1. Introduction
Pelvic organ prolapse (POP) is a common condition among adult women and represents a major contributor to impaired quality of life. Epidemiological studies indicate that the lifetime risk of undergoing at least one surgical intervention for pelvic floor disorders is approximately 11% in earlier cohorts, rising to nearly 20% when stress urinary incontinence (SUI) and POP are considered together in contemporary analyses (1,2). With increasing life expectancy and a higher prevalence of modifiable risk factors such as obesity, the global burden of POP and the demand for durable surgical treatments are expected to rise further (3).
Rectocele, the prototypical posterior compartment defect resulting from attenuation of the rectovaginal septum, is particularly common in multiparous women and is strongly associated with defecatory dysfunction. Typical symptoms include obstructed defecation, incomplete evacuation, vaginal bulge, manual splinting, and anorectal pain, all of which significantly affect daily functioning, sexual health, and psychological well-being (4). The pathogenesis is multifactorial: childbirth-related levator ani trauma, pudendal neuropathy, connective tissue remodelling, and age-related alterations in collagen and elastin contribute to rectovaginal wall weakness. Established risk factors such as advancing age, multiparity, and obesity play key roles not only in the development of rectocele but also in its recurrence following repair (5–7).
Conservative treatments including dietary regulation, pelvic floor physiotherapy, and pessary use remain first-line options. However, when symptoms persist, surgical repair becomes necessary. Native-tissue posterior colporrhaphy continues to be widely performed, although recurrence rates remain high, and overcorrection can lead to dyspareunia or obstructed defecation (8). To improve the durability of repair, mesh augmentation was introduced. Although mesh reinforcement offers additional structural support, concerns regarding mesh exposure, erosion, infection, and dyspareunia have generated ongoing debate, culminating in the 2019 U.S. Food and Drug Administration order to discontinue transvaginal mesh devices for POP repair (9).
Evidence on the effectiveness of mesh-augmented rectocele repair remains heterogeneous. While several prospective and randomised studies report superior anatomical outcomes compared with native-tissue repair, long-term durability, quality-of-life impact, complication rates, and sexual function outcomes remain controversial (10,11). Furthermore, real-world studies have seldom explored how patient-specific factors particularly age, multiparity, and obesity modify surgical outcomes and recurrence risk. Ageing affects tissue integrity and oestrogen status; multiparity predisposes to levator ani avulsion and neuromuscular injury; and obesity increases intra-abdominal pressure and impairs wound healing. These elements warrant focused evaluation in preoperative counselling and surgical planning (5–7,12).
This study aimed to evaluate the efficacy and safety of transvaginal Prolene mesh–augmented rectocele repair in a tertiary pelvic-floor centre. The primary endpoints included changes in validated patient-reported measures such as the Constipation Scoring System (CSS), Obstructed Defecation Syndrome (ODS) score, Patient Assessment of Constipation Quality of Life (PAC-QOL), and visual analogue scale (VAS) for pelvic and rectal pain at twelve months postoperatively. Secondary endpoints included perioperative complications and exploratory analyses assessing the influence of age, body mass index (BMI), and multiparity on outcomes.
2. Materials and Methods
2.1. Study Design and Setting
This retrospective, single-centre cohort study included 120 consecutive female patients who underwent transvaginal rectocele repair with Prolene® mesh (Ethicon, Johnson & Johnson, Somerville, NJ, USA) at the Department of General Surgery, Akdeniz University Faculty of Medicine, between January 2020 and December 2023.
The study protocol was approved by the Akdeniz University Medical Research Ethics Committee (approval no: TBAEK-630, date: 05 September 2024). Written informed consent was obtained from all patients for surgery and for the subsequent use of anonymised data for research purposes, in accordance with the Declaration of Helsinki.
2.1. Patient Selection
Eligible patients met the following criteria:
Clinical diagnosis of rectocele confirmed by symptoms, physical examination, and defecography;
Age ≥ 18 years;
Availability of complete preoperative and postoperative data (minimum follow-up: 12 months).
Exclusion criteria were:
Previous rectocele repair,
Incomplete medical records,
Concomitant major pelvic surgery (e.g., hysterectomy, anterior compartment repair, or sacrocolpopexy).
2.2. Preoperative Assessment
Demographic data, including age, body mass index (BMI), and obstetric history (parity and mode of delivery), as well as comorbid medical conditions, were systematically recorded for all patients. The severity of rectocele was assessed through standardised clinical examination and confirmed using radiological imaging (defecography or pelvic floor MRI).
