Introduction
Epilepsy is one of the most common multiethiologic non-communicable chronic neurological disorder of the brain [
1] affecting around 50 million people worldwide [
2], characterized by recurrent epileptic seizures and requiring long-term Anti-Seizure Drug (ASD) treatment. Complete seizure control can be achieved in up to 65-70% of patients on adequate management with ASD treatment; in remaining 30-35% of patients the seizures are highly difficult to manage even with the correct pharmacological treatment regimen. Such cases are considered as a drug resistant epilepsy (DRE) [
3] and epilepsy surgery is considered to be the most effective alternative ways to achieve seizure control. However, epilepsy surgery is an option only for eligible candidates with a DRE, and after presurgical evaluation, only a quarter of them are considered for surgery [
3].
The success of epilepsy surgery depends on the early identification of potential surgical candidates and selecting from them ideal cases destined to have a postoperative seizure-free outcome [
4]. In the first stage, evaluation of good candidates for epilepsy surgery requires long-term scrupulous investigations and multidisciplinary analyzes according to the noninvasive protocol for pre-surgical evaluation (video-EEG, Multimodal Imaging, Neuropsychological Testing) [
5]. The key data of pre-surgical assessment in DRE is the identification of the Seizure Onset Zone (SOZ) for its subsequent surgical resection and the determination of the trajectory of further propagation of the epileptic activity, for the evaluation of which the crucial modality is video-EEG monitoring and analysis of ictal / interictal EEG-patterns by the qualified clinical neurophysiologist. However, 10-40% of patients who undergo pre-surgical evaluation have seizures that are not localized by the use of scalp EEG, multimodal imaging and MEG, while many of these patients undergo Intracranial-EEG (IEEG) recording with grid and depth electrodes [
6]. At the same time the increasing numbers of electrodes implanted by neurosurgeons in recent years often totalling over 100 per patient have not always produced greater clarity. Sampling of the SOZ may still be incomplete and traditional criteria for the estimation of IEEG seizure onset may be inadequate for many patients [
6]. Currently, there are no diagnostic tools that can unambiguously outline the SOZ, so its assessment must be carried out on the basis of various additional diagnostic measures.
Recent studies suggest that high frequency neural oscillations (HFO) such as “ripples” (<250 Hz) [
7] and “fast ripples” (>250 Hz), are considered to be an unmistakable biomarker of early detection of epileptic foci and epileptic seizures in the brain [
8,
9,
10,
11]. Nevertheless, rapid oscillations are still considered to be the most sensitive electrical biomarkers in terms of detecting the localization of epileptic SOZ more specific than spikes, especially in MRI-negative cases [
12,
13]. The most complex and distinct approach to this issue is nonparametric estimation method by Granger Causality [
14].
Granger Causality (GC) is a statistical method for assessing directional influences between simultaneously recorded time series which involves the process of matrix factorization. There are data indicating that GC is a highly effective method in cases involving dipole localization of seizures [
15].
So far, Wilson’s algorithm (WA) for matrix spectral factorization [
16] has been the most commonly used approach in the neuroscience community for nonparametric GC.
At the same time there is another alternative matrix spectral factorization method that was developed by Janashia and Lagvilava (1999) on the basis of a long tradition of mathematical research in Georgia. The advantage of this method is that it avoids standard and effectively utilizes a certain class of unitary matrix functions which is a core idea of this method. Currently this method is known in the literature as the "Janashia-Lagvilava Matrix spectral factorization algorithm" (JLA) [
17]. The advantages of this algorithm over WA have been well demonstrated in recent publications [
18,
19]. Therefore, it can be assumed that the JLA can be effective in determining the accuracy of the SOZ, although its capabilities have never been explored in clinical issue.
In this study, we analyzed scalp EEG recordings from patients with drug-resistant epilepsy using an alternative nonparametric GC, which relies on spectral density matrix factorization based on the JLA.
The aim of our study was to determine the SOZ and trajectory of its propagation by the GC using JLA method and to evaluate the possibilities of JLA method in clinical practice.
Methods
This cohort study is ongoing in the Epilepsy Prevention and Control Center of the Institute of Neurology and Neuropsychology (Tbilisi, Georgia). On the first stage we analyze preliminary data of the six adult patients with DRE who underwent presurgical evaluation and were selected as a candidate for epilepsy surgery. In all cases interdisciplinary investigations were conducted by the non-invasive protocol (phase 1a) [
5].
Seizure Semiology
The Seizure semiology was evaluated by the ILAE 2017 classification of seizures [
1]. All study participants had focal seizures.
MRI
3T MRI scans were collected in all cases by the epilepsy protocol. For improving sensitivity and specificity in identification possible structural abnormalities that underlie epileptic seizures there were used T1, FLAIR, T2, SWI, DWI/ASC, and additional sequences, namely DIR, T1-weighted C+ and DTI [
20].
