1. Introduction
Worldwide, neovascular age-related macular degeneration (nAMD) is a leading cause of central blindness in older adults[
1,
2,
3]. Intravitreal vascular endothelial growth factor (VEGF) therapy is currently the first-line treatment for nAMD[
4].
Drugs such as ranibizumab[
5], aflibercept 2 mg[
2], brolucizumab[
6], and faricimab[
7] are currently available on the market. Among these, aflibercept 2 mg has been used extensively and remains the most widely used drug. Aflibercept is a recombinant fusion protein consisting of portions of the VEGF receptor A and B extracellular domains fused to the Fc portion of human immunoglobulin G, which blocks placental growth factor (PLGF) [
8].
In 2023, aflibercept (8 mg) was approved as a new anti-VEGF drug following a phase 3 clinical trial, the PULSAR trial[
9]. The PULSAR trial demonstrated that injections of aflibercept 8 mg every 12 or 16 weeks were non-inferior to injections of aflibercept 2 mg every 8 weeks in terms of improvements in best-corrected visual acuity (BCVA) and central foveal thickness (CFT) [
9]. Additionally, during the evaluation up to week 16 of the loading phase, the fluid control effect of aflibercept 8 mg was significantly superior to aflibercept 2 mg. Many reports have shown that switching agents can improve exudative changes in patients previously treated with anti-VEGF agents[
10,
11,
12,
13,
14]. It is of particular interest to determine how effectively the newly approved aflibercept 8 mg performs in this context. However, given the increasing number of drug options, it has become difficult in practice to evaluate which agent is more effective when switching treatments. If the therapeutic effect is inadequate, switching back to a prior agent remains an option.
In this study, we retrospectively evaluated the initial response to exudative changes and the functional outcomes of intravitreal aflibercept 8 mg for nAMD in real-world settings.
2. Materials and Methods
This study was approved by the Ethics Committee of the Osaka University Graduate School of Medicine (approval number 10039) and followed the tenets of the Declaration of Helsinki. The need for informed consent was waived due to the retrospective design of the study.
