1. Introduction
Retinal disorders constitute a major contributor to ocular morbidity and vision impairment. According to research conducted in both hospitals and the general public, the most prevalent retinal issues among the vitreoretinal illnesses are age related macular degeneration (AMD), diabetic retinopathy, and retinal vein occlusion edema (RVO) [
1].
The pathophysiological hallmarks of AMD, DME, and RVO edema often intersect, involving oxidative stress, inflammatory cascades, and abnormal angiogenesis. Collectively, these factors converge on VEGF and Ang2 which orchestrates pathological vessel growth and elevates vascular permeability [
2,
3]. Co-expression of Ang-2 and VEGF-A has been linked to accelerated neovascularization [
4]. VEGF-A facilitates angiogenesis by encouraging the movement, survival, and development of endothelial cells. Ang-2 has been demonstrated to increase proinflammatory signals in endothelial cells and is implicated in vascular leakage and aberrant vessel shape [
4].
The treatment of retinal disorders has changed over the past ten years due to the introduction of anti-vascular endothelial growth factors (anti-VEGFs), which are currently the first-line treatment [
5]. Nowadays, anti-VEGF medication is unquestionably a powerful and successful tool in the fight against RVO, DR complications, and wet AMD. Three VEGF inhibitors are currently used to treat retinal disorder: bevacizumab, aflibercept, and ranibizumab, the latter is used off-label [
6].
In an attempt to address the multifactorial nature of retinal vascular diseases, new therapies that are more comprehensive than anti-VEGF-A monotherapies alone are being searched after. This is because intravitreal anti-VEGF-A therapeutics appear to have a ceiling on vision improvement and require frequent injections to maintain clinical benefits. Faricimab (faricimab-svoa; VabysmoTM), a new bispecific antibody that targets both VEGF-A and Ang-2, was recently developed by Roche/Genentech to treat DME and nAMD. Faricimab binds and neutralizes Ang-2, a key regulator of vascular stability, as well as VEGF-A, a major contributor to neovascularization and vascular permeability. This means that, in contrast to current VEGF-A inhibitors, the simultaneous blockage of both pathways offers the possibility of comprehensive therapeutic efficacy [
7].
The purpose of this study is to provide real-world assessment after switching to intravitreal faricimab in patients with wet AMD or macular edema due to diabetes mellitus or retinal vein occlusion edema who received previously repeated intravitreal anti-VEGF therapy with NO or poor response.
2. Materials and Methods
2.1. Study Design
This study was designed as prospective, interventional clinical investigation to assess the anatomical and visual responses after switching to Faricimab in patients with refractory diabetic macular edema (DME), refractory retinal vein occlusion edema (RVO) and refractory neovascular age-related macular degeneration (nAMD) who were non-responsive to anti-VEGF treatments. This study was carried out at a specialized ophthalmic center in Baghdad, Iraq. Over 12 months the research team maintained full compliance with Good Clinical Practice guidelines and adhered to the ethical principles of the Declaration of Helsinki. Institutional Review Board approval obtained (03.37/2025). The study is officially registered in the ClinicalTrials.gov database under the identifier number (NCT07093385). All participants provided written informed consent after being thoroughly informed about the purpose, risks, procedures, and potential benefits of the research.
2.2. Inclusion and Exclusion Criteria
Participants were adult (>18 years) with confirmed diagnosis persistent DME, RVO edema, or active nAMD by clinical evidence, optical coherence tomography OCT and angiography (OCTA). Participants Show no significant anatomical or visual improvement to previous anti-VEGF, show willingness and ability to attend follow-up appointments and comply with treatment protocol. Only previously treated patients with: minimum of 6 monthly bevacizumab injections for DME or RVO edema within the past 8 months, or 3 injections for nAMD within the past 4 months or a minimum of 5 monthly aflibercept injections for DME or RVO edema within the past 6 months, or 3 injections for nAMD within the past 4 months.
