Submitted:
23 October 2025
Posted:
27 October 2025
You are already at the latest version
Abstract
Systematic reviews and original research articles should have a structured abstract of Background & objectives: Malnutrition is a prevalent condition linked to increased morbidity and mortality among hospitalized adult patients. This study assessed the effectiveness of three Nutrition Risk Screening (NRS) tools i.e. NRS-2002, Malnutrition Universal Screening Tool (MUST), and Malnutrition Screening Tool (MST) across Kenyatta National Hospital, Moi Teaching and Referral Hospital, and Kisii National Teaching and Referral Hospital in Kenya. Materials and Methods: A cross-sectional analytical design involved 420 adult inpatients screened within 24 hours of admission, using body mass index (BMI) as the reference standard. Results: Among hospitalized adults in three Kenyan referral hospitals, participants were predominantly middle-aged (median = 35.5 years). The NRS-2002 identified the highest malnutrition risk prevalence (85.7%), followed by MST (71.7%) and MUST (51.0%), whereas BMI classified only 23.8% as at risk. Both NRS-2002 and MUST showed perfect sensitivity (1.000) but MUST demonstrated higher specificity (0.65) and superior diagnostic accuracy (AUC = 0.82). Agreement with BMI was low across all tools, though MUST showed the best overall consistency and balance between sensitivity and specificity. Conclusion: The study revealed significant variation in malnutrition risk detection across NRS tools. While NRS-2002 was highly sensitive, it tended to overestimate risk. The MUST demonstrated the best overall balance of sensitivity, specificity, and diagnostic accuracy. Adoption of the MUST as the standard screening tool in Kenyan referral hospitals is recommended to enhance early malnutrition detection and improve patient care outcomes.
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Design and Variables
2.2. Study Location and Target Population
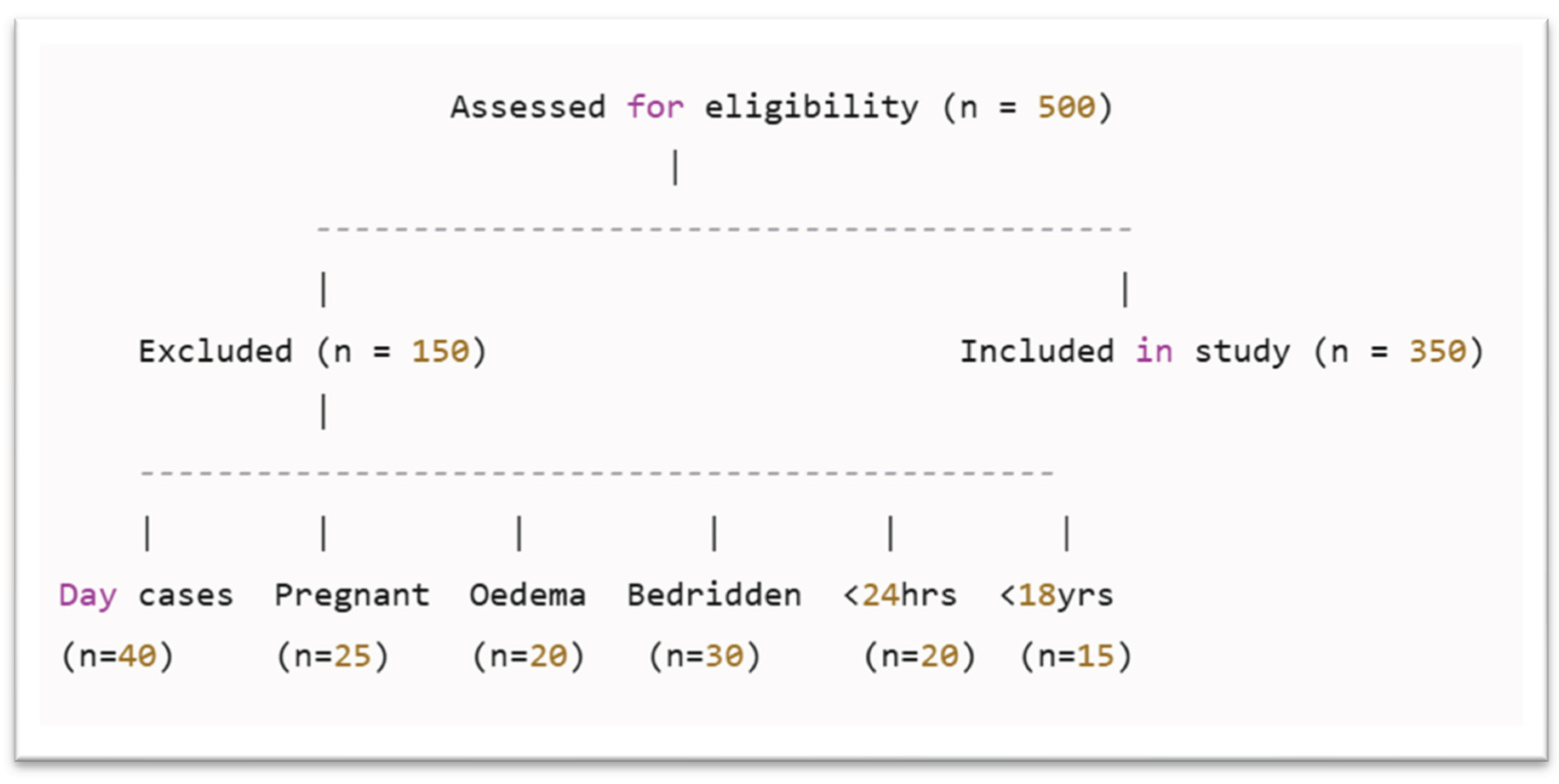
2.3. Exclusion Criteria
- Twenty-five women who were pregnant or breastfeeding were excluded.
