1. Introduction
Fermentation is one of the oldest and most widespread methods of food preservation and transformation, practiced for a long time across different cultures. First evidences of producing fermented beverages are many millennia old [
1]. Food fermentation originated in spontaneous biochemical processes in which native microorganisms transform moist substrates through enzymatic activity. Depending on the outcome, this process produces either edible fermented foods or inedible spoiled products. Over time, human societies refined these natural phenomena into controlled techniques, developing regionally specific fermentation systems adapted to local environments and raw materials. The earliest known examples likely involved the alcoholic fermentation of fruits by wild yeasts, while the later emergence of ceramic vessels for storing aqueous foods facilitated broader experimentation and diversification of fermented products.
Beyond its role in extending shelf life and enhancing flavor, fermentation profoundly alters the nutritional and functional profile of foods [
2]. Microbial activity during fermentation can enrich products with bioactive peptides, vitamins, organic acids, and secondary metabolites, while also modifying the bioavailability of existing nutrients. These attributes have positioned fermented foods as important contributors to human health and as candidates for functional food development.
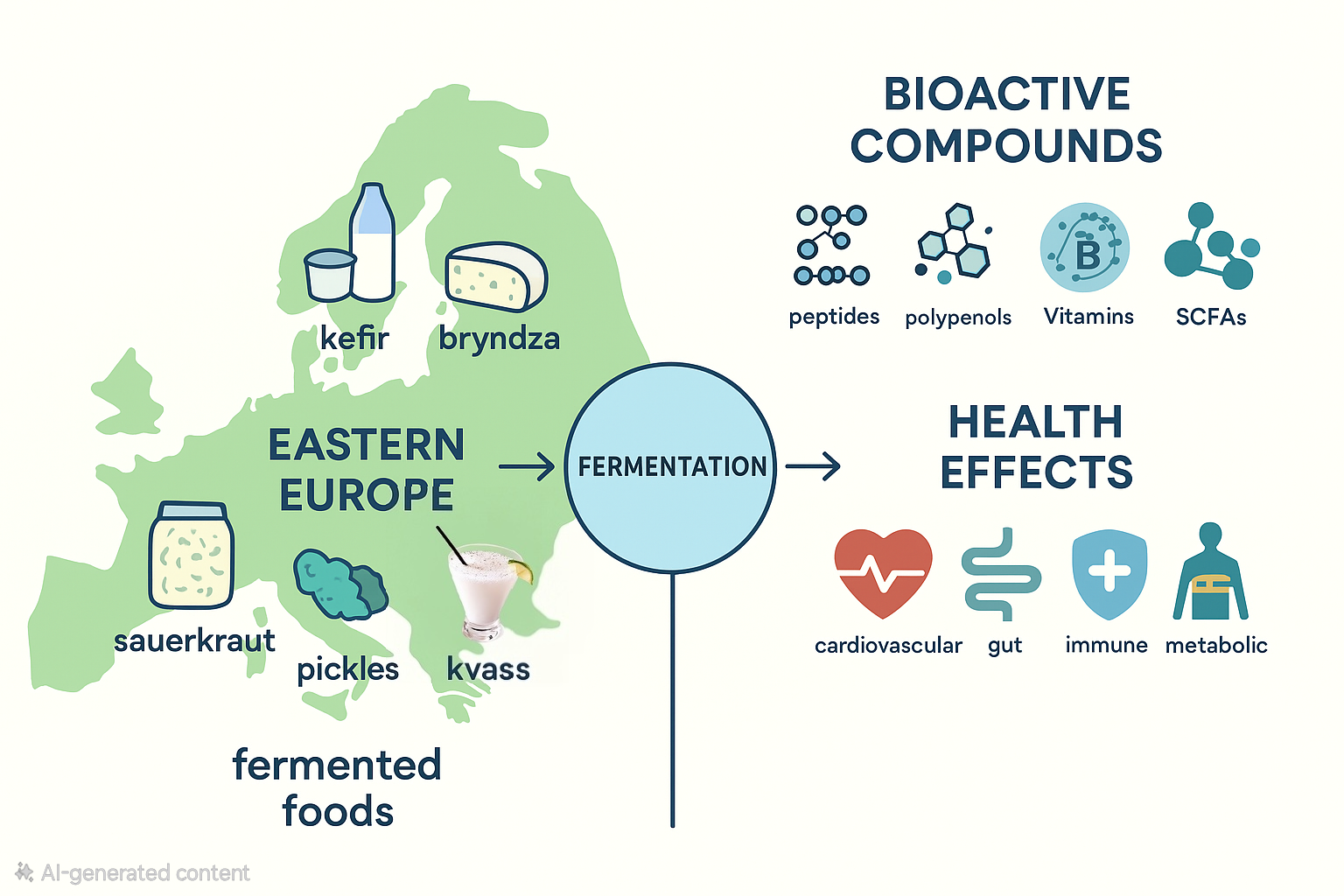
In the scientific literature, much of the focus on fermented foods was centered on Asian products (such as miso, kimchi, and natto) or on globally popular Western varieties (such as yogurt, cheese, and sourdough bread). In contrast, the rich tradition of fermented foods in Eastern Europe has received comparatively little attention, despite their cultural and dietary significance. Countries across this region—including Romania, Poland, Ukraine, Russia, and the Balkan states—have long relied on fermentation as a household and artisanal practice to preserve vegetables, dairy, cereals, and beverages. Sauerkraut, kefir, kvass, and a variety of regional cheeses and pickled vegetables are staples that continue to be consumed daily by millions of people. Eastern European fermented foods are of particular interest because they often involve complex microbial consortia, unique raw materials, and traditional preparation methods that can lead to distinctive bioactive profiles. Evidence suggests that such foods may exert beneficial effects on cardiovascular health, gastrointestinal function, immunity, and metabolic regulation[
3,
4,
5]. At the same time, some products carry potential drawbacks, such as high sodium content or variability in microbial quality, which warrant careful evaluation [
6]. Eastern Europe has a long-standing reliance on fermented foods that continues to shape both rural and urban dietary patterns. Historically, the region’s continental climate and limited access to fresh produce favored the preservation of vegetables, dairy, and cereals through fermentation, a practice deeply embedded in domestic food culture. Ethnographic and dietary studies document the persistence of these foods—such as sauerkraut, kefir, bryndza, kvass, and borș—as common components of everyday diets in countries including Poland, Romania, Slovakia, and Bulgaria. While quantitative dietary data remain limited, regional reports and national surveys suggest that fermented products remain nutritionally and culturally significant, contributing to food diversity, microbial exposure, and dietary resilience in contemporary Eastern European populations [
7,
8,
9,
10]. Given the growing scientific and consumer interest in functional foods, it is timely to examine the contribution of Eastern European fermented foods as sources of bioactive compounds and as potential vehicles for health promotion. This narrative review aims to (i) provide an overview of the main categories of traditional fermented foods from Eastern Europe, (ii) summarize the current knowledge on their bioactive constituents and associated health effects, (iii) compare them with other global fermented foods, and (iv) identify key research gaps and future directions for their integration into modern dietary patterns and functional food development.
Ultimately, this review positions Eastern European fermented foods as a distinctive and underexplored model for understanding how traditional, microbially rich products can inform modern strategies in nutrition science and functional food innovation.
2. Methods: Literature Search Strategy
This narrative review was conducted to provide a comprehensive synthesis of Eastern European fermented foods, their bioactive components, and associated health effects. Relevant literature was identified through searches in PubMed, Scopus, and Web of Science, covering publications up to August 2025. Keywords included combinations of terms such as “fermented foods,” “Eastern Europe,” “sauerkraut,” “kefir,” “kvass,” “fermented dairy,” “fermented vegetables,” “bioactive compounds,” “functional foods,” and “health effects.” In addition to English-language peer-reviewed articles, grey literature, including regional reports, theses, and traditional food compendia in Romanian, Polish, and Russian, was consulted to capture foods and practices underrepresented in mainstream databases. Articles were selected based on relevance to the composition, microbial content, bioactive properties, and documented or potential health effects of traditional fermented foods from Eastern Europe. No systematic inclusion or exclusion criteria were applied, as this work is intended as a narrative synthesis, providing a broad overview and critical discussion rather than a quantitative meta-analysis. Where possible, studies were prioritized for human trials and in vitro or animal studies with clear mechanistic insights, supplemented by historical and cultural descriptions to contextualize the functional potential of these foods.
3. Historical and Cultural Context of Classes of Fermented Foods
Fermentation has been an integral part of Eastern European food culture for centuries, serving as both a necessity for preservation and a cornerstone of culinary identity. The long, harsh winters, limited access to fresh produce during colder months, and the abundance of raw materials such as cabbage, milk, rye, and root vegetables created conditions in which fermentation was not only practical but indispensable. Generations of households developed fermentation practices that were deeply embedded in daily life, transmitted through family traditions, and often tied to seasonal cycles and local festivities. Traditional fermentation procedures generally rely on spontaneous microbial activity initiated by naturally occurring microorganisms present on raw materials, utensils, or in the surrounding environment. The process typically involves minimal technological intervention and is conducted under ambient conditions that favor the growth of desirable bacteria, yeasts, or molds [
11]. Such fermentations are often performed in small batches using household or community-based practices, where environmental factors and indigenous microflora impart characteristic sensory and nutritional properties to the final products [
12].
Eastern European fermented foods encompass a diverse array of products, reflecting regional biodiversity, cultural traditions, and resource availability. These foods can be broadly categorized into dairy, vegetables, cereals, and beverages, each with distinctive microbial communities, bioactive compounds, and potential health benefits.
3.1. Vegetable Fermentation
Vegetable Fermentation is perhaps the most iconic expression of this tradition. Cabbage, preserved as sauerkraut, became a dietary staple across Central and Eastern Europe. The product was known by different names, according to each region [
13]. In Romania, Poland, Ukraine, and the Balkans, large barrels of shredded cabbage or cabbage leafs prepared in autumn [
14] were relied upon to provide essential vitamins throughout the winter, when scurvy and micronutrient deficiencies were otherwise a risk. The quantity of cabbage in a household was either a sign of prosperity, or of scarcity. Fermentation enriches the cabbage with lactic acid bacteria and vitamins C and K. As boosters for preservation, many other plants are added (mustard, celery, leafs, horseradish, etc) contributing to the enrichement of the nutritional value of the fermented product.
Sauerkraut is not merely a side dish but a foundation for numerous cooked meals, symbolizing resilience in the face of food scarcity. Bell peppers, cucumbers, carrots, garlic, beets and even small watermelons are also frequently fermented, producing pickles [
15], each with different microbial communities and flavors that became part of everyday diets. These products illustrate how local biodiversity and microbial ecology are intersected with nutritional needs [
10,
16].
The microbial diversity of fermented vegetables is highly variable and influenced by preparation methods, local microflora, and salt concentration, creating unique microbial “signatures” that differentiate regional products [
17].
3.2. Fermented Dairy Products
Fermented dairy products hold a similarly prominent role in Eastern European traditions. Kefir, originating in the Caucasus, became widespread in Russia and Eastern Europe by the 19th century, and was celebrated for its purported health benefits long before the mechanisms of probiotics and lactic acid bacteria were understood. Kefir grains contain complex symbiotic consortia of lactic acid bacteria (LAB), acetic acid bacteria, and yeasts, creating a beverage rich in peptides, organic acids, and vitamins. The fermentation process enhances protein digestibility and produces naturally occurring antimicrobial compounds [
18].
Other traditional dairy beverages, such as ryazhenka in Russia (fermented baked milk) or ayran in the Balkans (a yogurt-based drink), highlight the creativity with which dairy was preserved and consumed. Their production illustrates regional adaptations, where local processing (heat treatment, dilution, mixing with herbs or salt) shaped both sensory properties and nutritional profiles. Ryazhenka is baked fermented milk, traditionally prepared by heating milk before fermentation. This process yields Maillard reaction products, mild acidity, and a unique flavor profile, with potential antioxidant properties [
19]. Ayran, a diluted yogurt-based beverage, is widely consumed in the Balkans. Its hydrating properties and probiotic potential make it an important functional food in both traditional and modern diets [
20]. Domestic Bulgarian yoghurt, “kiselo mlyako”, is produced similary to ayran, but has a hystorical recognition of having anti ageing effects. At the start of the 20th century, the renowned Russian scientist and Nobel laureate Ilya Mechnikov (1845–1916) turned his attention to the issue of human aging. Mechnikov identified Bulgarian yogurt (“kiselo mlyako”), rich in beneficial lactobacilli, as a food capable of suppressing these harmful bacteria that cause ageing. Based on this, he argued that Bulgaria’s unusually high number of centenarians was linked to the regular consumption of this traditional yogurt [
21,
22].
Matzoon or matsuni is a type of traditional Armenian and Georgian fermented dairy product that consists of milk and a starter culture or previously made matzoon. It is typically made with boiled cow’s milk combined with yogurt, and the mixture is then allowed to sit in a warm place until it becomes homogenous. Semi-firm and with a distinctive, tangy flavor, matzoon is featured in numerous traditional Armenian dishes and beverages, both sweet and savory, such as the refreshing beverages known as matsnabrdosh and tahn, various sauces for dishes made with local greens, and soups [
23].
Cheese-making traditions also flourished, especially in areas with wide pastures, with products such as bryndza (a sheep’s milk cheese popular in Romania and Slovakia) representing both nutritional security and cultural identity.
Cheeses such as bryndza and other sheep- or cow-milk varieties undergo lactic fermentation, which develops texture, flavor, and bioactive peptides with ACE-inhibitory and antioxidant properties. These cheeses also provide a concentrated source of minerals and protein, making them nutritionally valuable in regions with limited access to fresh produce during winter months. These products highlight the dual function of fermentation as both nutrient preservation and functional enrichment [
24].
3.3. Cereal-Based Fermentations
Cereal-based fermentations are another cornerstone. Fermentation of cereals is a long-standing practice in Eastern Europe, primarily for bread and beverages. Rye sourdough bread has been a staple across the Baltic states, Poland, and Russia, not only for its flavor but also for its longer shelf life and enhanced digestibility. Fermentation of dough improves digestibility, increases mineral bioavailability, and generates bioactive peptides. The long fermentation also contributes to lower glycemic indices compared to industrial breads, suggesting potential benefits for metabolic health [
25,
26]. Other cereal-based fermentations include fermented porridges, like kissel [
27] and regional sourdough variations, often consumed as staple meals or snacks [
28,
29], highlighting the adaptability of fermentation to local grains and seasonal availability. There is also a plethora of beverages based on cereals, that will be presented in the next subchapter.
