Submitted:
21 October 2025
Posted:
22 October 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Cardioprotective Effects
2.1. Coronary Artery Protection
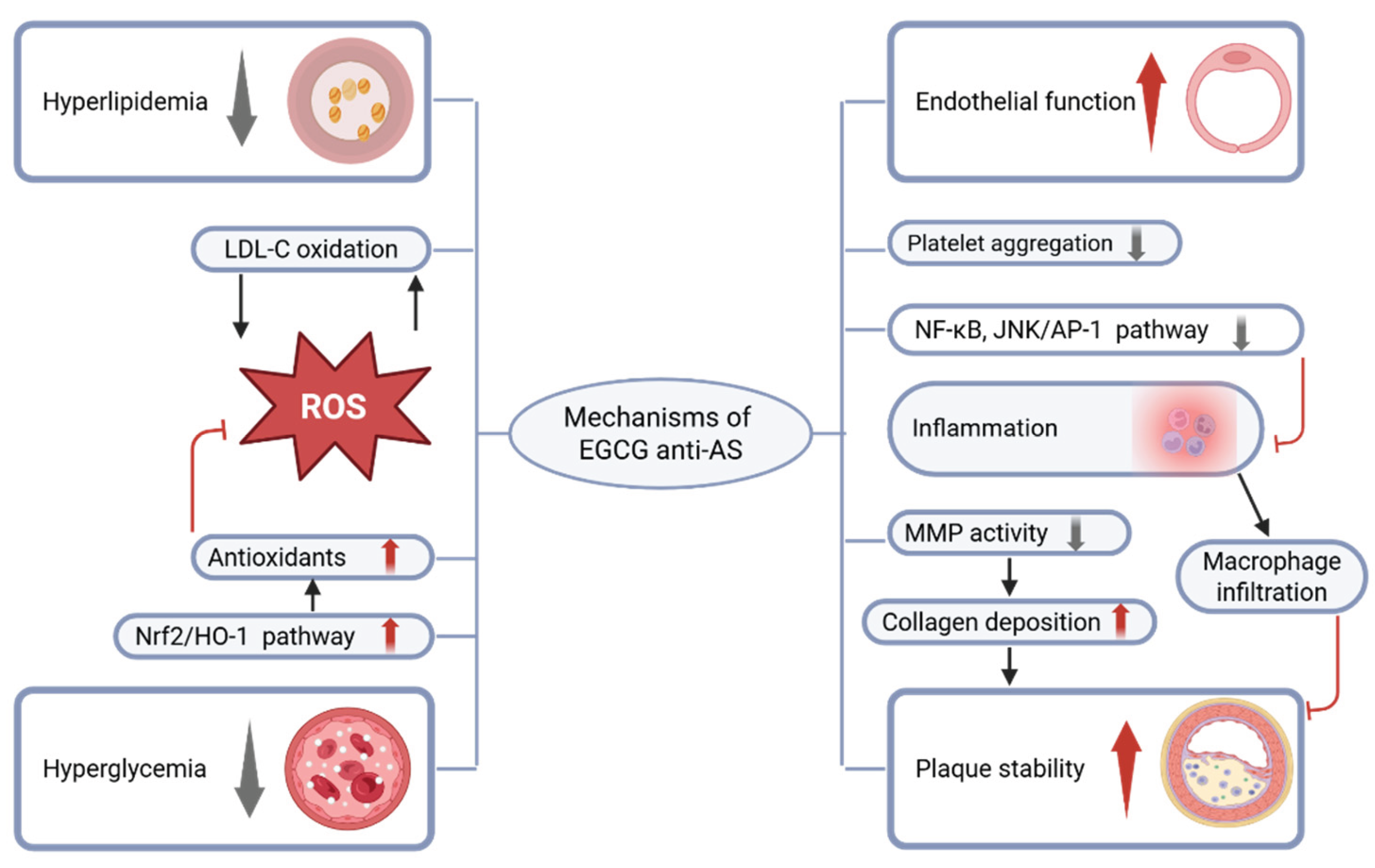
2.1.1. Improve Metabolic Disorders
| Study population | Dose | Effect | References |
|---|---|---|---|
| 88 obese male patients aged 40-65 years |
400 mg of EGCG (po, bid) for 8 weeks |
DBP ↓ NS between groups: HOMA-IR index, OGTT TG, TC, HDL-C, LDL-C |
[27] |
| 56 obese patients with hypertension |
379 mg of GTE (po, qd) for 3 months |
SBP, DBP ↓ HOMA-IR index, FBG ↓ TNF-α, CRP ↓ T-AOC ↑ TC, TG, LDL-C ↓ HDL-C ↑ |
[22] |
| 102 women with central obesity |
856.8mg of EGCG (po, qd) for 12 weeks |
TC, LDL-C ↓ | [23] |
| 30 obese patients | 150mg of EGCG (po, bid) for 8 weeks |
Serum kisspeptin, TG ↓ | [24] |
| 30 obese patients | 150mg of EGCG (po, bid) for 8 weeks |
SBP, DBP, MAP ↓ | [26] |
| 68 T2DM patients with obesity |
1,500 mg of GTE (po, qd) for 16 weeks (Supplement to routine medication) |
NS between groups: HOMA-IR index, FBG, HbA1c Within GTE group: Compared to baseline HbA1c ↓ |
[30] |
| 77 T2DM patients with LA |
500 mg of GTE (po, tid) for 16 weeks (Supplement to routine medication) |
NS between groups: HOMA-IR index, FBG, HbA1c TG, TC, HDL-C, LDL-C Within GTE group: Compared to baseline GLP-1 ↑ HOMA-IR ↓ |
[31] |
| 326 pregnant women diagnosed with GDM during third trimester |
500 mg of EGCG (po, qd) until full term |
Maternal diabetic parameters ↑ Cases of neonatal complications ↓ |
[34] |
| 20 T2DM patients | 400 mg of GTE (po, qd) for 12 weeks |
Improved arterial stiffness | [39] |
| 50 T2DM patients | 300 mg of EGCG (po, bid) for 2 months (Supplement to routine medication) |
MAP, DBP ↓ TC, TG, AIP ↓ T-AOC ↑ |
[33] |
| 120 South Indian male smokers |
100 ml of GT (po, tid) for 1 year |
Improved LA | [25] |
| 52 patients with early AS |
30 ml olive oil with 280 mg of EGCG (po, qd) for 4 months (Supplement to routine medication) |
Endothelial function ↑ | [40] |
| 42 CAD patients | 150 mg of EGCG (po, bid) for 2 weeks (Supplement to routine medication) |
Endothelial function ↑ | [41] |
| 19 patients with ATTR-CM |
GT and/or GTE (Exposure factors) for 1 year (Supplement to routine medication) |
Left ventricular mass ↓ TC, LDL-C ↓ |
[42] |
| 25 male patients with wtATTR-CM |
600 mg of EGCG (po, qd) for 1 year (Supplement to routine medication) |
Left ventricular mass ↓ Extracellular volume fraction ↓ TC ↓ |
[13,43] |
2.1.2. Alleviate Endothelial Dysfunction
| Injury Models | Dosage regimen | Results | References |
|---|---|---|---|
| Atherogenic diet for 45 days in male Wistar rats |
EGCG 100 mg/kg (ip, qd) for the last 14 days |
CRP, ESR ↓ | [44] |
| High-fat diet for 15 weeks P. gingivalis (iv, tiw) for 3 weeks in ApoE-deficient mice |
Drinking water with EGCG (0.2 g/L) for 7 weeks |
AS areas ↓ CRP, IL-8, MCP-1 ↓ HO-1 ↑ oxidized LDL-C ↓ |
[45] |
| High-fat diet for 30 days in Wistar rats |
EGCG 100 mg/kg (ip, qd) for 6/12 days |
TC, TG, LDL-C ↓ HDL-C ↑ Antioxidants ↑ Lipid peroxidation ↓ |
[46] |
| High-fat diet for 16 weeks in ApoE-deficient mice |
EGCG 10 mg/kg (ip, qd) for 16 weeks |
TNF-α, IL-6, MCP-1, INF-γ ↓ EMMPRIN, MMP-2, MMP-9 ↓ Plaque stability ↑ |
[48] |
| High-fat diet for 6 weeks in ApoE-deficient mice |
EGCG 10, 20, 40 mg/kg (po, qd) for 6 weeks |
TC, TG, LDL-C, ↓ HDL-C ↑ VEGFA, MMP-2 ↓ SOD, Nrf2/HO-1 pathway ↑ ROS ↓ |
[49] |
| T2DM in db/db mice |
Diet with EGCG (10 g/ kg) for 10 weeks |
FBG ↓ Plasma insulin ↑ Number of pancreatic islets ↑ |
[35] |
| NAM 100 mg/kg (ip) 20 min later STZ 55 mg/kg (ip) in male Wistar rats |
After induction of DM EGCG 2 mg/kg (po, qod) for 1 month |
HOMA-IR index, FBG, HbA1c ↓ TG, TC, LDL-C, VLDL-C ↓ HDL-C ↑ SOD, CAT, GSH ↑ ROS ↓ IL- 1β, IL-6, TNF-α, ICAM-1, VCAM-1 ↓ cTnT, CK-MB, LDH, AST ↓ Histopathological injury ↓ Apoptosis ↓ Fibrosis area ↓ |
[36] |
| STZ 65 mg/kg (ip) in male SD rats |
After induction of DM EGCG 10, 20, 40 mg/kg (po, qd) for 12 weeks |
FBG ↓ TG, TC, LDL-C ↓ HDL-C ↑ Fibrosis area, COL-I, COL-III ↓ |
[37] |
| Senium (24-26 months of age) in albino Wistar rats |
EGCG 200 mg/kg for 30 days |
Nrf2 ↑ ROS ↓ NF-κB ↓ TGFβ, TNFα ↓ Apoptosis ↓ COL ↓ |
[50] |
| AAC for 4 weeks in male SD rats |
EGCG 25, 50 mg/kg (po, qd) for 4 weeks |
NF-κB activation, CTGF ↓ Fibrosis area ↓ |
[51] |
| TAC for 4 weeks in male C57BL/6 mice |
EGCG 20, 40, 80 mg/kg (po, qd) for 4 weeks |
HW/BW, HW/TL, COL ↓ AKT/mTOR pathway ↓ |
[52] |
| COL (10 µg/mL) for 5 min with washed platelets from male SD rats |
Preincubated with 1, 5, 10, 30, 50 μM EGCG for 3 min |
Platelet aggregation ↓ | [53] |
| 100 nM Ang II for 24 h with CFs of adult rats |
Preincubated with 1, 10 μM EGCG for 1 h |
CFs proliferation ↓ NF-κB, CTGF ↓ COL-I, COL-III ↓ |
[51] |
| 10nM Ang II for 4h with CFs of adult rats |
At the same time EGCG 1, 10,100 μM for 4h |
JNK/AP-1 ↓ Endoglin ↓ CFs proliferation ↓ |
[54] |
| ADP 6.5 μM or COL 3.2μg/ml for 6 min with blood samples from people taking antiplatelet drugs |
EGCG 50, 100, 200 μM preincubated for 30 min |
Platelet aggregation ↓ | [55] |
| Human primary T cells incubated with P/I for 20 h |
EGCG 10, 20 μM preincubated for 4 h |
AP-1 binding activity ↓ IL-2, IL-4, INF-γ, TNF-α ↓ |
[47] |
| TGF-β2 10 ng/mL IL-1β 1 ng/mL for 24 h with HUVECs |