The study included a consecutive series of patients with nAMD who had been previously treated with other anti-VEGF drugs and switched to an intravitreal injection of aflibercept (8 mg) at Osaka University Hospital between May and October 2024. Among them, patients with exudative changes (subretinal fluid [SRF], intraretinal fluid [IRF], or serous pigment epithelial detachment [sPED]) within a 6 mm x 6 mm macular cube at the time of switching to aflibercept 8 mg, and who experienced no change in dosing intervals before and after the switch (within ±7 days), were included in the analysis.
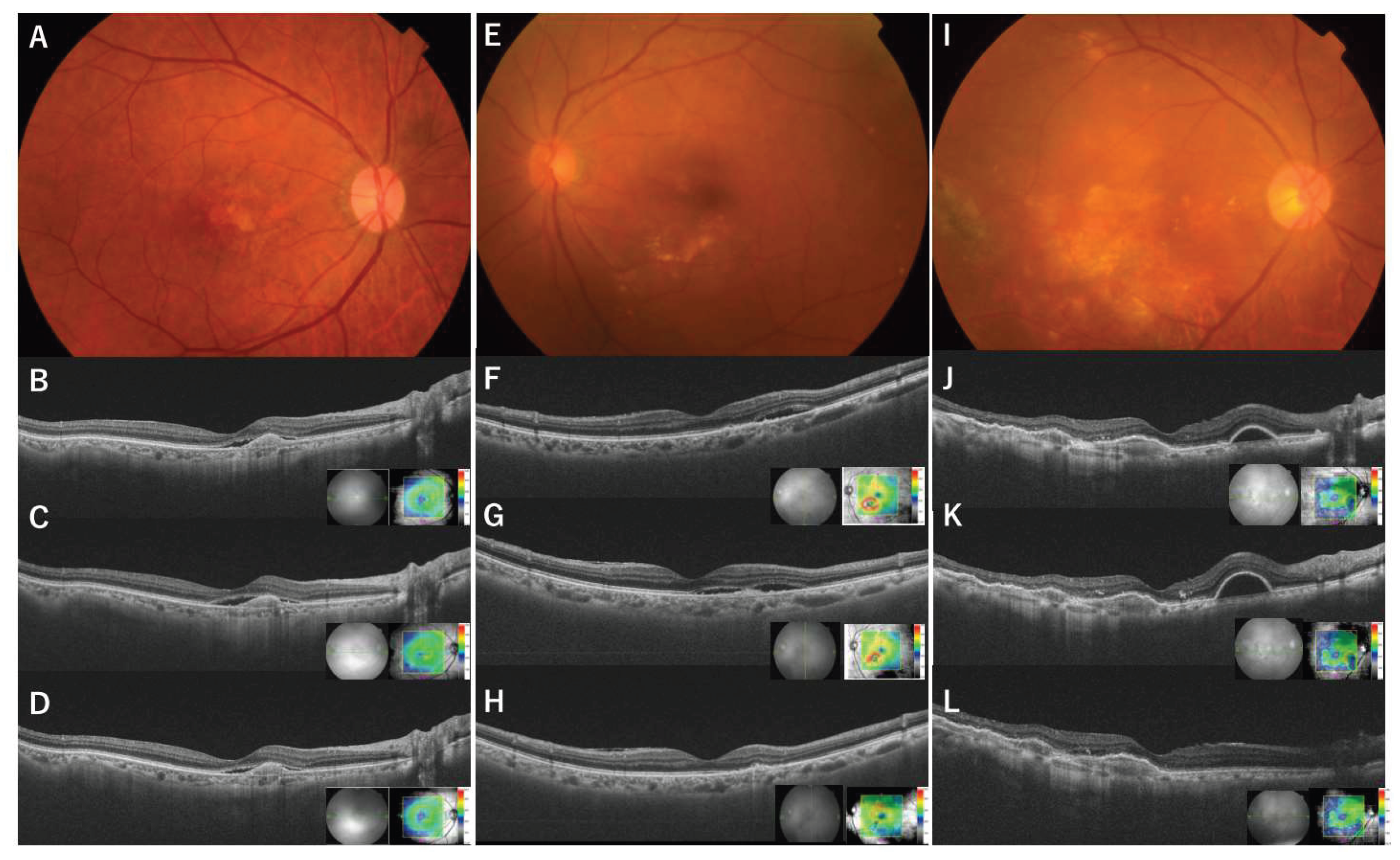
All patients underwent a comprehensive ocular examination, including the measurement of BCVA using Landolt C charts, color fundus photography, and spectral-domain and swept-source optical coherence tomography (SD-OCT; Cirrus HD-OCT, Carl Zeiss Meditec Inc., Dublin, CA, and SS-OCT; DRI-SS-OCT, Topcon Inc., Tokyo, Japan) before and after switching to aflibercept 8 mg.
The outcomes included changes in BCVA, CFT (from the internal limiting membrane and the presumed Bruch membrane at the fovea) using SD-OCT and SS-OCT, and exudative changes (SRF, IRF, and sPED within the macular cube) at the time of switching and at the first follow-up visit. Improvement, partial improvement, or no change was judged by three retinal specialists (CH, KS, and YF) based on the OCT findings.
Statistical Analysis
For statistical analyses, BCVA was converted to the logarithm of the minimum angle of resolution (logMAR). One-way analysis of variance was used to assess changes in BCVA and CRT. All statistical analyses were performed using the JMP Pro version 17 software (SAS Institute Inc., Cary, NC, USA). Statistical significance was set at p < 0.05.
3. Results
A total of 201 eyes from 196 patients with nAMD were switched to aflibercept 8 mg from other anti-VEGF drugs during the study period. One hundred and two eyes from 98 patients (61 male and 37 female) met the inclusion criteria (patients with exudative changes [SRF, IRF or sPED] within a 6 mm x 6 mm macular cube at the time of switching and no difference in dosing intervals before and after the change [within ±7 days]).
