1. Introduction
At its core, the nation’s health care issues center on the growing crisis of chronic disease and, more specifically, multimorbidity (MM), the concurrent presence of two or more chronic conditions. The dimensions of the issue are enormous. According to data from the Centers for Disease Control and Prevention (CDC) issued recently, almost six of every ten American adults have at least one chronic disease, and 51.4%, representing over 130 million people, have multiple chronic conditions (MCCs) [
1]. This population is estimated to account for 90% of the nation’s
$4.9 trillion annually in health care expenditure [
2]. The cost is not only additive, but the comorbidity of diseases makes clinical management difficult, resulting in increased utilisation of health resources, higher expenses, and a greatly reduced quality of life. As the incidence of MM accelerates, especially among young adults, the pattern of national spending on health is widely recognized to be unsustainable, and there is a need for better and better-focused management approaches [
3].
While chronic disease has historically been viewed as a condition of aging, recent evidence shatters this perception. From 2013 until 2023, MCC incidence among adults aged 18 to 34 rose significantly from 21.8% to 27.1%. The increasing pattern, largely due to rising rates of obesity and depression, is a landmark change in the national health picture [
1]. The increasing incidence is compounded by inherent clinical problems of treating MM. The presence of multiple conditions complicates clinical management, increases the risk of polypharmacy and adverse drug events, and leads to conflicting and/or time-intensive treatment regimens that can also impact patients’ treatment adherence [
4,
5]
While conventional clinical endpoints have objective parameters, such as morbidity and mortality, a full assessment of MM requires measuring the humanistic burden, which quantifies the subjective patient experience throughout the course of disease, and the treatment intervention, on the other hand, becomes more pertinent to the evaluation of care. These can be predominantly evaluated by Patient-Reported Outcomes, such as the Physical and Mental Health-Related Quality of Life (HRQL), which are central variables of the current study. Alongside this humanistic toll is the direct financial burden placed on patients. This is usually measured by out-of-pocket (OOP) costs, a payment highly concentrated among individuals with more serious conditions and lower health status, so that individuals with the largest need for services tend to have the lowest ability to pay for them [
6,
7].
However, while the high cost of chronic disease is well-documented, most research provides a static, cross-sectional view of this burden. This approach fails to capture the dynamic nature of MM in a longitudinal scope, leaving a critical gap in our understanding of how the economic and humanistic consequences evolve over time. As a result, policymakers and health systems lack granular, longitudinal evidence on the heterogeneity within the MM population, specifically, which combinations of conditions drive the most significant costs and quality of life reductions in the long term. Without this longitudinal perspective, efforts to design effective, value-based care models that target the highest-need, highest-cost patients are hindered. This study aims to address that knowledge gap by characterizing and comparing the four-year trajectories of economic and humanistic burdens across distinct, clinically defined clusters of MM. Using longitudinal data from the 2019 – 2022 Medical Expenditure Panel Survey (MEPS), we analyze how total health care expenditures, OOP spending, and HRQL change over time for different patient profiles to inform the development of more precise and efficient value-based interventions.
2. Materials and Methods
2.1. Study Design and Data Source
This study was a retrospective, longitudinal cohort analysis using the 2019–2022 MEPS Panel 24 longitudinal data file. MEPS is a nationally representative survey of the U.S. civilian noninstitutionalized population, administered by the Agency for Health care Research and Quality (AHRQ), which collects detailed information on health status, health care utilization, and expenditures.
2.2. Study Population and Multimorbidity Clusters
The study population included all 5,565 individuals in the Panel 24 cohort. At baseline (Year 1:2019), individuals were categorized into eight mutually exclusive MM clusters based on clinically diagnosed chronic conditions: cancer (any type), cardiometabolic disease (diabetes, high blood pressure, coronary heart disease, or high cholesterol), and respiratory disease (asthma or emphysema). The eight clusters were defined as follows: no targeted conditions (Cluster 0); respiratory only (Cluster 1); cardiometabolic only (Cluster 2); cancer only (Cluster 3); cardiometabolic and respiratory (Cluster 4); cancer and respiratory (Cluster 5); cancer and cardiometabolic (Cluster 6); and all three condition types: cardiometabolic, cancer, and respiratory (Cluster 7).