Exclusion criteria include inconsistent treatment history (missed a dose of anti-VEGF), failed to complete the three loading doses of Faricimab, ocular comorbidities that could confound outcomes (Visually significant cataract as Grade 2+ or more, corneal opacity, uncontrolled glaucoma), severe baseline vision loss (BCVA < 6/60), Transient responders to anti-VEGF therapy that initially demonstrate anatomical and functional improvement following treatment but subsequently exhibit a decline in therapeutic efficacy, characterized by worsening visual acuity and increased retinal fluid or thickness despite ongoing injections.
2.3. Treatment Protocol
The study focused exclusively on patients who were deemed non-responsive to Bevacizumab and/or Aflibercept, thus offering a highly selective cohort to assess the impact of Faricimab therapy. To ensure accuracy in Faricimab evaluation, only primary non-responder to current anti-VEGF agents were included. These patients show persistent subretinal or intraretinal fluid with no improvement of visual acuity (VA) nor a decrease in central retinal thickness (CRT) after repeated injections of Bevacizumab and Aflibercept (CRT is the same or increased after injections, or a decrease of less than 10% of CRT before treatment, with worsening in BCVA from baseline evaluation or remain the same).
All patients followed a standardized treatment protocol consisting of 3 monthly loading doses of intravitreal faricimab (6.0 mg/0.05 mL). The injection procedure took place in sterile environment by experienced retina specialist.
2.4. Data Collection
The data collected for analysis include age, gender, and medical history of hypertension and diabetes mellitus, baseline Best-Corrected Visual Acuity (BCVA) measurement, baseline intraretinal fluid (IRF), baseline subretinal fluid (SRF), baseline central retinal thickness (CRT), baseline pigmented epithelial detachment (PED) in AMD cases and baseline intraocular pressure (IOP). Patient's observation and examination were repeated posttreatment to compare the results and to assess treatment response.
2.5. Statistical Analysis
