Submitted:
06 November 2025
Posted:
07 November 2025
Read the latest preprint version here
Abstract
Keywords:
Introduction
2. Lipid Nanoparticles for mRNA Delivery: Biological Properties and Effects on Cellular Systems
2.1. Factors Influencing Nanoparticle Bioactivity
2.2. LNP Biodistribution
2.3. Mechanisms of Uptake
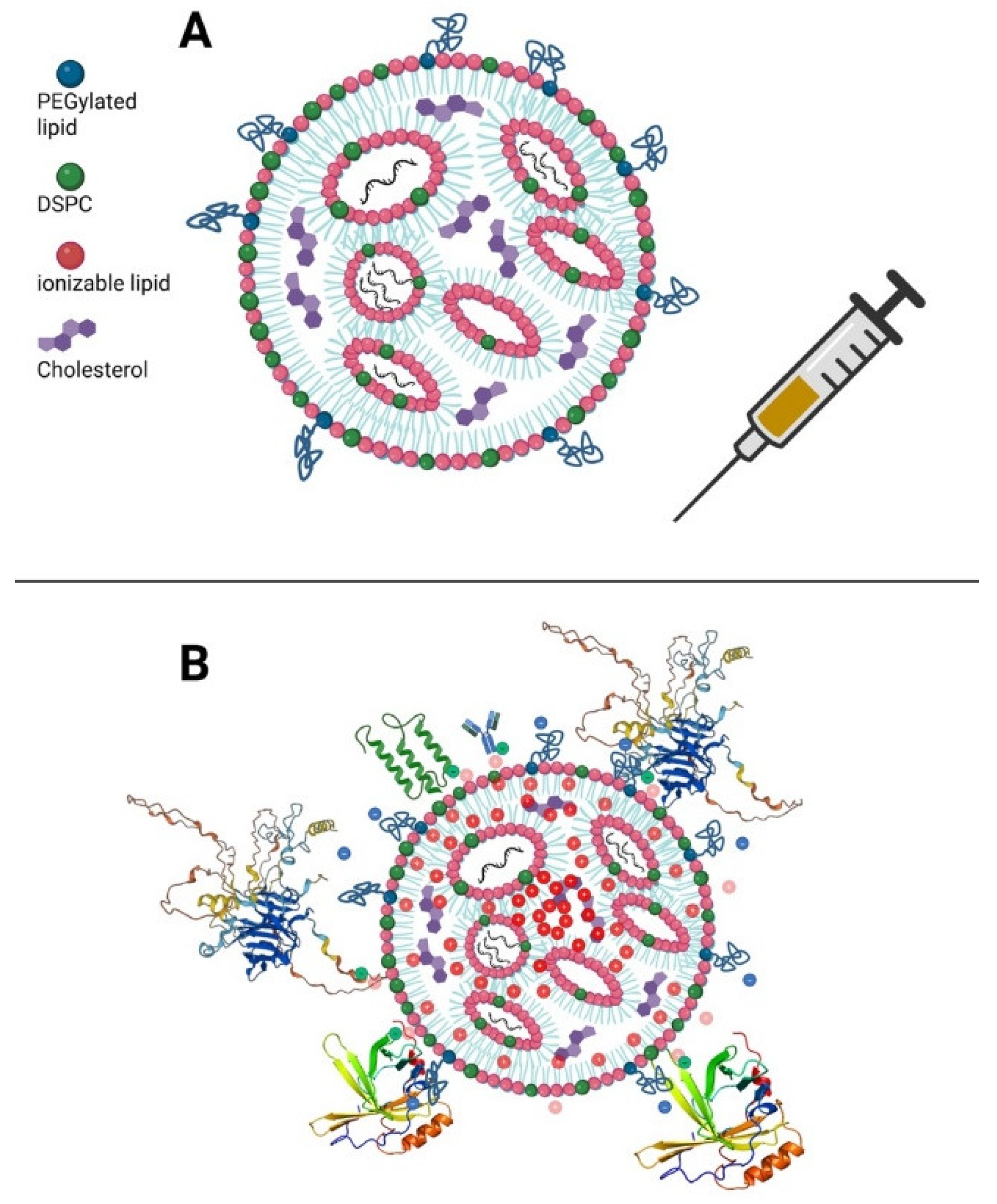
2.4. Endosomal Escape and Membrane Destabilization Due to Ionizable Lipids
2.5. Spread to Distant Sites via Exosomes
2.6. LNP Metabolism Leads to Oxidative Stress and Signaling Cascades
2.7. Activation of the Immune System
3. The Principles Behind How LNP-modRNA Was Thought to Work
4. Omics: Evidence for Membrane Dysfunction Secondary to LNP Transfection
4.1. Ndeupen et al. - a Pioneering Omics Study
| Pathway | Direction of NES | Consequences/Biological Function |
|---|---|---|
| Mismatch repair | Downregulated | DNA repair inefficiency; Promotion of tumorigenesis [138] |
| Phagosome | Upregulated | Induction of phagocytosis and autophagy |
| Necroptosis | Upregulated | Inflammatory form of cell death associated with many human diseases [139] |
| Apoptosis | Upregulated | Induction of programmed cell death |
| Metabolism of xenobiotics by cytochrome P450 | Downregulated | Impaired cytochrome P450 activity in the liver may lead to increased drug toxicity [140] |
| NF-κB | Upregulated | A key regulator of the immune system, inflammation, cell survival, and stress responses [141] |
| TNF | Upregulated | A powerful pro-inflammatory agent that regulates many facets of macrophage function [142] |
| IL-17 | Upregulated | Promotes proinflammatory cytokine production, neutrophil recruitment, tissue remodeling, and antimicrobial defenses [143] |
| Toll-like receptors, RIG-1-like receptors, Nod-like receptors | Upregulated | These receptors activate inflammatory and immune responses [144] |
| TCA cycle | Downregulated | Impaired TCA cycle is a feature of Alzheimer’s disease [145] |
| Circadian rhythm | Downregulated | Dysregulation of circadian rhythms increases cancer susceptibility [146] |
| Hematopoetic cell lineage | Upregulated | Increased clonal hematopoiesis leads to hematological malignancy [147] |
