduct extract (peel–pomace; water, no added solvents) showed superior cardiovascular actions in rodent models and vascular ex vivo assays [
21].
A randomized, double-blind, placebo-controlled clinical trial of an oral pomegranate extract (standardized peel/fruit polyphenols from solvent extraction) improved skin wrinkles and biophysical features while modulating the gut–skin axis [
73]. In patients with type-2 diabetes, a standardized pomegranate peel extract (punicalagin-rich; solvent-extracted) improved plasma lipid profile, fatty-acid balance and blood pressure [
74]. A comprehensive review of clinical studies corroborates cardiometabolic and anti-inflammatory benefits of pomegranate preparations rich in punicalagin and related ellagitannins [
75].
Neuroinflammation models further support efficacy: a new peel-extract formulation (punicalagin-dominated ellagitannins; solvent extraction) alleviated disease severity in mice with experimental autoimmune encephalomyelitis [
76]. Photoprotection findings with an aged pomegranate extract extend to clinical setting. A randomized, double- blind, placebo-controlled clinical trial evaluated the impact of an orally-administered standardized pomegranate extract on UV- induced erythema, melanin content, skin hydration, and lightness. The extract demonstrated high antioxidant capacity and significantly reduced reactive oxygen species and inflammatory cytokine levels in vitro. Clinical findings showed significant reductions in UV- induced erythema and melanin levels, with concurrent improvements in skin hydration and lightness compared to placebo [
77].
Computational predictions converge with these outcomes: docking and absorption, distribution, metabolism, excretion, toxicity analyses indicate that punicalagin and other peel metabolites hit multiple protein targets with antidiabetic relevance (e.g., α-amylase and α-glucosidase), supporting glucose- and lipid-regulatory activity [
78].
Overall, punicalagin-rich pomegranate extracts, also obtained via HC in water or conventional hydroalcoholic extraction, demonstrate clinical, in vivo and ex vivo benefits spanning vascular protection, dermal photoprotection, neuroinflammation mitigation and cardiometabolic control, with reviews synthesizing consistent human evidence [
79].
3.3. Abies Alba Extracts
Abies alba extracts have been tested in vivo and ex vivo. In vivo, a trunk-wood extract (polar solvent extraction; lignans, phenolic acids, flavonoids) protected guinea-pig arteries against atherogenic-diet damage; vasoprotective, antioxidant and anti-inflammatory actions were reported [
80]. Ex vivo, an Abies alba extract (wood/bark; polyphenols) reduced infarct size and improved post-ischemic recovery in isolated rat hearts (Langendorff model), showing cardioprotective and anti-lipid-peroxidation activity [
81]. An ex vivo Thiobarbituric Acid-Reactive Substance (TBARS) assay performed on a homogenized rat brain showed that an HC-based extract of silver fir twigs inhibited the lipid peroxidation better than extracts of
Picea abies twigs or bark [
82].
Mechanistic support came from a wood extract enriched in lignans (e.g., matairesinol, pinoresinol), obtained using solvent extraction with fractionation. Robust gastrointestinal stability and systemic antioxidant activity were demonstrated [
83]. Broader softwood work that included
Abies alba bark/needle preparations (hydroalcoholic/water extracts; with phenolics, flavonoids and terpenes) showed antioxidant and wound-healing bioactivity; in vitro and in vivo assays; pro-regenerative, anti-oxidative and anti-inflammatory functions [
84]. In skin-relevant ex vivo cell models, metabolomics on human keratinocytes exposed to softwood knot wood extracts comprising
Abies alba revealed a shift toward antioxidant and anti-inflammatory phenotypes [
85].
Overall, Abies alba extracts—obtained mainly via hydroalcoholic or aqueous techniques from bark, trunk/wood, branches, twigs and needles—feature lignans, proanthocyanidins, phenolic acids, flavonoids and terpenes as dominant classes and consistently express antioxidant, anti-inflammatory, cardioprotective, vasoprotective and wound-healing bioactivities across clinical, in vivo and ex vivo studies.
4. Direct Blending of DPI with HC-Based Bioactive Extracts
HC-based dry extracts, such as spray-dried IntegroPectin, can be physically blended with commercial DPI. Obvious technical benefits include process simplicity, dose flexibility, and use of existing dry blenders. Functional benefits include, at least, the increase in overall antioxidant or anti-inflammatory activity of the blended mixture compared to pure DPI, similar to gluten-free biscuits with 15% lemon IntegroPectin substitution, which showed 300% higher antioxidant activity (ORAC assay) [
86], or gluten-free biscuits supplemented with extracts of pomegranate byproducts, which substantially extended shelf-life [
87]. Whole wheat bread supplemented with extracts of silver fir needles, as well as spruce, pine and fir twigs, showed remarkably increased antioxidant activity [
88,
89].
Assessing the effective amount of the further bioactive ingredients which, when added to DPI, potentially result in significant biological effects, could help standardization and resource and cost containment. The following calculations were performed assuming the usually recommended serving of commercial whey or plant-based DPIs of about 30 g daily, which agrees with the recommendations of the International Society of Sports Nutrition aimed at maximally stimulating muscle protein synthesis [
90]. Moreover, when a clinically relevant dose of a specific bioactive compound or phytocomplex was not available, but in vivo trials provided effective doses for animals, dose translation from different animals to human were performed according to consolidated conversion factors [
91], assuming a human average weight of 70 kg.
Based on an in vivo effective dose of an HC-based integral extract of red orange waste peel (IntegroPectin) of 20 mg/kg daily in mice concerning hepatoprotective and neuroprotective effects, equivalent to approximately 200 mg per day for humans (about 5 mg daily of hesperidin), an effective proportion of red orange IntegroPectin to DPI would be 1:150 w/w, i.e., 1 g of red orange IntegroPectin per 150 g of whey DPI. The in vivo effective dose of an HC-based integral extract of grapefruit waste peel (IntegroPectin) was 135 mg/kg in rats concerning anti-ischemic cardioprotective activity [
6], equivalent to approximately 1.5 g daily for humans. This means an effective proportion of grapefruit IntegroPectin to DPI of 1:20 w/w.
A phytocomplex extracted using HC from pomegranate byproducts showed an in vivo effective dose of 100 mg/kg in rats concerning chronic hypotensive activity, along with anti-inflammatory and anti-fibrotic activities [
21], equivalent to approximately 1.1 g daily for humans (about 55 mg of punicalagins). This means an effective proportion of whole pomegranate extract to DPI of 1:27 w/w. Clinical doses for pomegranate extracts span 250 mg/day of standardized peel extract (about 75 mg punicalagins, oral supplementation), for skin outcomes (significant improvements in several biophysical properties, wrinkles, and shifts in the skin microbiome) [
73], and 250 mg twice a day (total punicalagins about 35 mg, hydroalcoholic extraction, oral supplementation) in type-2 diabetes [
74], which translate into an effective proportion of pomegranate extract to DPI of 1:120 and 1:240 w/w, respectively.
Overall, a relatively small amount of extracts of citrus and pomegranate fruit byproducts would be needed to effectively functionalize both animal- and plant-based DPIs (1/20 to less than 1:200 w/w), depending on the origin and nature of the phytocomplex and the target functionality.
Extracts of forestry byproducts, including
Abies alba extracts, represent another valuable resource for the functionalization of DPIs through direct blending. In a human meal-challenge, one capsule of Belinal
®, containing 200 mg of
Abies alba wood extract, assumed concomitantly with 100 g of white bread, significantly reduced post-prandial glycemia with effects comparable to the positive control acarbose [
92]. This means an effective proportion of
Abies alba extract to DPI of 1:150 w/w. Guinea pigs were fed for 8 weeks with an atherogenic diet, basic diet or atherogenic diet supplemented with an
Abies alba trunk extract obtained by means of a two step procedure (water at 70 °C followed by ethyl acetate), with an intake of about 10 mg/kg/day, equivalent to a human dose of about 150 mg/day. The addition of the
Abies alba extract to the atherogenic diet significantly improved the aorta relaxation response compared to that of the atherogenic diet without the extract and significantly decreased the number of atherosclerotic plaques compared to the atherogenic group [
93]. This dose translates into an effective proportion of
Abies alba extract to DPI of 1:200 w/w. Lignans dominated the composition of extracts of
Abies alba branches and twigs, with about 10% w/w [
94], although with a remarkable variability due to a steep content gradient from proximal to distal sections [
95].
