1. Introduction
Worldwide, the agri-food industry generates more than 190 million tons of by-products annually. This biomass, commonly referred to as waste, constitutes a valuable source of compounds such as sugars, dietary fiber, lipids, proteins, minerals, and bioactive molecules including phenolic acids, tannins, flavonoids and carotenoids. However, it is frequently discarded in landfills or other waste disposal sites, where its slow and progressive degradation contributes to the release of greenhouse gases, air, water, and soil contamination, and the occupation of vast areas that could be used for agriculture. The above highlights the importance of developing strategies for their appropriate disposal, recycling, or valorization. Among the most common by-products are those derived from the harvesting of oilseeds, vegetables, and horticultural crops, as well as residues obtained from plants such as grapes, pomegranate, melon, and particularly citrus fruits [
1,
2,
3].
Citrus fruits are recognized for their elevated content of vitamin C and carotenoids with pro-vitamin A activity, particularly β-cryptoxanthin [
4], positioning them as one of the most valued fruit categories due to their substantial nutritional and pharmacological relevance. Their distinctive aroma and flavor further contribute to their global importance, making them one of the most extensively cultivated crops, with an annual production exceeding 100 million tons, concentrated primarily in six countries: China (46.6 Mt), Brazil (18.8 Mt), India (14.3 Mt), Mexico (8.8 Mt), and the United States (6.2 Mt) [
5,
6,
7]. Given the magnitude of this industry, citrus cultivation and processing generate considerable quantities of non-edible by-products, estimated in 40 million tons of biomass per year, thereby underscoring the importance of developing efficient strategies for the valorization and sustainable management of these residues [
8].
Citrus production is essential for the Mexican economy, contributing with a 34.89% of the national agricultural Gross Domestic Product (GDP). In 2016, oranges represented the perennial crop with the largest cultivated area, covering 335,336 hectares. Citrus fruits are not only widely consumed domestically but also contribute significantly to exports, positioning Mexico as one of the leading countries in the global citrus market, particularly for sweet oranges (
Citrus sinensis L. Osbeck), limes (
C. aurantifolia,
Swingle and C.
latifolia Tanaka), and lemons (
C. limon L.) [
5,
9]. In addition, other species, although cultivated on a smaller scale, have important cultural and culinary relevance in Mexican gastronomy, as the case of
Citrus aurantium.
Commonly known as sour orange or bitter orange,
C. aurantium belongs to the Ru-taceae family, and its leaves, flowers, and fruits have been widely used in traditional Chinese medicine [
10]. In Mexico, sour orange is primarily cultivated in the Yucatán Peninsula, where it plays a central role in the preparation of traditional dishes such as pibikek’en (“Cochinita pibil”, Yucatan-style slow-roasted pork) and xréenteja jetel k’ek’en (“Frijol con Puerco”, pork and beans) [
11]. Although its cultivation is largely confined to backyard orchards, vulnerable communities in the region often grow it as livelihood, selling the fruit to local companies for the extraction of juice and essential oils [
12]. This practice generates substantial amounts of residues, mainly consisting of albedo, flavedo, pulp, and seeds, which are commonly disposed to the environment for natural degradation or alternatively sold as forage. In either case, this biomass is frequently undervalued, despite representing a rich source of bioactive compounds that could be harnessed for value-added applications.
Among the wide variety of bioactive compounds present in citrus by-products, polyphenols are currently among the most valued and extensively studied due to their broad spectrum of therapeutic effects against various diseases and their ability to prevent the onset of neurodegenerative disorders such Alzheimer and Parkinson’s disease [
13]. However, their extraction is not a straightforward task, as these compounds are highly sensitive to factors as pH, light and heat, which can promote degradation and consequently reduce their functionality. Therefore, the selection of an appropriate extraction method becomes a crucial step [
14]. In this context, green chemistry has emerged as an environmentally friendly alternative to conventional extraction techniques where there are disadvantages as the use of polluting solvents, energy consumption, long extraction times, toxicity and/or degradation of thermo-sensitive compounds, and offer advantages such as recognition as Generally Recognized as Safe (GRAS), shorter extraction times, biodegradability, and reduced costs [
15,
16].
Natural Deep Eutectic Solvents (NADES) are considered a GRAS extraction system, as they are composed of metabolites naturally produced by plants, such as sugars, organic acids, and amino acids. They consist of mixtures of two or three components that do behave as hydrogen bond donor (HBD) and hydrogen bond acceptor (HBA) interacting through hydrogen bonding and resulting in a melting point lower than that of each individual component when combined in the appropriate molar ratio [
17]. Due to the wide range of possible combinations, NADES exhibit selective extraction capacity; in other words, depending on their composition and molar proportion, a given NADES can be adjusted to efficiently extract specific compounds [
18]. Furthermore, since they are non-toxic and safe for human consumption, NADES represent a highly promising alternative for the extraction of bioactive compounds from agro-industrial by-products [
19].
To the best of our knowledge, the use of NADES for the extraction of bioactive compounds from by-products of
C. aurantium harvested under the distinctive conditions of the Yucatán Peninsula, mainly Leptosol soils [
20] and warm sub-humid climate [
21], after the extraction of juices and essential oils, has not yet been reported. Therefore, the aim of this study was to evaluate different NADES combinations on the polyphenolic profile of