All analyses were conducted using SPSS v.26. Data was represented as tables and figures. Age was normally distributed and summarized as mean ± standard deviation (Mean±SD). Visual acuity and central retinal thickness were non-normally distributed, so they were reported as median with interquartile range (IQR). For within-group comparisons of these non-normally distributed variables, we used the Wilcoxon signed-rank test instead of the paired t-test. Intraretinal fluid and subretinal fluid data were reported as frequencies and percentages, for within group comparison fisher exact test were used. Categorical data were reported as frequencies and percentages. P value < 0.05 was considered statistically significant.
3. Results
3.1. Demographic Data of Patients and Medical Background
A total of 35 patients were included in the study, with a mean age of 66.74±9.422 years range in between (46-83). Males were 19 (54.3%) and females were 16 (45.7%). Past medical history (PMH) was positive among 28 (80.0%) patients, with hypertension (HT) among 13 (37.1%) and diabetes mellitus (DM) among 20 (57.1%).
Table 1.
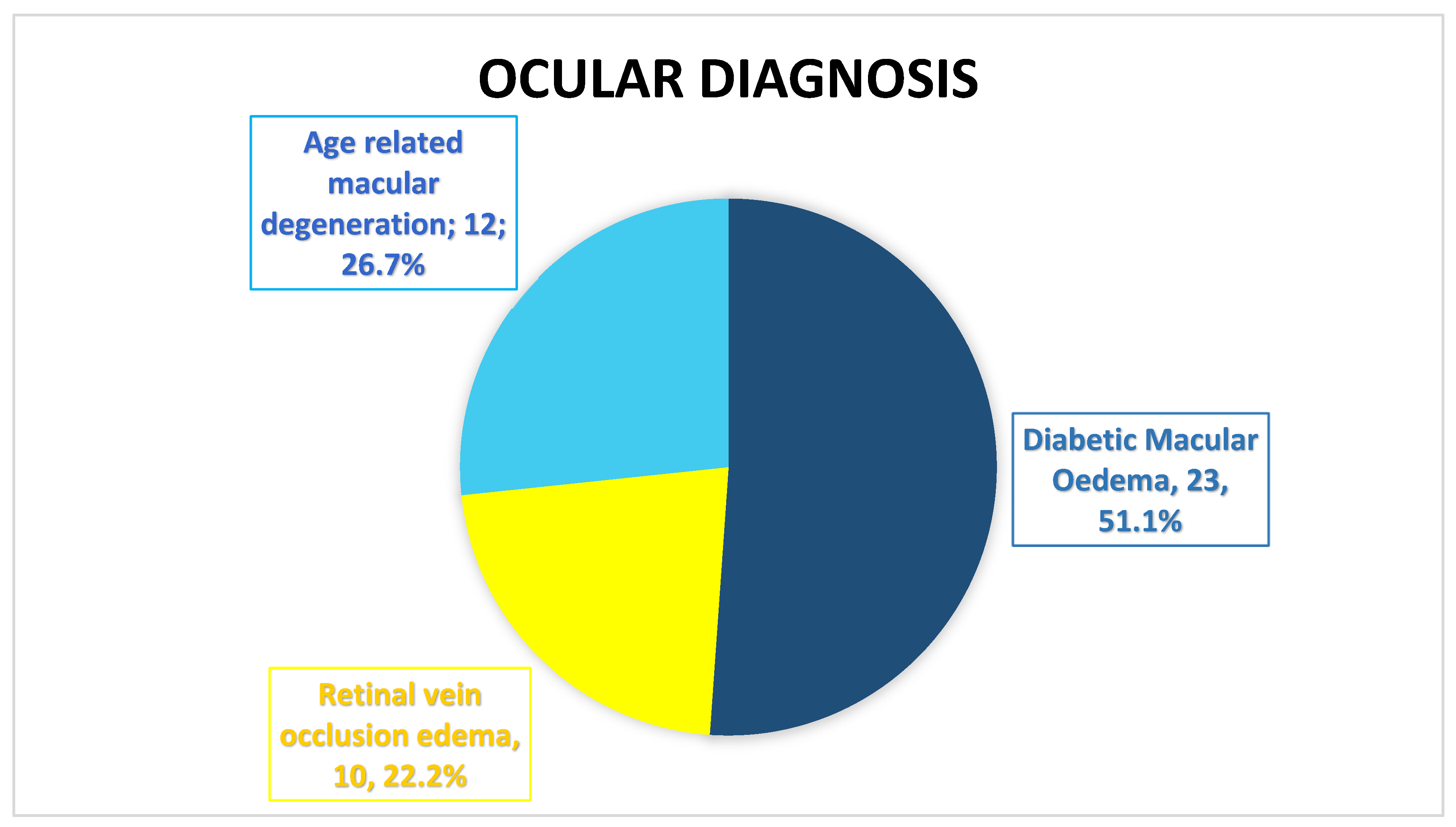
Of the total patients (n=35); there were 45 eyes with ocular disease treated with intravitreal faricimab, out of them; 23 (51.1%) eyes with Diabetic Macular Edema (DME), 10 (22.2%) eyes with Retinal vein occlusion edema (RVO), and 12 (26.7%) eyes with Age related macular degeneration (nAMD).
Figure 1.
There were 23 (51.1%) right eyes and 22 (48.9%) left eyes. previous Anti-VEGF injection were Intravitreal Eylea among 26 (57.8%) eyes, Intravitreal Avastin 16 (35.6%), and mix Intravitreal Eylea + Intravitreal Avastin among 3 (6.7%) eyes; with median dose of 7.0 (3.0).
Table 2.
3.2. Comparison of Clinical Parameters Before and After Starting Faricimab for All Eyes
The comparison of clinical parameters before and after starting Faricimab was detailed in
Table 3.
Visual acuity and central retinal thickness show a significant improvement (P<0.001). The median visual acuity reduced from 0.6 (0.4) at baseline to 0.3 (0.4) after Faricimab loading dose and median central retinal thickness reduced from 411.0 (201.0) to 289.0 (112.50).
Intraretinal fluid and subretinal fluid show significant reduction (P<0.001). The proportion of eyes with SRF decreased from 24 (53.33%) pretreatment to 3 (6.66%) posttreatment, while the proportion of eyes with IRF decreased from 35 (77.77%) to 16 (35.55%). Intraocular pressure remained stable over the course of the study (p=0.35).