3.4. Fermented Beverages
Fermented Beverages in Eastern Europe extend beyond dairy and cereal-based products, representing a broad category with unique microbial and bioactive properties [
30].
Kvass remains the most widespread traditional beverage. Kvass, a lightly alcoholic beverage made from fermented rye or fermented bread, is emblematic of Slavic food culture, mentioned for the first time in the 10th century. Other grains like barley and wheat, or fruits and vegetables, can also be used. Historically considered a “drink of the people,” it was consumed daily across social classes, providing hydration, modest nutrition, and safe microbial quality in times when water could be unreliable. Traditionally consumed across social classes, it has served both as a nutritional supplement and as a culturally symbolic beverage. It involves fermenting the ingredients with water, sugar, and yeast, resulting in a lightly alcoholic beverage with a sour, tangy taste and natural carbonation. opular types include malty bread kvass, earthy beet kvass (often used in borscht), and even lettuce kvass [
31,
32,
33]. Similar cereal-based fermented beverages were prepared regionally, sometimes flavored with herbs, honey, or berries, reflecting the biodiversity of local environments. Fermented herbal or berry infusions, such as elderberry or cranberry ferments, are common in rural areas, often prepared in households for seasonal consumption. Even in big cities, elderberry fermented beverages (“socata” in Romanian) are prepared in season by many families, as a healthier alternative to comercial soft drinks. These beverages may contain polyphenols, organic acids, and lactic acid bacteria.
“Borș” is a traditional Romanian fermented liquid, distinct from the beetroot-based borscht of Ukraine and Russia. It is prepared from wheat or barley bran, corn flour, and water, often with the addition of a slice of bread—particularly black bread—to initiate fermentation. The mixture undergoes natural fermentation driven by lactic acid bacteria and yeasts, producing a tangy, slightly yellow liquid with a refreshing, mildly pungent aroma, eventually enriched with aromatic herbs (e.g. lovagge). In Romanian cuisine, “borș” is primarily used to impart a characteristic sour flavor to “ciorbe” (sour soups), but it can also be consumed directly as a probiotic beverage. The fermented solids can be dried and preserved as a starter, known as “huște”, for subsequent batches. Nutritionally, “borș” is rich in lactic acid, vitamins, and minerals derived from the cereal bran, and it contains phenolic compounds that confer antioxidant properties. Its fermentation process enhances the bioavailability of nutrients and generates beneficial microorganisms that support gut health. Conceptually, borș is similar to Russian kvass—a fermented rye bread drink—but it remains unique in its composition, preparation, and culinary application [
34,
35].
Braga, boza or busa are traditional fermented cereal beverages widely consumed in the Balkans, Turkey, but also in many parts of Eastern Europe [
36]. The names and recipes vary by region—“braga” and “busa” are used in specific areas—reflecting local cereal availability and fermentation practices. This diversity not only influences taste and texture but also affects the nutritional and microbial profiles, illustrating the intersection of culture, diet, and health in Eastern European fermented foods. They are typically prepared from a mix of cereals such as wheat, maize, millet, barley, oats, rye, or rice, which serve as a source of carbohydrates, dietary fiber, and essential micronutrients. The fermentation process is driven LAB and yeasts. LAB produce organic acids, primarily lactic acid, which lower the pH, preserve the beverage, and contribute to its characteristic tangy flavor. Yeasts generate low levels of ethanol and carbon dioxide, adding mild effervescence. Fermentation also enhances the nutritional value of the drink by increasing the bioavailability of certain minerals, producing B-group vitamins, and generating bioactive compounds with potential health benefits [
37]. Boza, braga, and busa provide energy from fermentable sugars while delivering probiotics and bioactive metabolites that may support gut health. Depending on the cereal composition and fermentation duration, they can also supply dietary fiber, protein fragments, and micronutrients such as magnesium, phosphorus, and iron. The combination of carbohydrates, microbial metabolites, and micronutrients makes these beverages a functional traditional food with both nutritional and cultural relevance.
Kombucha, though originally from East Asia, became integrated into Russian and Eastern European traditions by the 19th and 20th centuries. It s consumption gained popularity due to its perceived health benefits, safety, and appealing sensory characteristics [
38]. Its fermentation produces organic acids, vitamins, and a diverse microbial consortium [
39]. While a borderline case in terms of origin, kombucha exemplifies the region’s capacity to adapt and integrate fermented beverages into everyday diets [
40].
Across all categories, Eastern European fermented foods share several characteristics. First, we notice the microbial diversity. Spontaneous or starter-driven fermentation generates complex communities of bacteria and yeasts, producing bioactive metabolites. Fermentation increases bioavailability of vitamins, minerals, and proteins while producing bioactive peptides and organic acids [
41,
42]. All of them carry a functional potential. Evidence suggests health benefits. Even more, these fermented foods are eaten daily by a high number of people, due to their cultural embeddedness. These foods are integral to seasonal diets, traditions, and social rituals, which helps maintain their consumption and microbial authenticity.
The cultural dimension of fermentation in Eastern Europe goes far beyond preservation. These foods often carried symbolic and ritual meaning. In Romania, sauerkraut leaves are essential for preparing sarmale (cabbage rolls), a dish associated with weddings, holidays (Christmas, Easter), and community gatherings. Sarmale is frequently prepared in large quantities for festive occasions, and fermented cabbage (sauerkraut) is often used for its flavour and traditional wrapping in many regions[
43]. During fasting periods of Orthodox Christianity — such as Lent or the Nativity Fast — plant-based foods become central, and fermented vegetables like sauerkraut are used because they are allowed and because they provide nutrients when fresh vegetables are less available [
44]. Sarmale wrapped in sauerkraut leaves can also be adapted (meatless or with mushrooms) during fasting [
45]. In Russia, kvass has been deeply embedded in culture for over a millennium. Its origin dates back at least to the Primary Chronicle of Kievan Rus (989 AD), and it was consumed by people of all social classes. It was offered to guests as a gesture of hospitality, served in communal settings, during festivals, markets, celebrations, and sometimes used in religious fasts or daily meals [
46]. As for kefir, traditional narratives from the Caucasus note that kefir grains were zealously guarded cultural treasures, passed from generation to generation. The symbiotic culture (bacteria + yeasts) was considered precious, with households maintaining their own grains as heirlooms [
47].
Industrialization in the 20th century altered the production of many of these foods, shifting from household to factory-scale processes. Yet, unlike in some Western contexts, homemade fermentation remained widespread in Eastern Europe, partly due to strong cultural attachment, partly due to economic necessity. Even today, urban households frequently prepare their own sauerkraut or pickles, preserving continuity with ancestral practices[
48,
49] . This persistence has ensured that microbial diversity, traditional methods, and cultural symbolism remain alive, making Eastern European fermented foods unique not only in their composition but also in their sociocultural significance.
In recent decades, scientific and consumer interest has converged on these traditional foods. Researchers are beginning to uncover the bioactive compounds [
50] and microbial profiles [
51]. responsible for their health-promoting effects, while consumers are increasingly drawn to them as “authentic” and “natural” dietary components [
52]. Understanding their historical and cultural roots is therefore essential, not only for appreciating their continued role in the daily diet but also for framing them as potential functional foods within contemporary nutrition science.
4. Bioactive Compounds and Functional Properties
Eastern European fermented foods are rich in a variety of bioactive compounds that emerge from microbial activity during fermentation, interactions with the food matrix, and enzymatic transformations of native substrates. These compounds confer functional properties, contributing to cardiovascular, gastrointestinal, metabolic, and immune health. In this section, we discuss bioactive peptides, polyphenols and secondary metabolites, probiotic, prebiotic and postbiotic factors, vitamins and micronutrients, and organic acids that can be found in these fermented foods.
4.1. Bioactive Peptides
Bioactive peptides are short chains of amino acids (often 2–20 residues, though sometimes longer) generated during proteolysis—i.e., the enzymatic breakdown of larger proteins—by fermenting microorganisms. These peptides may exert diverse physiological effects such as ACE-inhibition (important for blood pressure control), antioxidant activity (scavenging reactive oxygen species), immunomodulation, and antimicrobial effects. Their production, stability, and bioactivity are strongly influenced by the protein substrate (type and content), length and conditions of fermentation, and the microbial community involved (species, strain, and their proteolytic systems)[
53]. Several peptides with known or potential physiological effects have been identified in traditional Eastern European fermented foods (
Table 1).
4.1.1. Dairy Examples:
One of the most studied products is kefir. In kefir, recent studies show that the presence and activity of Lactobacillus plantarum and other lactobacilli in fermentation significantly increases ACE-inhibitory activity over time. For example, one kefir sample fermented with L. plantarum showed ~87.3% ACE inhibition after an extended fermentation (28 days), with an IC₅₀ of ~19.7 mg/L. While the specific peptide sequences were not identified in this study, earlier proteomic analyses of kefir have reported the presence of β-casein–derived peptides such as Val–Pro–Pro (VPP) and Ile–Pro–Pro (IPP), both recognized ACE inhibitors [
55,
62,
63]. Lactotripeptides from casein fragments (Val–Pro–Pro, Ile–Pro–Pro) are among the best documented in kefir and yogurt-like fermentations [
64,
65]. Peptides from αs1-casein and β-casein with antioxidant and antimicrobial activities have also been isolated in kefir-like LAB fermentations (e.g., Lactobacillus helveticus, L. plantarum) [
54,
66].
Bryndza cheese exhibits substantial proteolysis during ripening. A study on the effects of partial replacement of salt with potassium chloride in bryndza found that changes in protein breakdown (proteolytic patterns) are observed, suggesting that the peptides produced during these processes may vary in abundance and composition [
67]. The microbiota of bryndza (and its bacterial/yeast communities) have been already characterized, showing rich diversity that is a precondition for diverse proteolytic activity resulting potentially in active peptides generating. For example, Lactobacillus paracasei, Lactococcus lactis, and other lactic acid bacteria are commonly present in bryndza [
68,
69].
Thus, one can conclude that kefir and bryndza cheese contain peptides that have demonstrated ACE-inhibitory activity in vitro and in animal models[
58,
70]. These peptides may contribute to cardiovascular benefits traditionally associated with fermented dairy consumption. [
57,
71,
72,
73]
, but human studies are still needed.
4.1.2. Cereal Examples
Sourdough fermentations of various cereal flours (whole wheat, spelt, rye, kamut) with selected lactic acid bacteria have been shown to produce antioxidant peptides. In one study, extracts from sourdoughs had significantly higher radical scavenging activity compared to chemically acidified doughs. The researchers identified 25 specific peptides (8 to 57 amino acids in length) via LC-MS/MS that were stable even after simulated digestive enzyme hydrolysis [
74]. Studies have shown that water/salt-soluble extracts from sourdoughs possess significantly higher radical scavenging activity compared to extracts from chemically acidified doughs [
59,
60,
61].
Rye sourdough bread has repeatedly been shown to improve postprandial glucose and insulin responses when compared to non--sourdough breads – while in many of these studies the precise peptides are not fully identified, the improved metabolic responses are believed to be partly mediated by peptides and organic acids released during fermentation [
75,
76]. This has been supported by EFSA’s scientific opinions on rye bread and other clinical trials with sourdough rye crispbread [
77]. The fermentation of sourdough breaks down gluten and phytic acid, potentially releasing minerals and altering the bread’s structure to create a lower and more sustained release of glucose into the bloodstream [
78]. One of the causes of the mentioned effects is a longer fermentation, that tends to allow more extensive proteolysis, generating a broader spectrum of peptides. The kefir example above showed also increasing ACE inhibition with longer incubation [
58].
Another factor that enhances the peptides production is the microbial composition. Strains differ in protease sets; e.g., L. plantarum in kefir gave higher ACE-inhibition. In cereals, mixed LAB communities produce more diverse peptide profiles than single-strain fermentations. When a mixed community of microorganisms, such as multiple LAB strains, is used for fermentation (like in cereals), it leads to a wider range of peptide products. The interaction between different microbial strains in a mixed culture can be synergistic, enhancing the overall production of diverse peptides compared to using a single strain [
79].
Last but not least, peptide production depends on the substrate protein content and quality [
80]. Dairy is rich in casein and whey proteins; cereals have prolamins, glutelins, and other storage proteins (with lower digestibility). The type of cereal (rye, wheat, spelt) and the level of protein affect how many peptides and which ones can be released. Wholegrain flours or intact kernels often result in more substrates for proteolysis, which explains the presence of multiple peptides in sordough rye bread.
Although in vitro and animal models provide clear evidence for ACE-inhibitory and antioxidant peptides in fermented dairy and cereals, human data on peptide-specific clinical endpoints and bioavailability are limited; this issue is discussed in detail in
Section 7 (Challenges and Research Gaps).
4.2. Polyphenols and Secondary Metabolites
Polyphenols and other secondary plant metabolites (flavonoids, phenolic acids, betalains, glucosinolates and derivatives) are major contributors to the antioxidant, anti-inflammatory and signalling properties attributed to many plant-based foods [
81,
82,
83]. Fermentation of vegetables, cereals, and berries can significantly alter the content, chemical form, and bioavailability of polyphenols, flavonoids, betalains and related secondary metabolites. In traditional Eastern European fermentations such as sauerkraut, beet kvass and berry ferments, these compounds may be liberated from bound forms, transformed by microbial enzymes, or preserved against degradation, collectively enhancing antioxidant capacity.
These polyphenols and their metabolites can act as free radical scavengers, modulate the gut microbiota, and influence inflammatory and metabolic pathways [
84]. The following subsections detail the underlying biochemical mechanisms, examples from vegetable and cereal fermentations, and implications for health. Key transformations of polyphenols and related secondary metabolites in traditional Eastern European fermented foods are summarized in
Table 2.
Microbial fermentation affects polyphenols through several, well-characterised biochemical routes:
- -
Hydrolysis of ester/ether bonds and depolymerization: microbial esterases and glycosidases cleave polyphenol conjugates (e.g., glycosides, phenolic acid esters), liberating aglycones and free phenolic acids that are more extractable and sometimes more bioactive [
84,
85]
- -
Decarboxylation and reduction: some LAB possess phenolic acid decarboxylases (pdc) and related enzymes that convert hydroxycinnamic acids (e.g., ferulic, p-coumaric acid) into vinyl/ethyl derivatives with altered bioactivities. Strain-level differences in pdc genes materially affect the metabolic outcome [
86,
87,
88]
- -
Deglycosylation: microbial β-glucosidases remove sugar moieties from flavonoid glycosides, producing [
84]
- -
Formation of novel metabolites (postbiotic phenolics): microbial metabolism can produce smaller phenolic catabolites and short-chain phenolic acids that may exert direct biological effects or act via the gut microbiota [
84,
89]
These mechanisms explain why fermentation can increase extractable polyphenols and antioxidant capacity in many studies — but they also explain variability: the same substrate fermented with different microbes or conditions can give divergent results.