2.3. Outcome Variables
Five primary outcomes were analyzed to provide a comprehensive assessment of the burden of MM. Economic burden was measured by total annual health care expenditures and total annual out-of-pocket (OOP) spending, with both inflation-adjusted to 2022 U.S. dollars. Humanistic burden was assessed using physical and mental HRQL scores based on the Veterans RAND-12 (VR-12) health survey. Finally, health care utilization was measured by the annual number of inpatient hospital stays per person. All costs were converted to 2022 USD using the Medical Care deflator from the Bureau of Labor Statistics, applying year-specific multipliers.
2.4. Statistical Analysis
All statistical analyses were performed using SAS software, Version 9.4, and were weighted using MEPS longitudinal weights to produce nationally representative estimates. To examine longitudinal trajectories of the five outcomes, a set of Generalized Estimating Equation (GEE) models was utilized, which adjust for the correlation of repeated measures within the same subject over time. All models included the MM cluster, year, and their interaction (Cluster and Year) as the primary predictors, while controlling for baseline age, sex, and race/ethnicity. Specific statistical distributions (Gamma, Normal, and Negative Binomial) were chosen for each outcome based on its data characteristics. A gamma distribution with a log link was selected for the expenditure models (total and OOP), as it appropriately handles continuous, non-negative, and positively skewed cost data. Health-related quality of life scores were modeled using a normal distribution. The number of inpatient stays was modeled using a Negative Binomial distribution to account for the over-dispersed nature of the count data.