- Twenty patients with moderate to severe oedema were excluded.
- Thirty bedridden patients
- Twenty patients were excluded because their hospital stay was less than 24 hours
- Fifteen individuals under the age of 18 were excluded, as the study focused solely on adult patients and required the use of age-appropriate nutritional assessment tools.
2.4. Sample Size
- N: Desired sample size
- e: Desired margin of error/desired precision (0.05)
- Z: Standard normal deviate at 95% confidence level (1.96)
- p: The prevalence of hospital malnutrition/nutritional risk in the target population was assumed to (0.5).
2.5. Sampling Techniques
2.6. Study Instruments and Data Collection
2.7. Reference Standard for the Study
2.8. Data Quality Assurance
2.9. Data Analysis
- 1)
- 0.90 ≤ AUC ≤ 1.00: Excellent performance
- 2)
- 0.80 ≤ AUC < 0.90: Good performance
- 3)
- 0.70 ≤ AUC < 0.80: Fair performance
- 4)
- 0.60 ≤ AUC < 0.70: Poor performance
- 5)
- 0.50 ≤ AUC < 0.60: Failure
Ethical Considerations
3. Results
3.1. Characteristics of the Study Participants
3.2. Prevalence of Hospital Malnutrition Risk
- NRS-2002 identified the highest prevalence of malnutrition risk (85.7%),
- MST indicated 71.7%,
- MUST identified 51.0%,
- While BMI classified only 23.8% as at risk.
3.3. Evaluation of the Effectiveness of Nutritional Screening Tools
- Sensitivity: NRS-2002 and MUST both achieved perfect sensitivity (1.000), while MST showed slightly lower sensitivity (0.8725).
- Specificity: NRS-2002 had the lowest (0.1886), MST moderate (0.3333), and MUST the highest (0.6478).
- Predictive Values: Both NRS-2002 and MUST had perfect NPV (1.000), while MUST recorded the highest PPV (0.4766).
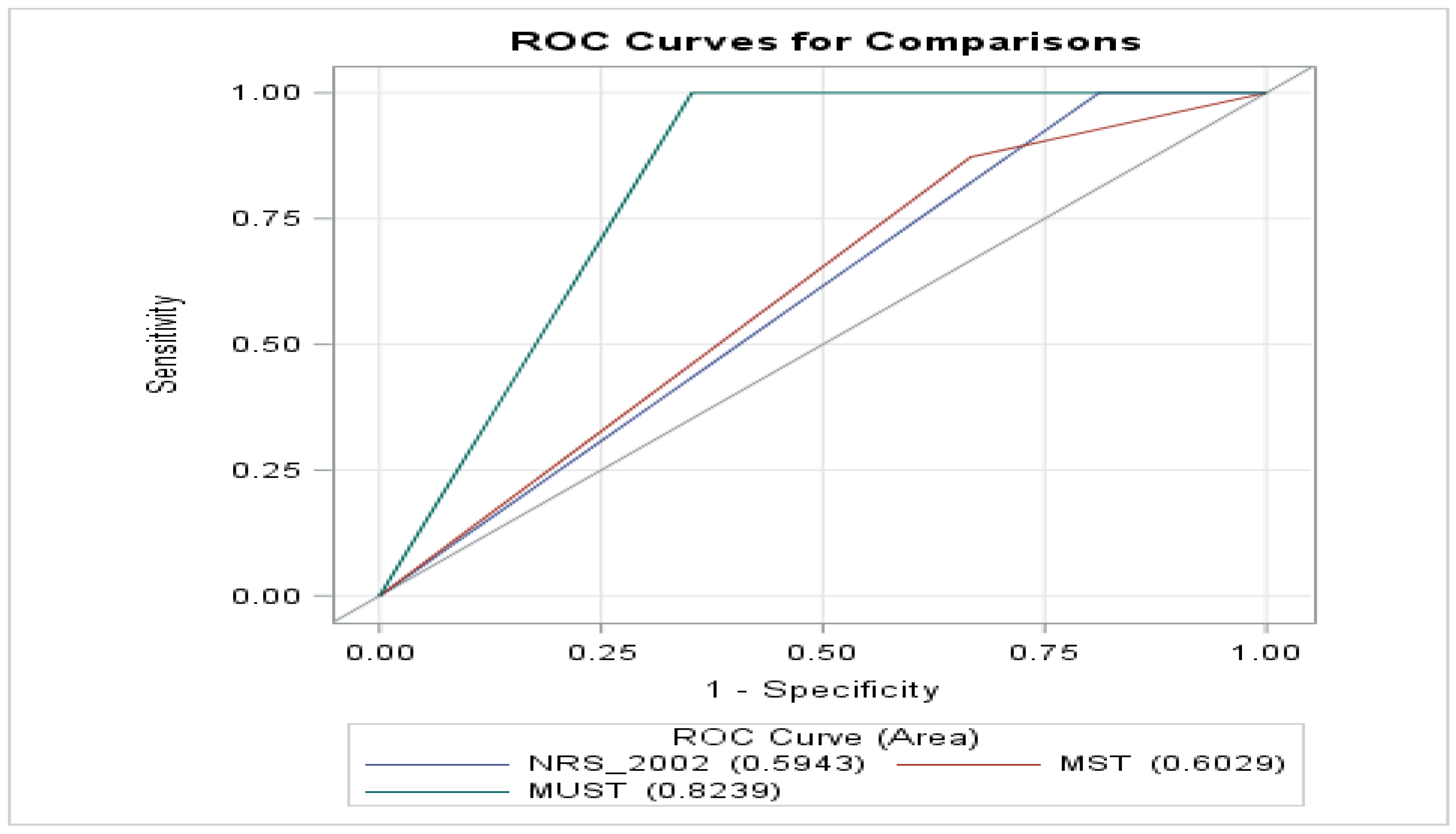
- ROC Analysis: MUST achieved the largest AUC (0.8239), indicating superior discriminative ability. MST (0.6029) and NRS-2002 (0.5943) performed comparatively lower (Figure 2).
3.4. ROC Contrast Estimation – Comparison of NRS-2002, MUST, and MST
- No significant difference was found between NRS-2002 and MST (p = 0.6996).
- MUST vs. NRS-2002: positive contrast estimate (0.2296), indicating better diagnostic accuracy.
- MUST vs. MST: positive estimate (0.2210, p < 0.05), showing significantly superior performance.These results further confirmed MUST’s higher AUC (0.8239) compared to MST (0.6029) and NRS-2002 (0.5943), reflecting its superior diagnostic capacity (Figure 2).
3.5. Agreement between NRS Tools and BMI
- NRS-2002: κ = 0.2115 (p = 0.001)
- MUST: κ = 0.2539 (p < 0.0001)
- MST: κ = 0.1254 (p < 0.001)
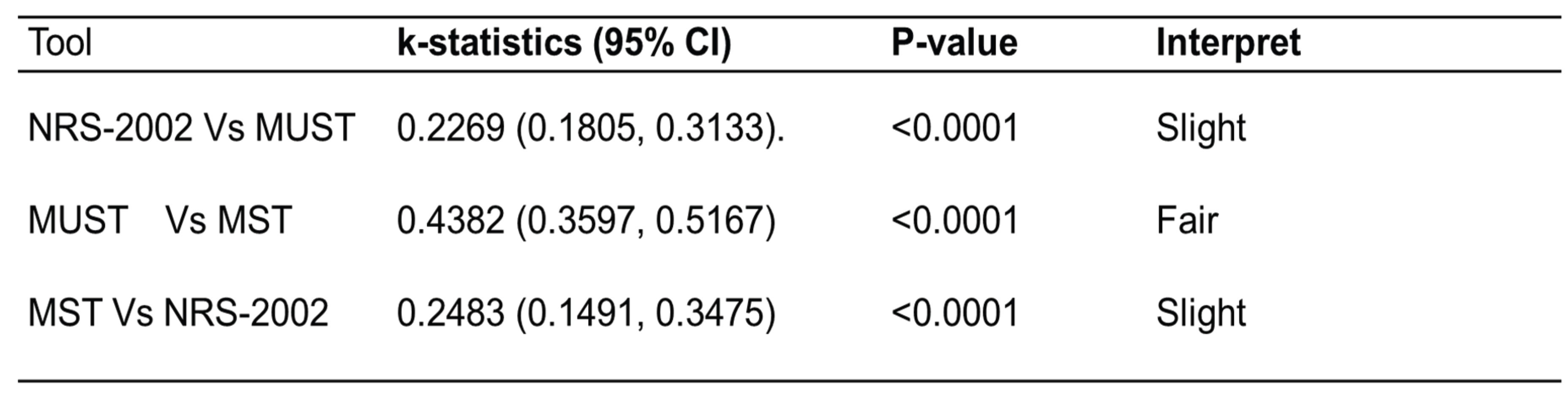
- NRS-2002 vs. MUST: κ = 0.2269
- MUST vs. MST: κ = 0.2483
- MST vs. NRS-2002: κ = 0.2269
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMI | Body Mass Index |
| KNH | Kenyatta National Hospital |
| KTRH | Kisii Teaching and Referral Hospital |
| MST | Malnutrition Screening Tool |