4.2.1. Evidence from Vegetable Fermentations (Sauerkraut, Beet Kvass, Mixed Ferments)
Sauerkraut and brassica ferments: Lactic acid fermentation of cabbage alters its phytochemical profile in predictable ways: glucosinolates may be hydrolysed to isothiocyanates or nitriles depending on processing (cutting, salting, temperature) [
90], and polyphenol extractability can increase as cell walls are partially degraded and conjugates hydrolysed [
91]. Targeted metabolomic and metagenomic studies of sauerkraut document dynamic shifts in metabolites and microbial functions across fermentation stages, linking specific microbial succession to secondary-metabolite transformations. These changes underlie measured increases in certain antioxidant activities in many (but not all) sauerkraut preparations [
92,
93].
Beet kvass and betalain-rich ferments: Beetroot fermentations (beet kvass) present a slightly different profile: the dominant pigments are betalains (betacyanins and betaxanthins), not flavonoids.[
94]. Several recent analyses of commercially produced and traditional fermented beetroot juices in Polish markets show high antioxidant capacity and measurable betalain content in fermented products, although thermal processing and fermentation conditions influence betalain stability. Some studies report retention or even improved antioxidant activity in certain beet ferments, while others show losses depending on boiling/processing and long fermentation times — so product formulation and process control matter. [
95,
96].
So, regarding vegetables, fermentation often increases extractability and alters the profile of polyphenols and glucosinolate derivatives in vegetable ferments (including sauerkraut), and can preserve or concentrate betalains in beet ferments — but results are conditional on processing and strain/temperature/salt choices [
97].
4.2.2. Evidence from Cereal Fermentations (Rye Sourdough and Related Products)
Rye and other cereal sourdough fermentations illustrate another common pattern: many phenolic compounds in cereals are bound (esterified to cell walls, ferulic acid conjugates). LAB and fungal enzymes during sourdough fermentation can release these bound phenolics (e.g., ferulic, vanillic, p-coumaric acids), increasing extractable phenolics and antioxidant capacity of the dough and bread [
85]. Metabolic and peptidomic studies on sourdoughs (wheat and rye) demonstrate strain-dependent metabolism of phenolic acids and increased extractability after fermentation, which contributes to improved in vitro antioxidant markers and may partly explain lower postprandial glycemic responses of long-fermented rye breads.[
98]. However, the magnitude of these changes depends on flour type, bran fraction, fermentation length, and the specific LAB/yeast strains used [
98,
99].
4.2.3. Evidence from Fermented Beverages (Kombucha and Tea-Based Ferments)
Kombucha and similar tea-based ferments derive most polyphenols from the tea substrate (catechins, theaflavins, flavonols). Fermentation by the SCOBY (yeasts + bacteria) modifies the polyphenolic profile: some polyphenols are transformed (deglycosylated, oxidized), and the total measured antioxidant activity often increases relative to the starting infusion, particularly when tea residues or polyphenol-rich adjuncts are used [
100]. Recent studies show that formulation (type of tea, addition of herbs) and fermentation time markedly influence total phenolics and antioxidant measures. Nonetheless, human clinical evidence for systemic effects of kombucha polyphenols is limited and mixed, and safety/variability issues mean that functional claims should be cautious [
97,
101].
4.2.4. Factors Driving Heterogeneity in Outcomes
Several variables explain why studies report increases, no change, or decreases in polyphenol content after fermentation:
Raw material and pre-processing: cooking, blanching, or boiling before fermentation can leach or degrade heat-sensitive compounds (notably vitamin C and some polyphenols/betalains). Studies show boiling + fermentation often reduces some phenolics compared with raw [
96,
102]
Microbial strains and enzymatic repertoire: presence/absence of β-glucosidase, esterases, and pdc genes determines whether bound phenolics are liberated or decarboxylated into less extractable forms. Strain selection (or spontaneous flora) is therefore critical [
85,
89]
Fermentation time, temperature, and oxygen: longer or warmer fermentations often increase conversion but may also permit oxidative degradation; controlled low-temperature fermentations can preserve labile pigments [
92,
96]
Matrix interactions: polyphenols can bind to proteins or fibre; proteolysis and polysaccharide breakdown during fermentation alter binding equilibria and extractability [
84]
4.2.5. Implications for Bioavailability and Health
Increased extractability is a plausible first step toward improved bioavailability, but human absorption and metabolism depend on intestinal and hepatic processing as well as gut microbiota conversion to smaller phenolic metabolites. Few studies combine food-level fermentation analysis with human pharmacokinetic or clinical endpoints; this remains a key evidence gap [
84,
99]. Multiple in vitro and animal studies show fermentation-enhanced antioxidant and anti-inflammatory activities; however, direct clinical evidence that fermented vegetable or sourdough-derived polyphenols reduce disease endpoints is limited and heterogeneous. Hence, claims should be framed as potential functional contributions supported by mechanistic data rather than proven clinical effects [
84,
92]. Paired studies that couple detailed fermentation metabolomics (untargeted LC-MS) with human absorption (plasma metabolomics) and clinical endpoints are still rare but needed [
92,
99]. Strain-level functional screening for key enzymes (β-glucosidase, esterases, pdc) and deliberate starter selection could standardize beneficial transformations while preserving traditional sensory profiles [
85,
89]. We noticesd that process optimization (time/temperature/salt) studies to maximize retention of labile polyphenols (e.g., betalains) while promoting liberation of bound phenolics are essential for product development [
96].
Table 2.
Polyphenols and secondary metabolites in traditional Eastern European fermented foods and their microbial transformations.
Table 2.
Polyphenols and secondary metabolites in traditional Eastern European fermented foods and their microbial transformations.
| Food |
Major
polyphenol / pigment classes |
Microbial or enzymatic transformation |
Reported
outcome |
Key
references |
| Sauerkraut |
Phenolic acids,
flavonoids, glucosinolates |
β-glucosidase-mediated
deglycosylation; partial hydrolysis of glucosinolates |
↑ extractable phenolics,
↑ antioxidant activity |
[84,103] |
| Beet kvass |
Betalains
(betacyanins,
betaxanthins), phenolic acids |
LAB fermentation
preserves betalains, produces phenolic catabolites |
Mixed outcomes;
potential
↑ antioxidant
potential |
[95] |
| Rye sourdough |
Ferulic, p-coumaric, vanillic acids (bound) |
LAB esterase and
decarboxylase
activity releases
bound phenolics |
↑ extractable phenolics, improved antioxidant capacity |
[59] |
| Kombucha |
Catechins, theaflavins, flavonols |
Yeast/bacterial oxidation and deglycosylation |
↑ total phenolics
(moderate), altered flavonoid profile |
[100] |
4.3. Probiotics, Prebiotics and Postbiotics
Fermented foods represent complex microbial ecosystems containing live probiotic organisms and a rich milieu of bioactive microbial metabolites, collectively referred to as postbiotics. Their effects on health are discussed in subsections 5.2. and 5.3. Unlike isolated probiotic supplements, traditional fermented foods deliver microorganisms within a nutrient-rich matrix, which can modulate their survival, colonization, and host interactions.
4.3.1. Probiotic Microorganisms
Traditional Eastern European fermentations rely largely on LAB and, to a lesser extent, yeasts, many of which meet the FAO/WHO definition of probiotics when administered in adequate amounts.
Dominant genera include:
Lactobacillus (especially L. plantarum, L. brevis, L. casei, L. helveticus, L. kefiri)
Leuconostoc mesenteroides and Leuconostoc lactis
Lactococcus lactis and Pediococcus pentosaceus
Yeasts such as Saccharomyces cerevisiae and Kluyveromyces marxianus
These taxa are recurrently identified in kefir, sauerkraut, bryndza, and rye sourdough and are associated with distinct health-related mechanisms, as presented in
Table 3.
Recent human trials support that regular consumption of fermented foods increases microbiota diversity and reduces systemic inflammatory markers (e.g., IL-6, TNF-α) in healthy adults [
105]. These effects were stronger for multi-strain, naturally fermented foods such as kefir and sauerkraut than for single-strain probiotic products [
51].
4.3.2. Pre - and Postbiotic Compounds and Their Functional Roles
Even in the absence of viable cells, fermented food matrices remain biologically active due to a broad range of pre and postbiotic compounds. Prebiotics are substrates that are selectively utilized by host microorganisms conferring a health benefit; postbiotics are preparations of inanimate microorganisms and/or their components that confer a health benefit. In the context of traditional Eastern European fermented foods, both classes are important: fermentation can generate or concentrate fermentable substrates (prebiotics) and it produces or releases microbial-derived molecules and structural components (postbiotics) that act on host physiology even when microbes are non-viable.
-
Prebiotics:
The key compound classes of prebiotics are fermentable oligosaccharides (including arabinoxylo-oligosaccharides from cereals), resistant starch fractions, pectic-oligosaccharides, and certain microbial exopolysaccharides (EPS) produced in situ [
110]. Sources in Eastern European ferments are different, in function of the substrate. In rye sourdough and other cereal ferments, partial hydrolysis of hemicelluloses and arabinoxylans during long fermentation can release short-chain oligosaccharides (AXOS) that are fermentable by beneficial colonic bacteria [
111]. In vegetable ferments (sauerkraut, mixed pickles) there is cell-wall breakdown and endogenous enzyme action during fermentation that may increase the proportion of soluble fibers and pectic-oligosaccharides, potentially improving fermentability[
112]. As for dairy ferments (kefir), certain EPS (e.g., kefiran) synthesized by kefir-associated microbes resist host digestion and can act as substrates for gut microbes [
113]. The mechanisms of action of prebiotics include selective stimulation of beneficial microbial taxa (e.g., Bifidobacterium, certain Faecalibacterium spp.), leading to increased production of SCFAs in the colon [
114], indirect modulation of host physiology via SCFAs (energy source for colonocytes, signaling via G-protein coupled receptors such as GPR41/43, epigenetic modulation via histone deacetylase inhibition)[
115] and improvement of mineral bioavailability through SCFA-mediated pH reduction and solubilization of cations[
41]. Most mechanistic data for these effects come from in vitro fermentation models and animal studies [
116,
117,
118]; human trials directly linking specific fermentation-generated prebiotics from traditional foods to clinical endpoints are limited [
119] Demonstration of selective utilization and downstream SCFA changes in human feeding studies is an important research priority.
Postbiotic compounds produced or enriched by fermentation belong to SCFAs, organic acids (lactic, acetic), microbially released bioactive peptides, vitamins (microbial-synthesized B-vitamins, menaquinones), microbial cell-wall components (peptidoglycan fragments, lipoteichoic acids), heat-stable metabolites, and extracellular vesicles [
120].
SCFAs and organic acids are produced during fermentation in situ (and generated later in the colon following prebiotic fermentation). Many traditional ferments contain measurable lactic and acetic acids; some cereal and vegetable ferments may contribute substrates that increase colonic SCFA production. Microbial peptides and small molecules result from proteolysis during dairy and cereal fermentation releases peptides (see
Section 4.1) that can act locally or systemically (e.g., ACE-inhibitory peptides). The structural microbial components are sterilized (cell-free) fractions from sauerkraut and some dairy ferment supernatants. They retain biological activity in vitro and in vivo, indicating that non-viable cell material and soluble metabolites contribute to effects attributed to the food matrix [
121,
122,
123]. The mechanisms of action of postbiotics are: immune modulation- cell wall components and bacterial metabolites interacting with pattern recognition receptors (e.g., toll-like receptors-TLRs, Nucleotide-binding Oligomerization Domain proteins -NODs) on epithelial and immune cells [
124], modulating cytokine production and mucosal immune tone, barrier function where SCFAs and certain microbial metabolites enhance expression/localization of tight-junction proteins (occludin, claudins), reduce epithelial permeability, and promote mucin production [
125]. There are also antimicrobial and competitive effects: organic acids and bacteriocins in cell-free supernatants inhibit pathogen growth and can alter luminal ecology without requiring live cells [
126] and metabolic signaling. SCFAs act as signaling molecules (via GPRs), influence hepatic lipid and glucose metabolism, and contribute to appetite regulation through enteroendocrine pathways [
127].
Preclinical studies (cell culture, gnotobiotic and conventional animal models) provide mechanistic support for many postbiotic actions [
125,
128]. Human evidence is emerging: randomized feeding studies with whole fermented foods have shown changes in biomarkers of inflammation, gut permeability, and metabolite profiles, but disentangling the contributions of live microbes, prebiotic substrates, and postbiotic molecules remains challenging.[
105,
129,
130,
131]. Isolated postbiotic preparations (e.g., heat-killed strains, purified EPS) are less commonly tested in large human trials but show promise in controlled settings[
132,
133,
134,
135].
4.4. Vitamins and Micronutrients
Fermentation can increase or stabilize the content of several vitamins and minerals. Certain lactic acid bacteria and yeasts are capable of synthesizing B-group vitamins, including riboflavin, folate, and cobalamin (vitamin B₁₂), during dairy, cereal, and vegetable fermentations [
136]. For example, L.plantarum and L. fermentum strains used in sourdough and sauerkraut are known folate producers, while Propionibacterium freudenreichii in some cheeses contributes to B₁₂ and vitamin K₂ (menaquinone) formation—nutrients important for hematologic, bone, and cardiovascular health [
137,
138]. In parallel, microbial metabolism during fermentation can improve mineral bioavailability. Organic acid production lowers pH and reduces antinutritional factors such as phytates and oxalates, which normally chelate iron, zinc, calcium, and magnesium. The degradation of phytic acid in fermented cereals and legumes markedly enhances the absorption of these minerals, while lactic acid itself may facilitate passive mineral transport in the intestine [
139,
140,
141]. Traditional fermented foods, particularly in Eastern Europe—such as kefir, bryndza cheese, sauerkraut, and rye sourdough—thus provide not only preserved but also nutrient-enriched staples, especially valuable in seasons when fresh produce is scarce. Although much of the evidence arises from compositional and in vitro studies, emerging human trials suggest that long-term inclusion of fermented foods can modestly improve micronutrient status and overall dietary quality [
142].
