1. Introduction
Until now, coated and coat-dependent vesicles were considered the final stage of transport vesicle formation, since vesicles that have undergone division are still coated and vesicles lose their coat only subsequently [
1,
2,
3,
4,
5,
6,
7,
8,
9,
10,
11,
12,
13,
14,
15]. Three coats, namely, COPII, COPI, and clathrin were proposed to be involved in the formation small (<100 nm) vesicles. COPII is present over COPII-coated buds, which are always hemispherical in shape and are visible on the granular ER [
16,
17,
18,
19]. COPI-coated buds are located on the ER exit site (ERES) structures, the cis-most Golgi cisterna and the medial Golgi cisternae, where COPI-coated structures usually have the shape of buds with a neck [
20,
21]. However, these organelles do not have clathrin-coated buds. Alternatively, trans-most cisternae, TGNs, endosomes, and plasma membrane lack COPII or COPI-coated buds but exhibit clathrin-coated buds [
22,
23,
24,
25,
26,
27,
28].
According to generally accepted models, AP1/Arf1 or AP2 (for clathrin-dependent vesicles formed on endosomes or PMs, respectively), GDP-Sar1 (for COPII-dependent vesicles), or DGP-Arf1-5 (for COPI-dependent buds) initially bind to membranes of the secretory pathway. In the case of COPII-dependent vesicles, GDP bound to Sar1 is replaced with GTP. Sec23/24 are then recruited, followed by Sec13/31. This results in the formation of the COPII coat. In the case of COPI-dependent buds, GDP-Arf1(or 2-5) first attaches to the membranes of the ERES or Golgi. ARFGEF then exchanges GDP for GTP. This leads to the recruitment of COPI. Once the coat is assembled, the vesicle undergoes fission. It is assumed that after this event, vesicles coated with either COPII or COPI persist in cells for some time. Finally, GTP hydrolysis triggers coat disassembly and uncoating [
14,
15,
16,
17,
18,
19,
20].
Indeed, many studies have claimed to have demonstrated the existence of COPI and COPII-coated vesicles. However, to date, no one has reported the existence of vesicles coated with either COPI or COPII in normal cells [
11,
12,
13,
14,
15]. Also, analysis of images presented in the literature allowed us to conclude that to date, no one has reported the existence of vesicles coated with either COPI or COPII in normal cells because in all these studies, where the authors claimed to have detected COPI-coated vesicles, three-dimensional electron microscopy was not used. Only thin sections or cryosections were used. Therefore, they were unable to distinguish COPI-coated buds from COPI-coated vesicles, especially in cryosections, where the COPI-coated structure could be separated from the clathrin coat with considerable difficulty [
28].
According to the current consensus, in the case of clathrin-dependent vesicles, initially AP1 (with the help of Arf1) attaches to the membrane surface of the trans-Golgi cisternae, trans-Golgi network, and endosomes whereas AP2 binds to the plasma membrane resulting in the assembly of a lattice of clathrin triskelium. The triskelium binds to AP1 or AP2 and form characteristic polyhedral closed cages, or fullerenes. which then forms clathrin-coated pits or buds. Next, in the case of clathrin-coated pits appeared on the plasma membrane, dynamin binds to the neck connecting the clathrin-coated sphere to the original membrane. When the thickness of this becomes sufficiently small, dynamin contracts, causing the coated sphere to split off or subjected to fission. Only after this step the clathrin coat does detach from the membrane [
20,
21].
It is claimed that clathrin-dependent vesicles formed from endosomes have a diameter of approximately 60 nm [
29]. However, three-dim3nswionql analysis of these “vesicles” was not performed. The presence of free clathrin-coated vesicles has been described previously [
30,
31], However, a complete set of serial images was not presented. We demonstrated serial tomo-images of the clathrin-coated 42 nm vesicle in
S. cerevisiae (see
Figure S1 in [
32]) and similar images of the 70-nm clathrin-coated vesicle [
33].
In the literatures, there are dozens of images of clathrin-coated buds on endosomes, the
trans-most cisternae and the TGN [
27,
34], and distensions of Golgi cisternae filled with procollagen I (see Figure 7D presented by Fusella et al. [
35]). Coated vesicles isolated from bovine adrenal cortex have diameters of 100 nm and 70 nm [
36]. The diameters of the round profiles near the basolateral PM ranges from 95 to 115 nm [
37,
38], and super-resolution images revealed clathrin-coated primordia near the PM with a diameter of 100–110 nm (see
Figure 2h in Shevchuk et al. [
39]). In early rat spermatids, the diameter of clathrin-coated round profiles was 103 nm [
28]. In
Figure 2C in Diao et al. [40, the diameter of a similar circular profile was 107 nm. In neurons, clathrin-coated vesicles localized near the basolateral plasma membrane have a diameter of 97 nm [
41]. The mechanism of fission of clathrin-coated buds formed on endosomes and TGN is unclear.
The functional role of cellular vesicles as transport carriers remains highly controversial. Both the vesicle- and cisterna maturation-dependent models of intracellular transport, which consider vesicles as carriers, have rather limited explanatory power [20,42. However, recent data by Sumya et al. [
43] have been interpreted in favor of this transport role of COPI-dependent vesicles. Furthermore, several studies describing the isolated vesicle fraction have reported an enrichment of this fraction in Golgi enzymes, but not in nucleotide-sugar transporters [
8,
44,
45,
46,
47,
48,
49,
50,
51,
52,
53,
54]. Vesicles are important for various models of intracellular transport [
20]. In the ongoing competition between different models of intracellular transport, the function of small coat-dependent vesicles represents an important argument. For example, the isolation from cells of the so-called vesicular fraction, enriched in Golgi resident proteins, is a major argument in favor of the cisternal maturation-progression model [
55].
In this study, we investigated the distribution of small vesicle diameters and their abundance under various conditions. For a long time, vesicle size remained virtually unnoticed. In this study, we demonstrated for the first time that vesicle size is highly specific and precise, which has important implications for understanding intracellular transport.
3. Results
Initially, using two step electron microscopy (EM) tomography and serial sections [
21,
32] we measured the distribution of diameters of small (<100 nm) membrane spheres (vesicles) in different cells, namely, COS7, HepG2, and HeLa cells. Representative examples of buds (
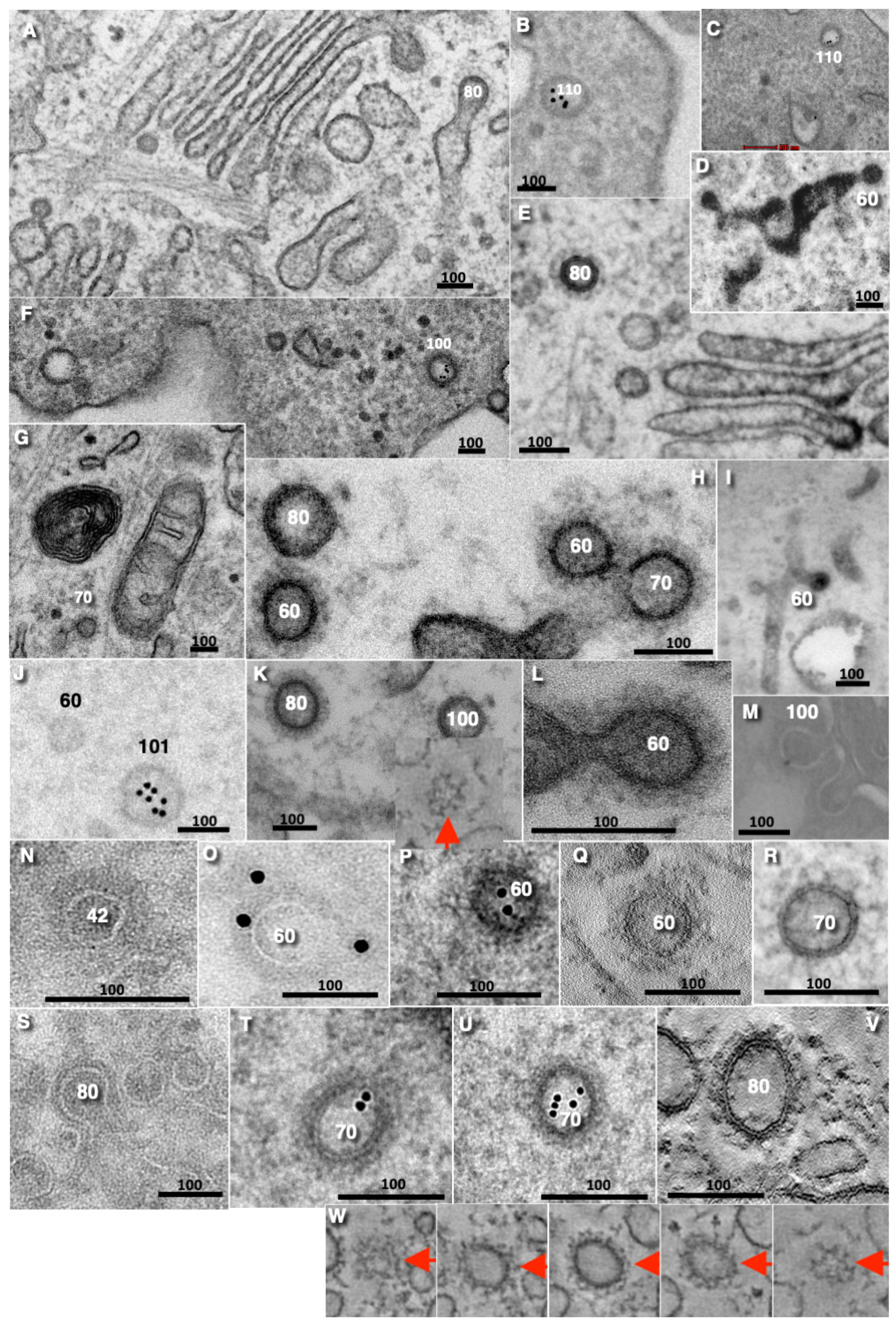
Figure 1A, D, E, I, L) and round profiles-vesicles (
Figure 1B, C, E, F, I, J, K, M-W) with different diameters obtained from in HeLa (
Figure 1A-F, I-V), HepG2 (
Figure 1G, H), and COS-7 (
Figure 1W) cells are demonstrated. For the preparation of cells, we used high pressure freezing. (
Figure 1A, E), labelling for EGF conjugated with 10 nm gold (
Figure 1B, C, F, J, T, U), labelling of endosomes with WGA-HRP (
Figure 1D, I), routine transmission EM (
Figure 1G, H, K, L, R), Tokuyasu cryosections (
Figure 1M, N, O, S), and EM tomography (
Figure 1Q, V-W). EM tomography allowed us to detect free vesicles (see serial tomo-images in
Figure 1W). Most of vesicles were present in the central part of the cells and near ER exit sites (
Figure S1). The peripheral parts of cells are mostly empty and do not contain vesicles. We compared the diameters of 52 nm near-Golgi vesicles after freezing and after chemical fixation (52.1±2.2 and 51.8±1.3 nm correspondingly) and concluded that both methods give comparable results.