4.2. Upregulation of Multiple Inflammatory Markers
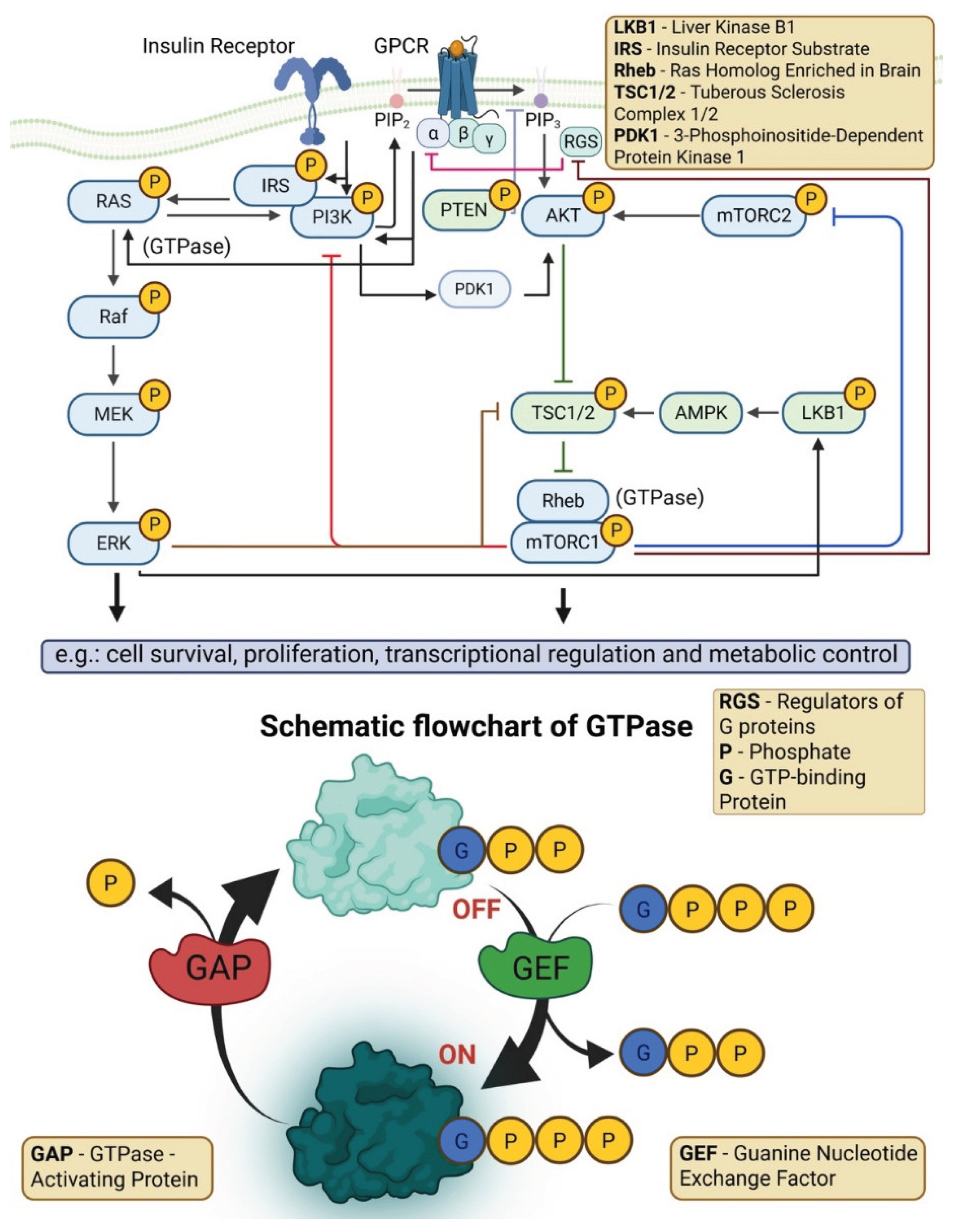
4.3. Downregulation of PPAR and AMPK Signaling
4.4. Downregulated Xenobiotic Metabolism by Cytochrome P450 Enzymes
4.5. Non-Canonical Transciptomics and Proteomic Alterations – Are the TLR4 Reactions Decoupled?
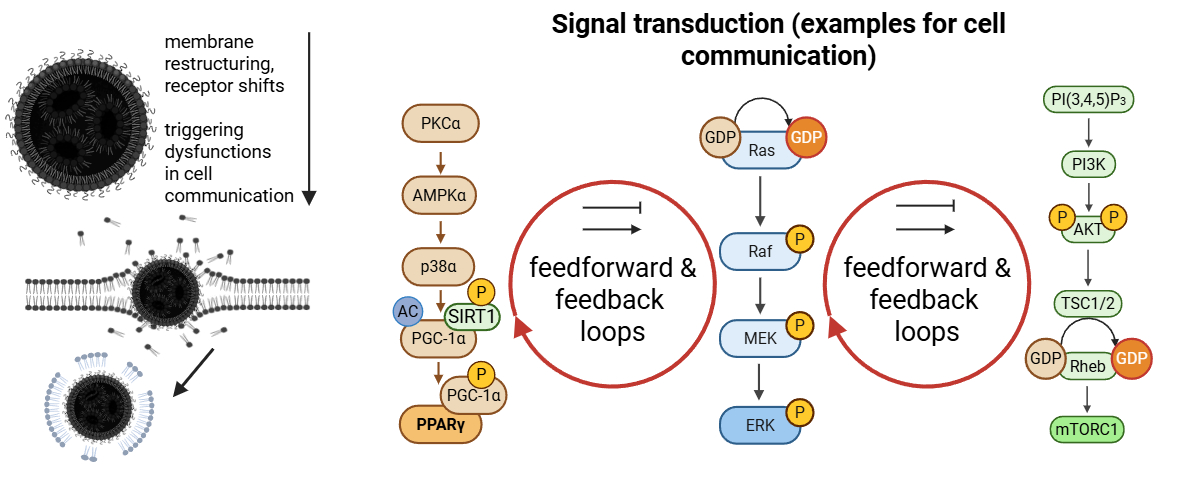
4.6. Dysregulation of MAPK/ERK, JAK-STAT, and Other Signaling Pathways
4.7. Further Studies Using Single Cell Analyses Revealing Gender-Based Differences
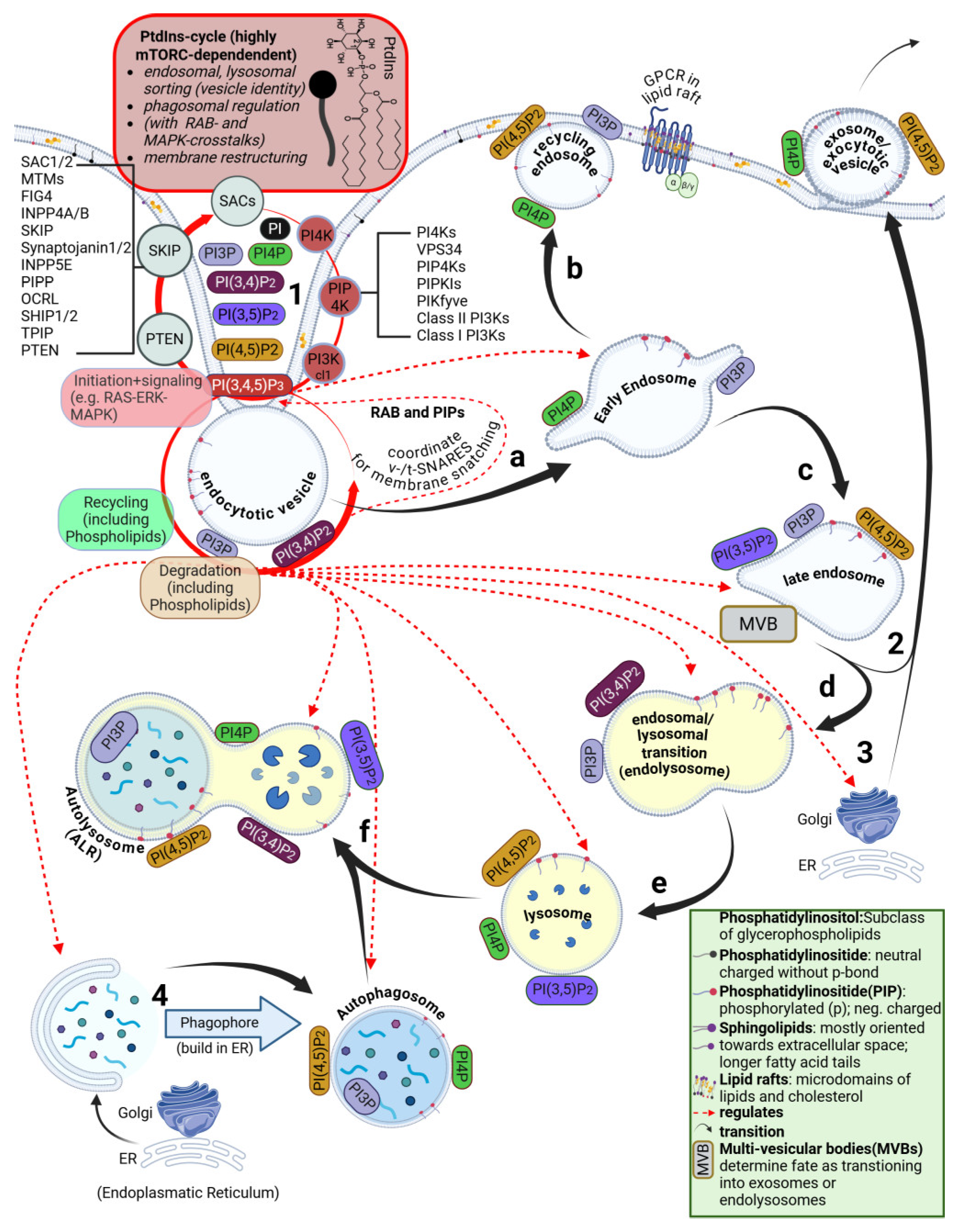
4.8. Disruption of the ESCRT Circuit and Phosphatidylinositol Signaling
4.9. Perturbations Originate at the Plasma Membrane and Disturb PtdIns Signaling Cascades
5. Breaching the Plasma Membrane: Important Roles for Phosphoinositides
5.1. Brief Overview of the Phosphatidylinositide Cycle
5.2. The Role of Lipid Rafts in LNP Uptake into Cells
5.3. Signaling Through Phosphorylation States of Phosphatidylinositols
5.4. Oxysterol-Binding Proteins (OSBs) and a Role for Cholesterol
5.5. How Does 1,2-distearoyl-sn-glycero-3-Phosphocholine (DSPC) Affect the PI Cycle?
5.6. A Role for Lipid Impurities
5.7. Small Perturbations Can Lead to Major Shifts in PIP Signaling
Discussion
Conclusions
Use of Generative AI
Author Contributions
Acknowledgements
Conflicts of Interest
List of Abbreviations
| 4HNE | 4-Hydroxynonenal |
| AKT | Protein Kinase B |
| ALC-0315 | ionizable lipid,[(4-hydroxybutyl)azanediyl]di(hexane-6,1-diyl) bis(2-hexyldecanoate) |
| ALR | Autophagic Lysosome Reformation |
| AMPK | AMP-Activated Protein Kinase |
| APC | Antigen-Presenting Cell |
| AhR | Aryl Hydrocarbon Receptor |
| ApoE | Apolipoprotein E |
| BUB1 | Budding Uninhibited by Benzimidazoles 1 |
| C57BL/6 | C57 black 6: a common inbred mouse strain |
| CARPA | Complement Activation-Related Pseudoallergy |
| CCL2/3/4/7 | Chemokine Ligands |
| CDC25A | Cell Division Cycle 25A |
| CDP-DAG | Cytidine Diphosphate Diacylglycerol |
| CRP | C-Reactive Protein |
| CSF2RB | Colony Stimulating Factor 2 Receptor Beta |
| CYP1A2, CYP2C9, CYP2C19, CYP3A4 | Cytochrome Isoenzymes |
| CYP | Cytochrome P450 |
| DC | Dendritic Cell |
| DEPs | Differentially Expressed Proteins |
| DLin-MC3-DMA | ionizable lipid, (6Z,9Z,28Z,31Z)-Heptatriaconta-6,9,28,31-tetraen-19-yl 4-(dimethylamino)butanoate |
| DSPC | 1,2-Distearoyl-sn-glycero-3-Phosphocholine |
| E2F/E2F1/E2F8 | E2F Transcription Factor Family |
| EMA | European Medicines Agency |
| ER | Endoplasmic Reticulum |
| ERK | Extracellular Signal-Regulated Kinase |
| ESCRT | Endosomal Sorting Complex Required for Transport |
| FDR | False Discovery Rate |
| GM-CSF | Granulocyte-Macrophage Colony-Stimulating Factor |
| GPCR | G-Protein Coupled Receptor |
| GSEA | Gene Set Enrichment Analysis |
| HDL | High-Density Lipoprotein |
| IFN-γ | Interferon-γ |
| IL-1β, IL-2, IL-6, IL-17 | Interleukins |
| IRF | Interferon Regulatory Factor |
| IgE, IgM, IgG | Immunoglobulin Isotypes |
| JAK-STAT | Janus Kinase - Signal Transducer and Activator of Transcription |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| KRAS | Kirsten Rat Sarcoma Oncogene |
| L-DMD | Lipid-Nanoparticle-Driven Membrane Dysfunction |
| LC3 | Microtubule-Associated Protein 1A/1B-Light Chain 3 |
| LDL-R | Low-Density Lipoprotein Receptor |
| LDL | Low-Density Lipoprotein |
| LNP | Lipid Nanoparticle |
| MAPK/ERK | The RAS–RAF–MEK–ERK Pathway |
| MAPK | Mitogen-Activated Protein Kinase |
| MC3 | ionizable lipid also known as D-Lin-MC3-DMA |
| MDDC | Monocyte-Derived Dendritic Cell |
| MSigDB | Molecular Signatures Database |
| MVB | Multivesicular Body |
| MyD88 | Myeloid Differentiation Primary Response 88 |
| NES | Normalized Enrichment Score |
| NF-κB | Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cells |
| NIK/RELA/RELB | NF-κB Pathway Subunits |
| NLRP3 | NOD-Like Receptor Family Pyrin Domain Containing 3 |
| NOD | Nucleotide-Binding Oligomerization Domain |
| OSBP/OSH | Oxysterol-Binding Protein/Yeast Ortholog |
| PA | Phosphatidic Acid |
| PBMC | Peripheral Blood Mononuclear Cell |
| PBS | Phosphate-Buffered Saline |
| PC | Phosphatidylcholine |
| PEG | Polyethylene Glycol |
| PEGylated | Covalently Modified with Polyethylene Glycol |
| PET-CT | Positron Emission Tomography–Computed Tomography |
| PI3K | Phosphatidylinositol-3-Kinase |
| PI3P, PI4P, PI (4,5) P2, PI (3,4,5) P3 | Phosphorylated PI Species |
| PIP | Phosphoinositide |
| PPAR | Peroxisome Proliferator-Activated Receptor |
| PTEN | Phosphatase and Tensin Homolog |
| PtdIns/PI | Phosphatidylinositol |
| RE | Recycling Endosome |
| RES | Reticuloendothelial System |
| RIG-I | Retinoic Acid–Inducible Gene I |
| ROS | Reactive Oxygen Species |
| RRM2 | Ribonucleotide Reductase Regulatory Subunit M2 |
| SM-102 | ionizable lipid:1-Octylnonyl 8-[(2-hydroxyethyl)[6-oxo-6-(undecyloxy) hexyl]amino]octanoate |
| SPARKLE | Strategic Peptide Anchored Retained Kept after Lipid Elimination |
| STAT3/STAT5 | Signal Transducer and Activator of Transcription 3/5 |
| TBK1 | Tank-Binding Kinase 1 |
| TCA | Tricarboxylic Acid Cycle |
| TGF-β | Transforming Growth Factor β |
| TLR2/3/4/7/8/9 | Specific Toll-Like Receptor Types |
| TLR | Toll-Like Receptor |
| TNF-α | Tumor Necrosis Factor Alpha |
| TRIF | TLR4 Signaling Adaptors |
| Tm | Transition Temperature |
| V-ATPase | Vacuolar ATPase |
| WIPI2 | WD Repeat Domain Phosphoinositide-Interacting Protein 2 |
| WT | Wild Type |
| mRNA | Messenger Ribonucleic Acid |
| mTOR/mTORC1/mTORC2 | Mechanistic Target of Rapamycin (Complex 1/2) |
| modRNA | Modified Messenger RNA |
| p53 | Tumour Suppressor Protein p53 |
| qPCR | Quantitative Polymerase Chain Reaction |
| siRNA | Small Interfering RNA |
| ζ | Zeta Potential (Surface Charge) |
References
- Khurana, A.; Allawadhi, P.; Khurana, I.; Allwadhi, S.; Weiskirchen, R.; Banothu, A.K.; Chhabra, D.; Joshi, K.; Bharani, K.K. Role of nanotechnology behind the success of mRNA vaccines for COVID-19. Nano Today 2021, 38, 101142. [Google Scholar] [CrossRef] [PubMed]
- Albertsen, C.H.; Kulkarni, J.A.; Witzigmann, D.; Lind, M.; Petersson, K.; Simonsen, J.B. The role of lipid components in lipid nanoparticles for vaccines and gene therapy. Adv. Drug Deliv. Rev. 2022, 188, 114416. [Google Scholar] [CrossRef] [PubMed]
- Swetha, K.; Kotla, N.G.; Tunki, L.; Jayaraj, A.; Bhargava, S.K.; Hu, H.; Bonam, S.R.; Kurapati, R. Recent Advances in the Lipid Nanoparticle-Mediated Delivery of mRNA Vaccines. Vaccines 2023, 11, 658. [Google Scholar] [CrossRef] [PubMed]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef]
- Ju, Y.; Carreño, J.M.; Simon, V.; Dawson, K.; Krammer, F.; Kent, S.J. Impact of anti-PEG antibodies induced by SARS-CoV-2 mRNA vaccines. Nat. Rev. Immunol. 2022, 23, 135–136. [Google Scholar] [CrossRef]
- Zhou, Z.-H.; Stone, C.A.; Jakubovic, B.; Phillips, E.J.; Sussman, G.; Park, J.; Hoang, U.; Kirshner, S.L.; Levin, R.; Kozlowski, S. Anti-PEG IgE in anaphylaxis associated with polyethylene glycol. J. Allergy Clin. Immunol. Pr. 2021, 9, 1731–1733.e3. [Google Scholar] [CrossRef]
- Kozma, G.T.; Shimizu, T.; Ishida, T.; Szebeni, J. Anti-PEG antibodies: Properties, formation, testing and role in adverse immune reactions to PEGylated nano-biopharmaceuticals. Adv. Drug Deliv. Rev. 2020, 154–155, 163–175. [Google Scholar] [CrossRef]
- Khalid, M.B.; Frischmeyer-Guerrerio, P.A. The conundrum of COVID-19 mRNA vaccine–induced anaphylaxis. J. Allergy Clin. Immunol. Glob. 2022, 2, 1–13. [Google Scholar] [CrossRef]
- Zelkoski, A.E.; Lu, Z.; Sukumar, G.; Dalgard, C.; Said, H.; Alameh, M.-G.; Mitre, E.; Malloy, A.M.W. Ionizable lipid nanoparticles of mRNA vaccines elicit NF-κB and IRF responses through toll-like receptor 4. npj Vaccines 2025, 10, 1–13. [Google Scholar] [CrossRef]
- Lee, Y.; Jeong, M.; Park, J.; Jung, H.; Lee, H. Immunogenicity of lipid nanoparticles and its impact on the efficacy of mRNA vaccines and therapeutics. Exp. Mol. Med. 2023, 55, 2085–2096. [Google Scholar] [CrossRef]
- Kimura, S.; Okada, K.; Matsubara, N.; Lyu, F.; Tsutsumi, S.; Kimura, Y.; Hashiya, F.; Inagaki, M.; Abe, N.; Abe, H. In vivo demonstration of enhanced mRNA delivery by cyclic disulfide-containing lipid nanoparticles for facilitating endosomal escape. RSC Med. Chem. 2025, 16, 4122–4137. [Google Scholar] [CrossRef]
- Gutschi LM, Seger F. Complexity, unpredictability and safety challenges of lipid nanoparticles. Zenodo preprint. Oct 13, 2025. [CrossRef]
- Lavington, S.; Watts, A. Lipid nanoparticle technologies for the study of G protein-coupled receptors in lipid environments. Biophys. Rev. 2020, 12, 1287–1302. [Google Scholar] [CrossRef]
- Sakurai, Y.; Watanabe, H.; Nishio, K.; Hashimoto, K.; Harada, A.; Gomi, M.; Suzuki, M.; Oyama, R.; Handa, T.; Sato, R.; et al. pH-Responsive Lipid Nanoparticles Achieve Efficient mRNA Transfection in Brain Capillary Endothelial Cells. Pharmaceutics 2022, 14, 1560. [Google Scholar] [CrossRef] [PubMed]
- Boros, L.G.; Kyriakopoulos, A.M.; Brogna, C.; Piscopo, M.; McCullough, P.A.; Seneff, S. Long-lasting, biochemically modified mRNA, and its frameshifted recombinant spike proteins in human tissues and circulation after COVID-19 vaccination. Pharmacol. Res. Perspect. 2024, 12, e1218. [Google Scholar] [CrossRef]
- Cordes, J.; Zhao, S.; Engel, C.M.; Stingele, J. Cellular responses to RNA damage. Cell 2025, 188, 885–900. [Google Scholar] [CrossRef] [PubMed]
- Kaludercic, N.; Deshwal, S.; Di Lisa, F. Reactive oxygen species and redox compartmentalization. Front. Physiol. 2014, 5, 285. [Google Scholar] [CrossRef]
- Kim, K.Q.; Burgute, B.D.; Tzeng, S.-C.; Jing, C.; Jungers, C.; Zhang, J.; Yan, L.L.; Vierstra, R.D.; Djuranovic, S.; Evans, B.S.; et al. N1-methylpseudouridine found within COVID-19 mRNA vaccines produces faithful protein products. Cell Rep. 2022, 40, 111300. [Google Scholar] [CrossRef]
- Röltgen, K.; Nielsen, S.C.; Silva, O.; Younes, S.F.; Zaslavsky, M.; Costales, C.; Yang, F.; Wirz, O.F.; Solis, D.; Hoh, R.A.; et al. Immune imprinting, breadth of variant recognition, and germinal center response in human SARS-CoV-2 infection and vaccination. Cell 2022, 185, 1025–1040.e14. [Google Scholar] [CrossRef]
- Kim, W.; Ly, N.K.; He, Y.; Li, Y.; Yuan, Z.; Yeo, Y. Protein corona: Friend or foe? Co-opting serum proteins for nanoparticle delivery. Adv. Drug Deliv. Rev. 2022, 192, 114635. [Google Scholar] [CrossRef]
- Suzuki, Y.; Ishihara, H. Difference in the lipid nanoparticle technology employed in three approved siRNA (Patisiran) and mRNA (COVID-19 vaccine) drugs. Drug Metab. Pharmacokinet. 2021, 41, 100424. [Google Scholar] [CrossRef]