C. aurantium by-products, with a view to their potential application in food, pharmaceutical, or cosmetic formulations.
2. Results
2.1. Evaluation of Total Polyphenols, Flavonoids, Ascorbic Acid, and Antioxidant Capacity in C. aurantium By-Products Extracts
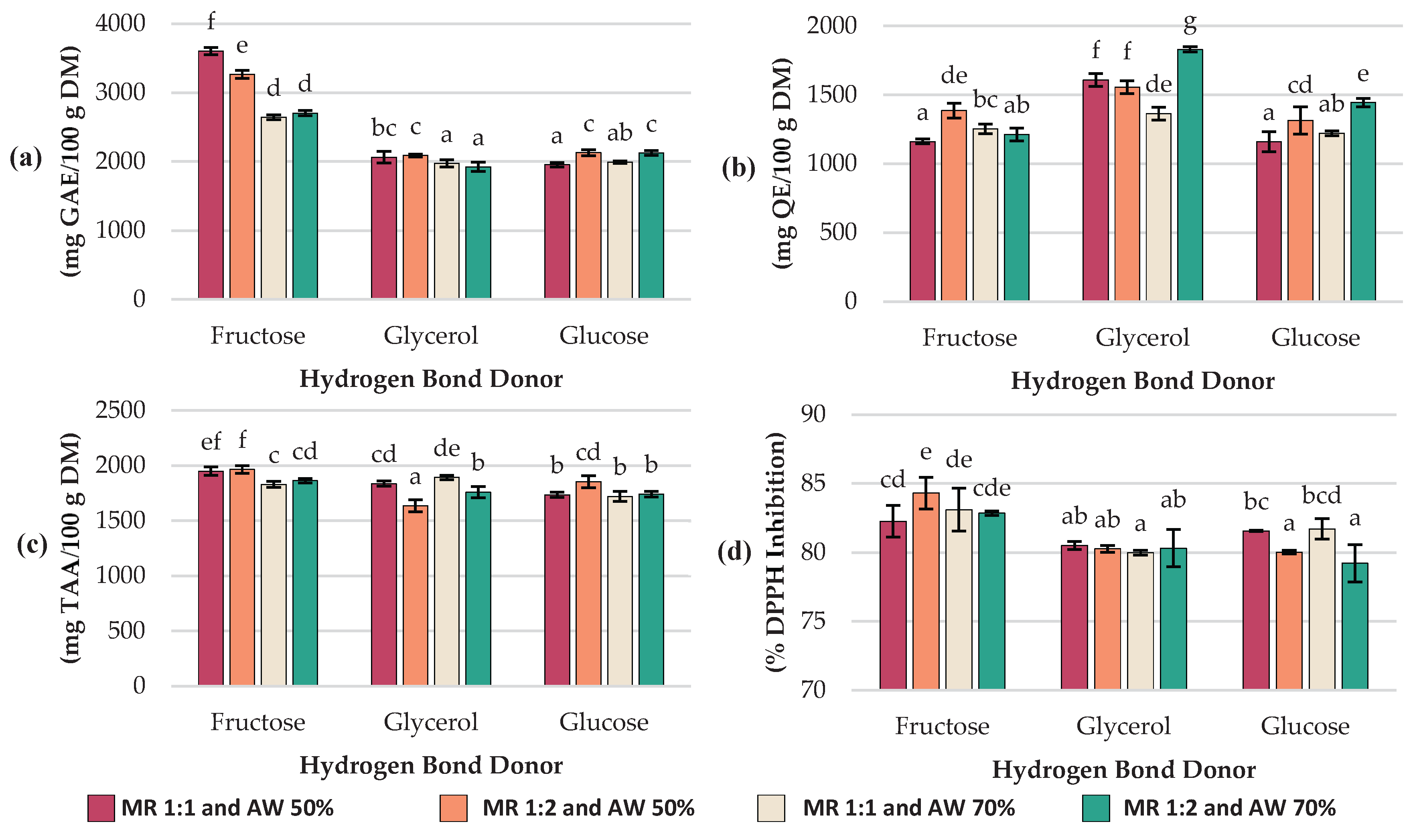
Total polyphenol content (TPC; expressed as GAE content), total flavonoid content (TFC; expressed as QE content), total ascorbic acid (TAA; expressed as ascorbic acid equivalent) and antioxidant capacity (Ax; expressed as %DPPH inhibition) results are summarized in
Figure 1. Additionally, all numerical data are provided in the
Supplementary Material (Table S1).
Significant differences (
p < 0.05) in TPC (
Figure 1a) were found among the experiments. The highest concentration of TPC was 3603.7 ± 52.9 mg GAE/100g DM and it was achieved with NADES based on fructose (MR 1:1 mol/mol and AW 50%). In contrast, the lowest concentrations: 1924.9 ± 69, 1955.2 ± 29.9, 1973.1 ± 53.5 and 1993.4 ± 16.9 mg GAE/100g DM were obtained with glycerol and glucose-based NADES at experiments #11 (HBD glycerol; MR 1:2 mol/mol; AW 70%), # 3 (HBD glucose; MR 1:1 mol/mol; AW 50%), #8 (HBD glycerol; MR 1:1 mol/mol; AW 70%) and #9 (HBD glucose; MR 1:1 mol/mol; AW 70%) respectively.
When comparing the effect of the HBD, fructose-based NADES showed consistently higher TPC values (2643.3–3603.7 mg GAE/100g DM) than glycerol (1924.9–2088 mg GAE/100g DM) and glucose (1955.2–2128.8 mg GAE/100g DM). Regarding the AW, most treatments exhibited higher values at 50% than at 70%. For example, Exp #1 (HBD fructose; MR 1:1 mol/mol; AW 50%) decreased from 3603.7 ± 52.9 to 2643.3 ± 33.8 mg GAE/100g DM at 70% AW (Exp #7), and Exp #2 (HBD glycerol; MR 1:1 mol/mol; AW 50%) decreased from 2062.7 ± 84 to 1973.1 ± 53.5 mg GAE/100g DM at 70% AW (Exp #8). A similar trend was observed for glucose-based NADES at Exp #6 (HBD glucose; MR 1:2 mol/mol; AW 50%) with 2128.8 ± 44.3 mg GAE/100g DM vs Exp #12 (HBD glucose; MR 1:2 mol/mol; AW 70%) with 2124.8 ± 34.7 mg GAE/100g DM (p > 0.05).
The total flavonoid content (TFC) was also determined in each experiment, and the results are summarized in
Figure 1b. The highest flavonoid concentration was 1829.8 ± 17.8 mg QE/100g DM and it was obtained in experiment #11 (HBD glycerol; MR 1:2 mol/mol; AW 70%), while Exp #1 (HBD fructose; MR 1:1 mol/mol; AW 50%), Exp #3 (HBD glucose; MR 1:1 mol/mol; AW 50%), Exp #9 (HBD glucose; MR 1:1 mol/mol; AW 70%), and Exp #10 (HBD fructose; MR 1:2 mol/mol; AW 70%) yielded the lowest values (1161.4 ± 17.5, 1159.6 ± 73.6, 1221.1 ± 17.7, and 1212.8 ± 46.2 mg QE/100g DM, respectively), with no significant statistical differences among them (
p > 0.05). Extracts obtained from glycerol-based NADES showed the highest flavonoid content (1363.4–1829.8 mg QE/100g DM), compared to those prepared with fructose (1161.4–1385 mg QE/100g DM) or glucose (1159.6–1443.8 mg QE/100g DM).
In fructose-based NADES, when the added water was 50%, flavonoid levels significantly increased as the molar ratio shifted from 1:1 mol/mol (Exp # 1; 1161.4 ± 17.5 mg QE/100g DM) to 1:2 mol/mol (Exp # 4; 1385 ± 52.9 mg QE/100g DM). A comparable trend was found in glucose-based NADES, where with 50% added water, flavonoid content rose from 1159.6 ± 73.6 mg QE/100g DM at MR 1:1 mol/mol (Exp #3) to 1313.9 ± 97.8 mg QE/100g DM at MR 1:2 mol/mol (Exp #6). In contrast, for glycerol-based NADES with 50% water, the opposite effect occurred: increasing the MR from 1:1 mol/mol (Exp #2) to 1:2 mol/mol (Exp #5) slightly reduced flavonoid content (1607.9 ± 46 to 1555.3 ± 46 mg QE/100g DM) (p > 0.05). Overall, except for fructose-based NADES, each hydrogen bond donor reached its highest flavonoid extraction capacity under conditions of MR 1:2 mol/mol and AW 70% (1443.8 ± 31.2 mg QE/100g DM for glucose and 1829.7 ± 17.8 mg QE/100g DM for glycerol).