Although further in vivo and clinical trials are urgently needed, the available evidence points to small relative quantities of Abies alba extracts needed to effectively functionalize both animal- and plant-based DPIs (1/150 to 1/200 w/w).
Table 3 summarizes the above figures for red orange waste peel, pomegranate waste peel and
Abies alba byproducts, with further information about the observed HC-based extraction yield.
The information presented in
Table 3 about the amount of fresh raw resource is relevant for the assessment of the operating expenditure (OPEX), which directly affects the cost of goods sold (COGS) and, in turn, the price of the functionalized DPI. Due to comparatively higher effective dose, high moisture level and limited extraction yield, the required amount of fresh pomegranate waste peel is approximately 2.5 to 12 times higher than fresh red orange waste peel or
Abies alba twigs.
Further information useful to assess the COGS is the energy consumption to obtain the dry bioactive extract. Based on data shown in a previous study [
14], the specific energy consumption (energy consumed per unit mass of dry extract) is critically dependent on the moisture of the raw resource and the content of the dry biomass, i.e., the water-to-biomass ratio (dw). Assuming for example a level of 10:1 for the latter ratio, the specific energy consumption would be 21.66, 47.15 and 46.34 kWh/kg of dry extract for red orange waste peel, pomegranate waste peel and
Abies alba twigs, respectively, the energy consumption to produce the amounts to be added to a daily serving of DPI would be approximately 4.3 kWh (red orange waste peel), 11.8 to 51.9 kWh (pomegranate waste peel) and 7.0 to 9.3 kWh (
Abies alba twigs). Based on the estimates of the needed amount of fresh raw resource and energy consumption, on the economic side, the most favorable resource could be orange waste peel, followed by
Abies alba twigs and, as potentially the most expensive, pomegranate waste peel. However, the unit cost of the raw resources, which will be related to the specific supply chains, and the consideration of specific target functionalities, might lead to a change in the score.
5. HC-Based Extraction of Vegetable Proteins
HC has rapidly moved from proof-of-concept to pilot- and large-scale reality for extracting and upgrading plant proteins, offering shorter process times, lower solvent usage, and straightforward scalability compared with UAE, high-pressure processing (HPP) or purely thermal/alkaline routes. Beyond yield, HC can improve functional quality by mitigating anti-nutritional factors (ANFs) and preserving or enhancing protein structure, delivering ingredients that perform well in foods and nutraceuticals.
In legumes, comparative studies on pea demonstrated that both HC and UAE outperform conventional extraction in recovery of protein isolates while better retaining structural integrity; however, HC is intrinsically scalable, whereas UAE faces geometric and energy-distribution limits at volume, making HC the pragmatic choice for industrial throughputs [
96]. Subsequent head-to-head work on ANF control showed HC to be the most effective technology for lowering trypsin inhibitor activity versus UAE and HPP, with phytic acid remaining the most persistent ANF—suggesting process windows that temper alkalinity or leverage near-neutral to mildly acidic media during or after HC to favor phytate solubilization or removal [
25]. Together, these findings indicate that HC can deliver pea protein isolates that are not only high-yielding but also digestion-friendly and formulation-ready.
The value of HC extends beyond soft seeds to tough secondary streams. On fiber-rich oat hulls, two HC reactor designs achieved higher protein extraction than conventional methods using either dilute alkali or water alone, while improving nutritional properties (higher in vitro digestibility and more favorable amino-acid metrics). This demonstrates that the intense micro-mixing, shockwaves and microjets generated by bubble collapse can compensate for limited solvent accessibility and reduce the need for harsh chemistries, as well as facilitate enzymatic digestion by reducing the particle size of the proteins [
97]. The same processes, which are exclusive to HC on a full scale, were deemed responsible for the higher phenolic content and antioxidant activity both in the extracts and post-digestion compared to conventional extraction.
Notably, both pea protein and oat hull isolates were obtained quite simply, adjusting pH to the isoelectric point of the protein of 4.5 and separating by centrifugation, obtaining a protein content of about 80% and 56%, respectively [
96,
97].
Bench-to-pilot translation in other crops is consistent. For faba bean, integrating Osborne fractionation with pilot-scale HC boosted protein recovery compared to conventional extraction while simplifying the unit-operation train—an important lever for energy and OPEX reduction when moving beyond laboratory volumes [
98]. In nuts, HC-based almond beverage processing showed that proteins can be liberated efficiently under water-based, short-residence conditions, with performance comparable to far more complex thermal/mechanical schemes; the authors noted that elevated temperatures and fast kinetics can cap ultimate recovery—pointing to the benefit of moderating the thermal load and extending residence time, if allowed by the flavor and microbiological and lipid stability constraints [
99].
Apple processing offers a practical blueprint for circular integration. A recent study introduced the extraction of proteins from apple seeds for waste valorization [
100], aligning naturally with HC pilot-scale work on apple pomace, where the same cavitation train already recovers pectin- and bioactives-rich streams [
101]. Co-processing seeds and pomace in a single HC workflow could therefore yield a defatted protein fraction from seeds, polyphenol- and pectin-rich aqueous extracts and a fiber coproduct mainly consisting of cellulose—an attractive three-product cascade that maximizes raw-material value while keeping water as the dominant solvent.
Mechanistically, HC promotes rapid cell disruption and protein solubilization through repeated compression/rarefaction cycles and micro-scale shear, while the intense micro-mixing shortens diffusion paths and improves mass transfer. When mild alkali is used, HC accelerates unfolding and solubilization without prolonged exposure to high pH; when water-only processing is feasible, as shown for oat hulls, HC’s physical effects alone could be sufficient to achieve competitive yields with improved functional attributes [
96,
97]. Importantly, unlike acoustic systems, hydraulic reactors (e.g., static linear reactors such as Venturi or orifice plate, or rotor–stator setups) scale linearly in flow and are compatible with inline heat-exchange, pH control and membrane clarification, enabling tight integration with downstream isolation and drying.
From a formulation standpoint, HC-processed plant proteins frequently exhibited better solubility and emulsification after ANF reduction and subtle structural rearrangements, along with higher phenolic content and antioxidant activity both in extracts and post-digestion. This facilitated their combination with HC-generated carbohydrate and phenolic streams to create protein–phytocomplex ingredients with superior techno-functional and potential biological performance—a direction already emerging for soy and pea systems processed under cavitation [
25,
102].
In sum, the evidence across legumes, cereals, nuts and fruit byproducts converges on a robust message: HC delivers high-quality vegetable proteins with fewer unit operations, lower chemical intensity, and credible industrial scalability. Priority optimizations for near-term deployment include:
Tuning pH/ionic strength to tackle phytate without harming digestibility.
Operating at moderate temperatures and solid loadings that preserve proteins while protecting flavor and lipids.
Designing cascaded HC lines that co-valorize proteins, polysaccharides and fibers from the same feedstock.
6. HC-Based Proteins-Polyphenols Conjugation
HC processing of citrus byproducts resulted in the stable conjugation of pectin and polyphenols, with the energy needed for the slightly endergonic reactions provided by the imploding cavitation bubbles [
103], with the processes showing a high degree of reproducibility and the generated phytocomplexes exhibiting remarkable standardization and enhanced bioactivity, primarily due to the synergy between pectin and flavonoids and the increase in bioavailability of flavonoids [
8,
104]. In the following, early evidence of the HC-driven conjugation of proteins and plant polyphenols is briefly reviewed, as it could positively affect the bioactivity of the resulting products.