| MTRH | Moi Teaching and Referral Hospital |
| MUST | Malnutrition Universal Screening Tool |
| NPV | Negative Predictive Value |
| NRS | Nutrition Risk Screening |
| NRS-2002 | Nutrition Risk Screening-2002 |
| PPV | Positive Predictive Value |
Appendix
- Appendix 1: Informed Consents for Patients
- Appendix 2: KU Graduate School Research Authorization
- Appendix 3: KU-ERC Research Approval
- Appendix 4: NACOSTI Research Approval License
- Appendix 5: KNH-UON ERC Research proposal approval
- Appendix 6: MTRH Authority to Conduct Research
- Appendix 7: MTRH Institutional Research Ethics Committee (IREC) Approval
- Appendix 8: KTRH Institutional Research Ethics Committee (IREC) authority
References
- Correia, M. I. T. D.; Waitzberg, D. L. The Impact of Malnutrition on Morbidity, Mortality, Length of Hospital Stay and Costs Evaluated through a Multivariate Model Analysis. Clinical Nutrition 2003, 22(3), 235–239. [Google Scholar] [CrossRef]
- Oflynn, J.; Peake, H.; Hickson, M.; Foster, D.; Frost, G. The Prevalence of Malnutrition in Hospitals Can Be Reduced: Results from Three Consecutive Cross-Sectional Studies. Clinical Nutrition 2005, 24(6), 1078–1088. [Google Scholar] [CrossRef]
- Correia, M. Isabel T. D.; Hegazi, Refaat A.; Higashiguchi, Takashi; Michel, Jean-Pierre; Reddy, B. Ravinder; Tappenden, Kelly A.; Uyar, Mehmet; Muscaritoli, Maurizio. Evidence-Based Recommendations for Addressing Malnutrition in Health Care: An Updated Strategy from the feedM.E. Global Study Group. Journal of the American Medical Directors Association 2014, 15(8), 544–550. [Google Scholar] [CrossRef]
- Behiry, Mervat E.; Salem, Marwa R. High Prevalence of Malnutrition among Hospitalized Patients in a Tertiary Care Hospital by Using Malnutrition Universal Screening Tool. The Egyptian Journal of Internal Medicine 2019, 31(3), 326–331. [Google Scholar] [CrossRef]
- van Vliet, Iris M. Y.; Gomes-Neto, Antonio W.; de Jong, Margriet F. C.; Bakker, Stephan J. L.; Jager-Wittenaar, Harriët; Navis, Gerjan J. Malnutrition Screening on Hospital Admission: Impact of Overweight and Obesity on Comparative Performance of MUST and PG-SGA SF. European Journal of Clinical Nutrition 2021, 75(9), 1398–1406. [Google Scholar] [CrossRef] [PubMed]
- van Vliet, Iris M. Y.; Gomes-Neto, António W.; de Jong, Margriet F. C.; Jager-Wittenaar, Harriët; Navis, Gerjan J. High Prevalence of Malnutrition Both on Hospital Admission and Predischarge. Nutrition 2020, 77, Article 110814. [Google Scholar] [CrossRef]
- Visser, Janicke; Cederholm, Tommy; Philips, Lauren; Blaauw, Renée. Prevalence and Related Assessment Practices of Adult Hospital Malnutrition in Africa: A Scoping Review. Clinical Nutrition ESPEN 2024, 63, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Neumann, S. A.; Miller, M. D.; Daniels, L.; Crotty, M. Nutritional Status and Clinical Outcomes of Older Patients in Rehabilitation. Journal of Human Nutrition and Dietetics 2005, 18(2), 129–136. [Google Scholar] [CrossRef]
