TOC
1. Introduction to modern drug delivery systems
2. Vesicular drug delivery systems
3. Nanoparticle-based drug delivery
4. Solid lipid systems
5. Innovative oral dosage forms
6. Controlled and sustained release technologies
1. Introduction to Modern Drug Delivery Systems
The ever-changing nature of pharmaceutical sciences shifted the emphasis of the conventional dosage forms to the new improved drug delivery systems which are aimed at maximizing therapeutic activity and reducing adverse effects. The goal of the modern drugs delivery is not only at transferring active agents in the systemic circulation, but also at targeting the right place of action, which enhances bioavailability and patient compliance [
1].
The development of targeted delivery strategies has become one of the key pillars of contemporary therapeutics since a rational design approach takes into account physiological barriers, disease pathology, and drug pharmacokinetics. The objective of targeting is to realize controlled localization of drugs, eliminate systemic exposure and an increase in therapeutic indices. A number of approaches including active targeting by use of ligands and passive targeting by carrier systems have been invented to address bio-limitation [
1].
Controlled drug delivery also serves as an addition to this strategy because it ensures steady-state plasma concentrations at extended intervals. These systems guarantee predictable pharmacological reactions by modulating release profiles and maximizing carrier interactions and reduce the dosing frequency and adverse effects [
2]. The combination of these targeting and control measures forms the basis of the present-day development of advanced nanocarrier and vesicular delivery technologies that prevail in the contemporary pharmaceutical market [
2].
2. Vesicular Drug Delivery Systems
One of the most diverse nanocarrier technologies that have been developed to improve bioavailability, stability, and controlled drug release is the vesicular drug delivery technology. These systems utilize lipid or surfactant bilayers to shield therapeutic agents, which is resistant to degradation and the delivery of therapeutic agents to a specific site is achieved by adjusting the composition and the size of vesicles [
3].
The idea of liposomes emerged as a result of the early experiment by Bangham et al., who proved the existence of phospholipid bi-layers which could entrap ions and molecules [
3]. Since this time, the liposomes have been widely categorized on the basis of size, lamellarity, and the mode of preparation into multilamellar, small unilamellar and large unilamellar vesicles. Currently, thin-film hydration, solvent injection, and microfluidic methods of preparation can be used to carefully regulate the characteristics of vesicles [
4].
The pharmaceutical uses of liposomes have been widely applied because of its biocompatibility and capability to accommodate both hydrophilic and lipophilic drugs. Their clinical performance in drugs like Doxil 2 is an indication of their future use in targeted cancer treatment and enhanced pharmacokinetics [
5]. The newer liposomal systems include surface modification with ligands or polymers to improve the circulation time and attain active targeting of a particular tissue [
6].
Besides liposomes, proniosomes and niosomes are also promising alternatives to liposomes, with better stability and cost-efficiency. Proniosomes specifically, are dry, free-flowing formulations that on hydration will form niosomes with the merits of vesicular encapsulation and the added storage stability [
7].
Moreover, the use of vesicles via naso-pulmonary routes has become popular in the treatment of respiratory as well as systemic diseases. Nanosystems based on lipid-vesicles that can be delivered via nasal or pulmonary routes may circumvent hepatic metabolism, deliver the drug as quickly as possible and result in localized deposition of the drug, which is ideal to treat respiratory diseases [
8].
Table 1.
Vesicular Drug Delivery Systems: Overview and Applications.
Table 1.