Validated symptom-assessment instruments were used to objectively evaluate preoperative and postoperative symptom burden and quality of life. These included the Constipation Scoring System (CSS) developed by Agachan et al., the Obstructed Defecation Syndrome (ODS) score described by Altomare et al., the Patient Assessment of Constipation Quality of Life (PAC-QOL) questionnaire, and the Visual Analogue Scale (VAS) for pelvic and rectal pain (13–16).
2.3. Surgical Technique
All operations were performed transvaginally under spinal or general anaesthesia by experienced pelvic floor surgeons. After dissection of the rectovaginal septum, a tailored Prolene® mesh was positioned to reinforce the posterior vaginal wall and secured with absorbable sutures to the levator ani and perineal body. Excess vaginal mucosa was excised, and layered closure of the vaginal wall was performed.
2.4. Postoperative Follow-up
Patients were evaluated at standardised follow-up visits at 6 and 12 months postoperatively. Assessments included physical examination, validated questionnaires (CSS, ODS, PAC-QOL, and VAS), and documentation of complications. Adverse events recorded included mesh exposure, retraction, infection, persistent vaginal pain, and recurrence. Recurrence was defined as the reappearance of symptoms or anatomical defects following rectocele repair.
2.5. Statistical Analysis
Data were analysed using R software (version 4.1; R Foundation for Statistical Computing, Vienna, Austria). Continuous variables were expressed as mean ± standard deviation (SD) or median (interquartile range, IQR) and compared using paired t-tests or Wilcoxon signed-rank tests, as appropriate. Categorical variables were analysed using χ² or Fisher’s exact tests. Independent associations between outcomes and potential risk factors (age, BMI, parity) were evaluated using multivariable logistic regression models. A two-tailed p-value < 0.05 was considered statistically significant.
3. Results
3.1. Cohort Characteristics
A total of 120 women underwent transvaginal Prolene mesh–augmented rectocele repair. The mean age was 49.5 ± 11.0 years and the mean BMI 27.2 ± 5.2 kg/m². Multiparity was common (75.0%), with 40.8% reporting two or more vaginal deliveries. Previous hysterectomy, prior pelvic floor surgery, and prior anal surgery were recorded in 15.0%, 13.3%, and 10.0% of cases, respectively (
Table 1). These characteristics are consistent with a typical tertiary referral population presenting with posterior compartment prolapse.
3.2. Symptoms and Quality-of-Life Outcomes
