Submitted:
28 October 2025
Posted:
30 October 2025
You are already at the latest version
Abstract
Mannans are structurally composed of β-(1→4)-linked mannose units, which are widely distributed in plant cell walls, yeast, and bacterial exopolysaccharides. Mannans have emerged as multipurpose biopolymers with significant industrial and biomedical potential. Celebrated mannans include guar gum, locust bean gum, konjac glucomannan, yeast mannans, and softwood glucomannans. This comprehensive review highlights the sources, structural diversity, extraction methods, physicochemical properties, and functional characteristics. The major bioactivities of mannans, including immunomodulatory, antioxidative, and prebiotic effects, reflect their relevance in biopharmaceutical applications. Moreover, mannans serve as valuable raw materials for developing biodegradable films, hydrogels, and nanocomposites applied in sustainable materials and drug delivery systems. Despite promising applications, challenges related to their large-scale production, standardization, and functional optimization remain to be investigated. Future perspectives focus on integrating advanced biotechnological approaches and chemical modifications to enhance the functional versatility of mannans. Overall, mannans represent a sustainable, multifunctional biopolymer with expanding applications across food, pharmaceutical, and biomedical industries.
Keywords:
1. Introduction
2. Methodology of Literature Review
3. Chemical Structure and Types of Mannans
4. Physicochemical Properties of Mannans
4.1. Solubility
4.2. Viscosity and Rheology
4.3. Gelling Properties
4.4. Biodegradability and Biocompatibility
4.5. Functional Modifications
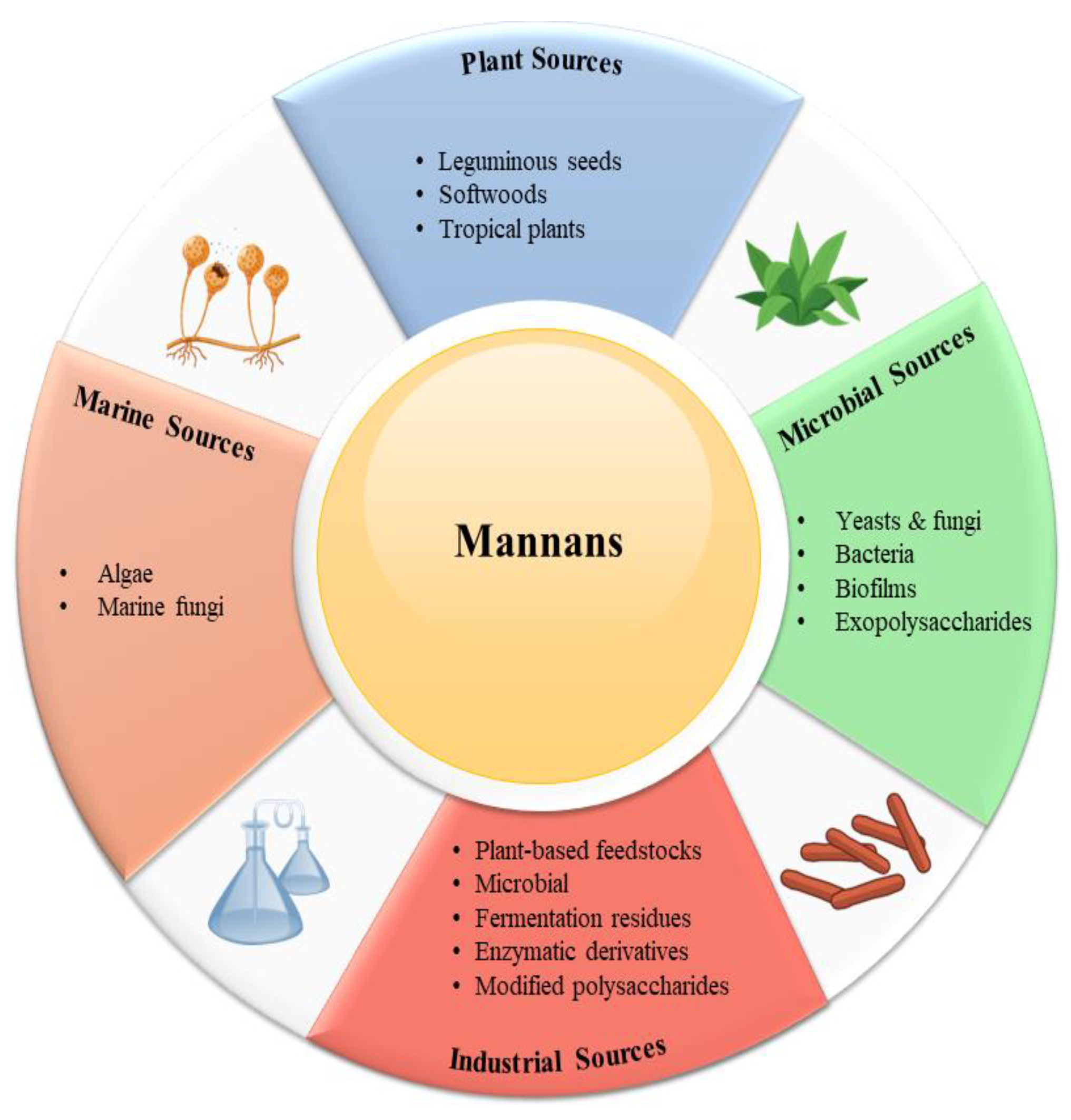
5. Natural Sources of Mannans
5.1. Plant Sources
5.2. Microbial Sources
5.3. Marine Sources
5.4. Industrial Sources
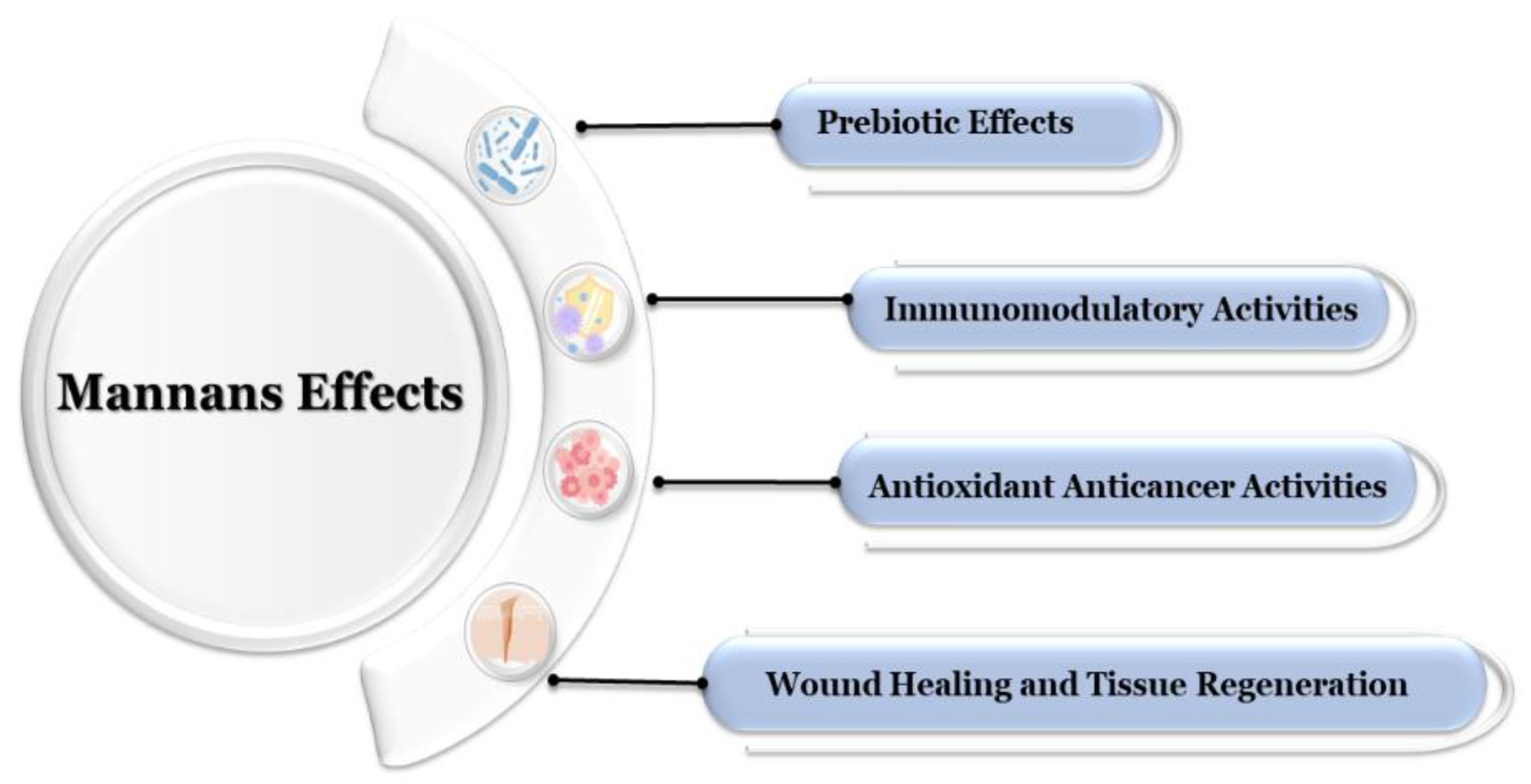
6. Biological Functions and Bioactivities
6.1. Prebiotic Effects
6.2. Immunomodulatory Activities
6.3. Antimicrobial Effects
6.4. Antioxidant and Anticancer Activities
6.5. Wound Healing and Tissue Regeneration
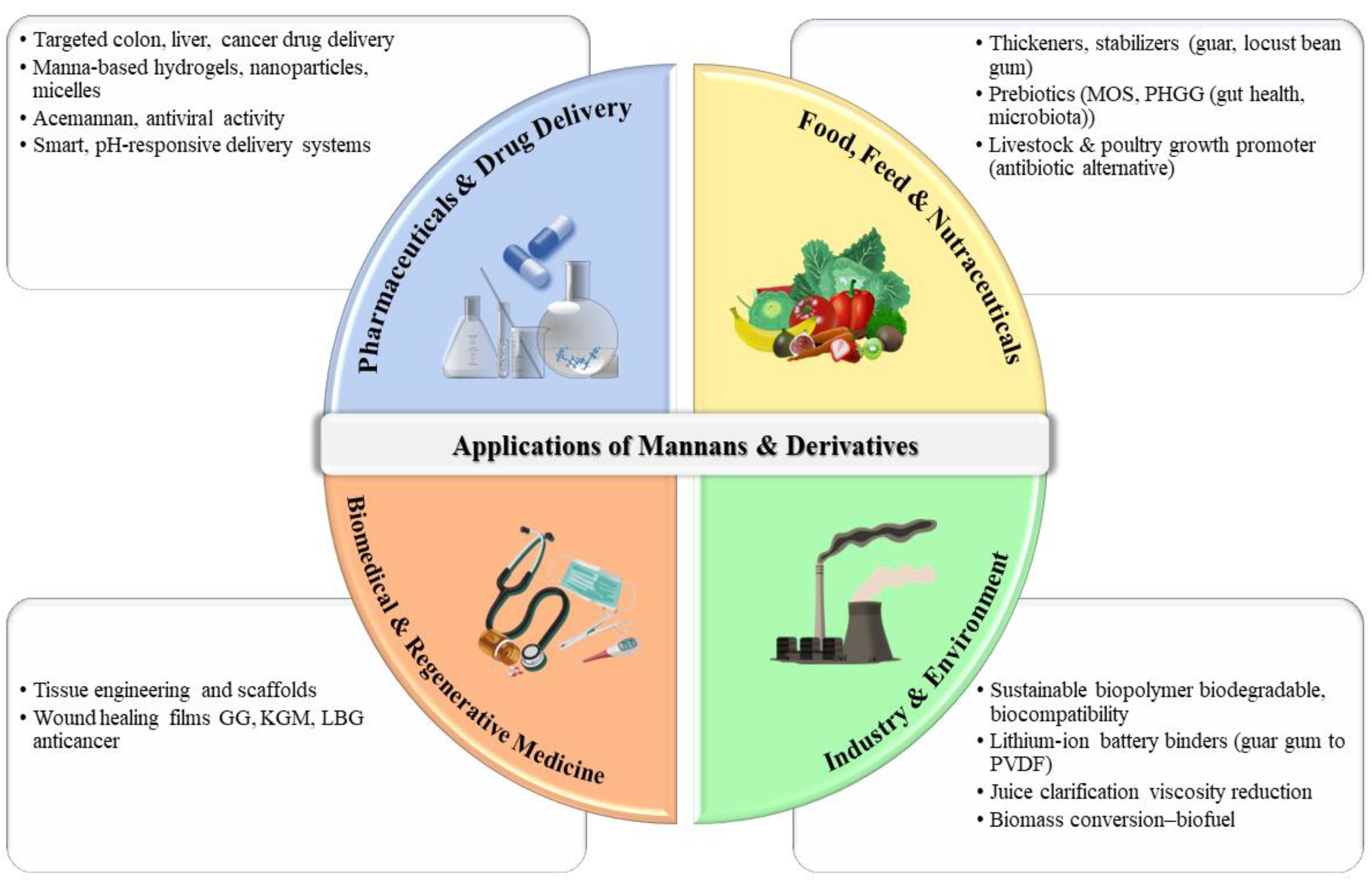
7. Industrial and Biomedical Applications of Mannans
7.1. Food, Feed, and Nutraceutical Industry
7.2. Pharmaceutical and Drug Delivery Systems
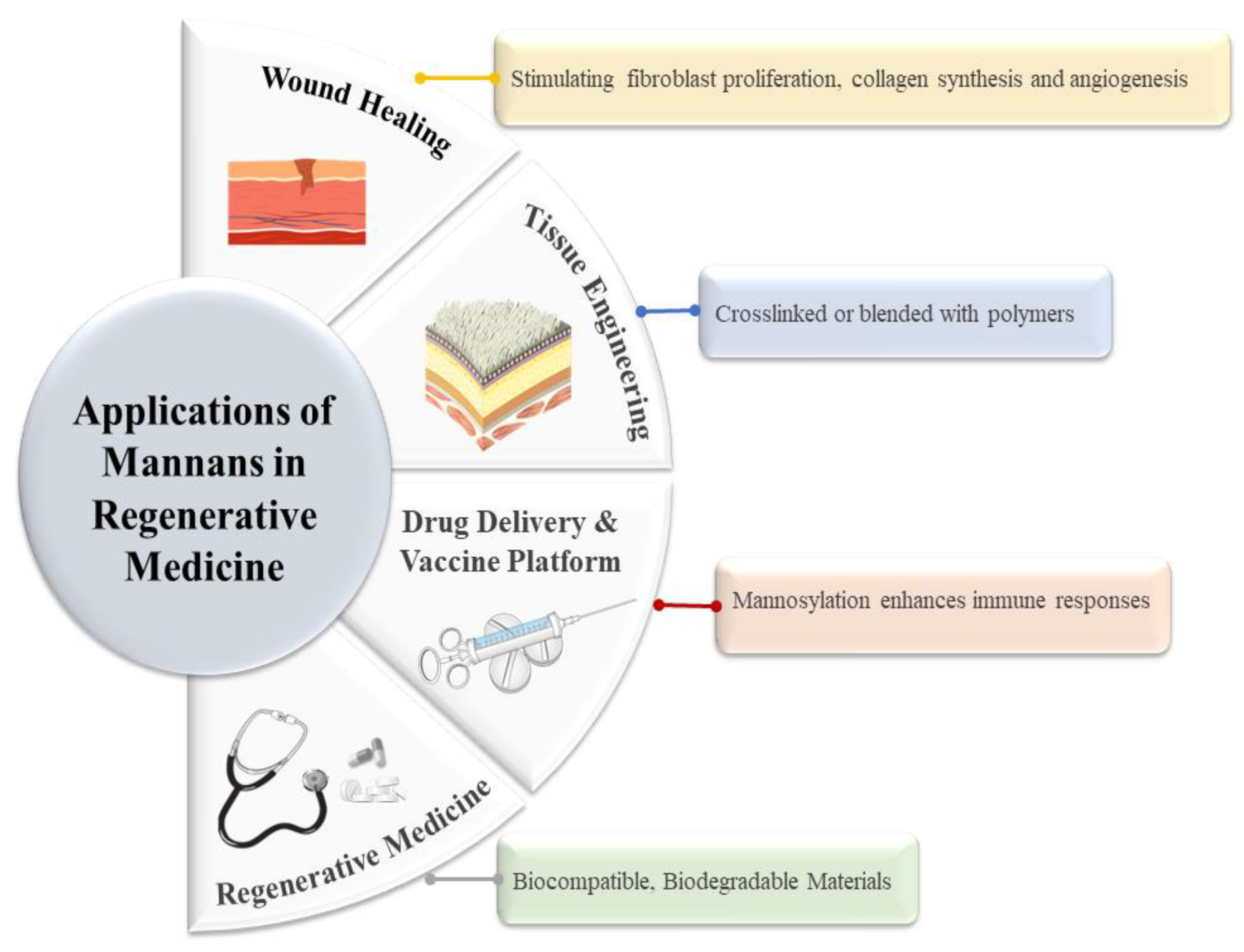
7.3. Biomedical and Tissue Engineering Applications
7.4. Environmental and Industrial Biotechnology