Total ascorbic acid (TAA) of the extracts varied depending on the HBD, MR, and AW, with values ranging from 1635.3 ± 52.8 to 1964.9 ± 33.7 mg/100g DM (
Figure 1c). The highest concentrations were obtained using fructose as HBD in Exp #4 (MR 1:2 mol/mol and AW 50%) with 1964.9 ± 33.7 mg/100g DM and Exp #1 (MR 1:1 mol/mol and AW 50%) with 1948.2 ± 38 mg/100g DM, while the lowest was observed with glycerol-based NADES in Exp #5 (MR 1:2 mol/mol and AW 50%) reaching 1635.2 ± 52.8 mg/100g DM. In general, extracts obtained with fructose yielded higher ascorbic acid values (1828.5–1964.8 mg/100g DM) compared to those obtained with glucose (1719.5–1854.9 mg/100g DM) and glycerol (1635.3–1892.5 mg/100g DM). For fructose and glucose-based NADES, increasing the molar ratio from 1:1 to 1:2 mol/mol at 50% AW slightly enhanced ascorbic acid recovery, whereas in glycerol-based NADES this change did not result in an improvement. Regarding the effect of water added, decreasing AW to 50%, increased ascorbic acid content in fructose-based NADES, whereas in glycerol-based NADES the opposite trend was observed, with lower TAA values at lower water content.
DPPH Radical Scavenging assay was used to determine antioxidant capacity of extracts (Ax). Ax of the extracts ranged from 79.22 ± 1.36% to 84.31 ± 1.15% among different experiments (
p < 0.05)(
Figure 1d). The highest Ax was obtained with fructose-based NADES at Exp #4 (MR 1:2 mol/mol and AW 50%) exhibiting 84.31 ± 1.15% inhibition, while the lowest were observed with Exp #12 (HBD glucose; MR 1:2 mol/mol; AW 70%) with 79.22 ± 1.36% inhibition, Exp #8 (HBD glycerol; MR 1:1 mol/mol; 70% AW) with 79.99 ± 0.17% inhibition, Exp #6 (HBD glucose; MR 1:2 mol/mol; 50% AW) with 80.03 ± 0.14% inhibition, Exp #5 (HBD glycerol; MR 1:2 mol/mol and AW 50%) with 80.27 ± 0.25% inhibition, Exp #11 (HBD glycerol; MR = 1:2 mol/mol; AW 70%) with 80.31 ± 1.36% inhibition and Exp #2 (HBD glycerol; MR 1:1 mol/mol; AW 50%) with 80.52 ± 0.29% inhibition. Overall, fructose-based NADES exhibited the strongest antioxidant capacity (82.26–84.31% inhibition), followed by glycerol (79.99–80.52% inhibition) and glucose (79.22–81.71% inhibition). At 50% AW, increasing the MR from 1:1 to 1:2 mol/mol slightly reduced antioxidant activity in glucose-based NADES from 81.57 (Exp #3) to 80.03% inhibition (Exp #6) (
p < 0.05) and glycerol-based NADES from 80.52 (Exp #2) to 80.27% inhibition (Exp #5) (
p > 0.05). In contrast, fructose-based NADES exhibited the opposite effect, with antioxidant activity increasing from 82.26 (Exp #1) to 84.31% inhibition (Exp #4) (
p < 0.05).
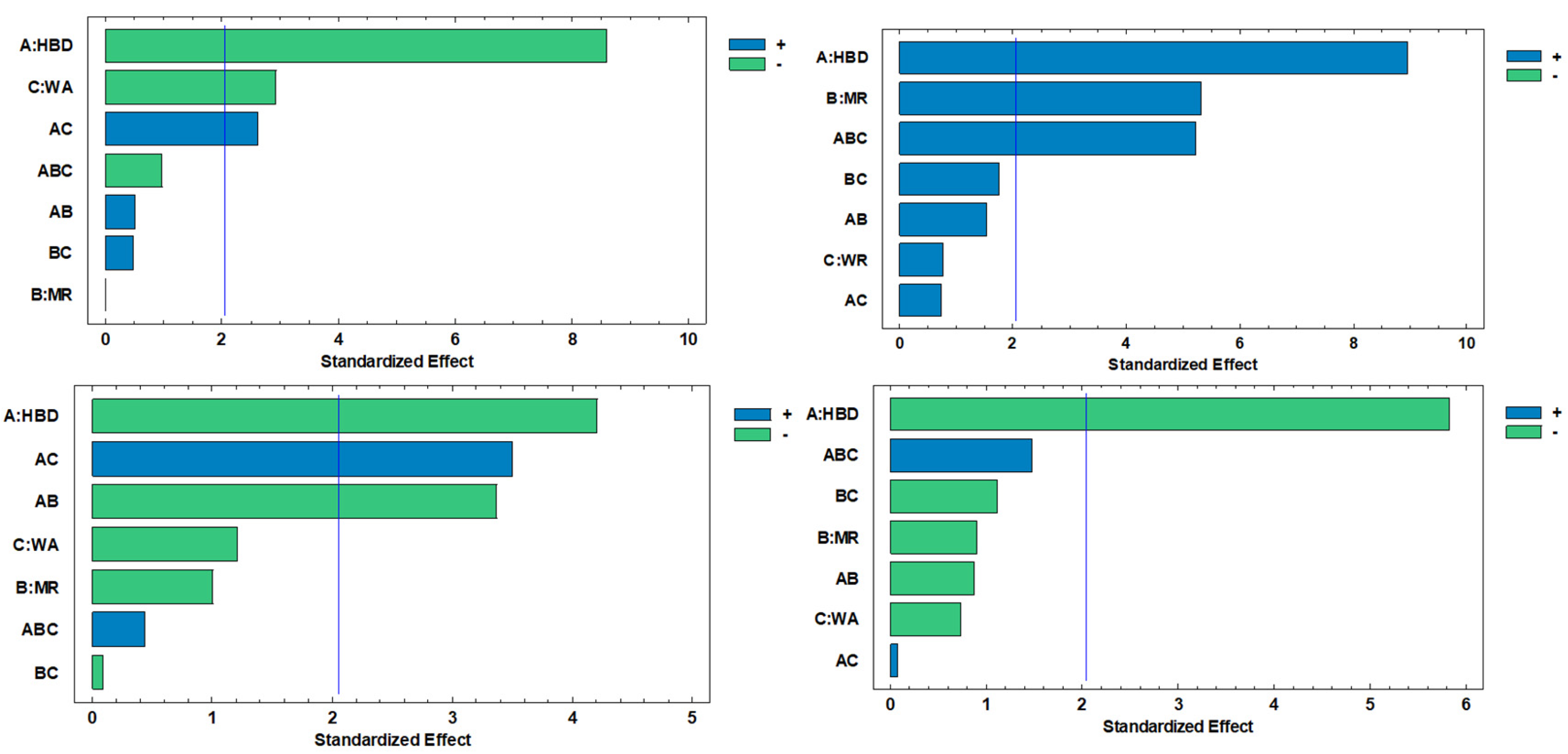
Figure 2 demonstrates the significant effect of all factors and interactions on different responses. HBD was the main factor influencing all response variables. For TPC (
Figure 2a), HBD (A) presented the largest standardized effect, followed by AW (C) and their interaction (AC).
In TFC (
Figure 2b), significant effects were observed for HBD and MR (B). Although, a triple interaction among three main factors was observed (ABC). For TAA (
Figure 2c), HBD was the dominant factor, with additional contributions from AC and AB interactions. Finally, in Ax (
Figure 2d), HBD was the only factor with a clear effect.