The mean age was 79.6±8.0 years. Forty-five eyes with polypoidal choroidal vasculopathy, 48 eyes with type 1 macular neovascularization (MNV), three eyes with type 2 MNV, and six eyes with type 3 MNV were included. The mean time from the first treatment to the switch to aflibercept 8 mg was 2172 ± 1467 days, and the mean number of anti-VEGF treatments during this period was 30.8±22.1 (aflibercept 2 mg: 27.8 ± 19.5, ranibizumab: 1.4 ± 4.5, faricimab: 0.9 ± 2.5, brolucizumab: 0.6 ± 2.9) treatments. Eighteen patients had a history of photodynamic therapy. Before switching to aflibercept 8 mg, 91, 5, and 6 eyes received injections of aflibercept (2 mg), faricimab, and brolucizumab, respectively. The mean interval from the intravitreal injection immediately prior to switching to the date of switching was 62.6 ± 19.8 days, and the mean interval from the date of the switching to the first visit after switching was 63.7 ± 20.0 days.
The logMAR BCVA at the time of the first aflibercept 8 mg injection was 0.27 ± 0.35,and remained unchanged at 0.27 ± 0.35 at the first visit after switching. The CFT significantly decreased from 289±115 µm to 265±114 µm. (p<0.0001)
Anatomical Outcomes
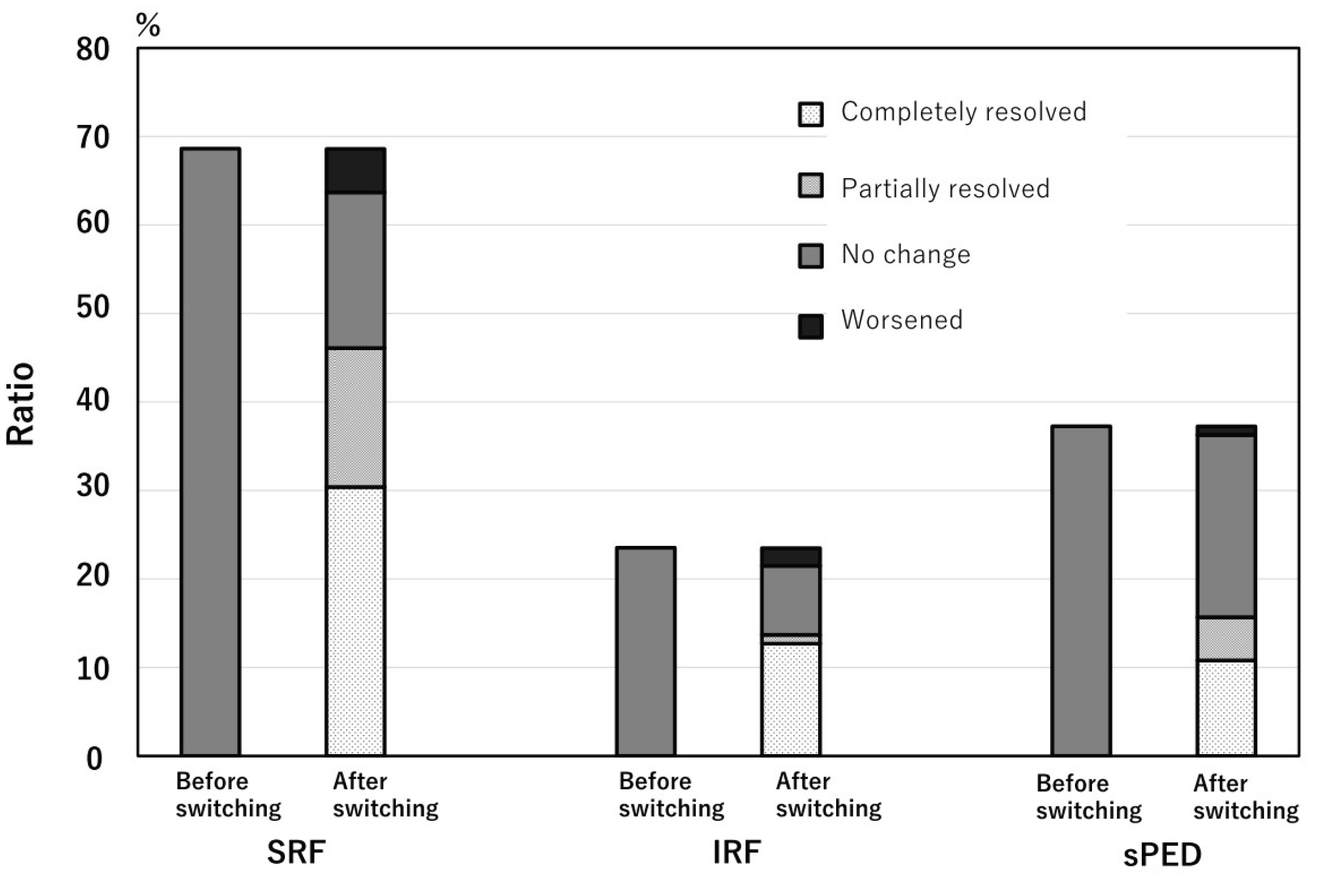