6.1. Early Evidence of HC-Driven Protein–Polyphenol Conjugation
HC promotes rapid unfolding of plant and dairy proteins and intense micro-mixing, enabling both non-covalent complexation (hydrogen bonding, hydrophobics) and covalent grafting to oxidized polyphenols (quinone-mediated Schiff base/Michael addition) within minutes. Recent HC studies with soy protein isolate (SPI) show formation of SPI–polyphenol complexes with enhanced structural order and interfacial activity, consistent with localized cavitation hotspots and shear-promoting exposure of reactive lysine and tyrosine side chains [
102,
105]. Cavitation microjets, shockwaves, and transient hotspots promote covalent protein–polyphenol grafting via phenolic-quinone/amine (Schiff-base and Michael-addition) pathways and can accelerate radical-initiated conjugation, without bulk harshness.
Processability is credible: whey-protein–pectin complexes have already been scaled from lab to a continuous technical-scale line [
106], indicating transferable unit operations for HC-assisted conjugates. Overall, HC shows the potential to uniquely couple fast protein exposure, phenolic oxidation, and mass transfer in water-only media, aligning with the green-extraction logic already established for polysaccharide–polyphenol conjugates.
6.2. Added Functionality of Protein–Polyphenol Conjugates
Stable protein–polyphenol conjugates unlock techno-functional gains directly relevant to nutraceuticals and beverages: higher water solubility and shifted isoelectric behavior with better dispersion at neutral pH, stronger interfacial films and emulsifying stability, and markedly improved oxidative protection in lipid and carotenoid systems. These outcomes are reported across whey and plant proteins—e.g., proanthocyanidin–WPI conjugates forming robust Pickering shells and protecting β-carotene [
107], enhanced resveratrol loading with preserved functionality via grafting [
108], and conjugates that maintain antioxidant activity through digestion [
109,
110]. Similar benefits track with numeric gains already mentioned in HC contexts for solubility, emulsification, and lipid-oxidation suppression in pea/soy/whey systems, reinforcing translational value for fortified foods and delivery systems. Together, these functionalities support cleaner labels (e.g., less surfactant/antioxidant), better bioaccessibility, and shelf-life extension—compelling complements to the HC polysaccharide-rich extracts highlighted in
Section 2.7 and
Section 4.
7. Conclusions
HC has progressed into a practical, water-centric unit operation that consistently delivers high-solids extraction and preserves functionality across citrus, pomegranate, and softwood matrices, among the others. Beyond extraction, two near-term, industry-ready routes stand out: direct blending of HC-derived dry phytocomplexes with DPI, enabling low-dose fortification with demonstrable antioxidant, anti-inflammatory, or cardiometabolic benefits; and HC-based extraction of vegetable proteins that reduces anti-nutritional factors and yields isolates suited for protein–polyphenol complexation, improving solubility, interfacial behavior, and bioaccessibility. These pathways leverage existing dry-mixing and separation trains, minimize additional unit operations, and align with circular use of all co-streams. While water management and drying remain important design levers, the decisive advantage of HC lies in its ability to couple scalable, water-only processing with ingredient performance and straightforward process integration—positioning HC for broader adoption in nutraceuticals, foods, and related materials, provided energy source and accounting, quality, and regulatory issues are properly addressed.
This review is narrative and may be subject to selection bias despite systematic intent; heterogeneity in reactor designs, operating conditions, and analytics limits direct cross-study comparisons. Energy metrics are often incompletely reported and few studies include solvent and byproduct mass balances, life cycle assessments or techno-economical analyses. Mechanistic claims for in-situ conjugation remain incompletely resolved. Finally, translational evidence at continuous, multi-ton scales is still sparse relative to pilot data.
Author Contributions
Conceptualization, F.M.; methodology, F.M., F.Z. and L.A.; validation, F.M., F.Z. and L.A.; formal analysis, F.M.; investigation, F.M.; data curation, F.Z. and L.A.; writing—original draft preparation, F.M.; writing—review and editing, F.Z. and L.A.; supervision, F.Z. and L.A.; project administration, F.M.; funding acquisition, F.M., F.Z. and L.A. All authors have read and agreed to the published version of the manuscript.
Funding
Part of this research was funded by the following projects: Ministry of Enterprises and Made in Italy (project “MeByC”, grant number CUP C77B24000000008 of 10 March 2022); PNRR (National Recovery and Resilience Plan)—Mission 4, Component 2, Investment 1.3, Italian Ministry of University and Research, funded by the European Union NextGenerationEU—Project PE0000003 (PE10 ON Foods: Research and innovation network on food and nutrition Sustainability, Safety, and Security—Working ON Foods; Spoke 2 “Smart and circular food system and distribution”), Concession Decree No. 1550 of 11 October 2022; CNR project “NUTRAGE,” FOE-2021 DBA.AD005.225.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created in this review article.
Acknowledgments
Clementina Falco and Francesca Martelli (CNR-IBE, Italy) are gratefully acknowledged for their effective management of projects “MeByC”, “ON Foods” and “NUTRAGE”.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ANF |
Anti-nutritional factors |
| COGS |
Cost of goods sold |
| CPW |
Citrus peel waste |
| DPI |
Dry protein isolate |
| EAE |
Enzyme-assisted extraction |
| GEP |
Green extraction principles |
| HC |
Hydrodynamic cavitation |
| HPP |
High-pressure processing |
| MAE |
Microwave-assisted extraction |
| NADES |
Natural Deep Eutectic Solvents |
| OPEX |
Operating expenditure |
| PEF |
Pulsed electric field |
| SPI |
Soy protein isolate |
| SWE |
Subcritical water extraction |
| TBARS |
Thiobarbituric Acid-Reactive Substance |
| UAE |
Ultrasound-assisted extraction |
| WPI |
Whey protein isolate |
References
- Andrade, M.A.; Barbosa, C.H.; Shah, M.A.; Ahmad, N.; Vilarinho, F.; Khwaldia, K.; Silva, A.S.; Ramos, F. Citrus By-Products: Valuable Source of Bioactive Compounds for Food Applications. Antioxidants 2023, 12, 38. [Google Scholar] [CrossRef]
- Meneguzzo, F.; Brunetti, C.; Fidalgo, A.; Ciriminna, R.; Delisi, R.; Albanese, L.; Zabini, F.; Gori, A.; Nascimento, L.B. dos S.; Carlo, A. De; et al. Real-Scale Integral Valorization of Waste Orange Peel via Hydrodynamic Cavitation. Processes 2019, 7, 581. [Google Scholar] [CrossRef]
- Cano-Lamadrid, M.; Martínez-Zamora, L.; Castillejo, N.; Artés-Hernández, F. From Pomegranate Byproducts Waste to Worth: A Review of Extraction Techniques and Potential Applications for Their Revalorization. Foods 2022, 11. [Google Scholar] [CrossRef]
- Tienaho, J.; Liimatainen, J.; Myllymäki, L.; Kaipanen, K.; Tagliavento, L.; Ruuttunen, K.; Rudolfsson, M.; Karonen, M.; Marjomäki, V.; Hagerman, A.E.; et al. Pilot Scale Hydrodynamic Cavitation and Hot-Water Extraction of Norway Spruce Bark Yield Antimicrobial and Polyphenol-Rich Fractions. Sep Purif Technol 2024, 360, 130925. [Google Scholar] [CrossRef]
- Šafranko, S.; Šubarić, D.; Jerković, I.; Jokić, S. Citrus By-Products as a Valuable Source of Biologically Active Compounds with Promising Pharmaceutical, Biological and Biomedical Potential. Pharmaceuticals 2023, 16, 1081. [Google Scholar] [CrossRef]
- Flori, L.; Albanese, L.; Calderone, V.; Meneguzzo, F.; Pagliaro, M.; Ciriminna, R.; Zabini, F.; Testai, L. Cardioprotective Effects of Grapefruit IntegroPectin Extracted via Hydrodynamic Cavitation from By-Products of Citrus Fruits Industry: Role of Mitochondrial Potassium Channels. Foods 2022, 11, 2799. [Google Scholar] [CrossRef] [PubMed]
- Nuzzo, D.; Scordino, M.; Scurria, A.; Giardina, C.; Giordano, F.; Meneguzzo, F.; Mudò, G.; Pagliaro, M.; Picone, P.; Attanzio, A.; et al. Protective, Antioxidant and Antiproliferative Activity of Grapefruit Integropectin on Sh-Sy5y Cells. Int J Mol Sci 2021, 22, 9368. [Google Scholar] [CrossRef]
- Vesci, L.; Martinelli, G.; Liu, Y.; Tagliavento, L.; Dell’Agli, M.; Wu, Y.; Soldi, S.; Sagheddu, V.; Piazza, S.; Sangiovanni, E.; et al. The New Phytocomplex AL0042 Extracted from Red Orange By-Products Inhibits the Minimal Hepatic Encephalopathy in Mice Induced by Thioacetamide. Biomedicines 2025, 13, 686. [Google Scholar] [CrossRef] [PubMed]
- Pozzo, L.; Raffaelli, A.; Ciccone, L.; Zabini, F.; Vornoli, A.; Calderone, V.; Testai, L.; Meneguzzo, F. Conifer By-Products Extracted Using Hydrodynamic Cavitation as a Convenient Source of Phenolic Compounds and Free Amino Acids with Antioxidant and Antimicrobial Properties. Molecules 2025, 30, 2722. [Google Scholar] [CrossRef]
- Yusuf, H.; Fors, H.; Galal, N.M.; Elhabashy, A.E.; Melkonyan, A.; Harraz, N. Barriers to Implementing Circular Citrus Supply Chains: A Systematic Literature Review. J Environ Manage 2025, 373, 123963. [Google Scholar] [CrossRef]
- Hussain, A.; Gulbadan Dar, N.; Paracha, G.M.; Akhter, S. Evaluation of Different Techniques for Extraction of Antioxidants as Bioactive Compounds from Citrus Peels (Industrial by Products). J. Agric. & Environ. Sci 2015, 15, 676–682. [Google Scholar]
- Moreira, M.M.; Morais, S.; Delerue-Matos, C. Environment-Friendly Techniques for Extraction of Bioactive Compounds From Fruits. In Soft Chemistry and Food Fermentation; Elsevier, 2017; pp. 21–47.