3.2. Evaluation of the Profile of Phenolic Compounds in C. aurantium By-Products Extracts
Among the 16 polyphenols examined in each extract from the factorial design (
Table S1), only 13 individual polyphenols were identified and quantified. Gallic acid, apigenin and diosmetin were not detected in any of the experiments (
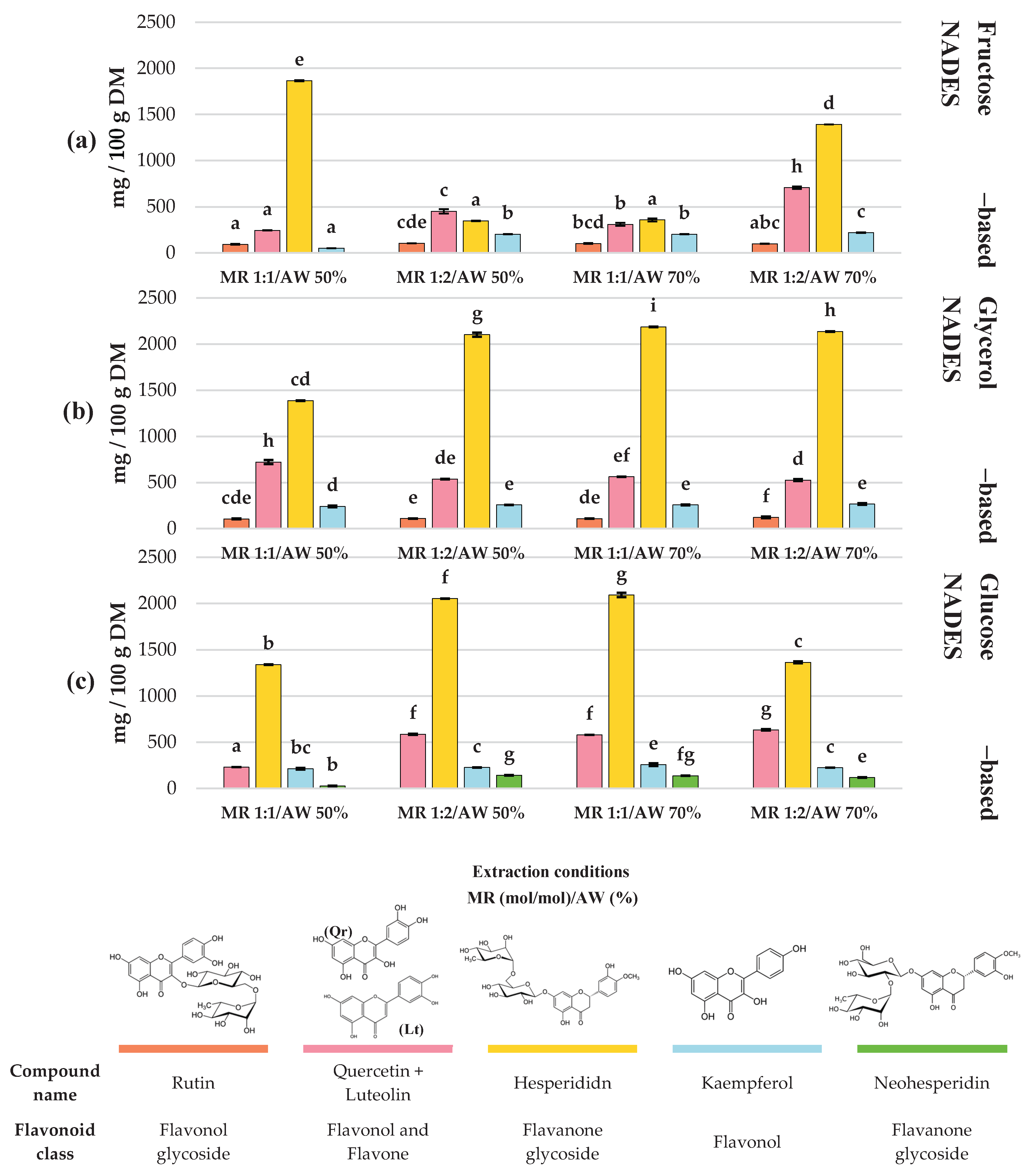
Table S2). Flavonoids (
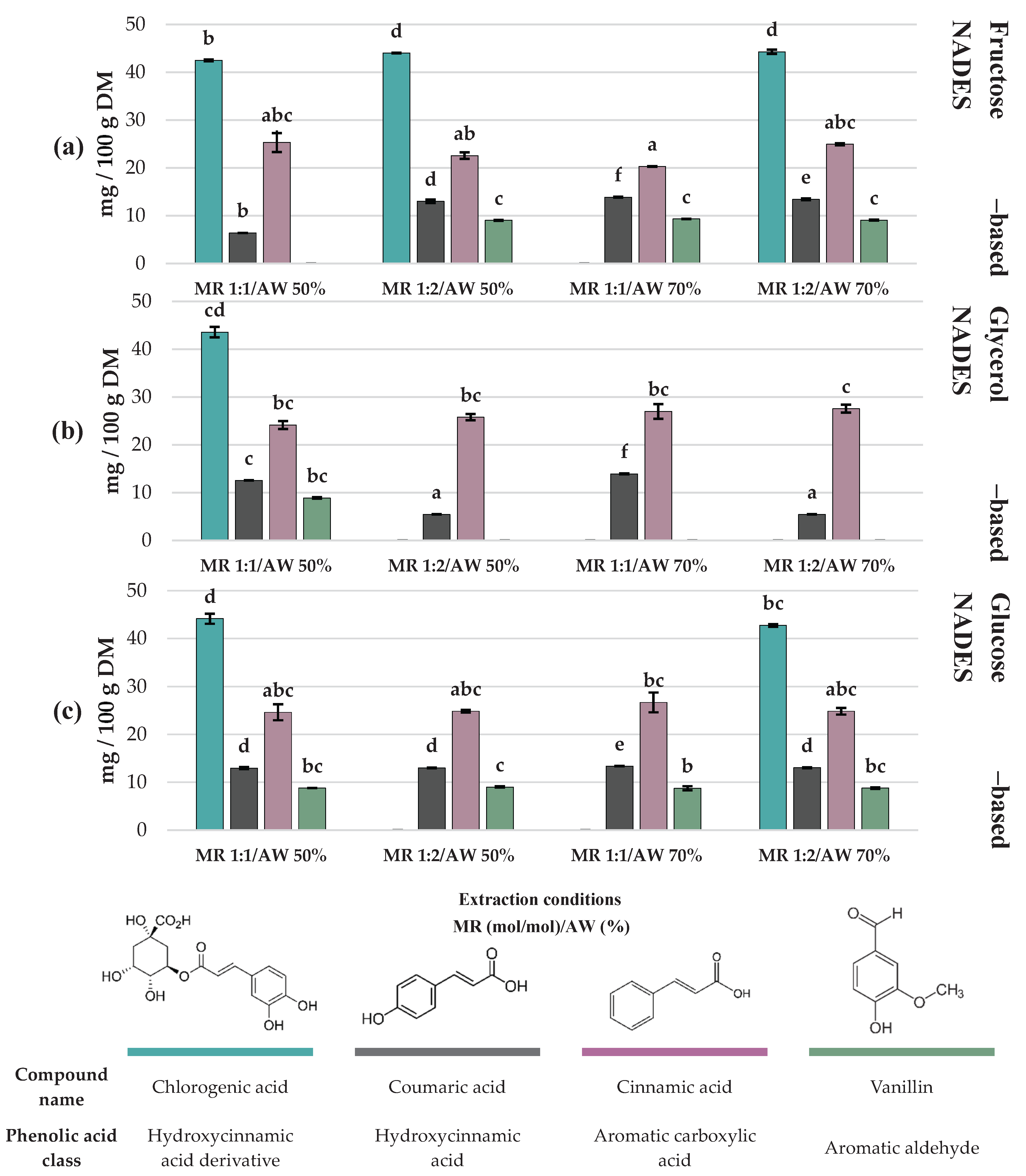
Figure 3) and phenolic acids (
Figure 4) contents are displayed as bar charts to enhance clarity and facilitate comparison among treatments.
