1. Introduction
The human ear is a biological and evolutionary masterpiece, responsible for converting sound vibrations into electrical signals that the brain interprets as sound. The central structure of the auditory system is the cochlea of the inner ear (
Figure 1A), a spiral-shaped organ designed for exquisite sensitivity to sound intensity and frequency discrimination [
1]. Maintaining the delicate balance of the cochlear environment, known as cochlear homeostasis, is critical for optimal auditory function. Yet, disruptions in this equilibrium are key to sensorineural hearing loss, affecting millions worldwide.
Sensorineural hearing loss (SNHL) encompasses a range of pathologies in which the cochlear sensory hair cells, supporting structures, and synaptic connections to the auditory nerve are compromised [
2]. Unlike conductive hearing loss, which impedes sound transmission through the outer or middle ear, SNHL is caused by cellular and molecular alterations in the cochlea due to altered homeostasis.
The term “electrochemical homeostasis” refers to the maintenance of ion gradients in the cochlea, specifically potassium (K⁺) and sodium (Na
+) levels, and the regulation of fluid composition, particularly the differential ionic makeup of the endolymph and perilymph [
3]. Endolymph, which fills the scala media, is rich in K⁺ and low in Na
+. In contrast, the perilymph contained in the scala tympani and scala vestibuli is a typical extracellular fluid, characterised by high Na and low K⁺ levels. This electrochemical separation facilitates rapid transduction of mechanical stimuli into receptor potentials and, consequently, the generation of nerve impulses in the auditory nerve.
Even subtle deviations in ionic composition or fluid balance can have a profound impact on hair cell function. Multiple factors, including genetic predisposition, ageing, noise exposure, ototoxic drug use, and metabolic disturbances, can perturb this delicate balance. The dysregulation of cochlear homeostasis ultimately results in cell damage, apoptosis, and permanent SNHL [
4,
5].
This review first delves into the cellular and molecular mechanisms that maintain homeostasis within the cochlea. We will then explore how disruptions in these mechanisms give rise to SNHL and discuss the complex interplay between environmental insults and the cochlear response to stress and injury. Finally, we will consider promising therapeutic interventions, from pharmacological and gene therapy to regenerative strategies to restore the cochlear environment and rescue function. By comparing cochlear homeostasis in health and disease, we aim to review the latest research on the molecular mechanisms and treatments for SNHL, from basic science to clinical innovation.
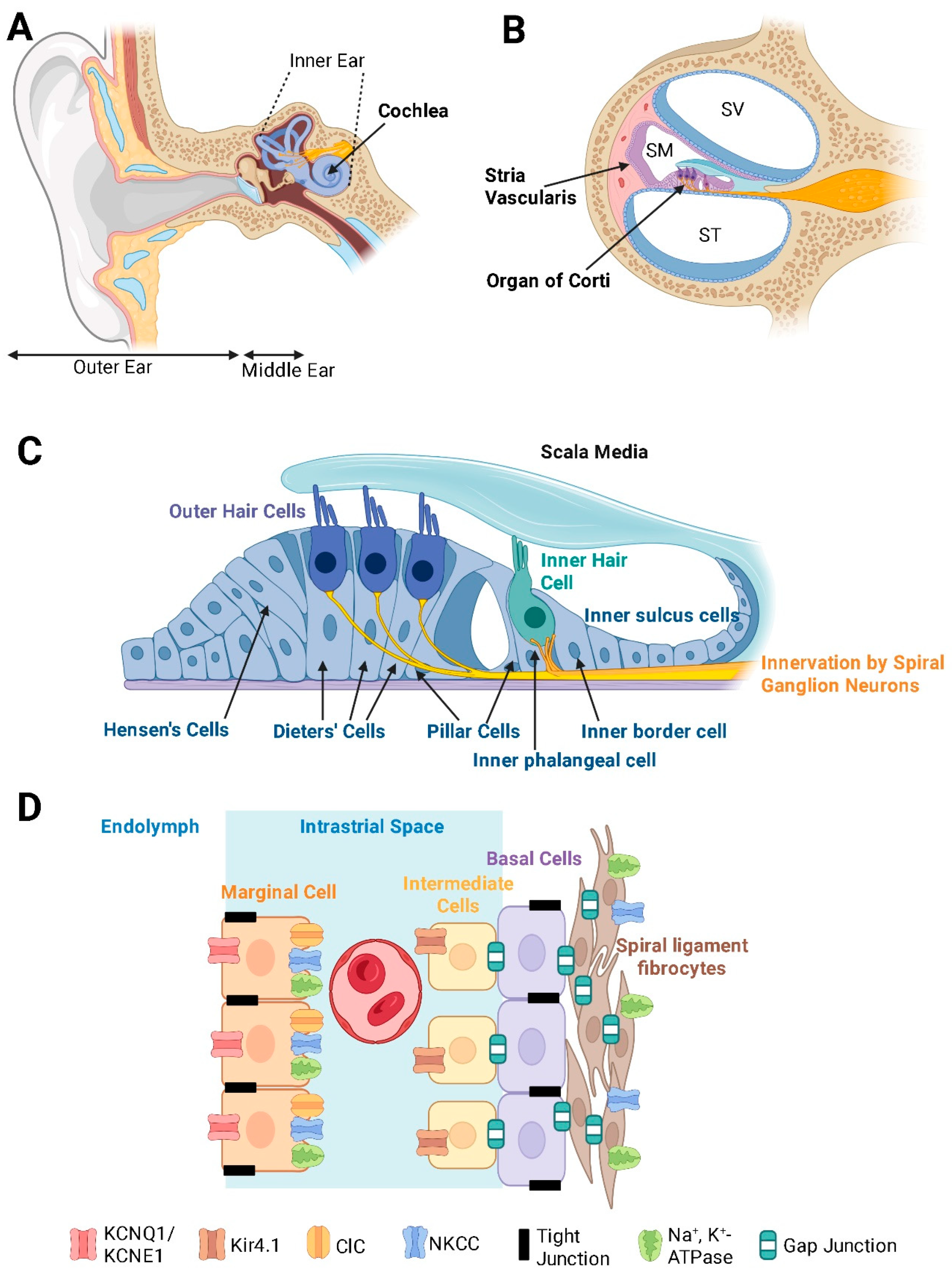
2. Structural Organisation of the Cochlea
The bony labyrinth of the cochlea contains three fluid-filled compartments: the scala vestibuli, scala media, and scala tympani (
Figure 1B). The scala media houses endolymph, a K⁺-rich fluid that fills the space around the apical surfaces of the sensory hair cells and supporting cells in the organ of Corti [
1]. In contrast, the scala vestibuli and scala tympani contain perilymph, which has an ionic composition similar to cerebrospinal fluid with high Na⁺ and low K⁺ levels. The distinct ionic compositions between these fluids underlie the endocochlear potential (EP), a key driving force for sensory transduction [
6].
The organ of Corti contains organised rows of inner and outer hair cells, each hair cell type serving specific roles in sound detection and amplification (
Figure 1C). Inner hair cells are the primary sensory cells sending sound information to the brain, whereas outer hair cells act as biological amplifiers by mechanically enhancing sound vibrations of the basilar membrane through electromotility [
7,
8].
The delicate structure of the organ of Corti is further upheld by a network of supporting cells, including Deiters’ cells, pillar cells, and Hensen’s cells [
1]. These cells provide both mechanical reinforcement and metabolic support to the sensory hair cells. They also contribute to K⁺ recycling, thus maintaining the consistent ionic concentration in endolymph necessary for sensory transduction [
9].
The interplay between the hair cells, supporting cells, and the stria vascularis is central to maintaining the balance of cochlear homeostasis. Any disruption from physical or metabolic stress can have progressive effects on the overall function of the auditory system.
2.1. The Critical Role of the Stria Vascularis in Maintaining Cochlear Electrochemical Homeostasis
The stria vascularis is one of the most metabolically active structures in the cochlea [
10]. This highly vascularised tissue ensures a constant supply of oxygen and nutrients to the inner ear to meet its substantial energy demands. The stria vascularis consists of three main cell types: marginal cells (facing the endolymph), intermediate cells, and basal cells (facing the spiral ligament) (
Figure 1D). Tight junction proteins within the marginal and basal cells of the stria vascularis are essential for maintaining the separation of endolymph from perilymph and preserving the distinct ionic environment in the stria vascularis required for K
+ secretion [
11].
Stria vascularis generates a small positive charge (the EP) in the endolymph by secreting K⁺ against a steep concentration gradient. This electrical potential is measured in the order of +80-100 mV [
6], and combined with the polarised hair cells, results in the rapid influx of K⁺ into hair cells during sensory transduction [
3,
12]. This specialised transduction is initiated by the hair cell mechanoelectrical transduction (MET) channels, which open when stereocilia are deflected by sound-induced fluid vibrations. This facilitates a rapid influx of K⁺ ions, depolarising the hair cells, opening of voltage-gated Ca
2+ channels, and triggering Ca
2+-dependent neurotransmitter (glutamate) release into the synaptic cleft. This electrical activity initiates action potentials in the spiral ganglion neurons, the primary afferent neurons in the cochlea [
13]. The generation of the EP by the stria vascularis incurs a high metabolic cost. Consequently, any compromise in strial function, such as vascular insufficiency or metabolic dysregulation, can lead to a collapse of the electrochemical gradients critical for sensory transduction [
14]. Age-related changes in strial metabolism associated with reduced vascularisation, chronic inflammation, and mitochondrial DNA damage can be important contributors to SNHL in older populations [
10,
15].
2.2. Ion Transport and K+ Recycling
The maintenance of a K⁺ gradient is a cornerstone of cochlear homeostasis [
3]. Once K⁺ ions enter the hair cells through MET channels, they must be efficiently recycled to sustain the EP and prevent their toxic accumulation around the basolateral surfaces of sensory and supporting cells. To enable this, the supporting cells in the organ of Corti, the fibrocytes in the spiral ligament and spiral limbus, the mesenchymal cells lining the scala vestibuli and the marginal and intermediate cells of the stria vascularis form an extensive intercellular communication network mediated by gap junctions. This network, comprising connexin hemichannels such as connexin 26 and connexin 30, creates a system for the rapid ion transport between cells, which ultimately facilitates a K⁺ recycling loop that redistributes the K
+ ions back to the stria vascularis [
16].
Ion pumps and channels located in the stria vascularis complement the function of these gap junctions in the K⁺ recycling loop (
Figure 1D). Intermediate cells express inwardly rectifying K⁺ channels, such as Kir4.1 (KCNJ10) [
17,
18]. The Kir4.1 channels are critical for the EP generation because they allow K⁺ to diffuse from intermediate cells into the intrastrial space. The Na⁺/K⁺-ATPase pump on the basolateral side of marginal cells, which uses intracellular ATP to drive the active transport of ions, is crucial for maintaining the high K
+ concentration inside the marginal cells of the stria vascularis [
3]. The Na⁺/K⁺/2Cl⁻ cotransporter (NKCC1) facilitates the further uptake of ions from the intrastrial space, consolidating the buildup of K⁺ in marginal cells [
19]. Potassium KCNQ1/KCNE1 channels on the apical membranes of marginal cells assist in establishing a pathway for K⁺ diffusion from marginal cells to endolymph [
3]. Mutations in either of these two subunits cause Jervell-Lange-Nielsen syndrome in humans [
20] characterised by hearing loss and cardiac arrhythmia. Precise regulation of these ion channels and transporters and efficient transport by gap junctions ensures the K
+ concentration gradient generated by active ion transport is maintained, which is an integral requirement for sensory transduction.