Neuropsychological Assessment
Neuropsychological Investigations were conducted in all cases by the Wechsler Adult Intelligence Scale, Fourth Edition [WAIS-IV (2008)] and Wechsler Memory Scale, Fourth Edition [WMS-IV (2008)].
By the WAIS-IV assessment 10 Subtests of Verbal Comprehension Index (Similarities, Vocabulary, Information), Perceptual Reasoning Index (Block Design, Matrix Reasoning, Visual Puzzles), Working Memory Index (Digit Span, Arithmetic) and Processing Speed Index (Symbol Search, Coding) were conducted.
For the analysis of the results and their interpretation were used The Subtest Scaled scores, Scale Composite Scores and Discrepancy Comparisons, also, individual’s strengths and weaknesses were determined.
Brief Cognitive Status Examination subtest for adults with 16-69 years of age and assessment 10 core subtests of 5 Indexes, namely Auditory Memory, Visual Memory, Visual Working Memory, Immediate Memory and Delayed Memory Indexes by the WMS-IV were conducted.
For the analysis of results and interpretation there were calculated the Index Scaled Scores, Subtest-level Differences within Indexes, Subset-level contrast Scaled Scores and Index-level contrast scaled scores. Also, Ability-Memory and contrast scaled scores analysis was conducted by comparison of WAIS-IV and WMS-IV Index scores.
Video-EEG Monitoring
Long-term video-EEG examinations during the 72 hours were performed in each patient using a non-invasive protocol (phase Ia) [
5] and according to all relevant requirements. Interictal/ictal epileptiform visual feature (spike and slow wave complexes), and slowing (rhythmic delta or theta activity) were defined according to the IFCN glossary of terms [
21].
The long term video EEG recordings were acquired at sampling rate of 1kHz using the Micromed EEG amplifiers (Mogliano Veneto, Italy). Video - EEG monitorings were carried out in the Department of Epilepsy Monitoring Laboratory of INN using a 32-channel device (Micromed BQ 3200 ACQDV / LTM32 EXPRESS PCI - 32 CHANNELS LTM DIGITAL VIDEO EEG SYSTEM; Italy), capable of detecting high frequency oscillations in variability 80- up to 500Hz. In the process of vEEG-recording all technical requirements were fully met; which allowed us to capture as many clinical or subclinical detections as possible.
During the analysis of EEG data, interictal and ictal epileptiform visual features (spike-and–slow-wave complexes) as well as slowing (rhythmic delta or theta activity) were identified according to the IFCN glossary of terms [
21].
From the video EEG recording, three different segments of the primary excitatory zone of seizure were extracted by the qualified epileptologist/clinical neurophysiologist. At list one electroclinical seizure was analyzed for each patient based on visual assessment.
According to our concept, SOZ obtained by mathematical processing of scalp video-EEG monitoring data should be correlated with SOZ obtained from seizure semiology, MRI data (3T), neuropsychological testing and epilepsy surgery outcome.
Preprocessing of the EEG Recordings
After video EEG monitoring, the epileptologist extracted six different segments of the EEG data from the primary hypothesized SOZ based on visual assessment: interictal, preictal (15, 9, and 6 seconds before the onset of the epileptic seizure), ictal, and postictal periods.
For the analysis of brainvawes in EEG, different time windows were selected, specifically 9.5-second, 6.5-second, and 3.0-second segments of pre-ictal spike activity. These segments serve as markers of the initial localization of the seizure onset zone (SOZ).
According to our hypothesis, SOZ obtained by mathematical processing of scalp video-EEG monitoring data would be correlated with SOZ obtained from seizure semiology, MRI data (3T), neuropsychological testing and epilepsy surgery outcome (in cases with epilepsy surgery).
A well-recognized technical limitation of scalp EEG recordings is the presence of movement-related (muscular) artifacts; which was also evident in our study. Such artifacts substantially degrade the quality of the EEG signal and may render accurate interpretation nearly impossible. Modern EEG systems incorporate built-in software tools (e.g., high- and low-pass filters, notch filters) to improve signal quality and facilitate data processing. Nevertheless, even with advanced filtering approaches, it remains impossible to completely eliminate artifacts, since filtering inevitably affects both noise and physiological or pathological activity within overlapping frequency ranges. This significantly complicates the reliable interpretation of EEG data.
For these reasons, the video-EEG recordings were subjected to extensive artifact removal procedures to ensure the highest possible data quality for subsequent mathematical processing, specifically:
A) EEG recordings were pre-processed using MATLAB (MATLAB and Statistics Toolbox Release 2010b, The MathWorks, Inc., Natick, MA, USA). The EEGLAB toolbox was employed to remove evident artifacts and corrupted segments, as well as to attenuate line noise originating from electrical devices [
22].