3.3. Comparison of Clinical Parameters Before and After Starting Faricimab Among Patients’ Eyes With DME
Comparison of clinical parameters among patients’ eyes with DME (n=23) before and after starting Faricimab was detailed in
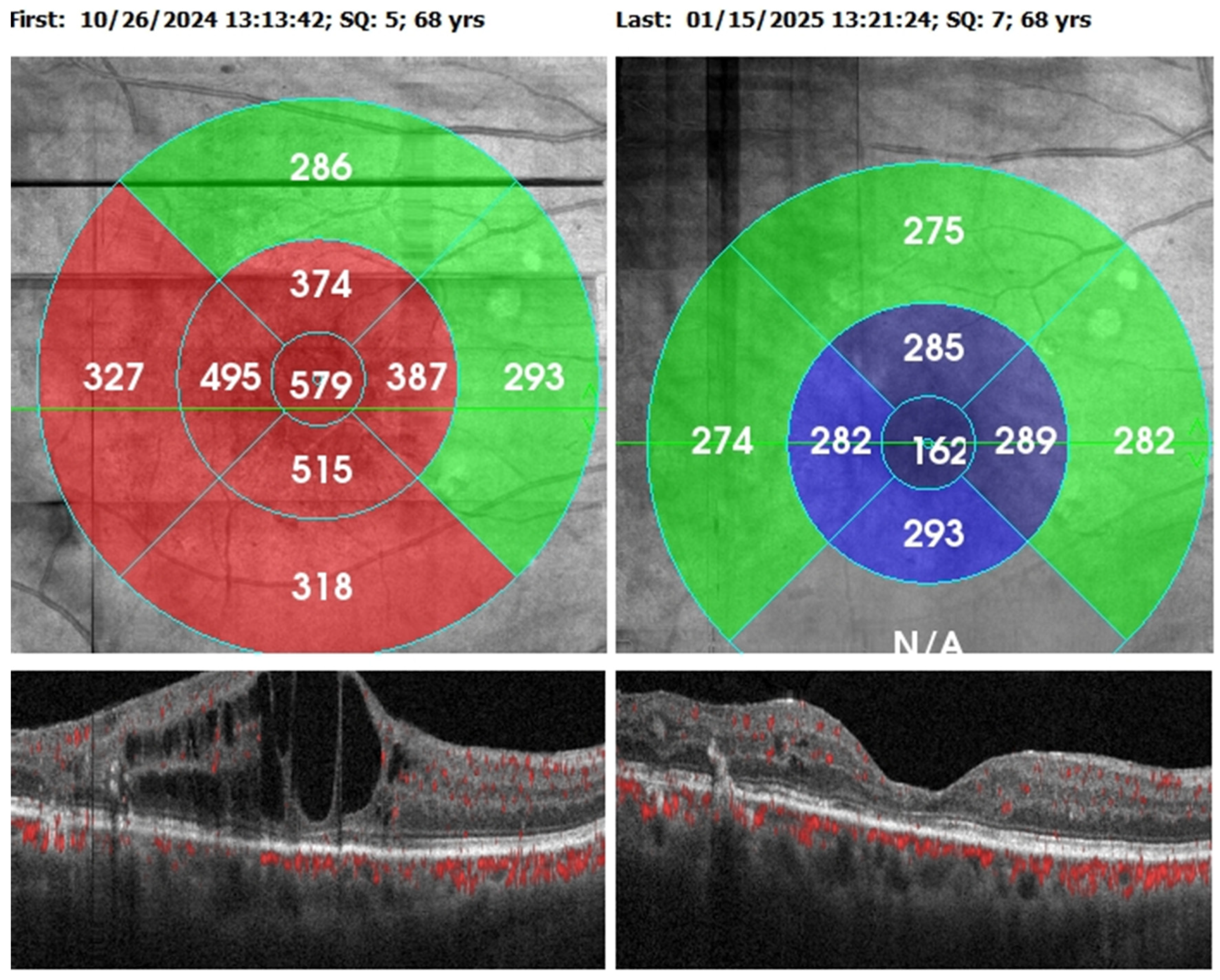
Table 4 and
Figure 2.
Visual acuity and central retinal thickness show a significant improvement (P<0.001). The median VA decreased from 0.5 (0.4) at baseline to 0.3 (0.4) following the Faricimab loading dosage and median CRT decreased from 435.0 (200.0) to 311.0 (132.50).