The diameter distribution formed the standard peaks with maximal sizes equal to 42 (not found in HeLa cells), 50-52, 60, 70, 80, 100-nm. Intermediate diameters with intermediate sizes were detected rarely. HeLa cells do not contain the 42-nm vesicles (
Figure 2A-F). The 50-52- and 100-nm vesicles were always uncoated (
Figure 2K-M) whereas the 42-, 60-, 70-, and 80-nm vesicles could be coated with clathrin or uncoated. Near ERES we found the 50-nm vesicles which were always uncoated and were negative for Sec22 (
Figure 2G). There are no vesicles with a diameter of 65-85 nm near ERES. COPII-coated buds on the ER always have the shape of hemisphere. Distributions of diameters of the ERES vesicles is like those of ERES COPI-coated buds, Golgi vesicles, and Golgi COPI-coated buds, but different from that of the ERES COPII-coated buds (
Figure 2E-I).
To find the source of clathrin-dependent vesicles, we incubated cells with the WGA lectin conjugated with horse radish peroxidase (HRP) to the medium. Also, we used WGA to discriminate the 60-, 70- and 80-nm uncoated vesicles presumably clathrin-dependent from COPII-dependent ones. The application of WGA conjugated with HRP or with the EGF protein combined with subsequent gold labelling allowed us to identified vesicles derived from the PM and endosomes because WGA could label the PM endosome and even the trans-Golgi network (TGN) and trans-most cisterna. This method potentially could discriminate the uncoated 70- and 80-nm vesicles as being derived from ERES (if these vesicles would be negative for WGA), or endosome and plasma membrane derived when the 70- and 80-nm vesicles will be positive for WGA. Stimulation of EGF receptor with EGF and labelling with gold would for a short time would allow us to discriminate clathrin-coated or clathrin-dependent vesicles derived from the basolateral plasma membrane.
To this end, COS-7 cells were incubated with WGA-HRP for 30 min. Accumulation of WGA-HRP in the trans-most-cisternae after incubation of cells with WGA-HRP was observed in 30 min (
Figure 2J). Incubation of cells with WGA-HRP induced labelling of endosomes and some of the vesicles and buds with diameters of 60, 70, 80 and 100 nm. Vesicles with diameter equal to 50-52 nm were filled with WGA-HRP (
Figure 1D, I). Some of these clathrin-coated vesicles have diameter equal to 42 nm (
Figure 3A: green arrows). In cells incubated with EGF conjugate with the 10-nm gold particles led to the appearance of gold particles inside these vesicles (
Figure 1J, P, T, U).
In cells incubated with WGA-HRP for 15 min and then with NEM on ice, washing and worming up to 37 ˚C and subsequent incubation for 1 min, a significant portion of vesicles were positive for WGA-HRP and some of them were coated with clathrin (green arrows in
Figure 3A, 4, 5P). However, when after treatment of cells with NEM and warming brefeldin A was added to the incubation medium the percentage of the 52 nm vesicles decreased (
Figure 3C). This was evident even after the NEM treatment the 3 min incubation with brefeldin A was prolonged up to 3 min (
Figure 3B, 4, 5P). Diameter of most vesicles accumulated after microinjection of antibodies against COG4 is larger than 55 nm (
Figure 3C).
To examine the effect of Golgi vesiculation on the content of the isolated vesicular fraction we used N-ethylmaleimide (NEM), which blocks membrane fusion. To accumulate free vesicles, we incubated cells with 1 mM NEM on ice for 15 min and after washed cells with DTT, cells were incubated at 37˚C for 1 or 3 min. Then we incubated cells with NEM. In agreement with our previous work [
35,
36,
81] In both COS-7 (
Figure 3A, E) and HeLa (
Figure 3B- D, F-J), the NEM treatment induced accumulation of vesicles. This is especially evident after incubation of cells with WGA-HRP for 15 min (to prevent labeling of the trans-most cisternae [69\0], subsequent treatment with NEM on ice and next incubation with 1 mg/ml brefeldin A to prevent formation of Golgi COPI-dependent vesicles) for one (
Figure 3A) of three (
Figure 3B) minutes, the 42 nm clathrin-coated vesicles and buds were visible better (
Figure 3A and 3B). Green arrows there show the clathrin-coated [completely or partially] round 42-nm round profiles. Also, accumulation of mostly WGA-HRP-positive vesicles were observed (
Figure 3A).
Microinjection of antibodies against COG4 also induced accumulation of mostly WGA-positive vesicles with diameter larger than 55 nm (
Figure 3C). The 52-nm vesicles accumulated under the action of NEM are positive for GS27 (
Figure 3D; nanogold), GS28 (
Figure 3E; nanogold) and SNAP29 (
Figure 3F; nanogold). Using IEM based on cryosections (
Figure 3G-I) or peroxidase-DAB-based labelling (
Figure 3J) we demonstrated that the 52-nm vesicles (red arrows) accumulated under the action of NEM are depleted in Golgi enzymes (
Figure 3G, H, J) and sugar transporters (
Figure 3G, I). The 52-nm vesicles accumulated under the action of NEM is positive for GS27 (
Figure 3D), GS8 (
Figure 3E) and SNAP29 (
Figure 3F).
Structure of the pellet formed after centrifugation of vesicular fractions obtained from cells incubated at 37ºC for 3 min after NEM treatment is shown in Figue 4A. Structure of the pellet formed after centrifugation of vesicular fractions was examined using routine transmission EM (
Figure 5A, H-O). After more intensive Golgi vesiculation the percentage of cisternal remnants in the vesicular fraction was significantly higher (
Figure 5P). The 52-nm uncoated vesicles are indicated in the red box. Membranes incubated with GTPgS were subjected to shaking to liberated COPI-coated vesicles and mixed with vesicular fraction formed after incubation of Golgi membranes with cytosol in the presence of GTP. Next, the pellet of the vesicular fraction was formed sung centrifugation.
In the mixture of vesicles formed in the presence of GTPgS or GTP the GTPgS-dependent COPI-coated vesicles are heavier than the uncoated ones and as a result were found on the very bottom of the pellet (
Figure 4B; COPI-coated vesicles are shown in green box).
Figure 4C demonstrated the pellet of the vesicular fraction obtained from normal cells. Cryosection of the pellet of the vesicular fraction obtained from cells incubated with NEM for 1 min. is shown in
Figure 4D. In the 52-nm round profiles visible in the pellet of the vesicular fraction we also observed a depletion of markers, namely, Golgi enzymes and nucleotide sugar transporters (
Figure 4D-G, I, M, N) and enrichment of GS27 (
Figure 4H, M, N), and GS28 (
Figure 4J, M, N) over the 52-nm round profiles. In the vesicular fraction the labelling for Sec22 is not observed over round profiles (
Figure 4K, L, M. N)
We used immune EM labelling was based on nanogold (
Figure 5B, F) and cryosections (
Figure 5C-E, G). The purity of the isolated Golgi. Is shown in
Figure 5A. In the vesicular fraction obtained from cells incubated for 3 min after the treatment with NEM, the 52-nm uncoated round profiles are GS27–positive (
Figure 5B). There is labelling for clathrin over the coat surrounding round profiles (
Figure 5C). In the vesicular fraction obtained from Golgi membranes, the 52-nm round profiles were negative for CMPST, the nucleotide sugar transporter (
Figure 5D) and for Myc6 (
Figure 5E). Man-II was depleted over round profiles but enrichment in other Golgi membranes (
Figure 5F, G). After isolation of the 52-mnm Golgi vesicles with magnetic beads coated with and antibodies against GS27, not only the 52-nm vesicles but also remnants of Golgi cisternae are obtained (
Figure 5H-K).
The vesicular fraction used for experiments with Arf1, and sequential binding was shown in
Figure 5L. Incubation of the Golgi membranes with cytosol induced formation of the 52-nm vesicles (
Figure 5M). After incubation of Golgi membranes with Arf1 at 37˚C) and then with COPI fraction oif cytosol on ice, the COPI-coated buds were not found (
Figure 5N). Incubation of the above-mentioned membranes at 37˚C for 2 min without addition of other proteins induced formation of COPI-coated buds (
Figure 5O). Thus, formation of COPI-coated buds and their fission potentiate uncoating of Golgi membranes.
