Submitted:
23 October 2025
Posted:
24 October 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Excipient Compatibility Studies
2.3. Long-Term ICH Stability Study
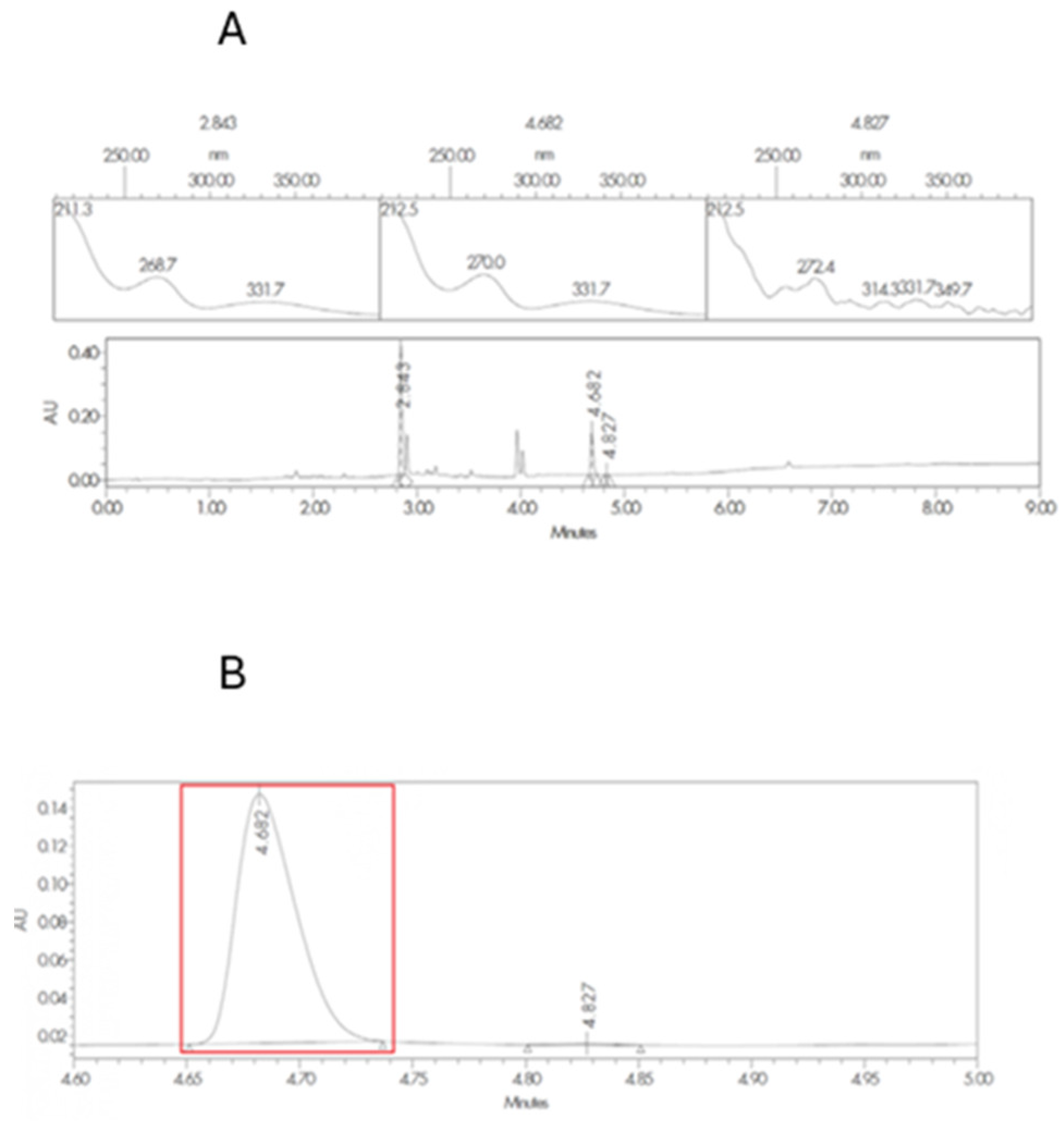
2.4. HPLC Quantification of SLD and Related Substances
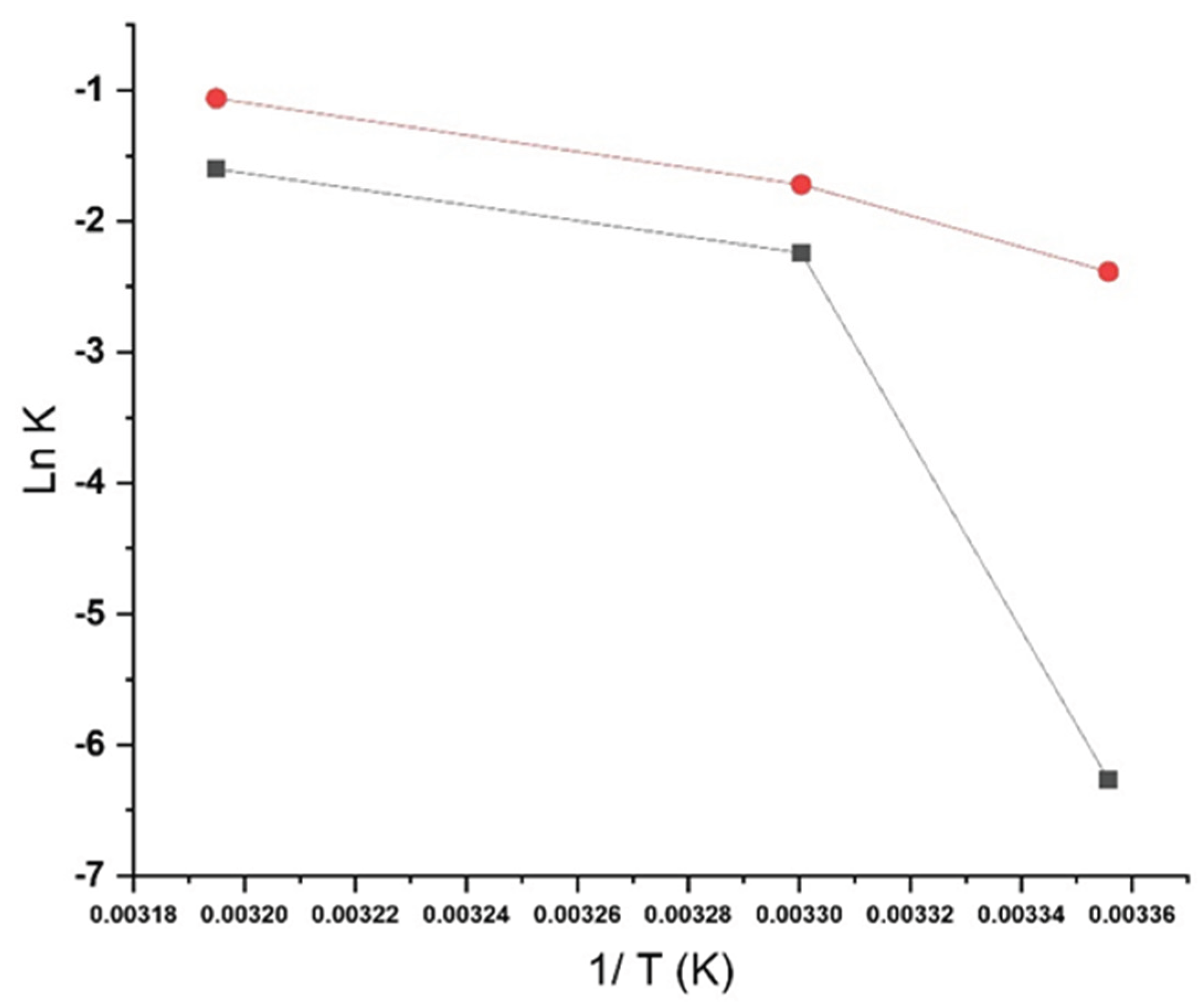
2.5. Stability Modeling
2.6. Permeability of Packing Materials
3. Results and Discussion
3.1. Excipient Compatibility Studies
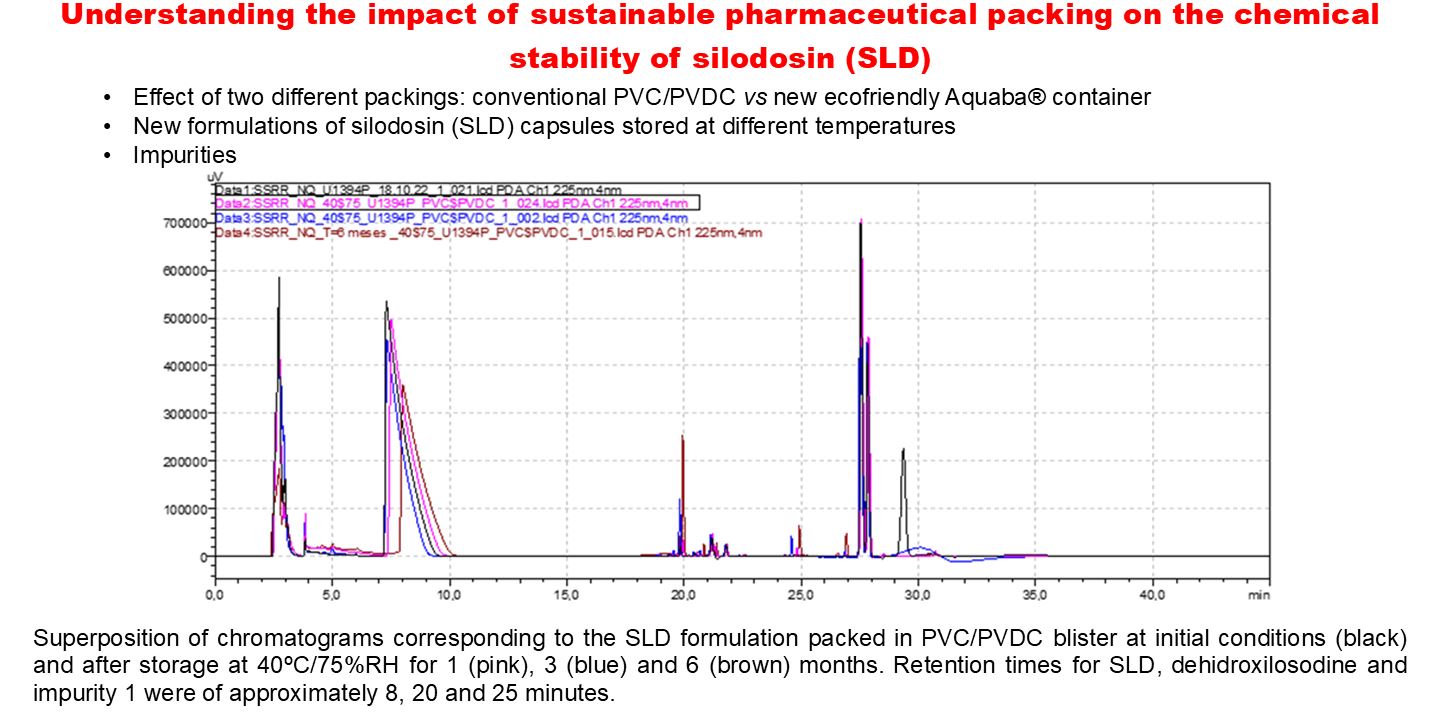
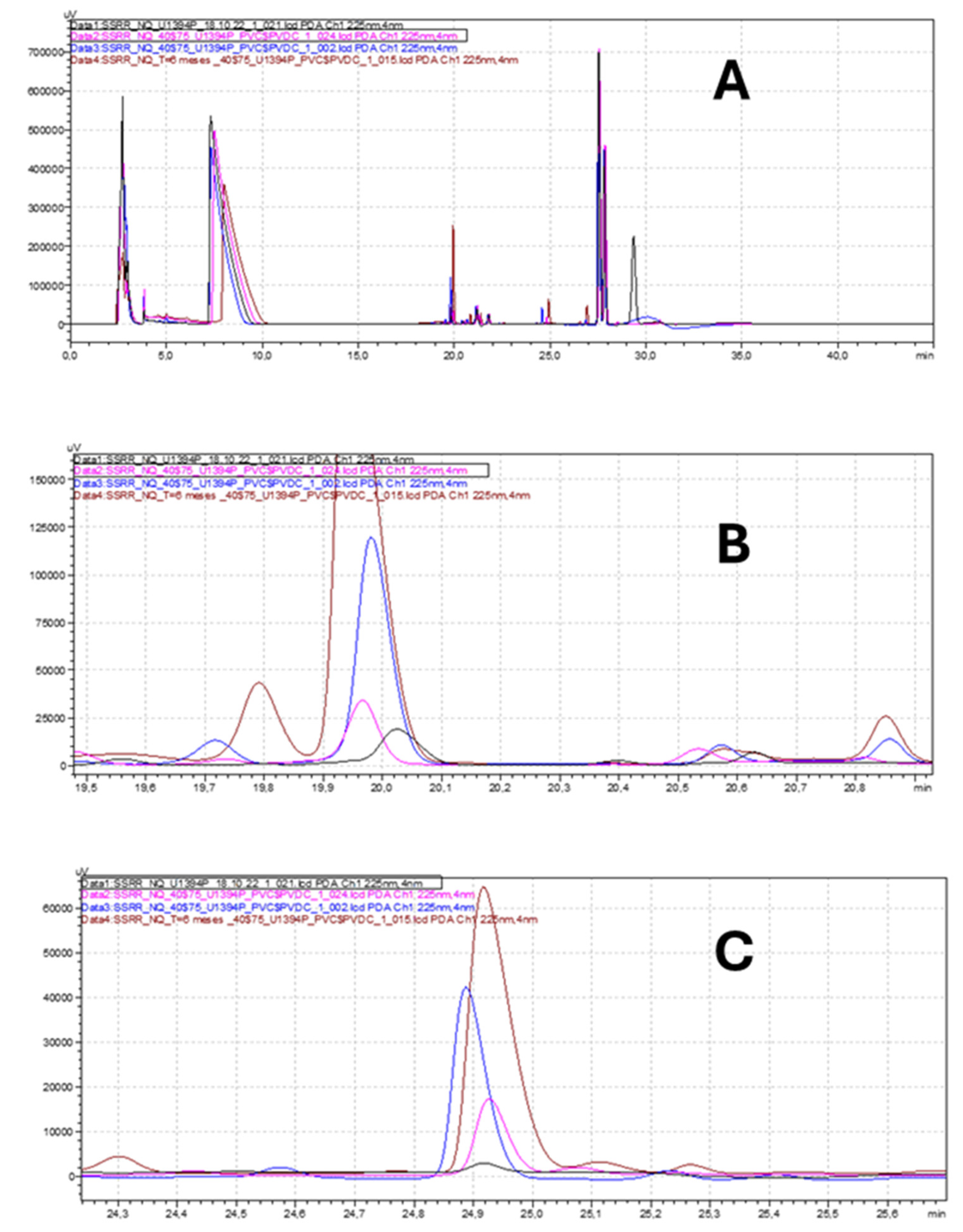
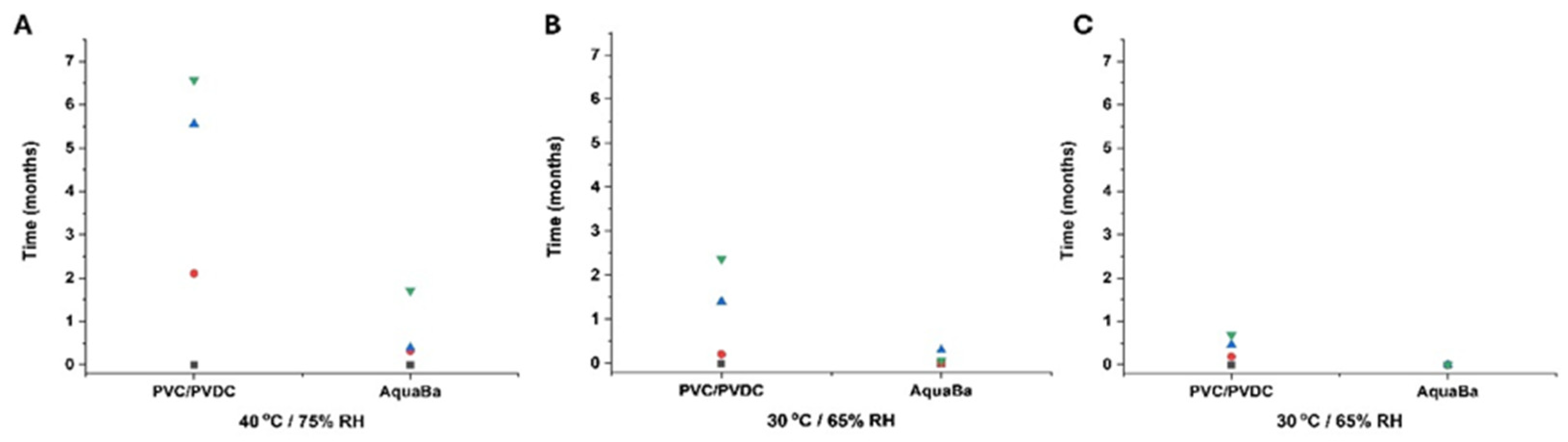