Missing data were addressed using Full Information Maximum Likelihood (FIML), the method that retains all cases by using all available observed data under the Missing at Random (MAR) assumption, reducing bias compared to listwise deletion.
3. Results
3.1. Baseline Characteristics of the Study Population
The study cohort included 5,565 individuals representing a weighted national population of 336.9 million U.S. civilians for the 2019-2022 period. The baseline demographic and clinical characteristics of the population, stratified by the defined MM clusters, are detailed in
Table 1. The mean age of the population at baseline (2019) was 36.9 years (95% CI, 35.7–38.1). The majority of the population (58.8%) was classified as having none of the targeted chronic conditions (Cluster 0). The most prevalent single condition group was cardiometabolic disease only (Cluster 2), representing 26.0% of the population. As expected, clusters representing more complex MM were substantially less common; for instance, individuals with a combination of cancer, cardiometabolic disease, and respiratory disease (Cluster 7) represented just 0.4% of the population.
3.2. Economic Burden: Total Health Care Expenditures
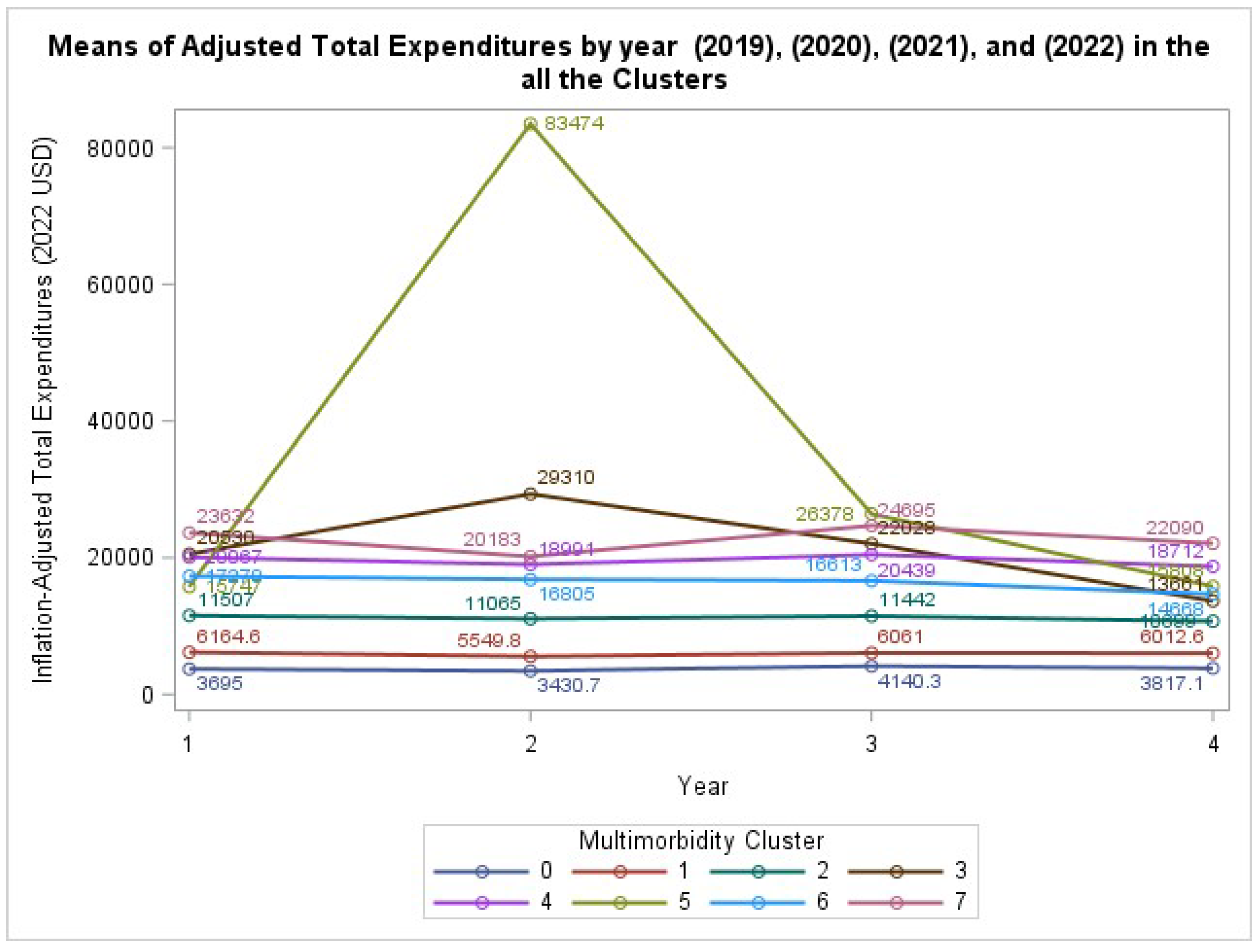