8. Future Prospects and Challenges of Mannans
9. Conclusions and Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gong, M.; Bassi, A. Carotenoids from microalgae: A review of recent developments. Biotechnol. Adv. 2016, 34, 1396–1412. [Google Scholar] [CrossRef] [PubMed]
- Liepman, A. H.; Nairn, C. J.; Willats, W. G.; Sørensen, I.; Roberts, A. W.; Keegstra, K. Functional genomic analysis supports conservation of function among cellulose synthase-like a gene family members and suggests diverse roles of mannans in plants. Plant Physiol. 2007, 143, 1881–1893. [Google Scholar] [CrossRef] [PubMed]
- Moreira, L.; Filho, E. An overview of mannan structure and mannan-degrading enzyme systems. Appl. Microbiol. Biotechnol.. 2008, 79, 165–178. [Google Scholar] [CrossRef]
- Srivastava, P. K.; Kapoor, M. Production, properties, and applications of endo-β-mannanases. Biotechnol. Adv. 2017, 35, 1–19. [Google Scholar] [CrossRef]
- Scheller, H. V.; Ulvskov, P. Hemicelluloses. Ann. Rev. Plant Biol. 2010, 61, 263–289. [Google Scholar] [CrossRef]
- Zhao, S.; Tariq, F.; Ma, C. Integrative dynamics of cell wall architecture and plant growth under salt stress. Front. Plant Sci. 2025, 16, 1644412. [Google Scholar] [CrossRef] [PubMed]
- Hall, R. A.; Gow, N. A. Mannosylation in C andida albicans: role in cell wall function and immune recognition. Mol. Microbiol. 2013, 90, 1147–1161. [Google Scholar] [CrossRef]
- Cottier, F.; Hall, R. A. Face/off: The interchangeable side of Candida albicans. Front.Cell. Infect. Microbiol. 2020, 9, 471. [Google Scholar] [CrossRef]
- Teli, S.; Deshmukh, K.; Khan, T.; Suvarna, V. Recent Advances in Biomedical Applications of Mannans and Xylans. Curr. Drug Targets. 2024, 25, 261–277. [Google Scholar] [CrossRef]
- Cerqueira, M.; Bourbon, A.; Pinheiro, A.; Martins, J.; Souza, B.; Teixeira, J.; Vicente, A. Galactomannans use in the development of edible films/coatings for food applications. Trends Food Sci. Technol.. 2011, 22, 662–671. [Google Scholar] [CrossRef]
- Voiniciuc, C. Modern mannan: a hemicellulose's journey. New Phytol. 2022, 234, 1175–1184. [Google Scholar] [CrossRef]
- Hu, Q.; Huang, G.; Huang, H. Extraction, structure, activity and application of konjac glucomannan. Ultrason. Sonochem. 2025, 107315. [Google Scholar] [CrossRef]
- Shen, L.; Haufe, J.; Patel, M. K. Product overview and market projection of emerging bio-based plastics PRO-BIP 2009. Report for European polysaccharide network of excellence (EPNOE) and European bioplastics. 2009, 243, 1–245. [Google Scholar]