- Kaur, S.; Miller, M. D.; Halbert, J.; Giles, L. C.; Crotty, M. Nutritional Status of Adults Participating in Ambulatory Rehabilitation. Asia-Pacific Journal of Clinical Nutrition 2008, 17(2), 199–207. [Google Scholar] [CrossRef] [PubMed]
- Nip, W. F. R.; Perry, L.; McLaren, S.; Mackenzie, A. Dietary Intake, Nutritional Status and Rehabilitation Outcomes of Stroke Patients in Hospital. Journal of Human Nutrition and Dietetics 2011, 24(5), 460–469. [Google Scholar] [CrossRef]
- Neumann, S. A.; Miller, M. D.; Daniels, L.; Crotty, M. Nutritional Status and Clinical Outcomes of Older Patients in Rehabilitation. Journal of Human Nutrition and Dietetics 2005, 18(2), 129–136. [Google Scholar] [CrossRef]
- Goiburu, M. E.; Goiburu, M. M. Jure; Bianco, H.; Díaz, J. Ruiz; Alderete, F.; Palacios, M. C.; Cabral, V.; Escobar, D.; López, R.; Waitzberg, D. L. The Impact of Malnutrition on Morbidity, Mortality and Length of Hospital Stay in Trauma Patients. Nutrición Hospitalaria 2006, 21(5), 604–610. [Google Scholar] [CrossRef]
- Barker, Lisa A.; Gout, Belinda S.; Crowe, Timothy C. Hospital Malnutrition: Prevalence, Identification and Impact on Patients and the Healthcare System. International Journal of Environmental Research and Public Health 2011, 8(2), 514–527. [Google Scholar] [CrossRef]
- Giner, Manuel; Laviano, Alessandro; Meguid, Michael M.; Gleason, John R. In 1995 a Correlation between Malnutrition and Poor Outcome in Critically Ill Patients Still Exists. In Nutrition 1996, 12(1), 23–29. [Google Scholar] [CrossRef]
- Banks, Merrilyn; Bauer, Judith; Graves, Nicholas; Ash, Susan. Malnutrition and Pressure Ulcer Risk in Adults in Australian Health Care Facilities. Nutrition 2010, 26(9), 896–901. [Google Scholar] [CrossRef]
- Planas, M.; Audivert, S.; Pérez-Portabella, C.; Burgos, R.; Puiggrós, C.; Casanelles, J. M.; Rosselló, J. Nutritional Status among Adult Patients Admitted to a University-Affiliated Hospital in Spain at the Time of Genoma. Clinical Nutrition 2004, 23(5), 1016–1024. [Google Scholar] [CrossRef] [PubMed]
- Deer, Rachel; McCall, Mackenzie; Volpi, Elena. Comparison of Malnutrition Screening Tools for Use in Hospitalized Older Adults (OR36-02-19). Current Developments in Nutrition 2019, 3 Suppl 1, OR36-02-19. [Google Scholar] [CrossRef]
- Deer, Rachel R.; Goodlett, Shawn; Volpi, Elena. Comparison of Four Malnutrition Screening Tools to the Subjective Global Assessment (SGA) in a Cohort of Acutely Ill Older Adults. The FASEB Journal 2017, 31 Suppl 1, 151.4. [Google Scholar] [CrossRef]
- Lim, Su L.; Ong, Kian C. B.; Chan, Yiong H.; Loke, Wai C.; Ferguson, Maree; Daniels, Lynne. Malnutrition and Its Impact on Cost of Hospitalization, Length of Stay, Readmission and 3-Year Mortality. Clinical Nutrition 2012, 31(3), 345–350. [Google Scholar] [CrossRef]
