1. Introduction
Brain metastases, particularly from lung cancer, represent a significant clinical challenge. Whole-brain radiotherapy (WBRT) remains a cornerstone of treatment for patients with multiple brain metastases, offering effective intracranial tumor control [
1]. Hippocampal-sparing whole-brain radiotherapy (HS-WBRT) or hippocampal avoidance in whole-brain radiation therapy (HA-WBRT) has emerged as an effective strategy to mitigate cognitive decline associated with traditional WBRT in patients with brain metastases [
2]. The hippocampus, specifically the subgranular zone of the dentate gyrus, is crucial for memory formation, and its radiation-induced injury has been implicated in early cognitive deterioration following WBRT [
3]. Studies have demonstrated that even low doses of radiation to the hippocampus can disrupt neurogenesis and impair memory recall [
4]. To address this, HA-WBRT techniques aim to deliver radiation to the brain while sparing the hippocampal neural stem-cell niche, thus preserving cognitive function [
5]. Clinical trials, such as RTOG 0933, have shown that HA-WBRT can significantly reduce hippocampal radiation exposure by up to 80%, leading to better memory preservation and quality of life compared to conventional WBRT[
6]. Additionally, combining HA-WBRT with neuroprotective agents, like memantine, has further improved cognitive outcomes in patients[
7]. A phase III trial (NRG Oncology CC001) is ongoing to validate these findings and explore the impact of HA-WBRT in combination with memantine on cognitive function in brain metastasis patients[
8]. Early results suggest that this approach not only preserves memory but also provides adequate control of brain metastases, making HA-WBRT a promising advancement in neuroprotective radiation therapy[
9].
The development of conformal radiation techniques has made HA-WBRT practically feasible. Three-dimensional conformal radiotherapy (3D-CRT) is a basic technique available in most radiation centers; however, its geometric limitations often make it suboptimal for sparing small, critical structures like the hippocampus. Intensity-modulated radiotherapy (IMRT) and volumetric modulated arc therapy (VMAT) are more advanced techniques that allow for improved dose conformity and sparing of organs at risk (OARs), particularly the hippocampi. Among these, VMAT has become the most widely used and preferred technique for HA-WBRT in many clinical settings. Its ability to deliver radiation in a continuous arc allows for improved dose distribution, better sparing of the hippocampus and other organs at risk (OARs), and shorter treatment times. Recent dosimetric studies and clinical planning investigations have consistently demonstrated VMAT’s advantages in target coverage and dose homogeneity for HA-WBRT, supporting its increasing adoption worldwide [
10,
11,
12].
However, access to advanced radiotherapy technologies like VMAT remains limited in many low- and middle-income countries, including Vietnam. In several hospitals across Vietnam, linear accelerators (LINACs) may only be equipped for IMRT, with no VMAT capabilities available [
13,
14,
15] . While IMRT is more accessible and technically feasible in such settings, its effectiveness and practicality in delivering HA-WBRT—compared to VMAT—need further evaluation.
This study aims to compare three radiotherapy techniques, 3D-CRT, IMRT, and VMAT, in the context of HA-WBRT, focusing on their dosimetric performance, treatment planning characteristics, and ability to meet clinical constraints for hippocampal avoidance. A key objective is to assess the feasibility and clinical relevance of using IMRT for HA-WBRT in resource-constrained environments like Vietnam, where VMAT may not be an available option. By identifying whether IMRT can provide acceptable hippocampal sparing and overall treatment quality, this study could help broaden access to neurocognitive-preserving radiotherapy for brain metastasis patients in settings with limited technology. Unlike previous studies that assume access to advanced radiotherapy systems, our study is distinct in assessing whether IMRT, which is more widely available in lower-resource hospitals, can deliver clinically acceptable HA-WBRT. This aspect is especially relevant for expanding hippocampal-sparing radiotherapy to underserved populations.
2. Materials and Methods
2.1. Patients
A group of 10 patients with brain metastases from lung cancer, who received palliative whole-brain radiotherapy (WBRT), were treated at Phuc Thinh General Hospital between June 2024 and January 2025. This group included two patients diagnosed with small-cell lung cancer (SCLC) and eight with non-small-cell lung cancer (NSCLC). All patients were in good general condition, alert, and compliant with medical instructions at the time of treatment.
2.2. Equipment
A comprehensive treatment process was implemented, from data collection to treatment delivery. CT images were acquired using a Discovery RT32 slice scanner (GE HealthCare, Chicago, USA). The CT data were transferred automatically to Monaco Planning Software (version 6.1.4.0) for radiation therapy planning and contouring.
For quality assurance (QA), both IMRT and VMAT plans were evaluated using the Matrix Resolution System and MyQA Accept Software (IBA) to ensure they met accuracy and safety standards before patient treatment.
The radiation therapy was delivered using the Elekta Synergy Linear Accelerator (LINAC) (Elekta Oncology Systems, Crawley, UK), equipped with an Agility head and a 160 multi-leaf collimator (MLC). Each leaf measures 0.5 cm in width and moves at a speed of 3.5 cm/s. The system supports both 6 MV and 10 MV photon beams, enhancing precision, optimizing dose delivery, and reducing treatment time, while improving normal tissue sparing during IMRT and VMAT radiotherapy.
2.3. Methodology
2.3.1. Patient Simulation CT Imaging and Volume Delineation Data
The patient was positioned supine and immobilized following the technician's guidance. A three-point mask was used to stabilize the head, and the laser center was aligned and marked as the simulation center. A simulation CT scan was performed using the GE Discovery RT 32-slice scanner (GE HealthCare, Chicago, USA) with a slice thickness of 2.5 mm. Although the RTOG 0933 protocol recommends a 1.25 mm slice thickness, this was not achievable due to equipment limitations. In addiation, MRI fusion was not performed, which we acknowledge as a limitation.
The CT data was analyzed by physicians to delineate the Planning Target Volume (PTV) and Organs at Risk (OARs)[
8]. Hippocampal contours were delineated in accordance with the RTOG 0933 atlas by experienced radiation oncologists.
CTV (Clinical Target Volume): Covers the entire brain up to C1 if there is no posterior fossa metastasis, or up to C2 if posterior fossa metastasis is present.