- Petitjean, M.; Isasi, J. R. Locust bean gum, a vegetable hydrocolloid with industrial and biopharmaceutical applications. Molecules. 2022, 27, 8265. [Google Scholar] [CrossRef]
- Li, F.; Zhao, J.; Wei, Y.; Jiao, X.; Li, Q. Holistic review of polysaccharides isolated from pumpkin: Preparation methods, structures and bioactivities. Int. J. Biol. Macromol. 2021, 193, 541–552. [Google Scholar] [CrossRef]
- Zhong, R.; Zhou, D.; Chen, L.; Rose, J. P.; Wang, B.C.; Ye, Z.H. Plant cell wall polysaccharide O-acetyltransferases. Plants. 2024, 13, 2304. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, R. A. R.; Matulewicz, M. C.; Ciancia, M. NMR spectroscopy for structural elucidation of sulfated polysaccharides from red seaweeds. Int. J. Biol. Macromol. 2022, 199, 386–400. [Google Scholar] [CrossRef] [PubMed]
- De Jesus Raposo, M. F.; De Morais, A. M. B.; De Morais, R. M. S. C. Marine polysaccharides from algae with potential biomedical applications. Marine drugs. 2015, 13, 2967–3028. [Google Scholar] [CrossRef]
- Pitkänen, L.; Heinonen, M.; Mikkonen, K. S. Safety considerations of plant polysaccharides for food use: a case study on phenolic-rich softwood galactoglucomannan extract. Food Funct. 2018, 9, 1931–1943. [Google Scholar] [CrossRef] [PubMed]
- Casillo, A.; Fabozzi, A.; Russo Krauss, I.; Parrilli, E.; Biggs, C. I.; Gibson, M. I.; Lanzetta, R.; Appavou, M.-S.; Radulescu, A.; Tutino, M. L. Physicochemical approach to understanding the structure, conformation, and activity of mannan polysaccharides. Biomacromolecules. 2021, 22, 1445–1457. [Google Scholar] [CrossRef]
- Xia, P.; Zheng, Y.; Sun, L.; Chen, W.; Shang, L.; Li, J.; Hou, T.; Li, B. Regulation of glycose and lipid metabolism and application based on the colloidal nutrition science properties of konjac glucomannan: A comprehensive review. Carbohydr. Polym. 2024, 331, 121849. [Google Scholar] [CrossRef]
- Buckeridge, M. S. Seed cell wall storage polysaccharides: models to understand cell wall biosynthesis and degradation. Plant physiol. 2010, 154, 1017–1023. [Google Scholar] [CrossRef]
- Stephen, A. Other plant polysaccharides. In The polysaccharides, Elsevier, 1983; pp 97-193.
- Reid, J. S. G. , and Edwards, M. Galactomannans and other cell wall storage polysaccharides in seeds. In Seed Development and Germination., In: Kigel, J. a. G., G. Ed.; Marcel Dekker., 1995.