- Arya, S.S.; More, P.R.; Ladole, M.R.; Pegu, K.; Pandit, A.B. Non-Thermal, Energy Efficient Hydrodynamic Cavitation for Food Processing, Process Intensification and Extraction of Natural Bioactives: A Review. Ultrason Sonochem 2023, 98, 106504. [Google Scholar] [CrossRef] [PubMed]
- Meneguzzo, F.; Zabini, F. Industrialization of Hydrodynamic Cavitation in Plant Resource Extraction. Curr Opin Chem Eng 2025, 48, 101140. [Google Scholar] [CrossRef]
- Novel Food Available online: https://www.efsa.europa.eu/en/topics/topic/novel-food.
- Chemat, F.; Rombaut, N.; Sicaire, A.-G.; Meullemiestre, A.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Ultrasound Assisted Extraction of Food and Natural Products. Mechanisms, Techniques, Combinations, Protocols and Applications. A Review. Ultrason Sonochem 2017, 34, 540–560. [Google Scholar] [CrossRef]
- Nayak, B.; Dahmoune, F.; Moussi, K.; Remini, H.; Dairi, S.; Aoun, O.; Khodir, M. Comparison of Microwave, Ultrasound and Accelerated-Assisted Solvent Extraction for Recovery of Polyphenols from Citrus Sinensis Peels. Food Chem 2015, 187, 507–516. [Google Scholar] [CrossRef]
- Peiró, S.; Luengo, E.; Segovia, F.; Raso, J.; Almajano, M.P. Improving Polyphenol Extraction from Lemon Residues by Pulsed Electric Fields. Waste Biomass Valorization 2019, 10, 889–897. [Google Scholar] [CrossRef]
- Meneguzzo, F.; Albanese, L. Intensification of the Dimethyl Sulfide Precursor Conversion Reaction: A Retrospective Analysis of Pilot-Scale Brewer’s Wort Boiling Experiments Using Hydrodynamic Cavitation. Beverages 2025, 11, 22. [Google Scholar] [CrossRef]
- Ciriminna, R.; Di Liberto, V.; Albanese, L.; Li Petri, G.; Valenza, C.; Angellotti, G.; Meneguzzo, F.; Pagliaro, M. Citrus IntegroPectin: A Family of Bioconjugates With Large Therapeutic Potential. ChemFoodChem 2025. [Google Scholar] [CrossRef]
- Benedetti, G.; Flori, L.; Spezzini, J.; Miragliotta, V.; Lazzarini, G.; Pirone, A.; Meneguzzo, C.; Tagliavento, L.; Martelli, A.; Antonelli, M.; et al. Improved Cardiovascular Effects of a Novel Pomegranate Byproduct Extract Obtained through Hydrodynamic Cavitation. Nutrients 2024, 16, 506. [Google Scholar] [CrossRef]
- Vojvodić, S.; Kobiljski, D.; Srđenović Čonić, B.; Torović, L. Landscape of Herbal Food Supplements: Where Do We Stand with Health Claims? Nutrients 2025, 17, 1571. [Google Scholar] [CrossRef]
- Fernandes, F.A.; Carocho, M.; Prieto, M.A.; Barros, L.; Ferreira, I.C.F.R.; Heleno, S.A. Nutraceuticals and Dietary Supplements: Balancing out the Pros and Cons. Food Funct 2024, 15, 6289–6303. [Google Scholar] [CrossRef]
- Liu, C.; Wang, R.; Wang, T.; Gu, C.; Zhang, L.; Meng, D.; Pan, M.; Yang, R. The Whey-Plant Protein Heteroprotein Systems with Synergistic Properties and Versatile Applications. J Agric Food Chem 2025, 73, 4440–4454. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.; Hu, Z.; Tang, J.; Das, R.S.; Sun, D.W.; Tiwari, B.K. Reducing Anti-Nutritional Factors in Pea Protein Using Advanced Hydrodynamic Cavitation, Ultrasonication, and High-Pressure Processing Technologies. Food Chem 2025, 488, 144834. [Google Scholar] [CrossRef]
- de Souza Mesquita, L.M.; Contieri, L.S.; e Silva, F.A.; Bagini, R.H.; Bragagnolo, F.S.; Strieder, M.M.; Sosa, F.H.B.; Schaeffer, N.; Freire, M.G.; Ventura, S.P.M.; et al. Path2Green: Introducing 12 Green Extraction Principles and a Novel Metric for Assessing Sustainability in Biomass Valorization. Green Chemistry 2024, 26, 10087–10106. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Cabal, J.; Avilés-Betanzos, K.A.; Cauich-Rodríguez, J.V.; Ramírez-Sucre, M.O.; Rodríguez-Buenfil, I.M. Recent Developments in Citrus Aurantium L.: An Overview of Bioactive Compounds, Extraction Techniques, and Technological Applications. Processes 2025, 13, 120. [Google Scholar] [CrossRef]
- Ge, M.; Zhang, G.; Petkovšek, M.; Long, K.; Coutier-Delgosha, O. Intensity and Regimes Changing of Hydrodynamic Cavitation Considering Temperature Effects. J Clean Prod 2022, 338, 130470. [Google Scholar] [CrossRef]
- Acciardo, E.; Tabasso, S.; Cravotto, G.; Bensaid, S. Process Intensification Strategies for Lignin Valorization. Chemical Engineering and Processing - Process Intensification 2022, 171, 108732. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.-G.; Meullemiestre, A.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Ultrasound Assisted Extraction of Food and Natural Products. Mechanisms, Techniques, Combinations, Protocols and Applications. A Review. Ultrason Sonochem 2017, 34, 540–560. [Google Scholar] [CrossRef]
- Papoutsis, K.; Pristijono, P.; Golding, J.B.; Stathopoulos, C.E.; Bowyer, M.C.; Scarlett, C.J.; Vuong, Q. V. Screening the Effect of Four Ultrasound-Assisted Extraction Parameters on Hesperidin and Phenolic Acid Content of Aqueous Citrus Pomace Extracts. Food Biosci 2018, 21, 20–26. [Google Scholar] [CrossRef]
- Vathsala, V.; Singh, S.P.; Bishnoi, M.; Varghese, E.; Saurabh, V.; Khandelwal, A.; Kaur, C. Ultrasound-Assisted Extraction (UAE) and Characterization of Citrus Peel Pectin: Comparison between Pummelo (Citrus Grandis L. Osbeck) and Sweet Lime (Citrus Limetta Risso). Sustain Chem Pharm 2024, 37. [Google Scholar] [CrossRef]
- Kaur, S.; Panesar, P.S.; Chopra, H.K. Citrus Processing By-Products: An Overlooked Repository of Bioactive Compounds. Crit Rev Food Sci Nutr 2023, 63, 67–86. [Google Scholar] [CrossRef]
- Peiró, S.; Luengo, E.; Segovia, F.; Raso, J.; Almajano, M.P. Improving Polyphenol Extraction from Lemon Residues by Pulsed Electric Fields. Waste Biomass Valorization 2019, 10, 889–897. [Google Scholar] [CrossRef]
- Chatzimitakos, T.; Athanasiadis, V.; Kalompatsios, D.; Mantiniotou, M.; Bozinou, E.; Lalas, S.I. Pulsed Electric Field Applications for the Extraction of Bioactive Compounds from Food Waste and By-Products: A Critical Review. Biomass 2023, 3, 367–401. [Google Scholar] [CrossRef]