PTV (Planning Target Volume): Defined as CTV + 3mm, excluding the hippocampal margin.
OARs (Organs at Risk): Includes the eyeballs, lenses, optic nerves (left and right), optic chiasm, hippocampus, and hippocampal avoidance zone (hippocampus + 5mm margin).
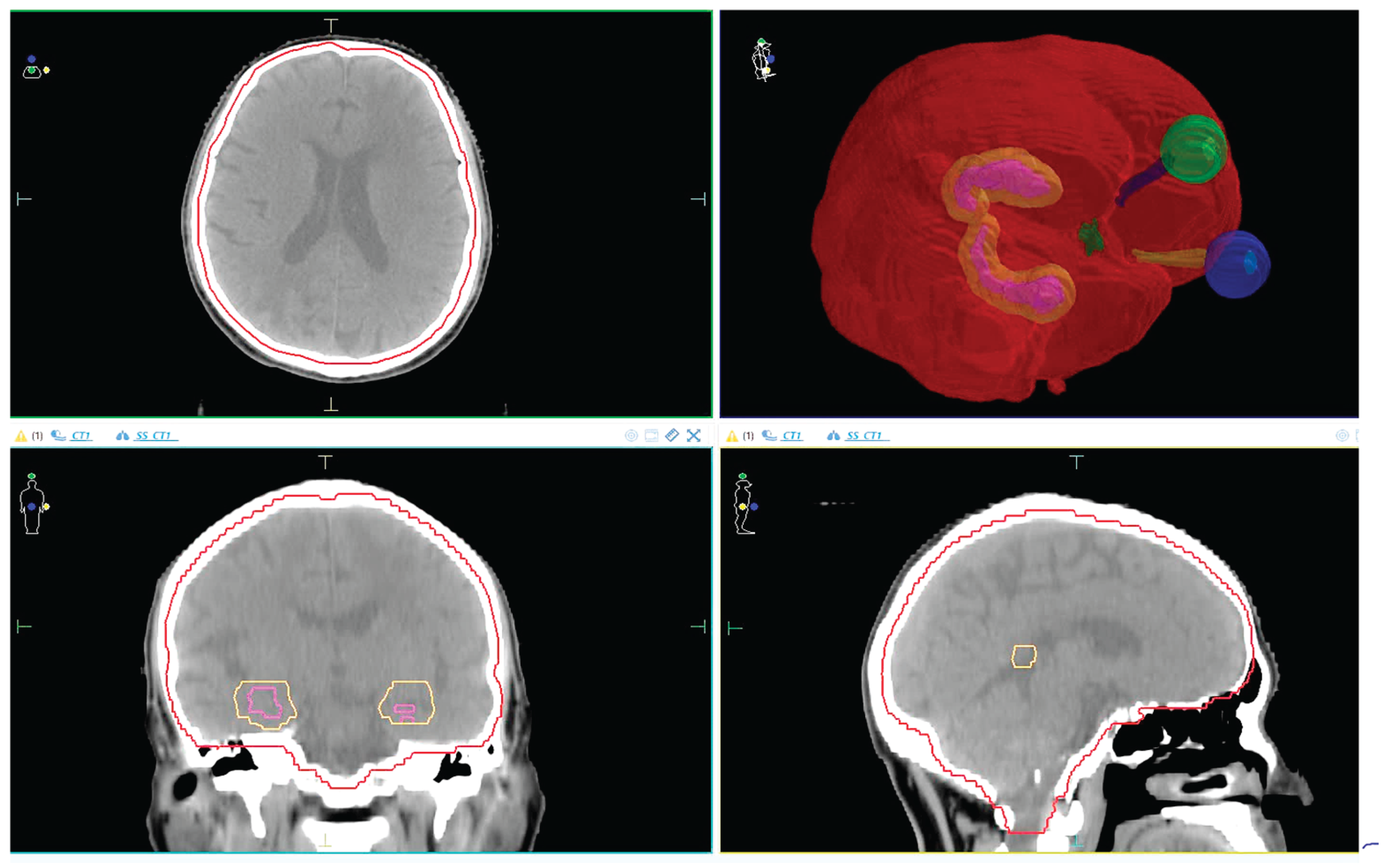
The anatomical relationship between the PTV and surrounding OARs, including the hippocampus and avoidance zone, is illustrated in
Figure 1. This visual reference helps clarify spatial planning constraints and margin definitions used in this study.
2.3.2. Radiotherapy Dose Prescription
According to RTOG 0933, the total dose for WBRT is 30Gy, delivered in 10 fractions. The evaluation criteria are outlined in the
Table 1 and
Table 2.
2.3.3. Treatment Planning
Each patient underwent treatment planning using three different techniques 3D-CRT, IMRT, and VMAT all conducted on the Monaco software.
3D-CRT Planning: Designed with two opposed lateral fields at gantry angles of 90° and 270°, with a collimator rotation of 5° to optimize eye shielding. The field size was extended to cover the entire PTV. The Collapsed Cone algorithm was used for dose calculation.
Hippocampal-Sparing WBRT using IMRT: This plan utilized nine fields, each with a collimator angle of 45°. In our study, the 45° collimator angle was selected based on institutional clinical practice and experience, as it helps reduce the tongue-and-groove effect and facilitates dose modulation in concave anatomical regions such as the hippocampus. The Monte Carlo algorithm was applied.
Hippocampal-Sparing WBRT using VMAT: The plan incorporated a single beam consisting of three full arcs (360° rotation), with the collimator set to 45°.