- Buckley, M.; Araínga, M.; Maiorino, L.; Pires, I.S.; Kim, B.; Michaels, K.K.; Dye, J.; Qureshi, K.; Zhang, Y.J.; Mak, H.; et al. Visualizing lipid nanoparticle trafficking for mRNA vaccine delivery in non-human primates. Mol. Ther. 2025, 33, 1105–1117. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Sato, Y.; Okuda, K.; Iwakawa, K.; Harashima, H. mRNA-Loaded Lipid Nanoparticles Targeting Dendritic Cells for Cancer Immunotherapy. Pharmaceutics 2022, 14, 1572. [Google Scholar] [CrossRef] [PubMed]
- Hassett, K.J.; Rajlic, I.L.; Bahl, K.; White, R.; Cowens, K.; Jacquinet, E.; Burke, K.E. mRNA vaccine trafficking and resulting protein expression after intramuscular administration. Mol. Ther. - Nucleic Acids 2023, 35, 102083. [Google Scholar] [CrossRef] [PubMed]
- Maugeri, M.; Nawaz, M.; Papadimitriou, A.; Angerfors, A.; Camponeschi, A.; Na, M.; Hölttä, M.; Skantze, P.; Johansson, S.; Sundqvist, M.; et al. Linkage between endosomal escape of LNP-mRNA and loading into EVs for transport to other cells. Nat. Commun. 2019, 10, 1–15. [Google Scholar] [CrossRef]
- Inam, W.; Bhadane, R.; Akpolat, R.N.; Taiseer, R.A.; Filippov, S.K.; Salo-Ahen, O.M.H.; Rosenholm, J.M.; Zhang, H. Interactions between polymeric nanoparticles and different buffers as investigated by zeta potential measurements and molecular dynamics simulations. View 2022, 3, 20210009. [Google Scholar] [CrossRef]
- Simak, J.; De Paoli, S. The effects of nanomaterials on blood coagulation in hemostasis and thrombosis. WIREs Nanomed. Nanobiotechnol. 2017, 9. [Google Scholar] [CrossRef]
- Brooks, D.; Seaman, G. The effect of neutral polymers on the electrokinetic potential of cells and other charged particles. J. Colloid Interface Sci. 1973, 43, 670–686. [Google Scholar] [CrossRef]
- Ilinskaya, A.N.; Dobrovolskaia, M.A. Nanoparticles and the blood coagulation system. Part II: Safety concerns. Nanomedicine 2013, 8, 969–981. [Google Scholar] [CrossRef]
- Omo-Lamai, S.; Zamora, M.E.; Patel, M.N.; Wu, J.; Nong, J.; Wang, Z.; Peshkova, A.; Majumder, A.; Melamed, J.R.; Chase, L.S.; et al. Physicochemical Targeting of Lipid Nanoparticles to the Lungs Induces Clotting: Mechanisms and Solutions. Adv. Mater. 2024, 36, e2312026. [Google Scholar] [CrossRef]
- Bekal, S.; Husari, G.; Okura, M.; A Huang, C.; Bukari, M.S.; Huang, C.A.; Bukari, S. Thrombosis Development After mRNA COVID-19 Vaccine Administration: A Case Series. Cureus 2023, 15, e41371. [Google Scholar] [CrossRef]
- Kent, S.J.; Li, S.; Amarasena, T.H.; Reynaldi, A.; Lee, W.S.; Leeming, M.G.; O’connor, D.H.; Nguyen, J.; Kent, H.E.; Caruso, F.; et al. Blood Distribution of SARS-CoV-2 Lipid Nanoparticle mRNA Vaccine in Humans. ACS Nano 2024, 18, 27077–27089. [Google Scholar] [CrossRef]
- Ren, Y.; Lin, L.; Abdallah, M.; Zhu, X.; Liu, H.; Fabb, S.A.; Payne, T.J.; Pouton, C.W.; Johnston, A.P.; Trevaskis, N.L. Impact of ionizable lipid type on the pharmacokinetics and biodistribution of mRNA-lipid nanoparticles after intravenous and subcutaneous injection. J. Control. Release 2025, 384, 113945. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Molbay, M.; Chen, Y.; Horvath, I.; Kadletz, K.; Kick, B.; Zhao, S.; Al-Maskari, R.; Singh, I.; Ali, M.; et al. Nanocarrier imaging at single-cell resolution across entire mouse bodies with deep learning. Nat. Biotechnol. 2025, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Di, J.; Du, Z.; Wu, K.; Jin, S.; Wang, X.; Li, T.; Xu, Y. Biodistribution and Non-linear Gene Expression of mRNA LNPs Affected by Delivery Route and Particle Size. Pharm. Res. 2022, 39, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.K.; Nougarède, A.; Clément, F.; Fournier, C.; Jouvin-Marche, E.; Escudé, M.; Jary, D.; Navarro, F.P.; Marche, P.N. Tuning the Immunostimulation Properties of Cationic Lipid Nanocarriers for Nucleic Acid Delivery. Front. Immunol. 2021, 12. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, R.; Chen, Y.; Wang, M.; Du, J. Crosstalk between Oxidative Stress and Exosomes. Oxidative Med. Cell. Longev. 2022, 2022, 3553617. [Google Scholar] [CrossRef]
- Wei C, Zhu Y, Lu X, Goodier KD, Yu D, Liu X, Choy J, Calderón AT, Ma J, Su Y, Lin J, Li S, Schneck JP, Murphy SC, Mao H-Q. Systemic trafficking of mRNA lipid nanoparticle vaccine following intramuscular injection generates potent tissue-specific T cell response. bioRxiv Preprint. April 24, 2025. [CrossRef]
- Balcorta, H.V.; Corral, M.Y.M.; Gallegos, A.; Chavez, J.; Perez, J.; Balivada, S.; Natividad-Diaz, S.L.; Poon, W. Development of Chemical Tags for Universal Lipid Nanoparticle Visualization and Tracking in 2D and 3D Imaging. Nano Lett. 2025, 25, 7682–7689. [Google Scholar] [CrossRef]
- Yamamoto, K.; Mashiba, T.; Takano, K.; Suzuki, T.; Kami, M.; Takita, M.; Kusumi, E.; Mizuno, Y.; Hamaki, T. A Case of Exacerbation of Subclinical Hyperthyroidism after First Administration of BNT162b2 mRNA COVID-19 Vaccine. Vaccines 2021, 9, 1108. [Google Scholar] [CrossRef]
- Hummel, A.; Oniszczuk, J.; Kervella, D.; Charbit, M.; Guerrot, D.; Testa, A.; Philipponnet, C.; Chauvet, C.; Guincestre, T.; Brochard, K.; et al. Idiopathic nephrotic syndrome relapse following COVID-19 vaccination: A series of 25 cases. Clin. Kidney J. 2022, 15, 1574–1582. [Google Scholar] [CrossRef]
- Malayala, S.V.; Papudesi, B.N.; Sharma, R.; Vusqa, U.T.; Raza, A. A Case of Idiopathic Thrombocytopenic Purpura After Booster Dose of BNT162b2 (Pfizer-Biontech) COVID-19 Vaccine. Cureus 2021, 13. [Google Scholar] [CrossRef]
- Shumnalieva, R.; Ravichandran, N.; Hannah, J.; Javaid, M.; Darooka, N.; Roy, D.; Gonzalez, D.E.; Velikova, T.; Milchert, M.; Kuwana, M.; et al. Characteristics of emerging new autoimmune diseases after COVID-19 vaccination: A sub-study by the COVAD group. Int. J. Rheum. Dis. 2024, 27, e15178. [Google Scholar] [CrossRef]
- Broachwala, M.; Banks, D.W.; Jevotovsky, D.S.; Oehlermarx, W.; Durbhakula, S. Burning Mouth Syndrome Following Covid Vaccination: A Case Report. Clin. Case Rep. 2025, 13, e70329. [Google Scholar] [CrossRef]
- Fraiman, J.; Erviti, J.; Jones, M.; Greenland, S.; Whelan, P.; Kaplan, R.M.; Doshi, P. Serious adverse events of special interest following mRNA COVID-19 vaccination in randomized trials in adults. Vaccine 2022, 40, 5798–5805. [Google Scholar] [CrossRef] [PubMed]
- Faksova, K.; Walsh, D.; Jiang, Y.; Griffin, J.; Phillips, A.; Gentile, A.; Kwong, J.; Macartney, K.; Naus, M.; Grange, Z.; et al. COVID-19 vaccines and adverse events of special interest: A multinational Global Vaccine Data Network (GVDN) cohort study of 99 million vaccinated individuals. Vaccine 2024, 42, 2200–2211. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, M.-H.; Choi, M.G.; Chun, E.M. 1-year risks of cancers associated with COVID-19 vaccination: A large population-based cohort study in South Korea. Biomark. Res. 2025, 13, 1–4. [Google Scholar] [CrossRef]
- Abbasi, R.; Shineh, G.; Mobaraki, M.; Doughty, S.; Tayebi, L. Structural parameters of nanoparticles affecting their toxicity for biomedical applications: A review. J. Nanoparticle Res. 2023, 25, 1–35. [Google Scholar] [CrossRef]
- Yuan, Z.; Yan, R.; Fu, Z.; Wu, T.; Ren, C. Impact of physicochemical properties on biological effects of lipid nanoparticles: Are they completely safe. Sci. Total. Environ. 2024, 927, 172240. [Google Scholar] [CrossRef]
- Szebeni, J.; Kiss, B.; Bozó, T.; Turjeman, K.; Levi-Kalisman, Y.; Barenholz, Y.; Kellermayer, M. Insights into the Structure of Comirnaty Covid-19 Vaccine: A Theory on Soft, Partially Bilayer-Covered Nanoparticles with Hydrogen Bond-Stabilized mRNA–Lipid Complexes. ACS Nano 2023, 17, 13147–13157. [Google Scholar] [CrossRef]
- Brader, M.L.; Williams, S.J.; Banks, J.M.; Hui, W.H.; Zhou, Z.H.; Jin, L. Encapsulation state of messenger RNA inside lipid nanoparticles. Biophys. J. 2021, 120, 2766–2770. [Google Scholar] [CrossRef]
- Münter, R.; Larsen, J.B.; Andresen, T.L. The vast majority of nucleic acid-loaded lipid nanoparticles contain cargo. J. Colloid Interface Sci. 2024, 674, 139–144. [Google Scholar] [CrossRef]
- Li, S.; Hu, Y.; Li, A.; Lin, J.; Hsieh, K.; Schneiderman, Z.; Zhang, P.; Zhu, Y.; Qiu, C.; Kokkoli, E.; et al. Payload distribution and capacity of mRNA lipid nanoparticles. Nat. Commun. 2022, 13, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ye, Y.; Li, M.; Zuo, T.; Xie, Z.; Ke, Y.; Cheng, H.; Hong, L.; Liu, Z. Structural characterization of mRNA lipid nanoparticles (LNPs) in the presence of mRNA-free LNPs. J. Control. Release 2025, 386, 114082. [Google Scholar] [CrossRef] [PubMed]
- Rampado, R.; Crotti, S.; Caliceti, P.; Pucciarelli, S.; Agostini, M. Recent Advances in Understanding the Protein Corona of Nanoparticles and in the Formulation of “Stealthy” Nanomaterials. Front. Bioeng. Biotechnol. 2020, 8, 166. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, Y.; Rehman, M.; Wang, Y.-F.; Guo, S. Protein Corona of Nanoparticles: Isolation and Analysis. Chem Bio Eng. 2024, 1, 757–772. [Google Scholar] [CrossRef]
- Voke, E.; Arral, M.L.; Squire, H.J.; Lin, T.-J.; Zheng, L.; Coreas, R.; Lui, A.; Iavarone, A.T.; Pinals, R.L.; Whitehead, K.A.; et al. Protein corona formed on lipid nanoparticles compromises delivery efficiency of mRNA cargo. Nat. Commun. 2025, 16, 1–16. [Google Scholar] [CrossRef]
- Sebastiani, F.; Yanez Arteta, M.; Lerche, M.; Porcar, L.; Lang, C.; Bragg, R.A.; Elmore, C.S.; Krishnamurthy, V.R.; Russell, R.A.; Darwish, T.; et al. Apolipoprotein E Binding Drives Structural and Compositional Rearrangement of mRNA-Containing Lipid Nanoparticles. ACS Nano 2021, 15, 6709–6722. [Google Scholar] [CrossRef]
- Liu, K.; Nilsson, R.; Lázaro-Ibáñez, E.; Duàn, H.; Miliotis, T.; Strimfors, M.; Lerche, M.; Ribeiro, A.R.S.; Ulander, J.; Lindén, D.; et al. Multiomics analysis of naturally efficacious lipid nanoparticle coronas reveals high-density lipoprotein is necessary for their function. Nat. Commun. 2023, 14, 1–16. [Google Scholar] [CrossRef]
- Hosseini-Kharat, M.; Bremmell, K.E.; Prestidge, C.A. Why do lipid nanoparticles target the liver? Understanding of biodistribution and liver-specific tropism. Mol. Ther. - Methods Clin. Dev. 2025, 33, 101436. [Google Scholar] [CrossRef]
- Therapeutic Goods Administration. Nonclinical Evaluation Report BNT162b2 [mRNA] COVID-19 vaccine (COMIRNATY). Health, Ed.; Department of Health and Aged Care. 2021; Vol. FOI 2389. Available online: https://www.tga.gov.au/sites/default/files/foi-2389-06.pdf.