- Dakia PA, K.N. R. a. N. S. Physicochemical properties and functional characteristics of konjac glucomannan. Food Chem. 2008, 107, 1481–1487. [Google Scholar]
- Stephen, A. M.; Phillips, G. O. Food polysaccharides and their applications; CRC press, 2016.
- Shao, J.; Pu, J.; Chen, F.; Liu, Y.; Song, J. Konjac glucomannan-based hydrogels with tunable mechanical strength and frictional resistance for biomedical applications. International Journal of Biological Macromolecules. 2025, 295, 139612. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, C.; Zhang, W.; Wang, X.; Zhao, Z.; Wang, Z.; Zhang, L. Multifunctional self-healing and pH-responsive hydrogel dressing based on cationic guar gum and hyaluronic acid for on-demand drug release. Int. J. Biol. Macromol. 2025, 140326. [Google Scholar] [CrossRef]
- Nagano, T.; Higashimura, Y.; Nakano, M.; Nishiuchi, T.; Lelo, A. P. High-viscosity dietary fibers modulate gut microbiota and liver metabolism to prevent obesity in high-fat diet-fed mice. Int. J.Biol. Macromol. 2025, 298, 139962. [Google Scholar] [CrossRef]
- Sittikijyothin, W.; Torres, D.; Gonçalves, M. Modelling the rheological behaviour of galactomannan aqueous solutions. Carbohydr. Polym. 2005, 59, 339–350. [Google Scholar] [CrossRef]
- Fu, K.; Wang, H.; Pan, T.; Cai, Z.; Yang, Z.; Liu, D.; Wang, W. Gel-forming polysaccharides of traditional gel-like foods: Sources, structure, gelling mechanism, and advanced applications. Food Res. Int. 2024, 198, 115329. [Google Scholar] [CrossRef]
- Huynh, N.; Fliri, L.; Valle-Delgado, J. J.; Österberg, M. Exploiting the high affinity between cellulose nanofibrils and Aloe vera acemannan to develop elastic, crosslinker-free, all-polysaccharide hydrogels. Int J. Biol. Macromol. 2025, 304, 140853. [Google Scholar] [CrossRef] [PubMed]
- Dakia, P. A.; Blecker, C.; Robert, C.; Wathelet, B.; Paquot, M. Composition and physicochemical properties of locust bean gum extracted from whole seeds by acid or water dehulling pre-treatment. Food Hydrocoll. 2008, 22, 807–818. [Google Scholar] [CrossRef]
- Wang, P.; Pei, X.; Zhou, W.; Zhao, Y.; Gu, P.; Li, Y.; Gao, J. Research and application progress of microbial β-mannanases: a mini-review. World J. Microbiol Biotechnol. 2024, 40, 169. [Google Scholar] [CrossRef]
- Ferreira, S. A.; Oslakovic, C.; Cukalevski, R.; Frohm, B.; Dahlbäck, B.; Linse, S.; Gama, F. M.; Cedervall, T. Biocompatibility of mannan nanogel—safe interaction with plasma proteins. Biochim. Biophys. Acta Gen. Subj. 2012, 1820, 1043–1051. [Google Scholar] [CrossRef]
- Paliya, B. S.; Sharma, V. K.; Sharma, M.; Diwan, D.; Nguyen, Q. D.; Aminabhavi, T. M.; Rajauria, G.; Singh, B. N.; Gupta, V. K. Protein-polysaccharide nanoconjugates: Potential tools for delivery of plant-derived nutraceuticals. Food Chem. 2023, 428, 136709. [Google Scholar] [CrossRef]
- Wu, J.; Liu, Y.; Liu, R.; Xiao, C.; Xuan, L.; Wu, L.; Qian, J.; Qin, X.; Hou, Y.; Xie, M. Fishing out AIEC with FimH capturing microgels for inflammatory bowel disease treatment. Nat. Commun. 2025, 16, 7924. [Google Scholar] [CrossRef]
- Salehi, F. Effect of common and new gums on the quality, physical, and textural properties of bakery products: A review. J. Texture Stud. 2020, 51, 361–370. [Google Scholar] [CrossRef]
- Willför, S.; Sundberg, K.; Tenkanen, M.; Holmbom, B. Spruce-derived mannans–A potential raw material for hydrocolloids and novel advanced natural materials. Carbohydr. Polym. 2008, 72, 197–210. [Google Scholar] [CrossRef]
- Sang, J.; Zhao, G.; Koidis, A.; Wei, X.; Huang, W.; Guo, Z.; Wu, S.; Huang, R.; Lei, H. Isolation, structural, biological activity and application of Gleditsia species seeds galactomannans. Carbohydr. Polym. 2024, 334, 122019. [Google Scholar] [CrossRef] [PubMed]
- Malgas, S.; van Dyk, J. S.; Pletschke, B. I. A review of the enzymatic hydrolysis of mannans and synergistic interactions between β-mannanase, β-mannosidase and α-galactosidase. World J. Microbiol. Biotechnol. 2015, 31, 1167–1175. [Google Scholar] [CrossRef]
- Yu, W.; Shen, L.; Qi, J.; Hu, T. Conjugation with loxoribine and mannan improves the immunogenicity of Mycobacterium tuberculosis CFP10-TB10. 4 fusion protein. Eur. J. Pharm. Biopharm. 2022, 172, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, J.; Li, Q.; Wang, Y.; Wang, C. Two natural glucomannan polymers, from Konjac and Bletilla, as bioactive materials for pharmaceutical applications. Biotechnol. Lett. 2015, 37, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Granato, D.; Reshamwala, D.; Korpinen, R.; Azevedo, L.; do Carmo, M. A. V.; Cruz, T. M.; Marques, M. B.; Wen, M.; Zhang, L.; Marjomäki, V. From the forest to the plate–Hemicelluloses, galactoglucomannan, glucuronoxylan, and phenolic-rich extracts from unconventional sources as functional food ingredients. Food Chem. 2022, 381, 132284. [Google Scholar] [CrossRef] [PubMed]
- Kilpatrick, D. Mannan-binding lectin and its role in innate immunity. Transfus. Med. 2002, 12, 335–352. [Google Scholar] [CrossRef] [PubMed]
- Schiavone, M.; François, J. M.; Zerbib, D.; Capp, J.-P. Emerging relevance of cell wall components from non-conventional yeasts as functional ingredients for the food and feed industry. J. Curr. Res. Food Sci. 2023, 7, 100603. [Google Scholar] [CrossRef]
- Méndez-Líter, J. A.; de Eugenio, L. I.; Nieto-Domínguez, M.; Prieto, A.; Martínez, M. J. Hemicellulases from Penicillium and Talaromyces for lignocellulosic biomass valorization: A review. Bioresour. Technol. 2021, 324, 124623. [Google Scholar] [CrossRef]
- Wang, Z. A.; Li, L. X.; Doering, T. L. Unraveling synthesis of the cryptococcal cell wall and capsule. Glycobiology. 2018, 28, 719–730. [Google Scholar] [CrossRef]
- Zou, Y.; Liu, M.; Lai, Y.; Liu, X.; Li, X.; Li, Y.; Tang, Q.; Xu, W. The glycoside hydrolase gene family profile and microbial function of Debaryomyces hansenii Y4 during South-road dark tea fermentation. Front. Microbiol. 2023, 14, 1229251. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, Q.; Wu, X.; Algharib, S. A.; Gong, F.; Hu, J.; Luo, W.; Zhou, M.; Pan, Y.; Yan, Y. Structure, preparation, modification, and bioactivities of β-glucan and mannan from yeast cell wall: A review. Int. J. Biol Macromol. 2021, 173, 445–456. [Google Scholar] [CrossRef]
- Shibata, N.; Suzuki, A.; Kobayashi, H.; Okawa, Y. Chemical structure of the cell-wall mannan of Candida albicans serotype A and its difference in yeast and hyphal forms. Biochem. J. 2007, 404, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Larsbrink, J.; Tuveng, T. R.; Pope, P. B.; Bulone, V.; Eijsink, V. G.; Brumer, H.; McKee, L. S. Proteomic insights into mannan degradation and protein secretion by the forest floor bacterium Chitinophaga pinensis. J Proteom. 2017, 156, 63–74. [Google Scholar] [CrossRef]
- Deniaud-Bouët, E. , Hardouin, K., Potin, P., Kloareg, B., & Hervé, C. A review about brown algal cell walls and fucanase-driven action for biotechnological applications. Adv. Bot. Res. 2014, 71, 91–148. [Google Scholar]
- Grice, I.; Mariottini, G. Glycans with antiviral activity from marine organisms. Marine organisms as model systems in biology and medicine. 2018; 439–475. [Google Scholar]