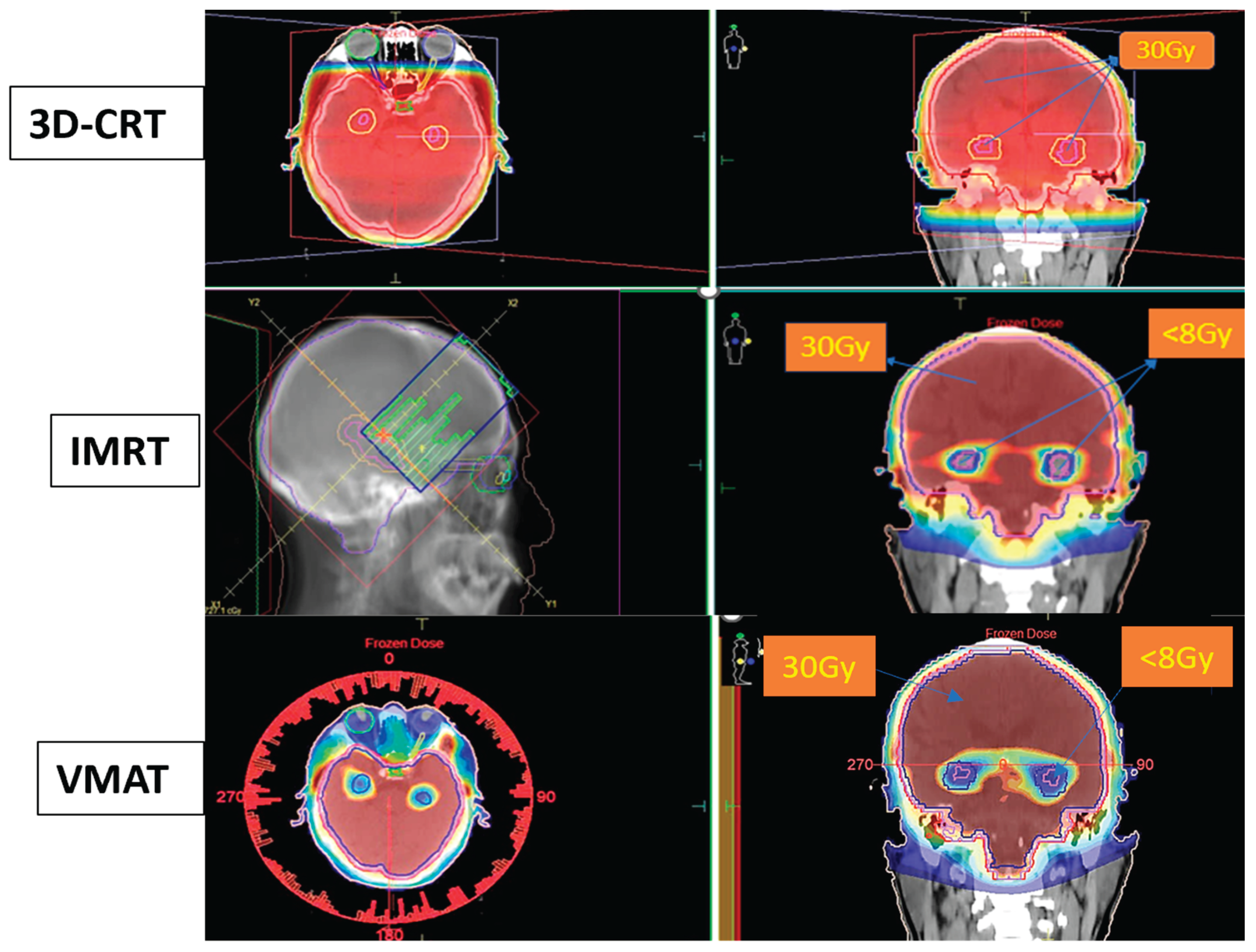
Figure 2.
Dose distribution for Hippocampal-Sparing Whole-Brain Radiotherapy using 3D-CRT, IMRT, and VMAT techniques.
Figure 2.
Dose distribution for Hippocampal-Sparing Whole-Brain Radiotherapy using 3D-CRT, IMRT, and VMAT techniques.
2.3.4. Treatment Plan Evaluation Criteria
In addition to tumor coverage percentage, homogeneity index (HI) and conformity index (CI) are two other critical parameters for evaluating the effectiveness of a treatment plan in tumor management [
16,
17,
18].
Homogeneity Index:
where:
D2% is the absorbed dose value close to the Dmax, received by 2% of the PTV volume.
D98% is the absorbed dose value close to the Dmin, received by 98% of the PTV volume.
Drx is the prescribed dose to the PTV volume.
The HI approaching 0 indicates a uniform dose distribution within the target volume. The closer HI is to 0, the better the radiotherapy quality.
Conformity Index: indicates the ratio between the volume covered by the reference isodose line (typically 95% according to ICRU) and the prescribed PTV volume.
where:
The CI has an ideal value of 1, with values closer to 1 indicating higher dose conformity to the designated target volume [
19].