- Agarwal, Ekta; Ferguson, Maree; Banks, Merrilyn; Batterham, Marijka; Bauer, Judith; Capra, Sandra; Isenring, Elisabeth. Malnutrition and Poor Food Intake Are Associated with Prolonged Hospital Stay, Frequent Readmissions, and Greater in-Hospital Mortality: Results from the Nutrition Care Day Survey 2010. Clinical Nutrition 2013, 32(5), 737–745. [Google Scholar] [CrossRef] [PubMed]
- Sharma, Yogesh; Miller, Michelle; Kaambwa, Billingsley; Shahi, Rashmi; Hakendorf, Paul; Horwood, Chris; Thompson, Campbell. Malnutrition and Its Association with Readmission and Death within 7 Days and 8–180 Days Postdischarge in Older Patients: A Prospective Observational Study. BMJ Open 2017, 7(11), e018443. [Google Scholar] [CrossRef]
- Blaauw, Renée; Achar, Esther; Dolman, Robin C.; Harbron, Janetta; Moens, Merel; Munyi, Faith; Nyatefe, Dzifa; Visser, Janicke. The Problem of Hospital Malnutrition in the African Continent. Nutrients 2019, 11(9), Article 2028. [Google Scholar] [CrossRef]
- Doganay, Mutlu; Halil, Meltem G.; Uyar, Mehmet; Kocatakan, Pinar; Dikmeer, Ayse; Cakir, Burcu Kelleci; Kilicturgay, Sadik; et al. Prevalence of Malnutrition Risk in Hospitalized Patients: A Large Nationwide Study. Journal of Health, Population, and Nutrition 2025, 44(1), 137. [Google Scholar] [CrossRef]
- Coats, Gamble; Katherine, G.; Morgan, Sarah L.; Bartolucci, Alfred A.; Weinsier, Roland L. Hospital-Associated Malnutrition: A Reevaluation 12 Years Later. Journal of the American Dietetic Association 1993, 93(1), 27–33. [Google Scholar] [CrossRef]
- Ferguson, M. Uncovering the Skeleton in the Hospital Closet. What Next? Australian Journal of Nutrition & Dietetics 2001, 58(2), 83–84. [Google Scholar]
- Elia, M.; Zellipour, L.; Stratton, R. J. To Screen or Not to Screen for Adult Malnutrition? Clinical Nutrition 2005, 24(6), 867–884. [Google Scholar] [CrossRef]
- Watterson, C.; Fraser, A.; Banks, M.; Isenring, E.; Miller, M.; Silvester, C.; Hoevenaars, R.; Bauer, J.; Vivanti, A. P.; Ferguson, M. Evidence Based Practice Guidelines for the Nutritional Management of Malnutrition in Adult Patients across the Continuum of Care. Nutrition and Dietetics 2009, 66(s3), S1–S34. [Google Scholar] [CrossRef]
- Ahmed, Nadim; Haboubi, N. Assessment and Management of Nutrition in Older People and Its Importance to Health. Clinical Interventions in Aging 2010, 5, 207–216. [Google Scholar] [CrossRef]
- Skipper, Annalynn; Ferguson, Maree; Thompson, Kyle; Castellanos, Victoria H.; Porcari, Judy. Nutrition Screening Tools: An Analysis of the Evidence. Journal of Parenteral and Enteral Nutrition 2012, 36(3), 292–298. [Google Scholar] [CrossRef] [PubMed]
- Kirkland, Lisa L.; Shaughnessy, Erin. Recognition and Prevention of Nosocomial Malnutrition: A Review and a Call to Action! The American Journal of Medicine 2017, 130(12), 1345–1350. [Google Scholar] [CrossRef]