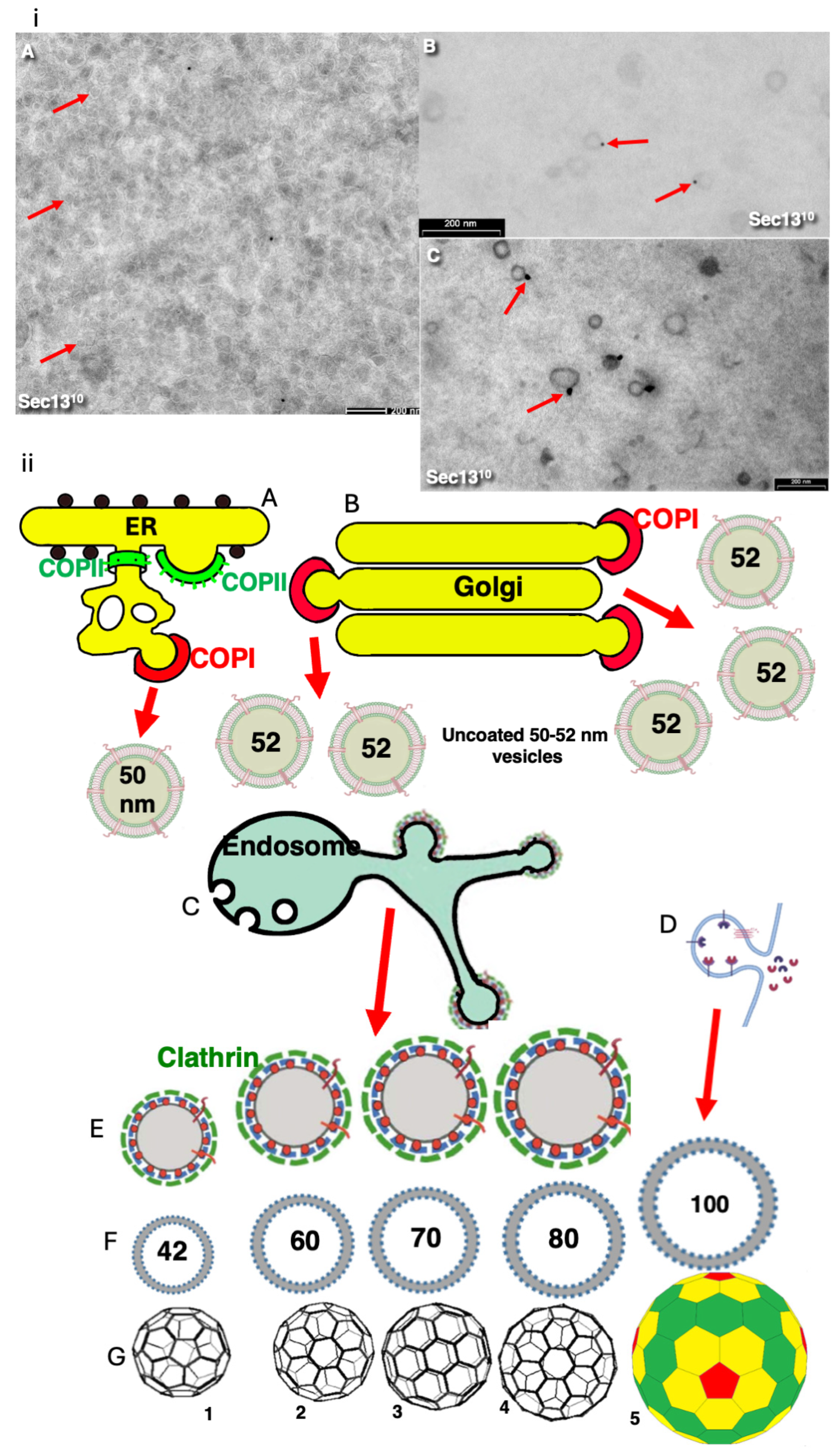
Finally, we tested whether COPI-dependent vesicles could recruit Sec13, the subunit of COPII. To this end, the 50-nm vesicles formed after incubation of endoplasmic reticulum microsomes with cytosol were isolated with magnetic beads conjugated with antibodies against GS27, we isolated COPI-dependent vesicles from the ER and incubated these vesicles with Sec13. The isolated with GS27/membrin-containing magnetic beads the 50-nm vesicles were not positive for Sec13. Labelling for Sec13 is visible over membrane structures larger than 60 nm (
Figure 6iA). After incubation of the vesicular fraction with cytosol, the 50-nm round profiles became positive for Sec13 (
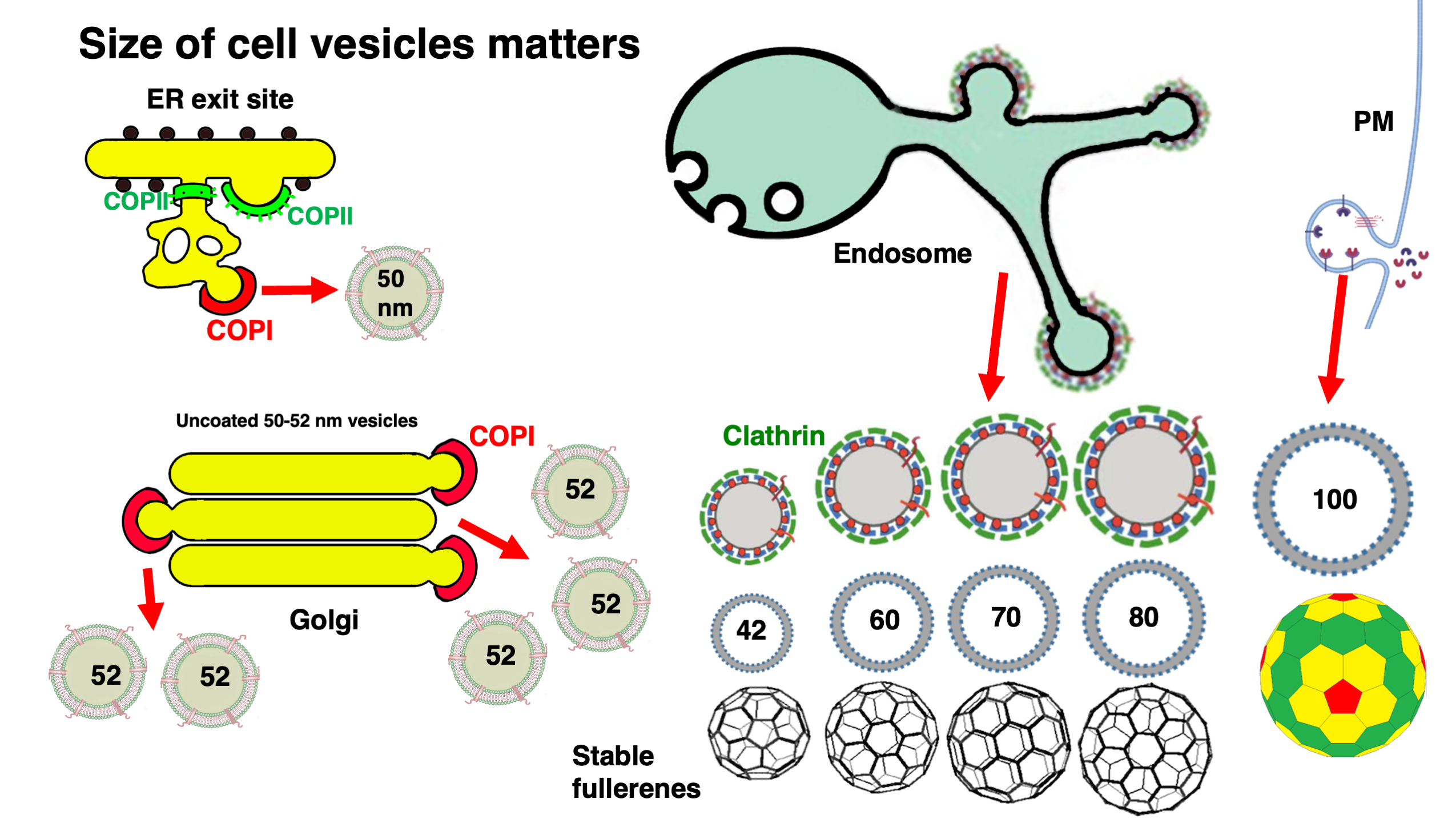
Figure 6iB, iC) whereas incubation with anti-albumin antibody the labeling was absent (not shown). Schemes of the formation of cell vesicles is shown in
Figure 6iiA-iF.
4. Discussion
Our analysis is aimed to study cell vesicles. The super-resolution methods are not suitable for the identification of small vesicles with sufficient details. We observed clear peaks on the diameter distribution graph: 42, 50, 60, 70, 80, 100 nm. We could not find the 42-nm vesicles in HeLa cells but found them in COS-7 cells. According to R
2 test, this difference is statistically significant (
p<0.05). The 42-nm vesicles were observed in absorptive enterocytes [
74] and neurons [
75], However, these 42-nm vesicles are not present in goblet cells [
21].