3.2. Long-Term ICH Stability Study
| Kinetic profile | 40 ºC / 75% RH | 30 ºC / 65% RH | 25 ºC / 60% RH | Average |
|---|---|---|---|---|
| Dehydrosilodosine | ||||
| Order 0 Order 1 order 2 Avrami Difussion |
0.85 1.00 0.99 0.96 0.90 |
0.87 0.88 0.87 0.75 0.85 |
0.87 0.88 0.87 0.75 0.80 |
0.86 0.92 0.91 0.82 0.85 |
|
Impurity 1 | ||||
| Order 0 Order 1 order 2 Avrami Difussion |
0.85 0.99 0.92 0.91 0.85 |
0.87 0.99 0.92 0.92 0.85 |
0.87 0.99 0.93 0.92 0.85 |
0.86 0.99 0.92 0.92 0.85 |
| Kinetic profile | 40 ºC / 75% RH | 30 ºC / 65% RH | 25 ºC / 60% RH | Average |
|---|---|---|---|---|
| Dehydrosilodosine | ||||
| Order 0 Order 1 order 2 Avrami Difussion |
0.98 0.98 0.99 0.99 0.64 |
0.84 0.96 0.92 0.86 0.72 |
* * * * * |
0.91 0.97 0.96 0.93 0.77 |
|
Impurity 1 | ||||
| Order 0 Order 1 order 2 Avrami Difussion |
0.91 0.99 0.92 0.91 0.85 |
0.9 0.99 0.93 0.92 0.85 |
0.91 0.99 0.93 0.92 0.85 |
0.91 0.99 0.93 0.92 0.85 |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Jindan, L., W. Xiao, and X. Liping, Evolving Role of Silodosin for the Treatment of Urological Disorders - A Narrative Review. Drug Des Devel Ther, 2022. 16: p. 2861-2884. [CrossRef]
- Gonzalez-Alvarez, I., et al., Exploring a Bioequivalence Failure for Silodosin Products Due to Disintegrant Excipients. Pharmaceutics, 2022. 14(12). [CrossRef]
- Naharros-Molinero, A., et al., Shell Formulation in Soft Gelatin Capsules: Design and Characterization. Adv Healthc Mater, 2024. 13(1): p. e2302250. [CrossRef]
- ICH Q8 (R2) Pharmaceutical Development. Reference code: EMA/CHMP/ICH/167068/2004 (2014). https://www.ema.europa.eu/en/ich-q8-r2-pharmaceutical-development-scientific-guideline. Accessed date: 15/03/2025.