At twelve months postoperatively, all validated outcome measures showed statistically and clinically significant improvement (
Table 2). The Constipation Scoring System (CSS), Obstructed Defecation Syndrome (ODS) score, Patient Assessment of Constipation Quality of Life (PAC-QOL), and visual analogue scale (VAS) for pain all demonstrated meaningful reductions (all
p < 0.001). Collectively, these findings confirm marked postoperative improvement in bowel function, symptom burden, quality of life, and pelvic pain.
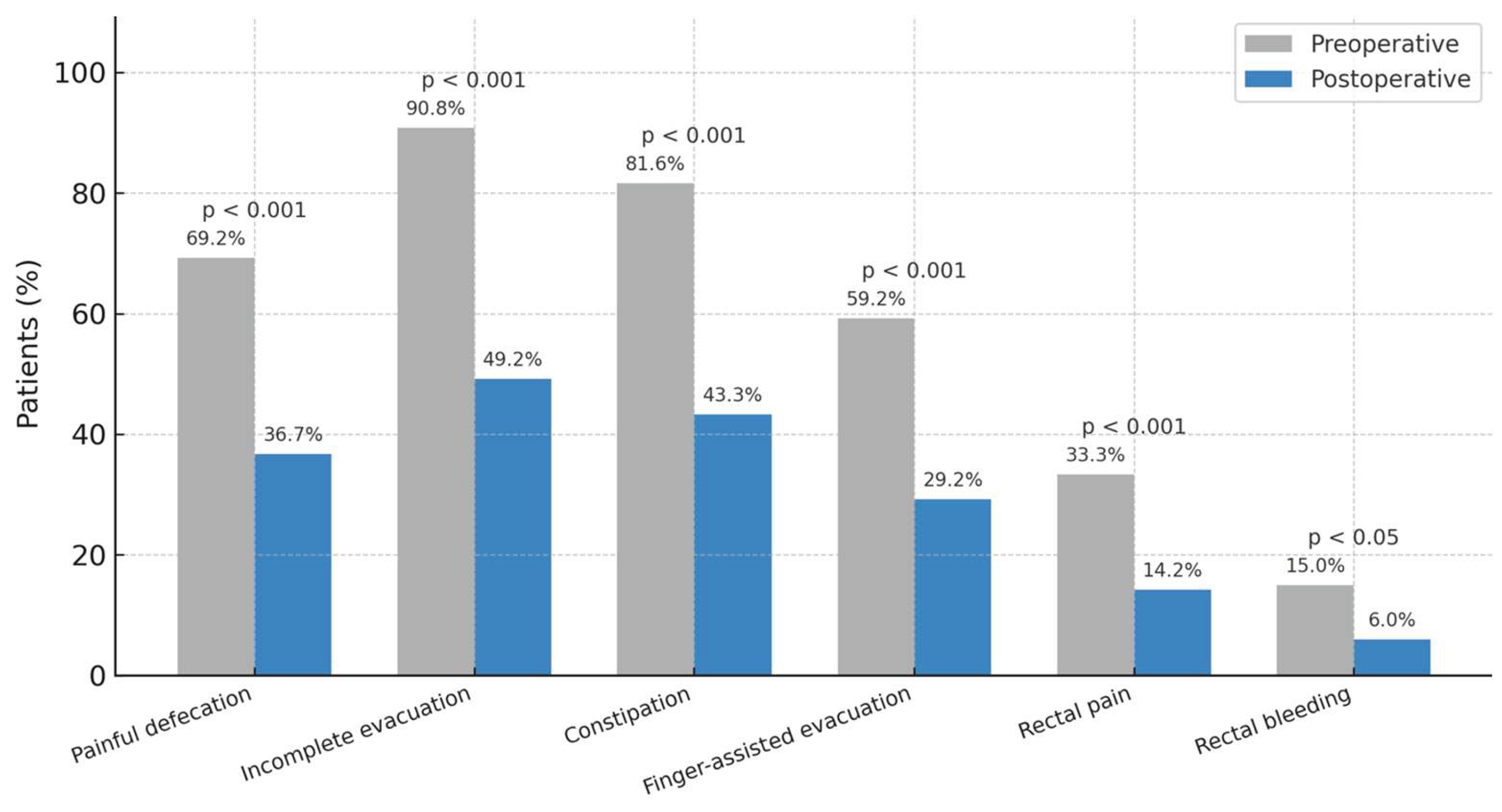
3.3. Symptom Prevalence
Improvements in continuous scores were mirrored by reductions in symptom prevalence (
Figure 1). The proportions of patients reporting painful defecation, incomplete evacuation, constipation, finger-assisted evacuation, rectal pain, and rectal bleeding all decreased significantly, highlighting the broad and consistent clinical benefit of surgery (
p < 0.001 for most domains)
3.4. Postoperative Complications
The overall complication rate was 11.7% (
Table 3). Mesh exposure occurred in 5.0% of patients, mesh retraction in 4.2%, and infection in 1.7%. One patient (0.8%) experienced isolated vaginal pain. No cases of fistula formation or deep infection were observed. Overall, these results indicate an acceptable safety profile, with complications limited to minor, manageable mesh-related issues.
4. Discussion
Pelvic organ prolapse (POP) is a common and often debilitating condition, and rectocele represents one of its most symptomatic forms. In this study, transvaginal Prolene mesh–augmented rectocele repair yielded significant short-term improvements in constipation, obstructed defecation, pelvic pain, and quality of life. These findings align with previous reports showing that symptom relief, rather than anatomic correction alone, is the principal determinant of patient satisfaction after rectocele surgery (4,8).
Age, parity, and body mass index emerged as key factors influencing recurrence risk. Ageing is associated with reduced connective-tissue integrity and oestrogen deficiency, both of which may compromise the durability of repair. Kayondo et al. (2021) found that women younger than 60 years and those experiencing postoperative infection had a higher risk of prolapse recurrence, underscoring the need for long-term surveillance across age groups (17). In the present cohort, the mean age of 49.5 years places most patients in the peri-menopausal period, when hormonal and structural changes may predispose to recurrence despite early symptomatic improvement.