- Yıldırım, Reyyan; Candaş, Bahar; Usta, M. Arif; Erkul, Oğuz; Türkyılmaz, Serdar; Güner, Ali. Comparison of Nutritional Screening Tools in Patients Undergoing Surgery for Gastric Cancer. Medical Bulletin of Haseki 2020, 58(2), 153–161. [Google Scholar] [CrossRef]
- Botero, Liliana; Banks, Merrilyn D.; Gordon, Emily H.; Bauer, Judy; Young, Adrienne M. Incidence and Outcomes of in-Hospital Nutritional Decline: A Prospective Observational Cohort Study in Adult Patients. Clinical Nutrition 2024, 43(5), 1057–1064. [Google Scholar] [CrossRef]
- Sunguya, B. F. P.; Koola, J. I.; Atkinson, S. Infections Associated with Severe Malnutrition among Hospitalised Children in East Africa. Tanzania Health Research Bulletin 2006, 8(3), 189–192. [Google Scholar] [CrossRef]
- Neelemaat, Floor; Meijers, Judith; Kruizenga, Hinke; van Ballegooijen, Hanne; van Bokhorst-de van der Schueren, Marian. Comparison of Five Malnutrition Screening Tools in One Hospital Inpatient Sample. Journal of Clinical Nursing 2011, 20(15–16), 2144–2152. [Google Scholar] [CrossRef]
- Cederholm, T.; Bosaeus, I.; Barazzoni, R.; Bauer, J.; Van Gossum, A.; Klek, S.; Muscaritoli, M.; et al. Diagnostic Criteria for Malnutrition—An ESPEN Consensus Statement. Clinical Nutrition 2015, 34(3), 335–340. [Google Scholar] [CrossRef]
- Al-Subaihi, A. A. Sample Size Determination. Influencing Factors and Calculation Strategies for Survey Research. Saudi Medical Journal 2003, 24(4), 323–330. [Google Scholar]
- Boléo-Tomé, Carolina; Monteiro-Grillo, Isabel; Camilo, Maria; Ravasco, Paula. Validation of the Malnutrition Universal Screening Tool (MUST) in Cancer. British Journal of Nutrition 2012, 108(2), 343–348. [Google Scholar] [CrossRef]
- Gottschall, C.; Tarnowski, M.; Machado, P.; Raupp, D.; Marcadenti, A.; Rabito, E. I.; Silva, F. M. Predictive and Concurrent Validity of the Malnutrition Universal Screening Tool Using Mid-upper Arm Circumference Instead of Body Mass Index. Journal of Human Nutrition and Dietetics 2019, 32(6), 775–780. [Google Scholar] [CrossRef] [PubMed]
- Hassanin, Islam A.; Hassanein, Hazem; Elmenshawy, Pillar; El-Gameel, Dina; Elsheikh, Aya A.; El-Kobrosly, Aya; Samir, Bassant; Emil, Rafael; Alaa, Merihan; Selim, Dina H. Malnutrition Score and Body Mass Index as Nutritional Screening Tools for Hemodialysis Patients. Clinical Nutrition ESPEN 2021, 42, 403–406. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.Mary M. Reliability of Nutritional Screening and Assessment Tools. Nutrition 2004, 20(3), 307–311. [Google Scholar] [CrossRef] [PubMed]
- Coutu, T. Nutrition Screening in the Adult Stroke Population Using the Canadian Nutrition Screening Tool in Comparison with the Subjective Global Assessment”. Master’s thesis Electronic Thesis and Dissertation Repository, University of Western Ontario, 2017. Available online: https://ir.lib.uwo.ca/etd/4668.