The analysis of inflation-adjusted total health care expenditures revealed a highly significant interaction between MM cluster and year (GEE: χ² (21) = 2.39 x 10²⁴, P < .0001). This indicates that the trajectory of health care costs over the four-year period differed significantly across the defined MM clusters. As visualized in
Figure 1, while the healthy reference group (Cluster 0) maintained low and stable expenditures, all other clusters demonstrated significantly higher and more varied cost trajectories. Of special interest, those with both respiratory diseases and cancer (Cluster 5) had a sudden increase in mean spending during the second panel year, reflecting the severe economic burden involving this particular set of diseases.
3.3. Patient Financial Burden: OOP Expenditures
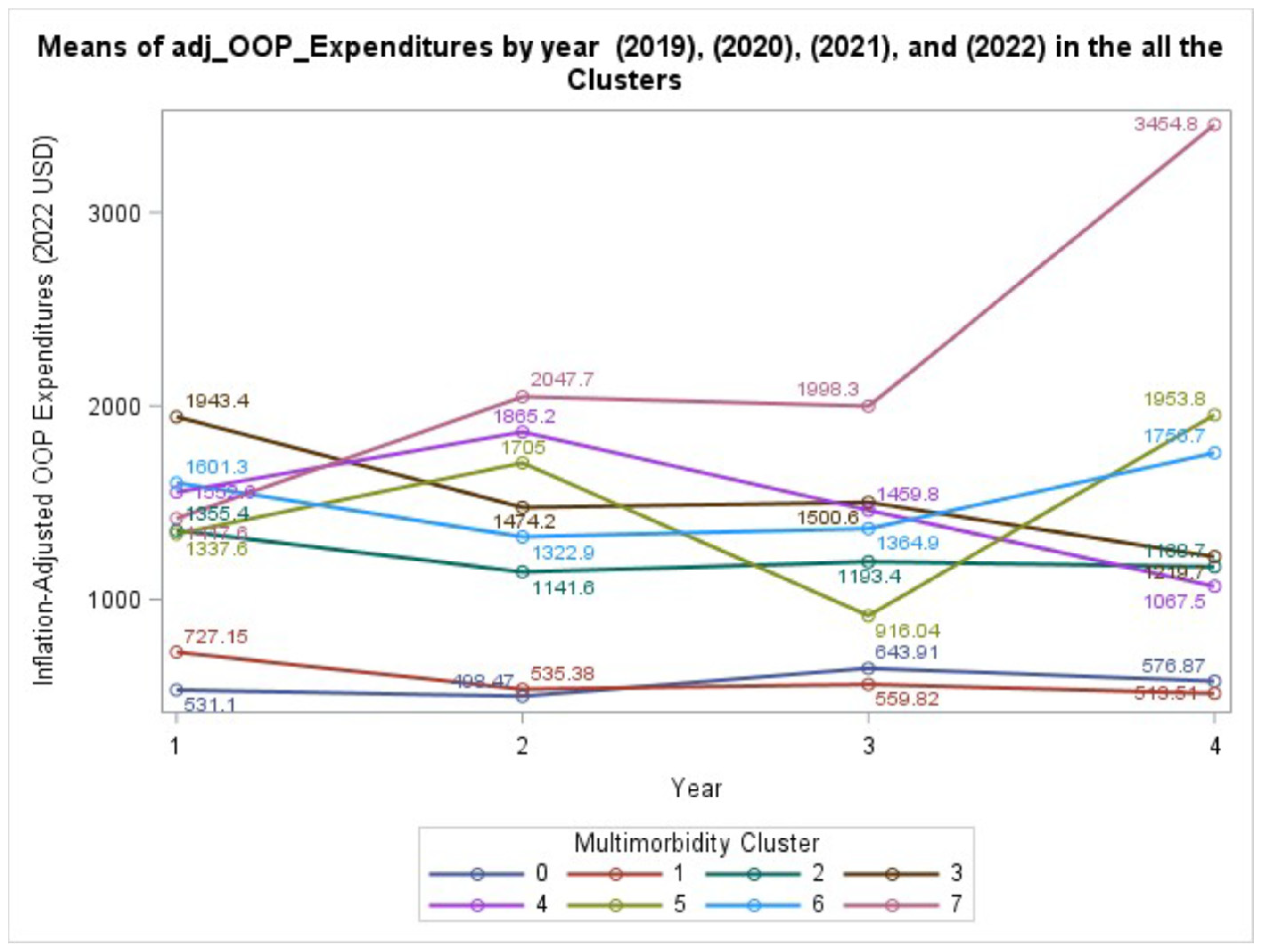
In the analysis of patient-borne financial burden, the interaction between MM cluster and year was not statistically significant (GEE: χ² (21) = 24.48, P = .2703). This suggests that while the level of OOP spending differs between groups, the trajectory of that spending over the four years does not significantly diverge.
Figure 2 illustrates these varied, but not statistically different, trajectories. The main effects of age, race/ethnicity, and sex were all significantly associated with OOP expenditures (P < .05 for all).
3.4. Humanistic Burden: Mental Health
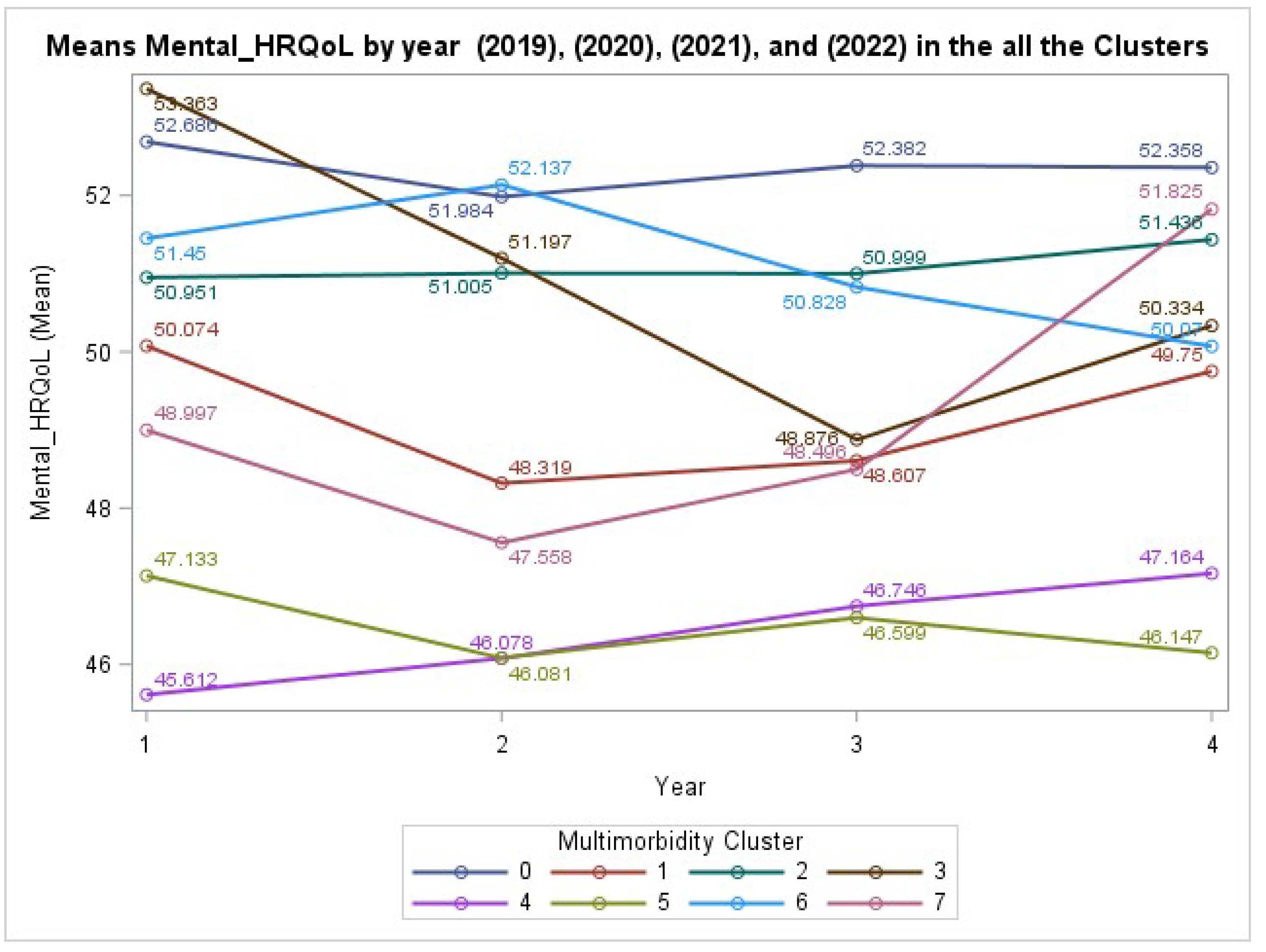
In contrast to physical health, the analysis of mental HRQL score also yielded a non-significant interaction between cluster and year (GEE: χ²(21) = 26.45, P = .1897). This suggests that the trajectories of mental health among the different clusters were not statistically divergent over the four-year period, as visualized in