Intraretinal fluid and subretinal fluid show significant reduction (P<0.05). The proportion of eyes with SRF decreased from 7 (30.43%) to 1 (4.34%) posttreatment, while the proportion of eyes with IRF decreased from 23 (100%) to 11 (47.82%).
3.4. Comparison of Clinical Parameters Before and After Starting Faricimab Among Patients’ Eyes With RVO.
Comparison of clinical parameters among patients’ eyes with RVO (n=10) before and after starting Faricimab was detailed in
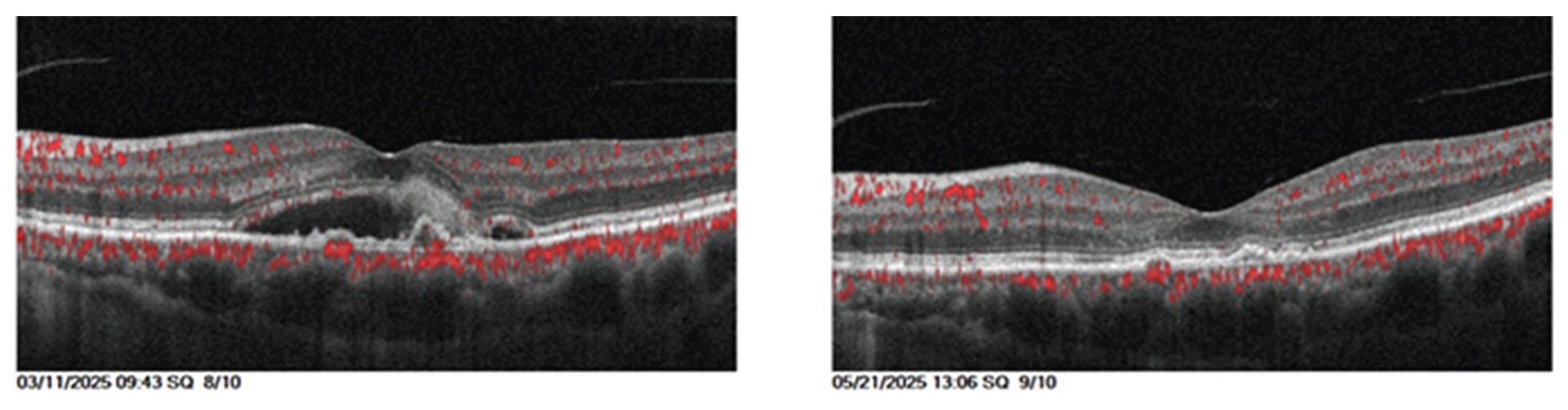
Table 5 and
Figure 3.
Visual acuity and central retinal thickness show a significant improvement (P<0.05). The median visual acuity reduced from 0.8 (0.43) at baseline to 0.45 (0.63) after Faricimab loading dose and median central retinal thickness reduced from 541.0 (327.0) to 293.0 (159.25).
Intraretinal fluid show significant reduction (P=0.033). The proportion of eyes with IRF decreased from 10 (100%) pretreatment to 5 (50%) posttreatment.
Even though the proportion of eyes with SRF dropped from 5 (50%) before therapy to 1 (10%) after, suggesting that some patients exhibit a notable decrease in retinal fluid, the limited sample size caused the p=0.141.