2.3.5. Treatment Plan Quality Assurance (QA Plan)
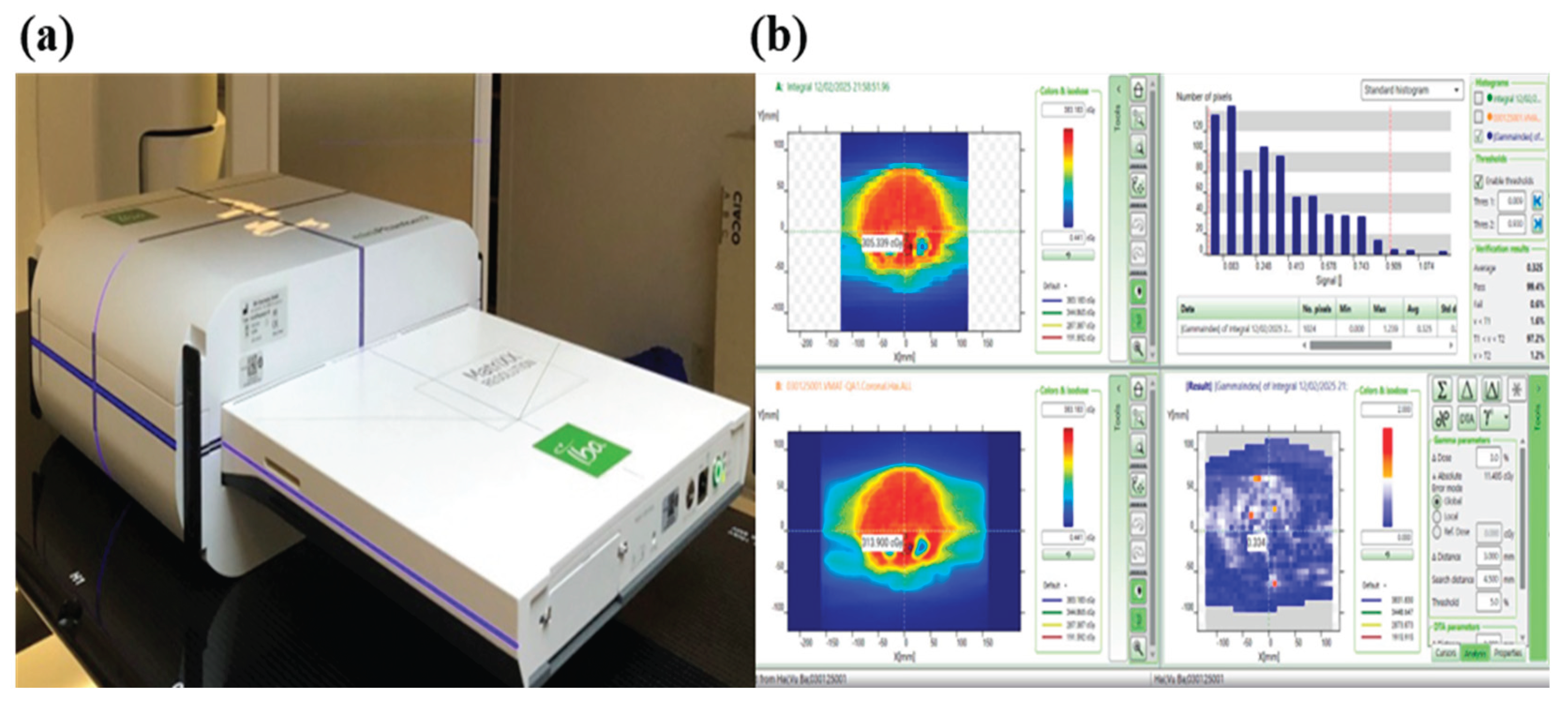
The equipment utilized to implement the QA plan is illustrated in
Figure 3, featuring the Matrixx Resolution system, Mini R phantom, and MyQA software. The Matrixx Resolution system is equipped with 1521 ionization chambers arranged in a 39 × 39 matrix, allowing for highly detailed dose distribution analysis while minimizing interpolation errors. With a detector spacing of just 6.5mm (center-to-center), this high-resolution setup ensures precise measurements, especially for advanced techniques like VMAT that involve steep dose gradients. Its expansive 25.3 × 25.3 cm
2 active measurement area enables efficient verification of large treatment fields.
The dose distribution of the Monaco-calculated plan was compared to the actual radiation dose using the gamma index method, with results expressed as the gamma pass rate [
20].
3. Results
3.1. QA Plan
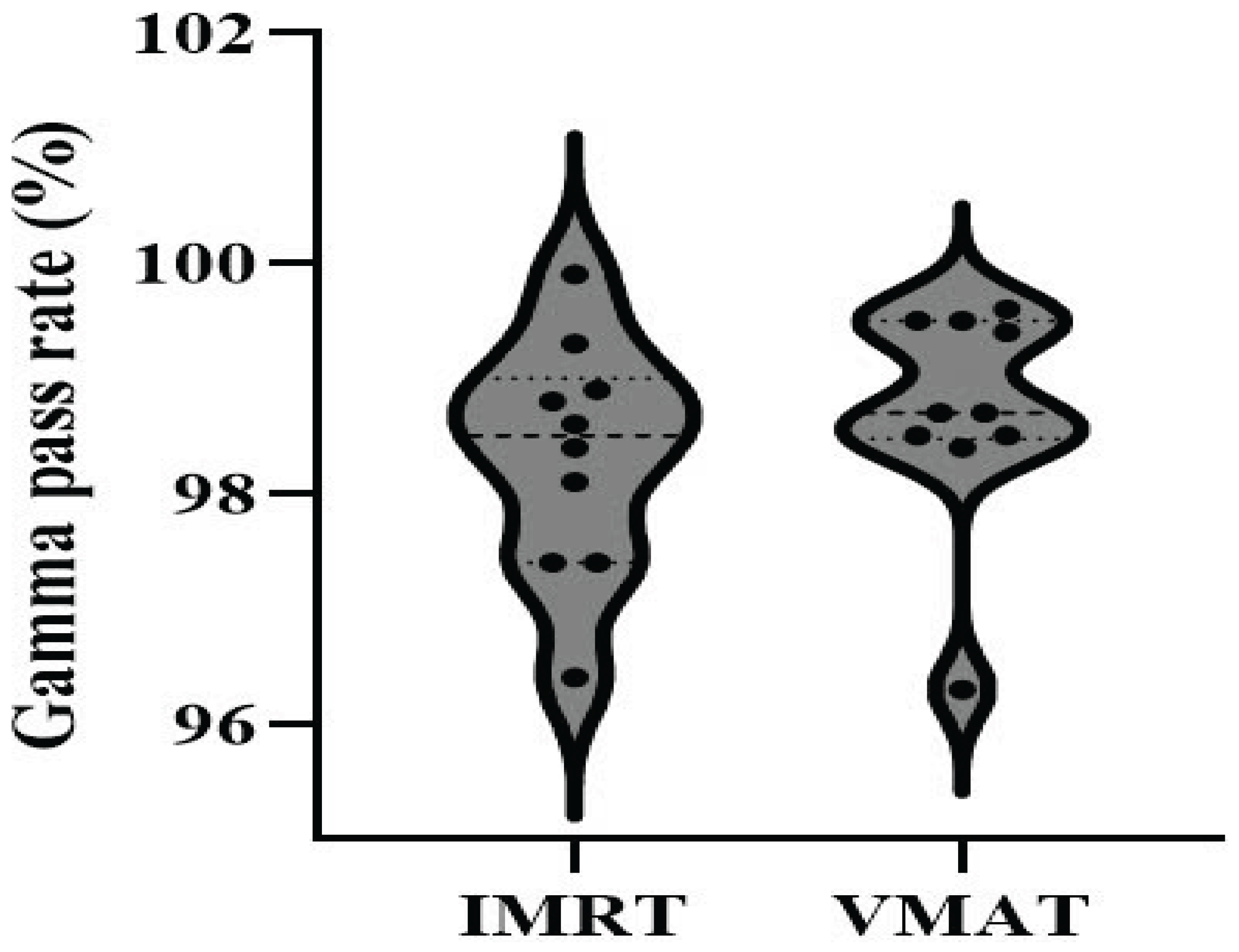
The
gamma analysis criterion of 3%/3mm was used for evaluation.