Vesicular Drug Delivery Systems: Overview and Applications.
| Aspect |
Description |
| Definition and Advantages |
Vesicular drug delivery systems employ lipid or surfactant bilayers to encapsulate therapeutic agents, enhancing bioavailability, stability, and controlled release while protecting drugs from degradation [3]. |
| Historical Background |
The concept of liposomes was pioneered by Bangham et al., demonstrating phospholipid bilayers capable of entrapping ions and molecules [3]. |
| Classification |
Liposomes are classified based on size, lamellarity, and preparation methods into multilamellar, small unilamellar, and large unilamellar vesicles. Modern preparation methods include thin-film hydration, solvent injection, and microfluidic approaches [4]. |
| Pharmaceutical Applications |
Liposomes carry both hydrophilic and lipophilic drugs. Clinically approved formulations like Doxil® demonstrate targeted cancer therapy and improved pharmacokinetics [5]. Advanced systems use surface modifications with ligands or polymers for prolonged circulation and active targeting [6]. |
| Non-Phospholipid Vesicles |
Proniosomes and niosomes offer improved stability and cost-effectiveness. Proniosomes are dry, free-flowing formulations that convert into niosomes upon hydration, combining vesicular encapsulation with enhanced storage stability [7]. |
| Naso-Pulmonary Applications |
Vesicular systems designed for nasal or pulmonary administration bypass hepatic metabolism, allow rapid absorption, and enable localized drug deposition, useful for treating respiratory and systemic disorders [8]. |
3. Nanoparticle-Based Drug Delivery
The nanoparticle-based drug delivery systems have transformed the pharmaceutics field since they have allowed the precise control of drug delivery, release and targeting. Nanoparticles, which are usually in the range of 10-1000 nm, can be made of polymers, lipids, or inorganic substances; and are also designed to enhance solubility, shield labile drugs and target specific action [
9]. Their small size that is in the nanoscale enables it to go through biological barriers with ease and interact with cellular constituents at the molecular scale, which provides a strong therapeutic benefit.
Nanoparticles based on polymers, i.e., polyethylene glycol, chitosan or polyethylene glycol are extensively studied in terms of controlled and sustained release formulations. These are biodegradable carriers that guarantee long-term stays and controllable kinetics of release. The use of inorganic nanoparticles such as gold and silica is used in imaging and targeted therapy particularly in oncology because these nanoparticles have special optical and magnetic features [
9]. New studies show that they are multifunctional, incorporating both diagnostic and therapeutic functionalities into a single platform [
10].
In spite of all these, oral delivery of nanoparticles containing proteins and peptides is a daunting task as it is affected by enzymatic degradation, low permeability, and gastrointestinal instabilities [
11]. The barriers have been circumvented by a number of formulation strategies such as mucoadhesive coatings, enzyme inhibitors, pH-sensitive carriers, etc. [
11].
Specific nanoparticles also maximize the therapeutic outcomes because, they target diseased tissues and reduce systemic toxicity. Active targeting is actively enabled by surface modification with ligands, antibodies or polymers and stimuli responsive nanoparticles can be used to release drugs in response to either pH, temperature or enzymatic signals. Such innovations are the beginning of more intelligent and responsive drug delivery systems and a transition to the personalized therapy and improved patient outcomes [
12].
Table 2.
Nanoparticle-Based Drug Delivery Systems.
Table 2.
Nanoparticle-Based Drug Delivery Systems.
| Aspect |
Description |
| Definition and Advantages |
Nanoparticle-based drug delivery systems enable precise control over drug distribution, release, and targeting. Their nanoscale dimensions (10–1000 nm) allow efficient navigation of biological barriers and molecular-level interactions, improving therapeutic outcomes [9]. |
| Composition |
Nanoparticles can be polymeric, lipid-based, or inorganic. Polymeric NPs include PLGA, chitosan, and PEG-based carriers for controlled and sustained release. Inorganic NPs, such as gold and silica, are employed for imaging and targeted therapy, especially in oncology [9]. |
| Multifunctionality |
Nanoparticles can integrate diagnostic and therapeutic functions in a single platform, supporting theranostic applications [10]. |
| Oral Delivery Challenges |
Oral delivery of proteins and peptides via nanoparticles is hindered by enzymatic degradation, poor permeability, and GI instability [11]. Strategies to overcome these include mucoadhesive coatings, enzyme inhibitors, and pH-sensitive carriers [11]. |
| Targeting and Stimuli-Responsiveness |
Surface modification with ligands, antibodies, or polymers enables active targeting to diseased tissues. Stimuli-responsive nanoparticles release drugs in response to pH, temperature, or enzymatic triggers, enhancing efficacy while minimizing systemic toxicity [12]. |
| Clinical Potential |
These systems represent intelligent, responsive drug delivery platforms, paving the way for personalized therapy and improved patient outcomes [12]. |
4. Solid Lipid Systems
Solid lipid based systems, including Solid Lipid Nanoparticles (SLNs) and Nanostructured Lipid carriers (NLCs) have become the new potential substitute of polymeric and liposomal carriers. These systems use lipids that are physiologically acceptable and can stay solid at room temperature and the body temperature so that they are very biocompatible and have controlled release properties [
13]. The major benefit of SLNs is that they are capable of trapping hydrophilic as well as lipophilic drugs, as well as protecting them against chemical breakdown and controlled release during prolonged periods of time [
13].
