Introduction
Intrahepatic Cholestasis is the consequence of impaired bile formation and decrease in bile flow, which is present in several chronic diseases and pregnancy [
1,
2,
3]. Several natural components and diorders exacerbate cholestasis [
4]. Intrahepatic Cholestasis of Pregnancy (ICP) represents the most common pregnancy-specific hepatic disorder characterized by impaired bile acid transport and subsequent hepatocellular dysfunction, leading to altered serum levels of bilirubin and albumin typically emerging in the third trimester. It is recognized as one of the most prevalent hepatic conditions associated with pregnancy [
5,
6,
7]. The incidence of ICP varies widely among populations, ranging from 0.6-0.7% in women of European ancestry to over 4% in certain indigenous South American populations, with reported rates of 1.75% in mainland China [
5,
7,
8]. Genetic predispositions, particularly mutations in hepatobiliary transport proteins, as well as hormonal changes during pregnancy, have been implicated in the development and severity of ICP, alongside potential environmental and dietary factors [
5,
6,
7].
ICP is characterized by pruritus and elevated serum bile acids, and is associated with an increased risk of adverse perinatal outcomes, including preterm birth, meconium-stained amniotic fluid, and stillbirth, and these risks are closely linked to maternal total bile acid levels exceeding 40 μmol/L [
5,
7,
8]. Biochemical markers, including aminotransferases, alkaline phosphatase, and bilirubin, are commonly elevated, although their specificity for ICP diagnosis is limited [
7,
8]. While total bile acids (TBA) remain the gold standard for diagnosis, there is a growing interest in cost-effective prognostic tools. The albumin-bilirubin (ALBI) score has recently shown promise for predicting disease severity, demonstrating positive correlations with TBA levels [
9]. However, its prognostic performance has not been directly compared to other readily available indices, such as the De Ritis ratio (AST/ALT), within the same ICP cohort. A head-to-head comparison is necessary to determine which marker offers superior utility for clinical risk stratification.
Despite these advances, accurate early identification of women at risk for adverse outcomes remains challenging, and there is a need for reliable, easily obtainable liver function indices to improve risk stratification in clinical practice [
6,
9]. Among these, the De Ritis ratio has been proposed as a simple indicator of hepatocellular injury that may complement existing markers [
6,
7]. Therefore, this study aimed to conduct a direct comparative analysis of the ALBI and the De Ritis ratio for predicting adverse perinatal outcomes in intrahepatic cholestasis of pregnancy, to determine which biomarker holds greater prognostic utility.
Materials and Methods
Study Design and Population
This retrospective study utilized a publicly available dataset published by CUI (2025) on Zenodo, titled “The relationship between the Albumin to Fibrinogen Ratio and Adverse perinatal outcomes in intrahepatic cholestasis of pregnancy” (DOI: 10.5281/zenodo.17060064) [
10]. The original dataset comprised clinical and laboratory data from 100 pregnant subjects diagnosed with ICP. For the purpose of this secondary analysis, we extracted the relevant variables necessary to calculate the ALBI grade and the De Ritis ratio for each subject. ALBI and De Ritis ratios are simple to calculate [
11,
12,
13]. The primary outcome was a composite of adverse perinatal outcomes associated with ICP, as defined in the original study’s outcomes.
Data Extraction and Variable Definition
The necessary variables were extracted directly from the provided dataset. These included serum albumin (g/L), total bilirubin (µmol/L), AST (U/L), and ALT (U/L). The ALBI score was calculated using the established formula: ALBI score = (log
10 bilirubin in µmol/L × 0.66) + (albumin in g/L × -0.085). The De Ritis ratio was calculated simply as AST (U/L) divided by ALT (U/L) [
11,
12,
13]. The primary endpoint for this analysis was a composite of adverse perinatal outcomes, as defined in the original dataset. This binary outcome (presence or absence of any adverse outcome) was used for all logistic regression and ROC analyses.
Statistical Analysis
Continuous variables are presented as mean ± standard deviation or median with interquartile range based on their distribution. Categorical variables are presented as numbers and percentages (n, %). Univariate and multivariable binary logistic regression analyses were performed to assess the associations between the ALBI score, De Ritis ratio, and the adverse outcome endpoint, presented as odds ratios (OR) with 95% confidence intervals (CI). The predictive performance of each marker and the combined models was evaluated using Receiver Operating Characteristic (ROC) curve analysis, reporting the area under the curve (AUC) and its 95% CI. The optimal cut-off value was determined using Youden’s Index (J). The DeLong test was used to compare AUCs. Model fit was assessed using the corrected Akaike Information Criterion (AICc). A two-tailed p-value < 0.05 was considered statistically significant. Analyses were performed using GraphPad Prism version 8.4.0 and MedCalc statistical software version 20.104.