Figure 4 displays the distribution of gamma pass rate values for both the IMRT and VMAT techniques.
It is clear that VMAT radiation plans exhibit higher gamma pass rates than IMRT, with 90% of VMAT plans exceeding a pass rate of 98%, compared to 70% for IMRT. However, all plans have a gamma pass rate greater than 95%, meeting the requirement for clinical treatment [
21].
3.2. Dosimetric Evaluation
The findings of the analysis on tumor coverage efficiency for 3D-CRT, VMAT, and IMRT are presented in
Table 3 and
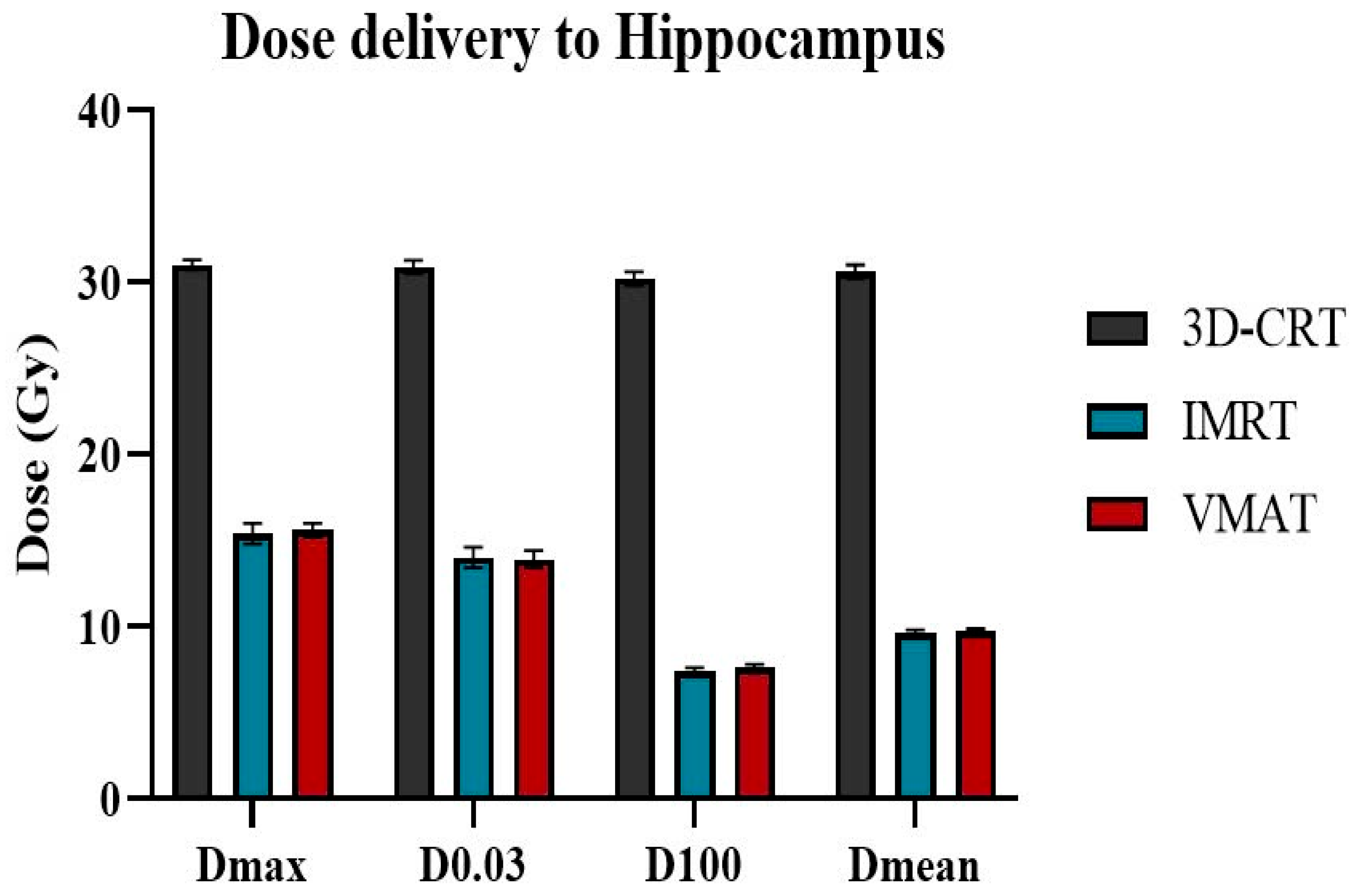
Table 4. It is evident that 3D-CRT demonstrates superior tumor coverage efficiency, with a D98% value of 29.2 ± 0.6 Gy, which exceeds that of both VMAT and IMRT by 12.3% and 15.4%, respectively. The V30Gy for both 3D-CRT and VMAT exceeds 95%, whereas for IMRT, it is 93.7%. In terms of hotspot control, 3D-CRT achieves the lowest D2% value (31.9 ± 0.4 Gy), while the D2% values for VMAT and IMRT, although greater than 35 Gy, remain significantly below the allowable limits set forth in the RTOG 0933 and NRG CC001 guidelines.
The absence of significant differences in the Homogeneity Index (HI) and Conformity Index (CI) between VMAT and IMRT, as shown in
Table 4, suggests that both techniques offer comparable dose uniformity and coverage within the tumor region. Additionally, the lower HI value observed for 3D-CRT supports its superior dose uniformity in the Planning Target Volume (PTV).