- Chi, Y.; Li, Y.; Ding, C.; Liu, X.; Luo, M.; Wang, Z.; Bi, Y.; Luo, S. Structural and biofunctional diversity of sulfated polysaccharides from the genus Codium (Bryopsidales, Chlorophyta): a review. Int. J. Biol. Macromol. 2024, 263, 130364. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, P. S.; Gupta, N. Insight into microbial mannosidases: a review. Crit. Rev. Biotechnol. 2017, 37, 190–201. [Google Scholar] [CrossRef]
- Dhawan, S.; Kaur, J. Microbial mannanases: an overview of production and applications. Crit. Rev. Biotechnol. 2007, 27, 197–216. [Google Scholar] [CrossRef] [PubMed]
- Galante, Y. M.; Merlini, L.; Silvetti, T.; Campia, P.; Rossi, B.; Viani, F.; Brasca, M. Enzyme oxidation of plant galactomannans yielding biomaterials with novel properties and applications, including as delivery systems. Appl. Microbiol. Biotechnol. 2018, 102, 4687–4702. [Google Scholar] [CrossRef]
- Faustino, M.; Durão, J.; Pereira, C. F.; Pintado, M. E.; Carvalho, A. P. Mannans and mannan oligosaccharides (MOS) from Saccharomyces cerevisiae–A sustainable source of functional ingredients. Carbohydr.Polym. 2021, 272, 118467. [Google Scholar] [CrossRef]
- Rana, M.; Jassal, S.; Yadav, R.; Sharma, A.; Puri, N.; Mazumder, K.; Gupta, N. Functional β-mannooligosaccharides: Sources, enzymatic production and application as prebiotics. Crit. Rev. Food Sci. Nutr. 2024, 64, 10221–10238. [Google Scholar] [CrossRef]
- Baurhoo, B.; Letellier, A.; Zhao, X.; Ruiz-Feria, C. Cecal populations of lactobacilli and bifidobacteria and Escherichia coli populations after in vivo Escherichia coli challenge in birds fed diets with purified lignin or mannanoligosaccharides. Poult. Sci. 2007, 86, 2509–2516. [Google Scholar] [CrossRef]
- Utama, G. L.; Oktaviani, L.; Balia, R. L.; Rialita, T. Potential application of yeast cell wall biopolymers as probiotic encapsulants. Polymers. 2023, 15, 3481. [Google Scholar] [CrossRef]
- Al-Asmari, F.; Abdelshafy, A. M.; Neetoo, H.; Zhang, Y. Natural gums (gum Arabic, guar gum and xanthan gum) as a promising source of prebiotics: a review on their functional roles and food applications. Int. J. Biol. Macromol. 2025, 318, 145101. [Google Scholar] [CrossRef]
- Jana, U. K.; Suryawanshi, R. K.; Prajapati, B. P.; Kango, N. Prebiotic mannooligosaccharides: Synthesis, characterization and bioactive properties. Food Chem. 2021, 342, 128328. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, D. U.; Sharma, H.; Maheshwari, R.; Pareek, A.; Gaur, M.; Prajapati, B. G.; Castro, G. R.; Thanawuth, K.; Suttiruengwong, S.; Sriamornsak, P. Konjac glucomannan: A comprehensive review of its extraction, health benefits, and pharmaceutical applications. Carbohydr.polym. 2024, 339, 122266. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Xi, Y.; Wang, Q.; Liu, J.; Li, P.; Meng, X.; Liu, K.; Chen, W.; Liu, X.; Liu, Z. Mannan oligosaccharide attenuates cognitive and behavioral disorders in the 5xFAD Alzheimer's disease mouse model via regulating the gut microbiota-brain axis. Brain, Behav. Immun. 2021, 95, 330–343. [Google Scholar] [CrossRef] [PubMed]
- Tang, N.; Wang, X.; Yang, R.; Liu, Z.; Liu, Y.; Tian, J.; Xiao, L.; Li, W. Extraction, isolation, structural characterization and prebiotic activity of cell wall polysaccharide from Kluyveromyces marxianus. Carbohydr. Polym. 2022, 289, 119457. [Google Scholar] [CrossRef]
- Wang, J.; Ke, S.; Strappe, P.; Ning, M.; Zhou, Z. Structurally orientated rheological and gut microbiota fermentation property of mannans polysaccharides and oligosaccharides. Foods. 2023, 12, 4002. [Google Scholar] [CrossRef]
- Kwak, S.; Robinson, S. J.; Lee, J. W.; Lim, H.; Wallace, C. L.; Jin, Y.-S. Dissection and enhancement of prebiotic properties of yeast cell wall oligosaccharides through metabolic engineering. Biomaterials. 2022, 282, 121379. [Google Scholar] [CrossRef]
- Fernandes, A.; Nair, A.; Kulkarni, N.; Todewale, N.; Jobby, R. Exploring mushroom polysaccharides for the development of novel prebiotics: A review. Int. J. Med. Mushrooms. 2023, 25. [Google Scholar] [CrossRef]
- Panwar, D.; Shubhashini, A.; Kapoor, M. Complex alpha and beta mannan foraging by the human gut bacteria. Biotechnol. Adv. 2023, 66, 108166. [Google Scholar] [CrossRef]
- Dev, K.; Akbar Mir, N.; Biswas, A.; Kannoujia, J.; Begum, J.; Kant, R. Dietary Mannan-oligosaccharides potentiate the beneficial effects of Bifidobacterium bifidum in broiler chicken. Lett Appl. Microbiol. 2020, 71, 520–530. [Google Scholar] [CrossRef]
- Corrigan, A.; McCooey, P.; Taylor-Pickard, J.; Stockdale, S.; Murphy, R. Breaking the Cycle: A Yeast Mannan-Rich Fraction Beneficially Modulates Egg Quality and the Antimicrobial Resistome Associated with Layer Hen Caecal Microbiomes under Commercial Conditions. Microorganisms. 2024, 12, 1562. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, L.; Ma, Z.; He, W.; Huang, E.; Meng, L.; Li, L.; Tong, T.; Yang, H.; Liu, Y. Effects of Mannan Oligosaccharides on Growth, Antioxidant and Immune Performance, and mTOR Signaling Pathway in Juvenile Tilapia (Oreochromis niloticus). Animals. 2025, 15, 2459. [Google Scholar] [CrossRef] [PubMed]
- Brown, G. D.; Gordon, S. Immune recognition of fungal β-glucans. Cell. Microbiol. 2005, 7, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Ju, J.; Xu, H.; Wang, Y. Intestinal fungi and antifungal secretory immunoglobulin A in Crohn’s disease. Frontiers in Immunology. 2023, 14, 1177504. [Google Scholar] [CrossRef]
- Ma, Z.; Ensley, H. E.; Lowman, D. W.; Kruppa, M. D.; Williams, D. L. Recent advances in chemical synthesis of phosphodiester linkages found in fungal mannans. Carbohydr. Res. 2025, 547, 109325. [Google Scholar] [CrossRef]
- Pan, X.; Zong, Q.; Liu, C.; Wu, H.; Fu, B.; Wang, Y.; Sun, W.; Zhai, Y. Konjac glucomannan exerts regulatory effects on macrophages and its applications in biomedical engineering. Carbohydr. Polym. 2024, 345, 122571. [Google Scholar] [CrossRef]
- Song, X.; Lei, T.; Cui, N.; Jin, X.; Huang, Y.; Shi, Y.; Zhao, Z. A preliminary investigation on the protective effects of β-glucan and mannan induced trained immunity in pufferfish Takifugu obscurus. Fish Shellfish Immunol. 2025, 156, 110035. [Google Scholar] [CrossRef]
- Witvrouw, M.; De Clercq, E. Sulfated polysaccharides extracted from sea algae as potential antiviral drugs. General Pharmacology: The Vascular System. 1997, 29, 497–511. [Google Scholar] [CrossRef]
- Wang, W.; Wang, S.X.; Guan, H.S. The antiviral activities and mechanisms of marine polysaccharides: an overview. Mar. Drugs. 2012, 10, 2795–2816. [Google Scholar] [CrossRef]
- Mardani, R.; Bahmanje, A.; Kazeroni, Y. C.; Khoshroo, F.; Roshanaie, B.; Sadeghche, T.; Pajaie, K.; Hosseini, S. N.; Doroud, D.; Shahali, M. Oxidized Mannan: A Novel Adjuvant Candidate for Enhancing Immune Responses in Veterinary Rabies Vaccine. Vaccines (Basel). 2025, 13, 25. [Google Scholar] [CrossRef] [PubMed]
- Kim, H. W.; Ko, M.-K.; Park, S. H.; Shin, S.; Kim, G. S.; Kwak, D. Y.; Park, J.H.; Kim, S.M.; Lee, J.S.; Lee, M. J. D-galacto-D-mannan-mediated Dectin-2 activation orchestrates potent cellular and humoral immunity as a viral vaccine adjuvant. Front. Immunol. 2024, 15, 1330677. [Google Scholar] [CrossRef]
- Xing J; Zhao X; Li X; Fang R; Sun M; Zhang Y; N., S. Song N. Recent advances in vaccine adjuvants: molecular design and clinical translation. Front. Immunol. 2025, 16, 1557415.