The extraction profiles of the main flavonoids in C. aurantium by-products showed clear differences depending on the type of NADES and the extraction conditions. Overall, hesperidin was consistently the predominant compound across all treatments, reaching its maximum concentration when glycerol-based NADES were used at a 1:1 mol/mol MR and 70% AW (Exp #8), obtaining 2186 ± 2.06 mg/100 g DM.
In general, glycerol-based NADES provided the highest hesperidin recovery (1386.74 – 2186.08 mg/100 g DM). Conversely, neohesperidin was the least abundant flavonoid among the main ones, where a glucose-based NADES showed the greatest efficiency extracting this compound, reaching 141.99 ± 2.62 mg/100 g DM under experiment #6 (HBD glucose; MR = 1:2 mol/mol; AW 50%).
Regarding the remaining flavonoids, quercetin + luteolin represented the second main flavonoids, with concentrations ranging from 229.69 to 721.32 mg/100 g DM across treatments. The highest yields (721.32 ± 23.05 and 707.20 ± 13.17 mg/100 g DM ) were observed in Exp #2 (HBD glycerol; MR 1:1 mol/mol; AW 50%) and Exp #10 (HBD fructose; MR 1:2 mol/mol; AW 70%) respectively (p > 0.05). Kaempferol and rutin were also present in all treatments. Kaempferol showed its highest recoveries in Exp #9 (HBD glucose; MR 1:1 mol/mol; AW 70%), #Exp 5 (HBD glycerol; MR 1:2 mol/mol; AW 50%), Exp #8 (HBD glycerol; MR 1:1 mol/mol; AW 70%), and Exp #11 (HBD glycerol; MR 1:2 mol/mol; AW 70%), with values of 256.65 ± 3.30, 256.79 ± 0.77, 257.08 ± 3.03, and 266.88 ± 8.44 mg/100 g DM, respectively (p > 0.05). Meanwhile, rutin reached its maximum concentration (121.16 ± 9.10 mg/100 g DM) under experiment #11 (HBD glycerol; MR 1:2 mol/mol; AW 70%). Overall, NADES formulated with glycerol as HBD stood out as the most effective for extracting the different flavonoids identified.
The extraction of phenolic acids from C. aurantium by-products also varied significantly depending on the type of NADES and extraction conditions. Chlorogenic acid was mainly recovered in treatments with fructose and glucose-based NADES, reaching the highest concentration in Exp #4 (HBD fructose; MR 1:2 mol/mol; AW 50%), Exp #10 (HBD fructose; MR 1:2 mol/mol; AW 70%), Exp #3 (HBD glucose; MR 1:1 mol/mol; AW 50%) and Exp #2 (HBD glycerol; MR 1:1 mol/mol; AW 50%) with 44.01 ± 0.05, 44.28 ± 0.44, 44.14 ± 1.05 and 43.60 ± 1.10 mg/100 g DM respectively (p > 0.05). Cinnamic acid displayed a similar trend, even though its maximum concentration reached 27.59 ± 0.83 mg/100 g DM, there was no statical difference among 10 out of 12 treatments. Coumaric acid was consistently found at lower concentrations across treatments, with the highest extraction obtained at Exp #7 (13.86 ± 0.08 mg/100 g DM) and Exp #8 (13.93 ± 0.08 mg/100g DM), both at a 1:1 MR mol/mol and 70% of AW using as HBD fructose and glycerol respectively.
Regarding vanillin, its extraction was limited to specific treatments. The highest concentration was obtained with fructose-based NADES, specifically Exp #4 (MR 1:2 mol/mol and AW 50%), Exp #7 (MR 1:1 and AW 70%), and Exp #10 (MR 1:2 and AW 70%), with values of 9.02 ± 0.10, 9.32 ± 0.01 and 9.07 ± 0.06 mg/100g DM respectively (p > 0.05). In general, fructose and glucose as HBDs favored the extraction of chlorogenic acid and vanillin, whereas glycerol-based NADES were less efficient for these specific compounds but still enabled the recovery of cinnamic acid at comparable levels.
Other minor flavonoids and phenolic acids were also found in the extracts, but its extraction was very selective (
Table S2), for example, protocatechuic acid and catechin only were found in fructose-based NADES, both reaching their maximum at experiment #10 (MR 1:2 mol/mol and AW 70%) obtaining 62.82 ± 7.6 and 23.09 ± 5.6 mg/100g DM respectively. Naringenin, another characteristic flavonoid in citrus species, was also found in sugars-based NADES, reaching its highest concentration at Exp #4 (HBD fructose; MR 1:2 mol/mol; AW 50%) and Exp #3 (HBD glucose; MR 1:1 mol/mol; AW 50%) reaching 25.59 ± 1.10 and 25.07 ± 0.05 mg/100g DM respectively (
p > 0.05).
Table 1 presents the p-values of the main factors and their interactions. HBD (A) showed a significant effect on all the individual polyphenols evaluated. In addition, the molar ratio (B) and added water (C) also influenced the extraction of most compounds, except for chlorogenic acid (only B showed no effect), rutin (only C showed no effect), cinnamic acid, hesperidin, vanillin, and naringenin. Regarding interactions, although double interactions (AB, AC, and BC) were observed, a triple interaction (ABC) was particularly evident for most individual polyphenols, except for chlorogenic acid, coumaric acid, and quercetin + luteolin.