The solid lipids that make SLNs are usually glycerol monostearate, stearic acid, or tripalmitin which is stabilized by surfactants. They offer better drug stability, less burst release and better patient compliance. Nevertheless, the properties of the drug loading, potential gelation during storage and storage constraints have prompted the development of second-generation lipid carriers Nanostructured Lipid Carriers (NLCs) [
13].
NLCs are prepared through a mixture of solid and liquid lipids in small proportions to form an imperfect crystal framework improving drug loading and avoiding loss in storage due to expulsion. Such structural change leads to increased stability, entrapment effectiveness, and controlled release kinetics than SLNs [
13]. Moreover, the recent technological developments have perfected the preparation processes of hot homogenization, high-pressure emulsification, and solvent evaporation, which guarantee scalability and reproducibility of such lipid carriers [
14].
These lipid-based systems are also flexible and thus applicable in different routes of administration such as oral, topical and parenteral. They are also naturally compatible with biological membranes and can be surface modified with polymers or ligands thus amplifying their target effect increasing the targeting power of SLNs and NLCs as an important constituent in future drug delivery studies [
14].
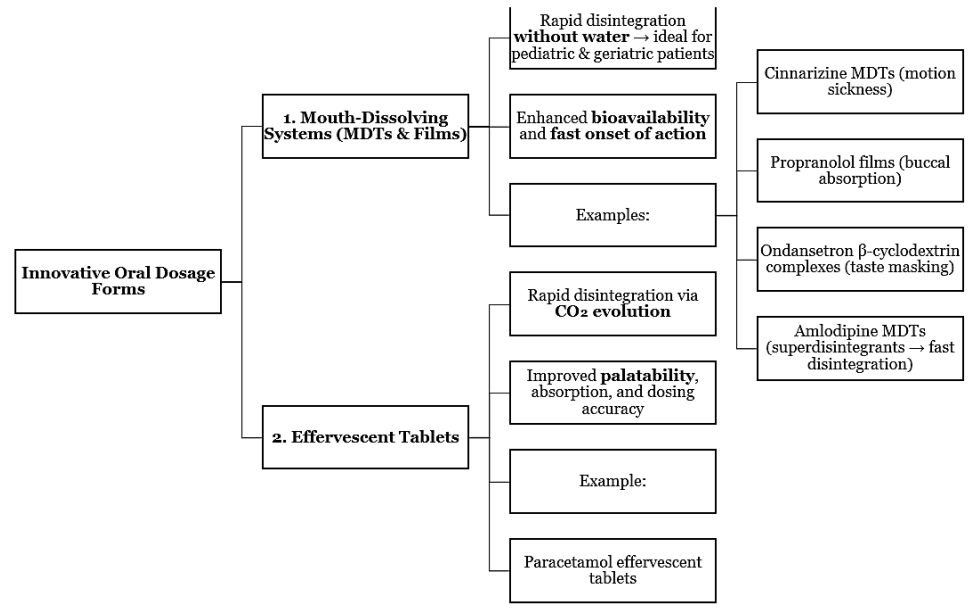
5. Innovative Oral Dosage Forms
The most preferred route of administration is oral drug delivery because it is easily administered, safe, and patients obey it. Nevertheless, novel dosage forms like oral dosages have emerged owing to the progress of formulation science to overcome drawbacks that are linked with the conventional tablets and capsules. Some of them are mouth--dissolving, effusible, chewable, and pulsatile systems, which are significant technological advancements that can increase bioavailability and patient acceptability [
15].