Results
Baseline Characteristics
The mean maternal age was 29.5 ± 4.2 years. At the time of analysis, the mean gestational age was 38.0 ± 1.2 weeks. Liver function profiles revealed elevated hepatobiliary enzymes; the mean ALT and AST levels were 75.7 U/L (±90.8) and 60.4 U/L (±62.9), respectively, with median values of 29.6 U/L and 29.2 U/L indicating a right-skewed distribution. Similarly, mean alkaline phosphatase (ALP) and gamma-glutamyl transferase (GGT) levels were 226.6 U/L (±115.2) and 42.3 U/L (±81.2), respectively. The mean total bilirubin level was 10.8 µmol/L (±6.3), while the mean albumin level was 31.5 g/L (±3.2). The median ALBI score was -2.04 (mean: -2.04 ± 0.30), and the median De Ritis ratio was 1.06 (mean: 1.29 ± 0.69).
Univariate Logistic Regression Analysis
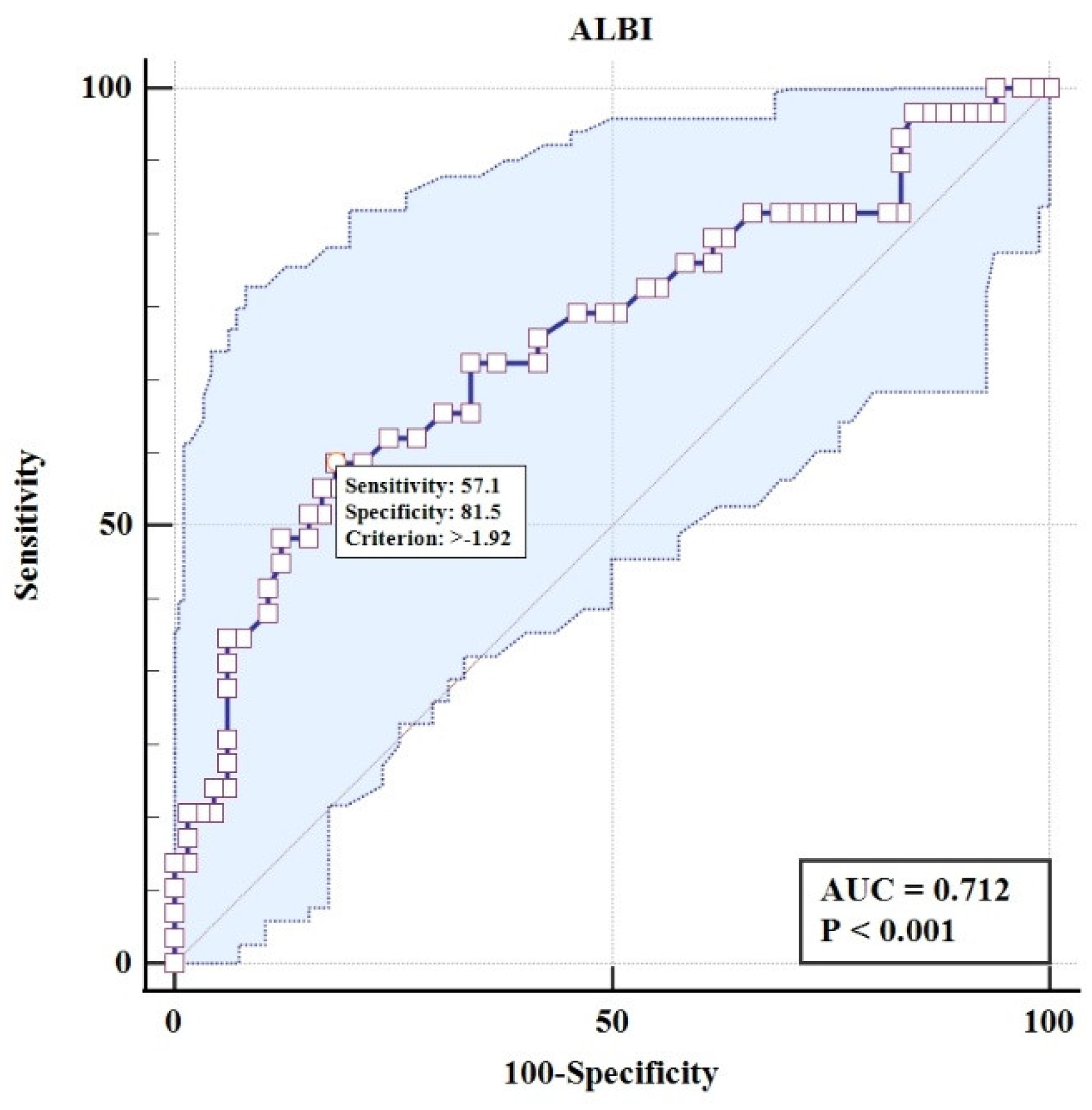
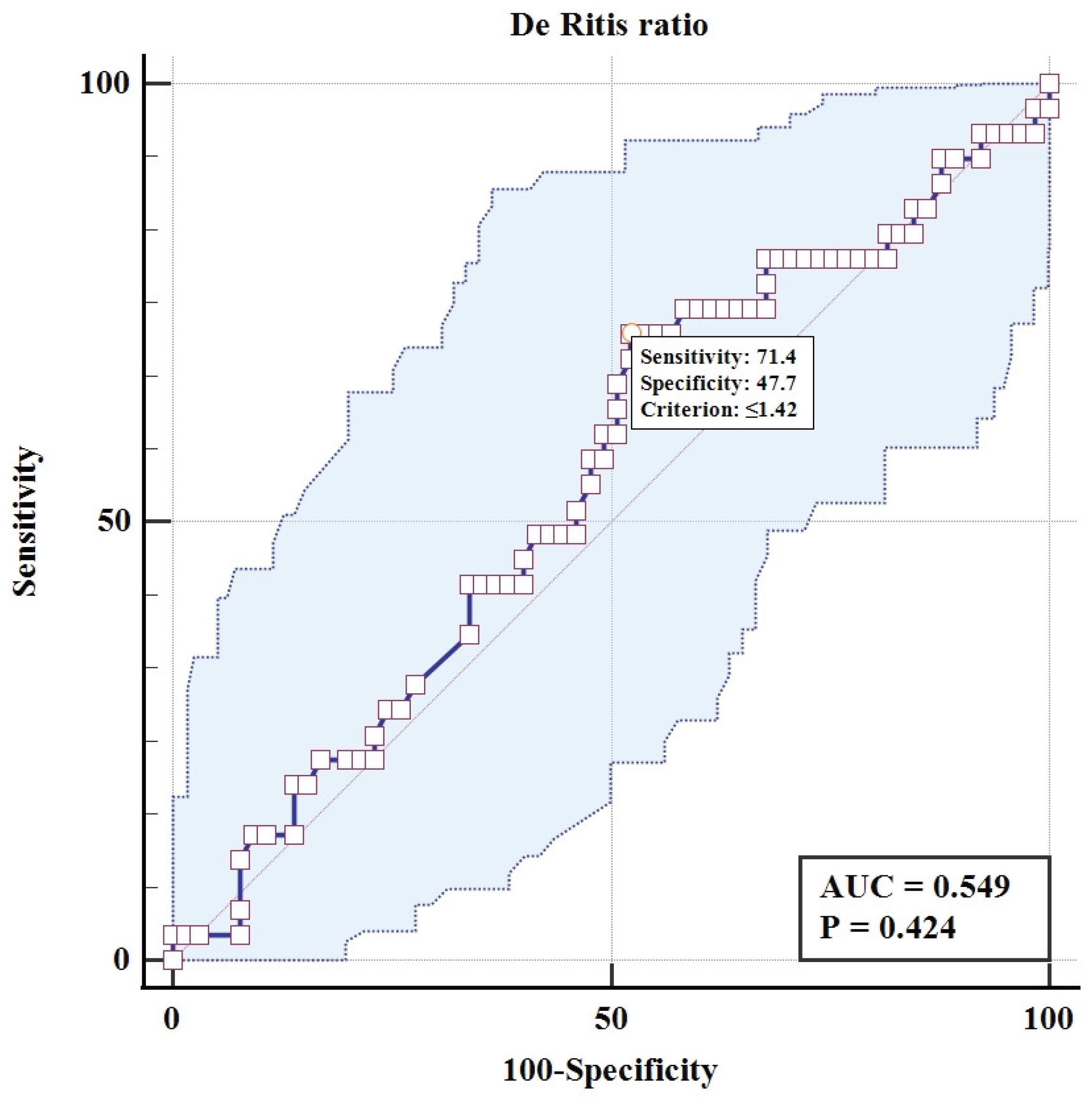
Univariate logistic regression analysis was performed to assess the association of the De Ritis ratio and the ALBI score with ICP (
Table 1). The analysis revealed a stark contrast in the predictive utility of the two biomarkers. The De Ritis ratio was not a significant predictor of adverse perinatal outcomes (OR = 0.78, 95% CI: 0.41 to 1.42; p = 0.42), with its model demonstrating poor discriminatory power, evidenced by an area under the ROC curve of 0.549 and a classification accuracy of only 65%. In direct contrast, the ALBI score was a highly significant predictor (OR = 20.54, 95% CI: 3.96 to 139.5; p<0.001). The model for ALBI showed substantially better performance, with a significantly larger AUC of 0.712 and a higher overall classification accuracy of 73%, confirming its superior prognostic utility for identifying adverse outcomes in this cohort of subjects.