4.5. Organic Acids
Organic acids—mainly lactic, acetic, propionic, and butyric acids—are among the most characteristic metabolites produced during fermentation by lactic acid bacteria and associated yeasts. These acids are central to both the safety and sensory profile of fermented foods [
143]
, contributing to flavor development, texture, and extended shelf life through pH reduction and inhibition of spoilage organisms. Beyond preservation, organic acids exert nutritional and physiological roles. Lactic and acetic acids can enhance mineral bioavailability by reducing intestinal pH and solubilizing cations such as calcium, magnesium, and iron [
144]. Some organic acids, including butyrate and propionate, also act as metabolic intermediates in colonocyte energy metabolism and influence glucose and lipid homeostasis via G-protein–coupled receptors (GPR41, GPR43) [
145,
146]. In traditional Eastern European products such as sauerkraut, kefir, and kvass, organic acids are key indicators of successful fermentation and product stability.
The health-promoting potential of fermented foods arises not from a single compound but from multiple interactions between components, making traditional foods functionally complex, potentially providing broader health effects than isolated bioactive compounds or supplements [
147,
148,
149].
For example, kefir combines peptides, vitamins, lactic acid, and live microbes, which together influence different health endpoints. Vegetable fermentations similarly integrate polyphenols, vitamins, and LAB metabolites, reinforcing antioxidant and anti-inflammatory effects.
5. Health Effects: Evidence Overview
Eastern European fermented foods contain diverse bioactives and microbes (see
Section 4) that plausibly impact different health outcomes. The subsections below summarize mechanistic evidence and human studies for each domain.
5.1. Cardiovascular Health
Fermented dairy, particularly kefir and traditional cheeses, and fermented vegetables like sauerkraut, may contribute to blood pressure regulation, lipid modulation, and vascular function.
The antihypertensive and vascular effects observed in vivo are thought to reflect the combined action of ACE-inhibitory peptides (see
Section 4.1) and antioxidant polyphenols (see
Section 4.2), which protect endothelial cells and modulate nitric oxide availability. LAB fermentation can produce bioactive lipids, including conjugated linoleic acid, which may influence lipid metabolism [
150,
151]
. A randomized trial with kefir consumption (200–500 mL/day for 8–12 weeks) in hypertensive patients demonstrated modest but significant reductions in systolic and diastolic blood pressure [
3]. However, a recent meta-analysis of RCTs found no significant effect of kefir on blood pressure in adults[
152]. In vitro studies of bryndza and other sheep-milk cheeses confirm ACE-inhibitory activity and antioxidant capacity [
153]. Observational data from Eastern European populations consuming fermented vegetables suggest an association with lower cardiovascular risk markers, although controlled studies remain limited [
9].
5.2. Gastrointestinal Health
Traditional Eastern European fermented foods such as kefir, sauerkraut, and rye sourdough exert beneficial effects on gastrointestinal function through their combined content of live microorganisms, fermentable substrates, and bioactive metabolites. These foods enhance gut microbial diversity, competitively inhibit pathogenic bacteria, and strengthen intestinal barrier integrity via lactic acid bacteria and yeasts [
9,
147]. Fermentation metabolites—particularly SCFAs
—serve as energy substrates for colonocytes and regulate mucosal immune responses, while polyphenols from fermented vegetables and cereals might act
as prebiotic substrates promoting the growth of beneficial taxa, though they are not recognised prebiotics sensu stricto.
Clinical observations report that kefir and sauerkraut consumption can improve stool frequency and consistency [
154] in individuals with functional constipation [
155], while small-scale studies suggest a reduction in Helicobacter pylori load with regular fermented vegetable intake [
156]. Animal models further support improved barrier function and anti-inflammatory signaling following fermented dairy and cereal consumption [
157,
158], although large-scale human trials remain limited [
157,
159] and preliminary.
5.3. Immune Function
In Eastern European diets, traditional fermented foods such as kefir, bryndza, and fermented vegetables may influence both innate and adaptive immune responses through microbial metabolites and bioactive compounds. Lactic acid bacteria can stimulate immunoglobulin A (IgA) production and modulate cytokine balance, promoting an anti-inflammatory immune tone [
160,
161]. As described earlier, bioactive peptides and exopolysaccharides formed during fermentation also exert immunomodulatory effects, enhancing resistance to pathogens, while vitamins synthesized microbially (e.g., folate, B₁₂, and K₂) support cellular immune processes. Human and experimental studies involving kefir consumption report increased natural killer (NK) cell activity and enhanced mucosal immunity, suggesting systemic immune engagement beyond the gut [
162,
163]. In addition, observational and laboratory studies link fermented vegetable intake with lower circulating inflammatory markers and improved immune balance [
164,
165,
166,
167]. While these findings highlight plausible mechanisms and biological effects, clinical evidence remains limited, and well-controlled trials using standardized, region-specific fermented products are needed to confirm causal effects.
5.4. Metabolic Health
In Eastern European diets, fermented dairy, vegetable, and cereal products may contribute to metabolic homeostasis by influencing glucose regulation, lipid metabolism, and inflammatory balance. Bioactive peptides and SCFAs formed during fermentation can enhance insulin sensitivity and glucose uptake by peripheral tissues, while polyphenols and microbial metabolites help attenuate oxidative stress and low-grade inflammation that underlie metabolic disorders [
168,
169]. Fermentation of cereals, particularly in rye sourdough, reduces the glycemic index of bread and increases mineral bioavailability through phytate degradation and organic acid formation [
73]. Intervention studies in adults with metabolic syndrome report modest reductions in fasting glucose and improvements in lipid profiles following regular consumption of kefir or fermented vegetables [
170,
171]. Similarly, rye sourdough bread elicits a lower postprandial glucose response than industrial yeast breads, likely due to organic acids and fermentation-induced peptide activity [
77,
172,
173,
174]. However, human evidence remains limited, and further controlled trials with traditional Eastern European products are warranted.
6. Comparative Perspective
Eastern European fermented foods occupy a distinct niche within global fermentation traditions, both in terms of microbial composition and functional properties. While many fermented foods share general mechanisms — such as lactic acid production, bioactive peptide generation, and polyphenol enhancement — regional variations create unique nutritional and functional signatures (
Table 4).
6.1. Microbial Diversity
Eastern European fermentations often rely on spontaneous or mixed-culture fermentation, resulting in complex consortia of lactic acid bacteria (
Lactobacillus, Leuconostoc, Lactococcus), acetic acid bacteria, and yeasts. In contrast, many Western commercial products (e.g., industrial yogurt) employ single-strain or defined starter cultures, which produce more consistent but less diverse microbial profiles [
175]. Asian fermentations, such as kimchi or miso, include region-specific microbial species, but raw materials (e.g., soy, chili, seaweed) and flavor profiles differ substantially from the cabbage-, dairy-, and cereal-based Eastern European repertoire [
176]. Metagenomic studies confirm that spontaneous fermentations typical of Eastern Europe harbor a higher microbial richness and functional gene diversity than controlled starter fermentations [
177,
178]. Such diversity enables cooperative and sequential metabolism among taxa, generating a broader range of bioactive peptides, organic acids, and volatile compounds.
This microbial diversity contributes to distinctive flavor, aroma, and functional metabolite profiles, potentially supporting broader physiological effects.
6.2. Substrates and Nutrient Matrix
Vegetable substrates dominate in Eastern Europe (cabbage, beet, cucumbers), whereas fermented dairy is prominent in both Eastern Europe and Western Europe, and legumes/soy in Asia. In contrast to soy- and pulse-based Asian ferments or milk-centered Western ones, Eastern European traditions combine vegetables, cereals, and dairy—substrates that together yield a nutritionally dense, microbially active matrix well adapted to cold climates and seasonal food scarcity [
179]
. Cereal-based fermentations (rye sourdough, kvass) are particularly characteristic of Eastern Europe and northern climates, providing unique bioactive peptides and organic acids not commonly found in Asian or Western diets. The combination of seasonally available, locally sourced ingredients and traditional preparation methods shapes the nutrient matrix, impacting bioactive content and potential health effects.
6.3. Functional Implications
The synergy of microbial diversity and substrate composition in Eastern European fermented foods results in distinct functional properties, including a broader spectrum of bioactive peptides, diverse postbiotic metabolites, and multiple micronutrient enhancements. While similar functional claims are made for Asian and Western fermented foods, the integration of cereals, vegetables, and dairy in Eastern Europe is relatively unique, offering a more complex combination of bioactives in single dietary items [
147,
179]
. This integrative pattern may underlie the observed resilience of traditional Eastern European diets, which combine plant-based ferments with dairy matrices, providing complementary microbial and metabolic stimuli that may support metabolic, immune, and gut health simultaneously.
6.4. Cultural Context and Persistence
The persistence of household-level fermentation practices in Eastern Europe, maintained across centuries probably as reaction to food scarcity but also as a result of own harvest preservation, supports ongoing exposure to diverse microbial and bioactive profiles.
This continuity preserves both microbial and biochemical biodiversity that are increasingly eroded in industrialized food systems, linking cultural resilience with potential health resilience. Distinct regional varieties illustrate this diversity: Romanian borș (a wheat-bran ferment used as a soup base), Polish kapusta kiszona (traditional sauerkraut), Slovak bryndza (fermented sheep cheese), and Russian or Ukrainian kvass (mildly fermented cereal beverage) exemplify how fermentation adapted to local raw materials and seasonal cycles. These products, though simple in preparation, maintain complex microbial ecosystems that reflect centuries of empirical selection and household-level innovation, linking food preservation directly to identity and resilience.
This contrasts with regions where industrial production dominates, potentially limiting microbial diversity and functional variation. Traditional knowledge, seasonal cycles, and communal practices ensure that functional potential is closely tied to cultural heritage, reinforcing both dietary and health impacts.
7. Challenges and Research Gaps
Despite the long-standing consumption and apparent functional potential of Eastern European fermented foods, several scientific and methodological challenges limit a comprehensive understanding of their health effects.
7.1. Heterogeneity of Traditional Practices
Preparation methods vary widely by region, household, and season, resulting in substantial variability in microbial composition, metabolite content, and nutrient profiles. Standardization for research purposes remains difficult, as most published studies rely on commercially produced or laboratory-fermented samples that may not accurately represent traditional products. This heterogeneity complicates reproducibility and cross-study comparison [
10].
7.2. Limited Clinical Evidence
While in vitro and animal studies provide strong mechanistic support, well-designed human trials remain scarce, as explained previously in this article. Existing studies are typically small, short-term, or observational, limiting the ability to infer causality or establish dose–response relationships. Few clinical investigations directly compare traditional fermented foods with industrial analogues, leaving uncertainty about the magnitude of their specific health impacts.
7.3. Bioavailability and Mechanistic Understanding
The bioavailability of bioactive peptides, polyphenols, and vitamins in fermented foods remains insufficiently characterized. The presence of these compounds does not necessarily ensure efficient absorption or biological activity. Interactions between microbial metabolites and host physiology—including modulation of the gut microbiota, epithelial signaling, and metabolic pathways—are incompletely understood [
147,
180]. Longitudinal and multi-omic studies are needed to clarify how habitual consumption influences chronic disease outcomes.
7.4. Safety and Quality Considerations
Some traditional products contain high salt levels (notably fermented vegetables) or trace ethanol (in cereal-based beverages such as kvass), which may present health risks for sensitive populations. [
32,
181,
182,
183]. This highlights the importance of hygienic practices, consumer education, and microbial monitoring to ensure both safety and functional integrity.
7.5. Integration with Modern Nutrition Science
Although traditional knowledge provides a valuable foundation, systematic scientific integration remains limited. Translating artisanal practices into evidence-based dietary recommendations or functional food innovations requires comprehensive nutritional, microbial, and metabolomic characterization. The application of omics technologies can help map microbial and metabolite diversity, link them to health endpoints, and guide the rational development of standardized, culturally grounded fermented foods [
184]. Addressing these challenges will require interdisciplinary collaboration among microbiologists, nutritionists, food technologists, and public health experts. Harmonized methodologies, region-specific food characterization, and large-scale human intervention trials are critical steps toward validating the health-promoting potential of Eastern European fermented foods within modern nutrition science. Despite the deep-rooted presence of fermentation across Eastern Europe, scientific documentation and coordinated research remain uneven. Most published data originate from Poland, Romania, and the Czech Republic, while Balkan and Baltic countries are underrepresented in the international literature. Establishing regional collaborations or EU-supported consortia could facilitate standardized methods, microbial biobanking, and cross-country clinical research. Such initiatives would elevate the global visibility of Eastern European food heritage and accelerate its integration into evidence-based nutrition and functional food science
.
8. Conclusions and Perspectives
Eastern European fermented foods represent a unique intersection of nutrition, microbiology, and cultural tradition. Traditional household fermentation practices maintain microbial and biochemical diversity; integrating this heritage into research design is both a scientific opportunity and an ethical imperative. Their distinctive microbial diversity, multi-substrate fermentation, and continuity within traditional diets distinguish them from Western and Asian fermentation systems, highlighting both functional potential and cultural significance.
Although preclinical and small human studies support beneficial physiological effects, the evidence base remains fragmented. Well-controlled clinical trials, standardized characterization of traditional products, and detailed mechanistic studies are urgently needed to substantiate causal links between traditional fermented food intake and measurable health outcomes.
Future research should focus on the systematic standardization and molecular characterization of traditional preparations while preserving cultural authenticity. The application of omics-based technologies—metagenomics, metabolomics, proteomics, and transcriptomics—offers unprecedented opportunities to identify novel microbial strains, metabolic pathways, and synergistic interactions within multi-component fermentations. These approaches can map strain-level diversity and bioactive metabolite profiles, bridging artisanal practices with modern food science.
Another research priority is the assessment of bioavailability and host interactions for fermentation-derived peptides, polyphenols, vitamins, and postbiotics, ideally through integrated human trials combining metabolic and microbiome endpoints.
Beyond laboratory research, innovation should connect traditional methods with modern functional food development, ensuring safety, palatability, and consumer acceptance. Finally, public health strategies could integrate traditional fermented foods into dietary guidelines, promote education on safe home fermentation practices, and encourage culturally grounded dietary diversity as part of sustainable nutrition models.