- Neves, A.R.; Queiroz, J.F.; Lima, S.A.C.; Figueiredo, F.; Fernandes, R.; Reis, S. Cellular uptake and transcytosis of lipid-based nanoparticles across the intestinal barrier: Relevance for oral drug delivery. J. Colloid Interface Sci. 2016, 463, 258–265. [Google Scholar] [CrossRef]
- Haghighi, E.; Abolmaali, S.S.; Dehshahri, A.; Shaegh, S.A.M.; Azarpira, N.; Tamaddon, A.M. Navigating the intricate in-vivo journey of lipid nanoparticles tailored for the targeted delivery of RNA therapeutics: A quality-by-design approach. J. Nanobiotechnology 2024, 22, 1–55. [Google Scholar] [CrossRef]
- Khare, P.; Edgecomb, S.X.; Hamadani, C.M.; Tanner, E.E.; Manickam, D.S. Lipid nanoparticle-mediated drug delivery to the brain. Adv. Drug Deliv. Rev. 2023, 197, 114861. [Google Scholar] [CrossRef]
- Chen, J.; Xu, Y.; Zhou, M.; Xu, S.; Varley, A.J.; Golubovic, A.; Lu, R.X.Z.; Wang, K.C.; Yeganeh, M.; Vosoughi, D.; et al. Combinatorial design of ionizable lipid nanoparticles for muscle-selective mRNA delivery with minimized off-target effects. Proc. Natl. Acad. Sci. 2023, 120. [Google Scholar] [CrossRef]
- Younis, M.A.; Sato, Y.; Elewa, Y.H.; Kon, Y.; Harashima, H. Self-homing nanocarriers for mRNA delivery to the activated hepatic stellate cells in liver fibrosis. J. Control. Release 2022, 353, 685–698. [Google Scholar] [CrossRef] [PubMed]
- Naasani, I. Establishing the Pharmacokinetics of Genetic Vaccines is Essential for Maximising their Safety and Efficacy. Clin. Pharmacokinet. 2022, 61, 921–927. [Google Scholar] [CrossRef] [PubMed]
- European Medical Assessment. 2020. Available online: https://www.ema.europa.eu/en/documents/assessment-report/comirnaty-epar-public-assessment-report en.pdf.
- Akhter, H.; Khalilullah, H.; Gupta, M.; Alfaleh, M.A.; Alhakamy, N.A.; Riadi, Y.; Md, S. Impact of Protein Corona on the Biological Identity of Nanomedicine: Understanding the Fate of Nanomaterials in the Biological Milieu. Biomedicines 2021, 9, 1496. [Google Scholar] [CrossRef] [PubMed]
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Brown, D.; Alkilany, A.M.; Farokhzad, O.C.; Mahmoudi, M. Cellular uptake of nanoparticles: Journey inside the cell. Chem. Soc. Rev. 2017, 46, 4218–4244. [Google Scholar] [CrossRef]
- Chatterjee, S.; Kon, E.; Sharma, P.; Peer, D. Endosomal escape: A bottleneck for LNP-mediated therapeutics. Proc. Natl. Acad. Sci. 2024, 121. [Google Scholar] [CrossRef]
- Sengottiyan, S.; Mikolajczyk, A.; Jagiełło, K.; Swirog, M.; Puzyn, T. Core, Coating, or Corona? The Importance of Considering Protein Coronas in nano-QSPR Modeling of Zeta Potential. ACS Nano 2023, 17, 1989–1997. [Google Scholar] [CrossRef]
- Fell, V.H.K.; Kramer, T.; Heindl, A.; Merkel, O.M. Prediction of the Apparent pKa Value of Lipid Nanoparticles by Density Functional Theory. ACS Mater. Au 2025, 5, 451–457. [Google Scholar] [CrossRef]
- Johansson, J.M.; Du Rietz, H.; Hedlund, H.; Eriksson, H.C.; Blenke, E.O.; Pote, A.; Harun, S.; Nordenfelt, P.; Lindfors, L.; Wittrup, A. Cellular and biophysical barriers to lipid nanoparticle mediated delivery of RNA to the cytosol. Nat. Commun. 2025, 16, 1–20. [Google Scholar] [CrossRef]
- Ermilova, I.; Swenson, J. Ionizable lipids penetrate phospholipid bilayers with high phase transition temperatures: Perspectives from free energy calculations. Chem. Phys. Lipids 2023, 253, 105294. [Google Scholar] [CrossRef]
- Er-Rafik, M.; Ferji, K.; Combet, J.; Sandre, O.; Lecommandoux, S.; Schmutz, M.; Le Meins, J.-F.; Marques, C.M. Tear of lipid membranes by nanoparticles. Soft Matter 2022, 18, 3318–3322. [Google Scholar] [CrossRef] [PubMed]
- Pilkington, E.H.; Suys, E.J.; Trevaskis, N.L.; Wheatley, A.K.; Zukancic, D.; Algarni, A.; Al-Wassiti, H.; Davis, T.P.; Pouton, C.W.; Kent, S.J.; et al. From influenza to COVID-19: Lipid nanoparticle mRNA vaccines at the frontiers of infectious diseases. Acta Biomater. 2021, 131, 16–40. [Google Scholar] [CrossRef]
- Müller, J.A.; Schäffler, N.; Kellerer, T.; Schwake, G.; Ligon, T.S.; Rädler, J.O. Kinetics of RNA-LNP delivery and protein expression. Eur. J. Pharm. Biopharm. 2024, 197, 114222. [Google Scholar] [CrossRef]
- Aliakbarinodehi, N.; Niederkofler, S.; Emilsson, G.; Parkkila, P.; Olsén, E.; Jing, Y.; Sjöberg, M.; Agnarsson, B.; Lindfors, L.; Höök, F. Time-Resolved Inspection of Ionizable Lipid-Facilitated Lipid Nanoparticle Disintegration and Cargo Release at an Early Endosomal Membrane Mimic. ACS Nano 2024, 18, 22989–23000. [Google Scholar] [CrossRef]
- Schlich, M.; Palomba, R.; Costabile, G.; Mizrahy, S.; Pannuzzo, M.; Peer, D.; Decuzzi, P. Cytosolic delivery of nucleic acids: The case of ionizable lipid nanoparticles. Bioeng. Transl. Med. 2021, 6, e10213. [Google Scholar] [CrossRef]
- Sabnis, S.; Kumarasinghe, E.S.; Salerno, T.; Mihai, C.; Ketova, T.; Senn, J.J.; Lynn, A.; Bulychev, A.; McFadyen, I.; Chan, J.; et al. A Novel Amino Lipid Series for mRNA Delivery: Improved Endosomal Escape and Sustained Pharmacology and Safety in Non-human Primates. Mol. Ther. 2018, 26, 1509–1519. [Google Scholar] [CrossRef]
- Paramasivam, P.; Franke, C.; Stöter, M.; Höijer, A.; Bartesaghi, S.; Sabirsh, A.; Lindfors, L.; Arteta, M.Y.; Dahlén, A.; Bak, A.; et al. Endosomal escape of delivered mRNA from endosomal recycling tubules visualized at the nanoscale. J. Cell Biol. 2021, 221. [Google Scholar] [CrossRef]
- Sahay, G.; Querbes, W.; Alabi, C.; Eltoukhy, A.; Sarkar, S.; Zurenko, C.; Karagiannis, E.; Love, K.; Chen, D.; Zoncu, R.; et al. Efficiency of siRNA delivery by lipid nanoparticles is limited by endocytic recycling. Nat. Biotechnol. 2013, 31, 653–658. [Google Scholar] [CrossRef]
- Bitounis, D.; Jacquinet, E.; Rogers, M.A.; Amiji, M.M. Strategies to reduce the risks of mRNA drug and vaccine toxicity. Nat. Rev. Drug Discov. 2024, 23, 281–300. [Google Scholar] [CrossRef]
- Moghimi, S.M.; Simberg, D. Pro-inflammatory concerns with lipid nanoparticles. Mol. Ther. 2022, 30, 2109–2110. [Google Scholar] [CrossRef] [PubMed]
- Bates, S.M.; Munson, M.J.; Trovisco, V.; Pereira, S.; Miller, S.R.; Sabirsh, A.; Betts, C.J.; Blenke, E.O.; Gay, N.J. The kinetics of endosomal disruption reveal differences in lipid nanoparticle induced cellular toxicity. J. Control. Release 2025, 114047. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Song, Y.; Zhu, Z.; Huang, X.; Fan, J.; Qiao, J.; Mao, F. Cell–cell communication: New insights and clinical implications. Signal Transduct. Target. Ther. 2024, 9, 1–52. [Google Scholar] [CrossRef]
- Bansal, S.; Perincheri, S.; Fleming, T.; Poulson, C.; Tiffany, B.; Bremner, R.M.; Mohanakumar, T. Cutting Edge: Circulating Exosomes with COVID Spike Protein Are Induced by BNT162b2 (Pfizer–BioNTech) Vaccination prior to Development of Antibodies: A Novel Mechanism for Immune Activation by mRNA Vaccines. J. Immunol. 2021, 207, 2405–2410. [Google Scholar] [CrossRef]
- Kammerer USV, Steger K. BioNTech RNA-based COVID-19 injections contain large amounts of residual DNA including an SV40 promoter/enhancer Sequence. Journal of Science, Public Health Policy and the Law 2024, v5.2019-2024.
- Sahin, U.; Karikó, K.; Türeci, Ö. mRNA-based therapeutics—Developing a new class of drugs. Nat. Rev. Drug Discov. 2014, 13, 759–780. [Google Scholar] [CrossRef]
- Jörgensen, A.M.; Wibel, R.; Bernkop-Schnürch, A. Biodegradable Cationic and Ionizable Cationic Lipids: A Roadmap for Safer Pharmaceutical Excipients. Small 2023, 19, e2206968. [Google Scholar] [CrossRef]
- Knaggs, K.L.M.; Sun, Y.; Walz, B.A.; Pang, J.; Khan, O.F. The role of excipients in lipid nanoparticle metabolism: Implications for enhanced therapeutic effect. Ther. Deliv. 2025, 16, 687–700. [Google Scholar] [CrossRef]
- Packer, M.; Gyawali, D.; Yerabolu, R.; Schariter, J.; White, P. A novel mechanism for the loss of mRNA activity in lipid nanoparticle delivery systems. Nat. Commun. 2021, 12, 1–11. [Google Scholar] [CrossRef]
- Moderna. Moderna Science and Technology Day. 2022. Available online: https://s29.q4cdn.com/435878511/files/doc_presentations/2022/05/Science-Day-2022-Master-Slides-FINAL-(05.17_7am).pdf (accessed on 28 February 2023).
- USFDA. Letter to Pfizer: Children’s vaccination, authorization of formulation change. US Food and Drug Administration, 2021. Available online: https://cacmap.fda.gov/media/150386/download.
- Maelfait, J.; Liverpool, L.; Rehwinkel, J. Nucleic Acid Sensors and Programmed Cell Death. J. Mol. Biol. 2020, 432, 552–568. [Google Scholar] [CrossRef]
- Fritz, K.S.; Petersen, D.R. An overview of the chemistry and biology of reactive aldehydes. Free Radic. Biol. Med. 2013, 59, 85–91. [Google Scholar] [CrossRef]
- Dalleau, S.; Baradat, M.; Guéraud, F.; Huc, L. Cell death and diseases related to oxidative stress:4-hydroxynonenal (HNE) in the balance. Cell Death Differ. 2013, 20, 1615–1630. [Google Scholar] [CrossRef]
- Hashiba, K.; Taguchi, M.; Sakamoto, S.; Otsu, A.; Maeda, Y.; Ebe, H.; Okazaki, A.; Harashima, H.; Sato, Y. Overcoming thermostability challenges in mRNA–lipid nanoparticle systems with piperidine-based ionizable lipids. Commun. Biol. 2024, 7, 1–13. [Google Scholar] [CrossRef]
- Wang, W.; Deng, S.; Lin, J.; Ouyang, D. Modeling on in vivo disposition and cellular transportation of RNA lipid nanoparticles via quantum mechanics/physiologically-based pharmacokinetic approaches. Acta Pharm. Sin. B 2024, 14, 4591–4607. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Li, Q.; Wang, J.; Yu, Y.; Wang, Y.; Zhou, Q.; Li, P. Reactive Oxygen Species-Related Nanoparticle Toxicity in the Biomedical Field. Nanoscale Res. Lett. 2020, 15, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Atianand, M.K.; A Fitzgerald, K. Molecular Basis of DNA Recognition in the Immune System. J. Immunol. 2013, 190, 1911–1918. [Google Scholar] [CrossRef] [PubMed]
- Szebeni, J.; Simberg, D.; González-Fernández, A.; Barenholz, Y.; Dobrovolskaia, M.A. Roadmap and strategy for overcoming infusion reactions to nanomedicines. Nat. Nanotechnol. 2018, 13, 1100–1108. [Google Scholar] [CrossRef]
- Bakos, T.; Mészáros, T.; Kozma, G.T.; Berényi, P.; Facskó, R.; Farkas, H.; Dézsi, L.; Heirman, C.; de Koker, S.; Schiffelers, R.; et al. mRNA-LNP COVID-19 Vaccine Lipids Induce Complement Activation and Production of Proinflammatory Cytokines: Mechanisms, Effects of Complement Inhibitors, and Relevance to Adverse Reactions. Int. J. Mol. Sci. 2024, 25, 3595. [Google Scholar] [CrossRef]
- European Medicines Agency. Onpattro: European Public Assessment Report (EPAR) EMA/554262/2018. CHMP, Ed.; Amsterdam, NL, 2018.
- Wang, J.; Ding, Y.; Chong, K.; Cui, M.; Cao, Z.; Tang, C.; Tian, Z.; Hu, Y.; Zhao, Y.; Jiang, S. Recent Advances in Lipid Nanoparticles and Their Safety Concerns for mRNA Delivery. Vaccines 2024, 12, 1148. [Google Scholar] [CrossRef]
- Zhang, L.; Ren, F.; Zhang, X.; Wang, X.; Shi, H.; Zhou, L.; Zheng, S.; Chen, Y.; Chen, D.; Li, L.; et al. Peroxisome proliferator-activated receptor alpha acts as a mediator of endoplasmic reticulum stress-induced hepatocyte apoptosis in acute liver failure. Dis. Model. Mech. 2016, 9, 799–809. [Google Scholar] [CrossRef]
- Popovics, H.; Mikone, K.; Mozes, M.; Kwon, J.; Hansmann, G.; Kokeny, G. P0721PPAR-GAMMA ACTIVATION INHIBITS TGF-BETA INDUCED RENAL COMPLEMENT AND GALECTIN-3 EXPRESSION IN VIVO AND IN VITRO. Nephrol. Dial. Transplant. 2020, 35. [Google Scholar] [CrossRef]
- Kong, Y.; Wang, N.; Tong, Z.; Wang, D.; Wang, P.; Yang, Q.; Yan, X.; Song, W.; Jin, Z.; Zhang, M. Role of complement factor D in cardiovascular and metabolic diseases. Front. Immunol. 2024, 15, 1453030. [Google Scholar] [CrossRef] [PubMed]
- Pfizer/BioNTech. R&D STUDY REPORT No. R-20-0112 Characterizing the immunophenotype in spleen and lymph node of mice treated with SARS-CoV2 vaccine candidates. Report released by FOIA 1/2/23. 2020 Sep 17. Available online: https://phmpt.org/wp-content/uploads/2023/02/125742_S1_M4_4.2.1-r-20-0112.pdf (accessed on 5 October 2025).