Multivariable Logistic Regression Analysis
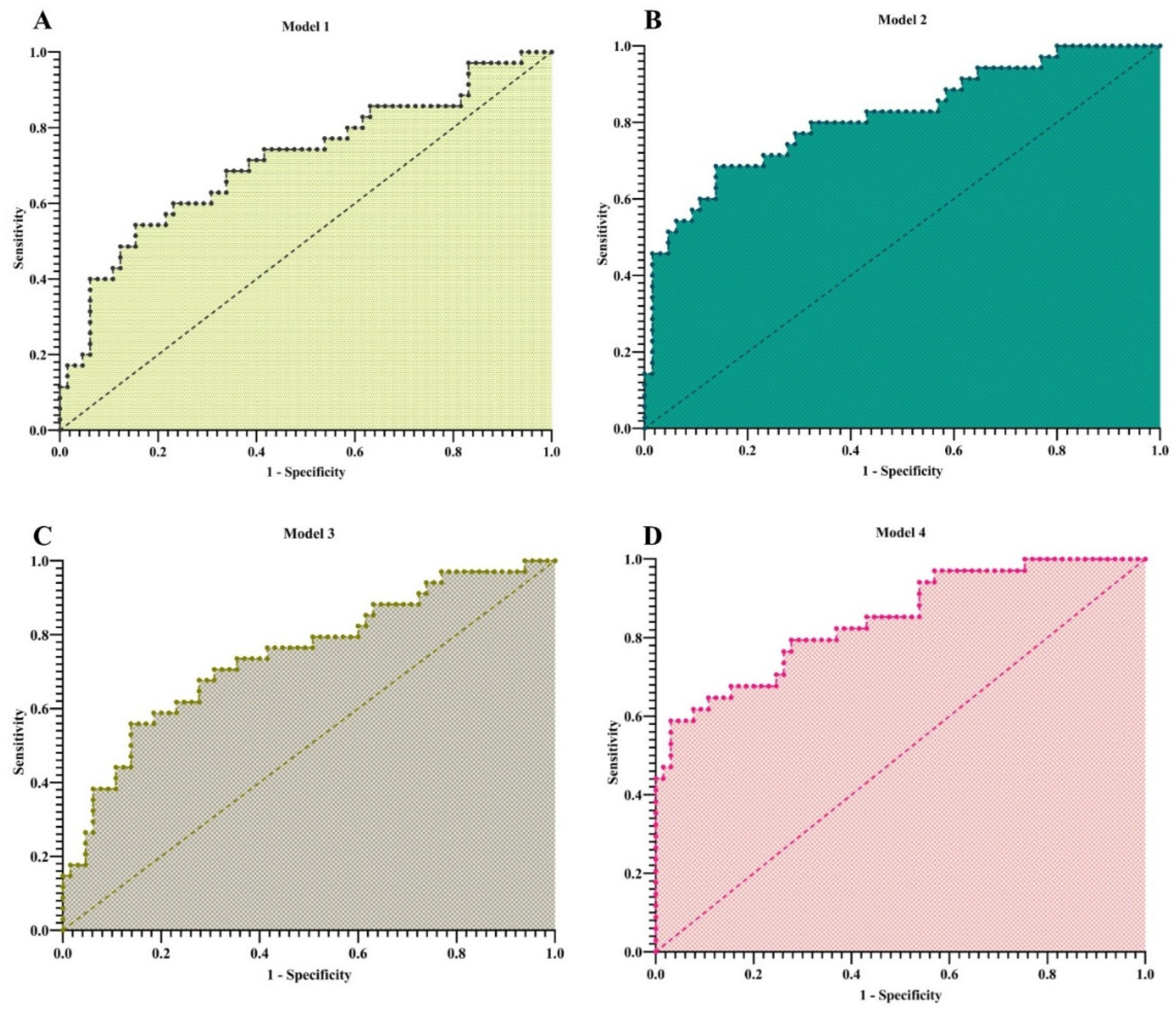
Four sequential multivariable models were constructed (
Table 2). In Model 1 (including only the two scores), the ALBI score remained a strong, significant predictor (OR = 20.88, 95% CI: 3.85–149.1; p=0.001), while the De Ritis ratio was non-significant (OR=1.03, p=0.937). The model AUC was 0.714.