The 42, 60, 70, 80 nm vesicles can be coated with clathrin. Being dependent on the clathrin and sometimes coated with it, these vesicles clearly corresponded to stable fullerenes: C₆₀, C₇₂, C₈₄, C₉₆, and C₁₃₄. Schemes of stable fullerenes are shown in
Figure 6iiG. 1. The fullerene C
60 corresponds to a soccer ball; the diameter of the inscribed sphere = 42 nm; 2. C
72: the diameter of the inscribed sphere = 60 nm; 3. C
84; the diameter of the inscribed sphere = 70 nm; 4. C
96; the diameter of the inscribed sphere = 80 nm; 5. C
134; the diameter of the inscribed sphere = 100 nm.
These vesicles are achievable for the WGA lectin conjugated with peroxidase and added to the culture medium. The 50-52 nm vesicles were always uncoated. Similar observations regarding vesicles of 50–52 nm have been previously reported [
66,
78]. We compared the size of the buds and vesicles. There is a higher chance that ER exit site (ERES) vesicles are formed from COPI-coated buds. In the cells and vesicular fractions, vesicles were depleted in Sec22, GGE, NSTs, GS27 is present in the 50 nm vesicles.
In intestinal absorptive enterocytes, there are no COPII-coated buds and COPII-dependent vesicles [
74]. In goblet cells, ERES are extremely small [
51,
76] COPII-coated buds and COPII-dependent vesicles were not found there [
21]). Also in the regenerating endothelium, endothelial cells being in G2 phase contain ERES [
77].
Clathrin-coated vesicles were achievable for WGA lectin added outside. Uncoated vesicles with diameters of 42, 60, 70, 80, and 100 nm were also available for external addition of WGA. After 30 minutes of cell incubation with WGA-HRP and subsequent membrane fusion blocking, vesicles with wavelengths of 42, 60, 70, 80, and 100 nm, but not 50–52 nm, were labeled with the lectin. In vesicles with diameter equal to 42, 60, 70, and 80 nm, the concentration of Sec22 was significantly lower than in the ERES. These findings indicate that 50 nm vesicles are of a different origin than other vesicles. Clathrin-coated 100 nm vesicles were not found. Therefore, the hypothesis that clathrin-coated buds on the plasma membrane can also transform into clathrin-coated vesicles is either incorrect or a very rare occurrence.
Several authors have stated that they have demonstrated COPII- or COPI-coated vesicles, but none of them have used 3D EM. For example, in
Figure 1A presented by [
29], the so-called “clearly visible COP layer” is an indistinct structure without a clearly visible white membrane line. In
Figure 1C also shown in the same location, the “Man II positive vesicle with visible COP coating” is coated with clathrin, as the coating thickness exceeds 15 nm in diameter.
Figure 2 shows a circular profile without evidence that this profile represents a vesicle.
It is more straightforward to identify COPII dependency by labeling Sec22, a marker that was already identified in the research by Rexach et al. [
79]. However, such labeling has not been performed or shown in the images presented by Bednarek and colleagues [
73]. The so-called COPII-coated vesicles shown in
Figure 2D and COPI-coated vesicles in
Figure 2E from Bednarek et al [
73] are identical in both size and morphology. Their structural characteristics closely resemble those of COPI coats depicted in the images provided by Orci et al [
78]. COPI binds to the ER proteins using the dilysine motif [
80].
In the images published by [
16,
17,
18,
19] obtained from quickly frozen samples, the transverse diameter of hemispheres coated with COPII ranged from 70 to 85 nanometers on the ER. This makes it difficult to form spheres from these hemispheres, as excess membranes are formed. For instance, the surface area of a sphere with a diameter of 50 nanometers is twice that of a sphere with a diameter of 70 nm, and nearly three times that of a sphere with diameters between 85 and 86 nm. If we assume that the COPII vesicles originated from a coated bud of such a large size, a mechanism will need to exist to remove excess membrane during the formation of the vesicle. Buds observed by Bannykh et al. [
81] were also larger than 65 nm.
In most studies, vesicles of 52 nm do not contain Golgi resident proteins [
35,
56,
82,
83,
84,
85]. Also, during mitosis, these vesicles have fewer Golgi enzymes compared to Golgi cisternae [
86,
87]. Martinez-Menarguez et al. [
88] observed a slight increase in ManII-GFP content in circular profiles coated with COPI, although this protein was overexpressed.
Golgi glycosylation enzymes, nucleotide sugar transporters, and Sec22 are not present in 52 nm vesicles but are positive for GS27. Specifically, the concentration of Golgi resident proteins in these 52 nm vesicles is significantly lower compared to above the tanks or tank residues located at the bottom of the sedimentary fraction obtained after centrifugation. Furthermore, the structural characteristics of NSTs indicate their involvement in COPI-dependent retrograde transport vesicles. Indeed, NSTs contain ten transmembrane domains and form homo-oligomers ranging from dimers to hexamers [
89,
90,
91,
92,
93]. The width of the NST dimer with 20 trans-membrane domains is 20 nanometers, and the size of hexamers is even higher [
93]. Due to these dimensions, it is highly unlikely that NSTs could be effectively encapsulated in membrane of the 52 nanometer COPI vesicles.
The formation of COPI-buds coated with COPI and subsequent detachment o0f vesicles may serve as a mechanism for the removal of COPI from the Golgi membranes. It has been established that COPI is not only present in buds coated with this protein, but also along the periphery of Golgi cisternae, where these buds function as a means for collecting COPI [
94]. During our investigations, we did not observe vesicles positive for Sec22, which should be in putative COPII vesicles [
21,
79,
95].
To demonstrate that COPI-dependent bud formation leads to membrane opening, a three-stage experimental approach was employed. Initially, myristoylated Arf1 was incorporated into the membranes of Golgi cisternae. At 0 °C, COPI binds to Arf1, a temperature that decreases membrane fluidity but does not disrupt the Arf1-COPI interaction. The inhibition of the formation of COPI-coated vesicles when heated in the presence of coatomer complexes without e-COPI support this mechanism, thus confirming the hypothesis that the formation of these vesicles helps to remove the COPI coat. The sequential attachment of Arf1 to the Golgi membrane, then binding of COPI to Arf1 and subsequent heating, resulted in a decrease in the labeling of COPI on these membranes. This suggests that the formation of 50–52 nm vesicles could induce the removal of the COPI coat from the Golgi membranes.
Clathrin-coated vesicles, the size of which was consistent with our data, were identified by several research groups (see the Introduction). One potential explanation for the presence of these distinct peaks observed in clathrin-coated vesicles is that their formation is guided by the physical principles that underlie the geometry of fullerenes. It is well known that clathrin triskelions, composed of three heavy chains and three light chains, self-assemble into multifaceted structures. The thickness of the clathrin coat is 18.3 nanometers, and the length of the edges of the polyhedral structure is approximately 16 nanometers. Notably, the structure of C
60 fullerene, also known as a truncated icosahedron, comprises 20 hexagonal faces and 12 pentagonal faces, forming a spherical structure with 60 vertices, mirroring the geometry of a coat for a soccer ball. If we consider a synaptic vesicle with an outer diameter of 41.6 nm, then the outer diameter of the clathrin coat of a football-shaped fullerene would be 77.6 nm. This is calculated by adding the 41.6 nm of the vesicle and doubling the 18.3 nm of the clathrin coat. Thus, the membrane sphere that fits within the geometry of a cell has a diameter of approximately 42 nm. The geometry of clathrin coats provides a physical justification for the consistent size of clathrin-coated vesicles. The stable relationship between the vesicle diameter and the football-shaped structure supports the idea that the assembly of the coat may be challenging from a physical perspective. C₆₀ fullerenes, consisting of 60 carbon atoms and forming a truncated icosahedron, geometrically correspond to clathrin frameworks that can accommodate a membrane sphere with a diameter of ~42 nm. This size is like that of synaptic vesicles. A membrane sphere with a diameter of 60 nanometers (nm) could be inserted into the C
96 fullerene molecule, while spheres with diameters of 70 and 80 nm correspond to C138 and C180 fullerenes, respectively. These fullerenes (C96, C138, and C180) are among the most stable and geometrically ideal due to their lack of adjacent pentagonal contacts, a well-known destabilizing factor in fullerene structures. In contrast, smaller fullerenes with pentagonal structures, such as C76, are energetically less favorable and structurally less stable [
96,
97].
These diameters of the polyhedron that form the clathrin coating correspond to more energetically favorable configurations for the formation of the clathrin lattice [
98,
99,
100]. Additionally, a barrel-shaped hexagonal fullerene can accommodate spheres with a diameter of 30 nanometers (nm), and even smaller mini-shell-like fullerenes composed entirely of pentagons may correspond to tiny vesicles observed in
S. cerevisiae [
101,
102]. Therefore, our findings once again demonstrate that biology follows physical laws, specifically, mathematics and fullerene physics dictate biological structure.
Our data explain why the vesicle fraction used for analysis of COPI vesicles contain significant amount of Golgi glycosylation enzymes. One possible explanation is that the vesicle fractions contain not only intact vesicles but also cisternal remnants [
56]. Importantly, according to the methods described by Gilchrist et al, [
49] samples prepared for electron microscopy was additionally purified using gradient centrifugation whereas this procedure was not used for samples prepared for biochemical analysis. Moreover, in the vesicular fraction analyzed by Gilchrist et al, [
49], four Golgi resident proteins, ERGIC32, STX5, p115, and p24 were found in these vesicles in lower concentrations compared to their levels in cisternal membranes. This suggests against the role of COPI vesicles as retrograde carriers.