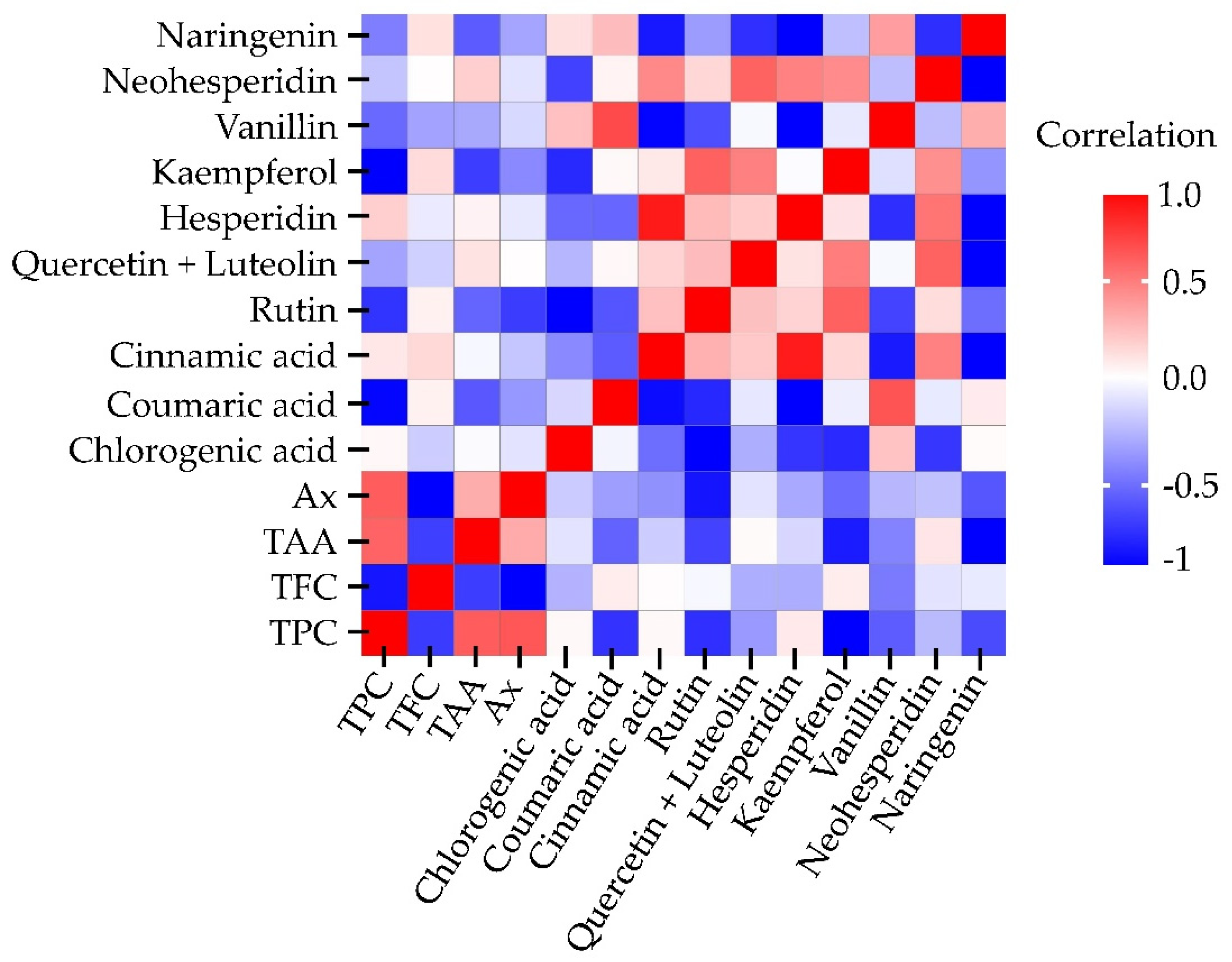
2.3. Pearson Correlation
The Pearson correlation coefficient is a statistical measure that quantifies the strength and direction of relationship between two quantitative variables.
Figure 5 presents the heatmap of Pearson correlations obtained from all response variables in the 3 × 2 × 2 experimental design.
A positive correlation was observed between TPC and TAA (r = 0.7120), as well as between TPC and Ax (r = 0.7275). Additionally, some individual polyphenols displayed strong associations, such as hesperidin with cinnamic acid (r = 0.9108) and vanillin with coumaric acid (r = 0.7559). In contrast, certain secondary metabolites exhibited negative correlations; for instance, naringenin with kaempferol (r = –0.8021) and naringenin with cinnamic acid (r = –0.7211). Overall, most correlations showed no statistical relevance, with coefficients below 0.7.
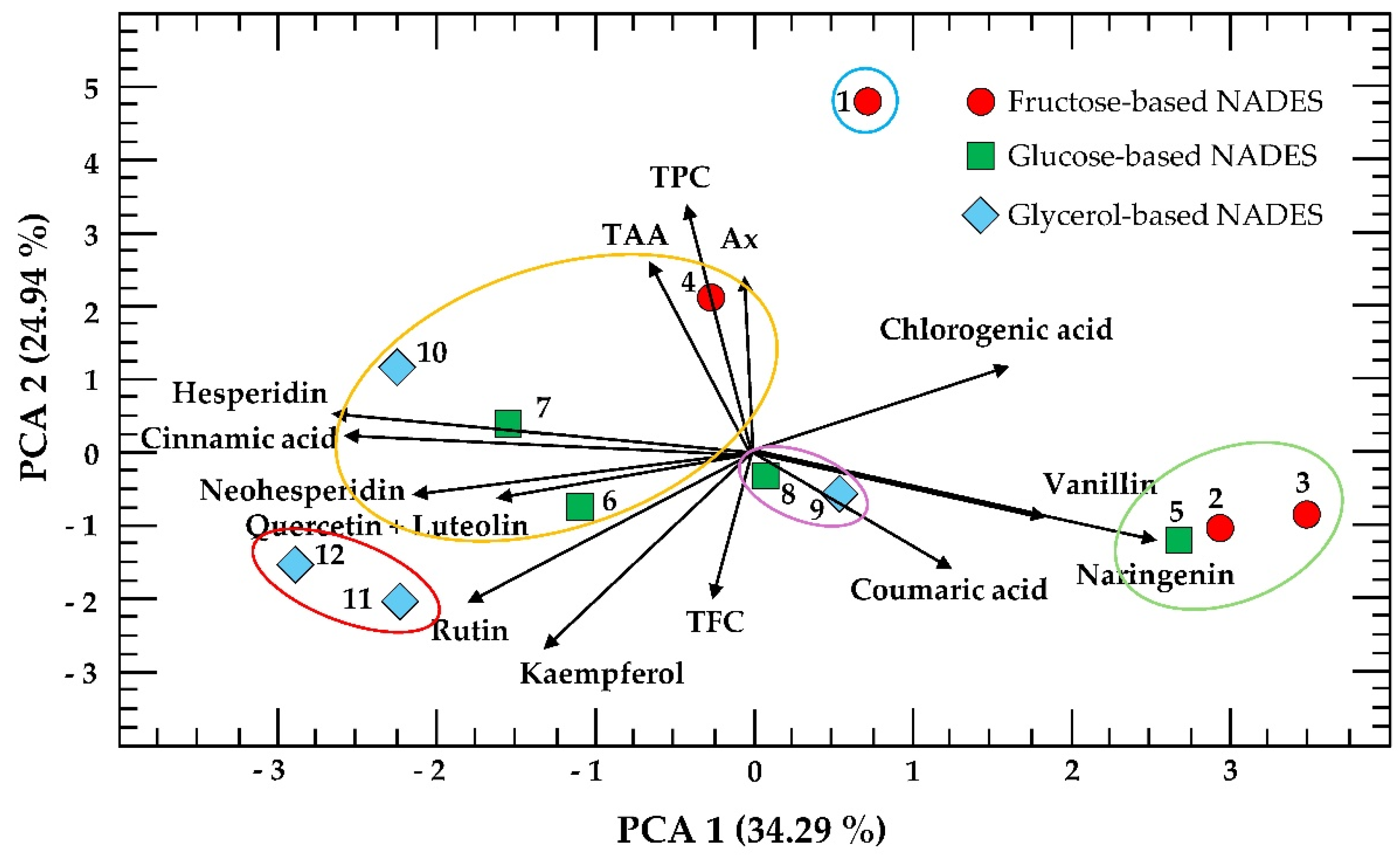
3.4. Principal Component Analysis
Principal component analysis (PCA) with K-means clustering was performed on the experimental conditions of the 3 × 2 × 2 factorial design (
Figure 6). PCA shows that the two principal components accounted for 59.23% of the total variance are presented as a biplot with K-means clustering, simultaneously displaying the scores, loadings, and cluster groupings of the first two principal components. However, the three main components accounted for 73% of the total variance and complementary biplots of PC1 vs PC3, PC2 vs PC3 and PC1 vs PC2 vs PC3 are provided in
Figure S1 (Supplementary Material), allowing further visualization of patterns across all three principal components.