Multiparity was highly prevalent (75%) and remains a well-established risk factor for posterior compartment prolapse. Vaginal birth can cause levator ani trauma and pudendal neuropathy, leading to rectovaginal-septum weakness. Dietz (2022) demonstrated that even partial levator ani avulsion strongly correlates with posterior compartment prolapse (5), while van Gruting et al. (2021) reported persistent levator ani muscle avulsion in 13% of women four years after first delivery, with subsequent births exacerbating the defect (18). These observations reinforce the importance of incorporating detailed obstetric history into preoperative counselling. Obesity also contributed to recurrence risk. Increased intra-abdominal pressure, impaired wound healing, and systemic inflammation are recognised mechanisms linking obesity to pelvic floor failure. Long-term follow-up data show that obese women have a significantly higher likelihood of reoperation for recurrent prolapse (19), and meta-analytic evidence confirms BMI as a strong predictor of both POP severity and recurrence (20). Weight optimisation should therefore be considered part of preoperative management to improve outcomes.
The overall complication rate of 11.7% in our series, including mesh exposure (5.0%) and retraction (4.2%), is comparable with the 4–12% range reported in contemporary studies of posterior mesh repair (10,12). Notably, no cases of fistula formation or deep infection occurred, indicating an acceptable safety profile when the procedure is performed in specialised centres by experienced surgeons. Since the 2019 FDA restrictions on transvaginal mesh, there has been growing emphasis on careful patient selection, surgical expertise, and shared decision-making. Our findings support the continued use of mesh-augmented posterior repair in well-selected cases where functional improvement is the primary goal.
This study has several limitations. Its retrospective, single-centre design limits generalisability, and the follow-up period of 12 months may not fully capture late recurrences or mesh-related complications. Despite these limitations, the study provides valuable real-world evidence supporting the short-term safety and efficacy of mesh-augmented posterior repair in appropriately selected patients.
Overall, transvaginal mesh–augmented rectocele repair provides meaningful short-term improvements in bowel function, pain, and quality of life with acceptable morbidity. However, recurrence remains influenced by patient factors particularly multiparity and obesity highlighting the importance of individualised risk assessment, lifestyle optimisation, and long-term follow-up. Future prospective, stratified multicentre studies with extended surveillance are needed to refine patient selection criteria, validate long-term durability, and optimise outcomes for posterior compartment prolapse.
5. Conclusions
Transvaginal Prolene mesh–augmented rectocele repair provides significant short-term improvements in bowel function, pelvic pain, and quality of life, with an acceptable complication rate when performed in specialised centres. Multiparity and obesity were identified as key predictors of recurrence, emphasising the need for careful preoperative counselling, weight optimisation, and tailored follow-up. Although long-term data are required, these findings support mesh-augmented repair as a viable option for selected patients with symptomatic posterior compartment prolapse seeking durable functional improvement.
Author Contributions
Conceptualization, G.A.; methodology, G.A., Y.E.S., M.O. and Z.D.; software, G.A.; validation, G.A.; formal analysis, G.A.; investigation, G.A.; resources, G.A.; data curation, G.A.; writing—original draft preparation, G.A.; writing—review and editing, G.A., MO, O.D.; visualization, G.A.; supervision, O.D.; project administration, O.D.; funding acquisition, O.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (Ethics Committee) of Akdeniz University Faculty of Medicine (protocol code TBAEK-630, date of approval: 5 September 2024).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patients to publish this paper.
Data Availability Statement
The original contributions presented in this study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author(s).
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| BMI |
Body mass index |
| CSS |
Constipation Scoring System |
| ODS |
Obstructed Defecation Syndrome |
| PAC-QOL |
Patient Assessment of Constipation Quality of Life |
| VAS |
Visual Analogue Scale |
| QoL |
Quality of Life |
| POP |
Pelvic Organ Prolapse |
| LAM |
Levator Ani Muscle |
| SD |
Standard Deviation |
| FDA |
Food and Drug Administration |
References
- Olsen A, Smith V, Bergstrom J, Colling J, Clark A. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstetrics & Gynecology. 1997 Apr;89(4):501–6. [CrossRef]
- Wu JM, Matthews CA, Conover MM, Pate V, Jonsson Funk M. Lifetime Risk of Stress Urinary Incontinence or Pelvic Organ Prolapse Surgery. Obstetrics & Gynecology. 2014 Jun;123(6):1201–6. [CrossRef]
- Nygaard I. Prevalence of Symptomatic Pelvic Floor Disorders in US Women. JAMA. 2008 Sep 17;300(11):1311. [CrossRef]
- Bharucha AE, Rao SSC. An Update on Anorectal Disorders for Gastroenterologists. Gastroenterology. 2014 Jan;146(1):37-45.e2.