The oral cavity has received much attention over mouth-dissolving tablets (MDTs) which offer a perfect solution to geriatric and pediatric patients as they dissolve quickly without the use of water. Cinnarizine formulations with superdisintegrants showed better dissolution and quicker action onset and better therapeutic effect in motion sickness [
15,
16]. On the same note, propranol hydrochloride was developed by using mouth-dissolving films that registered high systemic absorption through buccal mucosa causing better bioavailability and patient convenience [
17]. Addition of 2-cyclodextrin complexes to ondansetron preparations improved solubility and taste masking which further aided patient compliance [
18]. MDTs based on Amlodipine have also been optimized to use different superdisintegrants resulting in a faster disintegration time as well as a steady profile of releasing [
19].
Effervescent pills like those that have been developed to be used in paracetamol have the benefit of both fast dissolution and better palatability due to the development of carbon dioxide on exposure to water. These formulations increase the rate of absorption, offer quick relief and dose precision in the pediatric and adult patients [
20].
Another innovative category that helps on-the-go delivery and increase compliance is chewable tablets, which are most effective in children and when treating older patients. Their development should be based on strict balancing of hardness, texture and taste so that they can be acceptable by the patients and still retain drug stability [
21].
Controlled oral drug delivery has been transformed through the use of pulsatile and multiparticulate systems which enable site-specific and time-dependent release. These systems have been used to produce low-density multiparticulates of meloxicam which have the ability to release drugs in sync with circadian rhythms and this has enhanced therapeutic effects of the drug in diseases where chronotherapy is required [
22]. In addition, microencapsulation and other similar methods have been incorporated in oral dosage delivery; thereby, attaining sustained release, active agent protection, and targeted delivery, which represents a major advancement in the use of oral pharmaceutical technology [
23].
6. Controlled and Sustained Release Technologies
Controlled and sustained release technologies have become an essential part of contemporary pharmaceutics that provide a high level of accuracy in the kinetics of drug release and the maintenance of therapeutic concentration during prolonged periods. The aim of these systems is to address the limitations associated with traditional dosage forms, including variable plasma concentrations and repeated dosage which will improve therapeutic effect and adherence to the drug [
24].
Here the basis of controlled release technology is to create matrices or carriers that can be used to control the rate of diffusion or degradation of drugs. The natural and synthetic polymers are important in these systems and allow a variety of release mechanisms such as diffusion, erosion and osmotic control. The release rates can be designed by adjusting the parameters of polymer type and formulations to obtain long-term therapeutic effects at minimal side effects [
24].
Recently, trends in the use of vesicular and nanoparticulate systems have greatly increased the range of controlled release. The liposomal vesicular carriers, e.g., have a dual purpose: firstly they increase bioavailability, and secondly they prolong the period of action of the drug since it is encased in lipid bilayers. The ability to create a tunable composition and a surface modification enables the targeting and controlled delivery of the drugs to a target tissue or cell [
25]. Such systems are being used more often in delivery of anticancer agents, peptides, and vaccines, where a controlled drug concentration is highly important to be effective.
A paradigm shift in the pharmaceutical formulation design is the introduction of controlled and sustained release technologies in association with novel advanced carriers, such as liposomes, nanoparticles, and polymeric matrices. These breakthroughs not only guarantee superior treatment processes, but also create new possibilities of customized and receptive drug delivery systems [
25].
Table 3.
Controlled and Sustained Release Technologies.
Table 3.