B) The pre-processed EEG data were further analyzed in EEGLAB using Independent Component Analysis (ICA). This procedure decomposed the signals into independent frequency components, enabling the separation of neural oscillations from non-neural sources (e.g., muscular artifacts, line noise, ocular and cardiac artifacts). The non-brain components were removed, after which the EEG data were reconstructed.
C) In the next step, EEG channels identified as probable seizure onset zones (SOZs) were digitized and prepared for subsequent mathematical processing.
The
Figure 1 and
Figure 2 show examples of EEG time series before and after preprocessing performed in MATLAB/EEGLAB using ICA.
Regions in all cases were selected right or left temporal lobe channels because all of them had hippocampal sclerosis based on MRI.
Mathematical Justification (Data Analyzes by the GC and JLA)
A mathematical validation of the conclusions drawn from visual tests regarding the epilepsy onset zones and their propagation, as discussed in the previous sections, is one of the main contributions of this paper. GC is one of the leading statistical techniques for inferring directions of neural interactions and information flow from data [
23]. Describing the method in a few words, when one has two simultaneously acquired time series
and
, if the autoregressive prediction of the first time series at present time can be improved by including the past information of the second time series, it is said that
has a causal influence on
. Expressed quantitively, if
and
are autoregressive and joint representations of and, respectively, then the Granger causality measure is (see [DCB06], Eq. (7))
Nonparametric estimation of (1) relies on factorization of power spectral density matrix of the stationary stochastic process as it is described in the recent paper [
24].
We employ recent developments in GC that take multi-time ahead prediction step (L) into account in causal relations. Namely, we consider multi-step ahead prediction representations in (1) and (2) and define -lag causal relation between and
where
and
The quantity in (5) measures the proportion of the influence of time series on that is distributed within milliseconds and it can also be obtained from the spectral factorization (4) of the power spectral density (see EV2025).
Ethical Issue
The Ethics Committee of the INN (INN-005/2023) approved the project proposal. The study followed the principles outlined in the WMA Declaration of Helsinki. Before enrolling, all study patients agreeing to participate in the study provided written informed consent.
Results
Demographics