Another problem of the vesicle isolation is the use of GTPgS instead of GTP [
103]. For instance, Sonnichsen et al. [
104] observed giantin on COPI vesicles using cryosections. However, to obtain the vesicles they used GTPγS. On the other hand, in several papers, where GTP was used, ARFGAP was not included in their incubation medium. These experimental conditions resemble those based on GTPgS because when GTP hydrolysis is inhibited in the presence GTPgS, or in the presence of GTP but in the absence of ARFGAP, vesicles are unable to detach from the membrane [
105]. Adolf et al. [
8] used incubation with COPI subunits, myristoylated ARF1, GTP but in the absence of ARFGAP and observed in vesicular fractions an increase in the levels of ManI and GalNAcT2, enzymes commonly found in the cis- and trans-Golgi. They claim that ARFGAP is not necessary for the release of COPI vesicles. However, g-COP was present on their COPI vesicles, suggesting that these vesicles are coated with COPI. This situation resembles that when GTPgS is used. Moreover, the size of vesicles visible in the vesicula fraction is unusual.
Figure 6 presented by Adolf et al. [
8] shows that the diameter of these COPI vesicles is approximately 90 nm. Alternatively, in
Figure 1D from Adolf et al. [
106], the diameters of round profiles, judging by the scale bar, range from 17 to 43 nm. Our data suggest that during the isolation procedure, COPII components could have bound nonspecifically to COPI-dependent vesicles, like the nonspecific interactions described by Aridor et al. [
1]. Thus, it is not possible to exclude that GGEs are present in the vesicular fractions are derived from cisternal remnants.
We have previously shown [
35,
56,
70] that during intensive Golgi vesiculation, many cisternal remnants appear in the cell after their detachment from the stacks. The remnants contaminate the vesicular fraction. Their entry into the vesicular fraction explains why, when Golgi vesicle function is blocked, many remnants appear in the vesicular fraction that contain a significant amount of Golgi enzyme.
Indeed, after treatment of cells with N-ethylmaleimide (NEM), especially after prolonged treatment, cisternal remnants were visible in the bottom of our vesicular fraction after its centrifugation more often. Also, in cells from which lacking the COG complex was suddenly eliminated, the Golgi appeared irregular in shape (“ugly”) and contain cisternal remnants [
107,
108]. These remnants containing a higher concentration of GGE [
56] have accumulated in the vesicular fraction, potentially creating the impression that Golgi vesicles are enriched in GGE. This mechanism may provide an alternative explanation for observations reported by the Lupashin’s group [
43,
55]. This observation provides an alternative explanation for data presented by Lupashin’s group [
43,
55] who observed that after Golgi vesiculation then amount of Golgi enzymes in the vesicular fraction became higher than without Golgi vesiculation. However, they did not examine the pellet of the vesicular fraction at EM level Gilchrishst et al. [
49] also took a non-pellet precipitate and a membrane fraction enclosed between the density gradients of sucrose solutions whereas the entire vesicular fraction was biochemically examined.
However, this sharp increase in the number of vesicles following acute inactivation of the COG function reported by Sumya et al [
43,
55] appears to be a transient phenomenon [
109,
110]. Sumya et al. [
43,
55] isolated vesicles using antibodies against giantin and GS15, but the concentration of GS15 and giantin was almost the same in Golgi cisternae and COPI vesicles [
85]. Furthermore, our observations indicate that even when magnetic beads conjugated with antibodies against GS27 or GS28 are used to increase the specificity of isolation, infection with remnants of Golgi cisterna cannot be completely ruled out.
A vesicle fraction obtained in the presence of GTP and analyzed by Gilchrist et al. [
49], was enriched in Rab1A, Rab1B, Rab2A, and Rab14 proteins were enriched. This suggest that COG able to bind Rabs could function as a tether fir COPI-dependent vesicle and explains why in the absence of COG the number of 50-nm vesicles increased. Indeed, in the absence of tether-like strings vesicles could diffuse out of Golgi zone.
Finally, in experiments conducted by Munro’s group [
111,
112,
113], Golgins were directed to the mitochondria, where they functioned to bind vesicles. The Golgi-97-dependent vesicles, which were approximately 30-80 nanometers in size and attached to mitochondria, did not contain GGE or NST, but did contain TGN46, CI-M6PR, and CD-M6PR [
111]. However, this study lacked systematic measurements of the diameter of the vesicles and immunolabeling for GGE, NST, GS27, and GS28, which are markers for COPI-dependent vesicles. Our measurements of the diameters of round profiles RPs attached to mitochondria after application of Golgin-97 probe in (Figure 7 presented by Wong and Munro [
112]) using the diameter of microtubules as a reference showed that the diameters ranged from 93 to 140 nanometers. This suggested that these vesicles were independent of COPI but likely depended on clathrin. In
Figure 3B presented by Welch et al. [
113], the vesicles near the mitochondria in insect cells had a diameter of approximately 30-40 nanometers. They worked with insects that have the 42 nm clathrin-dependent vesicles [
21,
32].
Therefore, the data collected by Munro’s team could have alternative explanation and thus, does not obligatory support the hypothesis that COPI-dependent vesicles serve as retrograde carriers.
Recently, we have shown that the articles by Kaiser and Schekman [
71] and Barlowe et al. [
72] have alternative explanations. Here we would like to show an alternative explanation for the paper by Bednarek et al. [
73]. We believe that the structures depicted in
Figure 2E presented by [
73] are COPI-coated buds. This interpretation is supported by several lines of evidence. We measured the diameter of the two coated buds depicted in
Figure 2A,D presented by [
73]. Their diameters are equal to 56 and 48 nm respectively. These measurements correspond precisely to the 50–52 nm vesicle sizes we reported, supporting our interpretation. Notably, we did not detect vesicles with diameter equal to 70–85 nm near ERES. However, Bednarek et al. [
73] observed coated buds on the nuclear envelope in the absence of ARF1 but in the presence of COPI.
Conversely, the addition of Arf1 resulted in the formation of a minimal number of coated buds (see
Figure 1E in [
73]). For the membrane isolation Bednarek et al [
73] used method described by Rexach and Schekman [114\97], which does not include a washing step. Moreover,
Figure 2A presented by Bednarek and colleagues [
73] clearly demonstrates proteins within the nuclear pores. This suggests that the nuclear envelope has not been washed. Also, in the earlier work of the Schekman group [
72], the authors employed ER membranes containing a significant amount of g-COP.
Considering that the nuclei in the experiments conducted by Bednarek and others [
73] were not treated with urea or high-concentrated potassium, it is plausible that their samples contain some erosion typically observed on the nuclear membrane. However, in the absence of a specific amount of Arf1, which is typically absent in ERESs, COPI would not be capable of forming the curvature of the membrane [
115,
116]. The addition of Arf1 allows COPI, which was attached to the membrane of nuclear envelopes, to migrate into ERESs, bind to Arf1, and form COPI-coated bud.
Figure 1.
Examples of vesicles with different diameters in HeLa (A-F, I-V), COS-7 (W-Z), and HepG2 (G, H) cells. (A, E) High pressure freezing. (B, C, F, J, T, U) Labelling for EGF conjugated with 10 nm gold. (D, I) Labelling of endosomes with WGA conjugated with peroxidase. (G, H, K, L, R) Routine TEM. (M, N, O, S) Cryosections. (Q, V, W) EM tomography. Diameters of round profiles are indicated on images. Diameter of gold particles is equal to 10 nm. The thickness of clathrin coat in all images is equal to 18 nm. arrow in [Z]) is equal to 24.5 nm. (W) Serial tomo-slices of the 70-nm clathrin-coated vesicle (red arrows) in COS-7 cell. Scale bars: 100 nm (size is indicated on images).
Figure 1.
Examples of vesicles with different diameters in HeLa (A-F, I-V), COS-7 (W-Z), and HepG2 (G, H) cells. (A, E) High pressure freezing. (B, C, F, J, T, U) Labelling for EGF conjugated with 10 nm gold. (D, I) Labelling of endosomes with WGA conjugated with peroxidase. (G, H, K, L, R) Routine TEM. (M, N, O, S) Cryosections. (Q, V, W) EM tomography. Diameters of round profiles are indicated on images. Diameter of gold particles is equal to 10 nm. The thickness of clathrin coat in all images is equal to 18 nm. arrow in [Z]) is equal to 24.5 nm. (W) Serial tomo-slices of the 70-nm clathrin-coated vesicle (red arrows) in COS-7 cell. Scale bars: 100 nm (size is indicated on images).
Figure 2.
Size of different cell vesicles. (A, B) Distribution of diameters of the cell vesicles in HeLa (A) and COS-7 (B) cells. The 42-nm vesicles in HeLa cells are present whereas in COS-7 cells these vesicles are very rarely seen. (C) Treatment of cells with NEM, subsequent washing, warming and incubation at 37ºC for 3 min, the proportion of the 52-nm vesicles increased. (D) In cells incubated after the NEM treatments in the presence of brefeldin A, the speed of the formation of the 52-nm vesicle became lower than that of other vesicles. (E) The section of ERES. Nanogold labelling. The absence of Sec22 nano-gold labelling over the ERES 52 nm round profiles (red arrows, see also
Figure S4C). (F-J) Distribution of diameters of ERES free vesicles and buds. (F) ERES COPI-coated buds. (G) ERES COPI-coated buds. (I), Golgi free vesicles. (J) Golgi COPI-coated buds in HeLa cells. (K) Accumulation of WGA-HRP in the trans-most-cisternae after incubation of cells with WGA-HRP for 30 min. (L) In cells, treated with NEM and examined in 1 min, there is accumulation of the 52-nm vesicles mostly near the Golgi (red arrow) and in less extent near ERES (green arrow). (M) Cells treated with WGA-HRP for 30 min, then incubated with NEM and next examined in 1 min after the NEM washout. Red arrows show clathrin-coated the 42-nm vesicle. (N) In cells, treated with NEM and examined in 3 min in the presence of IKA blocking endocytosis, the Golgi is completely transformed into the 52-nm vesicles. (O) Cells processed as in (M) but incubated after the NEM treatment in the presence of brefeldin A. Accumulation of WGA–positive vesicles and tubulation of the Golgi. Scale bars (nm): 600 (J, L-O); 1000 (K); size is shown in images.