Controlled and Sustained Release Technologies.
| Aspect |
Description |
| Definition and Advantages |
Controlled and sustained release systems offer precise control over drug release kinetics and maintain therapeutic concentrations over extended periods, reducing dosing frequency and improving patient compliance [24]. |
| Mechanisms of Drug Release |
Release is modulated by diffusion, erosion, or osmotic control. Polymers (natural and synthetic) form matrices or carriers that regulate drug diffusion/degradation rates, enabling tailored sustained release [24]. |
| Role of Polymers |
Polymers are critical in controlling drug release profiles. Their composition, molecular weight, and crosslinking influence the rate and duration of drug release [24]. |
| Vesicular and Nanoparticulate Integration |
Liposomal carriers and nanoparticles enhance bioavailability while enabling prolonged drug action. Surface modifications and tunable compositions allow targeted and controlled delivery to specific tissues or cells [25]. |
| Applications |
Particularly useful for anticancer agents, peptides, and vaccines where maintaining therapeutic drug levels is essential for efficacy [25]. |
| Impact on Drug Delivery |
Integration of controlled release technologies with advanced carriers (liposomes, nanoparticles, polymeric matrices) enables personalized, responsive, and high-efficacy drug delivery platforms [25]. |
Conclusion
The development of the science of drug delivery through the traditional dosage delivery to the sophisticated controlled delivery depicts a spectacular fusion of chemistry, biology as well as material science. Specific delivery methods have enhanced the therapeutic accuracy and minimized systemic toxicity and enhanced drug bioavailability. Vesicular carriers including liposomes, niosomes and proniosomes have also provided flexible frameworks within which a wide range of therapeutic agents can be encapsulated, and nanocarriers, including polymeric, inorganic and lipid-based nanoparticles, have helped to redefine the concept of site-specific and sustained drug action. The use of solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs) has improved the stability of the formulation, loading efficiency of the drug and patient safety.
Simultaneously, new oral dose agents including mouth-dissolving, effervescent and chewable tablets have enhanced patient compliance and treatment flexibility. Implementation of pulsatile and multiparticulate systems is another example of the shift to physiological synchronization of the drug release. It has been on controlled and sustained release technologies, which have been backed by the use of microencapsulation and the use of polymeric design, that have become the pillars in supporting the maintenance of constant therapeutic levels and reduction of the dosing frequency.
All these innovations demonstrate how the pharmaceutical industry is changing towards being precise and patient-centric in therapy. The future of drug delivery is in the union of nanotechnology, biomaterials and personalized medicine, where smart, responsive and biocompatible systems will dominate the new era of clinical care. Further research and translation function will play a key role in ensuring that these technologies pass through the transition phase between the laboratory design and the general clinical use.
References
- Sengar, A. Targeting methods: A short review including rationale, goal, causes, strategies for targeting. International Journal of Research Publication and Reviews 2023, 4, 1379–1384. [Google Scholar]
- Vyas, S.P.; Khar, R.K. (2002). Controlled drug delivery: concepts and advances. Vallabh Prakashan.