At the time of the initial aflibercept 8 mg injection, SRF was observed in 70 of the 102 eyes (68.6%), IRF was observed in 24 eyes (23.5%), and sPED was observed in 38 (37.3%) within a 6mm x 6mm macular cube. All eyes exhibited exudative changes during switching. At the first visit after switching, SRF completely resolved in 31 of 70 eyes (44%), partially resolved in 17 eyes (24%), remained unchanged in 18 eyes (25%), and worsened in 5 eyes (7%). IRF completely resolved in 13 of 24 eyes (54%), partially resolved in one eye (4%), remained unchanged in eight eyes (33%), and worsened in two eyes (8%). sPED completely resolved in 11 of 38 eyes (29%), partially resolved in five eyes (13%), remained unchanged in 21 eyes (55%), and worsened in one eye (3%). In 53 eyes (52%), all SRF- and IRF-only exudative changes completely resolved, and in 37 eyes (36%), all exudative changes, including sPED, resolved. There was a significant reduction in all types of exudative changes (SRF, IRF, and sPED) with p-values < 0.001 (chi-square test) (
Figure 1).
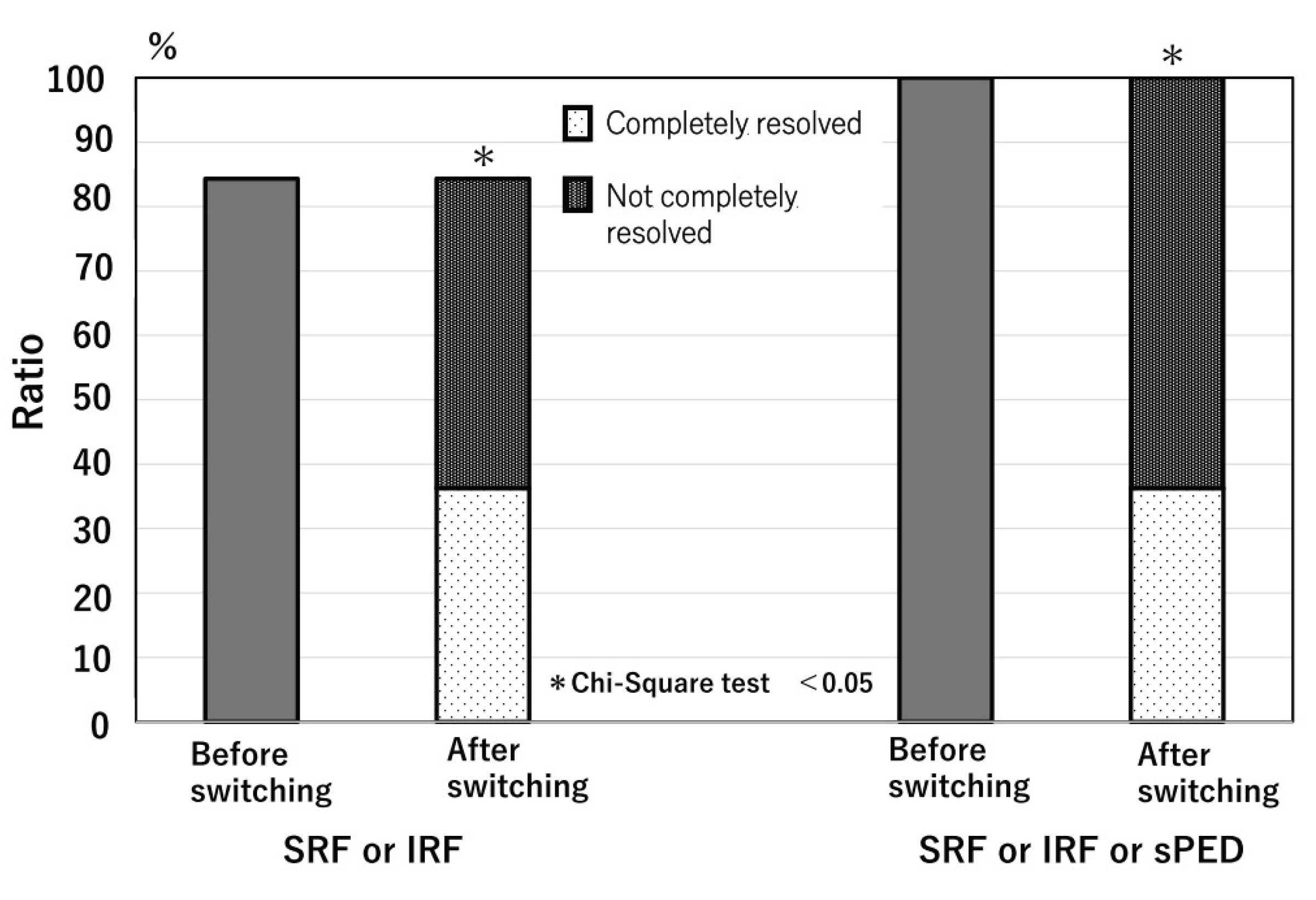
Eighty-five eyes had either SRF, IRF, or both at the time of switching, which completely resolved in 38 eyes (44%) after the switch. Complete resolution of all types of exudative changes was observed in 37 eyes (36%) (
Figure 2 and
Figure 3). In the comparison of the characteristics of cases with and without complete resolution of exudative change, the cases with complete resolution of exudative change were significantly older than those without complete resolution, both comparison of cases with either SRF or IRF or both (82.3±7.3 vs 78.1±8.2; p=0.012) and comparison of cases with all exudations including PED (81.7±7.3 vs 78.3±8.2; p=0.033). Other factors (sex, year, treatment period, number of anti-VEGF treatments, MNV type, logMAR BCVA at switching, interval between injections, drug type before switching, and history of PDT) were not significantly different between patients with and without complete resolution of the exudative change (
Table 1 and
Table 2).
Adverse Effect
Ocular and systemic adverse effects, including intraocular inflammatory findings, were not observed during the period of switching to aflibercept 8 mg.
4. Discussion
In this study, we evaluated the initial effects of switching to aflibercept 8 mg in patients with nAMD treated with other anti-VEGF drugs. Several reports have investigated the therapeutic effect of switching anti-VEGF drugs [
11,
12,
13,
14,
15]; however, it is difficult to accurately evaluate these effects for a number of reasons. For example, there are differences in terms of the observation and injection of switching drugs, and whether and how often the same drug is used (and if the effect of the switching drug is insufficient). Therefore, in the present study, to minimize bias as much as possible, the same pre- and post-dose intervals as the previous drug were used, and exudative changes after a single dose were examined.