Figure 2.
Size of different cell vesicles. (A, B) Distribution of diameters of the cell vesicles in HeLa (A) and COS-7 (B) cells. The 42-nm vesicles in HeLa cells are present whereas in COS-7 cells these vesicles are very rarely seen. (C) Treatment of cells with NEM, subsequent washing, warming and incubation at 37ºC for 3 min, the proportion of the 52-nm vesicles increased. (D) In cells incubated after the NEM treatments in the presence of brefeldin A, the speed of the formation of the 52-nm vesicle became lower than that of other vesicles. (E) The section of ERES. Nanogold labelling. The absence of Sec22 nano-gold labelling over the ERES 52 nm round profiles (red arrows, see also
Figure S4C). (F-J) Distribution of diameters of ERES free vesicles and buds. (F) ERES COPI-coated buds. (G) ERES COPI-coated buds. (I), Golgi free vesicles. (J) Golgi COPI-coated buds in HeLa cells. (K) Accumulation of WGA-HRP in the trans-most-cisternae after incubation of cells with WGA-HRP for 30 min. (L) In cells, treated with NEM and examined in 1 min, there is accumulation of the 52-nm vesicles mostly near the Golgi (red arrow) and in less extent near ERES (green arrow). (M) Cells treated with WGA-HRP for 30 min, then incubated with NEM and next examined in 1 min after the NEM washout. Red arrows show clathrin-coated the 42-nm vesicle. (N) In cells, treated with NEM and examined in 3 min in the presence of IKA blocking endocytosis, the Golgi is completely transformed into the 52-nm vesicles. (O) Cells processed as in (M) but incubated after the NEM treatment in the presence of brefeldin A. Accumulation of WGA–positive vesicles and tubulation of the Golgi. Scale bars (nm): 600 (J, L-O); 1000 (K); size is shown in images.
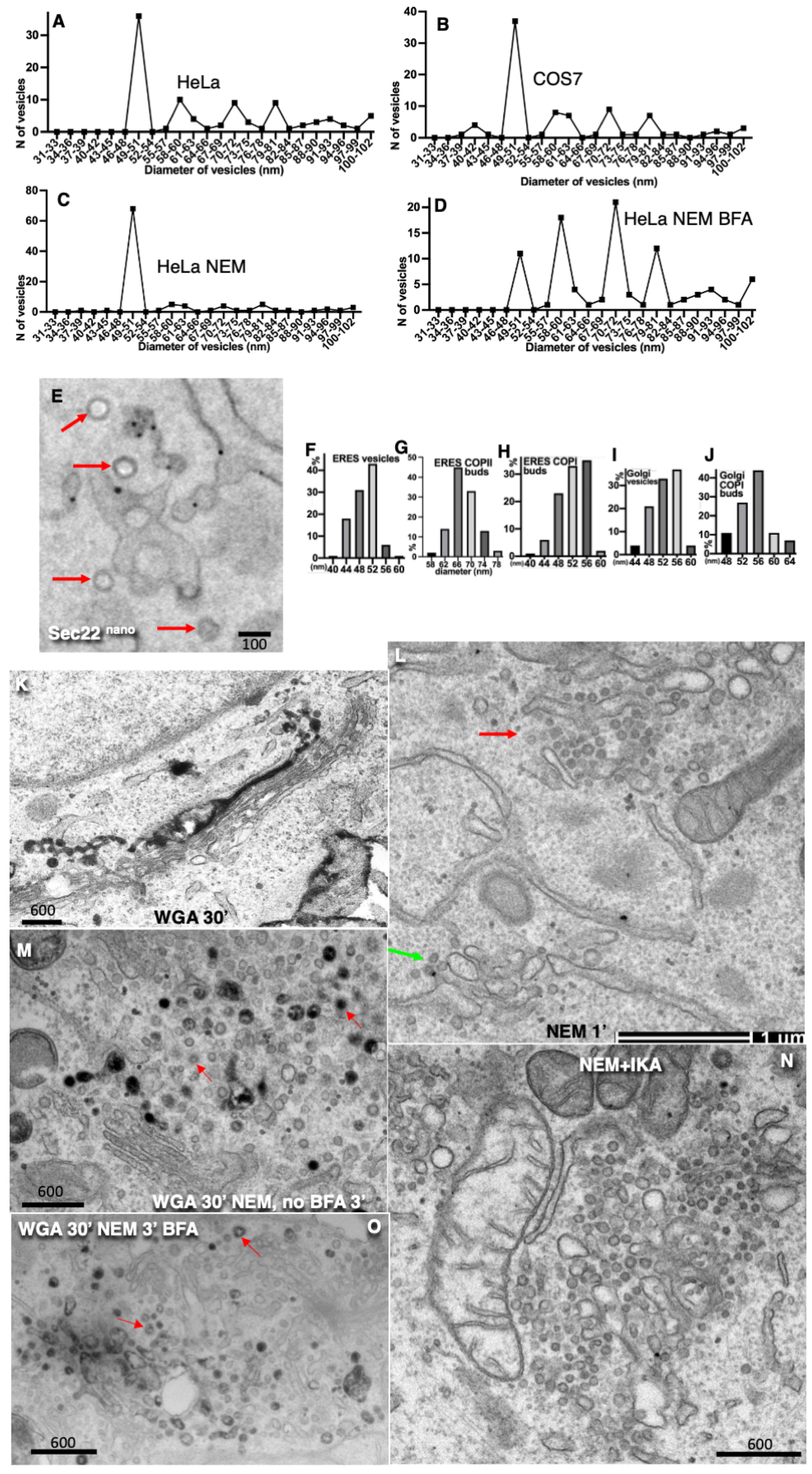
Figure 3.
Vesicles accumulated after the NEM treatment in COS-7 (A, E) and HeLa (others) cells are depleted in Golgi enzymes and nucleotide sugar transporters but enriched in GS27. resident proteins. (A) Accumulation of WGA-HRP (DAB)-positive vesicles after incubation with WGA-HRP (15 min), then NEM and next BFA. Green arrows show the 42 nm clathrin-coated vesicles and buds. (B) Accumulation of WGA-HRP (DAB)-positive vesicles after incubation with WGA-HRP (15 min) and NEM and next brefeldin A. Green arrows show the clathrin-coated (completely and partially) round profiles. (C) Microinjection of antibodies against COG4. Low level of the accumulation of the 52-nm vesicles. Diameter of most vesicles is larger than 55 nm. (D-F) The 52-nm vesicles accumulated under the action of NEM is positive for GS27 (D; nanogold), GS8 (E; nanogold) and SNAP29 (F; nanogold). (G-J) The 52-nm vesicles accumulated (red arrows) under the action of NEM are depleted in Golgi enzymes (G, H, J) and sugar transporters (G, I). Cryosection based IEM (G-I) and peroxidase-based IEM (J). Markers and gold size are shown on images. Scale bars (nm): 200 (A-H), 100 (I, J); size is shown in images.
Figure 3.
Vesicles accumulated after the NEM treatment in COS-7 (A, E) and HeLa (others) cells are depleted in Golgi enzymes and nucleotide sugar transporters but enriched in GS27. resident proteins. (A) Accumulation of WGA-HRP (DAB)-positive vesicles after incubation with WGA-HRP (15 min), then NEM and next BFA. Green arrows show the 42 nm clathrin-coated vesicles and buds. (B) Accumulation of WGA-HRP (DAB)-positive vesicles after incubation with WGA-HRP (15 min) and NEM and next brefeldin A. Green arrows show the clathrin-coated (completely and partially) round profiles. (C) Microinjection of antibodies against COG4. Low level of the accumulation of the 52-nm vesicles. Diameter of most vesicles is larger than 55 nm. (D-F) The 52-nm vesicles accumulated under the action of NEM is positive for GS27 (D; nanogold), GS8 (E; nanogold) and SNAP29 (F; nanogold). (G-J) The 52-nm vesicles accumulated (red arrows) under the action of NEM are depleted in Golgi enzymes (G, H, J) and sugar transporters (G, I). Cryosection based IEM (G-I) and peroxidase-based IEM (J). Markers and gold size are shown on images. Scale bars (nm): 200 (A-H), 100 (I, J); size is shown in images.