- ICH: Q 1 A (R2): Stability testing of new drug substances and products. Reference code CPMP/ICH/2736/99 (2003). https://www.ema.europa.eu/en/ich-q1a-r2-stability-testing-new-drug-substances-drug-products-scientific-guideline. Accessed date: 15/03/2025.
- ICH: Q 3 A (R2): Impurities in new drug substances. Reference code: CPMP/ICH/2737/99 (2006). https://www.ema.europa.eu/en/ich-q3a-r2-impurities-new-drug-substances-scientific-guideline. Accessed daet: 15/03/2025.
- Zadbuke, N., et al., Recent trends and future of pharmaceutical packaging technology. J Pharm Bioallied Sci, 2013. 5(2): p. 98-110.
- AquaBa pahrmaceutical packing material. Available at: https://kaatimex.bg/wp-content/uploads/2015/12/Bilcare_Aquaba.pdf. Accessed date: 15/03/2025.
- Pharmaceutical Pakcing material PVC. Available at: https://www.rijerplastic.com/news/pharmaceutical-packaging-material-pvc/. Accessed date: 15/03/2025.
- Van Dooren, A.A., PVC as pharmaceutical packaging material. A literature survey with special emphasis on plasticized PVC bags. Pharm Weekbl Sci, 1991. 13(3): p. 109-18.
- Jaime, S., R. Alves, and P. Bocoli, Moisture and oxygen barrier properties of glass, PET and HDPE bottles for pharmaceutical products. Journal of Drug Delivery Science and Technology, 2022. 71( 103330).
- Pan, Z., et al., Water vapor transmission rate measurement for moisture barriers using infrared imaging. Materials Chemistry and Physics, 2023. 203(128289).
- WVTR of different packing materials. Available at: http://www.fortuneunion.net/materials-for-blister-packaging-film/. Accessed date: 16/03/2025.
- AquaBa packing material. Available at: https://kaatimex.bg/wp-content/uploads/2015/12/Bilcare_Aquaba.pdf. Accessed date: 15/03/2025.
- Serrano, D.R., et al., Designing Fast-Dissolving Orodispersible Films of Amphotericin B for Oropharyngeal Candidiasis. Pharmaceutics, 2019. 11(8).
- Serrano, D.R., et al., Optimising the in vitro and in vivo performance of oral cocrystal formulations via spray coating. Eur J Pharm Biopharm, 2018. 124: p. 13-27.
- Gonzalez-Gonzalez, O., et al., Application of Accelerated Predictive Stability Studies in Extemporaneously Compounded Formulations of Chlorhexidine to Assess the Shelf Life. Molecules, 2023. 28(23).
- Gonzalez-Gonzalez, O., et al., Drug Stability: ICH versus Accelerated Predictive Stability Studies. Pharmaceutics, 2022. 14(11).
- Fernandez-Garcia, R., et al., Targeted Oral Fixed-Dose Combination of Amphotericin B-Miltefosine for Visceral Leishmaniasis. Mol Pharm, 2025. 22(3): p. 1437-1448. [CrossRef]
- European Pharmacopoeia 11 edition. 2022.