In this representation, the points correspond to the observations (experimental conditions), illustrating their distribution and grouping in the multivariate space, while the vectors represent the response variables (TPC, TFC, TAA, Ax, and the polyphenols measured, excluding gallic acid, protocatechuic acid, catechin, apigenin, and diosmetin), indicating both, their contribution to the components and their correlations with each other. The superimposed clusters highlight groups of experimental conditions and variables that share similar profiles, facilitating the interpretation of how specific variables drive the separation or clustering within the dataset. Complementary biplots of PC1 vs PC3 and PC2 vs PC3 are provided in
Figure S1 (Supplementary Material), allowing further visualization of patterns across all three principal components.
As observed, the distance between the eigenvectors of TAA, TPC, and Ax is very small, indicating that samples with high TPC values also contain considerable levels of TAA and Ax. In contrast, TFC is positioned nearly opposite, suggesting that the first three variables and TFC are inversely related. Regarding the individual polyphenols, all acids except cinnamic acid are located on the right side of the PCA, while all flavonoids except naringenin are positioned on the left.
Finally, four distinct clusters of experimental conditions were observed. The first cluster (green ellipse) groups extractions 2, 3, and 5, which are associated with high levels of naringenin and vanillin. The second cluster (yellow ellipse) includes extractions 4, 6, 7, and 10, characterized by higher TPC, TAA, and Ax values, indicating a strong positive correlation among these variables. The third cluster (red ellipse) comprises extractions 11 and 12, which are associated with rutin, quercetin, and luteolin, while the fourth small cluster (purple ellipse) groups extractions 8 and 9 presented intermediate polyphenol profiles.
3. Discussion
The composition of NADES proved to be a determining factor in the extraction of phenolic compounds from
C. aurantium. The results for TPC, TFC, TAA, and Ax showed significant differences among treatments. In all cases, choline chloride was used as the common HBA, ensuring that the observed variations were solely due to the different HBDs, MR, and AW employed. The Folin–Ciocalteu method, widely used for quantifying polyphenols, is based on an electron transfer reaction in which the antioxidant species acts as an electron donor and the reagent serves as an oxidizing agent [
22].
Experiment #1 (HBD fructose; MR 1:1; AW 50%) achieved the highest TPC (3603.7 ± 52.9 mg GAE/100g DM), which is higher than the value reported by Edrisi et al., [
23], who extracted 785 mg GAE/100g DM using a NADES composed of choline chloride as the HBA and 1,4-butanediol as the HBD after an optimization process (optimal conditions: temperature of 40 °C, extraction time of 30 min, water content of 49.95%, and a solvent to biomass ratio of 18.97:1). These differences could be attributed to variations in the raw material and sample preparation methods. While Edrisi et al., [
23] used only the peel, the raw material in our study consisted of a mixture of different citrus fruit parts (albedo, flavedo, pulp, and seeds). In addition, the moisture removal method employed by those authors was air-drying at 25 °C, whereas in the present study, lyophilization was used. This technique can better preserve sensitive bioactive compounds due to the low temperatures and absence of light exposure [
24]. These results are comparable to those obtained using organic solvents. For instance, Abdallah et al., [
25] optimized a solid-to-liquid ratio of 2.4 g/100 mL, an acetone concentration of 80%, and a sonication time of 34.7 min, achieving an extraction yield of 3376 ± 563 mg GAE/100g DM. However, it is important to highlight that extracts obtained with organic solvents require purification steps to remove toxic residues, whereas NADES-based extracts, being made of natural plant metabolites, are considered safe and classified as GRAS [
16]. In contrast, the values obtained in the present study are lower than those reported by Amador-Luna et al., [
26], who developed an innovative three-step sequential extraction process: (1) Supercritical Fluid Extraction (SFE) for the nonpolar fraction, (2) Pressurized Liquid Extraction (PLE) using NADES for polar compounds, and (3) an antisolvent extraction step to recover compounds adsorbed in the PLE residue. The NADES extract, formulated with choline chloride and glycerol at a 1:2 mol/mol MR with 57.9% AW, yielded 6240 mg GAE/100g DM at semi-pilot scales. Although glycerol was also used in this study as HBD in a 1:2 mol/mol MR, the lower phenolic yields may be attributed to two main factors. First, the NADES used by Amador-Luna et al., [
26] were pressurized, which involves operating at high temperature and pressure (below critical point), enhancing solubility and mass transfer within the matrix and allowing rapid and efficient extraction with minimal solvent use [
27]. Second, the raw material used in the present study had already undergone essential oil removal (via freeze-drying extraction), and as reported by Amador-Luna et al., [
26] and other authors such as Alizadeh [
28], essential oils themselves contain phenolic compounds (664 and 4135 mg GAE/100g, respectively). However, the results obtained in this work confirm that even after essential oil extraction,
C. aurantium industrial by-products still retain significant amounts of these bioactive phenolic compounds.
In the case of total flavonoids, experiment #11 (HBD glycerol; MR 1:2; AW 70%) showed the highest TFC (1829.7 ± 17.8 mg QE/100g DM). These results are higher than those reported by Estrada-Sierra et al., [
29], who obtained a TFC concentration of 830 mg QE/100g DM from the by-products (seeds, pulp, and peel) of
C. aurantium juice extraction at the green maturity stage, using water as solvent for the extraction, being this difference attributed to the solvent used. Although water is a polar compound, flavonoids such as hesperidin are poorly water soluble [
30]. Conversely, NADES, being more complex mixtures, can extract a wider diversity of molecules [
17]. Also, the results obtained from by-products in this work are considerably lower than those reported by Liu et al., [
31], who extracted 16,816 ± 47 mg QE/100g of total flavonoids from
Aurantii Fructus Immaturus (AFI), the dried immature fruit of
C. aurantium. This high yield was achieved using ultrasound-assisted extraction with Natural Deep Eutectic Solvents (UAE-DES). Specifically, the optimal solvent selected was a betaine–urea (Bet:Ur) mixture, and the extraction conditions were optimized through Response Surface Methodology (RSM). The optimal parameters included a molar ratio of 1:3, a water content of 30%, a liquid-to-solid ratio of 30:1 mL/g, an extraction temperature of 50 °C, and an extraction time of 50 minutes.
The differences observed may be attributed to the use of betaine as the hydrogen bond acceptor (HBA), which has an amphiphilic and zwitterionic nature, modulated by pH, can form micelle-like self-assemblies that act as nanocontainers with dual affinity for both hydrophilic and lipophilic compounds [
32]. Furthermore, since the raw material used by Liu et al., [
31] had not undergone essential oil removal, the extraction likely included less polar compounds associated with these fractions. According to Shraim et al., [
33], such compounds can be quantified using the AlCl₃ which is not an specific colorimetric assay; in other words, a lot of compounds can be quantified if they complies at least one of the following structural requirements: a carbonyl group at C-4 conjugated with the ring and a hydroxyl group at C-3 or C-5; a catechol system (3′,4′-OH) on ring B; or other accessible phenolic –OH groups, although the latter may form less stable complexes. Therefore, since betaine-based NADES are highly effective at extracting diverse types of compounds, it is likely that the quantified phenolic content also includes non-flavonoid phenolic compounds.