After injury EGCG 1, 5, 10 μM for 24h |
ROS ↓ NF-κB, SMAD pathways↓ RhoA ↓ Cell migration ↓ EndMT ↓ |
[56] |
| 10 mM β-GP and 3 mM CaCl2 with HASMCs |
EGCG 20, 30 μM | JunB ↓ Osteogenic differentiation ↓ Mineral deposition ↓ |
[57] |
2.1.3. Prevent Coronary Thrombosis
2.2. Inhibition of Adverse Cardiac Remodeling
2.2.1. Inhibit Collagen Deposition
2.2.2. Inhibit Amyloid Deposition
2.3. Prevention of Cardiomyocyte Injury
| Injury Models | Dosage regimen | Results | References |
|---|---|---|---|
| CPB Bypass-time for 90 min Reperfusion for 2 h in domestic piglets (10–15 kg) |
Before CPB EGCG 10 mg/kg (iv) After CPB EGCG 10 mg/kg (iv) |
CK ↓ Nitrosative and oxidative stress ↓ Inflammation ↓ Apoptosis ↓ |
[66] |
| ISO 100 mg/kg (sc, qd) for 2 days in male Wistar rats |
After induction of MI EGCG 10, 20, 30 mg/kg (po, qd) for 21 days |
LDL-C, VLDL-C ↓ HDL-C ↑ AIP ↓ GSH, VC, VE, CER ↑ SOD, CAT ↑ MDA ↓ Mitochondrial damage ↓ Lysosomal enzymes ↓ CK, CK-MB, LDH, AST, ALT ↓ Histopathological injury ↓ |
[67,68,69,70,71,72] |
| ISO 100 mg/kg (sc, qd) for 2 days in male Wistar rats |
Before induction of MI EGCG 15 mg/kg (ip, qd) for 7days |
HW, HW/BW ↓ TC, TG, LDL-C ↓ HDL-C ↑ SOD, CAT ↑ MDA ↓ TNF-α ↓ CK-MB, LDH, ALT, ALP, cTnT ↓ DNA damage, Apoptosis ↓ |
[101] |
| LADO for 30 min Reperfusion for 2 h in male SD rats |
5 min before reperfusion EGCG 10 mg/kg (iv) |
PI3K/AKT pathway ↑ p38, JNK ↓ Infarct size ↓ |
[73] |
| LADO for 30 min Reperfusion for 2 h in male Wistar rats |
5 min before reperfusion EGCG 10 mg/kg (iv) |
PI3K/AKT pathway ↑ Plasma mtDNA, TNF--α, IL--6, IL--8 ↓ Incidence of ventricular arrhythmia ↓ Infarct size ↓ |
[74] |
| LADO for 30 min Reperfusion for 12 h in SD rats |
30 min before ischemia EGCG 10 mg/kg (iv) |
PI3K/AKT pathway ↑ miR-384 ↑ Beclin-1, Excessive autophagy ↓ cTnI ↓ Infarct size ↓ |
[75] |
| LADO for 45 min Reperfusion for 3 h in male C57BL/6 mice |
Before injury EGCG 250 mg/kg (po, qd) for 10 days |
LncRNA Gm4419 ↓ ERK1/2 ↓ Excessive autophagy ↓ Apoptosis ↓ Histopathological injury ↓ Infarct size ↓ |
[76] |
| H2O2 or HRI with MEFs or CMs of neonatal mice |
Before injury EGCG 20, 30, 40 μM for 1-3 h |
Self-cleavage of OMA1 ↓ Proteolysis of OPA1 ↓ Mitochondrial function ↑ Mitochondrial morphology ↑ Apoptosis ↓ |
[77] |
| miR30a knockdown cells Hypoxia for 24 h |
Exosomes from EGCG-Treated CMs |
miR30a ↑ Cell viability ↑ |
[78] |
| H2O2 100 μM for 24 h with CMs of neonatal mice |
EGCG (The dose is unknown) |
LncRNA Gm4419 ↓ ERK1/2 ↓ Excessive autophagy ↓ Apoptosis ↓ Cell viability ↑ LDH ↓ |
[76] |
| HL-1 cells Hypoxia for 18 h |
Before hypoxia EGCG 5, 25 μM for 8h |
GSH, GPX4 ↑ ROS ↓ miR-450b-5p ↑ ACSL4, Ferroptosis ↓ Cell viability ↓ |
[79] |
| H9c2 cells in 30 mM glucose Hypoxia for 2 h Reoxygenation for 4 h |
Before injury EGCG 20 μM for 24 h |
SIRT1 ↑ Mn-SOD ↑ MDA ↓ Apoptosis ↓ Cell viability ↑ LDH ↓ |
[80] |
| H9c2 cells Hypoxia for 6 h Reoxygenation for 12h |
Before injury EGCG 6.25, 25 μM for 4 h |
miR30a ↑ p53 ↓ Apoptosis ↓ CK-MB, LDH ↓ Cell viability ↑ ATP ↑ |
[78] |
| H9c2 cells Hypoxia for 6 h Reoxygenation for 12h |
Before injury EGCG 25 μM for 4 h |
PI3K/AKT pathway ↑ miR-384 ↑ Beclin-1, Excessive autophagy ↓ cTnI ↓ Cell viability ↑ |
[75] |
| H9c2 cells Hypoxia for 6 h Reoxygenation for 12h |
Before injury EGCG 8 mg/L for 24 h |
ROS ↓ ATG4C ↑ Excessive autophagy ↓ ATP ↑ Apoptosis ↓ Cell viability ↑ |
[81] |
| HL-1 cells Hypoxia for 2, 4, 8, 12 h Reoxygenation for 24h |
Before injury 5, 10, 20, 40, 80, 100 μM of EGCG for 3h |
LncRNA MEG3 ↓ TAF15 in cytoplasm ↓ AIM2 mRNA stability ↓ Pyroptosis ↓ Cell death rate ↓ Cell viability ↑ |
[82] |
| H9c2 cells Hypoxia for 3 h Reoxygenation for 2 h |
Before injury EGCG 10 μM for 48 h |
ROS, MDA ↓ 14–3-3η↑ Excessive autophagy ↓ Ferroptosis, Apoptosis ↓ Cell viability ↑ LDH ↓ |
[83] |
2.3.1. Alleviate Oxidative Stress and Inflammatory Responses
2.3.2. Alleviate Mitochondrial Dysfunction
2.3.3. Activate the Protective PI3K/Akt Pathway
2.3.4. Inhibit Regulated Cell Death
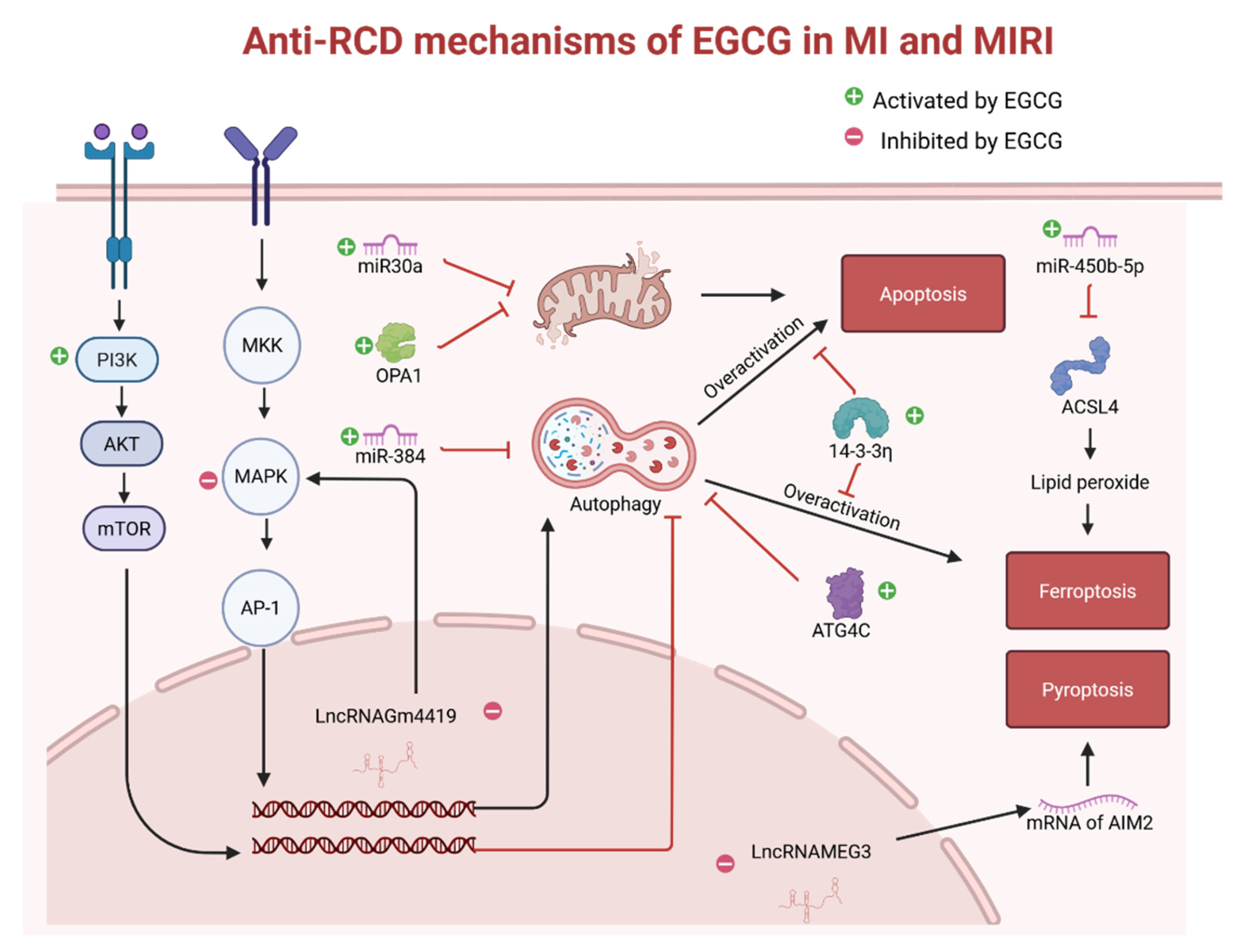
2.4. Preservation of Cardiac Function
| Injury Models | Dosage regimen | Results | References |
|---|---|---|---|
| Healthy male Wistar rats | 0.12 mg of EGCG (po, qd) for 28 days |
Mitochondrial function ↑ ATP ↑ Cardiomyocyte mechanics ↑ Calcium transient ↑ |
[89] |
| Transgenic mice (cTnI-R193H) |
EGCG 50 mg/kg (ip, qd) for 3 months |
Diastolic function ↑ | [85] |
| Senium C57BL/6 mice (16-18 months old) |
At the age of 16 months EGCG 50 mg/kg (ip, qd) for 8 weeks |
Diastolic function ↑ HDAC1, HDAC3 ↓ cTnI ↑ |
[86] |
| Mn-SOD-deficient mice | At the age of 8 week EGCG 10, 100mg/L in drinking water for 8 weeks |
Survival rate ↑ Cardiac dilatation ↓ Cardiac contraction ↑ Oxidative stress, Free fatty acids ↓ Telomerase activity ↓ Telomere length ↑ |
[87] |
| High-fat diet for 4 weeks STZ 30 mg/kg (ip) for 2 doses in 1 week in male SD rats |
After induction of DM EGCG 40, 80 mg/ kg (po, qd) for 8 weeks |
FBG ↓ CK-MB, cTnI ↓ Histopathological injury ↓ Autophagy, MMP2, MMP9 ↑ Fibrosis area, COL-I, COL-III ↓ LVSP, ±dp/dt max ↑ LVEDP ↓ |
[90] |
| TAC for 4 weeks in C57BL/6 mice |
EGCG 10 mg/kg (ip, qd) for 4 weeks |
Histopathological injury ↓ BNP ↓ Oxidative stress ↓ Inflammation ↓ Apoptosis ↓ LVEDD, LVESD ↓ LVEF ↑ TGF-β1/smad3 pathway ↓ COL-I, COL-III ↓ |
