Introduction
Biology is defined by a profound integration paradox: we possess an exquisitely detailed parts list of life, yet lack a principled, mechanistic framework to explain how these parts coalesce into stable, functioning wholes [
1,
2]. Network science has provided a powerful descriptive lens, yet its representations often abstract the physical nature of connections into topological links [
3]. This abstraction overlooks a fundamental biological reality: all interactions are physically instantiated within a structured, functional space. The interstice—the contiguous, structured medium linking all biological components—has been consistently undertheorized as a passive background rather than an active architectural director.
A convergence of discoveries now compels a paradigm shift. Evidence across scales reveals that these “empty” spaces are, in fact, functional entities. From biomolecular condensates [
4] and gap junctions [
5] to the body-wide interstitium [
6] and soil pore networks [
7], interstices are not voids but active mediators of biological processes. They constitute the relational fabric of life.
Building on our work on physical frameworks for emergence [
8] and the human interstitial system [
9], we formalize Biological Interstitialogy. This framework posits that interstices are the primary sites where local interactions are orchestrated and where novel system-level properties quantitatively emerge. We articulate a foundational, testable causal principle—Structure → Flow → Function—and demonstrate its mechanistic validity across the biological hierarchy. This paradigm shift moves biology from a science of parts to a science of coherent wholes.
Results
The S-F-F Principle: A Universal Causal Chain
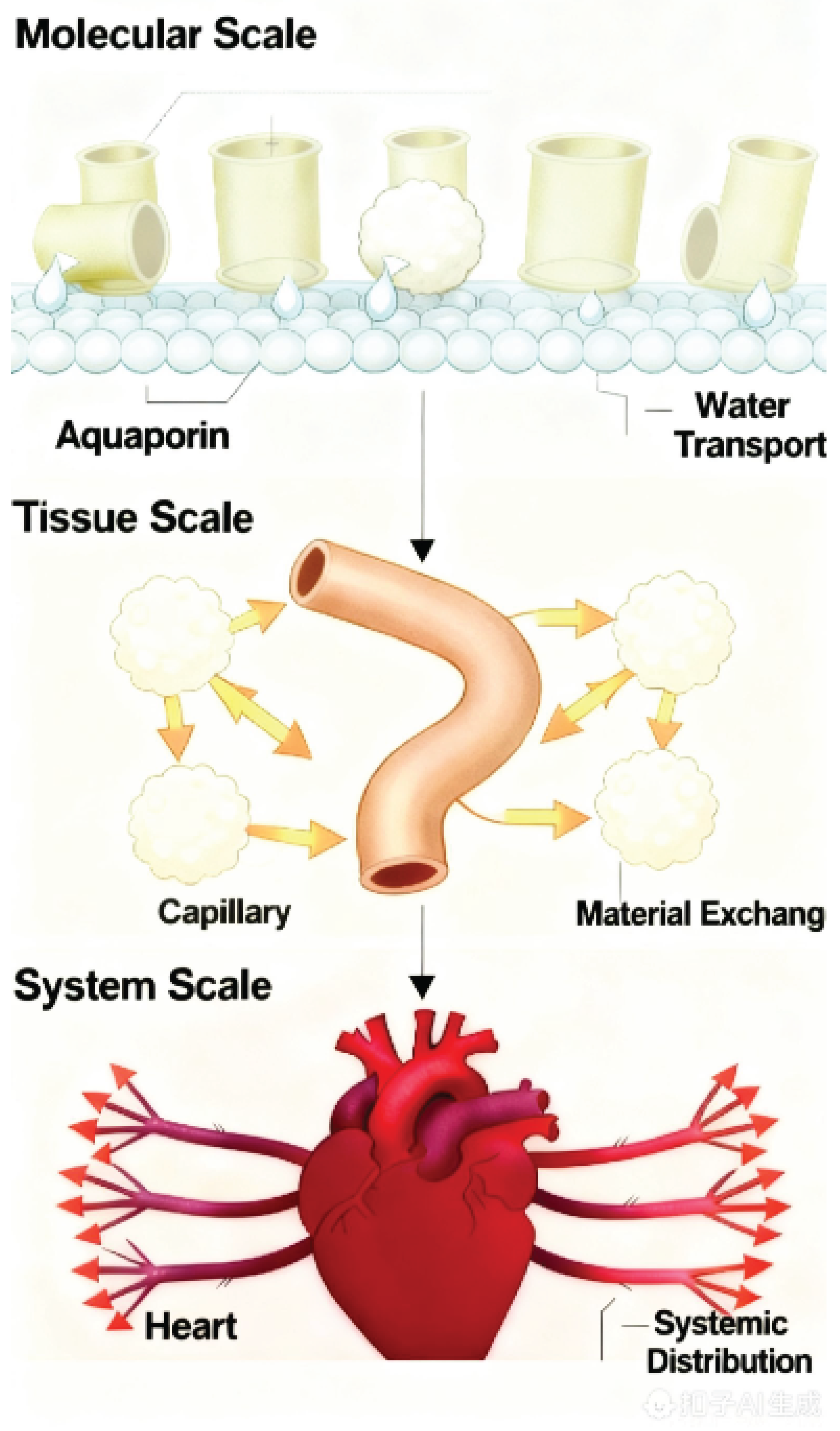
The core tenet of Biological Interstitialogy is a scale-free causal chain: the physical-chemical architecture of a biological interstice deterministically constrains spatiotemporal flow patterns, which in turn are the direct antecedents of system-level function (
Figure 1). We operationally define a functional interstice as a space that (i) physically separates recognized biological entities and (ii) whose intrinsic physico-chemical properties actively constrain and shape the patterns of flow within it.