Six patients with Drug-resistant focal epilepsy (aged 28-43 years, three females), who underwent pre-surgical investigations by the non-invasive protocol, and were conducted epilepsy surgery, included in the study; Demographic data, including age, epilepsy duration, presence of epileptic seizures, neuropsychological scores, MRI data and EEG-analyses are summarized in
Table 1.
As mentioned in the methodology, selected EEG segments were cleaned, processed, and digitized.
Figure 1 and
Figure 2 show images of the EEG data before and after cleaning, and recomposition.
The EEG image obtained after the re-composition of the purified EEG data is shown on
Figure 2, after which digitization and mathematical analysis were performed.
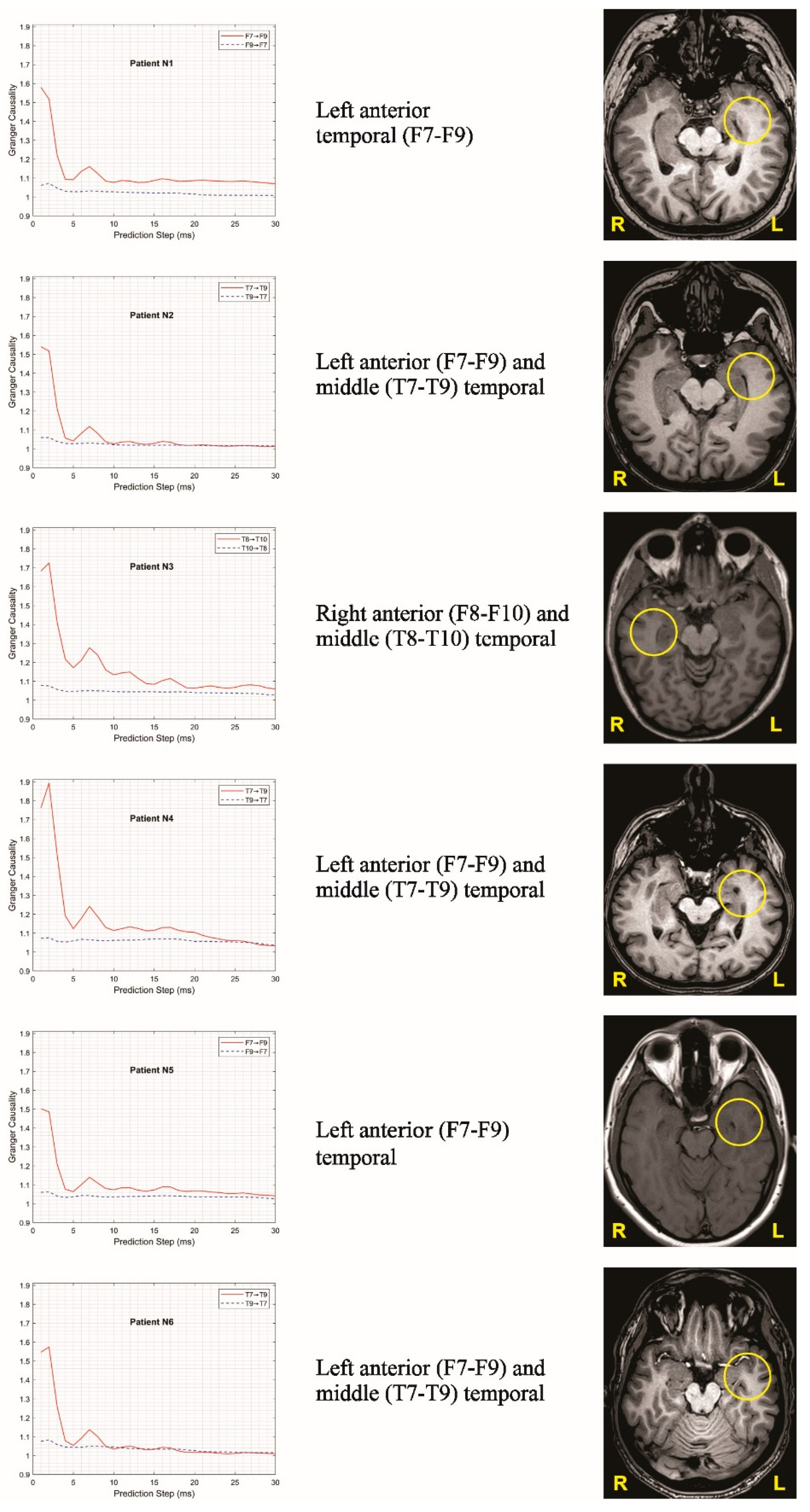
Figure 3 confirms the presence of a unidirectional spread of excitation in the EEG signals recorded between the corresponding brain regions. For illustration, six representative graphs were selected and presented in these figures. For all representatives, the EEG signals were processed over the same time interval relative to the reference point, defined as the clearly identifiable seizure onset observed during visual inspection.
After examining different options, 1-second signal segments recorded one second before the reference point were selected for demonstration. The selection of this interval involved evaluating numerous intervals of varying lengths and distances from the reference point. For each such interval, the power spectral density matrix was estimated using the multitaper method [
23], and spectral factorization (4) was then performed using JLA to evaluate (5). Since a large number of different matrices had to be factorized during this process, the Janashia–Lagvilava algorithm was extensively applied, and its advantages over Wilson’s algorithm, as described in [
18], were crucial for the successful completion of this task (see
Figure 3)
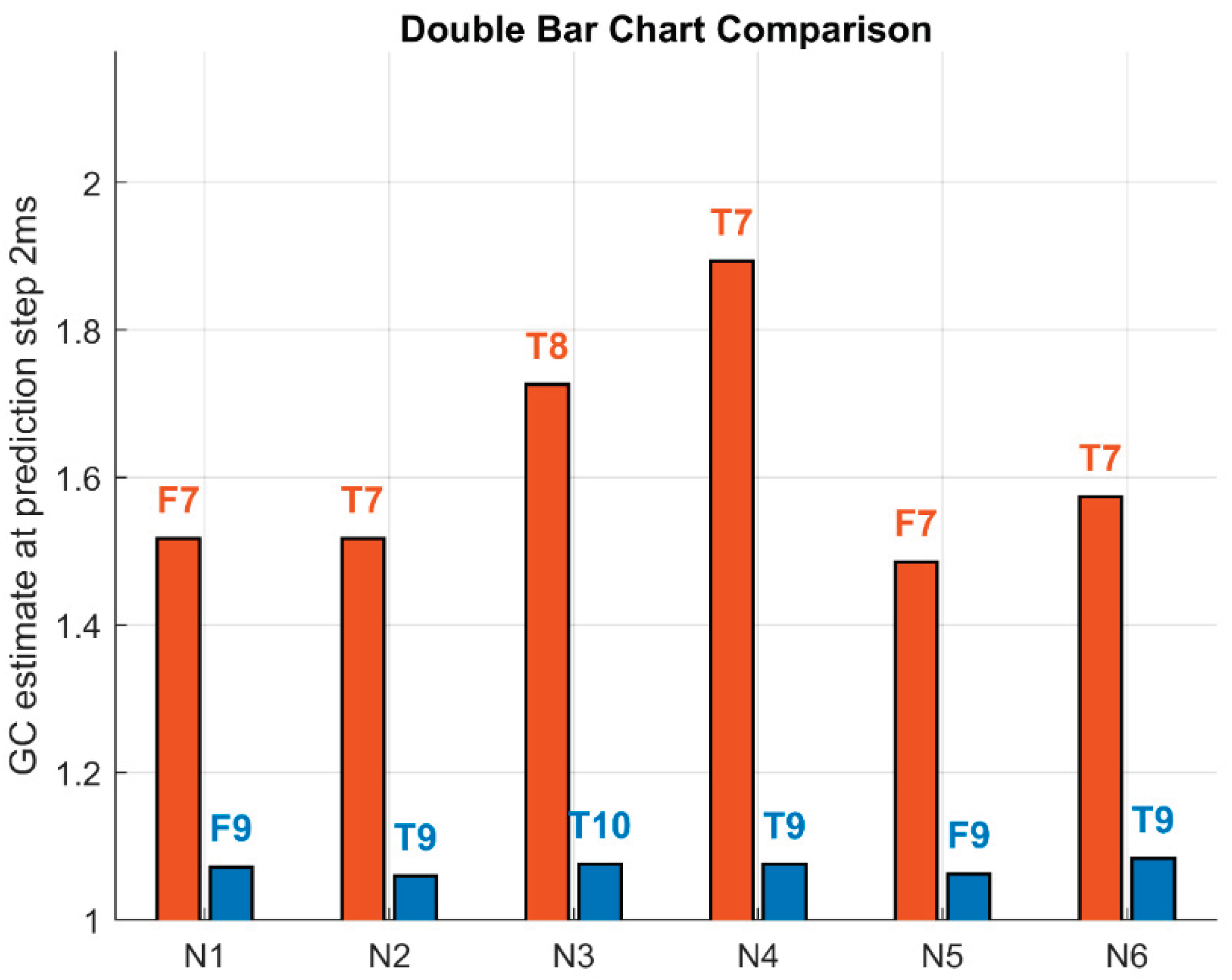
Because traditional Granger causality (GC) is based on one-step-ahead prediction, GC estimates at the one-step (2 ms) prediction horizon were isolated for comparison and are shown as double bar charts for the dominant (red) and reverse (blue) directions (see
Figure 4).
Discussion
In the present study, the JLA was applied for the first time to process real scalp-EEG data from six patients with drug-resistant epilepsy. In particular, the JLA was used to estimate L-lag-ahead GC with the nonparametric method. Our preliminary results showed that, in six patients who underwent surgical treatment, the SOZ and its propagation identified by scalp video-EEG analysis with traditional modalities were confirmed by the above-mentioned mathematical method.