In Model 2, adjusted for maternal age and gestational age (GA), GA was a significant independent predictor (OR = 0.32, 95% CI: 0.16–0.56; p=0.0003). The ALBI score retained its strong association (OR = 15.38, 95% CI: 2.23–136.1; p=0.008), and the model’s discriminatory power improved (AUC=0.815).
Model 3, adjusted for ALP and GGT, showed that neither enzyme was a significant predictor. In this context, the ALBI score demonstrated an even stronger independent effect (OR = 24.81, 95% CI: 4.25–197.1; p=0.0009).
The final, fully adjusted model (Model 4) confirmed that GA (OR = 0.27, 95% CI: 0.12–0.50; p=0.0002) and the ALBI score (OR = 21.65, 95% CI: 2.78–232.6; p=0.006) were the only significant independent predictors. The De Ritis ratio was consistently non-significant across all models. The full model achieved excellent discriminatory power (AUC = 0.845).
Discussion
This study demonstrates that the ALBI score is a significant and independent predictor of adverse perinatal outcomes in women with ICP, whereas the De Ritis ratio lacks prognostic utility in this context. The robust performance of the ALBI score, which persisted after adjusting for key clinical and biochemical confounders, underscores its potential as a valuable risk-stratification tool. These results indicate that ALBI is a robust marker for identifying women at risk for ICP complications, outperforming the simple AST/ALT ratio in our study [
14,
15].
Our findings regarding the prognostic utility of the ALBI score extend the existing literature, which has primarily focused on its diagnostic potential. For instance, Ozkavak et al. reported that ALBI scores were significantly elevated in ICP patients and demonstrated diagnostic value for identifying ICP [
9]. While their study focused on diagnosis, our results directly address prognosis, showing that a higher ALBI score is strongly associated with an increased risk of adverse perinatal events. This prognostic role is further supported by a recent prospective UKOSS study by Nana et al., which found that the worst ALBI score during pregnancy predicted preterm birth (AUROC=0.74) and maternal ICU admission in women with cirrhosis [
16]. Although their population had more advanced liver disease, it corroborates the value of ALBI in predicting obstetric complications rooted in hepatic dysfunction.
In contrast to our findings, some studies have suggested a diagnostic role for the De Ritis ratio. For example, Yılmaz et al. reported an AUC of 0.770 for detecting ICP itself [
17]. However, this contrasts with its lack of prognostic utility for adverse outcomes in our study, highlighting the critical distinction between diagnosing a condition and predicting its complications. While the De Ritis ratio is biologically plausible as a marker of hepatic stress, and some studies have reported correlations with disease severity markers [
14,
18,
19], it consistently failed to demonstrate significant predictive value for adverse perinatal outcomes in our analysis. This suggests that the simple balance between AST and ALT release is not a primary driver of the fetal complications in ICP.
The clinical implications of our study are noteworthy. Early identification of pregnant women at risk for ICP-related complications is crucial to optimize surveillance and intervention strategies, as conventional markers like fasting bile acids can be difficult or costly to measure routinely [
15,
18]. ALBI offers a simple, easily calculable index from standard laboratory tests that can inform risk stratification, potentially guiding closer monitoring, timely initiation of ursodeoxycholic acid therapy, or consideration for early delivery in high-risk cases [
9,
15,
18]. Furthermore, while ALBI correlates moderately with bile acid levels, it also integrates the patient’s nutritional and hepatic synthetic status through albumin, providing a broader picture of maternal hepatic function than bile acids alone [
9,
15].