Structure as the Architect. The interstice’s physical form—its geometry, topology, viscoelasticity, and chemical composition—defines the boundary and initial conditions for movement. The pore size and cross-linking density of the extracellular matrix (ECM), for instance, dictate cellular migratory potential and molecular diffusion rates [
10]. This structure is dynamic, capable of regulated phase transitions, as seen in biomolecular condensates that facilitate rapid component exchange via their liquid-like properties [
4].
Flow as the Constrained Dynamics. Flow is the movement—of ions, metabolites, cells, or forces—within this architectured space. Structure dictates selectivity, rate, directionality, and mode (e.g., diffusion vs. convection). The nanoscale architecture of a gap junction channel, for example, ensures selective permeation of specific ions and second messengers, enabling rapid intercellular synchronization [
5,
11].
Function as the Emergent Output. System-level function is the consequence of structured flow. Critically, identical components yield different functions if interstitial architecture is altered. The coordinated contraction of the heart emerges from rapid, structured ionic flow through cardiomyocyte gap junctions [
11]. Disrupt this flow, and the function fails.
Cross-Scale Validation of the S-F-F Principle
The universality of the S-F-F principle is demonstrated by convergent, causal evidence from disparate biological scales.
Molecular Scale: Biomolecular Condensates. Liquid-liquid phase separation creates interstices that concentrate reactants, accelerating biochemical reactions [
4]. The structure (e.g., composition) governs the flow (exchange rate). Perturbing the physical conditions to dissolve these interstices abrogates their catalytic function, establishing a direct causal link from structure → flow → function [
4].
Cellular Scale: Gap Junctions in Cancer. In triple-negative breast cancer, tumor cells form specific gap junctions with adipocytes [
12]. This structure enables the directional flow of cAMP from adipocytes to cancer cells, which directly functions to activate a critical pro-survival pathway [
12]. This illustrates how hijacking an interstitial structure dictates pathological outcomes.
Tissue Scale: Brain Clearance and ECM Mechanoregulation. The brain’s extracellular space (ECS) and glymphatic system form a system-wide interstitial network where structure (e.g., perivascular space) is essential for convective flow and waste clearance [
6]. Causal interventions show that modulating ECS flow reduces pathology and improves cognitive function [
13]. Similarly, ECM stiffness (structure) regulates the nuclear flow of YAP/TAZ, dictating stem cell function [
10].
Ecological Scale: Soil-Pore Networks. The physical structure of soil—its pore network—governs the flow of water, gases, and nutrients [
7]. This structured flow is a primary determinant of the ecosystem’s function, namely its productivity and stability.
Falsifiable Predictions and an Experimental Roadmap
We propose three decisive, quantitative experiments to challenge the theory:
Prediction 1 (Synthetic Morphogenesis): A pre-defined 3D stiffness gradient within a synthetic ECM, absent directional chemical cues, will be sufficient to guide mesenchymal stem cells to self-organize into a predicted branching network (with defined branch length/angle distributions). Falsification: Failure to form the predicted structure upon gradient imposition.
Prediction 2 (Cognitive Modulation): Targeted, non-invasive enhancement of hydraulic conductivity in the murine hippocampal ECS will increase interstitial fluid flow and yield a ≥20% improvement in long-term spatial memory consolidation (measured by latency in the Morris water maze). Falsification: Lack of a significant correlation between enhanced flow and memory improvement.