3. Adaptive Mechanisms in the Cochlea to Maintain Homeostasis
When exposed to cellular stress, the cochlea mounts compensatory responses aimed at restoring homeostasis. Upregulation of protective proteins, increased expression of antioxidant enzymes, and the activation of signalling pathways that promote cell survival are all part of the defence mechanism [
21,
22]. However, these compensatory mechanisms have limited capacity. When the intensity or duration of the insult exceeds these protective limits, compensatory responses become overwhelmed, leading to irreversible cellular damage. For example, long-lasting or repeated noise exposure progressively diminishes the cochlea’s capacity for repair, eventually culminating in widespread hair cell loss and permanent sensorineural hearing impairment [
23].
3.1. Adaptive Mechanisms Against Oxidative Stress
One of the leading causes of the disruption of cochlear homeostasis is oxidative stress. Noise trauma, ageing, and ototoxic drugs are all known to generate elevated levels of reactive oxygen species (ROS), which can damage cellular membranes, proteins, and DNA in the cochlea [
24]. Tissues with high metabolic demands, such as the stria vascularis and the outer hair cells, are particularly vulnerable to the damaging effects of ROS-induced oxidative stress [
25]. Excessive production of mitochondrial ROS causes oxidative damage to key mitochondrial components, such as mitochondrial DNA (mtDNA), mitochondrial membranes, and respiratory chain proteins, resulting in mtDNA mutations, lipid peroxidation, and protein oxidation [
25,
26]. The resultant oxidative damage can compromise the ion channels and pumps critical for maintaining the ion composition in endolymph, as previously demonstrated in the ageing brain [
27]. In addition, excessive ROS production leads to calcium influx into sensory hair cells, which in turn causes a surplus of glutamate release and excitotoxicity [
28].
Interestingly, there are also intrinsic protective mechanisms at play in the cochlea. The expression levels of several endogenous antioxidant enzymes (e.g., glutathione peroxidases and reductase, superoxide dismutase, catalase) increase in response to metabolic stress, noise exposure, or ototoxic drugs [
29]. These enzymes can attenuate the damaging effects of ROS and mitigate oxidative stress, a major cause of hair cell death.
3.2. Signalling Pathways Activated by Stress and Injury in the Cochlea
Specialised regulatory proteins and signal transduction pathways within the cochlea collaborate to sustain cellular viability and functionality. Mitogen-activated protein kinase (MAPK), phosphoinositide-3 kinase/protein kinase B (PI3K/Akt), Notch/Wnt/Atoh1, calcium channels, and ROS signalling pathways regulate the development and survival of auditory hair cells in response to environmental and metabolic challenges [
21]. Dysregulation in these signalling pathways can tip the balance from cell repair to cell death, contributing to SNHL [
21,
30].
Another receptor-mediated signalling pathway activated under stress is purinergic signalling via ATP-gated ion channels (P2X receptors) [
31]. The exposure to moderate noise levels, causing temporary threshold shift, elevates extracellular ATP in the endolymph and activates P2X2 receptors in the cochlear partition. The activation of P2X2 receptors provides a K
+ shunt conductance away from the endolymphatic compartment, which reduces the driving force for sound transduction and, consequently, hearing sensitivity in mice [
32]. This mechanism may contribute to protective hearing adaptation with sustained elevated sound levels that can cause permanent hearing loss [
5].
Adenosine receptor signalling is also a potent regulator of cochlear response to stress. Activation of adenosine A
1 receptors can mitigate cochlear injury caused by acoustic overexposure and ototoxic drugs (cisplatin and aminoglycosides) [
31,
33]. Similarly, inhibition of adenosine A
2A receptors can mitigate excitotoxic injury in organotypic tissue culture of the rat cochlea [
34] and delay the progression of age-related hearing loss in mice [
35]. The balance between A
1 and A
2A receptors appears critical for cochlear response to stress and injury [
31].
Furthermore, key growth factors, including brain-derived neurotrophic factor (BDNF) and neurotrophin-3 (NT-3), released from sensory and supporting cells of the organ of Corti, are involved in cellular repair processes within the cochlea [
2]. Administration of exogenous neurotrophins promotes synaptic regeneration of the ribbon synapses in sensory hair cells and enables hearing rescue in mice and guinea pigs following acoustic trauma [
36,
37]
3.3. Epigenetic Modifications and Homeostatic Regulation by MicroRNAs
Recent studies suggest that epigenetic factors, such as DNA methylation and histone modification, might play a crucial role in determining the susceptibility of cochlear cells to oxidative and metabolic stress [
38]. These epigenetic modifications can influence the differentiation, development, and protection of sensory hair cells in the cochlea, potentially leading to hair cell degeneration and hearing loss [
39,
40]. Manipulating the epigenetic status could be a tool to regulate the expression levels of critical protective proteins and ion channels, and may even alter the limited regenerative capacity of cochlear cells [
38,
40].
Other studies have shown that microRNAs (miRNAs), a class of short non-coding RNAs that regulate the expression of mRNA and protein targets, are important regulators of cellular senescence and ageing [
41]. These studies have raised the possibility that miRNAs are responsible for fine-tuning the expression of genes involved in protective and degenerative processes in the cochlea [
42]. The identification of hundreds of miRNAs in the auditory system and a better understanding of the function of many of these miRNAs hold promise for their use as inner ear therapeutics [
43,
44,
45].
3.4. The Cochlear Microenvironment, Intercellular Communication and Systemic Health
The cochlea is not an isolated system; it operates in concert with systemic physiological processes such as blood flow, immune regulation, and systemic metabolism. Intact and healthy vasculature and blood flow are critical for cochlear homeostasis [
46], and mounting evidence shows that the disruption to the blood-labyrinth barrier (BLB) function leads to hearing loss in models of ototoxic [
47], genetic [
48], and systemic diseases [
49]. Increased attention is now being focused on understanding how systemic factors, such as blood circulation, influence BLB and cochlear homeostasis. For instance, conditions like diabetes and hypertension are known to impair vascular supply and thereby disrupt the function of the stria vascularis [
50]. In addition, there is strong evidence that the cochlea is vulnerable to systemic inflammation [
51]. Such biomedical evidence of increased prevalence of hearing loss associated with poor health (e.g., diabetes-related) aligns with epidemiological observations in the human population [
52]. Therefore, therapeutic strategies that improve systemic health may have a beneficial impact on cochlear homeostasis and resilience to stress.
Intercellular communication via extracellular vesicles, such as exosomes, is another potential area of investigation. In other systems, these vesicles mediate communication between cells by transferring small RNAs, proteins, and other bioactive molecules [
53]. In the cochlea, heat stress stimulates exosome release, and these vesicles promote the survival of hair cells under ototoxic stress [
54]. The exosome signalling between hair cells, supporting cells, and immune cells may constitute a vital component of the adaptive response to maintain the homeostatic regulatory network in the cochlea [
55]. A better understanding of the exosome vesicular transport in the cochlea may lead to the use of exosomes containing therapeutic molecules (e.g., miRNA-21) [
56]. The research focusing on cellular distribution and trafficking of exosome vesicles could eventually lead to innovative biological therapies to restore cellular function in the cochlea.
4. Sensorineural Hearing Loss: Causes and Cellular Impact
SNHL is the most common form of permanent hearing loss arising from the loss of sensory hair cells, synaptic connections between sensory hair cells and the afferent terminals of the spiral ganglion neurons, with ultimate loss of primary auditory neurons in the spiral ganglion [
57]. Dysfunction of the central auditory pathways can also play an independent role in SNHL [
30]. SNHL manifests clinically as muffled hearing, reduced perception of high-pitched sounds, and difficulties with speech discrimination, particularly in noisy environments [
58]. The deterioration in auditory perception is typically irreversible because the sensory hair cells of the cochlea have a very limited capacity for regeneration in mammals. Hearing loss can range from mild to profound and is often accompanied by tinnitus, the perception of phantom sounds, further disrupting the quality of life for affected individuals.
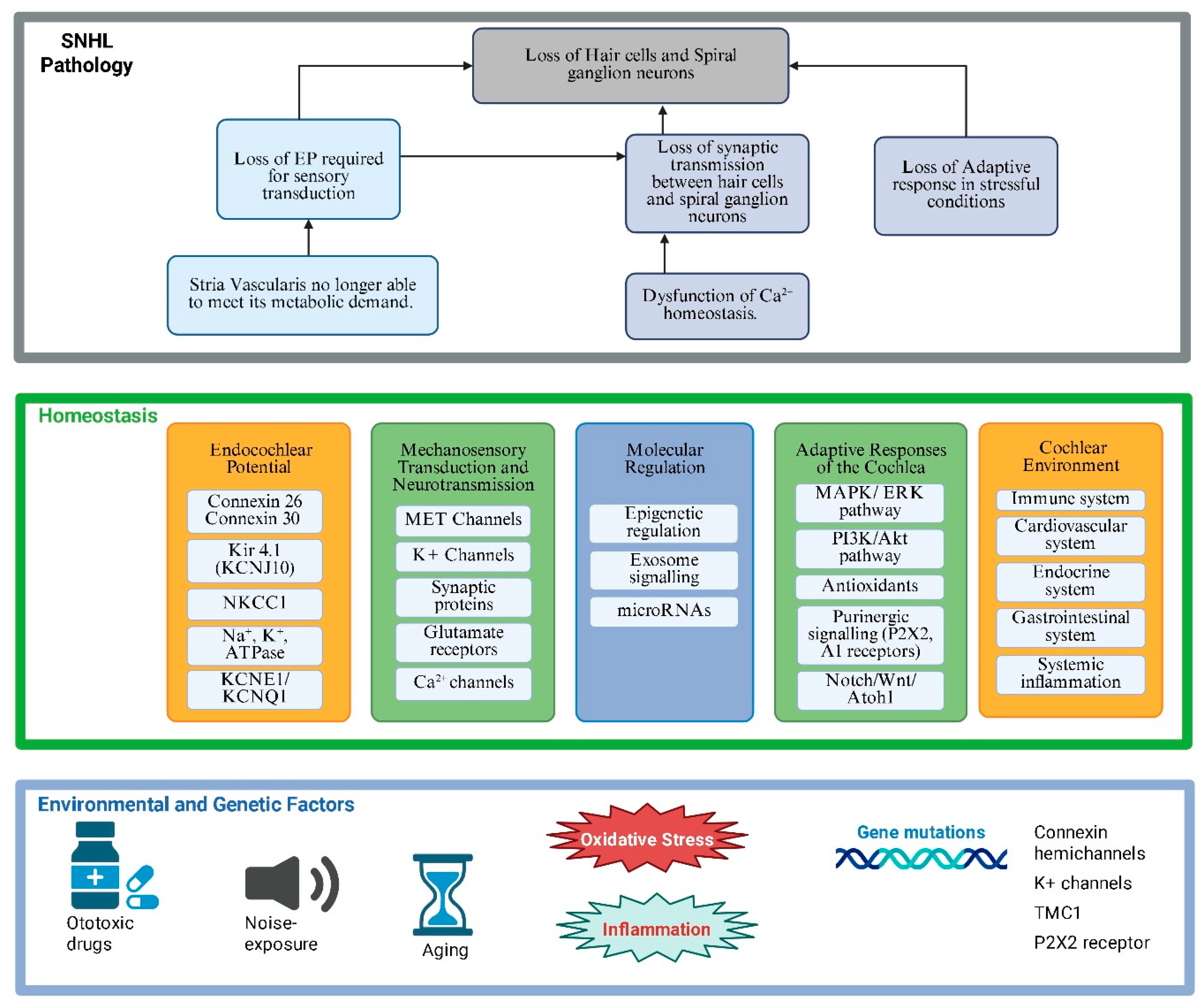
Multiple factors contribute to the onset of SNHL (
Figure 2). Genetic mutations affecting connexin proteins, ion channel function, or other critical regulatory molecules can predispose individuals to early-onset SNHL [
59]. Age-related hearing loss is linked to genetic risk factors but is also influenced by a lifelong cumulative effect of environmental stressors, such as noise, otological diseases, or ototoxic drugs [
15]. Acquired forms of SNHL may result from chronic noise exposure, common in industrialised and some recreational environments. Ototoxic medications, particularly aminoglycoside antibiotics and chemotherapeutic agents such as cisplatin, can also induce hair cell damage by directly activating intracellular pathways leading to apoptosis [
60,
61]. New evidence suggests that macrophage-induced inflammation also contributes to cellular damage following cisplatin treatment [
62]. Systemic conditions like diabetes, hypertension, and mitochondrial disorders have been linked to vascular or metabolic disruptions within the inner ear [
63,
64], further highlighting the critical role of cochlear homeostasis in the maintenance of normal auditory function.