[14] |
| TAC for 12 weeks in C57BL/6 mice |
After TAC EGCG 50 mg/kg (ip, qd) for 12 weeks |
Preventive effect on HF SERCA2a ↑ |
[91] |
| AAC for 4 weeks in rats |
After AAC EGCG (25, 50, 100 mg/kg/day) for 4 weeks |
GRK2 ↓ β1-AR ↑ HW/BW, Posterior wall thickness ↓ LVSP, ±dp/dt max ↑ LVEDP ↓ Histopathological injury ↓ |
[92] |
| TAC for 12 weeks in mice |
EGCG 50 mg/kg (ip, qd) for 12 weeks |
HDAC5 ↓ Cardiac enlargement ↓ Cardiac function ↑ |
[93] |
| AAC for 16 weeks in male SD rats |
8 weeks after AAC EGCG 100 mg/kg (ip, qd) for 8 weeks |
Cardiac function ↑ Myocardial hypertrophy, fibrosis ↓ Mitochondrial function ↑ |
[94] |
| LADO for 12h in male SD rats |
2h before induction of MI EGCG 10 mg/kg (iv) |
miR30a levels ↑ CK-MB, cTnI ↓ Histopathological injury ↓ Excessive autophagy ↓ Apoptosis ↓ LVEF, LVSP, ±dp/dt max ↑ LVEDP ↓ |
[78] |
| LADO for 18h in C57BL/6 mice |
30 min before induction of MI EGCG 5, 10, 20 mg/kg (iv) |
SOD ↑ MDA ↓ miR-450b-5p ↑ ACSL4, Ferroptosis ↓ LVEDD, LVESD ↓ LVEF, FS ↑ |
[79] |
| LADO for 4 weeks in C57BL/6 mice |
After induction of MI EGCG 50 mg/kg (po, qd) for 4 weeks |
CK-MB, LDH ↓ Histopathological injury ↓ LncRNA MEG3 ↓ Pyroptosis ↓ Cell death rate ↓ Infract size ↓ LVEF ↑ |
[82] |
| LADO for 14 days in adult Wistar rats |
After induction of MI EGCG 50 mg/kg (po, qd) for 14 days |
Endoglin ↓ HW/BW, Fibrosis area ↓ LVEDD, LVESD ↓ MAP, FS ↑ |
[54] |
| LADO for 4 weeks in C57BL/6 mice |
After induction of MI EGCG 50 mg/kg (po, qd) for 1 week |
1 week after MI: Snail (EndMT marker) ↓ MMP-2, MMP-9 ↓ COL-I, COL-III ↓ 4 weeks after MI: Apoptosis ↓ Infract size ↓ Fibrosis area ↓ Capillary density ↑ LVEF ↑ |
[56] |
| LADO for 30 min Reperfusion for 2 h in SD rats with DM |
Before injury EGCG 100 mg/kg (po, qd) for 2 weeks |
SIRT1 ↑ Mn-SOD ↑ MDA ↓ LDH ↓ Apoptosis ↓ Infarct size ↓ Fibrosis area ↓ LVSP, ±dp/dt max ↑ |
[80] |
| LADO for 30 min Reperfusion for 2 h In male SD rats |
10 min before reperfusion EGCG 10 mg/kg (iv) |
PI3K/AKT pathway ↑ Excessive autophagy ↓ CK-MB, LDH ↓ Nitric oxide ↑ Apoptosis ↓ Infarct size ↓ LVSP, ±dp/dt max ↑ LVEDP ↓ |
[95] |
| LADO for 30 min Reperfusion for 12 h in SD rats |
30 min before ischemia EGCG 10, 20 mg/kg (iv) |
miR30a ↑ p53 ↓ Apoptosis ↓ CK-MB, LDH ↓ Histopathological injury ↓ ATP ↑ LVEF, LVSP, ±dp/dt max ↑ LVEDP ↓ |
[96] |
| LADO for 60 min Reperfusion for 2 h in C57BL/6 mice |
Before injury EGCG 20 mg/kg (po, qd) for 6 weeks |
MDA ↓ Ferroptosis ↓ CK-MB, LDH ↓ Histopathological injury ↓ Infarct size ↓ LVEF ↑ |
[83] |
| LIHPS for hearts of male Wistar rats |
EGCG 1, 4 μM in perfusate |
LVSP, ±dp/dt max ↑ | [97] |
| LIHPS for hearts of Chinchilla rabbits Cardioplegia for 90 min Reperfusion for 1 h |
At the same time of cardioplegia EGCG 20 μM in cardioplegic solutions for 90 min |
Nitrosative and oxidative stress ↓ Apoptosis ↓ ATP ↑ LVSP ↑ |
[98] |
| LIHPS for hearts of male SHR Ischemia for 30 min Reperfusion for 2 h |
Before injury EGCG 200 mg/kg (po, qd) for 3 weeks |
Coronary flow ↑ Infarct size ↓ LVDP ↑ LVEDP ↓ |
[88] |
| LIHPS for hearts of guinea pigs Ischemia for 40 min Reperfusion for 40 min |
4 min before injury EGCG 30 μM in perfusate |
Mitochondrial Ca2+ elevation ↓ Apoptosis ↓ ATP ↑ LVEDP ↓ |
[99] |
| LIHPS for hearts of male Wistar rats Ischemia for 30 min Reperfusion for 2 h |
10 min before ischemia EGCG 1, 10 μM in perfusate for 40 min |
Infarct size ↓ LVDP, ±dp/dt max NS Mitochondrial KATP activity ↑ |
[100] |
| LIHPS for hearts of male Wistar rats Ischemia for 30 min Reperfusion for 2 h |
5 min before reperfusion EGCG 1, 10 μM in perfusate for 35 min |
Infarct size ↓ LVDP, ±dp/dt max ↑ |
[101] |
| LIHPS for hearts of male SD rats Ischemia for 20 min Reperfusion for 2 h |
10 min before injury EGCG 5 μM in perfusate for 130 min |
Mn-SOD, Cu/Zn-SOD ↑ Lipid peroxides ↓ Apoptosis ↓ Infarct size ↓ LVDP, ±dp/dt max ↑ LVEDP ↓ |
[102] |
| CMs of adult rats |
EGCG 2.5, 5 μM | Calcium transient ↑ FS ↑ |
[97] |
| CMs of C57BL/6 mice |
EGCG 10nM-100 μM | Calcium transient ↑ | [103] |
| Human cTn subunits with cTnT-Δ160E mutation |
EGCG 3 μM | Bind to the C-lobe of cTnC Binding of cTnI to cTnC ↑ Ca2+ sensitivity in myofilaments ↓ |
[104] |
| CMs of transgenic mice (cTnI-R193H) |
EGCG 5 μM | Ca2+ decay, Sarcomere relaxation ↑ | [85] |
| cTnT with mutations associated with HCM |
EGCG 100 μM | Restore the coupling between Ca2+ and cTnT |
[105] |
| Reconstituted TF with cTnC-G34S or cTnI-D127Y mutations |
EGCG 20 μM | Aggregation and elongation of TF ↑ Maximal myosin-S1-ATPase activity ↑ Ca2+ sensitivity in myofilaments ↓ |
[106] |
2.4.1. Better Cardiac Structure
2.4.2. More Cardiomyocyte Survival
2.4.3. Better Cardiomyocyte Function
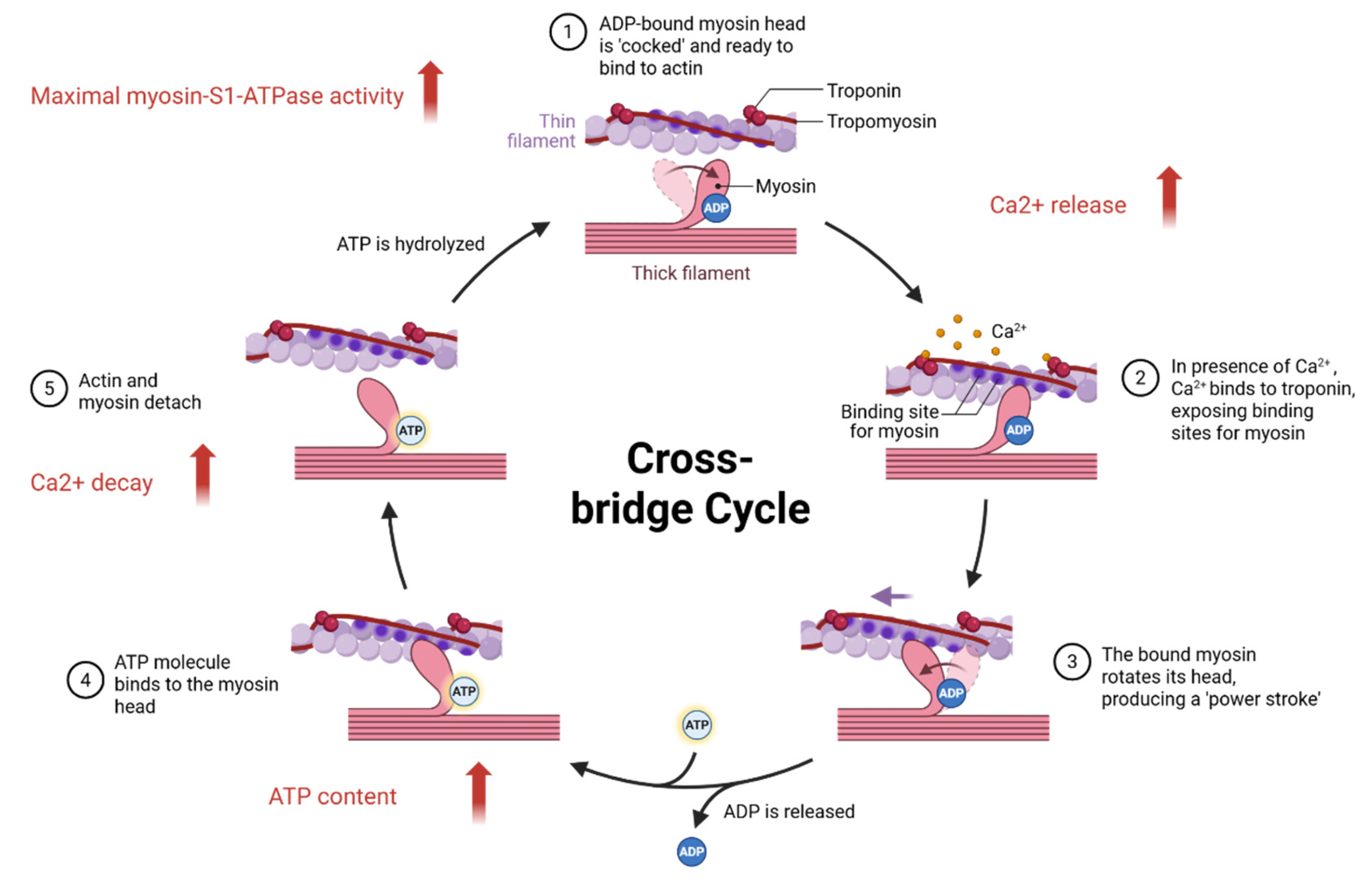
3. Adverse Reactions of Epigallocatechin Gallate
4. Conclusions and Future Perspectives
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| GT | Green tea |
| GTE | Green tea extract |
| EGCG | Epigallocatechin gallate |
| MS | Metabolic syndrome |
| T2DM | Type 2 diabetes mellitus |
| GDM | Gestational diabetes mellitus |
| DCM | Diabetic cardiomyopathy |
| ATTR-CM | Amyloidotic transthyretin cardiomyopathy |
| wtATTR-CM | wild-type transthyretin amyloid cardiomyopathy |
| AS | Atherosclerosis |
| CAD | Coronary artery disease |
| MI | Myocardial infarction |