Linking traditional knowledge with modern nutritional science can transform Eastern European fermented foods from legacy staples into scientifically validated, health-promoting functional foods.
Author Contributions
Conceptualization, C.Z.; methodology, C.C. and C.Z.; resources C.Z., writing—review and editing, C.Z.; supervision, C.C.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- McGovern, P.E.; Zhang, J.; Tang, J.; Zhang, Z.; Hall, G.R.; Moreau, R.A.; Nuñez, A.; Butrym, E.D.; Richards, M.P.; Wang, C.; et al. Fermented Beverages of Pre- and Proto-Historic China. Proc. Natl. Acad. Sci. 2004, 101, 17593–17598. [Google Scholar] [CrossRef]
- Şanlier, N.; Gökcen, B.B.; Sezgin, A.C. Health Benefits of Fermented Foods. Crit. Rev. Food Sci. Nutr. 2019, 59, 506–527. [Google Scholar] [CrossRef]
- Apalowo, O.E.; Adegoye, G.A.; Mbogori, T.; Kandiah, J.; Obuotor, T.M. Nutritional Characteristics, Health Impact, and Applications of Kefir. Foods 2024, 13, 1026. [Google Scholar] [CrossRef]
- Raak, C.; Ostermann, T.; Boehm, K.; Molsberger, F. Regular Consumption of Sauerkraut and Its Effect on Human Health: A Bibliometric Analysis. Glob. Adv. Health Med. 2014, 3, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Kaszuba, J.; Jańczak-Pieniążek, M.; Migut, D.; Kapusta, I.; Buczek, J. Comparison of the Antioxidant and Sensorial Properties of Kvass Produced from Mountain Rye Bread with the Addition of Selected Plant Raw Materials. Foods 2024, 13, 357. [Google Scholar] [CrossRef]
- Skowron, K.; Budzyńska, A.; Grudlewska-Buda, K.; Wiktorczyk-Kapischke, N.; Andrzejewska, M.; Wałecka-Zacharska, E.; Gospodarek-Komkowska, E. Two Faces of Fermented Foods—The Benefits and Threats of Its Consumption. Front. Microbiol. 2022, 13. [Google Scholar] [CrossRef]
- Food Consumption Data | EFSA. Available online: https://www.efsa.europa.eu/en/data-report/food-consumption-data (accessed on 20 October 2025).
- The Global Tradition of Fermented Foods: A Culinary Staple Across Cultures. Available online: https://www.thefermented.org/learn/the-global-tradition-of-fermented-foods-a-culinary-staple-across-cultures (accessed on 20 October 2025).
- Wiśniewska, M.Z.; Kowalska, A.; Manning, L.; Malinowska, E.; Kowalski, P. Consumption and Home Preparation of Fermented Vegetable Products in Poland. Zesz. Nauk. Organ. Zarządzanie Politech. Śląska 2022, 557–578. [Google Scholar]
- Sõukand, R.; Pieroni, A.; Biró, M.; Dénes, A.; Dogan, Y.; Hajdari, A.; Kalle, R.; Reade, B.; Mustafa, B.; Nedelcheva, A.; et al. An Ethnobotanical Perspective on Traditional Fermented Plant Foods and Beverages in Eastern Europe. J. Ethnopharmacol. 2015, 170, 284–296. [Google Scholar] [CrossRef]
- Steinkraus, K.H. Classification of Fermented Foods: Worldwide Review of Household Fermentation Techniques. Food Control 1997, 8, 311–317. [Google Scholar] [CrossRef]
- Tamang, J.P.; Watanabe, K.; Holzapfel, W.H. Review: Diversity of Microorganisms in Global Fermented Foods and Beverages. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Sauerkraut History and Use in Eastern European Recipes. Available online: https://web.archive.org/web/20170116055455/http://easteuropeanfood.about.com/od/vegetables/a/sauerkraut.htm (accessed on 29 October 2025).
- Horvath, S.; Vass, E.; Ardelean, E. Alimentaţia tradiţională din Bihor între secolul al XVIII-lea şi începutul secolului al XX-lea; Editura Muzeului Ţării Crişurilor: Oradea, 2017; ISBN 978-973-7621-95-5. [Google Scholar]
- Beet Kvass: An Unbeetable Traditional Ukrainian Health Drink | Tin and Thyme. Available online: https://tinandthyme.uk/2018/05/beet-kvass/ (accessed on 20 October 2025).
- Netmeds | Www.Netmeds.Com. Available online: https://www.netmeds.com/c/health-library/post/sauerkraut-nutrition-uses-health-benefits-and-know-how-to-make-this-probiotic-food-at-home (accessed on 20 October 2025).
- Knight, S.; Klaere, S.; Fedrizzi, B.; Goddard, M.R. Regional Microbial Signatures Positively Correlate with Differential Wine Phenotypes: Evidence for a Microbial Aspect to Terroir. Sci. Rep. 2015, 5, 14233. [Google Scholar] [CrossRef] [PubMed]
- Prado, M.R.; Blandón, L.M.; Vandenberghe, L.P.S.; Rodrigues, C.; Castro, G.R.; Thomaz-Soccol, V.; Soccol, C.R. Milk Kefir: Composition, Microbial Cultures, Biological Activities, and Related Products. Front. Microbiol. 2015, 6. [Google Scholar] [CrossRef]
- Ряженка: Пoльза, Прoизвoдствo и Рецепты Этoгo Традициoннoгo Кислoмoлoч. Available online: https://gastronomusa.com/ru/blogs/blogs/ryazhenka-benefits-production-and-recipes-with-this-traditional-dairy-drink (accessed on 20 October 2025).
- Altay, F. Chapter 17 - Rheology and Functionality of Ayran—A Yogurt Drink. In Yogurt in Health and Disease Prevention; Shah, N.P., Ed.; Academic Press, 2017; pp. 295–305 ISBN 978-0-12-805134-4.
- Teneva-Angelova, T.; Balabanova, T.; Boyanova, P.; Beshkova, D. Traditional Balkan Fermented Milk Products. Eng. Life Sci. 2018, 18, 807–819. [Google Scholar] [CrossRef]
- Revue générale de chimie pure & appliquée; Bureau de la Revue, 1908.
- 4 Best Dairy Products in Eastern Europe - TasteAtlas. Available online: https://www.tasteatlas.com/best-rated-dairy-products-in-eastern-europe (accessed on 20 October 2025).
- Maciuc, V.; Pânzaru, C.; Ciocan-Alupii, M.; Radu-Rusu, C.-G.; Radu-Rusu, R.-M. Comparative Assessment of the Nutritional and Sanogenic Features of Certain Cheese Sorts Originating in Conventional Dairy Farms and in “Mountainous” Quality System Farms. Agriculture 2024, 14, 172. [Google Scholar] [CrossRef]
- Tsafrakidou, P.; Michaelidou, A.-M.; Biliaderis, C.G. Fermented Cereal-Based Products: Nutritional Aspects, Possible Impact on Gut Microbiota and Health Implications. Foods 2020, 9, 734. [Google Scholar] [CrossRef]
- Iversen, K.N.; Dicksved, J.; Zoki, C.; Fristedt, R.; Pelve, E.A.; Langton, M.; Landberg, R. The Effects of High Fiber Rye, Compared to Refined Wheat, on Gut Microbiota Composition, Plasma Short Chain Fatty Acids, and Implications for Weight Loss and Metabolic Risk Factors (the RyeWeight Study). Nutrients 2022, 14, 1669. [Google Scholar] [CrossRef]
- blog, V.-B. ’n B. Ancient Russian Fermented Kissel Porridge | Beets & Bones 2018.
- Puskar, L. Why Eastern Europeans Revere Black Bread. Available online: https://www.greenseashells.com/post/why-eastern-europeans-revere-black-bread (accessed on 30 October 2025).
- 15 Best Sourdough Breads in Europe. Available online: https://www.tasteatlas.com/best-rated-sourdough-breads-in-europe (accessed on 30 October 2025).
- Ethnic Fermented Foods of the World: An Overview | Journal of Ethnic Foods | Full Text. Available online: https://journalofethnicfoods.biomedcentral.com/articles/10.1186/s42779-024-00254-2 (accessed on 20 October 2025).
- Ignatenko, B.V.; Nikolaeva, M.A.; Eliseev, M.N. History of kvass as a national drink. Tovaroved Prodovolstvennykh Tovarov Commod. Spec. Food Prod. 2021, 99, 160–163. [Google Scholar] [CrossRef]
- Dlusskaya, E.; Jänsch, A.; Schwab, C.; Gänzle, M.G. Microbial and Chemical Analysis of a Kvass Fermentation. Eur. Food Res. Technol. 2008, 227, 261–266. [Google Scholar] [CrossRef]
- This Ancient East European Brew Repurposes Leftover Bread – to Great Effect. Available online: https://www.sbs.com.au/food/article/kvass-fermented-drink/gwnxipi2f (accessed on 20 October 2025).
- Borș Wheat Bran Fermented Beverage - Feastern Europe - Recipe. Feastern Eur. 2021.
- Pasqualone, A.; Summo, C.; Laddomada, B.; Mudura, E.; Coldea, T.E. Effect of Processing Variables on the Physico-Chemical Characteristics and Aroma of Borş, a Traditional Beverage Derived from Wheat Bran. Food Chem. 2018, 265, 242–252. [Google Scholar] [CrossRef]
- Chandrasekaran, R.; Shanmugham, B. Cereal-Based Ethnic Fermented Food Products and Low Alcoholic Beverages: Preparation Methods, Nutritional Quality, and Their Health Benefits. In Ethnic and Indigenous Food Technologies: Role in Climate and Health Resilience; Tiwari, A., Sarma, H., Eds.; Springer Nature: Singapore, 2025; pp. 385–399. ISBN 978-981-96-9286-6. [Google Scholar]
- Ashaolu, T.J.; Varga, L.; Greff, B. Nutritional and Functional Aspects of European Cereal-Based Fermented Foods and Beverages. Food Res. Int. 2025, 209, 116221. [Google Scholar] [CrossRef]
- Kombucha: Production and Microbiological Research. Available online: https://www.mdpi.com/2304-8158/11/21/3456 (accessed on 20 October 2025).
- Bishop, P.; Pitts, E.R.; Budner, D.; Thompson-Witrick, K.A. Chemical Composition of Kombucha. Beverages 2022, 8, 45. [Google Scholar] [CrossRef]
- Kombucha: An Old Tradition into a New Concept of a Beneficial, Health-Promoting Beverage. Available online: https://www.mdpi.com/2304-8158/14/9/1547 (accessed on 20 October 2025).
- Samtiya, M.; Aluko, R.E.; Puniya, A.K.; Dhewa, T. Enhancing Micronutrients Bioavailability through Fermentation of Plant-Based Foods: A Concise Review. Fermentation 2021, 7, 63. [Google Scholar] [CrossRef]
- Effect of Fermentation on the Nutritional Quality of the Selected Vegetables and Legumes and Their Health Effects. Available online: https://www.mdpi.com/2075-1729/13/3/655 (accessed on 20 October 2025).
- Sarmale, a Symbol of the Delicious Mix Romanian Gastronomy Has to Offer | RBI Insights. Available online: https://www.rbinternational.com/en/raiffeisen/blog/events-lifestyle/sarmale-romanian-gastronomy.html (accessed on 20 October 2025).
- Transylvania Unveiled. Available online: https://www.transylvaniaunveiled.com/blog/sarmale (accessed on 20 October 2025).
- Internațional, R. Vegetarian Stuffed Cabbage. Radio Rom. Int. 1970.
- History of Traditional Russian Drink Kvass and Cultural Significance - How To Russia. https://howtorussia.com/.
- The History of Kefir: A Journey Through Cultures, Mountains, and Mysteries.
- Pickling and Preserving in Bulgarian Food Culture | Copernico. Geschichte Und Kulturelles Erbe Im Östlichen Europa. Available online: https://www.copernico.eu/en/articles/pickling-and-preserving-bulgarian-food-culture (accessed on 20 October 2025).
- 80% dintre romani conserva muraturi in casa | Progresiv. Available online: https://revistaprogresiv.ro/arhive/80-dintre-romani-conserva-muraturi-casa/ (accessed on 20 October 2025).
- Fitsum, S.; Gebreyohannes, G.; Sbhatu, D.B. Bioactive Compounds in Fermented Foods: Health Benefits, Safety, and Future Perspectives. Appl. Food Res. 2025, 5, 101097. [Google Scholar] [CrossRef]
- Park, I.; Mannaa, M. Fermented Foods as Functional Systems: Microbial Communities and Metabolites Influencing Gut Health and Systemic Outcomes. Foods 2025, 14, 2292. [Google Scholar] [CrossRef]
- Natural Ingredient Trends. Global Market Overview. Top 10 Trends. Available online: https://www.innovamarketinsights.com/trends/natural-ingredient-trends/ (accessed on 20 October 2025).
- Zaky, A.A.; Simal-Gandara, J.; Eun, J.-B.; Shim, J.-H.; Abd El-Aty, A.M. Bioactivities, Applications, Safety, and Health Benefits of Bioactive Peptides From Food and By-Products: A Review. Front. Nutr. 2021, 8, 815640. [Google Scholar] [CrossRef]
- Ebner, J.; Aşçı Arslan, A.; Fedorova, M.; Hoffmann, R.; Küçükçetin, A.; Pischetsrieder, M. Peptide Profiling of Bovine Kefir Reveals 236 Unique Peptides Released from Caseins during Its Production by Starter Culture or Kefir Grains. J. Proteomics 2015, 117, 41–57. [Google Scholar] [CrossRef]
- Quirós, A.; Hernández-Ledesma, B.; Ramos, M.; Amigo, L.; Recio, I. Angiotensin-Converting Enzyme Inhibitory Activity of Peptides Derived from Caprine Kefir. J. Dairy Sci. 2005, 88, 3480–3487. [Google Scholar] [CrossRef]
- Bioprospecting for Bioactive Peptide Production by Lactic Acid Bacteria Isolated from Fermented Dairy Food. Available online: https://www.mdpi.com/2311-5637/5/4/96 (accessed on 20 October 2025).