After switching, the mean CRT significantly decreased, and exudative changes significantly improved. SRF and IRF were completely resolved in approximately half of the eyes, and PED in 30% of eyes. In the PULSAR study[
9],
9 compared to patients treated with aflibercept 2 mg, the percentage of patients treated with aflibercept 8 mg who had no exudative changes at 16 weeks (8 weeks after the loading dose) was 51.6% for 2 mg and 63.3% for 8 mg, significantly higher than for 2 mg. The effectiveness of high-molar anti-VEGF agents has been previously reported. In the MARINA and ANCHOR trials, ranibizumab 0.5 mg showed an advantage over 0.3 mg in functional and anatomical effects[
5,
16]. The HAWK trial also showed similar results, with brolucizumab 6 mg reporting better anatomical outcomes compared to 3 mg brolucizumab[
6,
17].
In the treatment of nAMD with a treat-and-extend regimen, the treatment interval cannot be extended for patients with residual exudative changes despite continuous, frequent administration of anti-VEGF drugs[
18]. Although simple comparisons cannot be made, it is expected that fewer cases of exudation will remain even after a short time. The patients in this study were predominantly those with an average dosing interval of 62.6 days (approximately 9 weeks), making it difficult to extend the dosing interval. The disappearance of exudative changes in approximately half of the cases suggests that a switch to aflibercept 8 mg may extend the dosing interval. In the ALTIR study, which examined the aflibercept 2 mg dosing interval, approximately 40% of cases were treated with the shortest interval of 8 weeks; in contrast, in the PULSAR trial, which was a phase 3 clinical trial of aflibercept 8mg, the eyes treated with 8-week intervals was about 15% of cases[
9]. Although a simple comparison between these two trials cannot be made, the use of aflibercept 8 mg is expected to reduce the number of cases with residual exudative changes after repeated short-term treatment.
In this study, all types of exudative changes significantly disappeared after aflibercept 8 mg, and SRF and IRF improved in approximately half of the cases, whereas sPED improved in approximately 30% of cases. A previous report of a large number of patients who switched from 2 mg to 8 mg reported significant improvement in SRF and IRF after three doses, but no significant difference in PED[
15]. This result is similar to the trend observed in this study, and improvement can be expected for IRF and SRF.
Additionally, this study revealed that the patients with complete resolution of exudative changes were significantly older than those without. The difference is small (81.7±7.3 vs 78.3±8.2), and the reason is unclear. However, the older patients may have less exudative changes dependent on hyperpermeability of choroidal vessels, such as pachychoroid diseases, and more VEGF-dependent exudative changes[
20,
21,
22].
In this study, no systemic or ocular adverse effects were observed. Several reports call for caution regarding intraocular inflammation after switching to aflibercept 8mg[
11,
15,
23,
24,
25]. Considering that the intraocular inflammation in these reports was also mild in all cases and that no intraocular inflammation was observed in this study, the incidence of intraocular inflammation is low, and the risk of vision loss due to switching to aflibercept 8 mg-induced intraocular inflammation is considered to be very low due to the mild nature of the disease. However, some reported cases developed intraocular inflammation after the second or third injection of aflibercept 8 mg[
23,
24], and further attention should be paid to these cases.
The main limitations of this study are its retrospective, single-center nature and short-term outcomes. Furthermore, the non-comparative nature of this study introduces a selection bias and does not control for regression toward the mean; all participants were Japanese. However, to minimize any bias in this study, such as treatment intervals or discontinuation of therapy due to inadequate efficacy, only cases with equal intervals before and after the first injection at switching to aflibercept 8 mg were included. The anatomical response after the first injection of a new drug has a significant effect on whether treatment can be continued thereafter. In this cohort of patients, switching to aflibercept 8 mg significantly reduced the presence of SRF, IRF, and PED and improved CRT after the first injection. Although further prospective studies with larger sample sizes should involve long-term outcomes, this study is meaningful in evaluating the effects of switching from 2 to 8 mg in the real world over a short period.
5. Conclusions
In conclusion, switching from other anti-VEGF drugs to aflibercept 8 mg in patients with nAMD significantly reduced exudative changes, even after a single dose, and was particularly effective against SRF and IRF.