Nevertheless, certain limitations must be acknowledged. As a secondary analysis of an existing dataset, our study was constrained by the variables and outcome definitions available in the original source, which may not capture all relevant clinical endpoints. Our analysis was based on a relatively small retrospective study, and while the results are consistent with larger studies, validation in larger multicenter prospective studies is warranted. Additionally, the ALBI score does not directly measure bile acids, which remain the gold standard for ICP diagnosis; thus, ALBI should be considered complementary rather than a replacement marker. The De Ritis ratio, despite its low predictive value in our study, may still hold utility in specific subgroups or in combination with other liver-related scores such as APRI or MELD, as suggested by previous literature [
18,
19].
The ALBI score is a significant and independent predictor of adverse perinatal outcomes in intrahepatic cholestasis of pregnancy, demonstrating superior prognostic utility compared to the De Ritis ratio. Its calculation from readily available laboratory parameters makes it a practical and cost-effective tool for risk stratification. Incorporating the ALBI score into the clinical assessment of ICP patients could enhance management and improve perinatal outcomes.
The biological plausibility of our findings is strong. The ALBI score incorporates albumin, a marker of hepatic synthetic function and nutritional status, and bilirubin, which reflects excretory capacity. In ICP, where the primary pathology involves impaired biliary excretion, subsequent hepatocellular dysfunction can compromise synthetic function. Therefore, the ALBI score provides a composite snapshot of liver health that appears more prognostically relevant than isolated markers of cholestasis (like ALP) or hepatocellular injury (like aminotransferases or the De Ritis ratio).
The consistent lack of predictive value for the De Ritis ratio in our analysis suggests that the simple balance between AST and ALT release is not a primary driver of adverse outcomes in ICP. This may be because aminotransferase elevations in ICP are often heterogeneous and do not consistently reflect the severity of the underlying cholestatic process that dictates fetal risk.
The clinical implication of our study is that the ALBI score, derived from routine, low-cost tests, can help identify ICP patients at the highest risk for adverse outcomes. This could guide more intensive monitoring, earlier pharmacological intervention, or timely decision-making regarding delivery, particularly in settings where serial bile acid measurement is impractical.
Limitations
Our study has limitations. Its retrospective design and use of a single, pre-existing dataset limit our ability to control for all potential confounders or to validate the specific adverse outcomes comprising the endpoint. The sample size, while adequate for this initial comparison, is modest. This is reflected in the wide confidence intervals for the ALBI score’s odds ratio, and it limits the power for more extensive subgroup analyses.
Prospective, multi-center studies with standardized outcome definitions are needed to validate these findings and establish clinically actionable ALBI score thresholds.
Conclusion
In conclusion, the ALBI score demonstrates significant and independent prognostic utility for adverse perinatal outcomes in women with ICP, substantially outperforming the De Ritis ratio. Derived from routine laboratory parameters, it offers a practical and cost-effective tool for risk stratification, particularly in resource-limited settings. Future prospective, multicenter studies are warranted to validate these findings, establish definitive clinical cut-offs, and integrate the ALBI score into comprehensive management algorithms for ICP.
Author Contributions
MS and HPT: Reviewing the literature, Methodology, Investigation, Conceptualization, Formal analysis, Writing - the original draft, Writing - review & and editing; RA, MSD, HS, and SSS: Data curation, Formal analysis, Writing - review & and editing.
Data Availability Statement
Acknowledgments
During the preparation of a part of this work, we used AI, such as ChatGPT and DeepSeek, for paraphrasing and grammar checking. Following the use of this tool, the author thoroughly reviewed and edited the content as needed and take full responsibility for the final version of the publication.
Conflict of Interest
The author declare that they have no conflict of interest.
Animal Studies
Not applicable
Ethical Approval
This study is a second-analysis study. Dryad publishes all submitted data under the Creative Commons Attribution 4.0 International, which means that the public is able to see and use the data for their own purposes without use restriction. Using data from Zenodo (zenodo.org) in article is generally allowed without needing explicit permission, as Zenodo is an open-access repository that promotes the free reuse of research data. We analyzed publicly available data from CUI, Donghua (2025) on
The relationship between the Albumin to Fibrinogen Ratio and Adverse perinatal outcomes in intrahepatic cholestasis of pregnancy (Zenodo,
https://zenodo.org/records/17060064; accessed on September 12th, 2025).