Figure 3. However, the main effect of Cluster was highly significant (P < .0001), indicating that individuals in certain clusters (e.g., Cluster 4 and 5) reported significantly lower mental HRQL at baseline compared to the healthy reference group.
3.5. Humanistic Burden: Physical Health
The trajectory of physical HRQL, measured by the Physical Component Summary score, showed a non-significant interaction between cluster and year (GEE: χ²(21) = 16.46, P = .7432). However, both the main effect of Cluster (P < .0001) and Year (P = .2029, though not significant) were important. This indicates that while all groups followed a generally parallel decline in physical health, their starting points were significantly different.
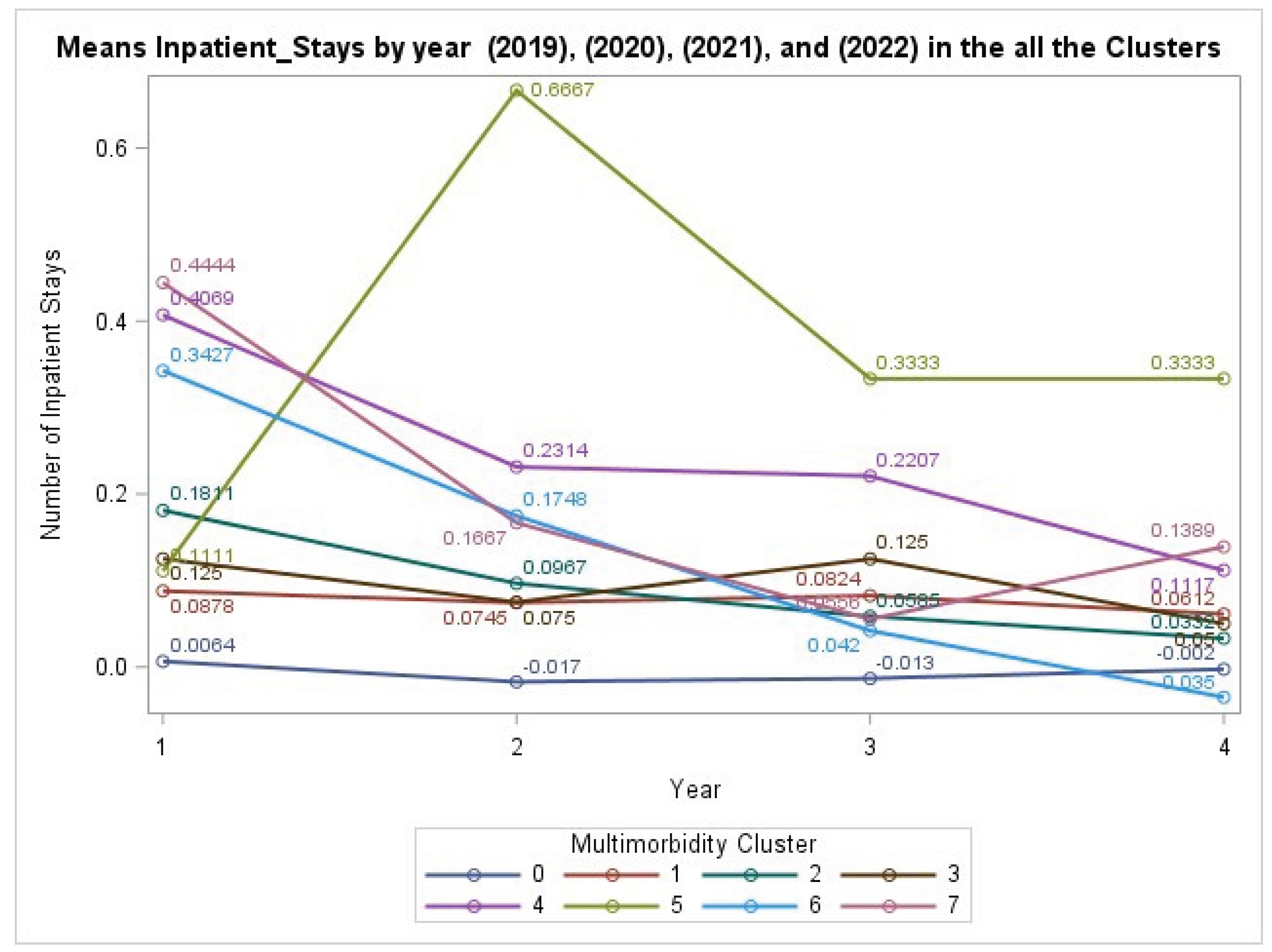
3.6. Health Care Utilization Burden: Inpatient Stays
The analysis of health care utilization, defined as the annual number of inpatient hospital stays, showed a non-significant interaction between MM cluster and year (GEE: χ²(21) = 19.07, P = .5809). This indicates that the trajectories of hospitalization rates did not significantly differ across the clusters over the four years. Nonetheless, the main effect of Cluster was highly significant (P < .0001), confirming that individuals with any chronic condition cluster had a significantly higher baseline rate of hospitalization compared to those with no conditions. The trends, including a notable spike for Cluster 5 in Year 2, are visualized in
Figure 4.
4. Discussion
In this nationally representative study, longitudinal analysis of the U.S. population, our primary finding was that the trajectory of total health care expenditures differed significantly depending on a patient’s specific combination of chronic conditions. This confirms that the system-level economic burden of MM is not static but follows divergent paths over time. Conversely, while the baseline patient-level financial burden, humanistic burden, and health care use (inpatient stays) were profoundly different across clusters, their trajectories were largely parallel over the four-year study period. Our primary finding that the trajectory of total health care expenditures differed significantly across MM clusters confirms that the system-level financial impact is dynamic and heterogeneous. Conversely, while individuals with complex conditions began with profoundly worse financial and quality-of-life burdens, the rate of change in these areas did not significantly differ from other groups over the four-year study period.
Implications for policy and practice
Invest earlier in prevention and secondary prevention to shift HRQL baselines before multimorbidity entrenches.
Maintain out-of-pocket caps while pairing with system-level cost controls to prevent payer-side cost escalation.
Target cancer + respiratory profiles for proactive case management and reduction of avoidable admissions.
Use cluster-based risk segmentation (beyond simple disease counts) in payment and care management algorithms.