4.1. Cellular Impact of Disruption in Homeostasis in Sensorineural Hearing Loss
Understanding how cochlear homeostasis is disrupted by environmental, metabolic or genetic factors provides critical insights into the pathophysiology of SNHL. When the cochlear homeostasis is disturbed, the resulting cascade of events precipitates cellular injury and permanent loss of function. The sensory hair cells, supporting cells, and secretory epithelial cells of the stria vascularis are extremely sensitive to disturbances in their microenvironment [
65,
66]. Numerous experimental models have highlighted the link between cochlear disorders and impaired homeostatic mechanisms. For example, in studies where the expression of gap junction proteins was genetically disrupted, a concomitant decrease in K
+ recycling efficiency was observed [
67]. In parallel, animal models subjected to oxidative stress exhibited significant dysregulation of ion pumps and various signalling pathways [
68], further highlighting the vulnerability of cochlear homeostasis in the face of environmental and metabolic challenges.
4.2. Metabolic Stress, Oxidative Damage, and Apoptosis
The cochlea is particularly vulnerable to energy deficits and oxidative stress due to its high metabolic demands. For instance, the temporary loss of blood supply to the cochlea may underlie the sudden onset SNHL due to energy deficits [
69]. Reduced vascularisation in the cochlea, cumulative oxidative stress, and low-grade cochlear inflammation play critical roles in the development of age-related hearing loss [
70]. Noise exposure, ischemia, and ototoxic drugs can all dramatically increase ROS production, overwhelming the cochlear antioxidant defences [
71]. Oxidative stress can undermine the functional integrity of membrane proteins vital for ion transport and signal transduction [
4]. These insults precipitate a cascade of intracellular events that may result in cell death by apoptosis or necrosis. Once hair cells are lost, the damage is irreversible, leading to permanent SNHL. Furthermore, damage to supporting cells can hinder the essential recycling of ions necessary for maintaining the EP, thereby amplifying the degenerative process [
72].
4.3. Immune Activation and Inflammation in the Cochlea
Inflammatory processes represent another critical component leading to the breakdown of cochlear homeostasis. Following cochlear injury by noise, drugs, or other insults, immune mediators such as cytokines and chemokines are released locally from infiltrating immune cells, such as macrophages [
73,
74,
75]. Macrophages are the primary innate immune cells in the cochlea, and they play important roles in cochlear immune homeostasis, repair, and pathology [
76]. There are several macrophage phenotypes present in the human cochlea from early in human inner ear development through to adulthood [
77,
78], where they have probable roles in cochlear homeostasis, trophic support and patterning, in addition to their well-characterised immune functions [
78]. The cochlea also contains resident macrophages in various cochlear regions, such as the basilar membrane, osseous spiral lamina, spiral ganglion, spiral ligament, and stria vascularis [
79,
80]. The inflammatory response involves the release and upregulation of inflammatory molecules from macrophages and other immune cells, including cytokines like IL-1β, TNF-α, TGF-β, and IL-10, chemokines like CX3CR1 and adhesion molecules (ICAM-1) [
81,
82]. Cytokines are signalling molecules that influence the activity of other immune cells and can either promote or resolve inflammation, whereas chemokines attract immune cells to the site of injury or inflammation [
83]. While these inflammatory responses may initially function in a protective capacity by mobilising repair mechanisms, chronic inflammation can exacerbate cellular dysfunction and damage and accelerate hearing deterioration [
81,
84]. The chronic activation of inflammatory cascades has been implicated in animal models of noise-induced, drug-induced, and age-related hearing loss [
15,
61,
62,
83]. Control of such inflammatory responses is emerging as a promising target for therapeutic interventions [
83,
84].
4.4. The Interplay between Genetic and Environmental Factors
While environmental factors, such as noise or exposure to ototoxic compounds, play a significant role in disrupting cochlear homeostasis, genetic predispositions can increase vulnerability to damage. Of over 150 genes identified to be associated with human SNHL, mutations in genes encoding ion channels (e.g.
KCNQ1, KCNQ4, TMC1, P2RX2, KCNJ10) [
85,
86,
87,
88], connexin hemichannels (e.g.
GJB2, GJB6) [
89,
90] or synaptic proteins (
OTOF) [
91] have also been associated with hereditary forms of SNHL. In individuals harbouring such mutations, even minor environmental insults may lead to a disproportionate collapse of cochlear homeostasis. For example, a mild genetic defect in ion channel function may maintain near-normal hearing in an affected individual until a significant noise insult pushes the system beyond its compensatory capacity [
92]. The disruption of cochlear homeostasis may thus lead to permanent damage of sensorineural tissues due to the combined effects of multiple environmental and genetic factors (
Figure 2).
Figure 2.
Sensorineural pathology and hearing loss resulting from dysfunctional cochlear homeostasis. This schematic diagram illustrates the essential elements of normal cochlear homeostasis (middle panel) and the environmental and genetic factors that can overwhelm adaptive responses in the cochlea, ultimately leading to hearing loss (bottom panel). Created with BioRender.com.
Figure 2.
Sensorineural pathology and hearing loss resulting from dysfunctional cochlear homeostasis. This schematic diagram illustrates the essential elements of normal cochlear homeostasis (middle panel) and the environmental and genetic factors that can overwhelm adaptive responses in the cochlea, ultimately leading to hearing loss (bottom panel). Created with BioRender.com.
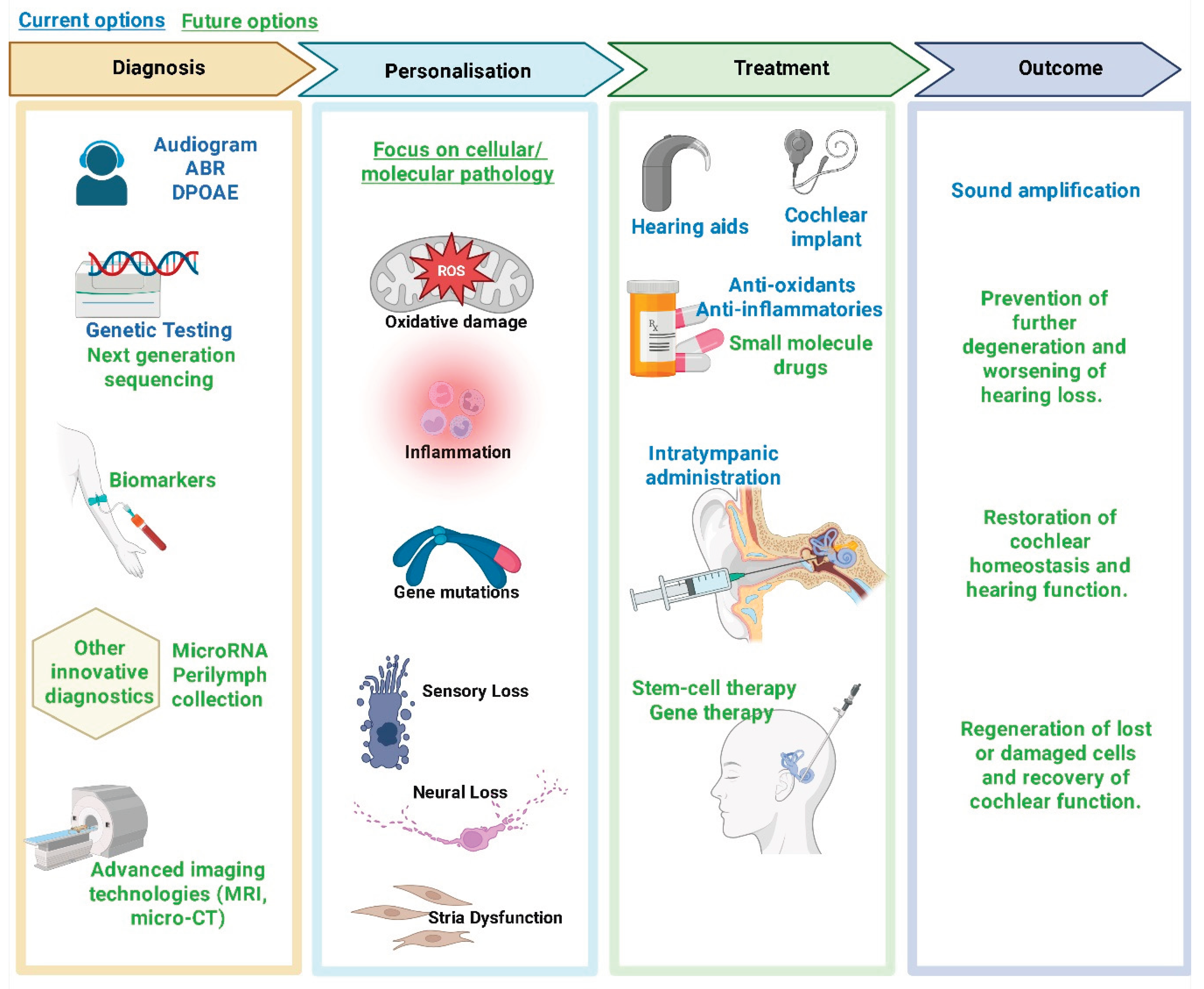
5. Current Research and Therapeutic Approaches
In response to the growing burden of SNHL, it is more important than ever to advance therapeutic strategies that target the fundamental cause of failed cochlear homeostasis, rather than treating the symptoms of dysfunction. The complexity of the cochlear microenvironment and the heterogeneous pathogenesis of sensorineural cochlear damage have prompted a range of innovative strategies aimed at maintaining cochlear homeostasis. These range from pharmacological interventions to gene therapy, cellular regeneration, cell replacement, and advanced biomolecular-bioengineering approaches.
5.1. Antioxidant and Anti-Inflammatory Therapies
Given the central role of oxidative stress in disrupting cochlear homeostasis, pharmacological strategies targeting ROS and promoting mitochondrial health have gained momentum. Antioxidants have been investigated for their ability to mitigate noise-, drug and age-related oxidative damage damage [
71,
93,
94]. Compounds that support mitochondrial function have also been tested in preclinical models with encouraging outcomes [
95,
96]. By stabilising mitochondrial membranes and reducing the production of ROS, these agents help preserve the delicate balance of ion transport and metabolic activity within the cochlea.
Inhibition of specific inflammatory mediators is also being explored to mitigate the adverse effects of chronic inflammation in the cochlea [
83,
97]. Several clinical trials assessed whether antioxidant and anti-inflammatory interventions can protect at-risk populations from progressive hearing loss, such as individuals chronically exposed to noise or those undergoing cisplatin chemotherapy [
98]. For example, the antioxidant sodium thiosulfate (STS) has been clinically approved to protect paediatric patients from cisplatin-induced hearing loss [
99]. While preclinical and limited clinical data suggest STS may also be effective in adults, more studies are needed to confirm its safety and efficacy in the adult population [
100]. This underscores the need to understand the entire range of perturbations to cochlear homeostasis in order to triage and stratify patient populations into trials that are most likely to result in improved function.
5.2. Gene Therapy to Restore Molecular Equilibrium
Gene therapy holds promise for correcting the genetic defects that underlie many forms of congenital SNHL, where the causal genes have been identified [
101]. Viral vectors such as adeno-associated viruses (AAV) are being optimised for delivery to the cochlea, targeting specific cell types [
102]. Therapeutic vectors can be delivered directly into the cochlear fluid space, allowing for even distribution in animal models [
102]. Innovative delivery approaches continue to be developed, including specialised dual lumen microneedles that simultaneously inject viral vectors whilst removing the same volume of perilymph [
103]. Such solutions confer significant benefits over routine intratympanic injections by facilitating the delivery of larger volumes of therapeutic agents to the inner ear, whilst minimising disruption to the intracochlear environment.