| MIRI | Myocardial ischemia-reperfusion injury |
| MINS | Myocardial injury after non-cardiac surgery |
| RCM | Restrictive cardiomyopathy |
| HCM | Hypertrophic cardiomyopathy |
| HF | Heart failure |
| ApoE | Apolipoprotein E |
| SD | Sprague-Dawley |
| SHR | Spontaneously hypertensive rats |
| HUVECs | Human umbilical vein endothelial cells |
| HASMCs | Human aortic smooth muscle cells |
| MEFs | Mouse embryonic fibroblasts |
| CMs | Cardiomyocytes |
| CFs | Cardiac fibroblasts |
| TF | Thin filament |
| P. gingivalis | Porphyromonas gingivalis |
| NAM | Nicotinamide |
| STZ | Streptozotocin |
| ISO | Isoprenaline |
| AAC | Abdominal aortic constriction |
| TAC | Transverse aortic constriction |
| LADO | Left anterior descending artery occlusion |
| LMCAO | Left main coronary artery occlusion |
| LIHPS | Langendorff isolated heart perfusion system |
| CPB | Cardiopulmonary bypass |
| Ang II | Angiotensin II |
| P/I | Phorbol 12-myristate 13-acetate and ionomycin |
| β-GP | β-Glucopyranosyl phosphate |
| HRI | Hypoxia-reoxygenation injury |
| LA | Lipid abnormality |
| TC | Total cholesterol |
| TG | Triglyceride |
| LDL-C | Low-density lipoprotein cholesterol |
| VLDL-C | Very low-density lipoprotein cholesterol |
| HDL-C | High-density lipoprotein cholesterol |
| AIP | Atherogenic index of plasma |
| HOMA-IR | Homeostasis model assessment of insulin resistance |
| OGTT | Oral glucose tolerance test |
| FBG | Fasting blood glucose |
| HbA1C | Hemoglobin A1C |
| GLP-1 | Glucagon-like peptide 1 |
| ROS | Reactive oxygen species |
| SOD | Superoxide dismutase |
| Mn-SOD | Manganese superoxide dismutase |
| CAT | Catalase |
| GPX4 | Glutathione peroxidase 4 |
| GSH | Glutathione |
| VC | Vitamin C |
| VE | Vitamin E |
| CER | Ceruloplasmin |
| T-AOC | Total antioxidant capacity |
| MDA | Malondialdehyde |
| CRP | C-reactive protein |
| ESR | Erythrocyte sedimentation rate |
| MPO | Myeloperoxidase |
| MCP-1 | Monocyte chemoattractant protein-1 |
| VEGFA | Vascular endothelial growth factor |
| ICAM-1 | Intercellular adhesion molecule-1 |
| VCAM-1 | Vascular cell adhesion molecule-1 |
| CTGF | Connective tissue growth factor |
| EMMPRIN | Extracellular matrix metalloproteinase inducer |
| MMPs | Matrix metalloproteinases |
| EndMT | Endothelial-to-mesenchymal transition |
| COL | Collagen |
| HW | Heart weight |
| BW | Body weight |
| TL | Tibia length |
| SIRT1 | Silent information regulator 1 |
| mtDNA | Mitochondrial DNA |
| SERCA2a | Sarcoplasmic reticulum Ca-ATPase |
| β1-AR | β1-adrenoceptors |
| cTn | Cardiac troponin |
| cTnC | Cardiac troponin C |
| cTnT | Cardiac troponin T |
| cTnI | Cardiac troponin I |
| CK | Creatine kinase |
| CK-MB | Creatine kinase-MB |
| LDH | Lactate dehydrogenase |
| ALP | Alkaline phosphatase |
| AST | Aspartate aminotransferase |
| ALT | Alanine transaminase |
| RCD | Regulated cell death |
| CO | Cardiac output |
| SAP | Systolic arterial pressure |
| MAP | Mean arterial pressure |
| DAP | Diastolic arterial pressure |
| LVEF | Left ventricular ejection fraction |
| LVEDD | Left ventricular end-diastolic dimension |
| LVESD | Left ventricular end-systolic dimension |
| LVSP | Left ventricular systolic pressure |
| LVEDP | Left ventricular end-diastolic pressure |
| LVDP | Left ventricular developed pressure |
| ±dp/dt max | Maximal left ventricular pressure variation rate |
| FS | Fractional shortening |
| TXA2 | Thromboxane A2 |
| ATG4C | Autophagy related 4C |
| HDAC | Histone deacetylase |
| KATP | ATP-sensitive potassium channels |
| NS | Not Significant |
References
- Saklayen, M. G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef]
- Al Hroob, A. M.; Abukhalil, M. H.; Hussein, O. E.; Mahmoud, A. M. Pathophysiological Mechanisms of Diabetic Cardiomyopathy and the Therapeutic Potential of Epigallocatechin-3-Gallate. Biomed. Pharmacother. Biomedecine Pharmacother. 2019, 109, 2155–2172. [Google Scholar] [CrossRef]
- Salari, N.; Morddarvanjoghi, F.; Abdolmaleki, A.; Rasoulpoor, S.; Khaleghi, A. A.; Hezarkhani, L. A.; Shohaimi, S.; Mohammadi, M. The Global Prevalence of Myocardial Infarction: A Systematic Review and Meta-Analysis. BMC Cardiovasc. Disord. 2023, 23, 206. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, S.; Li, H.; Su, F.; Huang, Y.; Mi, W.; Chinese Anaesthesiology Department Tracking Collaboration Group. Anaesthesiology in China: A Cross-Sectional Survey of the Current Status of Anaesthesiology Departments. Lancet Reg. Health West. Pac. 2021, 12, 100166. [Google Scholar] [CrossRef] [PubMed]
- Bello, C.; Rössler, J.; Shehata, P.; Smilowitz, N. R.; Ruetzler, K. Perioperative Strategies to Reduce Risk of Myocardial Injury after Non-Cardiac Surgery (MINS): A Narrative Review. J. Clin. Anesth. 2023, 87, 111106. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A. G.; Zotchev, S. B.; Dirsch, V. M.; International Natural Product Sciences Taskforce; Supuran, C. T. Natural Products in Drug Discovery: Advances and Opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Brody, H. Tea. Nature 2019, 566, S1. [Google Scholar] [CrossRef]
- Abudureheman, B.; Yu, X.; Fang, D.; Zhang, H. Enzymatic Oxidation of Tea Catechins and Its Mechanism. Mol. Basel Switz. 2022, 27, 942. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Mukhtar, H. Tea Polyphenols in Promotion of Human Health. Nutrients 2018, 11, 39. [Google Scholar] [CrossRef]