- Dietz H, Steensma A. The prevalence of major abnormalities of the levator ani in urogynaecological patients. BJOG. 2006 Feb 13;113(2):225–30. [CrossRef]
- DeLancey JOL. The hidden epidemic of pelvic floor dysfunction: Achievable goals for improved prevention and treatment. Am J Obstet Gynecol. 2005 May;192(5):1488–95. [CrossRef]
- Swift SE, Tate SB, Nicholas J. Correlation of symptoms with degree of pelvic organ support in a general population of women: what is pelvic organ prolapse? Am J Obstet Gynecol. 2003 Aug;189(2):372–7. [CrossRef]
- Maher C, Feiner B, Baessler K, Christmann-Schmid C, Haya N, Brown J. Surgery for women with apical vaginal prolapse. Cochrane Database of Systematic Reviews. 2016 Oct 1;2017(11). [CrossRef]
- U.S. Food and Drug Administration. https://www.fda.gov/medical-devices/urogynecologic-surgical-mesh-implants/pelvic-organ-prolapse-pop-surgical-mesh-considerations-and-recommendations. FDA orders manufacturers to stop selling all devices for transvaginal repair of pelvic organ prolapse. 2019. Available.
- Carey M, Higgs P, Goh J, Lim J, Leong A, Krause H, et al. Vaginal repair with mesh versus colporrhaphy for prolapse: a randomised controlled trial. BJOG. 2009 Sep 10;116(10):1380–6. [CrossRef]
- Altman D, Väyrynen T, Engh ME, Axelsen S, Falconer C. Anterior Colporrhaphy versus Transvaginal Mesh for Pelvic-Organ Prolapse. New England Journal of Medicine. 2011 May 12;364(19):1826–36. [CrossRef]
- Weintraub AY, Friedman T, Baumfeld Y, Neymeyer J, Neuman M, Krissi H. Long-term functional outcomes following mesh-augmented posterior vaginal prolapse repair. International Journal of Gynecology & Obstetrics. 2016 Oct 2;135(1):107–11. [CrossRef]
- Agachan F, Chen T, Pfeifer J, Reissman P, Wexner SD. A constipation scoring system to simplify evaluation and management of constipated patients. Dis Colon Rectum. 1996 Jun;39(6):681–5. [CrossRef]
- Altomare DF, Spazzafumo L, Rinaldi M, Dodi G, Ghiselli R, Piloni V. Set-up and statistical validation of a new scoring system for obstructed defaecation syndrome. Colorectal Disease. 2008 Jan 18;10(1):84–8. [CrossRef]
- Marquis P, De La Loge C, Dubois D, McDermott A, Chassany O. Development and validation of the Patient Assessment of Constipation Quality of Life questionnaire. Scand J Gastroenterol. 2005 May 8;40(5):540–51. [CrossRef]
- Scott J, Huskisson EC. Vertical or horizontal visual analogue scales. Ann Rheum Dis. 1979 Dec;38(6):560. [CrossRef]
- Kayondo M, Geissbüehler V, Migisha R, Kajabwangu R, Njagi J, Kato PK, et al. Risk factors for recurrence of pelvic organ prolapse after vaginal surgery among Ugandan women: a prospective cohort study. Int Urogynecol J. 2022 Jul 28;33(7):1933–9. [CrossRef]
- van Gruting IMA, van Delft KWM, Sultan AH, Thakar R. Natural history of levator ani muscle avulsion 4 years following childbirth. Ultrasound in Obstetrics & Gynecology. 2021 Aug 3;58(2):309–17. [CrossRef]
- Lallemant M, Giraudet G, Delporte V, Behal H, Rubod C, Delplanque S, et al. Long-Term Assessment of Pelvic Organ Prolapse Reoperation Risk in Obese Women: Vaginal and Laparoscopic Approaches. J Clin Med. 2022 Nov 21;11(22):6867. [CrossRef]
- Schulten SFM, Claas-Quax MJ, Weemhoff M, van Eijndhoven HW, van Leijsen SA, Vergeldt TF, et al. Risk factors for primary pelvic organ prolapse and prolapse recurrence: an updated systematic review and meta-analysis. Am J Obstet Gynecol. 2022 Aug;227(2):192–208. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).