- Neth, O.; Jack, D. L.; Dodds, A. W.; Holzel, H.; Klein, N. J.; Turner, M. W. Mannose-binding lectin binds to a range of clinically relevant microorganisms and promotes complement deposition. Infect. Immun. 2000, 68, 688–693. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Iji, P.; Choct, M. Dietary modulation of gut microflora in broiler chickens: a review of the role of six kinds of alternatives to in-feed antibiotics. World Poult. Sci. J. 2009, 65, 97–114. [Google Scholar] [CrossRef]
- Van Die, I.; Cummings, R. D. The mannose receptor in regulation of helminth-mediated host immunity. Front. Immunol. 2017, 8, 1677. [Google Scholar] [CrossRef]
- Leclercq, E.; Pontefract, N.; Rawling, M.; Valdenegro, V.; Aasum, E.; Andujar, L. V.; Migaud, H.; Castex, M.; Merrifield, D. Dietary supplementation with a specific mannan-rich yeast parietal fraction enhances the gut and skin mucosal barriers of Atlantic salmon (Salmo salar) and reduces its susceptibility to sea lice (Lepeophtheirus salmonis). Aquaculture. 2020, 529, 735701. [Google Scholar] [CrossRef]
- Bonde, C. S.; Drøhse, F. B.; Büdeyri Gökgöz, N.; Krych, L.; Nielsen, D. S.; Petersen, H. H.; Matthiesen, R.; Pedersen, N. R.; Geldhof, P.; Williams, A. R. Dietary supplementation with fermented rapeseed and seaweed modulates parasite infections and gut microbiota in outdoor pigs. Front.Vet. Sci. 2025, 12, 1565686. [Google Scholar] [CrossRef]
- Prasanphanich, N. S.; Mickum, M. L.; Heimburg-Molinaro, J.; Cummings, R. D. Glycoconjugates in host-helminth interactions. Front. Immunol. 2013, 4, 240. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, C.; Huo, K.; Cai, D.; Zhao, G. Antioxidant activity of yeast mannans and their growth-promoting effect on Lactobacillus strains. Food Funct. 2021, 12, 10423–10431. [Google Scholar] [CrossRef]
- Shwayekh, H. A.-J. I.; AL-Mosawi, R. H.; Mahrath, A. J. Potential Antidiabetic Activity of Ficus Carica: The Concept of Antioxidant Properties in a Rat Model. Hilla University College J. Med.Sci. 2024, 2, 66–71. [Google Scholar] [CrossRef]
- Liu, Y.; Du, C.; Yi, F.; Li, J.; Li, M. Enhancement on durability of cohesive soil by solidifying with modified guar gum. Int. J. Biol Macromol. 2025, 143003. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, X.; Wang, S.; Li, H.; Lu, Z.; Shi, J.; Xu, Z. Mannan-oligosaccharide modulates the obesity and gut microbiota in high-fat diet-fed mice. Food Funct. 2018, 9, 3916–3929. [Google Scholar] [CrossRef]
- Hoving, L. R.; Katiraei, S.; Heijink, M.; Pronk, A.; van der Wee-Pals, L.; Streefland, T.; Giera, M.; Willems van Dijk, K.; van Harmelen, V. Dietary mannan oligosaccharides modulate gut microbiota, increase fecal bile acid excretion, and decrease plasma cholesterol and atherosclerosis development. Mol. Nutr. Food Res. 2018, 62, 1700942. [Google Scholar] [CrossRef]
- De Araujo IW, R. J. , Vanderlei ES, et al.. Sulfated polysaccharides from marine algae with antioxidant and anticoagulant activity: a review. Mar. Drugs. 2020, 18, 435. [Google Scholar]
- Pomin, V. H. Marine non-glycosaminoglycan sulfated glycans as potential pharmaceuticals. Pharmaceuticals. 2015, 8, 848–864. [Google Scholar] [CrossRef]
- Shih, P.-C.; Lin, C.-H.; Chokkalingam, U.; Prakash, E.; Kao, C.-N.; Chang, C.-F.; Lin, W.-L. The Aloe vera acemannan polysaccharides inhibit phthalate-induced cell viability, metastasis, and stemness in colorectal cancer cells. Ecotoxicol Environ Saf. 2024, 288, 117351. [Google Scholar] [CrossRef]
- Choi, S.; Chung, M.-H. A review on the relationship between Aloe vera components and their biologic effects. In Seminars in integrative medicine, 2003; Elsevier: Vol. 1, pp 53-62.