There are three main types of viral vector-based gene therapy: gene replacement (including gene augmentation), gene editing and gene silencing [
101,
104]. Gene replacement therapy aims to deliver a functional gene to diseased or dysfunctional cells, essentially "correcting" the genetic defect. Gene augmentation introduces a functional copy of a gene into cells to compensate for a mutated or missing gene in diseases caused by loss-of-function mutations. Delivering functional copies of genes encoding critical structures in the cochlea, such as connexin hemichannels or ion channel subunits, can potentially restore the homeostatic mechanisms required for hearing [
101]. Historically, these techniques have been limited by the packing size of AAVs (~4.8 kb), however, advances in dual AAV strategies have allowed for the longer sequence of many deafness genes to be successfully delivered
in vivo [
104,
105]
Gene editing enables precise modification of genetic material
in situ, often utilising the CRISPR-Cas9 technology to remove a defective sequence, whilst preserving normal expression patterns [
104]. In contrast, gene silencing inhibits the translation of a disease-causing gene into a protein/s, for example, using antisense oligonucleotides, small interfering RNAs (siRNAs) or microRNAs to stop abnormal protein production [
104]. The type of gene therapy selected depends on the underlying pathology and the extent to which residual cochlear cytoarchitecture is available for reprogramming. This was demonstrated in early gene therapy studies, which clearly demonstrated the need for residual cells/structures for adequate expression of introduced genes [
106,
107].
The most popular gene therapy approaches for inner ear restoration thus far fall into the gene replacement category. In breakthrough clinical research, hearing recovery was reported in both a unilateral [
105] and bilateral [
108] gene therapy clinical trials, treating children with autosomal recessive deafness 9 (an otoferlin gene mutation). Results of these trials demonstrated both the safety and efficacy of the treatment, as measured by significant improvements in hearing thresholds, speech perception, and sound source localisation [
105,
108].
Additional gene therapy trials in the pipeline are directed at restoring MET channel function in hair cells, mutations to which cause permanent SNHL [
109,
110]. Promising preclinical experimental work has demonstrated the recovery of normal hearing function by multiple objective measures following a single injection of gene therapy via AAV targeted to the inner ear. Moreover, preclinical studies [
111,
112,
113] have demonstrated that gene therapy can partially restore ion channel or glutamate transport function in the cochlea. Some of these studies included murine models of Jervell-Lange-Nielsen syndrome and a type of deafness caused by a deficiency in vesicular glutamate transporter-3 (VGLUT3). Furthermore, gene therapy strategies are being developed to mediate protective responses in the context of ototoxic stress [
114,
115].
Fewer studies have aimed for gene augmentation or direct reprogramming for inner ear repair. However, these strategies may hold value when more extensive damage has occurred. A large part of the success of early gene therapies is likely due to the presence of residual structures in the organ of Corti. For instance, in otoferlin gene therapy trials, both the hair cells and auditory neurons remain present, at least for several years after birth.
These landmark studies mark the beginning of a new era in cochlear therapeutics. The successful delivery of new genes has been dramatically improved using cochlear perfusion [
116]. Although the safety and efficacy of gene therapies have been shown in the short term, the major challenges remaining include the longevity of the therapy, robust results across different gene mutations, and ensuring that the host immune response does not interfere with subsequent delivery of genes [
117]. Advances in the design of tailored viral vectors, including dual vector approaches as demonstrated in successful otoferlin trials [
105], and the exploration of non-viral delivery systems, such as nanoparticles, are promising paths that may further improve these therapies. This includes ongoing attempts to regenerate hair cells following their destruction, a therapy that holds considerable potential for reversing damage for the much larger numbers of patients experiencing SNHL [
117].
5.3. Regenerative Medicine and Stem Cell Therapies
Perhaps the most transformative approach for SNHL is the prospect of regenerative medicine. The cells of the inner ear, including both the sensory hair cells and neurons, are known for their limited regenerative capacity. Thus, advances in stem cell biology offer hope for their replacement after irreversible damage or death of these cells. Early investigations into stem cell therapy for hearing loss demonstrated the feasibility of engrafting stem cells into the mammalian cochlea to replace either sensory hair cells [
118] or spiral ganglion neurons [
119,
120]. Subsequent transplantation studies have reported the successful integration of human stem cell-derived auditory neural progenitors into a range of animal models, including mice [
121,
122], gerbils [
123], and guinea pigs [
124]. Building on this pre-clinical progress, these former “blue sky” approaches have received regulatory approval, with the UK-based company Rinri Therapeutics poised to commence first-in-human stem cell transplants designed to regenerate damaged auditory neurons [
125]. Patients in the trial have severe-to-profound SNHL and will receive both a cochlear implant and a cell transplant. Cochlear implants are the standard treatment option for these patients; therefore, the first trials aim to demonstrate safety.
Beyond transplantation, stem cells are also being used to generate
in vitro tissue models. These models hold promises for a better understanding of disease, correcting gene mutations, and as platforms for drug screening, prior to advancement into preclinical testing [
126,
127,
128]. The most advanced of these
in vitro models are inner ear organoids: three-dimensional, simplified and miniaturised versions of an organ that are grown using stem cells [
126]. Organoids comprise multiple cell types, arranged in a manner that partially mimics the structure and function of the corresponding real organ. This arrangement enables the replication of certain aspects of development, physiology, disease, and regenerative processes. Inner ear organoids have advanced significantly in recent years, with a landmark paper illustrating their similarity to human inner ear tissues using single-cell transcriptomics [
126]. Interestingly, these studies confirmed a preference for producing vestibular hair cells in inner ear organoids [
126], supporting earlier organoid studies drawing the same conclusions [
127].
Regenerative approaches have also explored the activation of latent supporting cells within the cochlea [
129]. The re-expression of transcription factors ATOH1, GFI1, and POU4F3 in the mature cochlea reprograms non-sensory cells into hair cells [
130,
131]. ATOH1 acts as a master switch, further guiding these progenitors towards a hair cell identity. In non-mammalian vertebrates, such as birds, fish and amphibians, supporting cells can transdifferentiate into hair cells following injury [
132,
133]. Unlocking the molecular switches that permit such plasticity in the mammalian cochlea is an active area of research. Small molecules and gene editing techniques that modulate pathways such as Notch and Wnt/β-catenin are at the forefront of attempts to induce cochlear cells back into a regenerative state [
134]. These attempts include more than just the hair cells, with advances made in the direct reprogramming of intracochlear non-neuronal cells into auditory neurons, when two transcription factors (ASCL1 and NEUROD1) were overexpressed [
135]. The direct reprogramming of non-neuronal cells into new auditory neurons may prove useful in pathological conditions where the hair cells remain functional, but the neurons are dysfunctional or missing (e.g., auditory neuropathy or hidden hearing loss).
5.4. Bioengineering Innovations
Bioengineering innovations are poised to offer complementary approaches to restore cochlear homeostasis. Advanced biomaterials, for example, are being developed to provide scaffolding for cell transplantation, ensuring proper alignment, survival, and integration of new cells [
136,
137]. Moreover, the use of nanotechnology to deliver ions, growth factors, and/or pharmacological agents directly to the inner ear represents an alternative strategy to enhance cochlear protection from stress and injury [
138].
Electrical stimulation devices, such as cochlear implants, have revolutionised the treatment for profound hearing loss. However, these devices do not restore natural cochlear homeostasis, and ongoing research aims to combine implant technology with regenerative approaches. This is evidenced by hybrid drug-eluting cochlear implant devices that integrate biomolecules with electronic functionality, which may eventually bridge the gap between prosthesis and organic repair, providing a natural and dynamic restoration of hearing [
139,
140].
In addition, clinical trials are underway that leverage the stimulating parameters of a cochlear implant to deliver growth factors to the inner ear. These growth factors support the long-term survival of auditory neurons and encourage their peripheral dendrites to grow towards the stimulating electrode. The trial, known as the BaDGE® platform, is led by the University of New South Wales [
141] and involves a novel proprietary technology that enables precision delivery of DNA / RNA therapeutics into the cochlea using an innovative pulsed electric lensing technology. The new DNA / RNA is delivered to the cochlea during cochlear implant surgery, driving directed regeneration of the auditory nerve fibres and ‘closing the neural gap’ between the nerve fibres and the electrode, thus improving device performance [
142].
5.5. Emerging Biomarkers and Personalised Medicine
One of the essential requirements for developing advanced biomolecular therapies for SNHL is the early identification of homeostatic disruption. Advances in molecular diagnostics have led to the discovery of several biomarkers, ranging from genetic polymorphisms and epigenetic signatures to proteomic profiles associated with oxidative stress or inflammation [
143]. For instance, increased levels of C-reactive protein (CRP) and IL-6 were found in blood samples of individuals with age-related hearing loss [
144], while a voltage-sensitive motor protein, prestin, is the main contender as a biomarker of outer hair cell damage [
145]. Circulating microRNAs are excellent candidates for the early detection of inner ear disease, as they are cell-independent and stable in many biological fluids [
146]. These biomarkers have the potential to identify individuals at increased risk of hearing loss before irreversible damage occurs, thereby opening a therapeutic window for early intervention. They may also be used to accurately assess the site of damage in the inner ear, thus improving patient management and treatment efficacy.
Personalised medicine approaches are particularly important to develop, given the heterogeneity of SNHL aetiologies. Next-generation sequencing is increasingly favoured for genetic diagnosis due to its capacity for large-scale genetic screening to identify gene variants that increase the risk of SNHL over time [
147]. By tailoring interventions to the specific genetic and health profiles of patients, clinicians are likely to be able to optimise therapeutic outcomes. Other examples are the use of ultrasharp microneedles for safe sampling of inner ear fluids [
148] and local drug delivery of gene therapy using nanoparticle vectors [
149]. Either through gene therapy, targeted pharmacologic strategies, regenerative medicine, or a combination of these techniques, the future of SNHL treatment ultimately rests in our ability to precisely detect and correct disturbances in cochlear homeostasis at an early stage [
149].
6. Integrative Perspectives on Cochlear Homeostasis and Hearing Loss
The active transport of ions, sound transduction by hair cells, and the ongoing metabolic support provided by the stria vascularis represent a delicate balance that is susceptible to both acute and chronic perturbations [
150]. This equilibrium is dynamic; it is continually adjusted in response to environmental challenges, metabolic demands, and age-associated alterations. The capacity of the cochlea to adapt to these changes through regulatory proteins, gap junctions, and repair mechanisms is a tribute to its evolutionary design. However, when the challenges exceed the homeostatic capacity, a loss of function can occur immediately, and this is often irreversible.
However, it is important to understand that SNHL rarely results from a single pathological process. Instead, multiple factors contribute to the disruption of cochlear homeostasis. For example, genetic predisposition may limit the efficiency of ion recycling; noise exposure and ototoxic compounds may induce oxidative damage; and age-related metabolic decline further compromises cochlear homeostasis. In many cases, it is the cumulative and/or combined effect of these stressors that precipitates the irreversible damage or loss of the critical cells in the cochlea. This multifactorial vulnerability necessitates a comprehensive therapeutic strategy that addresses both the cellular injury and the underlying disturbances in homeostasis (
Figure 3).