Table 5 and
Figure 5 highlight the superiority of IMRT and Volumetric Modulated Arc Therapy VMAT in minimizing radiation exposure to healthy organs during radiotherapy. The hippocampus exhibited the lowest D100% and D
mean values with IMRT, measuring 7.4 ± 0.2 Gy and 9.6 ± 0.2 Gy, respectively. VMAT plans produced nearly identical values, with D100 at 7.6 ± 0.2 Gy and D
mean at 9.7 ± 0.2 Gy, both well below the prescribed limits. In contrast, 3D-CRT resulted in significantly higher values for the hippocampus, with D100 at 30.2 ± 0.4 Gy and D
mean at 30.6 ± 0.4 Gy, which are nearly four times higher than the recommended limit for hippocampal protection. Regarding the hotspot area within the hippocampus, D
max and D100% values for both IMRT and VMAT were similar, ranging from 13.9 Gy to 15.6 Gy, exceeding the required dose limits of 16 Gy.
For the chiasm and both the left and right optic nerves, the lowest Dmax values were observed with VMAT, at 29.4 ± 0.3 Gy, 27.8 Gy, and 26.6 Gy, respectively. IMRT produced slightly higher values, though the difference was not statistically significant. The highest Dmax values were seen with the 3D-CRT technique, with all values exceeding 30 Gy. Although 3D-CRT resulted in the lowest dose indices for the lenses (L/R) and eyes (L/R), the doses from both VMAT and IMRT were still sufficiently low, meeting all the proposed safety criteria.
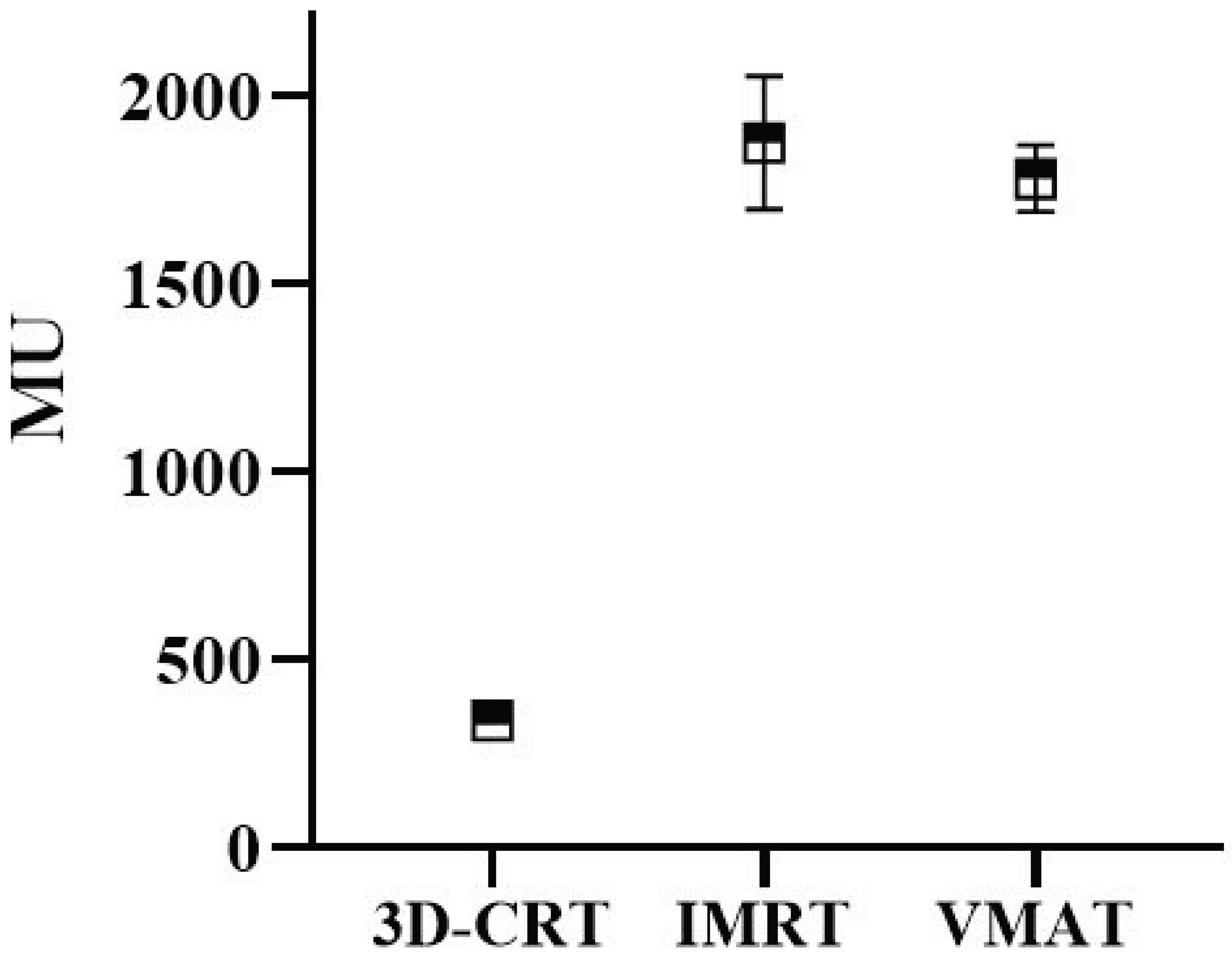
Figure 6 illustrates the calculated MU for treatment plans using 3D-CRT, IMRT and VMAT. 3D-CRT, possessing the least sophisticated dose modulation capabilities, exhibited the lowest mean MU (339). In contrast, IMRT had the highest average MU at 1875, while VMAT had a slightly lower average of 1781. As shown in
Figure 6, 3D-CRT exhibited the lowest mean MU (339) due to its simplicity, while IMRT required the highest (1,875) because of multiple fixed fields. VMAT, with a mean MU of 1,781, offered a balance between modulation and efficiency. The VMAT technique resulted in a mean treatment time of approximately 6 minutes, nearly half the 12 minutes required for IMRT. This finding supports existing literature indicating that VMAT reduces both MU and delivery time, thereby improving clinical workflow and patient throughput.
4. Discussion
This study demonstrated the superiority of IMRT and VMAT in preserving the hippocampus during whole-brain radiotherapy. Specifically, in the hippocampus region, the Dmax was reduced by approximately 49.6%, D0.03cm³ by 55%, D100% by 74.8%, and the Dmean by 68.3% when using VMAT and IMRT compared to the 3D-CRT technique. Notably, all treatment plans adhered to the criteria outlined in the RTOG 0933 guidelines, effectively mitigating the risk of radiation-induced damage to brain regions associated with memory function.