- Hwang, H.J.; Kim, H.J.; Ko, M.J.; Chung, M.S. Recovery of Hesperidin and Narirutin from Waste Citrus Unshiu Peel Using Subcritical Water Extraction Aided by Pulsed Electric Field Treatment. Food Sci Biotechnol 2021, 30, 217–226. [Google Scholar] [CrossRef]
- Luengo, E.; Álvarez, I.; Raso, J. Improving the Pressing Extraction of Polyphenols of Orange Peel by Pulsed Electric Fields. Innovative Food Science and Emerging Technologies 2013, 17, 79–84. [Google Scholar] [CrossRef]
- Carpentieri, S.; Režek Jambrak, A.; Ferrari, G.; Pataro, G. Pulsed Electric Field-Assisted Extraction of Aroma and Bioactive Compounds From Aromatic Plants and Food By-Products. Front Nutr 2022, 8. [Google Scholar] [CrossRef] [PubMed]
- García-Martín, J.F.; Feng, C.H.; Domínguez-Fernández, N.M.; Álvarez-Mateos, P. Microwave-Assisted Extraction of Polyphenols from Bitter Orange Industrial Waste and Identification of the Main Compounds. Life 2023, 13. [Google Scholar] [CrossRef]
- Nayak, B.; Dahmoune, F.; Moussi, K.; Remini, H.; Dairi, S.; Aoun, O.; Khodir, M. Comparison of Microwave, Ultrasound and Accelerated-Assisted Solvent Extraction for Recovery of Polyphenols from Citrus Sinensis Peels. Food Chem 2015, 187, 507–516. [Google Scholar] [CrossRef]
- Alvi, T.; Asif, Z.; Iqbal Khan, M.K. Clean Label Extraction of Bioactive Compounds from Food Waste through Microwave-Assisted Extraction Technique-A Review. Food Biosci 2022, 46. [Google Scholar] [CrossRef]
- Patra, A.; Abdullah, S.; Pradhan, R.C. Review on the Extraction of Bioactive Compounds and Characterization of Fruit Industry By-Products. Bioresour Bioprocess 2022, 9. [Google Scholar] [CrossRef]
- Li, B.B.; Smith, B.; Hossain, M.M. Extraction of Phenolics from Citrus Peels: II. Enzyme-Assisted Extraction Method. Sep Purif Technol 2006, 48, 189–196. [Google Scholar] [CrossRef]
- Marić, M.; Grassino, A.N.; Zhu, Z.; Barba, F.J.; Brnčić, M.; Rimac Brnčić, S. An Overview of the Traditional and Innovative Approaches for Pectin Extraction from Plant Food Wastes and By-Products: Ultrasound-, Microwaves-, and Enzyme-Assisted Extraction. Trends Food Sci Technol 2018, 76, 28–37. [Google Scholar] [CrossRef]
- Chávez-González, M.L.; López-López, L.I.; Rodríguez-Herrera, R.; Contreras-Esquivel, J.C.; Aguilar, C.N. Enzyme-Assisted Extraction of Citrus Essential Oil. Chemical Papers 2016, 70, 412–417. [Google Scholar] [CrossRef]
- Durmus, N.; Kilic-Akyilmaz, M. Bioactivity of Non-Extractable Phenolics from Lemon Peel Obtained by Enzyme and Ultrasound Assisted Extractions. Food Biosci 2023, 53. [Google Scholar] [CrossRef]
- Brezo-borjan, T.; Švarc-gaji, J.; Rodrigues, F. Chemical and Biological Characterisation of Orange. Processes 2023, 11, 1766. [Google Scholar] [CrossRef]
- Costa, J.M.; Strieder, M.M.; Saldaña, M.D.A.; Rostagno, M.A.; Forster-Carneiro, T. Recent Advances in the Processing of Agri-Food By-Products by Subcritical Water. Food Bioproc Tech 2023, 16, 2705–2724. [Google Scholar] [CrossRef]
- Ko, M.J.; Kwon, H.L.; Chung, M.S. Pilot-Scale Subcritical Water Extraction of Flavonoids from Satsuma Mandarin (Citrus Unshiu Markovich) Peel. Innovative Food Science and Emerging Technologies 2016, 38, 175–181. [Google Scholar] [CrossRef]
- Kim, D.S.; Lim, S. Bin Kinetic Study of Subcritical Water Extraction of Flavonoids from Citrus Unshiu Peel. Sep Purif Technol 2020, 250. [Google Scholar] [CrossRef]
- Lachos-Perez, D.; Baseggio, A.M.; Mayanga-Torres, P.C.; Maróstica, M.R.; Rostagno, M.A.; Martínez, J.; Forster-Carneiro, T. Subcritical Water Extraction of Flavanones from Defatted Orange Peel. Journal of Supercritical Fluids 2018, 138, 7–16. [Google Scholar] [CrossRef]
- Lachos-Perez, D.; Baseggio, A.M.; Torres-Mayanga, P.C.; Ávila, P.F.; Tompsett, G.A.; Marostica, M.; Goldbeck, R.; Timko, M.T.; Rostagno, M.; Martinez, J.; et al. Sequential Subcritical Water Process Applied to Orange Peel for the Recovery Flavanones and Sugars. Journal of Supercritical Fluids 2020, 160. [Google Scholar] [CrossRef]
- Panić, M.; Andlar, M.; Tišma, M.; Rezić, T.; Šibalić, D.; Cvjetko Bubalo, M.; Radojčić Redovniković, I. Natural Deep Eutectic Solvent as a Unique Solvent for Valorisation of Orange Peel Waste by the Integrated Biorefinery Approach. Waste Management 2021, 120, 340–350. [Google Scholar] [CrossRef]
- Mayanin, I. Evaluation of Polyphenol Profile from Citrus Peel Obtained by Natural Deep Eutectic Solvent / Ultrasound Extraction. Processes 2024, 12, 1–19. [Google Scholar] [CrossRef]
- Gómez-Urios, C.; Viñas-Ospino, A.; Puchades-Colera, P.; López-Malo, D.; Frígola, A.; Esteve, M.J.; Blesa, J. Sustainable Development and Storage Stability of Orange By-Products Extract Using Natural Deep Eutectic Solvents. Foods 2022, 11. [Google Scholar] [CrossRef]
- Ciriminna, R.; Scurria, A.; Pagliaro, M. Natural Product Extraction via Hydrodynamic Cavitation. Sustain Chem Pharm 2023, 33, 101083. [Google Scholar] [CrossRef]
- Arya, S.S.; More, P.R.; Ladole, M.R.; Pegu, K.; Pandit, A.B. Non-Thermal, Energy Efficient Hydrodynamic Cavitation for Food Processing, Process Intensification and Extraction of Natural Bioactives: A Review. Ultrason Sonochem 2023, 98, 106504. [Google Scholar] [CrossRef]
- Meneguzzo, F.; Brunetti, C.; Fidalgo, A.; Ciriminna, R.; Delisi, R.; Albanese, L.; Zabini, F.; Gori, A.; Nascimento, L.B. dos S.; Carlo, A. De; et al. Real-Scale Integral Valorization of Waste Orange Peel via Hydrodynamic Cavitation. Processes 2019, 7, 581. [Google Scholar] [CrossRef]
- Scurria, A.; Sciortino, M.; Garcia, A.R.; Pagliaro, M.; Avellone, G.; Fidalgo, A.; Albanese, L.; Meneguzzo, F.; Ciriminna, R.; Ilharco, L.M. Red Orange and Bitter Orange IntegroPectin: Structure and Main Functional Compounds. Molecules 2022, 27. [Google Scholar] [CrossRef] [PubMed]
- Ciriminna, R.; Angellotti, G.; Li Petri, G.; Meneguzzo, F.; Riccucci, C.; Carlo, G. Di; Pagliaro, M. Cavitation as a Zero-Waste Circular Economy Process to Convert Citrus Processing Waste into Biopolymers in High Demand. Journal of Bioresources and Bioproducts 2024, 9, 486–494. [Google Scholar] [CrossRef]