Author Contributions
Conceptualization, C.H. and Y.Y.; methodology, C.H.; investigation, C.H.; data curation, C.H., S.F, Y.F, K.S., K.N., K.M. S.S. and T.M ; writing—original draft preparation, C.H.; writing—review and editing, Y.F, and K.S.; visualization, C.H.; supervision, K.N.; project administration, K.N.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of the Osaka University Graduate School of Medicine (approval number 10039).
Informed Consent Statement
Patient consent was waived due to the retrospective design of the study.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| nAMD |
neovascular age-related macular degeneration |
| VEGF |
vascular endothelial growth factor |
| PLGF |
placental growth factor |
| BCVA |
best-corrected visual acuity |
| CFT |
central foveal thickness |
| SRF |
subretinal fluid |
| IRF |
intraretinal fluid |
| sPED |
serous pigment epithelial detachment |
| OCT |
optical coherence tomography |
| logMAR |
logarithm of the minimum angle of resolution |
| MNV |
macular neovascularization |
References
- Klaver, C.C.; Assink, J.J.; van Leeuwen, R.; et al. Incidence and progression rates of age-related maculopathy: the Rotterdam Study. Invest Ophthal Vis Sci 2001, 42, 2237–2241. [Google Scholar] [PubMed]
- Lim, L.S.; Mitchell, P.; Seddon, J.M.; Holz, F.G.; Wong, T.Y. Age-related macular degeneration. Lancet 2012, 379, 1728–1738. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, R.; Yasuda, M.; Song, S.J.; et al. The prevalence of age-related macular degeneration in Asians: a systematic review and meta-analysis. Ophthalmology 2010, 117, 921–927. [Google Scholar] [CrossRef]
- Fleckenstein, M.; Schmitz-Valckenberg, S.; Chakravarthy, U. Age-Related Macular Degeneration: A Review. Jama 2024, 331, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, P.J.; Brown, D.M.; Heier, J.S.; et al. Ranibizumab for neovascular age-related macular degeneration. NEJM 2006, 355, 1419–1431. [Google Scholar] [CrossRef]
- Dugel, P.U.; Singh, R.P.; Koh, A.; et al. HAWK and HARRIER: Ninety-Six-Week Outcomes from the Phase 3 Trials of Brolucizumab for Neovascular Age-Related Macular Degeneration. Ophthalmology 2021, 128, 89–99. [Google Scholar] [CrossRef]
- Heier, J.S.; Khanani, A.M.; Quezada Ruiz, C.; et al. Efficacy, durability, and safety of intravitreal faricimab up to every 16 weeks for neovascular age-related macular degeneration (TENAYA and LUCERNE): two randomised, double-masked, phase 3, non-inferiority trials. Lancet 2022, 399, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, N.; Martin, J.; Ruan, Q.; et al. Binding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF Trap, ranibizumab and bevacizumab. Angiogenesis 2012, 15, 171–185. [Google Scholar] [CrossRef]
- Lanzetta, P.; Korobelnik, J.F.; Heier, J.S.; et al. Intravitreal aflibercept 8 mg in neovascular age-related macular degeneration (PULSAR): 48-week results from a randomised, double-masked, non-inferiority, phase 3 trial. Lancet 2024, 403, 1141–1152. [Google Scholar] [CrossRef]
- Sim, S.Y.; Chalkiadaki, E.; Koutsocheras, G.; et al. Real-World 1-Year Outcomes of Treatment-Intensive Neovascular Age-Related Macular Degeneration Switched to Faricimab. Ophthalmology Retina 2025, 9, 22–30. [Google Scholar] [CrossRef]