International relevance
Although based on US data, the segmentation of multimorbidity into clinically interpretable clusters and the divergence in system-level cost trajectories are relevant to tax-funded and social insurance health systems internationally. Our results support early prevention and risk-stratified contracting for identifiable high-cost profiles, a strategy applicable across settings facing rising multimorbidity.
Our results extend a large body of cross-sectional research, including [
1] which established that 51.4% of U.S. adults with MCCs drive an estimated 90% of health care spending. Also, the MM was significantly associated with poorer HRQL, functional health status, and physical functioning when adjusting for relevant confounders, which is in accordance with findings by [
8]. By providing a longitudinal view, we demonstrate that this cost burden is not uniform. The significant interaction between MM cluster and year for total expenditures reveals that certain disease combinations, such as cancer co-occurring with respiratory conditions (Cluster 5), are associated with acute cost spikes that a static analysis would miss. This timing could mean that the combination of cancer and respiratory disease likely created an exceptionally vulnerable patient that are at risk for complications and high hospitalization bills. This finding provides a stark, data-driven indication of the pandemic’s catastrophic financial impact on specific high-risk populations.
The reason for this divergence is likely the synergistic effect of certain comorbidities. The presence of multiple conditions complicates clinical management, increases the risk of adverse events, and can lead to conflicting treatment regimens, resulting in higher health care utilization that is often greater than the sum of its parts. Research has consistently shown this “super-additive” effect, where the cost of treating a patient with multiple conditions exceeds the expected costs of treating each condition separately [
9]. Our finding of a cost spike for the expenditure for the cancer-respiratory combination cluster in the second year is an example of the phenomenon, possibly reflecting costly complications or intensive treatments required when these two conditions are managed concurrently, particularly during the height of the COVID-19 pandemic [
10].
This finding has critical implications for the design of value-based care models, suggesting that a “one-size-fits-all” approach to managing the MM population is inefficient. To contain costs, health systems and payers must move beyond simple disease counts and adopt more sophisticated patient segmentation strategies, like the clustering approach used here, to identify individuals at the highest risk for future high-cost events.
On the contrary, the parallel trajectories for OOP spending and health-related HRQL have significant findings. Parallel patterns of OOP spending, despite wildly divergent total costs, most likely reflect the benefit of the protective effects of insurance policies on maximum annual OOP. This policy is common in both public and private insurance plans, creating a “ceiling” that shields patients from the full financial consequences of high-cost events. While patients with MCCs still face a substantial financial burden, contributing to a medical debt crisis affecting millions of Americans, this insurance buffer prevents their personal costs from accelerating at the same catastrophic rate as the costs absorbed by the system [
11]. This finding underscores the dual reality of U.S. health care: insurance can mitigate personal financial ruin from a high-cost event, but it transfers that escalating fiscal pressure directly to public and private payers. Similarly, the parallel decline in HRQL suggests that the primary “damage” of MM to a patient’s well-being occurs early in the disease progression. This is in accord with systematic reviews, which similarly confirm that MM is strongly associated with a significantly lower quality of life [
12]. By the time patients are stratified into these complex clusters, they are already on distinct lower tracks of health status. The subsequent decline is persistent but occurs at a rate that is not statistically different from other groups. This reinforces the need for early preventive intervention, given the 5.3% rise in the incidence of MCCs among young adults, as previously mentioned [
1]. Without proactive management, future generations would start middle age with entrenched health deficits that are far more difficult and costly to reverse.
These findings carry significant implications for value-based care. The primary takeaway is that a “one-size-fits-all” approach to managing the multimorbid population is not ideal.
The divergent cost trajectories demand more sophisticated patient segmentation that moves beyond simple disease counts. Risk-adjustment models and payment systems should account for specific high-cost disease combinations. Identifying patients with high-risk profiles, like our Cluster 5 (Cancer + Respiratory), would allow for targeted, proactive care management to prevent costly complications.
The entrenched nature of quality-of-life deficits underscores the critical importance of primary and secondary prevention efforts to delay the onset and progression of chronic diseases.
Limitations
This work has a number of important strengths, such as its utilization of a nationally representative, large-scale longitudinal dataset covering the COVID-19 pandemic, giving a rare insight into patient trajectories during a phase of systemic stress. However, no work is perfect. Self-reported chronic conditions are subject to recall bias, and excluding the population in institutions may underestimate the true burden of MM. Additionally, although we adjusted for important demographics, there is an inherent risk of unmeasured confounding variables.