- Ntamo, Y.; Jack, B.; Ziqubu, K.; Mazibuko-Mbeje, S. E.; Nkambule, B. B.; Nyambuya, T. M.; Mabhida, S. E.; Hanser, S.; Orlando, P.; Tiano, L.; Dludla, P. V. Epigallocatechin Gallate as a Nutraceutical to Potentially Target the Metabolic Syndrome: Novel Insights into Therapeutic Effects beyond Its Antioxidant and Anti-Inflammatory Properties. Crit. Rev. Food Sci. Nutr. 2024, 64, 87–109. [Google Scholar] [CrossRef]
- Wang, L.; Tang, C.; Pan, Q. Mechanisms Underlying the Anti-Atherosclerotic Effects of EGCG. Curr. Mol. Med. 2025. [Google Scholar] [CrossRef]
- Wei, X.-Y.; Zeng, Y.-F.; Guo, Q.-H.; Liu, J.-J.; Yin, N.; Liu, Y.; Zeng, W.-J. Cardioprotective Effect of Epigallocatechin Gallate in Myocardial Ischemia/Reperfusion Injury and Myocardial Infarction: A Meta-Analysis in Preclinical Animal Studies. Sci. Rep. 2023, 13, 14050. [Google Scholar] [CrossRef]
- aus dem Siepen, F.; Bauer, R.; Aurich, M.; Buss, S. J.; Steen, H.; Altland, K.; Katus, H. A.; Kristen, A. V. Green Tea Extract as a Treatment for Patients with Wild-Type Transthyretin Amyloidosis: An Observational Study. Drug Des. Devel. Ther. 2015, 9, 6319–6325. [Google Scholar] [CrossRef]
- Chen, K.; Chen, W.; Liu, S. L.; Wu, T. S.; Yu, K. F.; Qi, J.; Wang, Y.; Yao, H.; Huang, X. Y.; Han, Y.; Hou, P. Epigallocatechingallate Attenuates Myocardial Injury in a Mouse Model of Heart Failure through TGF--β1/Smad3 Signaling Pathway. Mol. Med. Rep. 2018, 17, 7652–7660. [Google Scholar] [CrossRef]
- Fan, J.; Watanabe, T. Atherosclerosis: Known and Unknown. Pathol. Int. 2022, 72, 151–160. [Google Scholar] [CrossRef]
- Wang, R.-X.; Zhou, M.; Ma, H.-L.; Qiao, Y.-B.; Li, Q.-S. The Role of Chronic Inflammation in Various Diseases and Anti-Inflammatory Therapies Containing Natural Products. ChemMedChem 2021, 16, 1576–1592. [Google Scholar] [CrossRef]
- Reddy, P.; Lent-Schochet, D.; Ramakrishnan, N.; McLaughlin, M.; Jialal, I. Metabolic Syndrome Is an Inflammatory Disorder: A Conspiracy between Adipose Tissue and Phagocytes. Clin. Chim. Acta Int. J. Clin. Chem. 2019, 496, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liu, J.; Duan, H.; Li, R.; Peng, W.; Wu, C. Activation of Nrf2/HO-1 Signaling: An Important Molecular Mechanism of Herbal Medicine in the Treatment of Atherosclerosis via the Protection of Vascular Endothelial Cells from Oxidative Stress. J. Adv. Res. 2021, 34, 43–63. [Google Scholar] [CrossRef]
- Yamagata, K. Protective Effect of Epigallocatechin Gallate on Endothelial Disorders in Atherosclerosis. J. Cardiovasc. Pharmacol. 2020, 75, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J. L. Metalloproteinases in Atherosclerosis. Eur. J. Pharmacol. 2017, 816, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Ohishi, T.; Tanabe, H.; Miyoshi, N.; Nakamura, Y. Anti-Inflammatory Effects of Dietary Polyphenols through Inhibitory Activity against Metalloproteinases. Mol. Basel Switz. 2023, 28, 5426. [Google Scholar] [CrossRef]
- Bogdanski, P.; Suliburska, J.; Szulinska, M.; Stepien, M.; Pupek-Musialik, D.; Jablecka, A. Green Tea Extract Reduces Blood Pressure, Inflammatory Biomarkers, and Oxidative Stress and Improves Parameters Associated with Insulin Resistance in Obese, Hypertensive Patients. Nutr. Res. N. Y. N 2012, 32, 421–427. [Google Scholar] [CrossRef]
- Chen, I.-J.; Liu, C.-Y.; Chiu, J.-P.; Hsu, C.-H. Therapeutic Effect of High-Dose Green Tea Extract on Weight Reduction: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Clin. Nutr. Edinb. Scotl. 2016, 35, 592–599. [Google Scholar] [CrossRef]
- Chatree, S.; Sitticharoon, C.; Maikaew, P.; Pongwattanapakin, K.; Keadkraichaiwat, I.; Churintaraphan, M.; Sripong, C.; Sririwichitchai, R.; Tapechum, S. Epigallocatechin Gallate Decreases Plasma Triglyceride, Blood Pressure, and Serum Kisspeptin in Obese Human Subjects. Exp. Biol. Med. Maywood NJ 2021, 246, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Kanu, V. R.; Pulakuntla, S.; Kuruvalli, G.; Aramgam, S. L.; Marthadu, S. B.; Pannuru, P.; Hebbani, A. V.; Desai, P. P. D.; Badri, K. R.; Vaddi, D. R. Anti-Atherogenic Role of Green Tea (Camellia Sinensis) in South Indian Smokers. J. Ethnopharmacol. 2024, 332, 118298. [Google Scholar] [CrossRef]
- Wilasrusmee, K. T.; Sitticharoon, C.; Keadkraichaiwat, I.; Maikaew, P.; Pongwattanapakin, K.; Chatree, S.; Sririwichitchai, R.; Churintaraphan, M. Epigallocatechin Gallate Enhances Sympathetic Heart Rate Variability and Decreases Blood Pressure in Obese Subjects: A Randomized Control Trial. Sci. Rep. 2024, 14, 21628. [Google Scholar] [CrossRef]
- Brown, A. L.; Lane, J.; Coverly, J.; Stocks, J.; Jackson, S.; Stephen, A.; Bluck, L.; Coward, A.; Hendrickx, H. Effects of Dietary Supplementation with the Green Tea Polyphenol Epigallocatechin-3-Gallate on Insulin Resistance and Associated Metabolic Risk Factors: Randomized Controlled Trial. Br. J. Nutr. 2009, 101, 886–894. [Google Scholar] [CrossRef]