drug-resistant epilepsy leads to severe psycho-social, behavioral, health and economic consequences. Surgical intervention should be considered in a timely manner for patients who have drug-resistant focal epilepsy [
25]. If we considering that seizure freedom following epilepsy surgery is achieved by 60–80% in patients with focal epilepsy all approaches that will help improve the diagnostic methodologies for identification of SOZ, especially in extra-temporal and multifocal epilepsies, effectiveness of outcomes of epilepsy surgery and quality of life of such people should not be left unattended.
The presurgical evaluation and accurate localization of the SOZ as well as their propagations; which usually depends on the information obtained from minimal examinations such as ictal/interictal scalp video-EEG monitoring, MRI with epilepsy protocol and neuropsychological testing, is crucial for successful surgery and ultimate seizure freedom for patients [
26]. But this is not always enough and invasive video-EEG recordings for a long time (with intracranial electrodes, strips or grids) [
27] and different neuroimaging investigations are necessary; which significantly increases the cost of diagnostic. However invasive video-EEG recording, in addition to being relatively traumatic procedures for the patient, sometimes can even be a futile attempt; which further aggravates the psycho-social problems of the patient.
Although intracranial EEG (iEEG) studies are an effective way to identify the primary epileptogenic focus, especially in the presence of extratemporal, large, deep, or multiple foci [
28], invasive EEG monitoring entails risks of multiple adverse events, such as infection, hemorrhage, elevated intracranial pressure, etc. [
29,
30,
31].
To avoid such problems, HFOs as biomarkers of epileptogenicity have recently been actively used. They have shown to originate from small brain regions and can be recorded from the scalp [
32,
33].
For this reason, the analysis of high-frequency (>80 Hz) oscillations by nonparametric GC method has been reported to be successful [
6]. This autoregressive statistical method [
14] based on the theory of stochastic processes [
34] that calculates predictions from time series and is used effectively to analyze continuous series data. The concept of GC is as follows: If the data of current time series of one order is predicted by the data of the past time series of the second order, it is considered that the second order has a causal effect on the first.
Whereas longterm video-EEG monitoring is a study of continuous neuronal activity the GC method was easily adapted to EEG-modality and was effectively used for analysis of SOZ in patients with DRE by Dhamala and co-authors in 2008 [
23].