3.5. Clinical Parameters Before and After Starting Faricimab Among Patients’ Eyes With wet AMD.
Comparison of clinical parameters among patients’ eyes with AMD (n=12) before and after starting Faricimab was shown in
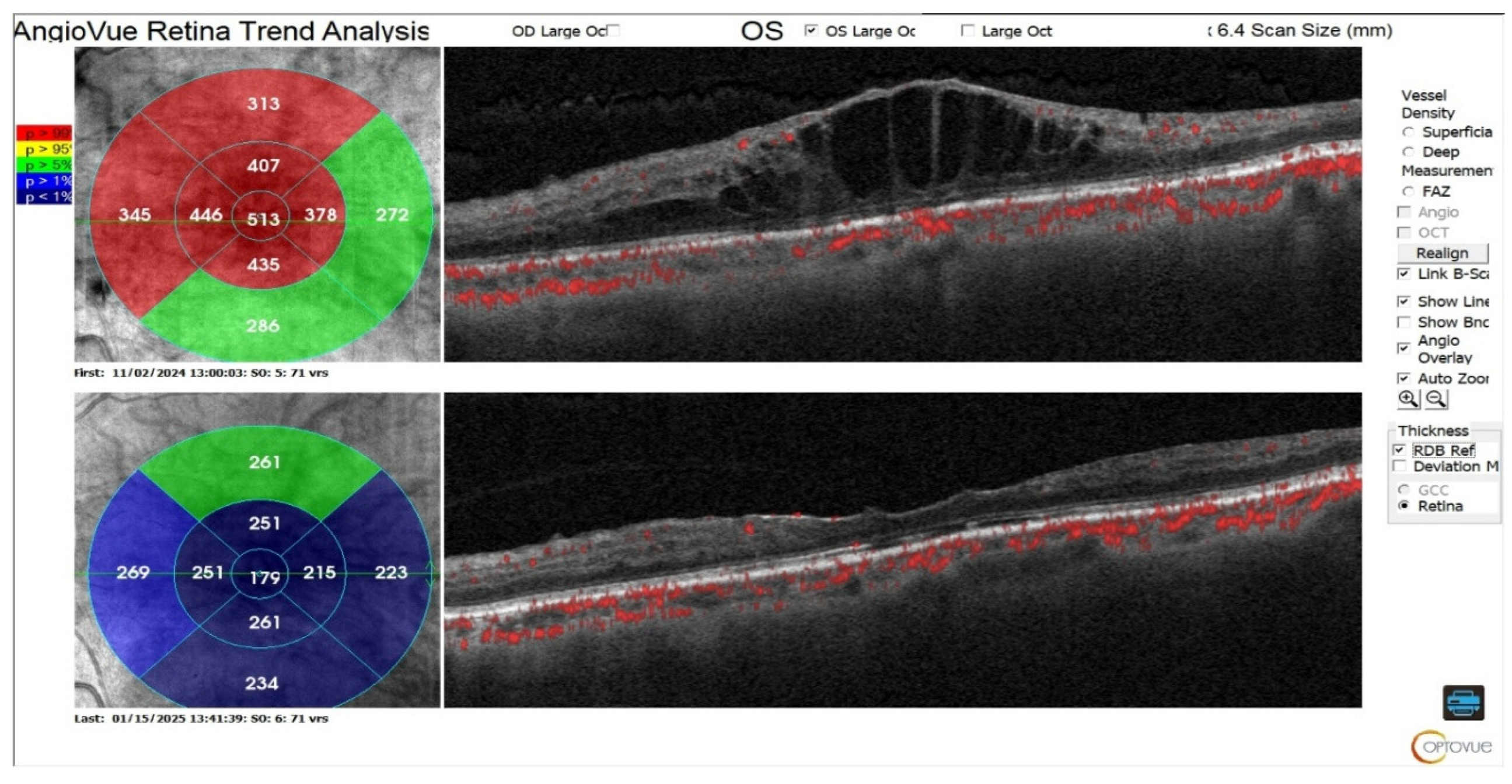
Table 6 and
Figure 4.
Visual acuity and central retinal thickness show a significant improvement (P<0.05). The median visual acuity reduced from 0.55 (0.20) at baseline to 0.3 (0.55) after Faricimab loading dose and median central retinal thickness reduced from 382.0 (87.75) to 247.5 (53.0).
Even though the median pigmented epithelial detachment both before and after treatment equal to 0, the IQR reduced from 382.0 to 81.0, indicating reduction in PED severity and a borderline significant improvement (p=0.06).
Subretinal fluid show significant reduction (P<0.001). The proportion of eyes with SRF decreased from 12 (100%) pretreatment to 1 (8.33%) posttreatment.
Even though the percentage of eyes with IRF dropped to 0 (0%) from 2 (16.66%) after therapy, suggesting that some patients exhibit a notable decrease in retinal fluid, the limited sample size caused the p=0.478.