- Momose, Y.; Maeda-Yamamoto, M.; Nabetani, H. Systematic Review of Green Tea Epigallocatechin Gallate in Reducing Low-Density Lipoprotein Cholesterol Levels of Humans. Int. J. Food Sci. Nutr. 2016, 67, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Asbaghi, O.; Fouladvand, F.; Moradi, S.; Ashtary-Larky, D.; Choghakhori, R.; Abbasnezhad, A. Effect of Green Tea Extract on Lipid Profile in Patients with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-H.; Liao, Y.-L.; Lin, S.-C.; Tsai, T.-H.; Huang, C.-J.; Chou, P. Does Supplementation with Green Tea Extract Improve Insulin Resistance in Obese Type 2 Diabetics? A Randomized, Double-Blind, and Placebo-Controlled Clinical Trial. Altern. Med. Rev. J. Clin. Ther. 2011, 16, 157–163. [Google Scholar]
- Liu, C.-Y.; Huang, C.-J.; Huang, L.-H.; Chen, I.-J.; Chiu, J.-P.; Hsu, C.-H. Effects of Green Tea Extract on Insulin Resistance and Glucagon-like Peptide 1 in Patients with Type 2 Diabetes and Lipid Abnormalities: A Randomized, Double-Blinded, and Placebo-Controlled Trial. PloS One 2014, 9, e91163. [Google Scholar] [CrossRef]
- Fryk, E.; Olausson, J.; Mossberg, K.; Strindberg, L.; Schmelz, M.; Brogren, H.; Gan, L.-M.; Piazza, S.; Provenzani, A.; Becattini, B.; Lind, L.; Solinas, G.; Jansson, P.-A. Hyperinsulinemia and Insulin Resistance in the Obese May Develop as Part of a Homeostatic Response to Elevated Free Fatty Acids: A Mechanistic Case-Control and a Population-Based Cohort Study. EBioMedicine 2021, 65, 103264. [Google Scholar] [CrossRef]
- Bazyar, H.; Hosseini, S. A.; Saradar, S.; Mombaini, D.; Allivand, M.; Labibzadeh, M.; Alipour, M. Effects of Epigallocatechin-3-Gallate of Camellia Sinensis Leaves on Blood Pressure, Lipid Profile, Atherogenic Index of Plasma and Some Inflammatory and Antioxidant Markers in Type 2 Diabetes Mellitus Patients: A Clinical Trial. J. Complement. Integr. Med. 2020, 18, 405–411. [Google Scholar] [CrossRef]
- Zhang, H.; Su, S.; Yu, X.; Li, Y. Dietary Epigallocatechin 3-Gallate Supplement Improves Maternal and Neonatal Treatment Outcome of Gestational Diabetes Mellitus: A Double-Blind Randomised Controlled Trial. J. Hum. Nutr. Diet. Off. J. Br. Diet. Assoc. 2017, 30, 753–758. [Google Scholar] [CrossRef]
- Ortsäter, H.; Grankvist, N.; Wolfram, S.; Kuehn, N.; Sjöholm, A. Diet Supplementation with Green Tea Extract Epigallocatechin Gallate Prevents Progression to Glucose Intolerance in Db/Db Mice. Nutr. Metab. 2012, 9, 11. [Google Scholar] [CrossRef]
- Othman, A. I.; El-Sawi, M. R.; El-Missiry, M. A.; Abukhalil, M. H. Epigallocatechin-3-Gallate Protects against Diabetic Cardiomyopathy through Modulating the Cardiometabolic Risk Factors, Oxidative Stress, Inflammation, Cell Death and Fibrosis in Streptozotocin-Nicotinamide-Induced Diabetic Rats. Biomed. Pharmacother. Biomedecine Pharmacother. 2017, 94, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Gui, L.; Wang, F.; Hu, X.; Liu, X.; Yang, H.; Cai, Z.; Qi, M.; Dai, C. Epigallocatechin Gallate Protects Diabetes Mellitus Rats Complicated with Cardiomyopathy through TGF-Β1/JNK Signaling Pathway. Curr. Pharm. Des. 2022, 28, 2758–2770. [Google Scholar] [CrossRef] [PubMed]
- Martin, M. A.; Goya, L.; Ramos, S. Protective Effects of Tea, Red Wine and Cocoa in Diabetes. Evidences from Human Studies. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2017, 109 Pt 1, 302–314. [Google Scholar] [CrossRef]
- Quezada-Fernández, P.; Trujillo-Quiros, J.; Pascoe-González, S.; Trujillo-Rangel, W. A.; Cardona-Müller, D.; Ramos-Becerra, C. G.; Barocio-Pantoja, M.; Rodríguez-de la Cerda, M.; Nérida Sánchez-Rodríguez, E.; Cardona-Muñóz, E. G.; García-Benavides, L.; Grover-Páez, F. Effect of Green Tea Extract on Arterial Stiffness, Lipid Profile and sRAGE in Patients with Type 2 Diabetes Mellitus: A Randomised, Double-Blind, Placebo-Controlled Trial. Int. J. Food Sci. Nutr. 2019, 70, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Widmer, R. J.; Freund, M. A.; Flammer, A. J.; Sexton, J.; Lennon, R.; Romani, A.; Mulinacci, N.; Vinceri, F. F.; Lerman, L. O.; Lerman, A. Beneficial Effects of Polyphenol-Rich Olive Oil in Patients with Early Atherosclerosis. Eur. J. Nutr. 2013, 52, 1223–1231. [Google Scholar] [CrossRef]
- Widlansky, M. E.; Hamburg, N. M.; Anter, E.; Holbrook, M.; Kahn, D. F.; Elliott, J. G.; Keaney, J. F.; Vita, J. A. Acute EGCG Supplementation Reverses Endothelial Dysfunction in Patients with Coronary Artery Disease. J. Am. Coll. Nutr. 2007, 26, 95–102. [Google Scholar] [CrossRef]
- Kristen, A. V.; Lehrke, S.; Buss, S.; Mereles, D.; Steen, H.; Ehlermann, P.; Hardt, S.; Giannitsis, E.; Schreiner, R.; Haberkorn, U.; Schnabel, P. A.; Linke, R. P.; Röcken, C.; Wanker, E. E.; Dengler, T. J.; Altland, K.; Katus, H. A. Green Tea Halts Progression of Cardiac Transthyretin Amyloidosis: An Observational Report. Clin. Res. Cardiol. Off. J. Ger. Card. Soc. 2012, 101, 805–813. [Google Scholar] [CrossRef] [PubMed]
- aus dem Siepen, F.; Buss, S. J.; Andre, F.; Seitz, S.; Giannitsis, E.; Steen, H.; Katus, H. A.; Kristen, A. V. Extracellular Remodeling in Patients with Wild-Type Amyloidosis Consuming Epigallocatechin-3-Gallate: Preliminary Results of T1 Mapping by Cardiac Magnetic Resonance Imaging in a Small Single Center Study. Clin. Res. Cardiol. Off. J. Ger. Card. Soc. 2015, 104, 640–647. [Google Scholar] [CrossRef]
- Ramesh, E.; Geraldine, P.; Thomas, P. A. Regulatory Effect of Epigallocatechin Gallate on the Expression of C-Reactive Protein and Other Inflammatory Markers in an Experimental Model of Atherosclerosis. Chem. Biol. Interact. 2010, 183, 125–132. [Google Scholar] [CrossRef]