The findings of this study align closely with previous research on hippocampal-sparing radiotherapy[
22,
23,
24,
25]. Mathew et al [
26] reported outcomes from a group of 12 patients treated with hippocampal-sparing whole-brain radiotherapy using the VMAT technique on the Elekta VersaHD system, achieving a median PTV V30Gy of 96.53% and a median D98% of 28.27 Gy. In comparison, our study showed slightly lower values of 95.1% and 26 Gy, respectively. For the hippocampus, Mathew's D100% values were 8.76 Gy and 8.86 Gy for the left and right sides, while our results were notably lower, with a mean value of 7.6 Gy across the entire region. Unfortunately, no data on the sparing of other organs at risk (OARs) were provided in that study, limiting further comparison. Alexander et al [
22] using IMRT, reported a PTV V30Gy of 92% and a D98% of 25.37 Gy. Our corresponding results were 93.7% and 24.7 Gy, respectively. Although the D98% of IMRT plans (24.7 Gy) was lower than that of VMAT (26.0 Gy), it still met the minimum requirement of ≥22.5 Gy as defined by NRG-CC001, thereby confirming its clinical acceptability. For hippocampal dose, our mean D100% was 7.4 Gy—significantly lower than the 8.37 Gy reported by Alexander—underscoring our focus on minimizing hippocampal exposure while maintaining clinically acceptable target coverage. Both Mathew and Alexander incorporated treatment table rotation to optimize plan quality. In contrast, our approach maintained a fixed table position throughout planning and delivery. While rotation can enhance hippocampal sparing, particularly in IMRT, it may extend treatment times and introduce potential setup uncertainties due to manual adjustments. To mitigate these concerns, we used a consistent 45° collimator rotation across all beam angles, which reduced the tongue-and-groove effect, improved dose distribution uniformity, and minimized dose variation—without compromising workflow efficiency [
27].
Specifically, an additional focus of this study is the clinical applicability of IMRT in hippocampal-protective whole-brain radiotherapy. As illustrated in
Table 3, IMRT demonstrated a 1.7% higher D2%, a 5% lower D98%, and a 1.5% lower V30Gy compared to VMAT. While IMRT showed a slight disadvantage in tumor coverage relative to VMAT, the observed differences remained within acceptable clinical thresholds. Furthermore, an assessment of dosimetric parameters for adjacent healthy organs (
Table 5) revealed minimal differences between IMRT and VMAT, with IMRT exhibiting a slight advantage in dose reduction to the hippocampus and the right eye. Overall, IMRT demonstrated satisfactory tumor coverage and organ-at-risk protection, supporting its clinical utility.
Nevertheless, a comprehensive evaluation of IMRT feasibility must consider factors such as monitor units (MU) and treatment duration. In this study, the total MU for IMRT was 1,875—comparable to VMAT—with nine treatment fields and an average treatment duration of 12 minutes, which is consistent with standard practice for head and neck radiotherapy cases [
28,
29]. In addition to comparable dosimetric outcomes, VMAT demonstrated clear advantages in treatment efficiency. The reduced MU and shorter beam-on time observed with VMAT (approximately 6 minutes) compared to IMRT (approximately 12 minutes) can significantly enhance clinical throughput and patient comfort. This is particularly valuable in high-volume or resource-constrained settings, where treatment time impacts machine availability and patient scheduling.
5. Conclusions
This study further supports the superiority of IMRT and VMAT in hippocampal-sparing whole-brain radiotherapy. All planning target volume (PTV) and organ-at-risk (OAR) parameters for IMRT and VMAT plans met the RTOG 0933 criteria, effectively minimizing the risk of cognitive impairment while preserving therapeutic efficacy. IMRT also demonstrated feasibility in achieving adequate tumor coverage alongside hippocampal and OAR sparing, making it a viable option for centers without access to VMAT technology.
Institutional Review Board Statement
Ethical review and approval were waived for this study because it involved only retrospective treatment planning data without any human participants, identifiable information, or animal experiments
Informed Consent Statement
Patient consent was waived because this study did not involve human participants or identifiable personal data
Data Availability Statement
Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sas-Korczynska B, Rucinska M. WBRT for brain metastases from non-small cell lung cancer: for whom and when?—Contemporary point of view. J Thorac Dis 2021;13:3246–57. [CrossRef]
- Gondi V, Pugh SL, Tome WA, Caine C, Corn B, Kanner A, et al. Preservation of Memory With Conformal Avoidance of the Hippocampal Neural Stem-Cell Compartment During Whole-Brain Radiotherapy for Brain Metastases (RTOG 0933): A Phase II Multi-Institutional Trial. J Clin Oncol 2014;32:3810–6. [CrossRef]
- Gondi V, Hermann BP, Mehta MP, Tomé WA. Hippocampal dosimetry predicts neurocognitive function impairment after fractionated stereotactic radiotherapy for benign or low-grade adult brain tumors. Int J Radiat Oncol Biol Phys 2012;83:e487-493. [CrossRef]
- Monje ML, Mizumatsu S, Fike JR, Palmer TD. Irradiation induces neural precursor-cell dysfunction. Nat Med 2002;8:955–62. [CrossRef]
- Gondi V, Tomé WA, Mehta MP. Why avoid the hippocampus? A comprehensive review. Radiother Oncol J Eur Soc Ther Radiol Oncol 2010;97:370–6. [CrossRef]