It is noteworthy that for today Wilson’s algorithm for spectral factorization [
16] dominated in neuroscience applications, however, an alternative JLA [
17] proved also to be effective [
19,
35].
Our findings demonstrate that this approach can accurately localize SOZs even from non-invasive EEG recordings in six study cases. These results highlight an exciting potential for no-ninvasive brain recordings in the diagnosis and planning of epilepsy surgery in people with drug resistant epilepsy.
Recent investigations show that the latter algorithm is more reliable for unstable matrices than the former one [
18], which was also confirmed by our preliminary study date. JLA can be used effectively with GC as an additional modality in presurgical evaluation of DRE by the non-invasive protocol to specify the SOZ and its propagation ultimately avoiding the invasive electrodes for video-EEG monitoring. This consideration is particularly relevant to our study, as pathological neuronal dynamics and their network interactions demonstrate considerable temporal instability, underscoring also the necessity of validating these findings in larger cohorts of individuals with drug-resistant epilepsy.
Strengths and Limitations
The primary strength of this study lies in the application of a powerful mathematical framework—the Janashia–Lagvilava matrix spectral factorization algorithm—within medical research for the first time. By integrating this advanced approach into nonparametric Granger causality analysis, we demonstrated its feasibility for identifying seizure onset zones using vEEG recordings under a non-invasive protocol, in combination with seizure semiology, MRI, and neuropsychological testing. This methodological innovation not only supports and validates visual assessments of ictal EEG patterns but also underscores the broader potential of mathematical algorithms in clinical neuroscience.
Nevertheless, several limitations should be acknowledged. The present study involved a relatively small patient cohort, and the findings require confirmation in larger and more diverse populations. Moreover, frequency-dependent Granger causality—which could offer a richer understanding of oscillatory interactions across different frequency bands—has not yet been implemented in the current analysis. Addressing this limitation represents an important direction for future research, with the potential to enhance diagnostic precision and expand clinical applicability.
Conclusions
The successful application of JLA-based spectral factorization to non-invasive EEG data opens new possibilities for precise localization of seizure onset zones without intracranial intervention. By integrating advanced mathematical spectral factorization with clinical EEG analysis, this approach bridges the gap between theoretical signal processing and practical neurology. Beyond its immediate clinical value in guiding epilepsy surgery, the JLA-based nonparametric Granger causality framework provides a powerful and scalable tool for studying directional information flow in complex neural systems. Its flexibility and robustness hold promise for broader applications in neuroengineering, brain–computer interfaces, and data-driven therapeutic planning.
Author Contributions
SK, GL and LE are the recipients of the research grant funding and receives salary under this grant, MD as the international supervisor of the study has travel and accommodation expenses covered to oversee and verify the quality of the study process on-site. They jointly developed the protocol and schedule of the study. SK is the principal investigator of this project and supervises the study, selects patients with DRE for the pre-surgical evaluations, conducts informed consent discussions with patients for preoperative examinations and surgical treatment. GL reviews and analyzes MRI study data, cleans and processes EEG data using EEG-MATLAB, digitizes data and prepares for mathematical analysis. LE performs mathematical analysis using the Granger Causality and JL-algorithm. GJ visually analyzed long-term vEEG data and on this base calculated SOZ. TG performed and analyzed neuropsychological examinations. TJ and MA coordinate patient's investigations according to study protocol at the Epilepsy Center, collect all study data and create databases for statistical analysis. SK, GL and LE, prepared the initial draft of the manuscript and after that all authors actively participated in the preparation of this scientific article. MD contributed to the study protocol data interpretation, participated in the preparation of this scientific article and provided critical manuscript revision. All approved the final version of the article.
Funding
This study was founded by the Fundamental Scientific Grant from Shota Rustaveli National Science Foundation of Georgia (Grant #FR-23-18000) and grant from Caucasus International University (#FR-01/69-2019), Tbilisi, Georgia.
Data Availability Statement
Data will be made available on request.
Acknowledgments
We want to express our gratitude to the Ketevan Silagadze (psychiatrist) as well as the technical Personness of EMU group – Gvantsa Museridze and Natia Todua for their invaluable support during the vEEG monitoring of the study participants. We also thank the Kutaisi International University students, Mariam Mamageishvili and Ani Okropiridze, for their assistance with the numerical simulations; We are grateful to all the people with drug Resistant Epilepsy who participated in the study and were given us permission to use their data in the aforementioned article.
Conflict of Interest
There is no conflict of interest to disclose concerning this work.
References
- Fisher, R.S.; Cross, J.H.; French, J.A.; Higurashi, N.; Hirsch, E.; Jansen, F.E.; Lagae, L.; Moshé, S.L.; Peltola, J.; Roulet Perez, E.; et al. Operational Classification of Seizure Types by the International League Against Epilepsy: Position Paper of the ILAE Commission for Classification and Terminology. Epilepsia 2017, 58, 522–530. [Google Scholar] [CrossRef]
- Epilepsy. Available online: https://www.who.int/news-room/fact-sheets/detail/epilepsy (accessed on 31 August 2025).