- Chaudhary, N.; Kasiewicz, L.N.; Newby, A.N.; Arral, M.L.; Yerneni, S.S.; Melamed, J.R.; LoPresti, S.T.; Fein, K.C.; Petersen, D.M.S.; Kumar, S.; et al. Amine headgroups in ionizable lipids drive immune responses to lipid nanoparticles by binding to the receptors TLR4 and CD1d. Nat. Biomed. Eng. 2024, 8, 1483–1498. [Google Scholar] [CrossRef] [PubMed]
- Means, N.; Elechalawar, C.K.; Chen, W.R.; Bhattacharya, R.; Mukherjee, P. Revealing macropinocytosis using nanoparticles. Mol. Asp. Med. 2022, 83, 100993. [Google Scholar] [CrossRef]
- Baimanov, D.; Wang, J.; Liu, Y.; Zheng, P.; Yu, S.; Liu, F.; Wang, J.; Boraschi, D.; Zhao, Y.; Chen, C.; et al. Identification of Cell Receptors Responsible for Recognition and Binding of Lipid Nanoparticles. J. Am. Chem. Soc. 2025, 147, 7604–7616. [Google Scholar] [CrossRef]
- Paunovska, K.; Sanchez, A.J.D.S.; Lokugamage, M.P.; Loughrey, D.; Echeverri, E.S.; Cristian, A.; Hatit, M.Z.C.; Santangelo, P.J.; Zhao, K.; Dahlman, J.E. The Extent to Which Lipid Nanoparticles Require Apolipoprotein E and Low-Density Lipoprotein Receptor for Delivery Changes with Ionizable Lipid Structure. Nano Lett. 2022, 22, 10025–10033. [Google Scholar] [CrossRef]
- Karikó, K.; Ni, H.; Capodici, J.; Lamphier, M.; Weissman, D. mRNA Is an Endogenous Ligand for Toll-like Receptor 3. J. Biol. Chem. 2004, 279, 12542–12550. [Google Scholar] [CrossRef]
- Karikó, K.; Muramatsu, H.; Welsh, F.A.; Ludwig, J.; Kato, H.; Akira, S.; Weissman, D. Incorporation of Pseudouridine Into mRNA Yields Superior Nonimmunogenic Vector With Increased Translational Capacity and Biological Stability. Mol. Ther. 2008, 16, 1833–1840. [Google Scholar] [CrossRef]
- Karikó, K.; Buckstein, M.; Ni, H.; Weissman, D. Suppression of RNA Recognition by Toll-like Receptors: The Impact of Nucleoside Modification and the Evolutionary Origin of RNA. Immunity 2005, 23, 165–175. [Google Scholar] [CrossRef]
- Monroe, J.; Eyler, D.E.; Mitchell, L.; Deb, I.; Bojanowski, A.; Srinivas, P.; Dunham, C.M.; Roy, B.; Frank, A.T.; Koutmou, K.S. N1-Methylpseudouridine and pseudouridine modifications modulate mRNA decoding during translation. Nat. Commun. 2024, 15, 1–11. [Google Scholar] [CrossRef]
- Przybylski, S.; Gasch, M.; Marschner, A.; Ebert, M.; Ewe, A.; Helmig, G.; Hilger, N.; Fricke, S.; Rudzok, S.; Aigner, A.; et al. Influence of nanoparticle-mediated transfection on proliferation of primary immune cells in vitro and in vivo. PLOS ONE 2017, 12, e0176517. [Google Scholar] [CrossRef]
- Guo, X.; Wang, H.; Li, Y.; Leng, X.; Huang, W.; Ma, Y.; Xu, T.; Qi, X. Transfection reagent Lipofectamine triggers type I interferon signaling activation in macrophages. Immunol. Cell Biol. 2018, 97, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Choe, U.; Yu, L.; Wang, T.T. Selected lipid-based transfection reagents activate NF-κB and MAP kinases signaling pathways, induced cytokines mRNA expression in human THP-1 macrophage. Anal. Biochem. 2019, 573, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Connors, J.; Joyner, D.; Mege, N.J.; Cusimano, G.M.; Bell, M.R.; Marcy, J.; Taramangalam, B.; Kim, K.M.; Lin, P.J.C.; Tam, Y.K.; et al. Lipid nanoparticles (LNP) induce activation and maturation of antigen presenting cells in young and aged individuals. Commun. Biol. 2023, 6, 1–13. [Google Scholar] [CrossRef]
- Korzun, T.; Moses, A.S.; Jozic, A.; Grigoriev, V.; Newton, S.; Kim, J.; Diba, P.; Sattler, A.; Levasseur, P.R.; Le, N.; et al. Lipid Nanoparticles Elicit Reactogenicity and Sickness Behavior in Mice Via Toll-Like Receptor 4 and Myeloid Differentiation Protein 88 Axis. ACS Nano 2024, 18, 24842–24859. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. Toll-like Receptors and Their Crosstalk with Other Innate Receptors in Infection and Immunity. Immunity 2011, 34, 637–650. [Google Scholar] [CrossRef]
- Ding, X.; Jin, S.; Tong, Y.; Jiang, X.; Chen, Z.; Mei, S.; Zhang, L.; Billiar, T.R.; Li, Q. TLR4 signaling induces TLR3 up-regulation in alveolar macrophages during acute lung injury. Sci. Rep. 2017, 7, srep34278. [Google Scholar] [CrossRef]
- Nouri-Shirazi, M.; Tamjidi, S.; Nourishirazi, E.; Guinet, E. Combination of TLR8 and TLR4 agonists reduces the degrading effects of nicotine on DC-NK mediated effector T cell generation. Int. Immunopharmacol. 2018, 61, 54–63. [Google Scholar] [CrossRef]
- Kim, H.-J.; Kim, H.; Lee, J.-H.; Hwangbo, C. Toll-like receptor 4 (TLR4): New insight immune and aging. Immun. Ageing 2023, 20, 1–11. [Google Scholar] [CrossRef]
- Alhamdan, F.; Bayarsaikhan, G.; Yuki, K. Toll-like receptors and integrins crosstalk. Front. Immunol. 2024, 15, 1403764. [Google Scholar] [CrossRef]
- Ghosh, T.K.; Mickelson, D.J.; Solberg, J.C.; Lipson, K.E.; Inglefield, J.R.; Alkan, S.S. TLR–TLR cross talk in human PBMC resulting in synergistic and antagonistic regulation of type-1 and 2 interferons, IL-12 and TNF-α. Int. Immunopharmacol. 2007, 7, 1111–1121. [Google Scholar] [CrossRef]
- Xu, X.H.; Shah, P.K.; Faure, E.; Equils, O.; Thomas, L.; Fishbein, M.C.; Luthringer, D.; Xu, X.-P.; Rajavashisth, T.B.; Yano, J.; et al. Toll-Like Receptor-4 Is Expressed by Macrophages in Murine and Human Lipid-Rich Atherosclerotic Plaques and Upregulated by Oxidized LDL. Circulation 2001, 104, 3103–3108. [Google Scholar] [CrossRef]
- Hovland, A.; Jonasson, L.; Garred, P.; Yndestad, A.; Aukrust, P.; Lappegård, K.T.; Espevik, T.; Mollnes, T.E. The complement system and toll-like receptors as integrated players in the pathophysiology of atherosclerosis. Atherosclerosis 2015, 241, 480–494. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Wall, A.A.; Tong, S.J.; Hung, Y.; Xiao, Z.; Tarique, A.A.; Sly, P.D.; Fantino, E.; Marzolo, M.-P.; Stow, J.L. TLR Crosstalk Activates LRP1 to Recruit Rab8a and PI3Kγ for Suppression of Inflammatory Responses. Cell Rep. 2018, 24, 3033–3044. [Google Scholar] [CrossRef] [PubMed]
- Köberlin, M.S.; Heinz, L.X.; Superti-Furga, G. Functional crosstalk between membrane lipids and TLR biology. Curr. Opin. Cell Biol. 2016, 39, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Kornilov, F.D.; Shabalkina, A.V.; Lin, C.; Volynsky, P.E.; Kot, E.F.; Kayushin, A.L.; Lushpa, V.A.; Goncharuk, M.V.; Arseniev, A.S.; Goncharuk, S.A.; et al. The architecture of transmembrane and cytoplasmic juxtamembrane regions of Toll-like receptors. Nat. Commun. 2023, 14, 1–12. [Google Scholar] [CrossRef]
- Ndeupen, S.; Qin, Z.; Jacobsen, S.; Bouteau, A.; Estanbouli, H.; Igyártó, B.Z. The mRNA-LNP platform’s lipid nanoparticle component used in preclinical vaccine studies is highly inflammatory. iScience 2021, 24, 103479. [Google Scholar] [CrossRef]
- Finney, D.J. The median lethal dose and its estimation. Arch. Toxicol. 1985, 56, 215–218. [Google Scholar] [CrossRef]
- Avila, A.M.; Bebenek, I.; Bonzo, J.A.; Bourcier, T.; Davis Bruno, K.L.; Carlson, D.B.; Dubinion, J.; Elayan, I.; Harrouk, W.; Lee, S.-L.; et al. An FDA/CDER perspective on nonclinical testing strategies: Classical toxicology approaches and new approach methodologies (NAMs). Regul. Toxicol. Pharmacol. 2020, 114, 104662. [Google Scholar] [CrossRef]
- Li, G.-M. Mechanisms and functions of DNA mismatch repair. Cell Res. 2007, 18, 85–98. [Google Scholar] [CrossRef]
- Molnár, T.; Mázló, A.; Tslaf, V.; Szöllősi, A.G.; Emri, G.; Koncz, G. Current translational potential and underlying molecular mechanisms of necroptosis. Cell Death Dis. 2019, 10, 1–21. [Google Scholar] [CrossRef]
- Park, B.; Pirmohamed, M.; Kitteringham, N.R. The role of cytochrome P450 enzymes in hepatic and extrahepatic human drug toxicity. Pharmacol. Ther. 1995, 68, 385–424. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Jin, Y.; Chen, X.; Ye, X.; Shen, X.; Lin, M.; Zeng, C.; Zhou, T.; Zhang, J. NF-κB in biology and targeted therapy: New insights and translational implications. Signal Transduct. Target. Ther. 2024, 9, 1–37. [Google Scholar] [CrossRef]
- Parameswaran, N.; Patial, S. Tumor Necrosis Factor-α Signaling in Macrophages. Crit. Rev. Eukaryot. Gene Expr. 2010, 20, 87–103. [Google Scholar] [CrossRef]
- Mills, K.H.G. IL-17 and IL-17-producing cells in protection versus pathology. Nat. Rev. Immunol. 2022, 23, 38–54. [Google Scholar] [CrossRef]
- Wicherska-Pawłowska, K.; Wróbel, T.; Rybka, J. Toll-Like Receptors (TLRs), NOD-Like Receptors (NLRs), and RIG-I-Like Receptors (RLRs) in Innate Immunity. TLRs, NLRs, and RLRs Ligands as Immunotherapeutic Agents for Hematopoietic Diseases. Int. J. Mol. Sci. 2021, 22, 13397. [Google Scholar] [CrossRef]
- Mohan, G.S.; Kumar, R. Impairment of Tricarboxylic Acid Cycle (TCA) Cycle in Alzheimer’s Disease: Mechanisms, Implications, and Potential Therapies. Aging Dis. 2025, 16, 2553–2574. [Google Scholar] [CrossRef]
- Lee, Y. Roles of circadian clocks in cancer pathogenesis and treatment. Exp. Mol. Med. 2021, 53, 1529–1538. [Google Scholar] [CrossRef]
- Dunn, W.G.; McLoughlin, M.A.; Vassiliou, G.S. Clonal hematopoiesis and hematological malignancy. J. Clin. Investig. 2024, 134. [Google Scholar] [CrossRef]
- Zheng, Y.; Sun, L.; Jiang, T.; Zhang, D.; He, D.; Nie, H. TNFαPromotes Th17 Cell Differentiation through IL-6 and IL-1βProduced by Monocytes in Rheumatoid Arthritis. J. Immunol. Res. 2014, 2014, 1–12. [Google Scholar] [CrossRef]
- Marasco, M.R.; Conteh, A.M.; Reissaus, C.A.; Cupit, J.E., V; Appleman, E.M.; Mirmira, R.G.; Linnemann, A.K. Interleukin-6 Reduces β-Cell Oxidative Stress by Linking Autophagy With the Antioxidant Response. Diabetes 2018, 67, 1576–1588. [Google Scholar] [CrossRef]
- Deretic, V.; Saitoh, T.; Akira, S. Autophagy in infection, inflammation and immunity. Nat. Rev. Immunol. 2013, 13, 722–737. [Google Scholar] [CrossRef] [PubMed]
- Chi, D.S.; Fitzgerald, S.M.; Pitts, S.; Cantor, K.; King, E.; A Lee, S.; Huang, S.-K.; Krishnaswamy, G. MAPK-dependent regulation of IL-1- and β-adrenoreceptor-induced inflammatory cytokine production from mast cells: Implications for the stress response. BMC Immunol. 2004, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.-T.; Cohen, P.; Rousseau, S. IL–1β-stimulated activation of ERK1/2 and p38α MAPK mediates the transcriptional up-regulation of IL–6, IL–8 and GRO-α in HeLa cells. Cell. Signal. 2008, 20, 375–380. [Google Scholar] [CrossRef]
- Tengesdal, I.W.; Dinarello, A.; Powers, N.E.; Burchill, M.A.; Joosten, L.A.B.; Marchetti, C.; Dinarello, C.A. Tumor NLRP3-Derived IL-1β Drives the IL-6/STAT3 Axis Resulting in Sustained MDSC-Mediated Immunosuppression. Front. Immunol. 2021, 12. [Google Scholar] [CrossRef]
- Li, H.; Wu, M.; Zhao, X. Role of chemokine systems in cancer and inflammatory diseases. Medcomm 2022, 3, e147. [Google Scholar] [CrossRef]
- Unver, N. Macrophage chemoattractants secreted by cancer cells: Sculptors of the tumor microenvironment and another crucial piece of the cancer secretome as a therapeutic target. Cytokine Growth Factor Rev. 2019, 50, 13–18. [Google Scholar] [CrossRef]
- Nakatsumi, H.; Matsumoto, M.; Nakayama, K.I. Noncanonical Pathway for Regulation of CCL2 Expression by an mTORC1-FOXK1 Axis Promotes Recruitment of Tumor-Associated Macrophages. Cell Rep. 2017, 21, 2471–2486. [Google Scholar] [CrossRef]
- Huda, N.; Khambu, B.; Liu, G.; Nakatsumi, H.; Yan, S.; Chen, X.; Ma, M.; Dong, Z.; Nakayama, K.I.; Yin, X.-M. Senescence Connects Autophagy Deficiency to Inflammation and Tumor Progression in the Liver. Cell. Mol. Gastroenterol. Hepatol. 2022, 14, 333–355. [Google Scholar] [CrossRef]