7. Concluding Remarks and Future Directions
Translating basic research on cochlear homeostasis into clinical therapies has made significant strides in recent years. Pre-clinical animal models have accelerated our understanding of the complexities of ion channel function, mitochondrial resilience, and regenerative potential in the inner ear. The advent of gene therapy, stem cell transplantation, and nanodelivery systems has offered substantial opportunities for clinical intervention, and both stem cell and gene therapies have now progressed to Phase I/II clinical trials. Future research is poised to address several key areas:
Continued efforts in bioengineering, such as the development of nanoparticle carriers and improvements to viral vectors, are expected to enhance the effectiveness, specificity, and safety of gene and drug delivery in the cochlea. In addition to refinements in surgical approach and the use of microneedles, these innovations will be essential for delivering therapeutics to the exact regions where homeostatic imbalance occurs.
High-throughput screening, such as next-generation sequencing and advanced imaging technologies, facilitates the discovery of new molecular targets involved in ion transport and the regulation of oxidative stress and inflammation. With the identification of novel targets, drugs can be designed to modulate their activity, providing another way to restore or preserve cochlear homeostasis. Similarly, new MRI techniques have been developed to quantify white matter fibre pathways in normal and abnormal hearing individuals [
151,
152], and these may provide additional objective measures for emerging cell and gene therapies.
Unlocking the regenerative potential of the cochlea remains one of the most exciting research avenues. Regenerative medicine may one day offer a permanent cure for SNHL, either through the reprogramming of supporting cells, the use of stem cell transplants, or the activation of latent regenerative pathways. A key focus is on ensuring that newly generated cells form synaptic connections with their targets, to re-establish the sensorineural circuitries essential for hearing.
Understanding the dual roles of inflammation, both as a repair mechanism in the cochlea and as a propagator of cell damage, will be necessary to design interventions that can modulate immune responses at the right time. Selective anti-inflammatory agents that preserve protective immune functions while suppressing chronic inflammation would be highly effective.
With advances in genomics and molecular diagnostics, the future of SNHL treatment is likely in personalised medicine. By adapting interventions to the genetic and environmental profiles of individual patients, clinicians can offer tailored therapies that target the precise disruptions in cochlear homeostasis before permanent damage occurs.
In summary, the maintenance of cochlear homeostasis is crucial for preserving the sensorineural function of the inner ear. Disruption of this delicate balance through genetic, environmental, or metabolic alterations sets the stage for irreversible SNHL. While current treatments for SNHL are largely palliative, the growing fields of gene and pharmacological therapies, as well as regenerative medicine, have begun to offer the promise of permanent hearing restoration. The multifaceted nature of cochlear homeostasis necessitates a comprehensive approach that not only addresses the symptoms of hearing loss but also targets the root causes at the cellular and molecular levels.
Author Contributions
Conceptualisation, methodology and writing: original draft preparation, SMV; review and editing, BAN and HSK; illustrations, HSK. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the MBIE program grant number PROP-1574-ENDRP-UOA.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Raphael, Y.; Altschuler, R.A. Structure and Innervation of the Cochlea. Brain Res Bull 2003, 60, 397–422. [Google Scholar] [CrossRef]
- Ma, Y.; Wise, A.K.; Shepherd, R.K.; Richardson, R.T. New Molecular Therapies for the Treatment of Hearing Loss. Pharmacol Ther 2019, 200, 190–209. [Google Scholar] [CrossRef] [PubMed]
- Wangemann, P. K+ Cycling and the Endocochlear Potential. Hear Res 2002, 165, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kurabi, A.; Keithley, E.M.; Housley, G.D.; Ryan, A.F.; Wong, A.C. . Cellular Mechanisms of Noise-Induced Hearing Loss. Hear Res 2017, 349, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Housley, G.D.; von Jonquieres, G.; Pinyon, J.L.; Matheson, J.T.; Pearson, L.J.; Salthouse, T.P.; Cederholm, J.M. Cochlear Homeostasis: A Molecular Physiological Perspective on Maintenance of Sound Transduction and Auditory Neurotransmission with Noise and Ageing. Current Opinion in Physiology 2020, 18, 106–115. [Google Scholar] [CrossRef]
- Von Bekesy, G. Resting Potentials Inside the Cochlear Partition of the Guinea Pig. Nature 1952, 169, 241–242. [Google Scholar] [CrossRef]
- Liberman, M.C.; Gao, J.; He, D.Z.Z.; Wu, X.; Jia, S.; Zuo, J. Prestin is Required for Electromotility of the Outer Hair Cell and for the Cochlear Amplifier. Nature 2002, 419, 300–304. [Google Scholar] [CrossRef]
- Kemp, D.T. Stimulated Acoustic Emissions from within the Human Auditory System. J Acoust Soc Am 1978, 64, 1386–1391. [Google Scholar] [CrossRef]
- Zdebik, A.A.; Wangemann, P.; Jentsch, T.J. Potassium Ion Movement in the Inner Ear: Insights from Genetic Disease and Mouse Models. Physiology (Bethesda) 2009, 24, 307–316. [Google Scholar] [CrossRef]
- Lang, H.; Noble, K.V.; Barth, J.L.; Rumschlag, J.A.; Jenkins, T.R.; Storm, S.L.; Eckert, M.A.; Dubno, J.R.; Schulte, B.A. The Stria Vascularis in Mice and Humans is an Early Site of Age-Related Cochlear Degeneration, Macrophage Dysfunction, and Inflammation. J Neurosci 2023, 43, 5057–5075. [Google Scholar] [CrossRef]
- Wang, B.; Hu, B.; Yang, S. Cell Junction Proteins within the Cochlea: A Review of Recent Research. J Otol 2015, 10, 131–135. [Google Scholar] [CrossRef]
- Wangemann, P. Supporting Sensory Transduction: Cochlear Fluid Homeostasis and the Endocochlear Potential. J Physiol 2006, 576, 11–21. [Google Scholar] [CrossRef]
- Magistretti, J.; Spaiardi, P.; Johnson, S.L.; Masetto, S. Elementary Properties of Ca2+ Channels and their Influence on Multivesicular Release and Phase-Locking at Auditory Hair Cell Ribbon Synapses. Front. Cell. Neurosci. 2015, 9. [Google Scholar] [CrossRef]
- Thulasiram, M.R.; Ogier, J.M.; Dabdoub, A. Hearing Function, Degeneration, and Disease: Spotlight on the Stria Vascularis. Front Cell Dev Biol 2022, 10, 841708. [Google Scholar] [CrossRef] [PubMed]
- Kociszewska, D.; Vlajkovic, S. Age-Related Hearing Loss: The Link between Inflammaging, Immunosenescence, and Gut Dysbiosis. Int J Mol Sci 2022, 23, 7348. [Google Scholar] [CrossRef] [PubMed]
- Jagger, D.J.; Forge, A. Connexins and Gap Junctions in the Inner Ear – It’s Not just about K+ Recycling. Cell Tissue Res 2015, 360, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhao, H. The Role of an Inwardly Rectifying K+ Channel (Kir4.1) in the Inner Ear and Hearing Loss. Neuroscience 2014, 265, 137–146. [Google Scholar] [CrossRef]
- Fracaro, S.; Hellies, F.; Marioni, G.; Brotto, D.; Franchella, S.; Zanoletti, E.; Albertin, G.; Astolfi, L. Role of Kir4.1 Channel in Auditory Function: Impact on Endocochlear Potential and Hearing Loss. Applied Sciences 2024, 14, 4985. [Google Scholar] [CrossRef]
- Hibino, H.; Nin, F.; Tsuzuki, C.; Kurachi, Y. How is the Highly Positive Endocochlear Potential Formed? the Specific Architecture of the Stria Vascularis and the Roles of the Ion-Transport Apparatus. Pflugers Arch - Eur J Physiol 2010, 459, 521–533. [Google Scholar] [CrossRef]
- Splawski, I.; Timothy, K.W.; Vincent, G.M.; Atkinson, D.L.; Keating, M.T. Molecular Basis of the Long-QT Syndrome Associated with Deafness. New England Journal of Medicine 1997, 336, 1562–1567. [Google Scholar] [CrossRef]
- Liu, Y.; Wei, M.; Mao, X.; Chen, T.; Lin, P.; Wang, W. Key Signaling Pathways Regulate the Development and Survival of Auditory Hair Cells. Neural Plasticity 2021, 2021, 5522717. [Google Scholar] [CrossRef]
- Yeo, X.Y.; Kwon, S.; Rinai, K.R.; Lee, S.; Jung, S.; Park, R. A Consolidated Understanding of the Contribution of Redox Dysregulation in the Development of Hearing Impairment. Antioxidants 2024, 13, 598. [Google Scholar] [CrossRef]
- Wang, Y.; Ren, C. Effects of Repeated "Benign" Noise Exposures in Young CBA Mice: Shedding Light on Age-Related Hearing Loss. J Assoc Res Otolaryngol 2012, 13, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Henderson, D.; Bielefeld, E.C.; Harris, K.C.; Hu, B.H. The Role of Oxidative Stress in Noise-Induced Hearing Loss. Ear and Hearing 2006, 27, 1. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.J.T.; Song, L. Role of Mitochondrial Dysfunction and Oxidative Stress in Sensorineural Hearing Loss. Hearing Research 2023, 434, 108783. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Yang, N. The Role and Research Progress of Mitochondria in Sensorineural Hearing Loss. Mol Neurobiol 2025, 62, 6913–6921. [Google Scholar] [CrossRef]
- Annunziato, L.; Pannaccione, A.; Cataldi, M.; Secondo, A.; Castaldo, P.; Di Renzo, G.; Taglialatela, M. Modulation of Ion Channels by Reactive Oxygen and Nitrogen Species: A Pathophysiological Role in Brain Aging? Neurobiol Aging 2002, 23, 819–834. [Google Scholar] [CrossRef]
- Waqas, M.; Gao, S.; Ali, M.K.; Ma, Y.; Li, W. Inner Ear Hair Cell Protection in Mammals Against the Noise-Induced Cochlear Damage. Neural Plast 2018, 2018, 3170801. [Google Scholar] [CrossRef]
- Pak, J.H.; Kim, Y.; Yi, J.; Chung, J.W. Antioxidant Therapy Against Oxidative Damage of the Inner Ear: Protection and Preconditioning. Antioxidants (Basel) 2020, 9, 1076. [Google Scholar] [CrossRef]
- Wong, A.C.Y.; Ryan, A.F. Mechanisms of Sensorineural Cell Damage, Death and Survival in the Cochlea. Front Aging Neurosci 2015, 7, 58. [Google Scholar] [CrossRef]
- Vlajkovic, S.M.; Thorne, P.R. Purinergic Signalling in the Cochlea. Int J Mol Sci 2022, 23, 14874. [Google Scholar] [CrossRef]
- Housley, G.D.; Morton-Jones, R.; Vlajkovic, S.M.; Telang, R.S.; Paramananthasivam, V.; Tadros, S.F.; Wong, A.C.Y.; Froud, K.E.; Cederholm, J.M.E.; Sivakumaran, Y.; et al. ATP-Gated Ion Channels Mediate Adaptation to Elevated Sound Levels. Proceedings of the National Academy of Sciences 2013, 110, 7494–7499. [Google Scholar] [CrossRef]