- Negi, D.; Bhavya, K.; Pal, D.; Singh, Y. Acemannan coated, cobalt-doped biphasic calcium phosphate nanoparticles for immunomodulation regulated bone regeneration. Biomater. Sci. 2024, 12, 3672–3685. [Google Scholar] [CrossRef]
- Kaur, S.; Santra, S. Application of Guar Gum and its Derivatives as Green Binder/Separator for Advanced Lithium-Ion Batteries. ChemistryOpen. 2022, 11, e202100209. [Google Scholar] [CrossRef]
- Moazzam, P.; Boroumand, Y.; Rabiei, P.; Baghbaderani, S. S.; Mokarian, P.; Mohagheghian, F.; Mohammed, L. J.; Razmjou, A. Lithium bioleaching: An emerging approach for the recovery of Li from spent lithium ion batteries. Chemosphere. 2021, 277, 130196. [Google Scholar] [CrossRef]
- Singh, S.; Singh, G.; Arya, S. K. Mannans: An overview of properties and application in food products. Int. J. Biol. Macromol. 2018, 119, 79–95. [Google Scholar] [CrossRef]
- Abik, F.; Palasingh, C.; Bhattarai, M.; Leivers, S.; Strom, A.; Westereng, B.; Mikkonen, K. S.; Nypelo, T. Potential of wood hemicelluloses and their derivates as food ingredients. J. Agric. Food Chem. 2023, 71, 2667–2683. [Google Scholar] [CrossRef] [PubMed]
- Yasukawa, Z.; Inoue, R.; Ozeki, M.; Okubo, T.; Takagi, T.; Honda, A.; Naito, Y. Effect of repeated consumption of partially hydrolyzed guar gum on fecal characteristics and gut microbiota: a randomized, double-blind, placebo-controlled, and parallel-group clinical trial. Nutrients. 2019, 11, 2170. [Google Scholar] [CrossRef] [PubMed]
- Beteri, B.; Barone, M.; Turroni, S.; Brigidi, P.; Tzortzis, G.; Vulevic, J.; Sekulic, K.; Motei, D.-E.; Costabile, A. Impact of Combined Prebiotic Galacto-Oligosaccharides and Bifidobacterium breve-Derived Postbiotic on Gut Microbiota and HbA1c in Prediabetic Adults: A Double-Blind, Randomized, Placebo-Controlled Study. Nutrients. 2024, 16, 2205. [Google Scholar] [CrossRef] [PubMed]
- Biswas, A.; Mohan, N.; Dev, K.; Mir, N. A.; Tiwari, A. K. Effect of dietary mannan oligosaccharides and fructo-oligosaccharides on physico-chemical indices, antioxidant and oxidative stability of broiler chicken meat. Sci. Rep. 2021, 11, 20567. [Google Scholar] [CrossRef]
- Kiarie, E. G.; Steelman, S.; Martinez, M.; Livingston, K. Significance of single β-mannanase supplementation on performance and energy utilization in broiler chickens, laying hens, turkeys, sows, and nursery-finish pigs: a meta-analysis and systematic review. Transl. Anim. Sci. 2021, 5, txab160. [Google Scholar] [CrossRef]
- Dar, A.; Singh, S.; Rahman, J.; Ahmad, S. The effects of probiotic Lactobacillus acidophilus and/or prebiotic mannan oligosaccharides on growth performance, nutrient utilization, blood metabolites, faecal bacteria, and economics of crossbred calves. Iranian J Vet Res. 2022, 23, 322. [Google Scholar]
- Grossi, S.; Dell’Anno, M.; Rossi, L.; Compiani, R.; Sgoifo Rossi, C. A. Supplementation of live yeast, mannan oligosaccharide, and organic selenium during the adaptation phase of newly arrived beef cattle: Effects on health status, immune functionality, and growth performance. Antibiotics. 2021, 10, 1114. [Google Scholar] [CrossRef]
- Abd El-Aziz, A. H.; Mota-Rojas, D.; Akinjute, O. F.; Abioja, M. O. Prebiotic Oligosaccharides as Potential Growth Promoter in Rabbits: A Review. J. Anim. Physiol. Anim. Nutr. 2025. [Google Scholar] [CrossRef]
- Lu, Z.Y.; Jiang, W.D.; Wu, P.; Liu, Y.; Kuang, S.Y.; Tang, L.; Yang, J.; Zhou, X.Q.; Feng, L. Mannan oligosaccharides supplementation enhanced head-kidney and spleen immune function in on-growing grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol. 2020, 106, 596–608. [Google Scholar] [CrossRef] [PubMed]
- Ghyselinck, J.; Verstrepen, L.; Rakebrandt, M.; Marynissen, S.; Daminet, S.; Marzorati, M. In vitro fermentation of yeast cell walls (mannan-oligosaccharide) and purified β-glucans modulates the colonic microbiota of dogs with inflammatory bowel disease and demonstrates protective effects on barrier integrity and anti-inflammatory properties. PLoS One. 2025, 20, e0322877. [Google Scholar] [CrossRef] [PubMed]
- Udaipuria, N.; Bhattacharya, S. Novel Carbohydrate Polymer-Based Systems for Precise Drug Delivery in Colon Cancer: Improving Treatment Effectiveness With Intelligent Biodegradable Materials. Biopolymers. 2025, 116, e23632. [Google Scholar] [CrossRef]
- Yadav, H.; Maiti, S. Research progress in galactomannan-based nanomaterials: Synthesis and application. Int. J. Biol. Macromol. 2020, 163, 2113–2126. [Google Scholar] [CrossRef]
- YIm, S. A.; Oh, S. T.; Song, S.; Kim, M. R.; Kim, D. S.; Woo, S. S.; Jo, T. H.; Park, Y. I.; Lee, C. K. Identification of optimal molecular size of modified Aloe polysaccharides with maximum immunomodulatory activity. Int. Immunopharmacol. 2005, 5, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Irfan, J.; Ali, A.; Hussain, M. A.; Haseeb, M. T.; Alsahli, T. G.; Naeem-ul-Hassan, M.; Tulain, U. R.; Hussain, S. Z.; Hussain, I.; Azhar, I. A superabsorbent and pH-responsive copolymer-hydrogel based on acemannan from Aloe vera (Aloe barbadensis M.): A smart material for drug delivery. Int. J. Biol. Macromol. 2024, 270, 132306. [Google Scholar] [CrossRef]
- Madrid, R. R.; Mathews, P. D.; Pramanik, S.; Mangiarotti, A.; Fernandes, R.; Itri, R.; Dimova, R.; Mertins, O. Hybrid crystalline bioparticles with nanochannels encapsulating acemannan from Aloe vera: Structure and interaction with lipid membranes. J. Colloid Interface Sci. 2024, 673, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Verma, D.; Sharma, S. K. Recent advances in guar gum based drug delivery systems and their administrative routes. Int. J. Biol. Macromol. 2021, 181, 653–671. [Google Scholar] [CrossRef]
- Amjed, N.; Zeshan, M.; Farooq, A.; Naz, S. Applications of guar gum polysaccharide for pharmaceutical drug delivery: A review. Int. J. Biol. Macromol. 2024, 257, 128390. [Google Scholar] [CrossRef]
- Lin, J.; Sun, Y.; Santos, H. O.; Găman, M.A.; Bhat, L. T.; Cui, Y. Effects of guar gum supplementation on the lipid profile: A systematic review and meta-analysis of randomized controlled trials. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 3271–3281. [Google Scholar] [CrossRef]
- Manna, S.; Karmakar, S.; Sen, O.; Sinha, P.; Jana, S.; Jana, S. Recent updates on guar gum derivatives in colon specific drug delivery. Carbohydr. Polym. 2024, 334, 122009. [Google Scholar] [CrossRef]
- Zhuang, K.; Shu, X.; Xie, W. Konjac glucomannan-based composite materials: Construction, biomedical applications, and prospects. Carbohydr. Polym. 2024, 344, 122503. [Google Scholar] [CrossRef] [PubMed]
- Illanes-Bordomás, C.; Landin, M.; García-González, C. A. Novel Core–Shell Aerogel Formulation for Drug Delivery Based on Alginate and Konjac Glucomannan: Rational Design Using Artificial Intelligence Tools. Polymers. 2025, 17, 1919. [Google Scholar] [CrossRef]