- Iii, J.F.; Nandi, D.; Kulkarni, A. mRNA-carrying lipid nanoparticles that induce lysosomal rupture activate NLRP3 inflammasome and reduce mRNA transfection efficiency. Biomater. Sci. 2022, 10, 5566–5582. [Google Scholar] [CrossRef]
- Na Shin, J.; Rao, L.; Sha, Y.; Fattah, E.A.; Hyser, J.; Eissa, N.T. p38 MAPK Activity Is Required to Prevent Hyperactivation of NLRP3 Inflammasome. J. Immunol. 2021, 207, 661–670. [Google Scholar] [CrossRef]
- Lv, J.-M.; Gao, Y.-L.; Wang, L.-Y.; Li, B.-D.; Shan, Y.-L.; Wu, Z.-Q.; Lu, Q.-M.; Peng, H.-Y.; Zhou, T.-T.; Li, X.-M.; et al. Inhibition of the P38 MAPK/NLRP3 pathway mitigates cognitive dysfunction and mood alterations in aged mice after abdominal surgery plus sevoflurane. Brain Res. Bull. 2024, 217, 111059. [Google Scholar] [CrossRef] [PubMed]
- Sozio, M.S.; Lu, C.; Zeng, Y.; Liangpunsakul, S.; Crabb, D.W. Activated AMPK inhibits PPAR-α and PPAR-γ transcriptional activity in hepatoma cells. Am. J. Physiol. Liver Physiol. 2011, 301, G739–G747. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhu, X.; Lin, W.; Zhou, Y.; Cai, W.; Qiu, L. Interactions of TLR4 and PPARγ, Dependent on AMPK Signalling Pathway Contribute to Anti-Inflammatory Effects of Vaccariae Hypaphorine in Endothelial Cells. Cell. Physiol. Biochem. 2017, 42, 1227–1239. [Google Scholar] [CrossRef] [PubMed]
- Harada, K.; Isse, K.; Kamihira, T.; Shimoda, S.; Nakanuma, Y. Th1 cytokine–induced downregulation of PPARγ in human biliary cells relates to cholangitis in primary biliary cirrhosis†. Hepatology 2005, 41, 1329–1338. [Google Scholar] [CrossRef]
- Yin, R.; Dong, Y.-G.; Li, H.-L. PPAR? phosphorylation mediated by JNK MAPK: A potential role in mac-rophage-derived foam cell formation. Acta Pharmacol. Sin. 2006, 27, 1146–1152. [Google Scholar] [CrossRef]
- Su, A.-C.; Zhang, L.-Y.; Zhang, J.-G.; Hu, Y.-Y.; Liu, X.-Y.; Li, S.-C.; Xian, X.-H.; Li, W.-B.; Zhang, M. The Regulation of Autophagy by p38 MAPK–PPARγ Signaling During the Brain Ischemic Tolerance Induced by Cerebral Ischemic Preconditioning. DNA Cell Biol. 2022, 41, 838–849. [Google Scholar] [CrossRef]
- Ballav, S.; Biswas, B.; Sahu, V.K.; Ranjan, A.; Basu, S. PPAR-γ Partial Agonists in Disease-Fate Decision with Special Reference to Cancer. Cells 2022, 11, 3215. [Google Scholar] [CrossRef]
- Zhao, M.; Ma, J.; Li, M.; Zhang, Y.; Jiang, B.; Zhao, X.; Huai, C.; Shen, L.; Zhang, N.; He, L.; et al. Cytochrome P450 Enzymes and Drug Metabolism in Humans. Int. J. Mol. Sci. 2021, 22, 12808. [Google Scholar] [CrossRef]
- Liu, F.; Aulin, L.B.S.; Manson, M.L.; Krekels, E.H.J.; van Hasselt, J.G.C. Unraveling the Effects of Acute Inflammation on Pharmacokinetics: A Model-Based Analysis Focusing on Renal Glomerular Filtration Rate and Cytochrome P450 3A4-Mediated Metabolism. Eur. J. Drug Metab. Pharmacokinet. 2023, 48, 623–631. [Google Scholar] [CrossRef]
- Stanke-Labesque, F.; Gautier-Veyret, E.; Chhun, S.; Guilhaumou, R. Inflammation is a major regulator of drug metabolizing enzymes and transporters: Consequences for the personalization of drug treatment. Pharmacol. Ther. 2020, 215, 107627. [Google Scholar] [CrossRef]
- Bayraktar, I.; Yalçın, N.; Demirkan, K. The potential interaction between COVID-19 vaccines and clozapine: A novel approach for clinical trials. Int. J. Clin. Pr. 2021, 75, e14441. [Google Scholar] [CrossRef]
- Imai, T.; Ochiai, S.; Ishimaru, T.; Daitoku, H.; Miyagawa, Y.; Fukuhara, R.; Boku, S.; Takebayashi, M. A case report: Clozapine-induced leukopenia and neutropenia after mRNA COVID-19 vaccination. Neuropsychopharmacol. Rep. 2022, 42, 238–240. [Google Scholar] [CrossRef] [PubMed]
- Thompson, D.; Delorme, C.M.; White, R.F.; Honer, W.G. Elevated clozapine levels and toxic effects after SARS-CoV-2 vaccination. J. Psychiatry Neurosci. 2021, 46, E210–E211. [Google Scholar] [CrossRef] [PubMed]
- Eiermann, B.; Engel, G.; Johansson, I.; Zanger, U.M.; Bertilsson, L. The involvement of CYP1A2 and CYP3A4 in the metabolism of clozapine. Br. J. Clin. Pharmacol. 1997, 44, 439–446. [Google Scholar] [CrossRef]
- Villemure, S.; Trenaman, S.C.; Goralski, K.B. The impact of COVID-19 infection on cytochrome P450 3A4-mediated drug metabolism and drug interactions. Expert Opin. Drug Metab. Toxicol. 2023, 19, 329–332. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.Y.M.; Al Bishtawi, B.; Lim, W. Role of Cytochrome P450 2C9 in COVID-19 Treatment: Current Status and Future Directions. Eur. J. Drug Metab. Pharmacokinet. 2023, 48, 221–240. [Google Scholar] [CrossRef]
- Szychowski, K.A.; Skóra, B.; Wójtowicz, A.K. Engagement of peroxisome proliferator-activated receptor gamma (PPARγ) and mammalian target of rapamycin (mTOR) in the triclosan-induced disruption of Cyp450 enzyme activity in an in vitro model of mouse embryo fibroblasts (3T3-L1). Toxicology 2024, 511, 154031. [Google Scholar] [CrossRef]
- Abdelmonem, B.H.; Abdelaal, N.M.; Anwer, E.K.E.; Rashwan, A.A.; Hussein, M.A.; Ahmed, Y.F.; Khashana, R.; Hanna, M.M.; Abdelnaser, A. Decoding the Role of CYP450 Enzymes in Metabolism and Disease: A Comprehensive Review. Biomedicines 2024, 12, 1467. [Google Scholar] [CrossRef]
- Koozi, H.; Lengquist, M.; Frigyesi, A. C-reactive protein as a prognostic factor in intensive care admissions for sepsis: A Swedish multicenter study. J. Crit. Care 2020, 56, 73–79. [Google Scholar] [CrossRef]
- Lenoir, C.; Rollason, V.; Desmeules, J.A.; Samer, C.F. Influence of Inflammation on Cytochromes P450 Activity in Adults: A Systematic Review of the Literature. Front. Pharmacol. 2021, 12. [Google Scholar] [CrossRef]
- Li, Q.; Zheng, Y.; Sun, Y.; Xu, G. Resveratrol attenuated fatty acid synthesis through MAPK-PPAR pathway in red tilapia. Comp. Biochem. Physiol. Part C: Toxicol. Pharmacol. 2023, 268, 109598. [Google Scholar] [CrossRef] [PubMed]
- Shveygert, M.; Kaiser, C.; Bradrick, S.S.; Gromeier, M. Regulation of Eukaryotic Initiation Factor 4E (eIF4E) Phosphorylation by Mitogen-Activated Protein Kinase Occurs through Modulation of Mnk1-eIF4G Interaction. Mol. Cell. Biol. 2010, 30, 5160–5167. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Bian, L.; Zhu, G.; Zhang, B. Vitronectin promotes proliferation and metastasis of cervical cancer cells via the epithelial-mesenchymal transition. Front. Oncol. 2024, 14, 1466264. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Lu, Y.; Tang, K.; Wang, W.; Wang, T.; Zhu, Y.; Zhao, J.; Mao, Y. Ficolin-1 ameliorates pulmonary fibrosis via directly binding to TGF-β1. J. Transl. Med. 2024, 22, 1–15. [Google Scholar] [CrossRef]
- Knabl, L.; Lee, H.K.; Wieser, M.; Mur, A.; Zabernigg, A.; Rauch, S.; Bock, M.; Schumacher, J.; Kaiser, N.; Furth, P.A.; et al. BNT162b2 vaccination enhances interferon-JAK-STAT-regulated antiviral programs in COVID-19 patients infected with the SARS-CoV-2 Beta variant. Commun. Med. 2022, 2, 1–10. [Google Scholar] [CrossRef]
- Burotto, M.; Chiou, V.L.; Lee, J.; Kohn, E.C. The MAPK pathway across different malignancies: A new perspective. Cancer 2014, 120, 3446–3456. [Google Scholar] [CrossRef]
- Panwar, V.; Singh, A.; Bhatt, M.; Tonk, R.K.; Azizov, S.; Raza, A.S.; Sengupta, S.; Kumar, D.; Garg, M. Multifaceted role of mTOR (mammalian target of rapamycin) signaling pathway in human health and disease. Signal Transduct. Target. Ther. 2023, 8, 1–25. [Google Scholar] [CrossRef]
- Yu, H.; Lin, L.; Zhang, Z.; Zhang, H.; Hu, H. Targeting NF-κB pathway for the therapy of diseases: Mechanism and clinical study. Signal Transduct. Target. Ther. 2020, 5, 1–23. [Google Scholar] [CrossRef]
- McCubrey, J.A.; Steelman, L.S.; Chappell, W.H.; Abrams, S.L.; Montalto, G.; Cervello, M.; Nicoletti, F.; Fagone, P.; Malaponte, G.; Mazzarino, M.C.; et al. Mutations and Deregulation of Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR Cascades Which Alter Therapy Response. Oncotarget 2012, 3, 954–987. [Google Scholar] [CrossRef]
- Nigam, M.; Punia, B.; Dimri, D.B.; Mishra, A.P.; Radu, A.-F.; Bungau, G. Reactive Oxygen Species: A Double-Edged Sword in the Modulation of Cancer Signaling Pathway Dynamics. Cells 2025, 14, 1207. [Google Scholar] [CrossRef]
- Cui, D.; Qu, R.; Liu, D.; Xiong, X.; Liang, T.; Zhao, Y. The Cross Talk Between p53 and mTOR Pathways in Response to Physiological and Genotoxic Stresses. Front. Cell Dev. Biol. 2021, 9. [Google Scholar] [CrossRef]
- Bassermann, F.; Eichner, R.; Pagano, M. The ubiquitin proteasome system — Implications for cell cycle control and the targeted treatment of cancer. Biochim. Biophys. Acta Bioenerg. 2014, 1843, 150–162. [Google Scholar] [CrossRef]
- Chen, Y.; Gong, W.; Dai, W.; Jiang, H.; Xu, X. E2F1/2/4 mRNA is associated with immune infiltration and are potential biomarkers for the prognosis of human gastric carcinoma. Transl. Cancer Res. 2021, 10, 2801–2811. [Google Scholar] [CrossRef]
- Daigh, L.H.; Saha, D.; Rosenthal, D.L.; Ferrick, K.R.; Meyer, T. Uncoupling of mTORC1 from E2F activity maintains DNA damage and senescence. Nat. Commun. 2024, 15, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hao, X.; Han, G.; Lu, Y.; Chen, Z.; Zhang, L.; Wu, J.; Wang, X. E2F1-mediated GINS2 transcriptional activation promotes tumor progression through PI3K/AKT/mTOR pathway in hepatocellular carcinoma. Am. J. Cancer Res. 2022, 12, 1707–1726. [Google Scholar]
- Calzone, L.; Gelay, A.; Zinovyev, A.; Radvanyi, F.; Barillot, E. A comprehensive modular map of molecular interactions in RB/E2F pathway. Mol. Syst. Biol. 2008, 4, 173. [Google Scholar] [CrossRef]
- Bertonnier-Brouty, L.; Andersson, J.; Kaprio, T.; Hagström, J.; Bsharat, S.; Asplund, O.; Hatem, G.; Haglund, C.; Seppänen, H.; Prasad, R.B.; et al. E2F transcription factors promote tumorigenicity in pancreatic ductal adenocarcinoma. Cancer Med. 2024, 13, e7187. [Google Scholar] [CrossRef]
- Wasserman, D.; Nachum, S.; Cohen, M.; Enrico, T.P.; Noach-Hirsh, M.; Parasol, J.; Zomer-Polak, S.; Auerbach, N.; Sheinberger-Chorni, E.; Nevenzal, H.; et al. Cell cycle oscillators underlying orderly proteolysis of E2F8. Mol. Biol. Cell 2020, 31, 725–740. [Google Scholar] [CrossRef]
- Timmers, C.; Sharma, N.; Opavsky, R.; Maiti, B.; Wu, L.; Wu, J.; Orringer, D.; Trikha, P.; Saavedra, H.I.; Leone, G. E2f1, E2f2, and E2f3 Control E2F Target Expression and Cellular Proliferation via a p53-Dependent Negative Feedback Loop. Mol. Cell. Biol. 2007, 27, 65–78. [Google Scholar] [CrossRef]
- Spitschak, A.; Dhar, P.; Singh, K.P.; Garduño, R.C.; Gupta, S.K.; Vera, J.; Musella, L.; Murr, N.; Stoll, A.; Pützer, B.M. E2F1-induced autocrine IL-6 inflammatory loop mediates cancer-immune crosstalk that predicts T cell phenotype switching and therapeutic responsiveness. Front. Immunol. 2024, 15, 1470368. [Google Scholar] [CrossRef]
- Li, J.; Ran, C.; Li, E.; Gordon, F.; Comstock, G.; Siddiqui, H.; Cleghorn, W.; Chen, H.-Z.; Kornacker, K.; Liu, C.-G.; et al. Synergistic Function of E2F7 and E2F8 Is Essential for Cell Survival and Embryonic Development. Dev. Cell 2008, 14, 62–75. [Google Scholar] [CrossRef]
- Zheng, J.; Huang, J.; Xia, J.; Zhou, W.; Dai, L.; Lin, S.; Gao, L.; Zou, C. Transcription factor E2F8 is a therapeutic target in the basal-like subtype of breast cancer. Front. Oncol. 2023, 13, 1038787. [Google Scholar] [CrossRef] [PubMed]