- Vlajkovic, S.M.; Housley, G.D.; Thorne, P.R. Auckland Hearing Science Discovery and Translation in Purinergic Signaling and Inner Ear Therapeutics. J R Soc N Z 2025, 55, 405–423. [Google Scholar] [CrossRef] [PubMed]
- Han, B.R.; Lin, S.C.; Espinosa, K.; Thorne, P.R.; Vlajkovic, S.M. Inhibition of the Adenosine A2A Receptor Mitigates Excitotoxic Injury in Organotypic Tissue Cultures of the Rat Cochlea. Cells 2019, 8, 877. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.; Pandya, M.; Espinosa, K.; Telang, R.; Boix, J.; Thorne, P.R.; Vlajkovic, S.M. Istradefylline Mitigates Age-Related Hearing Loss in C57BL/6J Mice. Int J Mol Sci 2021, 22, 8000. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, J.; Corfas, G.; Liberman, M.C. Round-Window Delivery of Neurotrophin 3 Regenerates Cochlear Synapses After Acoustic Overexposure. Sci Rep 2016, 6, 24907. [Google Scholar] [CrossRef]
- Sly, D.J.; Campbell, L.; Uschakov, A.; Saief, S.T.; Lam, M.; O'Leary, S.J. Applying Neurotrophins to the Round Window Rescues Auditory Function and Reduces Inner Hair Cell Synaptopathy After Noise-Induced Hearing Loss. Otol Neurotol 2016, 37, 1223–1230. [Google Scholar] [CrossRef]
- Xiao, Y.; Li, D. The Role of Epigenetic Modifications in Sensory Hair Cell Development, Survival, and Regulation. Front Cell Neurosci 2023, 17, 1210279. [Google Scholar] [CrossRef]
- Kwan, K.Y.; White, P.M. Understanding the Differentiation and Epigenetics of Cochlear Sensory Progenitors in Pursuit of Regeneration. Curr Opin Otolaryngol Head Neck Surg 2021, 29, 366–372. [Google Scholar] [CrossRef]
- Mittal, R.; Bencie, N.; Liu, G.; Nisenbaum, E.; Blanton, S.H.; Yan, D.; Mittal, J.; Dinh, C.T.; Young, J.I.; Gong, F.; et al. Recent Advancements in Understanding the Role of Epigenetics in the Auditory System. Gene 2020, 761, 144996. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, H.; Soukup, G.A.; He, D.Z.Z. Identifying MicroRNAs Involved in Aging of the Lateral Wall of the Cochlear Duct. PLOS ONE 2014, 9, e112857. [Google Scholar] [CrossRef]
- Mittal, R.; Liu, G.; Polineni, S.P.; Bencie, N.; Yan, D.; Liu, X.Z. Role of microRNAs in Inner Ear Development and Hearing Loss. Gene 2019, 686, 49–55. [Google Scholar] [CrossRef]
- Ushakov, K.; Rudnicki, A.; Avraham, K.B. MicroRNAs in Sensorineural Diseases of the Ear. Front. Mol. Neurosci. 2013, 6. [Google Scholar] [CrossRef]
- Xia, R.; Jin, C.; Fei, S.; Dong, T.; Wen, T.; Zhu, F.; Shi, Y.; Zhou, Q.; Tao, Y.; Peng, C. Therapeutic Restoration of miR-96 Prevents Hearing Loss in Mice through Modulation of Noise-Induced and Genetic Pathways. iScience 2025, 28. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jung, J.; Kang, H.; Park, K.; Lee, J.B.; Choi, S. Differential Expression and Regulation of FASLG by miR-5195/miR-3941 in Age-Related Hearing Loss. PLoS One 2025, 20, e0331661. [Google Scholar] [CrossRef] [PubMed]
- Shi, X. Pathophysiology of the Cochlear Intrastrial Fluid-Blood Barrier (Review). Hear Res 2016, 338, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Tan, J.; Chen, D.; Luo, J.; Li, P. Ethacrynic Acid Regulates Gentamicin Ototoxicity Via the Blood-Labyrinth Barrier. Hear Res 2025, 466, 109405. [Google Scholar] [CrossRef]
- Yang, Q.; Li, T.; Lu, Y.; Wang, T.; Chen, Z.; Xing, G.; Wei, Q.; Cao, X.; Yao, J. OSBPL2 Deficiency Impaired Cochlear Blood-Labyrinth Barrier Via Activation of NF-κB Signaling Pathway. Hearing Research 2025, 467, 109432. [Google Scholar] [CrossRef]
- Patel, A.; Pauzuolyte, V.; Ingham, N.J.; Leong, Y.C.; Berger, W.; Steel, K.P.; Sowden, J.C. Rescue of Cochlear Vascular Pathology Prevents Sensory Hair Cell Loss in Norrie Disease. Proc Natl Acad Sci U S A 2024, 121, e2322124121. [Google Scholar] [CrossRef]
- Samocha-Bonet, D.; Wu, B.; Ryugo, D.K. Diabetes Mellitus and Hearing Loss: A Review. Ageing Research Reviews 2021, 71, 101423. [Google Scholar] [CrossRef]
- Kociszewska, D.; Vlajkovic, S.M. The Association of Inflammatory Gut Diseases with Neuroinflammatory and Auditory Disorders. Front Biosci (Elite Ed) 2022, 14, 8. [Google Scholar] [CrossRef] [PubMed]
- Caballero-Borrego, M.; Andujar-Lara, I. Type 2 Diabetes Mellitus and Hearing Loss: A Prisma Systematic Review and Meta-Analysis. Otolaryngol. Head Neck Surg. 2025, n/a. [Google Scholar] [CrossRef] [PubMed]
- Pitt, J.M.; Kroemer, G.; Zitvogel, L. Extracellular Vesicles: Masters of Intercellular Communication and Potential Clinical Interventions. J Clin Invest 2016, 126, 1139–1143. [Google Scholar] [CrossRef] [PubMed]
- Müller, U. Exosome-Mediated Protection of Auditory Hair Cells from Ototoxic Insults. J Clin Invest 2020, 130, 2206–2208. [Google Scholar] [CrossRef]
- Breglio, A.M.; May, L.A.; Barzik, M.; Welsh, N.C.; Francis, S.P.; Costain, T.Q.; Wang, L.; Anderson, D.E.; Petralia, R.S.; Wang, Y.; et al. Exosomes Mediate Sensory Hair Cell Protection in the Inner Ear. J Clin Invest 2020, 130, 2657–2672. [Google Scholar] [CrossRef]
- Hao, F.; Shan, C.; Zhang, Y.; Zhang, Y.; Jia, Z. Exosomes Derived from microRNA-21 Overexpressing Neural Progenitor Cells Prevent Hearing Loss from Ischemia-Reperfusion Injury in Mice Via Inhibiting the Inflammatory Process in the Cochlea. ACS Chem Neurosci 2022, 13, 2464–2472. [Google Scholar] [CrossRef]
- Liberman, M.C.; Kujawa, S.G. Cochlear Synaptopathy in Acquired Sensorineural Hearing Loss: Manifestations and Mechanisms. Hear Res 2017, 349, 138–147. [Google Scholar] [CrossRef]
- McMahon, C.M.; Nieman, C.L.; Thorne, P.R.; Emmett, S.D.; Bhutta, M.F. The Inaugural World Report on Hearing: From Barriers to a Platform for Change. Clin Otolaryngol 2021, 46, 459–463. [Google Scholar] [CrossRef]
- Zhang, W.; Kim, S.M.; Wang, W.; Cai, C.; Feng, Y.; Kong, W.; Lin, X. Cochlear Gene Therapy for Sensorineural Hearing Loss: Current Status and Major Remaining Hurdles for Translational Success. Front. Mol. Neurosci. 2018, 11. [Google Scholar] [CrossRef]
- Fu, X.; Wan, P.; Li, P.; Wang, J.; Guo, S.; Zhang, Y.; An, Y.; Ye, C.; Liu, Z.; Gao, J.; et al. Mechanism and Prevention of Ototoxicity Induced by Aminoglycosides. Front Cell Neurosci 2021, 15, 692762. [Google Scholar] [CrossRef]
- Tan, W.J.T.; Vlajkovic, S.M. Molecular Characteristics of Cisplatin-Induced Ototoxicity and Therapeutic Interventions. Int J Mol Sci 2023, 24, 16545. [Google Scholar] [CrossRef]
- Sung, C.Y.W.; Hayase, N.; Yuen, P.S.T.; Lee, J.; Fernandez, K.; Hu, X.; Cheng, H.; Star, R.A.; Warchol, M.E.; Cunningham, L.L. Macrophage Depletion Protects Against Cisplatin-Induced Ototoxicity and Nephrotoxicity. Science Advances 2024, 10, eadk9878. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Zhang, A.; Zou, T.; Ding, R.; Chen, K.; Pan, Y.; Ji, P.; Ye, B.; Xiang, M. The Influence of Metabolic Syndrome on Age-Related Hearing Loss from the Perspective of Mitochondrial Dysfunction. Front. Aging Neurosci. 2022, 14. [Google Scholar] [CrossRef] [PubMed]
- Xipeng, L.; Ruiyu, L.; Meng, L.; Yanzhuo, Z.; Kaosan, G.; Liping, W. Effects of Diabetes on Hearing and Cochlear Structures. Journal of Otology 2013, 8, 82–87. [Google Scholar] [CrossRef]
- Liu, H.; Li, Y.; Chen, L.; Zhang, Q.; Pan, N.; Nichols, D.H.; Zhang, W.J.; Fritzsch, B.; He, D.Z.Z. Organ of Corti and Stria Vascularis: Is there an Interdependence for Survival? PLOS ONE 2016, 11, e0168953. [Google Scholar] [CrossRef]
- Fettiplace, R. Hair Cell Transduction, Tuning and Synaptic Transmission in the Mammalian Cochlea. Compr Physiol 2017, 7, 1197–1227. [Google Scholar] [CrossRef]
- Chen, P.; Wu, W.; Zhang, J.; Chen, J.; Li, Y.; Sun, L.; Hou, S.; Yang, J. Pathological Mechanisms of connexin26-Related Hearing Loss: Potassium Recycling, ATP-Calcium Signaling, Or Energy Supply? Front Mol Neurosci 2022, 15, 976388. [Google Scholar] [CrossRef]
- Fetoni, A.R.; Paciello, F.; Rolesi, R.; Paludetti, G.; Troiani, D. Targeting Dysregulation of Redox Homeostasis in Noise-Induced Hearing Loss: Oxidative Stress and ROS Signaling. Free Radical Biology and Medicine 2019, 135, 46–59. [Google Scholar] [CrossRef]
- Tsuzuki, N.; Wasano, K. Idiopathic Sudden Sensorineural Hearing Loss: A Review Focused on the Contribution of Vascular Pathologies. Auris Nasus Larynx 2024, 51, 747–754. [Google Scholar] [CrossRef]
- Wang, J.; Puel, J. Presbycusis: An Update on Cochlear Mechanisms and Therapies. Journal of Clinical Medicine 2020, 9, 218. [Google Scholar] [CrossRef]
- Maniaci, A.; La Via, L.; Lechien, J.R.; Sangiorgio, G.; Iannella, G.; Magliulo, G.; Pace, A.; Mat, Q.; Lavalle, S.; Lentini, M. Hearing Loss and Oxidative Stress: A Comprehensive Review. Antioxidants (Basel) 2024, 13, 842. [Google Scholar] [CrossRef]
- Monzack, E.L.; Cunningham, L.L. Lead Roles for Supporting Actors: Critical Functions of Inner Ear Supporting Cells. Hear Res 2013, 303, 20–29. [Google Scholar] [CrossRef]
- Frye, M.D.; Ryan, A.F.; Kurabi, A. Inflammation Associated with Noise-Induced Hearing Loss. J Acoust Soc Am 2019, 146, 4020–4032. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, K. Macrophage-Related Immune Responses in Inner Ear: A Potential Therapeutic Target for Sensorineural Hearing Loss. Front. Neurosci. 2024, 17. [Google Scholar] [CrossRef] [PubMed]
- Wood, M.B.; Zuo, J. The Contribution of Immune Infiltrates to Ototoxicity and Cochlear Hair Cell Loss. Front Cell Neurosci 2017, 11, 106. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Y.; Fu, X.; Wang, P.; Wang, Q.; Meng, W.; Wang, T.; Yang, J.; Chai, R. The Detrimental and Beneficial Functions of Macrophages After Cochlear Injury. Front Cell Dev Biol 2021, 9, 631904. [Google Scholar] [CrossRef]
- Steinacher, C.; Chacko, L.J.; Liu, W.; Rask-Andersen, H.; Bader, W.; Dudas, J.; Sergi, C.M.; Dhanaseelan, T.; Moreno, N.; Glueckert, R.; et al. Visualization of Macrophage Subsets in the Development of the Fetal Human Inner Ear. Front Immunol 2022, 13, 965196. [Google Scholar] [CrossRef]
- Deng, Y.; Ehiogu, B.; Luca, E.; Dabdoub, A.; Cao, K.L.; Wells, C.A.; Nayagam, B.A. Trophic and Temporal Dynamics of Macrophage Biology in Human Inner Ear Organogenesis. 2025, 2025.05.16.654631. [Google Scholar] [CrossRef]
- Hirose, K.; Discolo, C.M.; Keasler, J.R.; Ransohoff, R. Mononuclear Phagocytes Migrate into the Murine Cochlea After Acoustic Trauma. J Comp Neurol 2005, 489, 180–194. [Google Scholar] [CrossRef]
- Shi, X. Resident Macrophages in the Cochlear Blood-Labyrinth Barrier and their Renewal Via Migration of Bone-Marrow-Derived Cells. Cell Tissue Res 2010, 342, 21–30. [Google Scholar] [CrossRef]