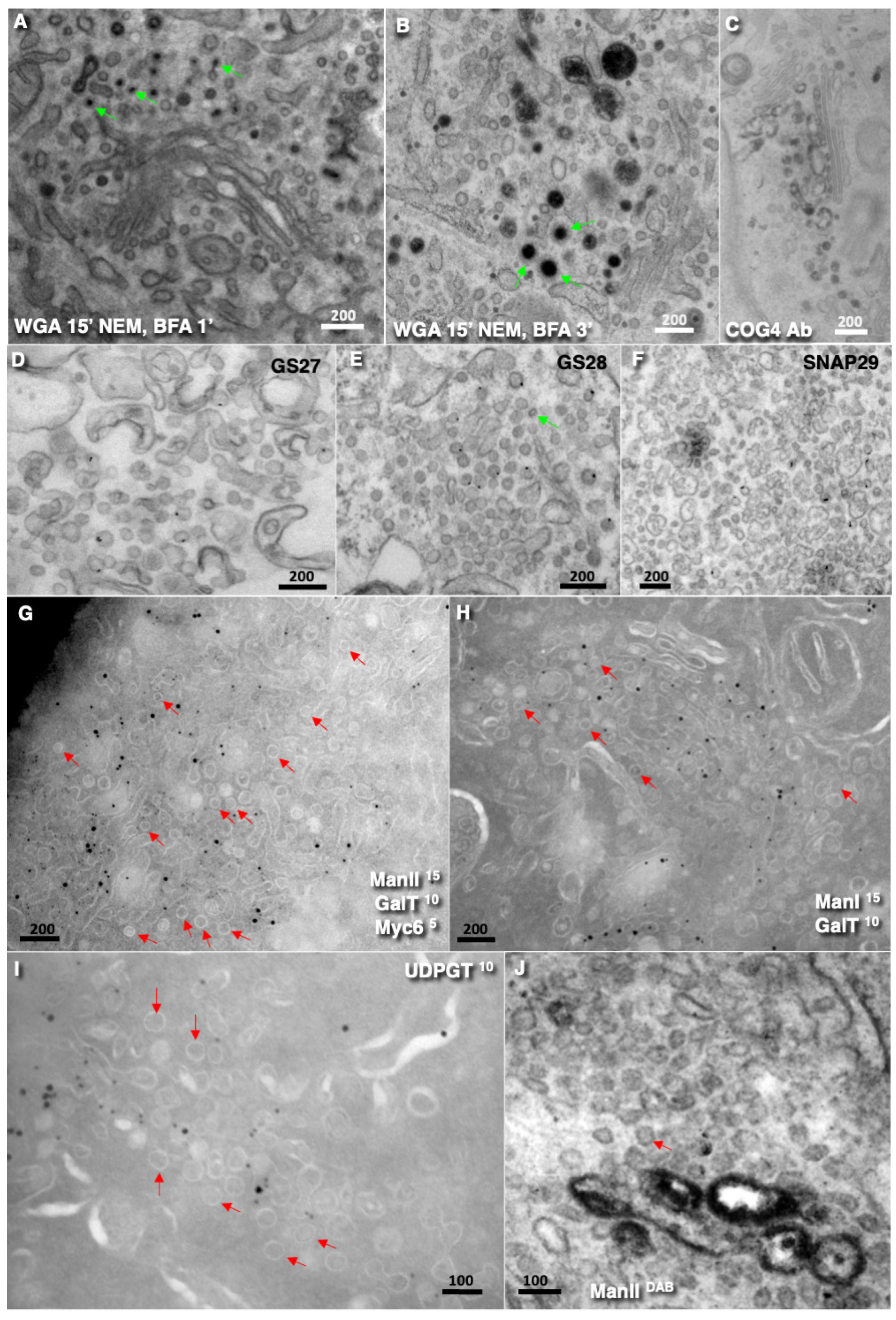
Figure 4.
Structure of the pellet formed after centrifugation of vesicular fractions. (A) The vesicular fraction from cells incubated for 3 min after NEM treatment with NEM. The 52-nm uncoated vesicles are indicated in the red box. (B) The mixture of vesicles formed in the presence of GTPgS and GTP. COPI-coated vesicles are shown in green box. (C) The pellet of the vesicular fraction obtained from normal cells. (D) The pellet of the vesicular fraction obtained from cells incubated with NEM for 1 min. Cryosection. (D-G, I) Depletion of markers, namely, Golgi enzymes and nucleotide sugar transporters, in the 52-nm round profiles. (H, J) Enrichment of GS27 (H), and GS28 (J) over the 52-nm round profiles. (K, L) No labelling for Sec22 is observed over round profiles in the vesicular fraction. (M, N) Labelling density of different markers in normal COS-7 cells (N) and in the vesicular fraction obtained from them (N). Gold labelling for Golgi enzymes and nucleotide sugar transporters (see indications on images: D-G, I, M, N), Sec22 (K, L, M. N), GS27 (H, M, N), and GS28 (J, M, N) over the 52-nm round profiles. Scale bars (nm): 200 (A); 100 (others); size is shown in images.
Figure 4.
Structure of the pellet formed after centrifugation of vesicular fractions. (A) The vesicular fraction from cells incubated for 3 min after NEM treatment with NEM. The 52-nm uncoated vesicles are indicated in the red box. (B) The mixture of vesicles formed in the presence of GTPgS and GTP. COPI-coated vesicles are shown in green box. (C) The pellet of the vesicular fraction obtained from normal cells. (D) The pellet of the vesicular fraction obtained from cells incubated with NEM for 1 min. Cryosection. (D-G, I) Depletion of markers, namely, Golgi enzymes and nucleotide sugar transporters, in the 52-nm round profiles. (H, J) Enrichment of GS27 (H), and GS28 (J) over the 52-nm round profiles. (K, L) No labelling for Sec22 is observed over round profiles in the vesicular fraction. (M, N) Labelling density of different markers in normal COS-7 cells (N) and in the vesicular fraction obtained from them (N). Gold labelling for Golgi enzymes and nucleotide sugar transporters (see indications on images: D-G, I, M, N), Sec22 (K, L, M. N), GS27 (H, M, N), and GS28 (J, M, N) over the 52-nm round profiles. Scale bars (nm): 200 (A); 100 (others); size is shown in images.
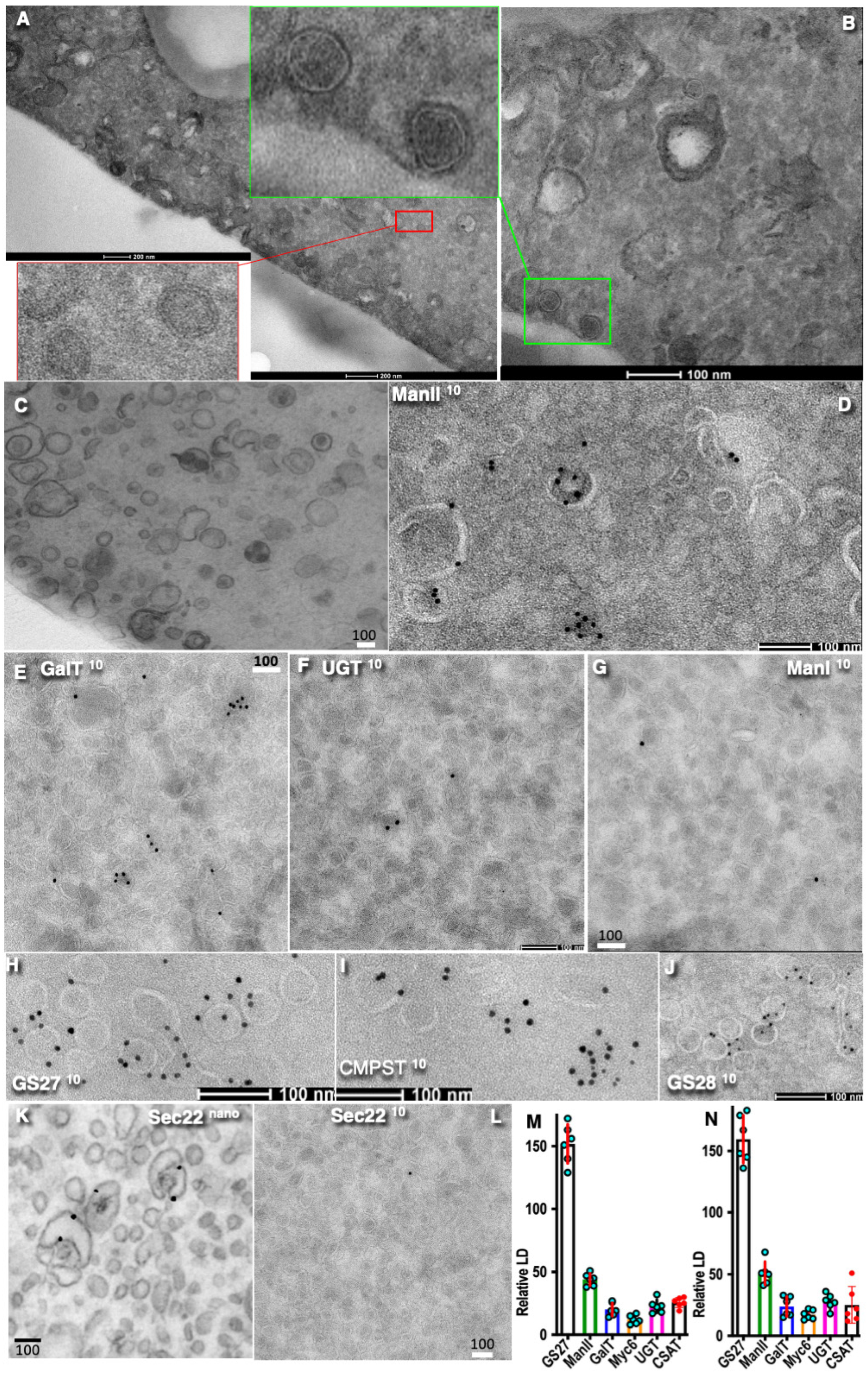
Figure 5.