- Monaghan, Thomas F.; Rahman, Syed N.; Agudelo, Christina W.; Wein, Alan J.; Lazar, Jason M.; Everaert, Karel; Dmochowski, Roger R. Foundational Statistical Principles in Medical Research: Sensitivity, Specificity, Positive Predictive Value, and Negative Predictive Value. Medicina 2021, 57(5), 503, Bookshelf: ID: NBK580496. [Google Scholar] [CrossRef] [PubMed]
- van Bokhorst-de van der Schueren, Marian A. E.; Guaitoli, Patrícia R.; Jansma, Elise P.; de Vet, Henrica C. W. Nutrition Screening Tools: Does One Size Fit All? A Systematic Review of Screening Tools for the Hospital Setting. Clinical Nutrition 2014, 33(1), 39–58. [Google Scholar] [CrossRef] [PubMed]
- McHugh, ML. Interrater reliability: the kappa statistic. Biochem Med (Zagreb) 2012, 22(3), 276–282. [Google Scholar] [CrossRef]
- Seid, Ahmed M.; Fentahun, Netsanet. Prevalence of Malnutrition among Old People in Africa: Systematic Review and Meta-analysis. BMJ Open 2022, 12(11), e065197. [Google Scholar] [CrossRef]
- Inciong, Jesus F. B.; Chaudhary, Adarsh; Hsu, Han-Shui; Joshi, Rajeev; Seo, Jeong-Meen; Trung, Lam Viet; Ungpinitpong, Winai; Usman, Nurhayat. Hospital Malnutrition in Northeast and Southeast Asia: A Systematic Literature Review. Clinical Nutrition ESPEN 2020, 39, 30–45. [Google Scholar] [CrossRef]
- Li, Tao; Zhang, Yalan; Gong, Cheng; Wang, Jing; Liu, Bao; Shi, L.; Duan, Jun. Prevalence of Malnutrition and Analysis of Related Factors in Elderly Patients with COVID-19 in Wuhan, China. European Journal of Clinical Nutrition 2020, 74(6), 871–875. [Google Scholar] [CrossRef]
- Yuan, Yajun; Wang, Nan; Ou, Xueqing. Caution Should Be Exercised for the Detection of SARS-CoV-2, Especially in the Elderly. Journal of Medical Virology 2020, 92(9), 1641–1648. [Google Scholar] [CrossRef]
- Susetyowati, Susetyowati; Sholikhati, Amalia S.; Cahyaningrum, Dinda K.; Rachmawati, Azizah I.; Handaya, Adeodatus Y. Comparison of Tools for Nutritional Assessment and Screening of Hospitalized Patients: A Study on Surgical Patients. Medeniyet Medical Journal 2023, 38(1), 70–77. [Google Scholar] [CrossRef]
- Wakahara, Toshitatsu; Shiraki, Makoto; Murase, Kayoko; Fukushima, Hideki; Matsuura, Katsuhiko; Fukao, Ayumi; Kinoshita, Sachiko; et al. Nutritional Screening with Subjective Global Assessment Predicts Hospital Stay in Patients with Digestive Diseases. Nutrition 2007, 23(9), 634–639. [Google Scholar] [CrossRef]
- Poulia, Kalliopi-Anna; Yannakoulia, Mary; Karageorgou, Dimitra; Gamaletsou, Maria; Panagiotakos, Demosthenes B.; Sipsas, Nikolaos V.; Zampelas, Antonis. Evaluation of the Efficacy of Six Nutritional Screening Tools to Predict Malnutrition in the Elderly. Clinical Nutrition 2012, 31(3), 378–385. [Google Scholar] [CrossRef] [PubMed]
- Poulia, Kalliopi-Anna; Klek, Stanislaw; Doundoulakis, Ioannis; Bouras, Emmanouil; Karayiannis, Dimitrios; Baschali, Aristea; Passakiotou, Marili; Chourdakis, Michael. The Two Most Popular Malnutrition Screening Tools in the Light of the New ESPEN Consensus Definition of the Diagnostic Criteria for Malnutrition. Clinical Nutrition 2017, 36(4), 1130–1135. [Google Scholar] [CrossRef]
- Wolters, Maike; Volkert, Dorothee; Streicher, Melanie; Kiesswetter, Eva; Torbahn, Gabriel; O’Connor, Eibhlís M.; O’Keeffe, Mary; MaNuEL consortium. Prevalence of Malnutrition Using Harmonized Definitions in Older Adults from Different Settings—A MaNuEL Study. Clinical Nutrition 2019, 38(5), 2389–2398. [Google Scholar] [CrossRef]
- Reber, Emilie; Gomes, Filomena; Vasiloglou, Maria F.; Schuetz, Philipp; Stanga, Zeno. Nutritional Risk Screening and Assessment. Journal of Clinical Medicine 2019, 8(7), Article 1065. [Google Scholar] [CrossRef]
- Silva, David Franciole Oliveira; Lima, Severina Carla Vieira Cunha; Sena-Evangelista, Karine Cavalcanti Mauricio; Marchioni, Dirce Maria; Cobucci, Ricardo Ney; de Andrade, Fábia Barbosa. Nutritional Risk Screening Tools for Older Adults with COVID-19: A Systematic Review. Nutrients 2020, 12(10), Article 2956. [Google Scholar] [CrossRef] [PubMed]