- Tan, W.J.T.; Thorne, P.R.; Vlajkovic, S.M. Characterisation of Cochlear Inflammation in Mice Following Acute and Chronic Noise Exposure. Histochem Cell Biol 2016, 146, 219–230. [Google Scholar] [CrossRef]
- Fujioka, M.; Kanzaki, S.; Okano, H.J.; Masuda, M.; Ogawa, K.; Okano, H. Proinflammatory Cytokines Expression in Noise-Induced Damaged Cochlea. J Neurosci Res 2006, 83, 575–583. [Google Scholar] [CrossRef]
- Kalinec, G.M.; Lomberk, G.; Urrutia, R.A.; Kalinec, F. Resolution of Cochlear Inflammation: Novel Target for Preventing Or Ameliorating Drug-, Noise- and Age-Related Hearing Loss. Front. Cell. Neurosci. 2017, 11. [Google Scholar] [CrossRef] [PubMed]
- Fujioka, M.; Okano, H.; Ogawa, K. Inflammatory and Immune Responses in the Cochlea: Potential Therapeutic Targets for Sensorineural Hearing Loss. Front Pharmacol 2014, 5, 287. [Google Scholar] [CrossRef] [PubMed]
- Homma, K. The Pathological Mechanisms of Hearing Loss Caused by KCNQ1 and KCNQ4 Variants. Biomedicines 2022, 10, 2254. [Google Scholar] [CrossRef] [PubMed]
- Imtiaz, A.; Maqsood, A.; Rehman, A.U.; Morell, R.J.; Holt, J.R.; Friedman, T.B.; Naz, S. Recessive Mutations of TMC1 Associated with Moderate to Severe Hearing Loss. Neurogenetics 2016, 17, 115–123. [Google Scholar] [CrossRef]
- Abdelhadi, O.; Iancu, D.; Stanescu, H.; Kleta, R.; Bockenhauer, D. EAST Syndrome: Clinical, Pathophysiological, and Genetic Aspects of Mutations in KCNJ10. Rare Diseases 2016, 4. [Google Scholar] [CrossRef]
- Yan, D.; Zhu, Y.; Walsh, T.; Xie, D.; Yuan, H.; Sirmaci, A.; Fujikawa, T.; Wong, ACY.; Loh, TL.; Du, L.; Grati, M.; Vlajkovic, SM.; Blanton, S.; Ryan, A.F.; Chen, Z.Y.; Thorne, P.R.; Kachar, B.; Tekin, M.; Zhao, H.B.; Housley, G.D.; King, M.C.; Liu, X.Z. Mutation of the ATP-Gated P2X2 Receptor Leads to Progressive Hearing Loss and Increased Susceptibility to Noise. PNAS 2013, 110, 2228–2233. [Google Scholar] [CrossRef]
- Martínez, A.D.; Acuña, R.; Figueroa, V.; Maripillan, J.; Nicholson, B. Gap-Junction Channels Dysfunction in Deafness and Hearing Loss. Antioxid Redox Signal 2009, 11, 309–322. [Google Scholar] [CrossRef]
- Wingard, J.C.; Zhao, H. Cellular and Deafness Mechanisms Underlying Connexin Mutation-Induced Hearing Loss – A Common Hereditary Deafness. Front. Cell. Neurosci. 2015, 9. [Google Scholar] [CrossRef]
- Vona, B.; Rad, A.; Reisinger, E. The Many Faces of DFNB9: Relating OTOF Variants to Hearing Impairment. Genes (Basel) 2020, 11, 1411. [Google Scholar] [CrossRef] [PubMed]
- Dror, A.A.; Avraham, K.B. Hearing Impairment: A Panoply of Genes and Functions. Neuron 2010, 68, 293–308. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto-Urata, M.; Urata, S.; Fujimoto, C.; Yamasoba, T. Role of Oxidative Stress and Antioxidants in Acquired Inner Ear Disorders. Antioxidants (Basel) 2022, 11, 1469. [Google Scholar] [CrossRef] [PubMed]
- Pisani, A.; Paciello, F.; Montuoro, R.; Rolesi, R.; Galli, J.; Fetoni, A.R. Antioxidant Therapy as an Effective Strategy Against Noise-Induced Hearing Loss: From Experimental Models to Clinic. Life (Basel) 2023, 13, 1035. [Google Scholar] [CrossRef]
- Fujimoto, C.; Yamasoba, T. Mitochondria-Targeted Antioxidants for Treatment of Hearing Loss: A Systematic Review. Antioxidants (Basel) 2019, 8, 109. [Google Scholar] [CrossRef]
- Luo, Y.; Wu, H.; Min, X.; Chen, Y.; Deng, W.; Chen, M.; Yang, C.; Xiong, H. SIRT1 Prevents Noise-Induced Hearing Loss by Enhancing Cochlear Mitochondrial Function. Cell Commun Signal 2025, 23, 160. [Google Scholar] [CrossRef]
- Li, T.; Yu, W.; Lei, W.; Zong, S.; Xiao, H. Targeting Inflammation to Prevent and Treat Sensorineural Hearing Loss. Chin Med J (Engl) 2025, 138, 1248–1250. [Google Scholar] [CrossRef]
- Le Prell, C.G. Otoprotectants: From Research to Clinical Application. Semin Hear 2019, 40, 162–176. [Google Scholar] [CrossRef]
- Brock, P.R.; Maibach, R.; Childs, M.; Rajput, K.; Roebuck, D.; Sullivan, M.J.; Laithier, V.; Ronghe, M.; Dall’Igna, P.; Hiyama, E.; et al. Sodium Thiosulfate for Protection from Cisplatin-Induced Hearing Loss. New England Journal of Medicine 2018, 378, 2376–2385. [Google Scholar] [CrossRef]
- Meijer, A.J.M.; Diepstraten, F.A.; Ansari, M.; Bouffet, E.; Bleyer, A.; Fresneau, B.; Geller, J.I.; Huitema, A.D.R.; Kogner, P.; Maibach, R.; et al. Use of Sodium Thiosulfate as an Otoprotectant in Patients with Cancer Treated with Platinum Compounds: A Review of the Literature. JCO 2024, 42, 2219. [Google Scholar] [CrossRef]
- Duhon, B.H.; Bielefeld, E.C.; Ren, Y.; Naidoo, J. Gene Therapy Advancements for the Treatment of Acquired and Hereditary Hearing Loss. Front. Audiol. Otol. 2024, 2. [Google Scholar] [CrossRef]
- Askew, C.; Chien, W.W. Adeno-Associated Virus Gene Replacement for Recessive Inner Ear Dysfunction: Progress and Challenges. Hear Res 2020, 394, 107947. [Google Scholar] [CrossRef] [PubMed]
- Hammer, D.R.; Voruz, F.; Aksit, A.; Breil, E.; Rousset, F.; Senn, P.; Ilmjärv, S.; Olson, E.S.; Lalwani, A.K.; Kysar, J.W. Novel Dual-Lumen Microneedle Delivers Adeno-Associated Viral Vectors in the Guinea Pig Inner Ear Via the Round Window Membrane. Biomed Microdevices 2025, 27, 27. [Google Scholar] [CrossRef] [PubMed]
- Petit, C.; Bonnet, C.; Safieddine, S. Deafness: From Genetic Architecture to Gene Therapy. Nat Rev Genet 2023, 24, 665–686. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Wang, H.; Cheng, X.; Chen, Y.; Wang, D.; Zhang, L.; Cao, Q.; Tang, H.; Hu, S.; Gao, K.; et al. AAV1-hOTOF Gene Therapy for Autosomal Recessive Deafness 9: A Single-Arm Trial. Lancet 2024, 403, 2317–2325. [Google Scholar] [CrossRef]
- Izumikawa, M.; Batts, S.A.; Miyazawa, T.; Swiderski, D.L.; Raphael, Y. Response of the Flat Cochlear Epithelium to Forced Expression of Atoh1. Hear Res 2008, 240, 52–56. [Google Scholar] [CrossRef]
- Atkinson, P.J.; Wise, A.K.; Flynn, B.O.; Nayagam, B.A.; Richardson, R.T. Hair Cell Regeneration After ATOH1 Gene Therapy in the Cochlea of Profoundly Deaf Adult Guinea Pigs. PLOS ONE 2014, 9, e102077. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Y.; Lv, J.; Cheng, X.; Cao, Q.; Wang, D.; Zhang, L.; Zhu, B.; Shen, M.; Xu, C.; et al. Bilateral Gene Therapy in Children with Autosomal Recessive Deafness 9: Single-Arm Trial Results. Nat Med 2024, 30, 1898–1904. [Google Scholar] [CrossRef]
- Kawashima, Y.; Kurima, K.; Pan, B.; Griffith, A.J.; Holt, J.R. Transmembrane Channel-Like (TMC) Genes are Required for Auditory and Vestibular Mechanosensation. Pflugers Arch - Eur J Physiol 2015, 467, 85–94. [Google Scholar] [CrossRef]
- Cho, S.H.; Yun, Y.; Lee, D.H.; Cha, J.H.; Lee, S.M.; Lee, J.; Suh, M.H.; Lee, J.H.; Oh, S.; Park, M.K.; et al. Novel Autosomal Dominant TMC1 Variants Linked to Hearing Loss: Insight into Protein-Lipid Interactions. BMC Medical Genomics 2023, 16, 320. [Google Scholar] [CrossRef]
- Vitry, S.; Mendia, C.; Maudoux, A.; El-Amraoui, A. Advancing Precision Ear Medicine: Leveraging Animal Models for Disease Insights and Therapeutic Innovations. Mamm Genome 2025. [Google Scholar] [CrossRef] [PubMed]
- Hahn, R.; Avraham, K.B. Gene Therapy for Inherited Hearing Loss: Updates and Remaining Challenges. Audiol Res 2023, 13, 952–966. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Zhao, L.; Guo, Y.; Yang, J. Gene Therapy for Hereditary Hearing Loss. Hearing Research 2025, 455, 109151. [Google Scholar] [CrossRef]
- Kawamoto, K.; Sha, S.; Minoda, R.; Izumikawa, M.; Kuriyama, H.; Schacht, J.; Raphael, Y. Antioxidant Gene Therapy can Protect Hearing and Hair Cells from Ototoxicity. Mol Ther 2004, 9, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Nassauer, L.; Staecker, H.; Huang, P.; Renslo, B.; Goblet, M.; Harre, J.; Warnecke, A.; Schott, J.W.; Morgan, M.; Galla, M.; et al. Protection from Cisplatin-Induced Hearing Loss with Lentiviral Vector-Mediated Ectopic Expression of the Anti-Apoptotic Protein BCL-XL. Molecular Therapy - Nucleic Acids 2024, 35, 102157. [Google Scholar] [CrossRef]
- Wei, C.; Gao, Z.; Knabel, M.; Ulbricht, M.; Senekowitsch, S.; Erfurt, P.; Maggi, N.; Zwick, B.; Eickner, T.; Matin-Mann, F.; et al. Development of a Drug Delivering Round Window Niche Implant for Cochlear Pharmacotherapy. Drug Deliv. 2024, 31, 2392755. [Google Scholar] [CrossRef]
- Jones, M.; Kovacevic, B.; Ionescu, C.M.; Wagle, S.R.; Quintas, C.; Wong, E.Y.M.; Mikov, M.; Mooranian, A.; and Al-Salami, H. The Applications of Targeted Delivery for Gene Therapies in Hearing Loss. Journal of Drug Targeting 2023, 31, 585–595. [Google Scholar] [CrossRef]
- Hildebrand, M.S.; Dahl, H.M.; Hardman, J.; Coleman, B.; Shepherd, R.K.; de Silva, M.G. Survival of Partially Differentiated Mouse Embryonic Stem Cells in the Scala Media of the Guinea Pig Cochlea. J Assoc Res Otolaryngol 2005, 6, 341–354. [Google Scholar] [CrossRef]
- Coleman, B.; Hardman, J.; Coco, A.; Epp, S.; de Silva, M.; Crook, J.; Shepherd, R. Fate of Embryonic Stem Cells Transplanted into the Deafened Mammalian Cochlea. Cell Transplant 2006, 15, 369–380. [Google Scholar] [CrossRef]
- Corrales, C.E.; Pan, L.; Li, H.; Liberman, M.C.; Heller, S.; Edge, A.S.B. Engraftment and Differentiation of Embryonic Stem Cell-Derived Neural Progenitor Cells in the Cochlear Nerve Trunk: Growth of Processes into the Organ of Corti. J Neurobiol 2006, 66, 1489–1500. [Google Scholar] [CrossRef]
- Chen, J.; Hong, F.; Zhang, C.; Li, L.; Wang, C.; Shi, H.; Fu, Y.; Wang, J. Differentiation and Transplantation of Human Induced Pluripotent Stem Cell-Derived Otic Epithelial Progenitors in Mouse Cochlea. Stem Cell Research & Therapy 2018, 9, 230. [Google Scholar] [CrossRef] [PubMed]
- Takeda, H.; Hosoya, M.; Fujioka, M.; Saegusa, C.; Saeki, T.; Miwa, T.; Okano, H.; Minoda, R. Engraftment of Human Pluripotent Stem Cell-Derived Progenitors in the Inner Ear of Prenatal Mice. Sci Rep 2018, 8, 1941. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Jongkamonwiwat, N.; Abbas, L.; Eshtan, S.J.; Johnson, S.L.; Kuhn, S.; Milo, M.; Thurlow, J.K.; Andrews, P.W.; Marcotti, W.; et al. Restoration of Auditory Evoked Responses by Human ES-Cell-Derived Otic Progenitors. Nature 2012, 490, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Juarez, A.; Lahlou, H.; Ripoll, C.; Cazals, Y.; Brezun, J.M.; Wang, Q.; Edge, A.; Zine, A. Engraftment of Human Stem Cell-Derived Otic Progenitors in the Damaged Cochlea. Mol Ther 2019, 27, 1101–1113. [Google Scholar] [CrossRef]
- UK MHRA Approval Received for First-in-Human Trial of Rincell-12025.