Regarding to ascorbic acid content, to the best of our knowledge, its quantification in NADES using spectrophotometry has not been previously reported. Fructose proved to be the most effective HBD, with experiments #1 (MR 1:1 mol/mol and AW 50%) and #4 (MR 1:2 mol/mol and AW 50%) showing the highest TAA levels with 1948.2 ± 38 and 1964.8 ± 33.7 mg/100g DM, respectively. These values are considerably higher than those reported in the literature for any citrus species. For instance, in
C. aurantium, values of 24 mg/100g have been reported for immature fruit juice [
34], while the peel of other citrus fruits such as lemon and grapefruit has shown maximum levels of 58.59 and 113.3 mg/100g DM, respectively [
35]. Such differences may be mainly attributed to the nature of the raw material used in this study, which consisted of a mixture of different parts of the fruit (albedo, flavedo, pulp, and seeds), potentially explaining the high ascorbic acid content. On the other hand, choline chloride–based NADES with sugar (e.g., fructose and glucose as HBD) have been reported to exhibit slightly acidic to near neutral pH values depending on their composition and water content [
36]. This intrinsic acidity and high polarity may contribute to improved extraction efficiency and to the enhanced stability of ascorbic acid under the conditions applied in this study. Indeed, vitamin C (with no presence of oxygen) is known to be more stable under acidic environments (pH 5 – 6), which further supports the observed high ascorbic acid content in the extracts [
37].
Antioxidant capacity was measured using the DPPH reagent, where antioxidants can act through two mechanisms: single electron transfer (SET), reducing the DPPH radical to its stable form, or hydrogen atom transfer (HAT), donating an H atom to the radical. In both cases, the radical is neutralized and the violet color fades to yellow [
38]. Fructose proved to be the most effective HBD, with experiments #4 (MR 1:2 mol/mol and AW 50%), #7 (MR 1:1 mol/mol and AW 70%), and #10 (MR 1:2 mol/mol and AW 70%) showing the highest values among the experiments (
p < 0.05), exhibiting inhibition percentages of 84.31% ± 1.15, 83.10% ± 1.56, and 82.85% ± 0.17 respectively (
p > 0.05). These results are slightly lower than those reported by Ramírez-Sucre et al., [
39], who achieved 100% DPPH radical inhibition in the peel of
C. aurantium. This difference could be attributed to the pretreatment process of the raw material, since the peel was manually separated and did not undergo any industrial extraction process (essential oils and juice extraction), allowing polyphenolic compounds, which are highly sensitive to factors such as light and heat [
14] to remain undegraded. The higher antioxidant capacity observed with fructose compared to glucose and glycerol could be attributed to its greater ability to extract phenolic acids. According to Gulcin et al., [
40], the stability of the DPPH radical is largely due to steric hindrance around the divalent nitrogen atom and to the “push–pull” effect, which limits the approach of bulky molecules. Thus, large molecules such as glycosylated flavonoids encounter greater steric hindrance when donating electrons or hydrogen atoms due to their restricted access to the radical center. In contrast, smaller and less hindered molecules, such as phenolic acids, can more easily approach and react rapidly, resulting in a higher percentage of DPPH radical inhibition.
Regarding the polyphenolic profile, hesperidin was the predominant flavonoid in the NADES extracts, reaching its highest concentration (
p < 0.05) in experiment #8 (HBD: glycerol; MR: 1:1 mol/mol; AW: 70%) with 2186.08 ± 2.06 mg/100 g DM. These results are similar than those obtained by Liu et al. [
31], who, under DES optimal extraction conditions (40% water in betaine/ethanediol (1:4) at 60 °C for 30 min heated extraction and a solid-to-liquid ratio of 1:100 g/mL), extracted 2872 ± 0.35 mg/100 g DM. However, it is important to note that under those conditions, they also extracted considerable amounts of naringin (3835 mg/100 g DM) and neohesperidin (8367 mg/100 g DM), the latter being much higher than the value obtained in the present study (141.99 mg/100 g DM). Glycerol proved to be the most effective HBD for flavonoid extraction, which, according to Qader et al., [
41], can be explained by the structural nature of both molecules. Since hesperidin is glycosylated, it has a relatively large molecular volume that hinders the formation of hydrogen bonds with bulky and hydroxyl groups rich HBDs such as sugars. In contrast, glycerol, being a smaller molecule with a reduced number of hydroxyl groups, offers greater conformational flexibility and lower steric hindrance, allowing it to interact more efficiently with the accessible sites of hesperidin.
A similar trend was observed for quercetin + luteolin, the second most abundant (glycosylated) flavonoids across experiments, whose maximum concentration (721.32 ± 23.05 mg/100g DM) was also obtained using glycerol as HBD (MR: 1:1 mol/mol; AW: 70%). These values are higher than those reported by Ramírez-Sucre et al., [
37], who extracted 160.99 ± 1.75 mg/100g from
C. aurantium peel using a NADES composed of glucose as HBD (MR: 1:2 mol/mol; AW: 70%).
Finally, in the case of phenolic acids, chlorogenic acid was reported in the highest amounts in experiments #4 (HBD fructose; MR 1:2 mol/mol; AW 50%), #10 (HBD fructose; MR 1:2 mol/mol; AW 70%), #3 (HBD glucose; MR 1:1 mol/mol; AW 50%) and #2 (HBD glycerol; MR 1:1 mol/mol; AW 50%) with 44.01 ± 0.05, 44.28 ± 0.44, 44.14 ± 1.05 and 43.60 ± 1.10 mg/100 g DM respectively (
p > 0.05). The three HBDs employed (glycerol, fructose, and glucose) showed similar extraction efficiencies (
p > 0.05) for this compound. This lack of selectivity can be explained by the same reason that makes glycerol the most effective HBD for flavonoid extraction [
41]. Due to their relatively small size and lower structural complexity, phenolic acids lack the steric hindrance that limits the solubilization of glycosylated flavonoids such as hesperidin. Their steric accessibility and the presence of hydroxyl groups in aromatic positions facilitate the formation of hydrogen-bond interactions with any of the tested HBDs. However, when analyzing each treatment individually, fructose with experiments #1 (MR 1:1 mol/mol and AW 50%) and #10 (MR 1:2 mol/mol and AW 70%) and glucose with experiments #3 (MR 1:1 mol/mol and AW 50%) and #12 (MR 1:2 mol/mol and AW 70%) enabled greater recovery in the main phenolic acids (chlorogenic acid, coumaric acid, cinnamic acid and vanillin), followed by glycerol in only experiment #2 (MR 1:1 mol/mol and AW 50%). This trend suggests that HBDs richer in –OH groups, such as sugars, may generate a denser network of interactions, favoring the solubilization of simple phenolic compounds, in contrast with glycerol, whose lower hydroxyl density makes it less competitive for this class of metabolites.