- Kwan, P.; Arzimanoglou, A.; Berg, A.T.; Brodie, M.J.; Allen Hauser, W.; Mathern, G.; Moshé, S.L.; Perucca, E.; Wiebe, S.; French, J. Definition of Drug Resistant Epilepsy: Consensus Proposal by the Ad Hoc Task Force of the ILAE Commission on Therapeutic Strategies: Definition of Drug Resistant Epilepsy. Epilepsia 2009, 51, 1069–1077. [Google Scholar] [CrossRef]
- Engel, J. The Current Place of Epilepsy Surgery. Curr Opin Neurol 2018, 31, 192–197. [Google Scholar] [CrossRef]
- Trinka, E.; Koepp, M.; Kalss, G.; Kobulashvili, T. Evidence Based Non-invasive Presurgical Evaluation for Patients with Drug Resistant Epilepsies. Curr Opin Neurol 2024, 37, 141–151. [Google Scholar] [CrossRef]
- Adhikari, B.M.; Epstein, C.M.; Dhamala, M. Localizing Epileptic Seizure Onsets with Granger Causality. Phys. Rev. E 2013, 88, 030701. [Google Scholar] [CrossRef] [PubMed]
- Bragin, A.; Engel, J.; Wilson, C.L.; Fried, I.; Buzsáki, G. High-Frequency Oscillations in Human Brain. Hippocampus 1999, 9, 137–142. [Google Scholar] [CrossRef]
- Cuello-Oderiz, C.; von Ellenrieder, N.; Dubeau, F.; Gotman, J. Influence of the Location and Type of Epileptogenic Lesion on Scalp Interictal Epileptiform Discharges and High-Frequency Oscillations. Epilepsia 2017, 58, 2153–2163. [Google Scholar] [CrossRef] [PubMed]
- Frauscher, B.; von Ellenrieder, N.; Zelmann, R.; Rogers, C.; Nguyen, D.K.; Kahane, P.; Dubeau, F.; Gotman, J. High-Frequency Oscillations in the Normal Human Brain. Ann Neurol 2018, 84, 374–385. [Google Scholar] [CrossRef] [PubMed]
- Gonen, O.M. High-Frequency Oscillations and Their Importance in Epilepsy. Journal of Neurological Disorders 2014, 02. [Google Scholar] [CrossRef]
- Zijlmans, M.; Jiruska, P.; Zelmann, R.; Leijten, F.S.S.; Jefferys, J.G.R.; Gotman, J. High-Frequency Oscillations as a New Biomarker in Epilepsy. Annals of Neurology 2012, 71, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Frauscher, B.; Bartolomei, F.; Kobayashi, K.; Cimbalnik, J.; van ‘t Klooster, M.A.; Rampp, S.; Otsubo, H.; Höller, Y.; Wu, J.Y.; Asano, E.; et al. High-Frequency Oscillations: The State of Clinical Research. Epilepsia 2017, 58, 1316–1329. [Google Scholar] [CrossRef]
- Sun, Y.-P.; Wang, Y.-P.; Wang, Z.-H.; Wu, F.-Y.; Tang, L.-O.; Zhang, S.-W.; Pei, H.-T.; Wang, Y.; Huang, Z.-Y.; Xue, Q.; et al. High-Frequency Oscillations and the Seizure Onset Zones in Neocortical Epilepsy. Chinese Medical Journal 2015, 128, 1724–1727. [Google Scholar] [CrossRef]
- Granger, C.W.J. Investigating Causal Relations by Econometric Models and Cross-Spectral Methods. Econometrica 1969, 37, 424. [Google Scholar] [CrossRef]
- Lu, Y.; Yang, L.; Worrell, G.A.; He, B. Seizure Source Imaging by Means of FINE Spatio-Temporal Dipole Localization and Directed Transfer Function in Partial Epilepsy Patients. Clin Neurophysiol 2012, 123, 1275–1283. [Google Scholar] [CrossRef]
- Wilson, G.T. The Factorization of Matricial Spectral Densities. SIAM J. Appl. Math. 1972, 23, 420–426. [Google Scholar] [CrossRef]
- Ephremidze, L.; Saied, F.; Spitkovsky, I.M. On the Algorithmization of Janashia-Lagvilava Matrix Spectral Factorization Method. IEEE Transactions on Information Theory 2018, 64, 728–737. [Google Scholar] [CrossRef]
- Ephremidze, L.; Gamkrelidze, A.; Spitkovsky, I. On the Spectral Factorization of Singular, Noisy, and Large Matrices by Janashia-Lagvilava Method. 2022, 176, 361–366.