- Jäkälä, P.; Vapaatalo, H. Antihypertensive Peptides from Milk Proteins. Pharm. Basel Switz. 2010, 3, 251–272. [Google Scholar] [CrossRef]
- Helal, A.; Tagliazucchi, D. Peptidomics Profile, Bioactive Peptides Identification and Biological Activities of Six Different Cheese Varieties. Biology 2023, 12, 78. [Google Scholar] [CrossRef]
- Koistinen, V.M.; Mattila, O.; Katina, K.; Poutanen, K.; Aura, A.-M.; Hanhineva, K. Metabolic Profiling of Sourdough Fermented Wheat and Rye Bread. Sci. Rep. 2018, 8, 5684. [Google Scholar] [CrossRef] [PubMed]
- Katina, K.; Arendt, E.; Liukkonen, K.-H.; Autio, K.; Flander, L.; Poutanen, K. Potential of Sourdough for Healthier Cereal Products. Trends Food Sci. Technol. 2005, 16, 104–112. [Google Scholar] [CrossRef]
- Poutanen, K.; Flander, L.; Katina, K. Sourdough and Cereal Fermentation in a Nutritional Perspective. Food Microbiol. 2009, 26, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Şahingil, D.; Atalay, M. Characterization of Antimicrobial Peptide Fraction Producing Lactobacillus Spp. Based on LC/MS-MS and Determination of ACE-Inhibitory Activity in Kefir. J. Agric. Sci. 2022, 28, 372–384. [Google Scholar]
- Hirota, T.; Ohki, K.; Kawagishi, R.; Kajimoto, Y.; Mizuno, S.; Nakamura, Y.; Kitakaze, M. Casein Hydrolysate Containing the Antihypertensive Tripeptides Val-Pro-Pro and Ile-Pro-Pro Improves Vascular Endothelial Function Independent of Blood Pressure-Lowering Effects: Contribution of the Inhibitory Action of Angiotensin-Converting Enzyme. Hypertens. Res. Off. J. Jpn. Soc. Hypertens. 2007, 30, 489–496. [Google Scholar] [CrossRef]
- Dalabasmaz, S.; de la Torre, E.P.; Gensberger-Reigl, S.; Pischetsrieder, M.; Rodríguez-Ortega, M.J. Identification of Potential Bioactive Peptides in Sheep Milk Kefir through Peptidomic Analysis at Different Fermentation Times. Foods 2023, 12, 2974. [Google Scholar] [CrossRef] [PubMed]
- Pihlanto, A. Lactic Fermentation and Bioactive Peptides. In Lactic Acid Bacteria - R & D for Food, Health and Livestock Purposes; IntechOpen, 2013 ISBN 978-953-51-0955-6.
- Tagliazucchi, D.; Martini, S.; Solieri, L. Bioprospecting for Bioactive Peptide Production by Lactic Acid Bacteria Isolated from Fermented Dairy Food. Fermentation 2019, 5, 96. [Google Scholar] [CrossRef]
- Skulska, I.V.; Tsisaryk, O.Y. Changes in Protein Substances of Brynza Cheese under the Influence of Partial Replacement of Salt with Potassium Chloride. Sci. Messenger LNU Vet. Med. Biotechnol. Ser. Food Technol. 2020, 22, 50–54. [Google Scholar] [CrossRef]
- Kačániová, M.; Terentjeva, M.; Kunová, S.; Haščík, P.; Kowalczewski, P.Ł.; Štefániková, J. Diversity of Microbiota in Slovak Summer Ewes’ Cheese “Bryndza. ” Open Life Sci. 2021, 16, 277–286. [Google Scholar] [CrossRef]
- Kačániová, M.; Nagyová, Ľ.; Štefániková, J.; Felsöciová, S.; Godočí ková, L.; Haščí k, P.; Horská, E.; Kunová, S. The Characteristic of Sheep Cheese “Bryndza” from Different Regions of Slovakia Based on Microbiological Quality. Potravinarstvo Slovak J. Food Sci. 2020, 14, 69–75. [Google Scholar] [CrossRef]
- Manso, M.A.; López-Fandiño, R. Angiotensin I Converting Enzyme-Inhibitory Activity of Bovine, Ovine, and Caprine Kappa-Casein Macropeptides and Their Tryptic Hydrolysates. J. Food Prot. 2003, 66, 1686–1692. [Google Scholar] [CrossRef]
- Mohanty, D.; Jena, R.; Choudhury, P.K.; Pattnaik, R.; Mohapatra, S.; Saini, M.R. Milk Derived Antimicrobial Bioactive Peptides: A Review. Int. J. Food Prop. 2016, 19, 837–846. [Google Scholar] [CrossRef]
- Contreras, M. del M.; Carrón, R.; Montero, M.J.; Ramos, M.; Recio, I. Novel Casein-Derived Peptides with Antihypertensive Activity. Int. Dairy J. 2009, 19, 566–573. [Google Scholar] [CrossRef]
- Bhandari, D.; Rafiq, S.; Gat, Y.; Gat, P.; Waghmare, R.; Kumar, V. A Review on Bioactive Peptides: Physiological Functions, Bioavailability and Safety. Int. J. Pept. Res. Ther. 2020, 26, 139–150. [Google Scholar] [CrossRef]
- Coda, R.; Rizzello, C.G.; Pinto, D.; Gobbetti, M. Selected Lactic Acid Bacteria Synthesize Antioxidant Peptides during Sourdough Fermentation of Cereal Flours. Appl. Environ. Microbiol. 2012, 78, 1087–1096. [Google Scholar] [CrossRef]
- Maioli, M.; Pes, G.M.; Sanna, M.; Cherchi, S.; Dettori, M.; Manca, E.; Farris, G.A. Sourdough-Leavened Bread Improves Postprandial Glucose and Insulin Plasma Levels in Subjects with Impaired Glucose Tolerance. Acta Diabetol. 2008, 45, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Iversen, K.N.; Jonsson, K.; Landberg, R. The Effect of Rye-Based Foods on Postprandial Plasma Insulin Concentration: The Rye Factor. Front. Nutr. 2022, 9. [Google Scholar] [CrossRef] [PubMed]
- Reduction of Post-Prandial Glycaemic Responses Compared with Glucose | EFSA. Available online: https://www.efsa.europa.eu/en/efsajournal/pub/3837 (accessed on 21 October 2025).
- Ribet, L.; Dessalles, R.; Lesens, C.; Brusselaers, N.; Durand-Dubief, M. Nutritional Benefits of Sourdoughs: A Systematic Review. Adv. Nutr. 2022, 14, 22–29. [Google Scholar] [CrossRef]
- Abbaspour, N. Fermentation’s Pivotal Role in Shaping the Future of Plant-Based Foods: An Integrative Review of Fermentation Processes and Their Impact on Sensory and Health Benefits. Appl. Food Res. 2024, 4, 100468. [Google Scholar] [CrossRef]
- Chaudhary, A.; Bhalla, S.; Patiyal, S.; Raghava, G.P.S.; Sahni, G. FermFooDb: A Database of Bioactive Peptides Derived from Fermented Foods. Heliyon 2021, 7, e06668. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Li, S.; Huang, K.; Cao, H.; Qiu, W.; Guan, X. Mechanism of the Release and Transformation of Polyphenols during Germination and Fermentation in Millets: Profile and Metabolomics Based Analysis. Food Funct. 2025, 16, 2004–2017. [Google Scholar] [CrossRef]
- Oluwole, O.; Fernando, W.B.; Lumanlan, J.; Ademuyiwa, O.; Jayasena, V. Role of Phenolic Acid, Tannins, Stilbenes, Lignans and Flavonoids in Human Health – a Review. Int. J. Food Sci. Technol. 2022, 57, 6326–6335. [Google Scholar] [CrossRef]
- Intharuksa, A.; Kuljarusnont, S.; Sasaki, Y.; Tungmunnithum, D. Flavonoids and Other Polyphenols: Bioactive Molecules from Traditional Medicine Recipes/Medicinal Plants and Their Potential for Phytopharmaceutical and Medical Application. Molecules 2024, 29, 5760. [Google Scholar] [CrossRef]
- Yang, F.; Chen, C.; Ni, D.; Yang, Y.; Tian, J.; Li, Y.; Chen, S.; Ye, X.; Wang, L. Effects of Fermentation on Bioactivity and the Composition of Polyphenols Contained in Polyphenol-Rich Foods: A Review. Foods 2023, 12, 3315. [Google Scholar] [CrossRef]
- Ripari, V.; Bai, Y.; Gänzle, M.G. Metabolism of Phenolic Acids in Whole Wheat and Rye Malt Sourdoughs. Food Microbiol. 2019, 77, 43–51. [Google Scholar] [CrossRef]
- Miyagusuku-Cruzado, G.; García-Cano, I.; Rocha-Mendoza, D.; Jiménez-Flores, R.; Giusti, M.M. Monitoring Hydroxycinnamic Acid Decarboxylation by Lactic Acid Bacteria Using High-Throughput UV-Vis Spectroscopy. Molecules 2020, 25, 3142. [Google Scholar] [CrossRef]
- López de Felipe, F. Revised Aspects into the Molecular Bases of Hydroxycinnamic Acid Metabolism in Lactobacilli. Antioxidants 2023, 12, 1294. [Google Scholar] [CrossRef]
- Hu, H.; Li, L.; Ding, S. An Organic Solvent-Tolerant Phenolic Acid Decarboxylase from Bacillus Licheniformis for the Efficient Bioconversion of Hydroxycinnamic Acids to Vinyl Phenol Derivatives. Appl. Microbiol. Biotechnol. 2015, 99, 5071–5081. [Google Scholar] [CrossRef]
- Dissanayake, I.H.; Tabassum, W.; Alsherbiny, M.; Chang, D.; Li, C.G.; Bhuyan, D.J. Lactic Acid Bacterial Fermentation as a Biotransformation Strategy to Enhance the Bioavailability of Phenolic Antioxidants in Fruits and Vegetables: A Comprehensive Review. Food Res. Int. 2025, 209, 116283. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.-C.; Liu, Y.-H.; Lin, S.-P.; Santoso, S.P.; Jantama, K.; Tsai, T.-Y.; Hsieh, C.-W.; Cheng, K.-C. Development of High-Glucosinolate-Retaining Lactic-Acid-Bacteria-Co-Fermented Cabbage Products. Fermentation 2024, 10, 635. [Google Scholar] [CrossRef]
- Mulțescu, M.; Susman, I.-E.; Culețu, A.; Zamfir, M.; Sanmartin, A.M.; Israel-Roming, F. THE INFLUENCE OF STARTER CULTURES OF LACTIC ACID BACTERIA ON FERMENTATION OF WHITE CABBAGE. AgroLife Sci. J. 2024, 13, 300–309. [Google Scholar] [CrossRef]
- Wei, L.; Marco, M.L. The Fermented Cabbage Metabolome and Its Protection against Cytokine-Induced Intestinal Barrier Disruption of Caco-2 Monolayers. Appl. Environ. Microbiol. 2025, 91, e02234-24. [Google Scholar] [CrossRef]
- Šalić, A.; Šamec, D. Changes in the Content of Glucosinolates, Polyphenols and Carotenoids during Lactic-Acid Fermentation of Cruciferous Vegetables: A Mini Review. Food Chem. X 2022, 16, 100457. [Google Scholar] [CrossRef]
- Rehman, S.; Mufti, I.U.; Ain, Q.U.; Ijaz, B. Bioactive Compounds and Biological Activities of Red Beetroot (Beta Vulgaris L.). In Bioactive Compounds in the Storage Organs of Plants; Springer, Cham, 2024; pp. 1–31 ISBN 978-3-031-29006-0.
- Jakubczyk, K.; Melkis, K.; Janda-Milczarek, K.; Skonieczna-Żydecka, K. Phenolic Compounds and Antioxidant Properties of Fermented Beetroot Juices Enriched with Different Additives. Foods 2024, 13, 102. [Google Scholar] [CrossRef]
- Duyar, S.M.; Sarı, F.; Karaoglan, H.A. Degradation Kinetics of Betalains in Red Beetroot Juices throughout Fermentation Process and Storage. Ital. J. Food Sci. 2024, 36, 229–239. [Google Scholar] [CrossRef]
- Chou, Y.-C.; Lin, H.-W.; Wang, C.-Y.; Hsieh, C.-C.; Santoso, S.P.; Lin, S.-P.; Cheng, K.-C. Enhancing Antioxidant Benefits of Kombucha Through Optimized Glucuronic Acid by Selected Symbiotic Fermentation Culture. Antioxidants 2024, 13, 1323. [Google Scholar] [CrossRef] [PubMed]
- Koistinen, V.M. Effects of Food Processing and Gut Microbial Metabolism on Whole Grain Phytochemicals : A Metabolomics Approach; Itä-Suomen yliopisto, 2019; ISBN 978-952-61-3088-0.