- Bangham, A. D. , Standish, M. M., & Watkins, J. C. Diffusion of univalent ions across the lamellae of swollen phospholipids. Journal of Molecular Biology 1965, 13, 238–252. [Google Scholar] [PubMed]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y. ... & Nejati-Koshki, K. Liposome: classification, preparation, and applications. Nanoscale Research Letters 2013, 8, 1–9. [Google Scholar]
- Torchilin, V.P. Recent advances with liposomes as pharmaceutical carriers. Nature Reviews Drug Discovery 2005, 4, 145–160. [Google Scholar] [CrossRef]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: from concept to clinical applications. Advanced Drug Delivery Reviews 2013, 65, 36–48. [Google Scholar] [CrossRef]
- Sengar, A.; Saha, S.; Sharma, L.; Hemlata Saindane, P.S.; Sagar, S.D. Fundamentals of proniosomes: Structure & composition, and core principles. World Journal of Pharmaceutical Research 2024, 13, 1063–1071. [Google Scholar]
- Sengar, A.; Jagrati, K.; Khatri, S. Enhancing therapeutics: A comprehensive review on naso-pulmonary drug delivery systems for respiratory health management. World Journal of Pharmaceutical Research 2024, 13, 1112–1140. [Google Scholar]
- Kaur, G.; Mehta, S.K. Developments of nanoparticles for drug delivery: a review. Materials Science and Engineering: C 2017, 76, 1276–1285. [Google Scholar]
- Prajapati, R.N.; Jagrati, K.; Sengar, A.; Prajapati, S.K. Nanoparticles: Pioneering the future of drug delivery and beyond. World Journal of Pharmaceutical Research 2024, 13, 1243–1262. [Google Scholar]
- Zhu, Q.; Chen, Z.; Paul, P.K.; Wu, W. Oral delivery of proteins and peptides: challenges and strategies. Acta Pharmaceutica Sinica B 2021, 11, 2396–2412. [Google Scholar] [CrossRef]
- Sengar, A.; Tile, S.A.; Sen, A.; Malunjkar, S.P.; Bhagat, D.T.; Thete, A.K. Effervescent tablets explored: Dosage form benefits, formulation strategies, and methodological insights. World Journal of Pharmaceutical Research 2024, 13, 1424–1435. [Google Scholar]
- Müller, R.H.; Radtke, M.; Wissing, S.A. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Advanced Drug Delivery Reviews 2002, 54, S131–S155. [Google Scholar] [CrossRef] [PubMed]
- Has, C.; Sunthar, P. A comprehensive review on recent preparation techniques of liposomes. Journal of Liposome Research 2020, 30, 336–365. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.P.; Patel, M.M. Formulation and evaluation of mouth dissolving tablets of cinnarizine. Indian Journal of Pharmaceutical Sciences 2007, 69, 568–570. [Google Scholar] [CrossRef]
- Mishra, B.; Patel, B. Formulation and evaluation of mouth dissolving tablets of cinnarizine. Indian Journal of Pharmaceutical Sciences 2007, 69, 568–570. [Google Scholar]
- Sengar, A.; Yadav, S.; Niranjan, S.K. Formulation and evaluation of mouth-dissolving films of propranolol hydrochloride. World Journal of Pharmaceutical Research 2024, 13, 850–861. [Google Scholar]
- Yadav, A.V.; Mote, H.H. Development of mouth dissolving tablets of ondansetron hydrochloride with β-cyclodextrin inclusion complex. Journal of Pharmacy Research 2008, 1, 101–105. [Google Scholar]
- Kamboj, S.; Saroha, K.; Goel, M. Formulation and evaluation of mouth dissolving tablets of amlodipine besylate using different superdisintegrants. International Journal of Pharmaceutical Sciences and Research 2013, 4, 649–657. [Google Scholar]
- Kumar, R.; Singh, S. Formulation and evaluation of effervescent tablets of paracetamol. International Journal of Pharmaceutical Sciences and Research 2012, 3, 218–223. [Google Scholar]
- Sengar, A.; Vashisth, H.; Chatekar, V.K.; Gupta, B.; Thange, A.R.; Jillella, M.S.R.S.N. From concept to consumption: A comprehensive review of chewable tablets. World Journal of Pharmaceutical Research 2024, 13, 176–189. [Google Scholar]
- Sharma, S.; Pawar, A. Low-density multiparticulate system for pulsatile release of meloxicam. International Journal of Pharmaceutics 2006, 313, 150–158. [Google Scholar] [CrossRef]
- Singh, M.N.; Hemant, K.S.Y.; Ram, M.; Shivakumar, H.G. Microencapsulation: a promising technique for controlled drug delivery. Research in Pharmaceutical Sciences 2010, 5, 65–77. [Google Scholar]
- Gupta, A.; Mishra, A.K. Recent trends of fast dissolving tablets: an overview of formulation technology. International Journal of Pharmaceutical & Biological Archives 2011, 2, 1–10. [Google Scholar]
- Jagrati, K.M.; Sengar, A. Liposomal vesicular delivery system: An innovative nano carrier. World Journal of Pharmaceutical Research 2024, 13, 1155–1169. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).