Research Involving Recombinant DNA
Not applicable
References
- Jüngst, C.; Berg, T.; Cheng, J.; Green, R.M.; Jia, J.; Mason, A.L.; Lammert, F. Intrahepatic cholestasis in common chronic liver diseases. Eur. J. Clin. Investig. 2013, 43, 1069–1083. [CrossRef]
- Hassanzadeh, M.; Sanat, Z.M.; Khayatian, S.; Sotoudeheian, M.; Shahbazian, A.; Hoseini, S. Acute sickle cell hepatopathy: A case report and literature review. J. Natl. Med Assoc. 2023, 116, 119–125. [CrossRef]
- Williamson C, Geenes V. Intrahepatic cholestasis of pregnancy. Obstetrics & Gynecology. 2014;124:120-33.
- Sotoudeheian, M.; Hoseini, S.; Mirahmadi, S.-M.; Farahmandian, N.; Pazoki-Toroudi, H. Oleuropein as a Therapeutic Agent for Non-alcoholic Fatty Liver Disease During Hepatitis C. Rev. Bras. de Farm. 2023, 33, 688–695. [CrossRef]
- Hague, W.“.; Williamson, C.; Beuers, U. Intrahepatic cholestasis of pregnancy: Introduction and overview 2024. Obstet. Med. 2024, 17, 138–143. [CrossRef]
- Smith DD, Rood KM. Intrahepatic cholestasis of pregnancy. Clinical obstetrics and gynecology. 2020;63:134-51. [CrossRef]
- Piechota, J.; Jelski, W. Intrahepatic Cholestasis in Pregnancy: Review of the Literature. J. Clin. Med. 2020, 9, 1361. [CrossRef]
- Jamshidi Kerachi A, Shahlaee MA, Habibi P, Dehdari Ebrahimi N, Ala M, Sadeghi A. Global and regional incidence of intrahepatic cholestasis of pregnancy: a systematic review and meta-analysis. BMC medicine. 2025;23:129. [CrossRef]
- Ozkavak, O.O.; Tanacan, A.; Haksever, M.; Sahin, R.; Serbetci, H.; Okutucu, G.; Aldemir, E.; Sahin, D. The utility of albumin–bilirubin score in patients with intrahepatic cholestasis of pregnancy: a retrospective comparative study. Front. Public Heal. 2024, 70, e20240860. [CrossRef]
- CUI D. The relationship between the Albumin to Fibrinogen Ratio and Adverse perinatal outcomes in intrahepatic cholestasis of pregnancy [Data set]. v1 ed. Zenodo: Zenodo; 2025.
- Sotoudeheian F, Sotoudeheian M, Toroudi HP, Azarbad R. The Albumin-Bilirubin Grade and Cognitive Function in Liver Cirrhosis; Animal Naming Test and Non-Invasive Liver Biomarkers. 2025.
- Sotoudeheian, M. Unveiling the Link between Albumin-Bilirubin Grade and Liver Fibrosis in Patients with a History of Gallstone and Gallbladder Surgery: A Focus on Metabolic Dysfunction-Associated Steatohepatitis. Korean J. Pancreas Biliary Tract 2025, 30, 10–18. [CrossRef]
- Shaikh SM, Varma A, Kumar S, Acharya S, Patil R. Navigating disease management: a comprehensive review of the De Ritis Ratio in clinical medicine. Cureus. 2024;16.