- Cai, Y.; Kurita-Ochiai, T.; Hashizume, T.; Yamamoto, M. Green Tea Epigallocatechin-3-Gallate Attenuates Porphyromonas Gingivalis-Induced Atherosclerosis. Pathog. Dis. 2013, 67, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Pan, J.; Zhou, X. Amelioration of Lipid Profile and Level of Antioxidant Activities by Epigallocatechin-Gallate in a Rat Model of Atherogenesis. Heart Lung Circ. 2014, 23, 1194–1201. [Google Scholar] [CrossRef]
- Huang, S.-C.; Kao, Y.-H.; Shih, S.-F.; Tsai, M.-C.; Lin, C.-S.; Chen, L. W.; Chuang, Y.-P.; Tsui, P.-F.; Ho, L.-J.; Lai, J.-H.; Chen, S.-J. Epigallocatechin-3-Gallate Exhibits Immunomodulatory Effects in Human Primary T Cells. Biochem. Biophys. Res. Commun. 2021, 550, 70–76. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, J.; Li, Y.; Shi, H.; Wang, H.; Chen, B.; Wang, F.; Wang, Z.; Yang, Z.; Wang, L. Green Tea Polyphenol Epigallocatechin-3-Gallate Increases Atherosclerotic Plaque Stability in Apolipoprotein E-Deficient Mice Fed a High-Fat Diet. Kardiol. Pol. 2018, 76, 1263–1270. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Chu, Y.; Ren, H.; Pang, X. Antioxidation Function of EGCG by Activating Nrf2/HO-1 Pathway in Mice with Coronary Heart Disease. Contrast Media Mol. Imaging 2022, 2022, 8639139. [Google Scholar] [CrossRef] [PubMed]
- Muhammed, I.; Sankar, S.; Govindaraj, S. Ameliorative Effect of Epigallocatechin Gallate on Cardiac Hypertrophy and Fibrosis in Aged Rats. J. Cardiovasc. Pharmacol. 2018, 71, 65–75. [Google Scholar] [CrossRef]
- Cai, Y.; Yu, S.-S.; Chen, T.-T.; Gao, S.; Geng, B.; Yu, Y.; Ye, J.-T.; Liu, P.-Q. EGCG Inhibits CTGF Expression via Blocking NF-κB Activation in Cardiac Fibroblast. Phytomedicine Int. J. Phytother. Phytopharm. 2013, 20, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Wang, Y.; Liu, G. Epigallocatechin Gallate (EGCG) Attenuates Myocardial Hypertrophy and Fibrosis Induced by Transverse Aortic Constriction via Inhibiting the Akt/mTOR Pathway. Pharm. Biol. 2021, 59, 1305–1313. [Google Scholar] [CrossRef]
- Ok, W.-J.; Cho, H.-J.; Kim, H.-H.; Lee, D.-H.; Kang, H.-Y.; Kwon, H.-W.; Rhee, M. H.; Kim, M.; Park, H.-J. Epigallocatechin-3-Gallate Has an Anti-Platelet Effect in a Cyclic AMP-Dependent Manner. J. Atheroscler. Thromb. 2012, 19, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-M.; Chang, H.; Wang, B.-W.; Shyu, K.-G. Suppressive Effect of Epigallocatechin-3-O-Gallate on Endoglin Molecular Regulation in Myocardial Fibrosis in Vitro and in Vivo. J. Cell. Mol. Med. 2016, 20, 2045–2055. [Google Scholar] [CrossRef] [PubMed]
- Joo, H. J.; Park, J.-Y.; Hong, S. J.; Kim, K.-A.; Lee, S. H.; Cho, J.-Y.; Park, J. H.; Yu, C. W.; Lim, D.-S. Anti-Platelet Effects of Epigallocatechin-3-Gallate in Addition to the Concomitant Aspirin, Clopidogrel or Ticagrelor Treatment. Korean J. Intern. Med. 2018, 33, 522–531. [Google Scholar] [CrossRef]
- Kim, S.; Lee, H.; Moon, H.; Kim, R.; Kim, M.; Jeong, S.; Kim, H.; Kim, S. H.; Hwang, S. S.; Lee, M. Y.; Kim, J.; Song, B.-W.; Chang, W. Epigallocatechin-3-Gallate Attenuates Myocardial Dysfunction via Inhibition of Endothelial-to-Mesenchymal Transition. Antioxid. Basel Switz. 2023, 12. [Google Scholar] [CrossRef]
- Li, T.; Fang, F.; Yin, H.; Zhang, Z.; Wang, X.; Wang, E.; Yu, H.; Shen, Y.; Wang, G.; He, W.; Liu, X. Epigallocatechin-3-Gallate Inhibits Osteogenic Differentiation of Vascular Smooth Muscle Cells through the Transcription Factor JunB. Acta Biochim. Biophys. Sin. 2024, 57, 901–915. [Google Scholar] [CrossRef]
- Scalise, R. F. M.; De Sarro, R.; Caracciolo, A.; Lauro, R.; Squadrito, F.; Carerj, S.; Bitto, A.; Micari, A.; Bella, G. D.; Costa, F.; Irrera, N. Fibrosis after Myocardial Infarction: An Overview on Cellular Processes, Molecular Pathways, Clinical Evaluation and Prognostic Value. Med. Sci. Basel Switz. 2021, 9, 16. [Google Scholar] [CrossRef]
- Sánchez-Hernández, C. D.; Torres-Alarcón, L. A.; González-Cortés, A.; Peón, A. N. Ischemia/Reperfusion Injury: Pathophysiology, Current Clinical Management, and Potential Preventive Approaches. Mediators Inflamm. 2020, 2020, 8405370. [Google Scholar] [CrossRef]
- Su, X.; Zhou, M.; Li, Y.; An, N.; Yang, F.; Zhang, G.; Xu, L.; Chen, H.; Wu, H.; Xing, Y. Mitochondrial Damage in Myocardial Ischemia/Reperfusion Injury and Application of Natural Plant Products. Oxid. Med. Cell. Longev. 2022, 2022, 8726564. [Google Scholar] [CrossRef]
- Mokra, D.; Joskova, M.; Mokry, J. Therapeutic Effects of Green Tea Polyphenol (‒)-Epigallocatechin-3-Gallate (EGCG) in Relation to Molecular Pathways Controlling Inflammation, Oxidative Stress, and Apoptosis. Int. J. Mol. Sci. 2022, 24, 340. [Google Scholar] [CrossRef]
- Del Re, D. P.; Amgalan, D.; Linkermann, A.; Liu, Q.; Kitsis, R. N. Fundamental Mechanisms of Regulated Cell Death and Implications for Heart Disease. Physiol. Rev. 2019, 99, 1765–1817. [Google Scholar] [CrossRef]
- Zhao, W.-K.; Zhou, Y.; Xu, T.-T.; Wu, Q. Ferroptosis: Opportunities and Challenges in Myocardial Ischemia-Reperfusion Injury. Oxid. Med. Cell. Longev. 2021, 2021, 9929687. [Google Scholar] [CrossRef]
- Liu, S.; Yao, S.; Yang, H.; Liu, S.; Wang, Y. Autophagy: Regulator of Cell Death. Cell Death Dis. 2023, 14, 648. [Google Scholar] [CrossRef]
- Deng, R.-M.; Zhou, J. The Role of PI3K/AKT Signaling Pathway in Myocardial Ischemia-Reperfusion Injury. Int. Immunopharmacol. 2023, 123, 110714. [Google Scholar] [CrossRef] [PubMed]