- Gondi V, Pugh SL, Tome WA, Caine C, Corn B, Kanner A, et al. Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): a phase II multi-institutional trial. J Clin Oncol Off J Am Soc Clin Oncol 2014;32:3810–6. [CrossRef]
- Brown PD, Pugh S, Laack NN, Wefel JS, Khuntia D, Meyers C, et al. Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: a randomized, double-blind, placebo-controlled trial. Neuro-Oncol 2013;15:1429–37. [CrossRef]
- Brown PD, Gondi V, Pugh S, Tome WA, Wefel JS, Armstrong TS, et al. Hippocampal Avoidance During Whole-Brain Radiotherapy Plus Memantine for Patients With Brain Metastases: Phase III Trial NRG Oncology CC001. J Clin Oncol Off J Am Soc Clin Oncol 2020;38:1019–29. [CrossRef]
- Brown PD, Ahluwalia MS, Khan OH, Asher AL, Wefel JS, Gondi V. Whole-Brain Radiotherapy for Brain Metastases: Evolution or Revolution? J Clin Oncol Off J Am Soc Clin Oncol 2018;36:483–91. [CrossRef]
- Sood S, Pokhrel D, McClinton C, Lominska C, Badkul R, Jiang H, et al. Volumetric-modulated arc therapy (VMAT) for whole brain radiotherapy: not only for hippocampal sparing, but also for reduction of dose to organs at risk. Med Dosim Off J Am Assoc Med Dosim 2017;42:375–83. [CrossRef]
- Liu R, Gong G, Meng K, Du S, Yin Y. Hippocampal sparing in whole-brain radiotherapy for brain metastases: controversy, technology and the future. Front Oncol 2024;14. [CrossRef]
- Xue J, Jin S, Zhang H, Zou K, Sheng J, Tang J, et al. A simplified non-coplanar volumetric modulated arc therapy for the whole brain radiotherapy with hippocampus avoidance. Front Oncol 2023;13:1143564. [CrossRef]
- Tai DT, Oanh LT, Son ND, Loan TTH, Chow JCL. Dosimetric and Monte Carlo verification of jaws-only IMRT plans calculated by the Collapsed Cone Convolution algorithm for head and neck cancers. Rep Pract Oncol Radiother 2019;24:105–14. [CrossRef]
- Thi Oanh L, Thanh Tai D, Thi Hong Loan T, Chow JC. Calculation of Jaws-only IMRT (JO-IMRT) dose distributions based on the AAPM TG-119 test cases using Monte Carlo simulation and Prowess Panther treatment planning system. Nucl Eng Technol 2021;53:4098–105. [CrossRef]
- Tai DT, Son ND, Loan TTH, Anson HPW. Quality assurance of the jaws only-intensity modulated radiation therapy plans for head-and-neck cancer. Phys Medica PM Int J Devoted Appl Phys Med Biol Off J Ital Assoc Biomed Phys AIFB 2017;38:148–52. [CrossRef]
- Tai DT, Phat LT, Ngoc Anh N, Sang HVT, Loc TM, Hai NX, et al. Dosimetric and radiobiological comparison between conventional and hypofractionated breast treatment plans using the Halcyon system. Front Oncol 2023;13.
- Tai DT, Oanh LT, Phuong PH, Sulieman A, Abolaban FA, Omer H, et al. Dosimetric and radiobiological comparison in head-and-neck radiotherapy using JO-IMRT and 3D-CRT. Saudi J Biol Sci 2022;29:103336. [CrossRef]
- Paddick I. A simple scoring ratio to index the conformity of radiosurgical treatment plans. Technical note. J Neurosurg 2000;93 Suppl 3:219–22.
- ICRU Report 83, Prescribing, Recording, and Reporting Intensity-Modulated Photon-Beam Therapy (IMRT) – ICRU n.d. https://www.icru.org/report/prescribing-recording-and-reporting-intensity-modulated-photon-beam-therapy-imrticru-report-83/ (accessed April 22, 2025).
- Tai DT, Omer H, Quoc LC, Hai NX, Minh TV, Sulieman A, et al. An open-source software for calculating 1D gamma index in radiation therapy. J King Saud Univ - Sci 2023;35:102937. [CrossRef]
- Ezzell GA, Burmeister JW, Dogan N, LoSasso TJ, Mechalakos JG, Mihailidis D, et al. IMRT commissioning: multiple institution planning and dosimetry comparisons, a report from AAPM Task Group 119. Med Phys 2009;36:5359–73. [CrossRef]
- Nevelsky A, Ieumwananonthachai N, Kaidar-Person O, Bar-Deroma R, Nasrallah H, Ben-Yosef R, et al. Hippocampal-sparing whole-brain radiotherapy using the Elekta equipment. J Appl Clin Med Phys 2013;14:113–20. [CrossRef]
- Lee K, Lenards N, Holson J. Whole-brain hippocampal sparing radiation therapy: Volume-modulated arc therapy vs intensity-modulated radiation therapy case study. Med Dosim Off J Am Assoc Med Dosim 2016;41:15–21. [CrossRef]
- Gondi V, Tolakanahalli R, Mehta MP, Tewatia D, Rowley H, Kuo JS, et al. Hippocampal-sparing whole-brain radiotherapy: a “how-to” technique using helical tomotherapy and linear accelerator-based intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys 2010;78:1244–52. [CrossRef]
- Zhao R, Kong W, Shang J, Zhe H, Wang Y-Y. Hippocampal-Sparing Whole-Brain Radiotherapy for Lung Cancer. Clin Lung Cancer 2017;18:127–31. [CrossRef]
- Goss MD, Rowles R, Spanovich L, Wegner RE, Hasan S, Horne ZD. Hippocampal-sparing whole brain volumetric modulated arc therapy (VMAT) planning in Monaco: a “How-to” not pull your hair out. Med Dosim Off J Am Assoc Med Dosim 2021;46:426–30. [CrossRef]
- Deng J, Pawlicki T, Chen Y, Li J, Jiang SB, Ma CM. The MLC tongue-and-groove effect on IMRT dose distributions. Phys Med Biol 2001;46:1039–60. [CrossRef]
- Van Khac P, Doanh VT, Thao MT, Tai DT, Sandwall P, Sulieman A, et al. Optimizing intensity-modulated radiation therapy for stage II nasopharyngeal cancer: A comparative study of 7-field and 9-field treatment plans. Radiat Phys Chem 2025:112700. [CrossRef]
- Phat LT, Thao MT, Kien TT, Tai DT, Sandwall P, Sulieman A, et al. Dosimetric analysis of beam number variations in IMRT for head-and-neck, breast, and pelvic cancers using Halcyon. Radiat Phys Chem 2025:112755. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).