- Vigo, E.; Müller, H.; Prosperini, E.; Hateboer, G.; Cartwright, P.; Moroni, M.C.; Helin, K. CDC25A Phosphatase Is a Target of E2F and Is Required for Efficient E2F-Induced S Phase. Mol. Cell. Biol. 1999, 19, 6379–6395. [Google Scholar] [CrossRef] [PubMed]
- Delgado, M.; Washam, C.L.; Urbaniak, A.; Heflin, B.; Storey, A.J.; Lan, R.S.; Mackintosh, S.G.; Tackett, A.J.; Byrum, S.D.; Chambers, T.C. Phosphoproteomics Provides Novel Insights into the Response of Primary Acute Lymphoblastic Leukemia Cells to Microtubule Depolymerization in G1 Phase of the Cell Cycle. ACS Omega 2021, 6, 24949–24959. [Google Scholar] [CrossRef] [PubMed]
- Knapp JD, Bhargava A. BNT162b2 mRNA vaccine-induced sex differences in the single-cell transcriptome of peripheral blood mononuclear cells in healthy adults bioRxiv Preprint. Oct 3, 2023. [CrossRef]
- Ezine, E.; Lebbe, C.; Dumaz, N. Unmasking the tumourigenic role of SIN1/MAPKAP1 in the mTOR complex 2. Clin. Transl. Med. 2023, 13, e1464. [Google Scholar] [CrossRef]
- Hickey, T.E.; Mudunuri, U.; Hempel, H.A.; Kemp, T.J.; Roche, N.V.; Talsania, K.; Sellers, B.A.; Cherry, J.M.; Pinto, L.A. Proteomic and serologic assessments of responses to mRNA-1273 and BNT162b2 vaccines in human recipient sera. Front. Immunol. 2025, 15, 1502458. [Google Scholar] [CrossRef]
- Savelsbergh, A.; Mohr, D.; Wilden, B.; Wintermeyer, W.; Rodnina, M.V. Stimulation of the GTPase Activity of Translation Elongation Factor G by Ribosomal Protein L7/12. J. Biol. Chem. 2000, 275, 890–894. [Google Scholar] [CrossRef]
- Chang, E.C.; Philips, M.R. Spatial Segregation of Ras Signaling—New Evidence from Fission Yeast. Cell Cycle 2006, 5, 1936–1939. [Google Scholar] [CrossRef]
- Fadhal, E. The Significance of Key Proteins in the RAS Signaling Pathway: Implications for Cancer and Therapeutic Targets. OBM Genet. 2024, 08, 1–15. [Google Scholar] [CrossRef]
- Lin, Y.; Parajón, E.; Yuan, Q.; Ye, S.; Qin, G.; Deng, Y.; Borleis, J.; Koyfman, A.; Iglesias, P.A.; Konstantopoulos, K.; et al. Ras-mediated dynamic and biphasic regulation of cell migration. Proc. Natl. Acad. Sci. 2025, 122. [Google Scholar] [CrossRef]
- Chippalkatti, R.; Parisi, B.; Kouzi, F.; Laurini, C.; Ben Fredj, N.; Abankwa, D.K. RAS isoform specific activities are disrupted by disease associated mutations during cell differentiation. Eur. J. Cell Biol. 2024, 103, 151425. [Google Scholar] [CrossRef]
- Wang, Y.-F.; Dong, Z.-K.; Jin, W.-L. Hijacking homeostasis: The brain-body neural circuitry in tumor pathogenesis and emerging therapeutic frontiers. Mol. Cancer 2025, 24, 206. [Google Scholar] [CrossRef]
- Peng, Q.; Li, X.; Fang, C.; Zhu, C.; Wang, T.; Yin, B.; Dong, X.; Guo, H.; Liu, Y.; Zhang, K. Disrupting calcium homeostasis and glycometabolism in engineered lipid-based pharmaceuticals propel cancer immunogenic death. Acta Pharm. Sin. B 2024, 15, 1255–1267. [Google Scholar] [CrossRef]
- Gulluni, F.; Martini, M.; Hirsch, E. Cytokinetic Abscission: Phosphoinositides and ESCRTs Direct the Final Cut. J. Cell. Biochem. 2017, 118, 3561–3568. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Thapa, N.; Choi, S.; Anderson, R.A. Emerging roles of PtdIns(4,5)P2 – beyond the plasma membrane. J. Cell Sci. 2015, 128, 4047–4056. [Google Scholar] [CrossRef] [PubMed]
- Ajazi, A.; Bruhn, C.; Shubassi, G.; Lucca, C.; Ferrari, E.; Cattaneo, A.; Bachi, A.; Manfrini, N.; Biffo, S.; Martini, E.; et al. Endosomal trafficking and DNA damage checkpoint kinases dictate survival to replication stress by regulating amino acid uptake and protein synthesis. Dev. Cell 2021, 56, 2607–2622.e6. [Google Scholar] [CrossRef] [PubMed]
- Hurley, J.H.; Coyne, A.N.; Miączyńska, M.; Stenmark, H. The expanding repertoire of ESCRT functions in cell biology and disease. Nature 2025, 642, 877–888. [Google Scholar] [CrossRef]
- Zhu, L.; Jorgensen, J.R.; Li, M.; Chuang, Y.-S.; Emr, S.D. ESCRTs function directly on the lysosome membrane to downregulate ubiquitinated lysosomal membrane proteins. eLife 2017, 6. [Google Scholar] [CrossRef]
- Jia, S.; Yang, Y.; Liu, J.; Wang, Z.; Bai, L. PPARγ controls ESCRT-dependent fibroblast-like synoviocyte exosome biogenesis and alleviates chondrocyte osteoarthritis mediated by exosomal ANXA1. J. Orthop. Transl. 2025, 53, 187–205. [Google Scholar] [CrossRef]
- Simonsen, J.B. A perspective on bleb and empty LNP structures. J. Control. Release 2024, 373, 952–961. [Google Scholar] [CrossRef]
- Schoenmaker, L.; Witzigmann, D.; Kulkarni, J.A.; Verbeke, R.; Kersten, G.; Jiskoot, W.; Crommelin, D.J. mRNA-lipid nanoparticle COVID-19 vaccines: Structure and stability. Int. J. Pharm. 2021, 601, 120586. [Google Scholar] [CrossRef]
- Liao, S.; Wang, S.; Wadhwa, A.; Birkenshaw, A.; Fox, K.; Cheng, M.H.Y.; Casmil, I.C.; Magana, A.A.; Bathula, N.V.; Ho, C.H.; et al. Transfection Potency of Lipid Nanoparticles Containing mRNA Depends on Relative Loading Levels. ACS Appl. Mater. Interfaces 2024, 17, 3097–3105. [Google Scholar] [CrossRef] [PubMed]
- Lonez, C.; Vandenbranden, M.; Ruysschaert, J.-M. Cationic liposomal lipids: From gene carriers to cell signaling. Prog. Lipid Res. 2008, 47, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Lonez, C.; Vandenbranden, M.; Ruysschaert, J.-M. Cationic lipids activate intracellular signaling pathways. Adv. Drug Deliv. Rev. 2012, 64, 1749–1758. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Bouteau, A.; Herbst, C.; Igyártó, B.Z. Pre-exposure to mRNA-LNP inhibits adaptive immune responses and alters innate immune fitness in an inheritable fashion. PLOS Pathog. 2022, 18, e1010830. [Google Scholar] [CrossRef]
- Sellegounder, D.; Ferrucci, L.; Anbazhagan, R.; Basisty, N. Editorial: Molecular crosstalk between endocrine factors, paracrine signals, and the immune system during aging. Front. Endocrinol. 2023, 14. [Google Scholar] [CrossRef]
- Rivolta, I.; Panariti, A.; Miserocchi, G. The effect of nanoparticle uptake on cellular behavior: Disrupting or enabling functions? Nanotechnology, Sci. Appl. 2012, 5, 87–100. [Google Scholar] [CrossRef]
- Voigt, J.; Christensen, J.; Shastri, V.P. Differential uptake of nanoparticles by endothelial cells through polyelectrolytes with affinity for caveolae. Proc. Natl. Acad. Sci. 2014, 111, 2942–2947. [Google Scholar] [CrossRef]
- Wang, T.; Bai, J.; Jiang, X.; Nienhaus, G.U. Cellular Uptake of Nanoparticles by Membrane Penetration: A Study Combining Confocal Microscopy with FTIR Spectroelectrochemistry. ACS Nano 2012, 6, 1251–1259. [Google Scholar] [CrossRef]
- Gerelli Y. Chapter Three: Exploring interactions between lipid membranes and nanoparticles through neutron and X-ray reflectometry techniques. In A. Iglič, M. Rappolt, & P. Losada-Pérez (Eds.), Advances in Biomembranes and Lipid Self-Assembly 2023; 38: 37–61. Academic Press. [CrossRef]
- Lavagna, E.; Barnoud, J.; Rossi, G.; Monticelli, L. Size-dependent aggregation of hydrophobic nanoparticles in lipid membranes. Nanoscale 2020, 12, 9452–9461. [Google Scholar] [CrossRef]
- Cooper, G.M. The Cell: A Molecular Approach, 2nd ed.; Sinauer Associates: Sunderland, MA, USA, 2000. [Google Scholar]
- Hammond, G.R.; Burke, J.E. Novel roles of phosphoinositides in signaling, lipid transport, and disease. Curr. Opin. Cell Biol. 2020, 63, 57–67. [Google Scholar] [CrossRef]
- Skotland, T.; Sandvig, K. The role of PS 18:0/18:1 in membrane function. Nat. Commun. 2019, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Heimburg, T. The excitable fluid mosaic. Biochim. et Biophys. Acta (BBA) - Biomembr. 2023, 1865, 184104. [Google Scholar] [CrossRef]
- Lupyan, D.; Mezei, M.; Logothetis, D.E.; Osman, R. A Molecular Dynamics Investigation of Lipid Bilayer Perturbation by PIP2. Biophys. J. 2010, 98, 240–247. [Google Scholar] [CrossRef]
- Pike, L.J. Lipid rafts: Bringing order to chaos. J. Lipid Res. 2003, 44, 655–667. [Google Scholar] [CrossRef] [PubMed]
- Levin, R.; Grinstein, S.; Schlam, D. Phosphoinositides in phagocytosis and macropinocytosis. Biochim. et Biophys. Acta (BBA) - Mol. Cell Biol. Lipids 2015, 1851, 805–823. [Google Scholar] [CrossRef] [PubMed]
- Weiner, O.D.; Neilsen, P.O.; Prestwich, G.D.; Kirschner, M.W.; Cantley, L.C.; Bourne, H.R. A PtdInsP3- and Rho GTPase-mediated positive feedback loop regulates neutrophil polarity. Nat. Cell Biol. 2002, 4, 509–513. [Google Scholar] [CrossRef]
- Guan, K.; Curtis, E.R.; Lew, D.J.; Elston, T.C. Particle-based simulations reveal two positive feedback loops allow relocation and stabilization of the polarity site during yeast mating. PLOS Comput. Biol. 2023, 19, e1011523. [Google Scholar] [CrossRef]
- Fallahi-Sichani, M.; Linderman, J.J. Lipid Raft-Mediated Regulation of G-Protein Coupled Receptor Signaling by Ligands which Influence Receptor Dimerization: A Computational Study. PLOS ONE 2009, 4, e6604. [Google Scholar] [CrossRef]
- Wang, X.; Shi, X.; Wang, R. Regulating mRNA endosomal escape through lipid rafts: A review. Int. J. Pharm. 2025, 675, 125571. [Google Scholar] [CrossRef]
- Posor, Y.; Jang, W.; Haucke, V. Phosphoinositides as membrane organizers. Nat. Rev. Mol. Cell Biol. 2022, 23, 797–816. [Google Scholar] [CrossRef] [PubMed]
- Van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane lipids: Where they are and how they behave. Nat. Rev. Mol. Cell Biol. 2008, 9, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Lolicato, F.; Nickel, W.; Haucke, V.; Ebner, M. Phosphoinositide switches in cell physiology - From molecular mechanisms to disease. J. Biol. Chem. 2024, 300, 105757. [Google Scholar] [CrossRef] [PubMed]
- Balla, T. Phosphoinositides: Tiny Lipids With Giant Impact on Cell Regulation. Physiol. Rev. 2013, 93, 1019–1137. [Google Scholar] [CrossRef]
- Kjos, I.; Vestre, K.; Guadagno, N.A.; Distefano, M.B.; Progida, C. Rab and Arf proteins at the crossroad between membrane transport and cytoskeleton dynamics. Biochim. et Biophys. Acta (BBA) - Mol. Cell Res. 2018, 1865, 1397–1409. [Google Scholar] [CrossRef]
- Koike, S.; Jahn, R. Rab GTPases and phosphoinositides fine-tune SNAREs dependent targeting specificity of intracellular vesicle traffic. Nat. Commun. 2024, 15, 1–14. [Google Scholar] [CrossRef]
- Eramo, M.J.; Mitchell, C.A. Regulation of PtdIns(3,4,5)P3/Akt signalling by inositol polyphosphate 5-phosphatases. Biochem. Soc. Trans. 2016, 44, 240–252. [Google Scholar] [CrossRef]
- Nishimura, T.; Gecht, M.; Covino, R.; Hummer, G.; Surma, M.A.; Klose, C.; Arai, H.; Kono, N.; Stefan, C.J. Osh Proteins Control Nanoscale Lipid Organization Necessary for PI(4,5)P2 Synthesis. Mol. Cell 2019, 75, 1043–1057.e8. [Google Scholar] [CrossRef]
- Heckle, L.A.; Kozminski, K.G. Osh-dependent and -independent Regulation of PI4P Levels During Polarized Growth ofSaccharomyces cerevisiae. Mol. Biol. Cell 2023, 34. [Google Scholar] [CrossRef]
- Raychaudhuri, S.; Prinz, W.A. The Diverse Functions of Oxysterol-Binding Proteins. Annu. Rev. Cell Dev. Biol. 2010, 26, 157–177. [Google Scholar] [CrossRef]
- Omo-Lamai S, Wang Y, Patel MN, Essien E-O, Shen M, Majumdar A, Espy C, Wu J, Channer B, Tobin M, Murali S, Papp TE, Maheshwari R, Wang L, Chase LS, Zamora ME, Arral ML, Marcos-Contreras OA, Myerson JW, Hunter CA, Tsourkas A, Muzykantov V, Brodsky I, Shin S, Whitehead KA, Gaskill P, Discher D, Parhiz H, Brenner JS. Lipid nanoparticle-associated inflammation is triggered by sensing of endosomal damage: Engineering endosomal escape without side effects. bioRxiv preprint. April 18, 2024. [CrossRef]