5. Conclusions
This study demonstrates that the longitudinal burden of MM in the United States is not monolithic. The economic burden on the health care system follows significantly different trajectories depending on a patient’s specific profile of chronic diseases. These findings emphasize the urgent need for risk-stratified, targeted health policy and clinical guidance to better serve the nation’s highest-need, highest-cost population to achieve the aims of a more efficient, equitable, and sustainable health system.
Author Contributions
Conceptualization, I.A.; methodology, I.A.; software, S.T.; validation, L.Y.; formal analysis, I.A. & S.T.; investigation, B.S.; resources, O.A.; data curation, I.A.; writing original draft preparation, I.A.; writing review and editing, B.S.; visualization, S.T.; supervision, L.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study because it used publicly available, de-identified data from the Medical Expenditure Panel Survey (MEPS).
Informed Consent Statement
Not applicable (secondary public data, MEPS). The study involved secondary analysis of de-identified public data and did not require informed consent.
Data Availability Statement
Acknowledgments
The author gratefully acknowledges the Jiann-Ping Hsu College of Public Health at Georgia Southern University for providing academic and research support that facilitated this work.
Conflicts of Interest
All authors declare no conflicts of interest in this paper.
Abbreviations
| CDC |
Centers for Disease Control and Prevention |
| HRQL |
Health Related Quality of Life |
| MCC |
Multiple chronic conditions |
| MEPS |
Medical Expenditure Panel Survey |
| MM |
Multimorbidity |
| OOP |
Out of Pocket |
| GEE |
Generalized Estimating Equation |
References
- Watson, K.B.; Wiltz, J.L.; Nhim, K. Trends in Multiple Chronic Conditions Among US Adults, By Life Stage, Behavioral Risk Factor Surveillance System, 2013–2023. Prev Chronic Dis 2025, 22. [Google Scholar] [CrossRef] [PubMed]
- Aborode, A.T.; Oginni, O.; Abacheng, M. Healthcare debts in the United States: a silent fight. Annals of Medicine & Surgery. 2025, 87, 663–672. [Google Scholar]
- Chen, C.; Zhou, W.; Cui, Y. Global, regional, and national characteristics of the main causes of increased disease burden due to the covid-19 pandemic: time-series modelling analysis of global burden of disease study 2021. BMJ. 2025, 390, e083868. [Google Scholar] [CrossRef] [PubMed]
- Félix, I.B.; Henriques, A. Medication adherence and related determinants in older people with multimorbidity: A cross--sectional study. Nurs Forum (Auckl). 2021, 56, 834–843. [Google Scholar] [CrossRef] [PubMed]
- Nobili, A.; Marengoni, A.; Tettamanti, M. Association between clusters of diseases and polypharmacy in hospitalized elderly patients: Results from the REPOSI study. Eur J Intern Med. 2011, 22, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Richard, P.; Walker, R.; Alexandre, P. The burden of out of pocket costs and medical debt faced by households with chronic health conditions in the United States. PLoS One. 2018, 13, e0199598. [Google Scholar] [CrossRef] [PubMed]
- Khera, R.; Valero-Elizondo, J.; Okunrintemi, V. Association of Out-of-Pocket Annual Health Expenditures With Financial Hardship in Low-Income Adults With Atherosclerotic Cardiovascular Disease in the United States. JAMA Cardiol. 2018, 3, 729. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.S.; Egede, L.E. The Association Between Multimorbidity and Quality of Life, Health Status and Functional Disability. Am J Med Sci. 2016, 352, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Soley-Bori, M.; Ashworth, M.; Bisquera, A. Impact of multimorbidity on healthcare costs and utilisation: a systematic review of the UK literature. British Journal of General Practice. 2021, 71, e39–e46. [Google Scholar] [CrossRef] [PubMed]
- Lai, A.G.; Pasea, L.; Banerjee, A. Estimated impact of the COVID-19 pandemic on cancer services and excess 1-year mortality in people with cancer and multimorbidity: Near real-time data on cancer care, cancer deaths and a population-based cohort study. BMJ Open 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Cox, C.; Ortaliza, J.; Wager, E. Health Policy 101, Health Care Costs and Affordability. 2024, 2024.
- Fortin, M.; Lapointe, L.; Hudon, C. Multimorbidity and quality of life in primary care: A systematic review. Health Qual Life Outcomes 2004, 2. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).