- Scurria, A.; Sciortino, M.; Albanese, L.; Nuzzo, D.; Zabini, F.; Meneguzzo, F.; Alduina, R.; Presentato, A.; Pagliaro, M.; Avellone, G.; et al. Flavonoids in Lemon and Grapefruit IntegroPectin. ChemistryOpen 2021, 10, 1055–1058. [Google Scholar] [CrossRef] [PubMed]
- Scurria, A.; Sciortino, M.; Presentato, A.; Lino, C.; Piacenza, E.; Albanese, L.; Zabini, F.; Meneguzzo, F.; Nuzzo, D.; Pagliaro, M.; et al. Volatile Compounds of Lemon and Grapefruit IntegroPectin. Molecules 2021, 26, 51. [Google Scholar] [CrossRef]
- Rubio-Senent, F.; Bermúdez-Oria, A.; Rodríguez-Gutiérrez, G.; Lama-Muñoz, A.; Fernández-Bolaños, J. Structural and Antioxidant Properties of Hydroxytyrosol-Pectin Conjugates: Comparative Analysis of Adsorption and Free Radical Methods and Their Impact on in Vitro Gastrointestinal Process. Food Hydrocoll 2025, 162, 110954. [Google Scholar] [CrossRef]
- Al Jitan, S.; Scurria, A.; Albanese, L.; Pagliaro, M.; Meneguzzo, F.; Zabini, F.; Al Sakkaf, R.; Yusuf, A.; Palmisano, G.; Ciriminna, R. Micronized Cellulose from Citrus Processing Waste Using Water and Electricity Only. Int J Biol Macromol 2022, 204, 587–592. [Google Scholar] [CrossRef]
- Iyer, G.; Pandit, A.B. Bridging Ingenuity and Utility in Cavitation─A Pioneer’s Predicament. Ind Eng Chem Res 2024, 63, 12265–12276. [Google Scholar] [CrossRef]
- Meneguzzo, F.; Ciriminna, R.; Zabini, F.; Pagliaro, M. Review of Evidence Available on Hesperidin-Rich Products as Potential Tools against COVID-19 and Hydrodynamic Cavitation-Based Extraction as a Method of Increasing Their Production. Processes 2020, 8, 549. [Google Scholar] [CrossRef]
- Minutolo, A.; Gismondi, A.; Chirico, R.; Di Marco, G.; Petrone, V.; Fanelli, M.; D’Agostino, A.; Canini, A.; Grelli, S.; Albanese, L.; et al. Antioxidant Phytocomplexes Extracted from Pomegranate (Punica Granatum L.) Using Hydrodynamic Cavitation Show Potential Anticancer Activity In Vitro. Antioxidants 2023, 12, 1560. [Google Scholar] [CrossRef] [PubMed]
- Albanese, L.; Bonetti, A.; D’Acqui, L.P.; Meneguzzo, F.; Zabini, F. Affordable Production of Antioxidant Aqueous Solutions by Hydrodynamic Cavitation Processing of Silver Fir (Abies Alba Mill.) Needles. Foods 2019, 8, 65. [Google Scholar] [CrossRef]
- Félix, J.; Díaz-Del Cerro, E.; Baca, A.; López-Ballesteros, A.; Gómez-Sánchez, M.J.; De la Fuente, M. Human Supplementation with AM3, Spermidine, and Hesperidin Enhances Immune Function, Decreases Biological Age, and Improves Oxidative–Inflammatory State: A Randomized Controlled Trial. Antioxidants 2024, 13, 1391. [Google Scholar] [CrossRef]
- Putri, D.D.P.; Maran, G.G.; Kusumastuti, Y.; Susidarti, R.A.; Meiyanto, E.; Ikawati, M. Acute Toxicity Evaluation and Immunomodulatory Potential of Hydrodynamic Cavitation Extract of Citrus Peels. J Appl Pharm Sci 2022, 12, 136–145. [Google Scholar] [CrossRef]
- Waheed, Z.A.; Aboud, H.K.; Al-Awadi, J.H.H.; Al-Mousawy, A.M.J.; Alzubaidi, khudhair R. khudhair Inhibition of α-Synuclein Aggregation by Hesperidin as a Potent Anti-Amyloidogenic Polyphenol: A Computational Approach and MM-PBSA /ADMET Analysis. Biochem Biophys Rep 2025, 44, 102318. [CrossRef]
- Rodrigues, C. V.; Pintado, M. Hesperidin from Orange Peel as a Promising Skincare Bioactive: An Overview. Int J Mol Sci 2024, 25, 1890. [Google Scholar] [CrossRef]
- Chakkalakal, M.; Nadora, D.; Gahoonia, N.; Dumont, A.; Burney, W.; Pan, A.; Chambers, C.J.; Sivamani, R.K. Prospective Randomized Double-Blind Placebo-Controlled Study of Oral Pomegranate Extract on Skin Wrinkles, Biophysical Features, and the Gut-Skin Axis. J Clin Med 2022, 11, 6724. [Google Scholar] [CrossRef]
- Grabež, M.; Škrbić, R.; Stojiljković, M.P.; Rudić-Grujić, V.; Paunović, M.; Arsić, A.; Petrović, S.; Vučić, V.; Mirjanić-Azarić, B.; Šavikin, K.; et al. Beneficial Effects of Pomegranate Peel Extract on Plasma Lipid Profile, Fatty Acids Levels and Blood Pressure in Patients with Diabetes Mellitus Type-2: A Randomized, Double-Blind, Placebo-Controlled Study. J Funct Foods 2020, 64. [Google Scholar] [CrossRef]
- Eghbali, S.; Askari, S.F.; Avan, R.; Sahebkar, A. Therapeutic Effects of Punica Granatum (Pomegranate): An Updated Review of Clinical Trials. J Nutr Metab 2021, 2021, 5297162. [Google Scholar] [CrossRef]
- Vallarino, G.; Salis, A.; Lucarini, E.; Turrini, F.; Olivero, G.; Roggeri, A.; Damonte, G.; Boggia, R.; Di Cesare Mannelli, L.; Ghelardini, C.; et al. Healthy Properties of a New Formulation of Pomegranate-Peel Extract in Mice Suffering from Experimental Autoimmune Encephalomyelitis. Molecules 2022, 27, 914. [Google Scholar] [CrossRef]
- Ikeda, Y.; Nasu, M.; Bruxer, J.; Díaz-Puertas, R.; Martínez-Godfrey, J.; Bulbiankova, D.; Herranz-López, M.; Micol, V.; Álvarez-Martínez, F.J. Photoprotective, Antioxidant and Anti-Inflammatory Effects of Aged Punica Granatum Extract: In Vitro and In Vivo Insights. Food Sci Nutr 2025, 13. [Google Scholar] [CrossRef]
- Gull, H.; Ikram, A.; Khalil, A.A.; Ahmed, Z.; Nemat, A. Assessing the Multitargeted Antidiabetic Potential of Three Pomegranate Peel-Specific Metabolites: An in Silico and Pharmacokinetics Study. Food Sci Nutr 2023, 11, 7188–7205. [Google Scholar] [CrossRef]
- Maqsood, M.; Saeed, R.A.; Rahman, H.U.U.; Khan, M.I.; Khalid, N. Pomegranate Punicalagin: A Comprehensive Review of Various In Vitro and In Vivo Biological Studies. ACS Food Science & Technology 2025, 5, 2064–2085. [Google Scholar] [CrossRef]
- Drevenšek, G.; Lunder, M.; Benković, E.T.; Mikelj, A.; Štrukelj, B.; Kreft, S. Silver Fir (Abies Alba) Trunk Extract Protects Guinea Pig Arteries from Impaired Functional Responses and Morphology Due to an Atherogenic Diet. Phytomedicine 2015, 22, 856–861. [Google Scholar] [CrossRef]
- Drevenšek, G.; Lunder, M.; Benković, E.T.; Štrukelj, B.; Kreft, S. Cardioprotective Effects of Silver Fir ( Abies Alba ) Extract in Ischemic-Reperfused Isolated Rat Hearts. Food Nutr Res 2016, 60, 29623. [Google Scholar] [CrossRef]