- Cheng, Y.; Zhao, E.; Yang, X.; Luo, C.; Zi, G.; Wang, R.; Xu, Y.; Peng, B. Entrapment of lipid nanoparticles in peripheral endosomes but not lysosomes impairs intracellular trafficking and endosomal escape. Int. J. Pharm. 2024, 669, 125024. [Google Scholar] [CrossRef]
- Puranik, A.; Lenehan, P.J.; Silvert, E.; Niesen, M.J.; Corchado-Garcia, J.; O’horo, J.C.; Virk, A.; Swift, M.D.; Gordon, J.E.; Speicher, L.L.; et al. Comparative effectiveness of mRNA-1273 and BNT162b2 against symptomatic SARS-CoV-2 infection. Med 2021, 3, 28–41.e8. [Google Scholar] [CrossRef]
- Granados-Riveron, J.T.; Aquino-Jarquin, G. Engineering of the current nucleoside-modified mRNA-LNP vaccines against SARS-CoV-2. Biomed. Pharmacother. 2021, 142, 111953. [Google Scholar] [CrossRef]
- Harvey, R.D.; Ara, N.; Heenan, R.K.; Barlow, D.J.; Quinn, P.J.; Lawrence, M.J. Stabilization of Distearoylphosphatidylcholine Lamellar Phases in Propylene Glycol Using Cholesterol. Mol. Pharm. 2013, 10, 4408–4417. [Google Scholar] [CrossRef]
- Skotland, T.; Kavaliauskiene, S.; Sandvig, K. The role of lipid species in membranes and cancer-related changes. Cancer Metastasis Rev. 2020, 39, 343–360. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Zhang, T.; Wang, C.; Huang, Z.; Luo, X.; Deng, Y. A review on phospholipids and their main applications in drug delivery systems. Asian J. Pharm. Sci. 2015, 10, 81–98. [Google Scholar] [CrossRef]
- McMaster, C.R. From yeast to humans – roles of the Kennedy pathway for phosphatidylcholine synthesis. FEBS Lett. 2017, 592, 1256–1272. [Google Scholar] [CrossRef]
- Cummings, R.; Parinandi, N.; Wang, L.; Usatyuk, P.; Natarajan, V. Phospholipase D/phosphatidic acid signal transduction: Role and physiological significance in lung. Mol. Cell Biochem. 2002, 234-235, 99–109. [Google Scholar] [CrossRef]
- Wagner, K.; Brezesinski, G. Phospholipase D Activity Is Regulated by Product Segregation and the Structure Formation of Phosphatidic Acid within Model Membranes. Biophys. J. 2007, 93, 2373–2383. [Google Scholar] [CrossRef]
- Bruntz, R.C.; Lindsley, C.W.; Brown, H.A. Phospholipase D Signaling Pathways and Phosphatidic Acid as Therapeutic Targets in Cancer. Pharmacol. Rev. 2014, 66, 1033–1079. [Google Scholar] [CrossRef]
- Semenkovich, C.F.; Goldberg, A.C.; Goldberg, I.J. Disorders of Lipid Metabolism. Chapter 37. In Textbook of Endocrinology, 13th ed.; Melmed, S., Polonsky, K.S., Larsen, P.R., Kroneneberg, H.M., Chamberlain, D., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 1660–1700. [Google Scholar] [CrossRef]
- Blunsom, N.J.; Cockcroft, S. Phosphatidylinositol synthesis at the endoplasmic reticulum. Biochim. Biophys. Acta Mol. Cell. Biol. Lipids 2020, 1865, 158471. [Google Scholar] [CrossRef]
- Pearce, B.; Jakobson, K.; Morrow, C.; Murphy, S. Phosphatidic acid promotes phosphoinositide metabolism and DNA synthesis in cultured cortical astrocytes. Neurochem. Int. 1994, 24, 165–171. [Google Scholar] [CrossRef]
- Quick, J.; Dos Santos, N.; Cheng, M.H.; Chander, N.; Brimacombe, C.A.; Kulkarni, J.; van der Meel, R.; Tam, Y.Y.C.; Witzigmann, D.; Cullis, P.R. Lipid nanoparticles to silence androgen receptor variants for prostate cancer therapy. J. Control. Release 2022, 349, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Jeschek, D.; Lhota, G.; Wallner, J.; Vorauer-Uhl, K. A versatile, quantitative analytical method for pharmaceutical relevant lipids in drug delivery systems. J. Pharm. Biomed. Anal. 2016, 119, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, S.; Blick, E.E.; Mineart, K.P.; Kelley, E.G. Chapter Three—Linking chemical degradation and physical instability of lipid vesicles. Advances in Biomembranes and Lipid Self-Assembly 2025, 41, 47–64. [Google Scholar] [CrossRef]
- Sarkar, S.; Carroll, B.; Buganim, Y.; Maetzel, D.; Ng, A.H.; Cassady, J.P.; Cohen, M.A.; Chakraborty, S.; Wang, H.; Spooner, E.; et al. Impaired Autophagy in the Lipid-Storage Disorder Niemann-Pick Type C1 Disease. Cell Rep. 2013, 5, 1302–1315. [Google Scholar] [CrossRef] [PubMed]
- Dall’ARmi, C.; Devereaux, K.A.; Di Paolo, G. The Role of Lipids in the Control of Autophagy. Curr. Biol. 2013, 23, R33–R45. [Google Scholar] [CrossRef]
- Zhang, S.; Peng, X.; Yang, S.; Li, X.; Huang, M.; Wei, S.; Liu, J.; He, G.; Zheng, H.; Yang, L.; et al. The regulation, function, and role of lipophagy, a form of selective autophagy, in metabolic disorders. Cell Death Dis. 2022, 13, 1–11. [Google Scholar] [CrossRef]
- Jarocki, M.; Turek, K.; Saczko, J.; Tarek, M.; Kulbacka, J. Lipids associated with autophagy: Mechanisms and therapeutic targets. Cell Death Discov. 2024, 10, 1–11. [Google Scholar] [CrossRef]
- Karim, M.; Mishra, M.; Lo, C.-W.; Saul, S.; Cagirici, H.B.; Gourdelier, M.; Ghita, L.; Ojha, A.; Tran, D.H.N.; Agrawal, A.; et al. PIP4K2C inhibition reverses autophagic flux impairment induced by SARS-CoV-2. Nat. Commun. 2025, 16, 1–18. [Google Scholar] [CrossRef]
- Haucke, V.; Kozlov, M.M. Membrane remodeling in clathrin-mediated endocytosis. J. Cell Sci. 2018, 131, jcs216812. [Google Scholar] [CrossRef]
- Rigby, R.E.; Rehwinkel, J. RNA degradation in antiviral immunity and autoimmunity. Trends Immunol. 2015, 36, 179–188. [Google Scholar] [CrossRef]
- Acevedo-Whitehouse, K.; Bruno, R. Potential health risks of mRNA-based vaccine therapy: A hypothesis. Med Hypotheses 2023, 171, 111015. [Google Scholar] [CrossRef]
- Fung, S.Y.S.; Xǔ, X.; Wu, M. Nonlinear dynamics in phosphoinositide metabolism. Curr. Opin. Cell Biol. 2024, 88, 102373. [Google Scholar] [CrossRef] [PubMed]
- Thiemicke, A.; Neuert, G. Rate thresholds in cell signaling have functional and phenotypic consequences in non-linear time-dependent environments. Front. Cell Dev. Biol. 2023, 11, 1124874. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Chen, Y.; Carrillo, N.D.; Cryns, V.L.; Anderson, R.A.; Sun, J.; Wang, S.; Chen, M. Phosphoinositide signaling at the cytoskeleton in the regulation of cell dynamics. Cell Death Dis. 2025, 16, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, H.; Cullen, P.J. Role of Phosphatidylinositol Phosphate Signaling in the Regulation of the Filamentous-Growth Mitogen-Activated Protein Kinase Pathway. Eukaryot. Cell 2015, 14, 427–440. [Google Scholar] [CrossRef]
- Wang, L.; Eghtesad, S.; Clemens, P.R. Migration of dendritic cells from murine skeletal muscle. Immunobiology 2011, 216, 195–199. [Google Scholar] [CrossRef]
- Woodland, D.L.; Kohlmeier, J.E. Migration, maintenance and recall of memory T cells in peripheral tissues. Nat. Rev. Immunol. 2009, 9, 153–161. [Google Scholar] [CrossRef]
- Zhang, Y.; Garcia-Ibanez, L.; Ulbricht, C.; Lok, L.S.C.; Pike, J.A.; Mueller-Winkler, J.; Dennison, T.W.; Ferdinand, J.R.; Burnett, C.J.M.; Yam-Puc, J.C.; et al. Recycling of memory B cells between germinal center and lymph node subcapsular sinus supports affinity maturation to antigenic drift. Nat. Commun. 2022, 13, 1–13. [Google Scholar] [CrossRef]
- Chazaud, B. Inflammation and Skeletal Muscle Regeneration: Leave It to the Macrophages! Trends Immunol. 2020, 41, 481–492. [Google Scholar] [CrossRef]
- Sackstein, R.; Schatton, T.; Barthel, S.R. T-lymphocyte homing: An underappreciated yet critical hurdle for successful cancer immunotherapy. Mod. Pathol. 2017, 97, 669–697. [Google Scholar] [CrossRef]
- Simonis, A.; Theobald, S.J.; E Koch, A.; Mummadavarapu, R.; Mudler, J.M.; Pouikli, A.; Göbel, U.; Acton, R.; Winter, S.; Albus, A.; et al. Persistent epigenetic memory of SARS-CoV-2 mRNA vaccination in monocyte-derived macrophages. Mol. Syst. Biol. 2025, 21, 341–360. [Google Scholar] [CrossRef]
- Ochoa, D.; Bradley, D.; Beltrao, P. Evolution, dynamics and dysregulation of kinase signalling. Curr. Opin. Struct. Biol. 2018, 48, 133–140. [Google Scholar] [CrossRef]
- Pelley, J.W. Membranes and Intracellular Signal Transduction. Chapter 5 in Pelley JW, Ed. Elsevier’s Integrated Biochemistry. Mosby, 2007, 37-46. [CrossRef]
- Grecco, H.E.; Schmick, M.; Bastiaens, P.I. Signaling from the Living Plasma Membrane. Cell 2011, 144, 897–909. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee A, Huang Y, Elgeti J, Oh S, Abreu JG, Neliat AR, Schüttler J, Su DD, Dupre C, Benites NC, Liu X, Peshkin L, Barboiu M, Stocker H, Kirschner MW, Basan M. Membrane potential mediates the cellular response to mechanical pressure. bioRxiv [Preprint]. 2024 Nov 13:2023.11.02.565386. [CrossRef]
- Szischik, C.L.; Szemere, J.R.; Balderrama, R.; de la Vega, C.S.; Ventura, A.C. Transient frequency preference responses in cell signaling systems. npj Syst. Biol. Appl. 2024, 10, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Cobb, M.; Goldsmith, E. Dimerization in MAP-kinase signaling. Trends Biochem. Sci. 2000, 25, 7–9. [Google Scholar] [CrossRef] [PubMed]
- Fossett, N. Signal transduction pathways, intrinsic regulators, and the control of cell fate choice. Biochim. et Biophys. Acta (BBA) - Gen. Subj. 2012, 1830, 2375–2384. [Google Scholar] [CrossRef]
- Landry, B.D.; Clarke, D.C.; Lee, M.J. Studying Cellular Signal Transduction with OMIC Technologies. J. Mol. Biol. 2015, 427, 3416–3440. [Google Scholar] [CrossRef]
- Vijay, S.; Gujral, T.S. Non-linear Deep Neural Network for Rapid and Accurate Prediction of Phenotypic Responses to Kinase Inhibitors. iScience 2020, 23, 101129. [Google Scholar] [CrossRef]
- Jiang, Y.; AkhavanAghdam, Z.; Li, Y.; Zid, B.M.; Hao, N. A protein kinase A–regulated network encodes short- and long-lived cellular memories. Sci. Signal. 2020, 13. [Google Scholar] [CrossRef]
- Ghomlaghi, M.; Hart, A.; Hoang, N.; Shin, S.; Nguyen, L.K. Feedback, Crosstalk and Competition: Ingredients for Emergent Non-Linear Behaviour in the PI3K/mTOR Signalling Network. Int. J. Mol. Sci. 2021, 22, 6944. [Google Scholar] [CrossRef]
- Veres, T.; Kerestély, M.; Kovács, B.M.; Keresztes, D.; Schulc, K.; Seitz, E.; Vassy, Z.; Veres, D.V.; Csermely, P. Cellular forgetting, desensitisation, stress and ageing in signalling networks. When do cells refuse to learn more? Cell. Mol. Life Sci. 2024, 81, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Rauch, N.; Rukhlenko, O.S.; Kolch, W.; Kholodenko, B.N. MAPK kinase signalling dynamics regulate cell fate decisions and drug resistance. Curr. Opin. Struct. Biol. 2016, 41, 151–158. [Google Scholar] [CrossRef]
- Lavoie, H.; Gagnon, J.; Therrien, M. ERK signalling: A master regulator of cell behaviour, life and fate. Nat. Rev. Mol. Cell Biol. 2020, 21, 607–632. [Google Scholar] [CrossRef]
- Parres-Gold, J.; Levine, M.; Emert, B.; Stuart, A.; Elowitz, M.B. Contextual computation by competitive protein dimerization networks. Cell 2025, 188, 1984–2002.e17. [Google Scholar] [CrossRef]
- Avraham, R.; Yarden, Y. Feedback regulation of EGFR signalling: Decision making by early and delayed loops. Nat. Rev. Mol. Cell Biol. 2011, 12, 104–117. [Google Scholar] [CrossRef]
- Wen, P.J.; Osborne, S.L.; Meunier, F.A. Phosphoinositides in neuroexocytosis and neuronal diseases. Curr Top Microbiol Immunol. 2012, 362, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Tsuruta, F. New insights into the functions of PtdIns(3,5)P2 in the pathogenisis of neurodegenerative disorders. Neural Regen. Res. 2016, 11, 240–241. [Google Scholar] [CrossRef] [PubMed]
- Houthaeve, G.; De Smedt, S.C.; Braeckmans, K.; De Vos, W.H. The cellular response to plasma membrane disruption for nanomaterial delivery. Nano Converg. 2022, 9, 1–17. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