- Henderson, J.A.; Dhamala, M.; Robinson, P.A. Brain Dynamics and Structure-Function Relationships via Spectral Factorization and the Transfer Function. Neuroimage 2021, 235, 117989. [Google Scholar] [CrossRef]
- Di Muzio, B.; Al Kabbani, A.; McArdle, D. Epilepsy Protocol (MRI). In Radiopaedia.org; Radiopaedia.org, 2015.
- Kane, N.; Acharya, J.; Benickzy, S.; Caboclo, L.; Finnigan, S.; Kaplan, P.W.; Shibasaki, H.; Pressler, R.; van Putten, M.J.A.M. A Revised Glossary of Terms Most Commonly Used by Clinical Electroencephalographers and Updated Proposal for the Report Format of the EEG Findings. Revision 2017. Clin Neurophysiol Pract 2017, 2, 170–185. [Google Scholar] [CrossRef] [PubMed]
- Barnett, L.; Seth, A.K. The MVGC Multivariate Granger Causality Toolbox: A New Approach to Granger-Causal Inference. Journal of Neuroscience Methods 2014, 223, 50–68. [Google Scholar] [CrossRef] [PubMed]
- Dhamala, M.; Rangarajan, G.; Ding, M. Analyzing Information Flow in Brain Networks with Nonparametric Granger Causality. NeuroImage 2008, 41, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Ephremidze, L.; Vatsadze, S. Stationary Processes, Wiener-Granger Causality, and Matrix Spectral Factorization, in Statistics of Random Processes and Optimal Control. Springer Nature volume dedicated to Albert Shiryaev 2025. [Google Scholar]
- Engel, J. Early Surgical Therapy for Drug-Resistant Temporal Lobe Epilepsy: A Randomized Trial. JAMA 2012, 307, 922. [Google Scholar] [CrossRef] [PubMed]
- Ryvlin, P.; Cross, J.H.; Rheims, S. Epilepsy Surgery in Children and Adults. The Lancet Neurology 2014, 13, 1114–1126. [Google Scholar] [CrossRef] [PubMed]
- Guery, D.; Rheims, S. Clinical Management of Drug Resistant Epilepsy: A Review on Current Strategies. NDT 2021, 17, 2229–2242. [Google Scholar] [CrossRef]
- Kovac, S.; Vakharia, V.N.; Scott, C.; Diehl, B. Invasive Epilepsy Surgery Evaluation. Seizure 2017, 44, 125–136. [Google Scholar] [CrossRef]
- Arya, R.; Mangano, F.T.; Horn, P.S.; Holland, K.D.; Rose, D.F.; Glauser, T.A. Adverse Events Related to Extraoperative Invasive EEG Monitoring with Subdural Grid Electrodes: A Systematic Review and Meta-analysis. Epilepsia 2013, 54, 828–839. [Google Scholar] [CrossRef]
- Eppler, M.B.; Sayegh, A.S.; Maas, M.; Venkat, A.; Hemal, S.; Desai, M.M.; Hung, A.J.; Grantcharov, T.; Cacciamani, G.E.; Goldenberg, M.G. Automated Capture of Intraoperative Adverse Events Using Artificial Intelligence: A Systematic Review and Meta-Analysis. J Clin Med 2023, 12, 1687. [Google Scholar] [CrossRef]
- Wong, G.; Gurevich, S.; Teti, S.; Guerrera, M.F.; Zelleke, T.; Gaillard, W.D.; Oluigbo, C.O. Invasive Intracranial Electroencephalography Monitoring in the Child with a Bleeding Disorder: Challenges and Considerations. Pediatr Neurosurg 2024, 60, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Noorlag, L.; Van Klink, N.E.C.; Kobayashi, K.; Gotman, J.; Braun, K.P.J.; Zijlmans, M. High-Frequency Oscillations in Scalp EEG: A Systematic Review of Methodological Choices and Clinical Findings. Clinical Neurophysiology 2022, 137, 46–58. [Google Scholar] [CrossRef]
- Zelmann, R.; Lina, J.M.; Schulze-Bonhage, A.; Gotman, J.; Jacobs, J. Scalp EEG Is Not a Blur: It Can See High Frequency Oscillations Although Their Generators Are Small. Brain Topogr 2014, 27, 683–704. [Google Scholar] [CrossRef]
- Bastos, A.M.; Schoffelen, J.-M. A Tutorial Review of Functional Connectivity Analysis Methods and Their Interpretational Pitfalls. Front. Syst. Neurosci. 2016, 9. [Google Scholar] [CrossRef] [PubMed]
- MacLaurin, J.N.; Robinson, P.A. Determination of Effective Brain Connectivity from Activity Correlations. Phys. Rev. E 2019, 99, 042404. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).