Structure of the pellet formed after centrifugation of different vesicular fractions. Routine transmission EM (A, H-O). Labelling with nanogold (B, F). Cryosections (C-E, G). (A) The purity of the isolated Golgi. (B) The vesicular fraction from cells incubated for 3 min after NEM treatment with NEM. The 52-nm uncoated vesicles are indicated in the red box. (B) Presence of GS27 over the 52-nm round profiles. (C) Labelling for clathrin over the coat surrounding round profile (green arrows). (D) Depletion of the NST, namely, CMPST over the 52-nm round profiles. (E) Depletion of the nucleotide sugar transporters tagged with Myc6 (see Methods) over the 52-nm vesicles. (F, G) Depletion of ManII over round profiles but enrichment in other Golgi membranes. (H-K) Isolation of the 52-mnm Golgi vesicles with magnetic beads (red arrows) and antibodies against GS27. Not only the 52-nm vesicles are isolated (green arrows) but also remnants of Golgi cisternae (blue arrows) are isolated. (L) The vesicular fraction used for experiments with ARf1 and sequential binds. (M) Incubation of the vesicular fraction with cytosol induced formation of the 52-nm vesicles (green arrows). (N) After incubation of Golgi mem brans with Arf1 (at 37˚C) and then with COPI on ice, the COOPI-coated buds were not found. (O) Incubation of the above-mentioned membranes at 37˚C without addition of proteins induced formation of COPI-coated buds (magenta arrows). (P) The percentage of cisternal remnants in the pellet of the vesicular fraction obtained after treatment of cells with NEM and consecutive incubation at 37 ˚C for the time indicated on the graph. After more prolonged incubation these cisternal remnants were observed more often. Scale bars (nm): the length of bars is shown in images.
Figure 5.
Structure of the pellet formed after centrifugation of different vesicular fractions. Routine transmission EM (A, H-O). Labelling with nanogold (B, F). Cryosections (C-E, G). (A) The purity of the isolated Golgi. (B) The vesicular fraction from cells incubated for 3 min after NEM treatment with NEM. The 52-nm uncoated vesicles are indicated in the red box. (B) Presence of GS27 over the 52-nm round profiles. (C) Labelling for clathrin over the coat surrounding round profile (green arrows). (D) Depletion of the NST, namely, CMPST over the 52-nm round profiles. (E) Depletion of the nucleotide sugar transporters tagged with Myc6 (see Methods) over the 52-nm vesicles. (F, G) Depletion of ManII over round profiles but enrichment in other Golgi membranes. (H-K) Isolation of the 52-mnm Golgi vesicles with magnetic beads (red arrows) and antibodies against GS27. Not only the 52-nm vesicles are isolated (green arrows) but also remnants of Golgi cisternae (blue arrows) are isolated. (L) The vesicular fraction used for experiments with ARf1 and sequential binds. (M) Incubation of the vesicular fraction with cytosol induced formation of the 52-nm vesicles (green arrows). (N) After incubation of Golgi mem brans with Arf1 (at 37˚C) and then with COPI on ice, the COOPI-coated buds were not found. (O) Incubation of the above-mentioned membranes at 37˚C without addition of proteins induced formation of COPI-coated buds (magenta arrows). (P) The percentage of cisternal remnants in the pellet of the vesicular fraction obtained after treatment of cells with NEM and consecutive incubation at 37 ˚C for the time indicated on the graph. After more prolonged incubation these cisternal remnants were observed more often. Scale bars (nm): the length of bars is shown in images.
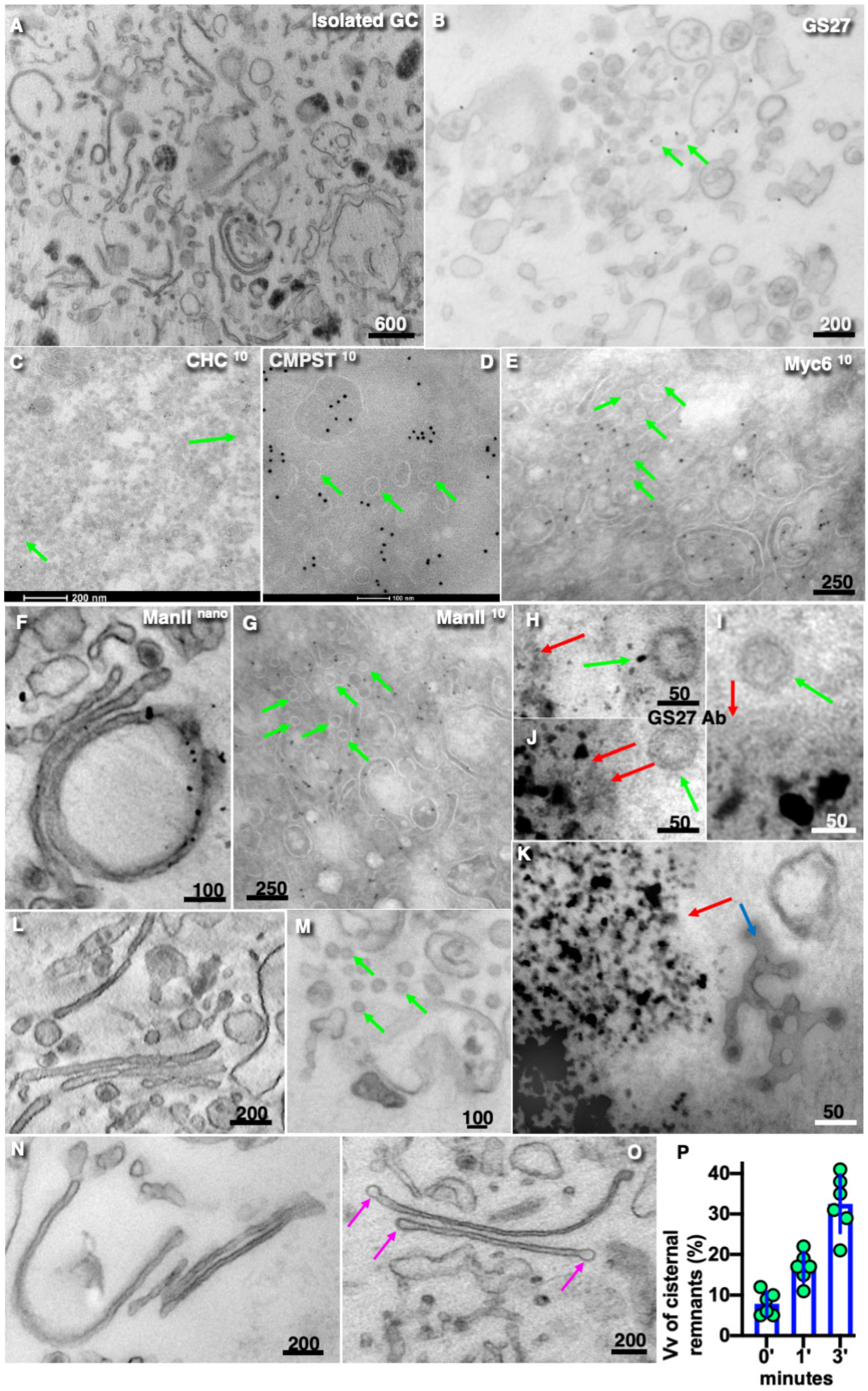
Figure 6.
Nonspecific binding of Sec13 to COPI-dependent vesicles and scheme of cell vesicles. (iA-iD) The 50-nm vesicles formed after incubation of microsomes with cytosol and isolated with magnetic beads can recruit COPII. (iA) The isolated with GS27/membrin-containing magnetic beads the 50-nm vesicles are not positive for Sec13. Labelling for Sec13 is visible over membrane structures larger than 60 nm. (iB, iC) After incubation of the vesicular fraction with cytosol, the 50-nm RPs are positive for Sec13. Nonspecific binding of Sec13 to COPI-dependent vesicles isolated from the ER after their incubation with cytosol. In the absence of COPI in cytosol these vesicles were not formed. (iiA-iiF) Schemes of the formation of cell vesicles. (iiG) Schemes of stable fullerenes. 1. C60 soccer ball; the diameter of the inscribed sphere = 42 nm; 2. C72: the diameter of the inscribed sphere = 60 nm; 3. C84; the diameter of the inscribed sphere = 70 nm; 4. C96; the diameter of the inscribed sphere = 80 nm; 5. C134; the diameter of the inscribed sphere = 100 nm. Scale bars (nm): the length of bars is shown in images (iA-iC).
Figure 6.
Nonspecific binding of Sec13 to COPI-dependent vesicles and scheme of cell vesicles. (iA-iD) The 50-nm vesicles formed after incubation of microsomes with cytosol and isolated with magnetic beads can recruit COPII. (iA) The isolated with GS27/membrin-containing magnetic beads the 50-nm vesicles are not positive for Sec13. Labelling for Sec13 is visible over membrane structures larger than 60 nm. (iB, iC) After incubation of the vesicular fraction with cytosol, the 50-nm RPs are positive for Sec13. Nonspecific binding of Sec13 to COPI-dependent vesicles isolated from the ER after their incubation with cytosol. In the absence of COPI in cytosol these vesicles were not formed. (iiA-iiF) Schemes of the formation of cell vesicles. (iiG) Schemes of stable fullerenes. 1. C60 soccer ball; the diameter of the inscribed sphere = 42 nm; 2. C72: the diameter of the inscribed sphere = 60 nm; 3. C84; the diameter of the inscribed sphere = 70 nm; 4. C96; the diameter of the inscribed sphere = 80 nm; 5. C134; the diameter of the inscribed sphere = 100 nm. Scale bars (nm): the length of bars is shown in images (iA-iC).