- Küçükyurt, A.K.; Atakul, N.; Solak, Y. Pregnancy cholestasis typically occurs in the third trimester of pregnancy and is a significant clinical condition. Arch. Gynecol. Obstet. 2024, 310, 2531–2539. [CrossRef]
- Triunfo, S. Exploring tests used in routine clinical practice for improving prediction, diagnosis and long-term prognosis of intrahepatic cholestasis of pregnancy. Arch. Gynecol. Obstet. 2024, 310, 3311–3312. [CrossRef]
- Nana, M.; Majewska, A.; Rahim, M.; Geenes, V.; Ovadia, C.; Knight, M.; Heneghan, M.; Williamson, C. Pregnancy Outcomes in Women With Liver Cirrhosis: A National Prospective Cohort Study Using the UK Obstetric Surveillance System. BJOG: Int. J. Obstet. Gynaecol. 2025, 132, 935–943. [CrossRef]
- Yılmaz, E.B.S.; Sağlam, E.; Yalcin, S.; Özler, M.R. The diagnostic and prognostic value of APRI and de Ritis ratio in intrahepatic cholestasis of pregnancy. BMC Pregnancy Childbirth 2025, 25, 1–11. [CrossRef]
- Karabay G, Şeyhanlı Z, Sucu ST, Tokgöz B, Aktemur G, Tonyalı NV, et al. Evaluation of Liver Function Indices in Intrahepatic Cholestasis of Pregnancy: Diagnostic Utility and Neonatal Outcomes. Ankara Eğitim ve Araştırma Hastanesi Tıp Dergisi. 2024;57:114-9. [CrossRef]
- Obut, M.; Kından, A.; Ibanoğlu, M.C.; Kahraman, N.Ç.; Arat, Ö.; Keleş, A.; Topkara, S.; Çakır, B.T.; Bucak, M.; İsKender, C.T. Liver damage parameters and peripheral blood parameters for prediction and diagnosis of intrahepatic cholestasis in pregnancy. J. Obstet. Gynaecol. Res. 2023, 50, 196–204. [CrossRef]
Table 1.
Univariate Logistic Regression Analysis of De Ritis Ratio and ALBI Score for Predicting ICP and Its Adverse Perinatal Outcomes.
Table 1.
Univariate Logistic Regression Analysis of De Ritis Ratio and ALBI Score for Predicting ICP and Its Adverse Perinatal Outcomes.
| Variable |
Odds Ratio (95% CI) |
P-value |
AUC (95% CI) |
P-value (AUC) |
Model Fit (AICc) |
Overall Correct Classification (%) |
| De Ritis ratio |
0.78 (0.41 - 1.42) |
0.42 |
0.549 (0.430 - 0.668) |
0.42 |
133.0 |
65.0 |
| ALBI score |
20.54 (3.96 - 139.5) |
< 0.001 |
0.712 (0.601 - 0.824) |
< 0.001 |
119.4 |
73.0 |
Abbreviations: ALBI, Albumin-Bilirubin; AUC, Area Under the Receiver Operating Characteristic Curve; AICc, Corrected Akaike Information Criterion; CI, Confidence Interval.
Note: A lower AICc value indicates a better-fitting model. Statistically significant values (p < 0.05) are presented in bold. |
Table 2.
Multivariable Logistic Regression Models for Predicting ICP and Its Adverse Perinatal Outcomes.
Table 2.
Multivariable Logistic Regression Models for Predicting ICP and Its Adverse Perinatal Outcomes.
| Variable |
Model 1: Only Scores |
Model 2: + Demographics |
Model 3: + Liver Enzymes |
Model 4: Full Model |
| |
aOR (95% CI) |
aOR (95% CI) |
aOR (95% CI) |
aOR (95% CI) |
| ALBI Score |
20.88 (3.85-149.1) |
15.38 (2.23-136.1) |
24.81 (4.25-197.1) |
21.65 (2.78-232.6) |
| De Ritis ratio |
1.03 (0.53-1.97) |
0.98 (0.47-2.05) |
0.97 (0.46-2.03) |
0.81 (0.34-1.88) |
| Gestational Age |
- |
0.32 (0.16-0.56) |
- |
0.27 (0.12-0.50) |
| Age |
- |
1.02 (0.90-1.16) |
- |
1.02 (0.89-1.17) |
| ALP |
- |
- |
1.00 (1.00-1.01) |
1.00 (1.00-1.01) |
| GGT |
- |
- |
0.99 (0.98-1.00) |
0.98 (0.96-1.00) |
| Model Fit |
|
|
|
|
| AICc |
121.5 |
105.2 |
119.6 |
101.1 |
| AUC (95% CI) |
0.714 (0.603-0.825) |
0.815 (0.723-0.907) |
0.743 (0.638-0.848) |
0.845 (0.763-0.928) |
| Classification % |
74.0 |
79.0 |
72.7 |
79.8 |
Abbreviations: aOR, adjusted Odds Ratio; CI, Confidence Interval; ALP, Alkaline Phosphatase; GGT, Gamma-Glutamyl Transferase; AICc, Corrected Akaike Information Criterion; AUC, Area Under the ROC Curve.
Note: Statistically significant results (p < 0.05) are presented in bold. The reference model for AICc comparison is the intercept-only model (AICc = 131.5). Model 3 and the Full Model (Model 4) were run on 99 observations due to one missing data point. Lower AICc values indicate a better-fitting model. |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).