- Pozzo, L.; Raffaelli, A.; Ciccone, L.; Zabini, F.; Vornoli, A.; Calderone, V.; Testai, L.; Meneguzzo, F. Conifer By-Products Extracted Using Hydrodynamic Cavitation as a Convenient Source of Phenolic Compounds and Free Amino Acids with Antioxidant and Antimicrobial Properties. Molecules 2025, 30, 2722. [Google Scholar] [CrossRef] [PubMed]
- Tavčar Benković, E.; Žigon, D.; Mihailović, V.; Petelinc, T.; Jamnik, P.; Kreft, S. Identification, in Vitro and in Vivo Antioxidant Activity, and Gastrointestinal Stability of Lignans from Silver Fir (Abies Alba) Wood Extract. Journal of Wood Chemistry and Technology 2017, 37, 467–477. [Google Scholar] [CrossRef]
- Geana, E.I.; Ciucure, C.T.; Tamaian, R.; Marinas, I.C.; Gaboreanu, D.M.; Stan, M.; Chitescu, C.L. Antioxidant and Wound Healing Bioactive Potential of Extracts Obtained from Bark and Needles of Softwood Species. Antioxidants 2023, 12, 1383. [Google Scholar] [CrossRef]
- Quin, O.; Bertrand, M.; Gerardin, P.; Gerardin, P.; Gerardin-Charbonnier, C.; Landon, C.; Pichon, C. Antioxidant Impact of Soft Knotwood Extracts on Human Keratinocytes Shown by NMR Metabolomic Analysis. J Proteome Res 2025, 24, 1745–1756. [Google Scholar] [CrossRef]
- Nuzzo, D.; Scurria, A.; Picone, P.; Guiducci, A.; Pagliaro, M.; Giuseppe; Pantaleo; Albanese, L.; Meneguzzo, F.; Ciriminna, R. A Gluten-Free Biscuit Fortified with Lemon IntegroPectin. ChemistrySelect 2022, 7, e202104247. [CrossRef]
- Breschi, C.; D’Agostino, S.; Meneguzzo, F.; Zabini, F.; Chini, J.; Lovatti, L.; Tagliavento, L.; Guerrini, L.; Bellumori, M.; Cecchi, L.; et al. Can a Fraction of Flour and Sugar Be Replaced with Fruit By-Product Extracts in a Gluten-Free and Vegan Cookie Recipe? Molecules 2024, 29, 1102. [Google Scholar] [CrossRef]
- Parenti, O.; Albanese, L.; Guerrini, L.; Zanoni, B.; Zabini, F.; Meneguzzo, F. Whole Wheat Bread Enriched with Silver Fir Needles (Abies Alba Mill.) Extract: Technological and Antioxidant Properties. J Sci Food Agric. [CrossRef]
- Fidelis, M.; Tienaho, J.; Meneguzzo, F.; Pihlava, J.-M.; Rudolfsson, M.; Järvenpää, E.; Imao, H.; Hellström, J.; Liimatainen, J.; Kilpeläinen, P.; et al. Spruce, Pine and Fir Needles as Sustainable Ingredients for Whole Wheat Bread Fortification: Enhancing Nutritional and Functional Properties. LWT 2024, 117055. [Google Scholar] [CrossRef]
- Kerksick, C.M.; Arent, S.; Schoenfeld, B.J.; Stout, J.R.; Campbell, B.; Wilborn, C.D.; Taylor, L.; Kalman, D.; Smith-Ryan, A.E.; Kreider, R.B.; et al. International Society of Sports Nutrition Position Stand: Nutrient Timing. J Int Soc Sports Nutr 2017, 14. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.; Jacob, S. A Simple Practice Guide for Dose Conversion between Animals and Human. J Basic Clin Pharm 2016, 7, 27. [Google Scholar] [CrossRef]
- Debeljak, J.; Ferk, P.; Čokolič, M.; Zavratnik, A.; Tavčar Benković, E.; Kreft, S.; Štrukelj, B. Randomised, Double Blind, Cross-over, Placebo and Active Controlled Human Pharmacodynamic Study on the Influence of Silver Fir Wood Extract (Belinal) on Post-Prandial Glycemic Response. Pharmazie 2016, 71, 566–569. [Google Scholar] [CrossRef] [PubMed]
- Drevenšek, G.; Lunder, M.; Benković, E.T.; Mikelj, A.; Štrukelj, B.; Kreft, S. Silver Fir (Abies Alba) Trunk Extract Protects Guinea Pig Arteries from Impaired Functional Responses and Morphology Due to an Atherogenic Diet. Phytomedicine 2015, 22, 856–861. [Google Scholar] [CrossRef]
- Vek, V.; Keržič, E.; Poljanšek, I.; Eklund, P.; Humar, M.; Oven, P. Wood Extractives of Silver Fir and Their Antioxidant and Antifungal Properties. Molecules 2021, 26. [Google Scholar] [CrossRef]
- Schoss, K.; Benedetič, R.; Kreft, S. The Phenolic Content, Antioxidative Properties and Extractable Substances in Silver Fir (Abies Alba Mill.) Branches Decrease with Distance from the Trunk. Plants 2022, 11, 333. [Google Scholar] [CrossRef]
- Tang, J.; Zhu, X.; Dong, G.; Hannon, S.; Santos, H.M.; Sun, D.W.; Tiwari, B.K. Comparative Studies on Enhancing Pea Protein Extraction Recovery Rates and Structural Integrity Using Ultrasonic and Hydrodynamic Cavitation Technologies. Lwt 2024, 200, 116130. [Google Scholar] [CrossRef]
- Tang, J.; Goksen, G.; Islam, M.S.; Ranade, V.; Hannon, S.; Sun, D.W.; Tiwari, B.K. Large-Scale Protein Extraction from Oat Hulls Using Two Hydrodynamic Cavitation Techniques: A Comparison of Extraction Efficiency and Protein Nutritional Properties. Food Chem 2025, 471, 142724. [Google Scholar] [CrossRef]
- Suchintita Das, R.; Zhu, X.; Hannon, S.; Mullins, E.; Alves, S.; Garcia-Vaquero, M.; Tiwari, B.K. Exploring Osborne Fractionation and Laboratory/Pilot Scale Technologies (Conventional Extraction, Ultrasound-Assisted Extraction, High-Pressure Processing and Hydrodynamic Cavitation) for Protein Extraction from Faba Bean (Vicia Faba L.). Innovative Food Science and Emerging Technologies 2023, 89, 103487. [Google Scholar] [CrossRef]
- Faraloni, C.; Albanese, L.; Zittelli, G.C.; Meneguzzo, F.; Tagliavento, L.; Zabini, F. New Route to the Production of Almond Beverages Using Hydrodynamic Cavitation. Foods 2023, 12, 935. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, S.; Bashir, I.; Wani, S.M.; Sofi, S.A.; Amin, T.; Malik, A.R.; Khan, F.; Murtaza, I.; Khan, I.; Ayaz, Q.; et al. Protein Extraction from Apple Seeds for Waste Valorization for Sustainable Food Systems. Sustainable Food Technology 2025. [Google Scholar] [CrossRef]
- Tagliavento, L.; Nardin, T.; Chini, J.; Vighi, N.; Lovatti, L.; Testai, L.; Meneguzzo, F.; Larcher, R.; Zabini, F. Sustainable Exploitation of Apple By-Products: A Retrospective Analysis of Pilot-Scale Extraction Tests Using Hydrodynamic Cavitation. Foods 2025, 14, 1915. [Google Scholar] [CrossRef]
- Wei, F.; Ren, X.; Huang, Y.; Hua, N.; Wu, Y.; Yang, F. Hydrodynamic Cavitation Induced Fabrication of Soy Protein Isolate–Polyphenol Complexes: Structural and Functional Properties. Curr Res Food Sci 2025, 10, 100969. [Google Scholar] [CrossRef]