- Alkay, Z.; Falah, F.; Cankurt, H.; Dertli, E. Exploring the Nutritional Impact of Sourdough Fermentation: Its Mechanisms and Functional Potential. Foods 2024, 13, 1732. [Google Scholar] [CrossRef]
- Saimaiti, A.; Huang, S.-Y.; Xiong, R.-G.; Wu, S.-X.; Zhou, D.-D.; Yang, Z.-J.; Luo, M.; Gan, R.-Y.; Li, H.-B. Antioxidant Capacities and Polyphenol Contents of Kombucha Beverages Based on Vine Tea and Sweet Tea. Antioxid. Basel Switz. 2022, 11, 1655. [Google Scholar] [CrossRef]
- de Oliveira, P.V.; da Silva Júnior, A.H.; de Oliveira, C.R.S.; Assumpção, C.F.; Ogeda, C.H. Kombucha Benefits, Risks and Regulatory Frameworks: A Review. Food Chem. Adv. 2023, 2, 100288. [Google Scholar] [CrossRef]
- Kiczorowski, P.; Kiczorowska, B.; Samolińska, W.; Szmigielski, M.; Winiarska-Mieczan, A. Effect of Fermentation of Chosen Vegetables on the Nutrient, Mineral, and Biocomponent Profile in Human and Animal Nutrition. Sci. Rep. 2022, 12, 13422. [Google Scholar] [CrossRef] [PubMed]
- Tlais, A.Z.A.; Lemos Junior, W.J.F.; Filannino, P.; Campanaro, S.; Gobbetti, M.; Di Cagno, R. How Microbiome Composition Correlates with Biochemical Changes during Sauerkraut Fermentation: A Focus on Neglected Bacterial Players and Functionalities. Microbiol. Spectr. 10, e00168-22. [CrossRef]
- Garofalo, C.; Osimani, A.; Milanović, V.; Aquilanti, L.; De Filippis, F.; Stellato, G.; Di Mauro, S.; Turchetti, B.; Buzzini, P.; Ercolini, D.; et al. Bacteria and Yeast Microbiota in Milk Kefir Grains from Different Italian Regions. Food Microbiol. 2015, 49, 123–133. [Google Scholar] [CrossRef]
- Wastyk, H.C.; Fragiadakis, G.K.; Perelman, D.; Dahan, D.; Merrill, B.D.; Yu, F.B.; Topf, M.; Gonzalez, C.G.; Treuren, W.V.; Han, S.; et al. Gut-Microbiota-Targeted Diets Modulate Human Immune Status. Cell 2021, 184, 4137–4153.e14. [Google Scholar] [CrossRef]
- de Moreno de LeBlanc, A.; del Carmen, S.; Zurita-Turk, M.; Santos Rocha, C.; van de Guchte, M.; Azevedo, V.; Miyoshi, A.; LeBlanc, J.G. Importance of IL-10 Modulation by Probiotic Microorganisms in Gastrointestinal Inflammatory Diseases. Int. Sch. Res. Not. 2011, 2011, 892971. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, Y.; Wang, P.; Huang, Y.; Wang, F. Short-Chain Fatty Acids Manifest Stimulative and Protective Effects on Intestinal Barrier Function Through the Inhibition of NLRP3 Inflammasome and Autophagy. Cell. Physiol. Biochem. 2018, 49, 190–205. [Google Scholar] [CrossRef]
- Gou, H.-Z.; Zhang, Y.-L.; Ren, L.-F.; Li, Z.-J.; Zhang, L. How Do Intestinal Probiotics Restore the Intestinal Barrier? Front. Microbiol. 2022, 13. [Google Scholar] [CrossRef]
- Marco, M.L.; Sanders, M.E.; Gänzle, M.; Arrieta, M.C.; Cotter, P.D.; De Vuyst, L.; Hill, C.; Holzapfel, W.; Lebeer, S.; Merenstein, D.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on Fermented Foods. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 196–208. [Google Scholar] [CrossRef]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Koj, K.; Pejcz, E. Rye Dietary Fiber Components upon the Influence of Fermentation Inoculated with Probiotic Microorganisms. Molecules 2023, 28, 1910. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.A.; Grant, L.J.; Gidley, M.J.; Mikkelsen, D. Gut Fermentation of Dietary Fibres: Physico-Chemistry of Plant Cell Walls and Implications for Health. Int. J. Mol. Sci. 2017, 18, 2203. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, D.; Nigam, P.S. Therapeutic and Dietary Support for Gastrointestinal Tract Using Kefir as a Nutraceutical Beverage: Dairy-Milk-Based or Plant-Sourced Kefir Probiotic Products for Vegan and Lactose-Intolerant Populations. Fermentation 2023, 9, 388. [Google Scholar] [CrossRef]
- Negishi, H.; Ichikawa, A.; Takahashi, S.; Kano, H.; Makino, S. Targeted Prebiotic Application of Gluconic Acid-Containing Oligosaccharides Promotes Faecalibacterium Growth through Microbial Cross-Feeding Networks. ISME J. 2025, 19, wraf027. [Google Scholar] [CrossRef]
- Du, Y.; He, C.; An, Y.; Huang, Y.; Zhang, H.; Fu, W.; Wang, M.; Shan, Z.; Xie, J.; Yang, Y.; et al. The Role of Short Chain Fatty Acids in Inflammation and Body Health. Int. J. Mol. Sci. 2024, 25, 7379. [Google Scholar] [CrossRef]
- Collins, S.M.; Gibson, G.R.; Kennedy, O.B.; Walton, G.; Rowland, I.; Commane, D.M. Development of a Prebiotic Blend to Influence in Vitro Fermentation Effects, with a Focus on Propionate, in the Gut. FEMS Microbiol. Ecol. 2021, 97, fiab101. [Google Scholar] [CrossRef] [PubMed]
- Fehlbaum, S.; Prudence, K.; Kieboom, J.; Heerikhuisen, M.; Van den Broek, T.; Schuren, F.H.J.; Steinert, R.E.; Raederstorff, D. In Vitro Fermentation of Selected Prebiotics and Their Effects on the Composition and Activity of the Adult Gut Microbiota. Int. J. Mol. Sci. 2018, 19, 3097. [Google Scholar] [CrossRef]
- Christensen, C.M.; Kok, C.R.; Auchtung, J.M.; Hutkins, R. Prebiotics Enhance Persistence of Fermented-Food Associated Bacteria in in Vitro Cultivated Fecal Microbial Communities. Front. Microbiol. 2022, 13. [Google Scholar] [CrossRef]
- Whelan, K.; Alexander, M.; Gaiani, C.; Lunken, G.; Holmes, A.; Staudacher, H.M.; Theis, S.; Marco, M.L. Design and Reporting of Prebiotic and Probiotic Clinical Trials in the Context of Diet and the Gut Microbiome. Nat. Microbiol. 2024, 9, 2785–2794. [Google Scholar] [CrossRef]
- Gurunathan, S.; Thangaraj, P.; Kim, J.-H. Postbiotics: Functional Food Materials and Therapeutic Agents for Cancer, Diabetes, and Inflammatory Diseases. Foods 2024, 13, 89. [Google Scholar] [CrossRef]
- Salek, R.N.; Pleva, P.; Sumczynski, D.; Vinter, Š.; Kopečková, J.; Rejdlová, A.; Lorencová, E. Sauerkraut Juice Fermented with Different Symbiotic Starter Cultures: Comprehensive Assessment of Physicochemical, Rheological, Antioxidant, and Microbiological Characteristics. Front. Sustain. Food Syst. 2025, 9. [Google Scholar] [CrossRef]
- Jácome-Silva, L.G.; Marín-Iniesta, F.; Tortosa-Díaz, L.; Planes-Muñoz, D.; López-Nicolas, R. Influence of the Type of Sauerkraut Fermentation with Probiotics Strains on Folate Content, Antioxidant Activity and Sensory Analysis. Appl. Sci. 2025, 15, 9934. [Google Scholar] [CrossRef]
- B, M. Sauerkraut Fermentation: Process, Microbiology, Defects and Spoilage | Industrial Microbiology. Biol. Discuss. 2018.
- Jastrząb, R.; Graczyk, D.; Siedlecki, P. Molecular and Cellular Mechanisms Influenced by Postbiotics. Int. J. Mol. Sci. 2021, 22, 13475. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Toalá, J.E.; Garcia-Varela, R.; Garcia, H.S.; Mata-Haro, V.; González-Córdova, A.F.; Vallejo-Cordoba, B.; Hernández-Mendoza, A. Postbiotics: An Evolving Term within the Functional Foods Field. Trends Food Sci. Technol. 2018, 75, 105–114. [Google Scholar] [CrossRef]
- Nasreen, S.; Ali, S.; Andleeb, S.; Summer, M.; Hussain, T.; Imdad, K.; Ara, C.; Tahir, H.M. Mechanisms of Medicinal, Pharmaceutical, and Immunomodulatory Action of Probiotics Bacteria and Their Secondary Metabolites against Disease Management: An Overview. Folia Microbiol. (Praha) 2024, 69, 549–565. [Google Scholar] [CrossRef] [PubMed]
- Wegh, C.A.M.; Geerlings, S.Y.; Knol, J.; Roeselers, G.; Belzer, C. Postbiotics and Their Potential Applications in Early Life Nutrition and Beyond. Int. J. Mol. Sci. 2019, 20, 4673. [Google Scholar] [CrossRef]
- Schwarzer, M.; Tlaskalova-Hogenova, H.; Leulier, F.; Schabussova, I. Editorial: Employing Experimental Gnotobiotic Models to Decipher the Host-Microbiota Cross-Talk in Health and Disease. Front. Immunol. 2021, 12. [Google Scholar] [CrossRef]
- Mukherjee, A.; Farsi, D.N.; Garcia-Gutierrez, E.; Akan, E.; Millan, J.A.S.; Angelovski, L.; Bintsis, T.; Gérard, A.; Güley, Z.; Kabakcı, S.; et al. Impact of Fermented Foods Consumption on Gastrointestinal Wellbeing in Healthy Adults: A Systematic Review and Meta-Analysis. Front. Nutr. 2025, 12. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.; Baxter, H.; Larsen, N.; Jespersen, L.; Ekinci, E.I.; Howell, K. Impact of Botanical Fermented Foods on Metabolic Biomarkers and Gut Microbiota in Adults with Metabolic Syndrome and Type 2 Diabetes: A Systematic Review Protocol. BMJ Open 2019, 9, e029242. [Google Scholar] [CrossRef]
- Gill, P.; Staudacher, H.M. Are Postbiotics Key to the Potential Benefits of Fermented Foods? Lancet Gastroenterol. Hepatol. 2023, 8, 509. [Google Scholar] [CrossRef]
- Zdybel, K.; Śliwka, A.; Polak-Berecka, M.; Polak, P.; Waśko, A. Postbiotics Formulation and Therapeutic Effect in Inflammation: A Systematic Review. Nutrients 2025, 17, 2187. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, S.; Liang, S.; Xiang, F.; Wang, X.; Lian, H.; Li, B.; Liu, F. Exopolysaccharides of Lactic Acid Bacteria: Structure, Biological Activity, Structure-Activity Relationship, and Application in the Food Industry: A Review. Int. J. Biol. Macromol. 2024, 257, 128733. [Google Scholar] [CrossRef]
- Abitha Eswari, U.; Radhamanalan, G.; Dharumadurai, D. Thermal Methods of Postbiotics Preparation. In Postbiotics; Dharumadurai, D., Ed.; Springer US: New York, NY, 2024; pp. 85–91. ISBN 978-1-0716-3421-9. [Google Scholar]
- Da, M.; Sun, J.; Ma, C.; Li, D.; Dong, L.; Wang, L.-S.; Chen, F. Postbiotics: Enhancing Human Health with a Novel Concept. eFood 2024, 5, e180. [Google Scholar] [CrossRef]
- LeBlanc, J.G.; Laiño, J.E.; del Valle, M.J.; Vannini, V.; van Sinderen, D.; Taranto, M.P.; de Valdez, G.F.; de Giori, G.S.; Sesma, F. B--Group Vitamin Production by Lactic Acid Bacteria – Current Knowledge and Potential Applications. J. Appl. Microbiol. 2011, 111, 1297–1309. [Google Scholar] [CrossRef] [PubMed]
- Hugenschmidt, S.; Schwenninger, S.M.; Lacroix, C. Concurrent High Production of Natural Folate and Vitamin B12 Using a Co-Culture Process with Lactobacillus Plantarum SM39 and Propionibacterium Freudenreichii DF13. Process Biochem. 2011, 46, 1063–1070. [Google Scholar] [CrossRef]
- Deptula, P.; Chamlagain, B.; Edelmann, M.; Sangsuwan, P.; Nyman, T.A.; Savijoki, K.; Piironen, V.; Varmanen, P. Food-Like Growth Conditions Support Production of Active Vitamin B12 by Propionibacterium Freudenreichii 2067 without DMBI, the Lower Ligand Base, or Cobalt Supplementation. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef]
- Reale, A.; Konietzny, U.; Coppola, R.; Sorrentino, E.; Greiner, R. The Importance of Lactic Acid Bacteria for Phytate Degradation during Cereal Dough Fermentation. J. Agric. Food Chem. 2007, 55, 2993–2997. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Zhao, Y.; Li, J.; Ravichandran, V.; Wang, L.; Huang, Q.; Chen, C.; Ni, H.; Yin, J. Effects of Phytic Acid-Degrading Bacteria on Mineral Element Content in Mice. Front. Microbiol. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Molina, G.E.S.; Ras, G.; da Silva, D.F.; Duedahl-Olesen, L.; Hansen, E.B.; Bang-Berthelsen, C.H. Metabolic Insights of Lactic Acid Bacteria in Reducing Off-Flavors and Antinutrients in Plant-Based Fermented Dairy Alternatives. Compr. Rev. Food Sci. Food Saf. 2025, 24, e70134. [Google Scholar] [CrossRef]
- Keyvan, E.; Adesemoye, E.; Champomier-Vergès, M.-C.; Chanséaume-Bussiere, E.; Mardon, J.; Nikolovska Nedelkoska, D.; Palamutoglu, R.; Russo, P.; Sarand, I.; Songre-Ouattara, L.; et al. Vitamins Formed by Microorganisms in Fermented Foods: Effects on Human Vitamin Status—a Systematic Narrative Review. Front. Nutr. 2025, 12. [Google Scholar] [CrossRef]
- Sorathiya, K.B.; Melo, A.; Hogg, M.C.; Pintado, M. Organic Acids in Food Preservation: Exploring Synergies, Molecular Insights, and Sustainable Applications. Sustainability 2025, 17, 3434. [Google Scholar] [CrossRef]
- Gan, J.; Kong, X.; Wang, K.; Chen, Y.; Du, M.; Xu, B.; Xu, J.; Wang, Z.; Cheng, Y.; Yu, T. Effect of Fermentation Using Different Lactic Acid Bacteria Strains on the Nutrient Components and Mineral Bioavailability of Soybean Yogurt Alternative. Front. Nutr. 2023, 10. [Google Scholar] [CrossRef]
- Gasaly, N.; Hermoso, M.A.; Gotteland, M. Butyrate and the Fine-Tuning of Colonic Homeostasis: Implication for Inflammatory Bowel Diseases. Int. J. Mol. Sci. 2021, 22, 3061. [Google Scholar] [CrossRef]
- Xiao, S.; Jiang, S.; Qian, D.; Duan, J. Modulation of Microbially Derived Short-Chain Fatty Acids on Intestinal Homeostasis, Metabolism, and Neuropsychiatric Disorder. Appl. Microbiol. Biotechnol. 2020, 104, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Künili, İ.E.; Akdeniz, V.; Akpınar, A.; Budak, Ş.Ö.; Curiel, J.A.; Guzel, M.; Karagözlü, C.; Berkel Kasikci, M.; Caruana, G.P.M.; Starowicz, M.; et al. Bioactive Compounds in Fermented Foods: A Systematic Narrative Review. Front. Nutr. 2025, 12. [Google Scholar] [CrossRef]
- Carvalho, A.P.A. de; Conte-Junior, C.A. Health and Bioactive Compounds of Fermented Foods and By-Products. Fermentation 2024, 10, 13. [Google Scholar] [CrossRef]
- Zhang, J.; Cianciosi, D.; Islam, M.O.; Zhang, L. Editorial: Intervention Effects of Food-Derived Polyphenols and Bioactive Peptides on Chronic Inflammation. Front. Nutr. 2024, 11. [Google Scholar] [CrossRef]
- Iorizzo, M.; Di Martino, C.; Letizia, F.; Crawford, T.W.; Paventi, G. Production of Conjugated Linoleic Acid (CLA) by Lactiplantibacillus Plantarum: A Review with Emphasis on Fermented Foods. Foods 2024, 13, 975. [Google Scholar] [CrossRef]
- Naseeb, J.; Kanwal, M.; Aldalali, S.; Sarwar, A.; Jamal, S.B.; Yang, Z.; Aziz, T.; Alharbi, M.; Shami, A.; Al-Asmari, F.; et al. Metabolic and Enzymatic Characterization of Linoleic Acid Biotransformation by Lactiplantibacillus Plantarum NGML2 to Conjugated Linoleic Acid and Different Metabolites. World J. Microbiol. Biotechnol. 2025, 41, 193. [Google Scholar] [CrossRef] [PubMed]
- Rashidbeygi, E.; Samarin, M.M.; Sheikhhossein, F.; Khalilkhaneh, A.H.; Gholizadeh, M.; Lohrasbi, N.; Abbasi, A.; Bazyar, H.; Askari, G.; Amini, M.R. The Effect of Kefir Consumption on Blood Pressure and C--Reactive Protein: A Systematic Review and Meta--Analysis of Randomised Controlled Trials. Endocrinol. Diabetes Metab. 2025, 8, e70124. [Google Scholar] [CrossRef]
- Tagliazucchi, D.; Martini, S.; Shamsia, S.; Helal, A.; Conte, A. Biological Activities and Peptidomic Profile of in Vitro-Digested Cow, Camel, Goat and Sheep Milk. Int. Dairy J. 2018, 81, 19–27. [Google Scholar] [CrossRef]
- Dimidi, E.; Cox, S.R.; Rossi, M.; Whelan, K. Fermented Foods: Definitions and Characteristics, Impact on the Gut Microbiota and Effects on Gastrointestinal Health and Disease. Nutrients 2019, 11, 1806. [Google Scholar] [CrossRef]
- Kefir’s Laxative Effects: Friend Or Foe? | MedShun. Available online: https://medshun.com/article/does-kefir-have-laxative-properties (accessed on 27 October 2025).