- Wang, Hongyue; Wang, Bokai; Zhang, Xunan; Feng, Changyong. Relations among Sensitivity, Specificity and Predictive Values of Medical Tests Based on Biomarkers. General Psychiatry 2021, 34(2), e100453. [Google Scholar] [CrossRef]
- Bellanti, Francesco; Lo Buglio, Aurelio; Quiete, Stefano; Pellegrino, Giuseppe; Dobrakowski, Michał; Kasperczyk, Aleksandra; Kasperczyk, Sławomir; Vendemiale, Gianluigi. Comparison of Three Nutritional Screening Tools with the New GLIM Criteria for Malnutrition and Association with Sarcopenia in Hospitalized Older Patients. Journal of Clinical Medicine 2020, 9(6), Article 1898. [Google Scholar] [CrossRef] [PubMed]
- Leuenberger, Michèle; Kurmann, Silvia; Stanga, Zeno. Nutritional Screening Tools in Daily Clinical Practice: The Focus on Cancer. Supportive Care in Cancer 2010, 18 Suppl 2, 17–27. [Google Scholar] [CrossRef]
- Tu, Mei-Yu; Chien, Tsair-Wei; Chou, Ming-Ting. Using a Nutritional Screening Tool to Evaluate the Nutritional Status of Patients with Colorectal Cancer. Nutrition and Cancer 2012, 64(2), 323–330. [Google Scholar] [CrossRef] [PubMed]
- Demirel, Birsen; Atasoy, Beste M. Comparison of Three Nutritional Screening Tools to Predict Malnutrition Risk and Detect Distinctions between Tools in Cancer Patients Receiving Radiochemotherapy. Nutrition and Cancer 2018, 70(6), 867–873. [Google Scholar] [CrossRef]
- Kesari, A.; Noel, J. Y. Nutritional Assessment. In StatPearls; StatPearls Publishing: Treasure Island, FL, 2023; Available online: https://www.ncbi.nlm.nih.gov/books/NBK580496/ (accessed on 1 Jul 2025).
- NCBI. Bookshelf: ID: NBK580496. [PubMed]
- Wolters, Maike; Volkert, Dorothee; Streicher, Melanie; Kiesswetter, Eva; Torbahn, Gabriel; O’Connor, Eibhlís M.; O’Keeffe, Mary; et al. Prevalence of Malnutrition Using Harmonized Definitions in Older Adults from Different Settings—A MaNuEL Study. Clinical Nutrition 2019, 38(5), 2389–2398. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Aguilar, Regina; Malih, Narges; Abbate, Manuela; Fresneda, Sergio; Yañez, Aina; Bennasar-Veny, Miquel. Validity of Nutrition Screening Tools for Risk of Malnutrition among Hospitalized Adult Patients: A Systematic Review and Meta-analysis. Clinical Nutrition 2024, 43(5), 1094–1116. [Google Scholar] [CrossRef] [PubMed]
| Validity | High | Moderate | Low |
| Sensitivity Specificity Negative predictive value Agreement (Cohens kappa) |
≥90% ≥90% ≥90% ≥0.8 |
70%–89% 70%–89% 70%–89% 0.6–0.79 |
<70% <70% <70% <0.6 |
| Tool | Category | Proportions of normal and at-risk cases per facility | |||
| Overall, N=420 | KTRH, N=140 | KNH, N=140 | MTRH, N=140 | ||
| NRS-2002 | Normal At-risk |
60 (14.3) 360 (85.7) |
19 (13.6) 121 (86.4) |
20 (14.3) 120 (85.7) |
21 (15.0) 119 (85.0) |
| MUST | Normal At-risk |
206 (49) 214 (51) |
64 (45.7) 76 (54.3) |
71 (50.7) 69 (49.3) |
71 (50.7) 69 (49.3) |
| MST | Normal At-risk |
119 (28.3) 301 (71.7) |
54 (38.6) 86 (61.4) |
41 (29.3) 99 (70.7) |
24 (17.1) 116 (82.9) |
| BMI | Normal At-risk |
320 (76.2) 100 (23.8) |
109 (77.9) 31 (22.1) |
101 (72.1) 39 (29.9) |
108 (77.1) 32 (22.9) |
| Comparison | P-Value |
| NRS vs. MST NRS vs. MST MUST vs. MST |
<0.0001 <0.0001 <0.0001 |
| NRS-2002 | MUST | MST | |
| Se Sp NPV PPV AUC |
1.0000 (0.9645–1.0000) 0.1886 (0.1457–0.2317) 1.0000 (0.9404–1.0000) 0.2833 (0.2373–0.3329) 0.5943 (0.5728–0.6159) |
1.0000 (0.9645–1.0000) 0.6478 (0.5925–0.7003) 1.0000 (0.9823–1.0000) 0.4766 (0.5953–0.7003) 0.8239 (0.7976–0.8502) |
0.8725 (0.8078–0.9373) 0.3333 (0.2817–0.3881) 0.8907 (0.8347–0.9468) 0.2956 (0.2441–0.3472) 0.6029 (0.5613–0.6445) |
| Tool | k-statistics | P-value | Interpret |
| NRS-2002 MUST MST |
0.2115 (0.1061–0.3169) 0.2539 (0.2008–0.3069) 0.1254 (0.0290–0.1822) |
0.0011 <0.0001 <0.0001 |
low low low |
 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