- Butera, V.; Ciriminna, R.; Valenza, C.; Petri, G.L.; Angellotti, G.; Barone, G.; Meneguzzo, F.; Di Liberto, V.; Bonura, A.; Pagliaro, M. Citrus IntegroPectin: A Computational Insight. Discover Molecules 2025, 2, 6. [Google Scholar] [CrossRef]
- Sano, C. Di; D’Anna, C.; Petri, G.L.; Angellotti, G.; Meneguzzo, F.; Ciriminna, R.; Pagliaro, M. Citrus Flavonoid-Pectin Conjugates: Towards Broad Scope Therapeutic Agents. Food Hydrocolloids for Health 2025, 8, 100246. [Google Scholar] [CrossRef]
- Hua, N.; Ren, X.; Yang, F.; Huang, Y.; Wei, F.; Yang, L. The Effect of Hydrodynamic Cavitation on the Structural and Functional Properties of Soy Protein Isolate–Lignan/Stilbene Polyphenol Conjugates. Foods 2024, 13, 3609. [Google Scholar] [CrossRef]
- Filla, J.M.; Hinrichs, J. Processing of Whey Protein-Pectin Complexes: Upscaling from Batch Lab Scale Experiments to a Continuous Technical Scale Process. J Food Eng 2023, 347, 111437. [Google Scholar] [CrossRef]
- Qin, X.; Di, X.; Li, Y.; Wang, Q.; Harold, C.; Liu, G. Bioactivity of Proanthocyanidin-Whey Protein Isolate Stabilized Pickering Emulsion with Encapsulated β-Carotene. Food Chem 2025, 493, 145756. [Google Scholar] [CrossRef]
- Manochai, T.; Kamthai, S.; Siriwoharn, T. Comparative Study of Free Radical Grafting and Alkaline Conjugation for Enhanced Resveratrol Incorporation and Whey Protein Functionalities. Foods 2025, 14, 2596. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Huang, F.; Xie, B.; Sun, Z.; McClements, D.J.; Deng, Q. Fabrication and Characterization of Whey Protein Isolates- Lotus Seedpod Proanthocyanin Conjugate: Its Potential Application in Oxidizable Emulsions. Food Chem 2021, 346, 128680. [Google Scholar] [CrossRef]
- Tang, C.; Tan, B.; Sun, X. Elucidation of Interaction between Whey Proteins and Proanthocyanidins and Its Protective Effects on Proanthocyanidins during In-Vitro Digestion and Storage. Molecules 2021, 26. [Google Scholar] [CrossRef] [PubMed]
Table 1.
Main advantages and drawbacks of CPW extraction methods.
Table 1.
Main advantages and drawbacks of CPW extraction methods.
| Method |
Advantages |
Drawbacks |
| UAE |
Water can be the only solvent;
Low working temperature, fast, low energy consumption. |
Scaling beyond pilot is challenging due to acoustic field attenuation;
Preservation of bioactive compounds sensitive to working temperature, amplitude, frequency and power. |
| MAE |
Low working temperature;
Low energy consumption;
High extraction yield;
Preservation of bioactive compounds including volatiles. |
Scalability not proven;
Generally a pre-treatment, needs further extraction technique downstream;
High cost of equipment at the real scale. |
| PEF |
Water as the only solvent;
Very short processing time. |
Generally a pre-treatment, needs further extraction technique downstream. |
| SWE |
Water as the only solvent;
Selective extraction; continuous flow of operation; short time. |
Difficult cleaning;
Possible degradation of bioactive compounds due to high temperature and pressure;
High cost of equipment; Energy intensive. |
| EAE |
High quality of recovered pectin;
As a pretreatment, allows UAE to increase the extraction yield of phenolic compounds. |
Lower recovery of phenolic compounds compared with conventional Soxhlet technique;
Selectivity of enzymes;
Long process time;
Difficult to scale up;
High cost of enzymes at the real scale. |
| NADES |
High selectivity of extracted bioactive compounds;
Low working temperature;
Simple equipment. |
Scalability not proven;
High cost of NADES;
NADES residues in the end product. |
| HC |
Water as the only solvent;
Low working temperature, fast, low energy consumption;
Creation of new stably conjugated, water-soluble phytocomplexes with higher bioavailability compared to individual compounds;
Insoluble residues with high technical value;
Straightforwardly scalable. |
Non-standard equipment;
Critical dependence of performance on construction details, hence the need for new skills. |
Table 2.
Assessment of the compliance of extraction technologies with the 12 GEPs (✓ likely compliant; ✗ likely non-compliant; — context-dependent) [
26].
Table 2.
Assessment of the compliance of extraction technologies with the 12 GEPs (✓ likely compliant; ✗ likely non-compliant; — context-dependent) [
26].
| GEP |
HC |
UAE |
MAE |
PEF |
SWE h
|
EAE |
NADES |
| 1. Use water/safe solvents a
|
✓ |
✓ |
✓ |
✓ |
✓ |
✗ |
✗ |
| 2. Non-denaturing conditions |
✓ |
✓ |
✓ |
✓ |
✗ |
✓ |
✓ |
| 3. Minimize biomass pre-treatment |
✓ |
✓ |
✓ |
✓ |
✓ |
✓ |
✓ |
| 4. Minimize energy consumption |
✓ |
✓ |
✓ |
✓ |
✗ |
✓ |
✓ |
| 5. Renewable energy sources b
|
✓ |
✓ |
✓ |
✓ |
✗ |
✓ |
✓ |
| 6. Minimize unit operations |
✓ |
✓ |
✗ |
✗ |
✓ |
✓ |
✓ |
| 7. Integration with downstream c
|
✓ |
✗ |
✗ |
✗ |
✗ |
✗ |
✗ |
| 8. Predictability and scalability |
✓ |
✗ |
✗ |
✓ |
✓ |
✗ |
✗ |
| 9. Automation d
|
✓ |
✓ |
✓ |
✓ |
✓ |
✗ |
✗ |
| 10. Safety and hygiene e
|
✓ |
✓ |
✓ |
✓ |
✗ |
✗ |
✗ |
| 11. Valorize all byproducts f
|
✓ |
✓ |
✗ |
✗ |
✗ |
✗ |
✗ |
| 12. Carbon footprint reduction g
|
✓ |
✓ |
✓ |
✓ |
✗ |
✗ |
✗ |
| Compliance Rate (%) |
100% |
83% |
67% |
75% |
42% |
42% |
42% |
Table 3.
Effective amounts of dry bioactive extracts per daily serving of DPI (30 g), reference molecule where available, HC-based yield of dry extract and the necessary quantity of fresh raw material.
Table 3.
Effective amounts of dry bioactive extracts per daily serving of DPI (30 g), reference molecule where available, HC-based yield of dry extract and the necessary quantity of fresh raw material.
| Raw resource |
Moisturea
(%) |
Daily amount
(mg) |
Reference molecule (amount in mg) |
Yieldb
(%) |
Fresh raw material
(g wet basis) |
| Red orange waste peel |
75 |
200 |
Hesperidin (5) |
30 |
2668 |
| Pomegranate waste peel |
72 |
250-1100 |
Punicalagin (35-75) |
13 |
6868-30.221 |
|
Abies alba byproducts |
30c
|
150-200 |
Lignans (9-24)d
|
11c
|
1949-2597 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).