- Vishnuvardhan, C., et al., LC-ESI-MS/MS evaluation of forced degradation behaviour of silodosin: In vitro anti cancer activity evaluation of silodosin and major degradation products. J Pharm Biomed Anal, 2017. 134: p. 1-10. [CrossRef]
- Pandeti, S., et al., Characterization of degradation products of silodosin under stress conditions by liquid chromatography/Fourier transform mass spectrometry. Rapid Commun Mass Spectrom, 2017. 31(6): p. 572-582. [CrossRef]
- Waterman, K.C. and R.C. Adami, Accelerated aging: prediction of chemical stability of pharmaceuticals. Int J Pharm, 2005. 293(1-2): p. 101-25. [CrossRef]
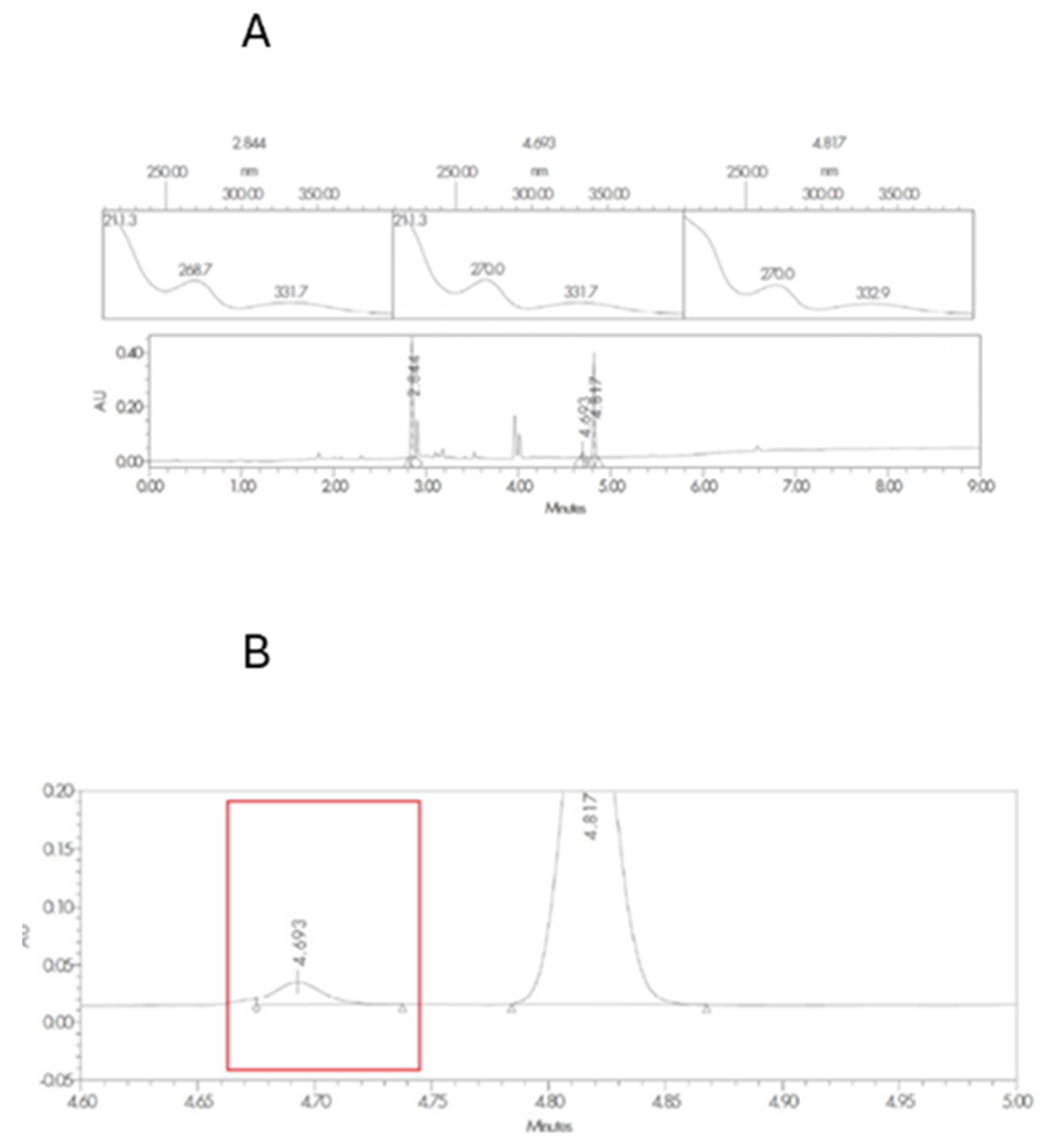
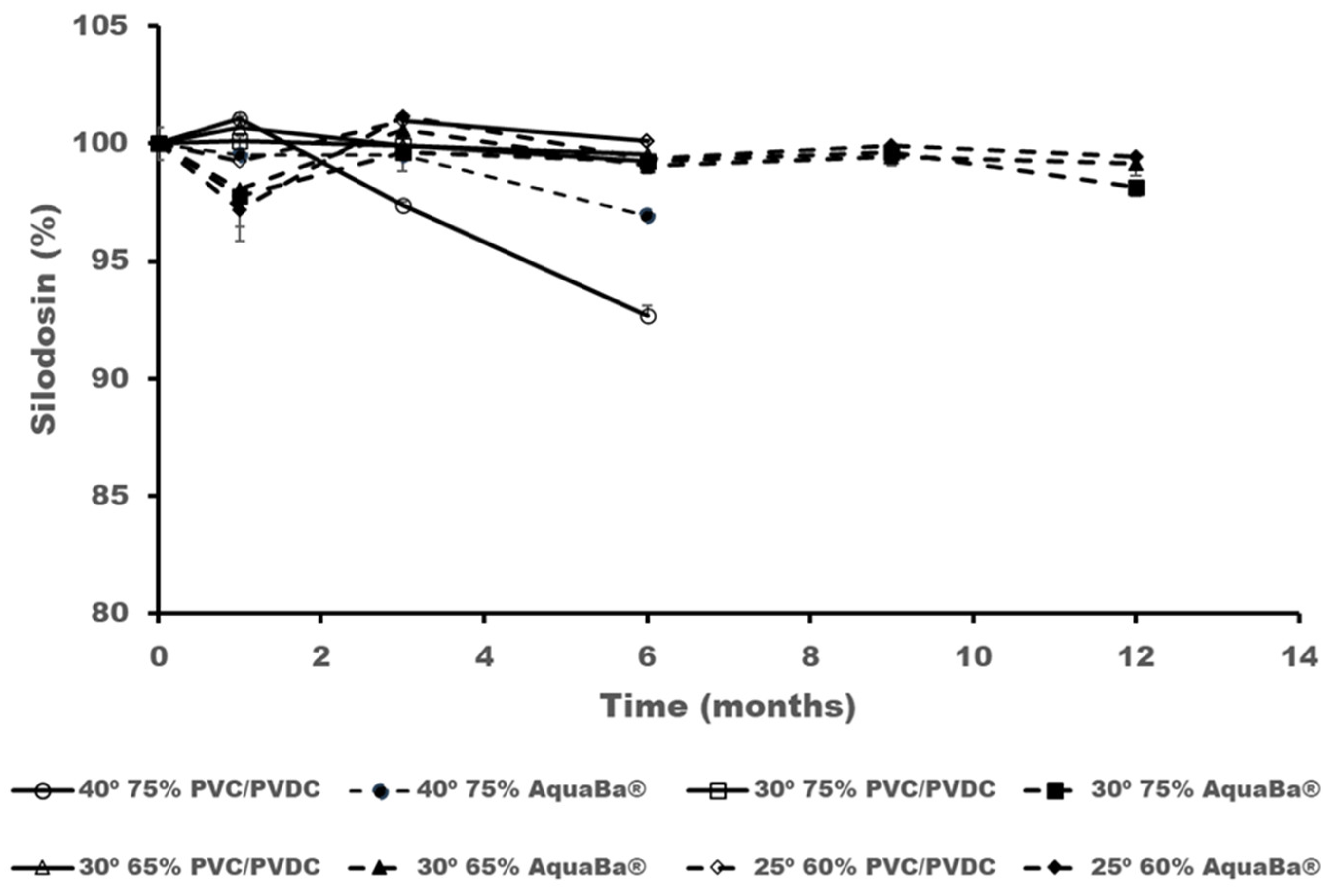
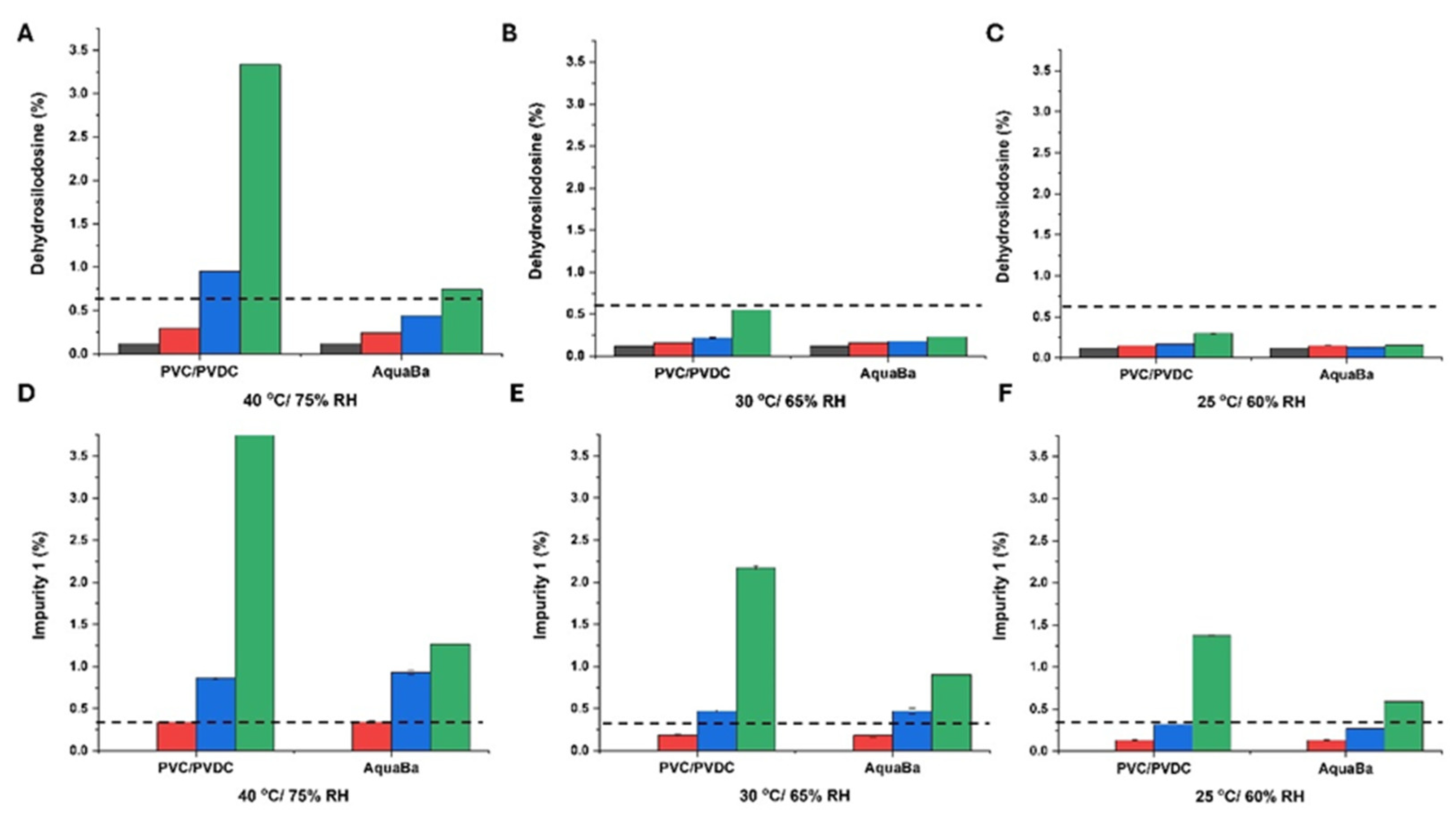
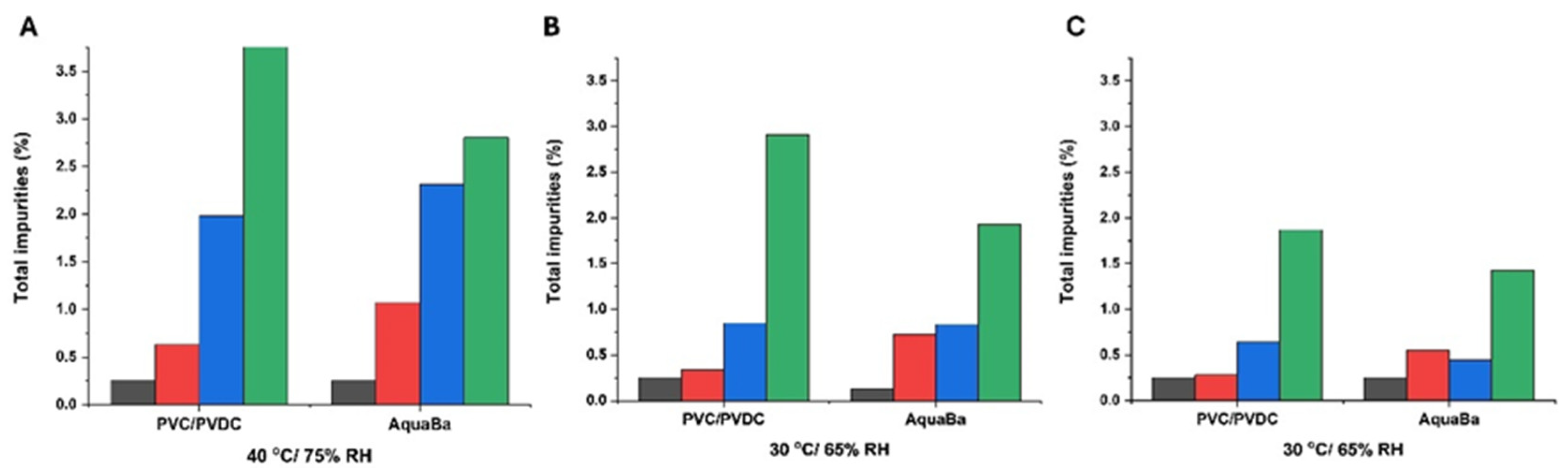
| Sample code | Component |
|---|---|
| 1 | SLD |
| 2 | Capryol 90 |
| 3 | Lauroyl macrogol-32 glycerides |
| 4 | BHT |
| 5 | SLD+Capryol 90 |
| 6 | SLD + Lauroyl macrogol-32 glycerides |
| 7 | SLD+BHT |
| 9 | SLD + Capryol 90+ Lauroyl macrogol-32 glycerides |
| 10 | SLD + Lauroyl macrogol-32 glycerides + BHT |
| 11 | Capryol 90 + Lauroyl macrogol-32 glycerides |
| 12 | Capryol 90 + Lauroyl macrogol-32 glycerides + BHT |
| Time (min) | % Mobile phase A | % Mobile phase B |
|---|---|---|
| 0.01 | 78 | 22 |
| 13.00 | 78 | 22 |
| 28.00 | 15 | 85 |
| 37.00 | 78 | 22 |
| 45.00 | 78 | 22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