4. Discussion
The current study evaluated the short-term response to Faricimab in patients who were switched from previous intravitreal treatments because of treatment resistance following multiple intravitreal injections of various medications. Treatment resistance was described as ongoing intraretinal fluid that affected BCVA even after taking anti-VEGF medication every four weeks. In order to assess the impact of intravitreal faricimab treatment, the study compared key clinical parameters in patients' eyes before and after therapy. All of these clinical parameters together offer a thorough understanding of the anatomical and functional function to faricimab, emphasizing how it can improve vision while preserving eye pressure stability and lowering retinal edema and fluid buildup. This was done in order to bridge the gap between clinical trials and real-world data.
The study indicating a strong correlation between retinal vascular disease (RVD) and aging. This finding aligns closely with a study conducted in Nepal, which reported a high prevalence (52.37%) of retinal abnormalities among individuals aged 60 years and older, with a mean age of 69.64 ± 7.31 years [
1].
The gender distribution in the present study was relatively balanced, this minor difference in the male-to-female ratio was not statistically significant and is consistent with the analysis reported by Shafiee, A., Juran, T [
8].
A considerable proportion of patients had pre-existing health issues, predominantly hypertension (37.1%) and diabetes mellitus (57.1%). Supporting this study, a study conducted by Owusu-Afriyie B, Baimur I in Papua New Guinea hypertension and diabetes are serious issues, with diabetes is the primary cause of DR and hypertension plays a role in the development and progression of DR as well as RVO [
9].
Data collected from this study indicate that DME patient most likely to develop Anti VEGF resistance followed by those with AMD and RVO. According to study performed by Rush RB, Rush SW 44% of patients with DME show persistent retinal fluid on OCT, Even after 2 years receiving aflibercept medication [
10]. In case of AMD, about 19.7% and 36.6% of patients who had aflibercept treatment every 4 and 8 weeks for a year, respectively, continued to have active exudation [
11]. For RVO the results of clinical trials and clinical practice differ, which suggests that VEGF-targeting medications may not be sufficient to completely treat this illness [
12].
The history of the baseline demographics, which includes several pretreatment doses of different intravitreal medicines, highlights the difficulty of treating refractory RVD and the urgent need for novel treatments like faricimab in this difficult to treat patient population.
A significant improvement in VA was observed during the study (P<0.001), as demonstrated by reduced median LogMAR values (0.6 to 0.3). The significant improvement in VA is consistent with Faricimab's dual mode of action, which targets both Ang-2 and VEGF to address the complex pathophysiology of nAMD, DME, and RVO-associated ME [
13].
For patients with DME, this study demonstrates a significant improvement in VA after switching to Faricimab. This finding aligns with real-world data reported by Rush RB [
14] but different from Deiters V.’s study that reported stable visual function with a negligible tendency for improvement [
15].
For patients with RVO, VA show significant improvement (P=0.01), indicating that the applied therapeutic technique was successful in improving patient sight.their eyesight had improved. This align with BALATON and COMINO study [
16].
For eyes receiving VEGF inhibitor treatment nAMD, treatment switching is a standard strategy to address nonresponse [
17]. Information on VA for patients with nAMD varies widely in the literatures. Some authors have documented improvement [
18] other documented stability [
19], while worsening is sometimes observed [
20]. The main causes of this could be different inclusion and exclusion criteria, different lengths of prior anti-VEGF treatment and different observation period of faricimab treatment. In the current study, the median visual acuity of individuals with wet AMD improved significantly from 0.55 to 0.3 (P 0.018).
According to published research, anti-VEGF medicines typically result in transient elevations in intraocular pressure (IOP) [
21,
22]. A study of the literature on the effects of different anti-VEGF on IOP was carried out by Hoguet et al [
23]. They came to the conclusion that, despite variations in design, all investigations on short-term IOP alterations after IVTs uniformly demonstrated an instantaneous rise in IOP, seen in 100% of eyes within one minute after injection. 92% to 97% of individuals showed an IOP below 30 mm Hg after 30 minutes following the surgery, indicating that this peak was temporary.
Data associated with Faricimab indicate that the pattern of IOP elevation closely resembles that documented in previous research for other anti-VEGF compounds with a noticeable rise in IOP immediately following injection and a recovery to below 21 mm Hg within 15 minutes [
23]. Regarding the safety profile of the treatment in this study, the stability of IOP after a period of time is comforting.