- Momenaei, B.; Yonekawa, Y.; Abril, P.; McCullough, R.; Abbey, A.M. Outcomes of Intravitreal Aflibercept 8 mg in Eyes With Neovascular Age-Related Macular Degeneration Previously Treated With Faricimab. OSLI 2025, 56, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Arcinue, C.A.; Ma, F.; Barteselli, G.; Sharpsten, L.; Gomez, M.L.; Freeman, W.R. One-year outcomes of aflibercept in recurrent or persistent neovascular age-related macular degeneration. Am J Ophthalmol 2015, 159, 426–436 e422. [Google Scholar] [CrossRef] [PubMed]
- Heussen, F.M.; Shao, Q.; Ouyang, Y.; Joussen, A.M.; Muller, B. Clinical outcomes after switching treatment from intravitreal ranibizumab to aflibercept in neovascular age-related macular degeneration. Graefe Arch Clin Exp Ophthalmol 2014, 252, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, K.; Itagaki, K.; Hashiya, N.; et al. Six-month outcomes of switching from aflibercept to faricimab in refractory cases of neovascular age-related macular degeneration. Graefe Arch Clin Exp Ophthalmol 2024, 262, 43–51. [Google Scholar] [CrossRef]
- Bala, S.; Barbosa, G.C.S.; Mohan, N.; et al. Initial Functional and Anatomical Outcomes of High-dose Aflibercept 8 mg in Exudative Neovascular Age-related Macular Degeneration. Ophthalmol. Retina 2025, 7, s2468–s6530. [Google Scholar] [CrossRef]
- Brown, D.M.; Kaiser, P.K.; Michels, M.; et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. NEJM 2006, 355, 1432–1444. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, X.; Gong, Y.; et al. Double-dose investigation of aflibercept in neovascular age-related macular degeneration (DIANA): a real-world study. BMC ophthalmol 2024, 24, 215. [Google Scholar] [CrossRef]
- Freund, K.B.; Korobelnik, J.F.; Devenyi, R.; et al. TREAT-AND-EXTEND REGIMENS WITH ANTI-VEGF AGENTS IN RETINAL DISEASES: A Literature Review and Consensus Recommendations. Retina 2015, 35, 1489–1506. [Google Scholar] [CrossRef] [PubMed]
- Ohji, M.; Takahashi, K.; Okada, A.A.; Kobayashi, M.; Matsuda, Y.; Terano, Y. Efficacy and Safety of Intravitreal Aflibercept Treat-and-Extend Regimens in Exudative Age-Related Macular Degeneration: 52- and 96-Week Findings from ALTAIR : A Randomized Controlled Trial. Adv Ther 2020, 37, 1173–1187. [Google Scholar] [CrossRef]
- Hata, M.; Yamashiro, K.; Ooto, S.; et al. Intraocular Vascular Endothelial Growth Factor Levels in Pachychoroid Neovasculopathy and Neovascular Age-Related Macular Degeneration. Inves Ophthal Vis Sci 2017, 58, 292–298. [Google Scholar] [CrossRef]
- Inoda, S.; Takahashi, H.; Inoue, Y.; et al. Cytokine profiles of macular neovascularization in the elderly based on a classification from a pachychoroid/drusen perspective. Graefe Arch Clin Exp Ophthalmol 2022, 260, 747–758. [Google Scholar] [CrossRef] [PubMed]
- Terao, N.; Koizumi, H.; Kojima, K.; et al. Distinct Aqueous Humour Cytokine Profiles of Patients with Pachychoroid Neovasculopathy and Neovascular Age-related Macular Degeneration. Sci Rep 2018, 8, 10520. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Hoshino, J.; Numaga, S.; Mimura, K.; Asatori, Y.; Akiyama, H. Retinal vasculitis after intravitreal aflibercept 8 mg for neovascular age-related macular degeneration. Jpn J Ophthal 2024, 68, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, L.; Michels, S.; Eandi, C.; Karam, M.A.; Figueiredo, E.C.O.; Hatz, K. Aflibercept high-dose (8mg) related intraocular inflammation (IOI) - a case series. BMC ophthalmol 2024, 24, 520. [Google Scholar] [CrossRef]
- Sisk, R.A. Occlusive Retinal Vasculitis After Aflibercept 8mg Injection for Wet Macular Degeneration. Retin Cases brief Rep 2025. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).