- Sana, S. S.; Raorane, C. J.; Venkatesan, R.; Roy, S.; Swain, S. K.; Kim, S.-C.; Al-Tabakha, M.; Bhandare, R. R.; Raj, V.; Lee, S. State-of-the-art progress on locust bean gum polysaccharide for sustainable food packaging and drug delivery applications: A review with prospectives. Int. J. Biol. Macromol. 2024, 275, 133619. [Google Scholar] [CrossRef]
- Kumar, D.; Malviya, R.; Sridhar, S. B.; Shareef, J.; Wadhwa, T. Extraction, Physicochemical Properties, and Biomedical Applications of Locust Bean Gum: A Comprehensive Review. Mini Rev. Med. Chem. 2025. [Google Scholar] [CrossRef]
- Luanda, A.; Mahadev, M.; Charyulu, R. N.; Badalamoole, V. Locust bean gum-based silver nanocomposite hydrogel as a drug delivery system and an antibacterial agent. Int. J. Biol. Macromol. 2024, 282, 137097. [Google Scholar] [CrossRef]
- Pontes, J. F.; Guerreiro, F.; Silva, J. P.; Almeida, M. P.; Rosso, A.; da Costa, A. M. R.; Agusti, G.; Lollo, G.; Gaspar, M. M.; Grenha, A. Locust bean gum (LBG)–A potential excipient for inhalation purposes: Excipient characterisation and in vitro and in vivo toxicological evaluation. Carbohydr.Polym. 2025, 123729. [Google Scholar] [CrossRef]
- Qi, M.; Yan, S.; Cui, Y.; Huang, Y.; Liu, Y.; Wu, W.; Yu, X.; Wang, P. Mannan-Containing Polymers from Hadal Bacterium Psychrobacter pulmonis: Preparation, Structural Analysis, Immunological Activity and Antitumor Effects. Mar. Drugs. 2025, 23, 326. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, Z.; Xia, N.; Zhao, Q. Carbohydrate-containing nanoparticles as vaccine adjuvants. Expert Rev. Vaccines. 2021, 20, 797–810. [Google Scholar] [CrossRef] [PubMed]
- Sharahi, M.; Bahrami, S. H.; Karimi, A. A comprehensive review on guar gum and its modified biopolymers: Their potential applications in tissue engineering. Carbohydr.Polym. 2025, 347, 122739. [Google Scholar] [CrossRef] [PubMed]
- Zachová, K.; Bartheldyová, E.; Hubatka, F.; Křupka, M.; Odehnalová, N.; Knötigová, P. T.; Vaškovicová, N.; Sloupenská, K.; Hromádka, R.; Paulovičová, E. The immunogenicity of p24 protein from HIV-1 virus is strongly supported and modulated by coupling with liposomes and mannan. Carbohydr Polym. 2024, 332, 121844. [Google Scholar] [CrossRef]
- Deesricharoenkiat, N.; Jansisyanont, P.; Chuenchompoonut, V.; Mattheos, N.; Thunyakitpisal, P. The effect of acemannan in implant placement with simultaneous guided bone regeneration in the aesthetic zone: a randomized controlled trial. Int. J. Oral Maxillofac. Surg. 2022, 51, 535–544. [Google Scholar] [CrossRef]
- Hao, Y.; Wang, J.; Zhang, H.; Liu, Q.; Wang, X.; Wei, Y.; Liang, Z.; Hu, Y.; Huang, D. Konjac glucomannan/Bletilla striata polysaccharide composite hydrogel: A promising anti-inflammatory dressing for accelerated wound healing. Carbohydr. Polym. 2025, 123639. [Google Scholar] [CrossRef]
- Zhong, Y.; Zhang, Y.; Liu, Y.; Zeng, K.; Fan, L.; Wang, Q.; Zhang, J. Adhesive hydrogel based on Konjac Glucomannan (KGM) loaded with siACTC1-exosomes for enhanced post-surgical keloid treatment. Int. J. Biol. Macromol. 2025, 145360. [Google Scholar] [CrossRef]
- Varguez-Catzim, P.; Hernández-Aburto, M.; Rodriguez-Canto, W.; Hunh-Ibarra, M.; Aguilar-Vega, M.; Claudio-Rizo, J. A.; González-Díaz, M. O. Tailoring membrane technology with galactomannan for enhanced biocompatibility and antibacterial action. Int. J. Biol. Macromol. 2025, 286, 138320. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.; Taj, M. B.; Carabineiro, S. A. C. Gum-based nanocomposites for the removal of metals and dyes from waste water. Environ. Sci. Pollut. Res. 2023, 30, 102027–102046. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Hwang, S.; Shi, S. Q. Guar gum, a low-cost sustainable biopolymer, for wastewater treatment: a review. Int. J. Biol. Macromol. 2023, 226, 368–382. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Huang, H.; Duan, L.; Xie, X.; Zhang, J.; Tang, J.; Liu, W.; Tong, C.; Pang, J.; Wu, C. Konjac glucomannan-based films and coatings for food packaging: Advances, applications, and future perspectives. Carbohydr. Polym. 2025, 123474. [Google Scholar] [CrossRef]
- Wei, K.; Zhang, L.; Li, N.; Gao, K.; Li, X.; Li, J.; Wang, S.; Mao, X. A colorimetric biosensor composed of split aptamers and mannan oligosaccharide nanozyme to monitor synthetic His-tagged food biomolecules. Food Chem. 2025, 466, 142108. [Google Scholar] [CrossRef]
- Research; Markets. Mannan-Oligosaccharide Market Report: Trends, Forecast and Competitive Analysis to 2031; Dublin, Ireland, 2025. https://www.researchandmarkets.com/reports/6086163/mannan-oligosaccharide-market-report-trends.
- Insights, P. B. Global Mannan Oligosaccharides Market: Growth, Trends, and Forecast (2025–2031) 2024. https://www.precisionbusinessinsights.com/market-reports/mannan-oligosaccharide-market.
- Research, P. Mannan oligosaccharides (MOS) market size, share, and trends analysis, 2024–2031; 2024. https://www.datamintelligence.com/research-report/mannan-oligosaccharide-market.
- Karimi, I.; Ghowsi, M.; Mohammed, L. J.; Haidari, Z.; Nazari, K.; Schiöth, H. B. Inulin as a Biopolymer; Chemical Structure, Anticancer Effects, Nutraceutical Potential and Industrial Applications: A Comprehensive Review. Polymers. 2025, 17, 412. [Google Scholar] [CrossRef]
| Name/PubChem CID | Molecular Weight g/mol | XLogP3-AA | Hydrogen Bond Donor Count | Hydrogen Bond Acceptor Count | Rotatable Bond Count | Topological Polar Surface Area Å | |
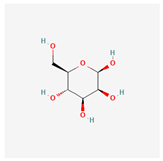 C6H12O6 C6H12O6
|
beta-D-mannopyranose, beta-D-Mannose/439680 |
180.16 | -2.6 | 5 | 6 | 1 | 110 |
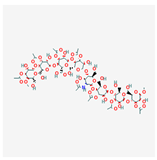 C66H101NO49 C66H101NO49
|
Acemannan, Cello gel, Acemannan (Aloe vera)/134129847 |
1692.5 | -12.8 | 17 | 49 | 39 | 711 |
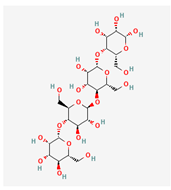 C24H42O21 C24H42O21
|
alpha-D-Mannan, Mannan, Mannoglycan/25147451 | 666.6 | -9 | 14 | 21 | 10 | 348 |
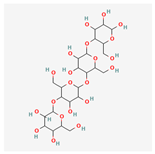 C24H42O21 C24H42O21
|
Amylotetraose; Fujioligo 450; alpha-1,4-Tetraglucose/870 | 666.6 | -9 | 14 | 21 | 10 | 348 |
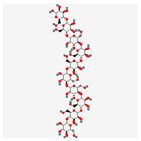 C60H102O51 C60H102O51
|
1,4-b-D-Mannan, GlyTouCan:G45304DG, G45304DG/53477899 | 1639.4 | -21.9 | 32 | 51 | 28 | 823 |
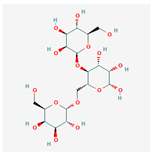 C18H32O16 C18H32O16
|
D-Galacto-d-mannan/439336 |
504.4 | -6.3 | 11 | 16 | 7 | 269 |
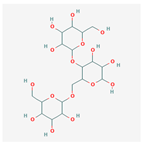 C18H32O16 C18H32O16
|
GlyTouCan:G55283BR, CAROB GALACTOMANNAN, 6-O-Glucosylmaltose, Aspergillus fumigatus galactomannan/3514701 | 504.4 | -6.3 | 11 | 16 | 7 | 269 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