Clinical parameter comparison for all eyes show a significant decline in intraretinal fluid (p<0.001), subretinal fluid (p<0.001) and central retinal thickness (p<0.001) after switching to Faricimab. These finding suggest that faricimab Ang-2 and VEGF-A inhibition combination improve the drying anatomical outcomes beyond VEGF-A inhibition alone [
15]. According to earlier research, faricimab effectively improves anatomical outcomes following a transition from other medications [
16,
24].
For patients with DME, the anatomical function of the eye shows significant improvement represented by significant reduction in CRT, IRF and SRF. This result supported by a study performed by Rush et al., patients with refractory DME who transitioned from aflibercept to faricimab demonstrated a notable improvements in CMT when compared to those who continued aflibercept medication with 37.5% of the patients had a CMT < 300 µm after switching to faricimab [
14]. IRF usually reacts favorably to anti-VEGF treatment [
25]. In this study IRF was detected in (100%) eyes in patients with DME at baseline. Following the administration of three loading doses of faricimab, (47.82%) of patients achieved complete dryness, while the remaining patients showed a considerable reduction in intraretinal fluid. These findings align with YOSEMITE and RHINE trials, 98.7% to 99.0% of eyes exhibited IRF at baseline. Two years later, IRF resolution was attained in 44–49% of the T&E group and 58–63% of the Q8W group. [
26] The YOSEMITE trial's Japanese subgroup showed IRF resolution rates of 17.3–29.0% in the T&E group and 55.7–70.8% in the Q8W group, indicating a higher propensity for recurrence in the latter group [
27].
Previous research for patients with RVO done by
Hirakata T, showed morphological improvements in both the naïve and switch groups with RVO, macular retinal fluid disappeared on OCT in 6 of 13 (46%) eyes in the switch group and 12 of 17 (71%) eyes in the naïve group one month following the initial IVF. Additionally, one month following the initial IVF, 12 of 13 (92%) eyes in the switch group and 13 of 17 (76%) eyes in the naïve group had CMTs below 325 μm [
28]. Additionally according to the BALATON and COMINO investigations, faricimab caused a rapid and significant decrease in retinal fluid from the baseline in patients with RVO, as seen by the decrease in CST [
16].These study results supported the current study result that shows a considerable morphological improvement exhibited as significant reduction in CRT (median -248.0, p 0.005) and IRF (p 0.033) . IRF dryness is seen in half of the patients, whereas a significant fluid loss is seen in the other half. For SRF 40% of eyes experience complete subretinal fluid dryness.
In the current study, faricimab improve anatomic function of the nAMD patient eyes, with a notable CRT median reduction (-135.0) (p=0.002) and a considerable proportion of patient (91.67%) without SRF following Faricimab treatment support the drying effect of faricimab on the eyes. These anatomical results are in line with a number of other real-world faricimab outcome studies in eyes that have already received treatment for nAMD [
19,
29,
30]. Although switching to faricimab lowers SRF, the probability ( p value) might not be suitable because of the small sample size. [
31] This study indicates that PED severity has decreased; however, due to the limited sample size, probability may not be appropriate. Faricimab counteracts the primary mechanisms behind the development of PEDs [
4,
32].
Our study demonstrates the effectiveness of faricimab in patients who had previously failed several lines of treatment in a practical context. Real-world investigations, as opposed to controlled clinical trials, frequently include a more varied patient group with a range of comorbidities and illness severity [
33]. This increases the therapeutic significance of our results and sheds light on how Faricimab is actually used in complicated situations.
Limitations: Small sample size, single center study, no control group, baseline demographic data differences and short follow up period, constitute limitation for our research. Since small sample size reduce statistical power and limit generalization for larger study. Baseline demographic data differences complicate the outcome. Short follow up period limit assessment of delayed effect and adverse event of treatment.
In conclusion, faricimab's dual blockage of VEGF and Ang-2 is a promising development in the management of refractory DME, RVO and nAMD. However, judgments about the long-term durability of Faricimab treatment and the possibility of interval extension are limited by the short 3-month follow-up period. Future research should examine Faricimab's long-term safety and effectiveness as well as how it can help improve treatment plans for RVD.