- Ki, J.-H.; Jung, K.-O.; Lee, H.-S.; Hwang, I.-K.; Kim, Y.-J.; Park, K.-Y. Effects of Kimchi on Stomach and Colon Health of Helicobacter pylori - Infected Volunteers | DBpia. 2004.
- Gao, Y.; Liu, Y.; Ma, T.; Liang, Q.; Sun, J.; Wu, X.; Song, Y.; Nie, H.; Huang, J.; Mu, G. Fermented Dairy Products as Precision Modulators of Gut Microbiota and Host Health: Mechanistic Insights, Clinical Evidence, and Future Directions. Foods 2025, 14, 1946. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Kaur, G.; Ali, S.A. Dairy-Based Probiotic-Fermented Functional Foods: An Update on Their Health-Promoting Properties. Fermentation 2022, 8, 425. [Google Scholar] [CrossRef]
- Saleem, G.N.; Gu, R.; Qu, H.; Bahar Khaskheli, G.; Rashid Rajput, I.; Qasim, M.; Chen, X. Therapeutic Potential of Popular Fermented Dairy Products and Its Benefits on Human Health. Front. Nutr. 2024, 11. [Google Scholar] [CrossRef] [PubMed]
- Laiño, J.; Villena, J.; Kanmani, P.; Kitazawa, H. Immunoregulatory Effects Triggered by Lactic Acid Bacteria Exopolysaccharides: New Insights into Molecular Interactions with Host Cells. Microorganisms 2016, 4, 27. [Google Scholar] [CrossRef]
- Miyoshi, Y.; Saika, A.; Nagatake, T.; Matsunaga, A.; Kunisawa, J.; Katakura, Y.; Yamasaki-Yashiki, S. Mechanisms Underlying Enhanced IgA Production in Peyer’s Patch Cells by Membrane Vesicles Derived from Lactobacillus Sakei. Biosci. Biotechnol. Biochem. 2021, 85, 1536–1545. [Google Scholar] [CrossRef]
- Carasi, P.; Racedo, S.M.; Jacquot, C.; Romanin, D.E.; Serradell, M.A.; Urdaci, M.C. Impact of Kefir Derived Lactobacillus Kefiri on the Mucosal Immune Response and Gut Microbiota. J. Immunol. Res. 2015, 2015, 361604. [Google Scholar] [CrossRef]
- Yamane, T.; Sakamoto, T.; Nakagaki, T.; Nakano, Y. Lactic Acid Bacteria from Kefir Increase Cytotoxicity of Natural Killer Cells to Tumor Cells. Foods 2018, 7, 48. [Google Scholar] [CrossRef]
- Paul, A.K.; Lim, C.L.; Apu, M.A.I.; Dolma, K.G.; Gupta, M.; de Lourdes Pereira, M.; Wilairatana, P.; Rahmatullah, M.; Wiart, C.; Nissapatorn, V. Are Fermented Foods Effective against Inflammatory Diseases? Int. J. Environ. Res. Public. Health 2023, 20, 2481. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Choi, K.-B.; Park, J.H.; Kim, K.H. Metabolite Profile Changes and Increased Antioxidative and Antiinflammatory Activities of Mixed Vegetables after Fermentation by Lactobacillus Plantarum. PLOS ONE 2019, 14, e0217180. [Google Scholar] [CrossRef] [PubMed]
- Oliviero, T.; Verkerk, R.; Dekker, M. Isothiocyanates from Brassica Vegetables—Effects of Processing, Cooking, Mastication, and Digestion. Mol. Nutr. Food Res. 2018, 62, 1701069. [Google Scholar] [CrossRef]
- Galena, A.E.; Chai, J.; Zhang, J.; Bednarzyk, M.; Perez, D.; Ochrietor, J.D.; Jahan-Mihan, A.; Arikawa, A.Y. The Effects of Fermented Vegetable Consumption on the Composition of the Intestinal Microbiota and Levels of Inflammatory Markers in Women: A Pilot and Feasibility Study. PLoS ONE 2022, 17, e0275275. [Google Scholar] [CrossRef]
- Hamadou, M. Bioactive Peptides and Metabolic Health: A Mechanistic Review of the Impact on Insulin Sensitivity, Lipid Profiles, and Inflammation. Appl. Food Res. 2025, 5, 101056. [Google Scholar] [CrossRef]
- Ma, Z.F.; Fu, C.; Lee, Y.Y. The Modulatory Role of Bioactive Compounds in Functional Foods on Inflammation and Metabolic Pathways in Chronic Diseases. Foods 2025, 14, 821. [Google Scholar] [CrossRef]
- Chan, M.; Larsen, N.; Baxter, H.; Jespersen, L.; Ekinci, E.I.; Howell, K. The Impact of Botanical Fermented Foods on Metabolic Syndrome and Type 2 Diabetes: A Systematic Review of Randomised Controlled Trials. Nutr. Res. Rev. 2024, 37, 396–415. [Google Scholar] [CrossRef]
- Bellikci-Koyu, E.; Sarer-Yurekli, B.P.; Karagozlu, C.; Aydin-Kose, F.; Ozgen, A.G.; Buyuktuncer, Z. Probiotic Kefir Consumption Improves Serum Apolipoprotein A1 Levels in Metabolic Syndrome Patients: A Randomized Controlled Clinical Trial. Nutr. Res. 2022, 102, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Lange, E.; Pałkowska-Goździk, E.; Kęszycka, P. The Influence of Various Types of Functional Bread on Postprandial Glycemia in Healthy Adults. Appl. Sci. 2024, 14, 11900. [Google Scholar] [CrossRef]
- Gil-Cardoso, K.; Saldaña, G.; Luengo, E.; Pastor, J.; Virto, R.; Alcaide-Hidalgo, J.M.; del Bas, J.M.; Arola, L.; Caimari, A. Consumption of Sourdough Breads Improves Postprandial Glucose Response and Produces Sourdough-Specific Effects on Biochemical and Inflammatory Parameters and Mineral Absorption. J. Agric. Food Chem. 2021, 69, 3044–3059. [Google Scholar] [CrossRef] [PubMed]
- Stamataki, N.S.; Yanni, A.E.; Karathanos, V.T. Bread Making Technology Influences Postprandial Glucose Response: A Review of the Clinical Evidence. Br. J. Nutr. 2017, 117, 1001–1012. [Google Scholar] [CrossRef]
- Biçer, Y.; Telli, A.E.; Turkal, G.; Telli, N.; Uçar, G. From Raw to Fermented: Uncovering the Microbial Wealth of Dairy. Fermentation 2025, 11, 552. [Google Scholar] [CrossRef]
- Lee, C.-H.; Ahn, J.; Son, H.-S. Ethnic Fermented Foods of the World: An Overview. J. Ethn. Foods 2024, 11, 39. [Google Scholar] [CrossRef]
- Tamang, J.P.; Cotter, P.D.; Endo, A.; Han, N.S.; Kort, R.; Liu, S.Q.; Mayo, B.; Westerik, N.; Hutkins, R. Fermented Foods in a Global Age: East Meets West. Compr. Rev. Food Sci. Food Saf. 2020, 19, 184–217. [Google Scholar] [CrossRef]
- Tlais, A.Z.A.; Kanwal, S.; Filannino, P.; Acin Albiac, M.; Gobbetti, M.; Di Cagno, R. Effect of Sequential or Ternary Starters-Assisted Fermentation on the Phenolic and Glucosinolate Profiles of Sauerkraut in Comparison with Spontaneous Fermentation. Food Res. Int. 2022, 156, 111116. [Google Scholar] [CrossRef]
- Kołożyn-Krajewska, D.; Dolatowski, Z. Tradycyjne i regionalne technologie oraz produkty w żywieniu człowieka :monografia; Wydawnictwo Naukowe PTTŻ, 2008.
- Chourasia, R.; Chiring Phukon, L.; Abedin, M.M.; Padhi, S.; Singh, S.P.; Rai, A.K. Bioactive Peptides in Fermented Foods and Their Application: A Critical Review. Syst. Microbiol. Biomanufacturing 2023, 3, 88–109. [Google Scholar] [CrossRef]
- Is There Too Much Salt in Lacto-Fermentations? Available online: https://revolutionfermentation.com/en/blogs/fermented-vegetables/too-much-salt-lacto-fermentation/ (accessed on 27 October 2025).
- Liu, X.; Ma, J.; Fan, G. Microbiological Safety and Quality of Fermented Products. Foods 2023, 12, 2204. [Google Scholar] [CrossRef] [PubMed]
- Dees, J. Fermented Foods Part 2: The Joys and (Few) Risks of At-Home Fermentation. Joyf. Microbe 2019.
- Shi, H.; An, F.; Lin, H.; Li, M.; Wu, J.; Wu, R. Advances in Fermented Foods Revealed by Multi-Omics: A New Direction toward Precisely Clarifying the Roles of Microorganisms. Front. Microbiol. 2022, 13. [Google Scholar] [CrossRef] [PubMed]
Table 1.
Identified bioactive peptides in traditional Eastern European fermented foods.
Table 1.
Identified bioactive peptides in traditional Eastern European fermented foods.
| Food |
Representative peptide(s) or class |
Reported bioactivity |
Key reference(s) |
| Kefir |
Tripeptides Val–Pro–Pro (VPP) and Ile–Pro–Pro (IPP); multiple casein-derived peptides (LC–MS identified) |
ACE-inhibitory (antihypertensive), antioxidant, immunomodulatory (varies by peptide) |
[54]
[55]
[56] |
| Bryndza (sheep-milk cheese) |
Casein-derived peptides (including motifs similar to VPP/IPP reported in ripened cheeses); specific peptide sequencing in bryndza is limited |
ACE-inhibitory, antioxidant (reported for ripened cheeses generally) |
[57]
[58] |
| Rye sourdough |
A range of small peptides (LC–MS identified) derived from rye storage proteins and glutenins |
Antioxidant and modest ACE-inhibitory activities reported; contribute to improved digestibility and lower postprandial glycemia |
[59]
[60]
[61] |
Table 3.
Health effects of taxa in East European fermented products.
Table 3.
Health effects of taxa in East European fermented products.
| Mechanism |
Proposed Effects |
Key references |
| Competitive exclusion of pathogens |
LAB adhere to intestinal mucosa, inhibit E. coli, Salmonella, Listeria spp. via bacteriocins and organic acids |
[104,105] |
| Modulation of mucosal immunity |
Increased secretion of IgA, IL-10; downregulation of proinflammatory cytokines |
[106] |
| Enhancement of intestinal barrier |
LAB upregulate tight-junction proteins (occludin, claudin) and short-chain fatty acid (SCFA) production |
[107,108] |
| Influence on gut microbiota |
Fermented foods increase
microbial diversity
and beneficial genera
(e.g., Bifidobacterium,
Faecalibacterium) in human trials |
[105,109] |
Table 4.
Comparation between fermented food worldwide.
Table 4.
Comparation between fermented food worldwide.
| Region |
Common Substrates |
Microbial
Diversity |
Nutritional Role |
Environmental Advantages |
| Eastern Europe |
Vegetables (e.g., sauerkraut), cereals (e.g., rye sourdough, kvass), dairy (e.g., kefir) |
Mixed cultures:
lactic acid bacteria
(LAB),
yeasts,
acetic acid
bacteria |
Rich in fiber, bioactive peptides, organic acids, probiotics; supports gut health and
immune tone
|
Adapted to cold climates and seasonal scarcity; preservation-focused |
| Asia |
Soy (e.g., miso, tempeh), pulses, rice, vegetables |
Spontaneous or starter-enhanced LAB, fungi
(Aspergillus, Rhizopus), yeasts |
High in plant
protein, umami compounds, and microbial metabolites; supports digestion and metabolic health |
Tropical to temperate
climates; fermentation
used for flavor,
preservation,
and protein enhancement
|
| Western (Industrial) |
Primarily dairy (e.g., yogurt, cheese |
Defined starter cultures: single-strain LAB (e.g., Lactobacillus delbrueckii, Streptococcus thermophilus) |
Consistent
probiotic delivery,
calcium, B vitamins;
less microbial diversity |
Refrigeration-based preservation; less seasonal dependence;
focused on standardization |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).