Prediction 3 (Agricultural State Transition): In degraded soil, introducing a burrowing keystone species to remodel porosity will trigger a shift in resource flow, leading to a ≥50% increase in microbial diversity and a ≥30% increase in plant biomass within two growth cycles. Falsification: Absence of such a transition.
Discussion
Our work establishes the S-F-F principle as a universal mechanistic chain, providing a physical substrate for emergence. Biological Interstitialogy resolves the mystery of how the whole becomes greater than the sum of its parts: through the constraints and opportunities afforded by the interstice. This framework moves beyond the descriptive adage of emergence to a predictive framework grounded in physical law.
This framework powerfully reframes pathophysiology as “intersticiopathies”—diseases of interstitial dysfunction. Fibrosis is the pathological solidification of tissue interstices; cancer involves the hijacking of interstitial communication [
12]; neurodegeneration involves failed interstitial clearance [
6,
13]. It reframes agricultural and ecological challenges as opportunities for “interstitial engineering.”
Our framework complements rather than replaces existing paradigms. Unlike network theory, which maps what is connected, Interstitialogy explains how the physical nature of the connection determines the functional outcome. It shifts the explanatory focus from node-centric properties to the relational spaces between them. By providing a mechanistic basis for emergence, it deepens concepts like self-organization, transforming them from philosophical descriptions into processes amenable to physical modeling and engineering.
Caveats and Future Directions
The S-F-F principle operates within complex biological contexts featuring redundancy. Future work must develop quantitative models (e.g., Function = Φ(Flow(Structure))) to achieve predictive power. The operational definition of a functional “interstice” will continue to evolve with new discoveries.
Conclusions
Biological Interstitialogy offers a paradigm shift from an entity-centric to a relation-centric view of biology. By focusing on the functional spaces between components, it provides a mechanistic, scale-free, and falsifiable framework to decode the logic of biological organization, paving the way for a new era of predicting and engineering life’s functions.
Methods
This theoretical synthesis integrates established empirical evidence from published literature across multiple disciplines, selected for its relevance in demonstrating causal S-F-F relationships. No new empirical data was generated.
Data Availability
All data supporting this theoretical study are available within the cited references.
Author Contributions
Q.W. conceived the framework, developed the theoretical model, and wrote the manuscript. Y.W. synthesized cross-scale evidence and critically revised the manuscript. Both authors approved the final version.
Competing Interests
The authors declare no competing interests.
Acknowledgements
We thank the interdisciplinary scientific community for foundational insights that enabled this synthesis.
References
- Noble, D. The Music of Life: Biology Beyond the Genome. (Oxford University Press, 2006).
- Koonin, E. V. The meaning of biological integration. PLoS Biol 14, e2000505 (2016).
- Barabási, A.-L. & Oltvai, Z. N. Network biology: understanding the cell’s functional organization. Nat Rev Genet 5, 101–113 (2004). [CrossRef]
- Shin, Y. & Brangwynne, C. P. Liquid phase condensation in cell physiology and disease. Science 357, eaaf4382 (2017). [CrossRef]
- Goodenough, D. A. & Paul, D. L. Gap junctions. Cold Spring Harb Perspect Biol 1, a002576 (2009). [CrossRef]
- Iliff, J. J. et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med 4, 147ra111 (2012). [CrossRef]
- Rillig, M. C., Muller, L. A. & Lehmann, A. Soil aggregates as massively concurrent evolutionary incubators. ISME J 11, 1943–1948 (2017). [CrossRef]
- Wang, Q. & Wang, Y. The Interstitial Integration Hypothesis: A Unified Physical Framework for Emergence and Self-Organization. Preprints 2025, 2025100923 (2025). [CrossRef]
- Wang, Q. & Wang, Y. A New Physiological Framework: The Human Interstitial System as the Body’s Relational Matrix. Preprints 2025, 2025101531 (2025). [CrossRef]
- Dupont, S. et al. Role of YAP/TAZ in mechanotransduction. Nature 474, 179–183 (2011). [CrossRef]
- Laird, D. W. The gap junction proteome and its relationship to disease. Trends Cell Biol 20, 92–101 (2010). [CrossRef]
- Wang, Y. et al. Adipocytes promote tumor progression and induce PD-L1 expression via gap junction communication. Cancer Lett 477, 52–60 (2020). [CrossRef]
- Yue, X. et al. Near-infrared light-triggered, targeted nanoagonist for multimodal therapy of Alzheimer’s disease. Adv Mater 35, e2207766 (2023).
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).