- van der Valk, W.H.; van Beelen, E.S.A.; Steinhart, M.R.; Nist-Lund, C.; Osorio, D.; de Groot, J.C.M.J.; Sun, L.; van Benthem, P.P.G.; Koehler, K.R.; Locher, H. A Single-Cell Level Comparison of Human Inner Ear Organoids with the Human Cochlea and Vestibular Organs. Cell Rep 2023, 42, 112623. [Google Scholar] [CrossRef]
- Mattei, C.; Lim, R.; Drury, H.; Nasr, B.; Li, Z.; Tadros, M.A.; D'Abaco, G.M.; Stok, K.S.; Nayagam, B.A.; Dottori, M. Generation of Vestibular Tissue-Like Organoids from Human Pluripotent Stem Cells using the Rotary Cell Culture System. Front Cell Dev Biol 2019, 7, 25. [Google Scholar] [CrossRef]
- Koehler, K.R.; Nie, J.; Longworth-Mills, E.; Liu, X.; Lee, J.; Holt, J.R.; Hashino, E. Generation of Inner Ear Organoids Containing Functional Hair Cells from Human Pluripotent Stem Cells. Nat Biotechnol 2017, 35, 583–589. [Google Scholar] [CrossRef]
- McGovern, M.M.; Cox, B.C. Hearing Restoration through Hair Cell Regeneration: A Review of Recent Advancements and Current Limitations. Hearing Research 2025, 461, 109256. [Google Scholar] [CrossRef]
- McGovern, M.M.; Hosamani, I.V.; Niu, Y.; Nguyen, K.Y.; Zong, C.; Groves, A.K. Expression of Atoh1, Gfi1, and Pou4f3 in the Mature Cochlea Reprograms Nonsensory Cells into Hair Cells. Proc Natl Acad Sci U S A 2024, 121, e2304680121. [Google Scholar] [CrossRef]
- McGovern, M.M.; Ghosh, S.; Dupuis, C.; Walters, B.J.; Groves, A.K. Reprogramming with Atoh1, Gfi1, and Pou4f3 Promotes Hair Cell Regeneration in the Adult Organ of Corti. PNAS Nexus 2024, 3, pgae445. [Google Scholar] [CrossRef]
- Cotanche, D.A. Regeneration of Hair Cell Stereociliary Bundles in the Chick Cochlea Following Severe Acoustic Trauma. Hear Res 1987, 30, 181–195. [Google Scholar] [CrossRef]
- Corwin, J.T.; Cotanche, D.A. Regeneration of Sensory Hair Cells After Acoustic Trauma. Science 1988, 240, 1772–1774. [Google Scholar] [CrossRef]
- Samarajeewa, A.; Jacques, B.E.; Dabdoub, A. Therapeutic Potential of Wnt and Notch Signaling and Epigenetic Regulation in Mammalian Sensory Hair Cell Regeneration. Mol Ther 2019, 27, 904–911. [Google Scholar] [CrossRef]
- Noda, T.; Meas, S.J.; Nogami, J.; Amemiya, Y.; Uchi, R.; Ohkawa, Y.; Nishimura, K.; Dabdoub, A. Direct Reprogramming of Spiral Ganglion Non-Neuronal Cells into Neurons: Toward Ameliorating Sensorineural Hearing Loss by Gene Therapy. Front. Cell Dev. Biol. 2018, 6. [Google Scholar] [CrossRef]
- Boufidis, D.; Garg, R.; Angelopoulos, E.; Cullen, D.K.; Vitale, F. Bio-Inspired Electronics: Soft, Biohybrid, and “Living” Neural Interfaces. Nat Commun 2025, 16, 1861. [Google Scholar] [CrossRef] [PubMed]
- Kharbikar, B.N.; Mohindra, P.; Desai, T.A. Biomaterials to Enhance Stem Cell Transplantation. Cell Stem Cell 2022, 29, 692–721. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zheng, J.; He, Y.; Lin, K.; Li, S.; Zhang, Y.; Song, P.; Zhou, Y.; Chen, X. Nanocarriers for Inner Ear Disease Therapy. Front Cell Neurosci 2021, 15, 791573. [Google Scholar] [CrossRef]
- Azees, A.A.; Thompson, A.C.; Ruther, P.; Ajay, E.A.; Zhou, J.; Aregueta Robles, U.A.; Garrett, D.J.; Quigley, A.; Fallon, J.B.; Richardson, R.T. Spatially Precise Activation of the Mouse Cochlea with a Multi-Channel Hybrid Cochlear Implant. J Neural Eng 2025, 22. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Maltby, T.; Moineddini, A.; Shi, D.; Wu, L.; Chen, J.; Yu, J.; Hung, J.; Viola, G.; Vilches, A.; et al. Piezoelectric Nanofiber–based Intelligent Hearing System. Science Advances 2025, 11, eadl2741. [Google Scholar] [CrossRef]
- BaDGE® Bionic Array Directed Gene Electrotransfer DNA/RNA Therapeutics Delivery Platform | School of Biomedical Sciences - UNSW Sydney, 2025.
- Pinyon, J.L.; von Jonquieres, G.; Crawford, E.N.; Abed, A.A.; Power, J.M.; Klugmann, M.; Browne, C.J.; Housley, D.M.; Wise, A.K.; Fallon, J.B.; et al. Gene Electrotransfer Via Conductivity-Clamped Electric Field Focusing Pivots Sensori-Motor DNA Therapeutics: "A Spoonful of Sugar Helps the Medicine Go Down". Adv Sci (Weinh) 2024, 11, e2401392. [Google Scholar] [CrossRef]
- Mahshid, S.S.; Higazi, A.M.; Ogier, J.M.; Dabdoub, A. Extracellular Biomarkers of Inner Ear Disease and their Potential for Point-of-Care Diagnostics. Adv Sci (Weinh) 2021, 9, 2104033. [Google Scholar] [CrossRef]
- Verschuur, C.A.; Dowell, A.; Syddall, H.E.; Ntani, G.; Simmonds, S.J.; Baylis, D.; Gale, C.R.; Walsh, B.; Cooper, C.; Lord, J.M.; et al. Markers of Inflammatory Status are Associated with Hearing Threshold in Older People: Findings from the Hertfordshire Ageing Study. Age Ageing 2012, 41, 92–97. [Google Scholar] [CrossRef]
- Parham, K.; Dyhrfjeld-Johnsen, J. Outer Hair Cell Molecular Protein, Prestin, as a Serum Biomarker for Hearing Loss: Proof of Concept. Otol Neurotol 2016, 37, 1217–1222. [Google Scholar] [CrossRef] [PubMed]
- Friedman, L.M.; Avraham, K.B. MicroRNAs and Epigenetic Regulation in the Mammalian Inner Ear: Implications for Deafness. Mamm Genome 2009, 20, 581–603. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, S.; Park, S.; Jung, S.H.; Yun, Y.; Choi, W.H.; Cha, J.H.; Yun, H.; Lee, S.; Suh, M.; et al. Comprehensive Genetic Profiling of Sensorineural Hearing Loss using an Integrative Diagnostic Approach. Cell Rep Med 2025, 6, 102206. [Google Scholar] [CrossRef] [PubMed]
- Peter, M.S.; Warnecke, A.; Staecker, H. A Window of Opportunity: Perilymph Sampling from the Round Window Membrane can Advance Inner Ear Diagnostics and Therapeutics. J Clin Med 2022, 11, 316. [Google Scholar] [CrossRef]
- Tavazzani, E.; Spaiardi, P.; Contini, D.; Sancini, G.; Russo, G.; Masetto, S. Precision Medicine: A New Era for Inner Ear Diseases. Front. Pharmacol. 2024, 15. [Google Scholar] [CrossRef]
- Bovee, S.; Klump, G.M.; Köppl, C.; Pyott, S.J. The Stria Vascularis: Renewed Attention on a Key Player in Age-Related Hearing Loss. International Journal of Molecular Sciences 2024, 25, 5391. [Google Scholar] [CrossRef]
- Zanin, J.; Dhollander, T.; Rance, G.; Yu, L.; Lan, L.; Wang, H.; Lou, X.; Connelly, A.; Nayagam, B.; Wang, Q. Fiber-Specific Changes in White Matter Microstructure in Individuals with X-Linked Auditory Neuropathy. Ear Hear 2020, 41, 1703–1714. [Google Scholar] [CrossRef]
- Zanin, J.; Dhollander, T.; Farquharson, S.; Rance, G.; Connelly, A.; Nayagam, B.A. Review: Using Diffusion-Weighted Magnetic Resonance Imaging Techniques to Explore the Microstructure and Connectivity of Subcortical White Matter Tracts in the Human Auditory System. Hear Res 2019, 377, 1–11. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).