- Salameh, A.; Dhein, S.; Mewes, M.; Sigusch, S.; Kiefer, P.; Vollroth, M.; Seeger, J.; Dähnert, I. Anti-Oxidative or Anti-Inflammatory Additives Reduce Ischemia/Reperfusions Injury in an Animal Model of Cardiopulmonary Bypass. Saudi J. Biol. Sci. 2020, 27, 18–29. [Google Scholar] [CrossRef]
- Devika, P. T.; Mainzen Prince, P. S. (-)-Epigallocatechin Gallate (EGCG) Prevents Isoprenaline-Induced Cardiac Marker Enzymes and Membrane-Bound ATPases. J. Pharm. Pharmacol. 2008, 60, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Devika, P. T.; Prince, P. S. M. Preventive Effect of (-)Epigallocatechin-Gallate (EGCG) on Lysosomal Enzymes in Heart and Subcellular Fractions in Isoproterenol-Induced Myocardial Infarcted Wistar Rats. Chem. Biol. Interact. 2008, 172, 245–252. [Google Scholar] [CrossRef]
- Devika, P. T.; Stanely Mainzen Prince, P. (-)Epigallocatechin-Gallate (EGCG) Prevents Mitochondrial Damage in Isoproterenol-Induced Cardiac Toxicity in Albino Wistar Rats: A Transmission Electron Microscopic and in Vitro Study. Pharmacol. Res. 2008, 57, 351–357. [Google Scholar] [CrossRef]
- Devika, P. T.; Stanely Mainzen Prince, P. (-)Epigallocatechingallate Protects the Mitochondria against the Deleterious Effects of Lipids, Calcium and Adenosine Triphosphate in Isoproterenol Induced Myocardial Infarcted Male Wistar Rats. J. Appl. Toxicol. JAT 2008, 28, 938–944. [Google Scholar] [CrossRef]
- Devika, P. T.; Stanely Mainzen Prince, P. Protective Effect of (-)-Epigallocatechin-Gallate (EGCG) on Lipid Peroxide Metabolism in Isoproterenol Induced Myocardial Infarction in Male Wistar Rats: A Histopathological Study. Biomed. Pharmacother. Biomedecine Pharmacother. 2008, 62, 701–708. [Google Scholar] [CrossRef]
- Devika, P. T.; Stanely Mainzen Prince, P. Preventive Effect of (-)Epigallocatechin Gallate on Lipids, Lipoproteins, and Enzymes of Lipid Metabolism in Isoproterenol-Induced Myocardial Infarction in Rats. J. Biochem. Mol. Toxicol. 2009, 23, 387–393. [Google Scholar] [CrossRef]
- Kim, S. J.; Li, M.; Jeong, C. W.; Bae, H. B.; Kwak, S. H.; Lee, S. H.; Lee, H. J.; Heo, B. H.; Yook, K. B.; Yoo, K. Y. Epigallocatechin-3-Gallate, a Green Tea Catechin, Protects the Heart against Regional Ischemia-Reperfusion Injuries through Activation of RISK Survival Pathways in Rats. Arch. Pharm. Res. 2014, 37, 1079–1085. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.-Y.; Zhang, H.-W.; Gu, J.; Xu, F.; Liang, H.-M.; Fan, K.-J.; Shen, J.-Y.; Xiao, Z.-H.; Zhang, E.-Y.; Hu, J. Mitochondrial DNA--induced Inflammatory Damage Contributes to Myocardial Ischemia Reperfusion Injury in Rats: Cardioprotective Role of Epigallocatechin. Mol. Med. Rep. 2017, 16, 7569–7576. [Google Scholar] [CrossRef]
- Zhang, C.; Liang, R.; Gan, X.; Yang, X.; Chen, L.; Jian, J. MicroRNA-384-5p/Beclin-1 As Potential Indicators For Epigallocatechin Gallate Against Cardiomyocytes Ischemia Reperfusion Injury By Inhibiting Autophagy Via PI3K/Akt Pathway. Drug Des. Devel. Ther. 2019, 13, 3607–3623. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; Wei, X.; He, Y.-L.; Chen, J.-X.; Lin, W.-T.; Xu, W.-X. EGCG Protects against Myocardial I/RI by Regulating lncRNA Gm4419-Mediated Epigenetic Silencing of the DUSP5/ERK1/2 Axis. Toxicol. Appl. Pharmacol. 2021, 433, 115782. [Google Scholar] [CrossRef] [PubMed]
- Nan, J.; Nan, C.; Ye, J.; Qian, L.; Geng, Y.; Xing, D.; Rahman, M. S. U.; Huang, M. EGCG Protects Cardiomyocytes against Hypoxia-Reperfusion Injury through Inhibition of OMA1 Activation. J. Cell Sci. 2019, 132, jcs220871. [Google Scholar] [CrossRef]
- Zhang, C.; Gan, X.; Liang, R.; Jian, J. Exosomes Derived From Epigallocatechin Gallate-Treated Cardiomyocytes Attenuated Acute Myocardial Infarction by Modulating MicroRNA-30a. Front. Pharmacol. 2020, 11, 126. [Google Scholar] [CrossRef]
- Yu, Q.; Zhang, N.; Gan, X.; Chen, L.; Wang, R.; Liang, R.; Jian, J. EGCG Attenuated Acute Myocardial Infarction by Inhibiting Ferroptosis via miR-450b-5p/ACSL4 Axis. Phytomedicine Int. J. Phytother. Phytopharm. 2023, 119, 154999. [Google Scholar] [CrossRef]
- Wu, Y.; Xia, Z.-Y.; Zhao, B.; Leng, Y.; Dou, J.; Meng, Q.-T.; Lei, S.-Q.; Chen, Z.-Z.; Zhu, J. (-)-Epigallocatechin-3-Gallate Attenuates Myocardial Injury Induced by Ischemia/Reperfusion in Diabetic Rats and in H9c2 Cells under Hyperglycemic Conditions. Int. J. Mol. Med. 2017, 40, 389–399. [Google Scholar] [CrossRef]
- Liu, P.; Huang, J.; Mei, W.; Zeng, X.; Wang, C.; Wen, C.; Xu, J. Epigallocatechin-3-Gallate Protects Cardiomyocytes from Hypoxia-Reoxygenation Damage via Raising Autophagy Related 4C Expression. Bioengineered 2021, 12, 9496–9506. [Google Scholar] [CrossRef]
- Qin, C.; Wang, T.; Qian, N.; Liu, J.; Xi, R.; Zou, Q.; Liu, H.; Niu, X. Epigallocatechin Gallate Prevents Cardiomyocytes from Pyroptosis through lncRNA MEG3/TAF15/AIM2 Axis in Myocardial Infarction. Chin. Med. 2023, 18, 160. [Google Scholar] [CrossRef]
- Hu, T.; Hu, F.-J.; Huang, H.; Zhang, Z.-Y.; Qiao, Y.-M.; Huang, W.-X.; Wang, Y.-C.; Tang, X.-Y.; Lai, S.-Q. Epigallocatechin-3-Gallate Confers Protection against Myocardial Ischemia/Reperfusion Injury by Inhibiting Ferroptosis, Apoptosis, and Autophagy via Modulation of 14-3-3η. Biomed. Pharmacother. Biomedecine Pharmacother. 2024, 174, 116542. [Google Scholar] [CrossRef]
- Othman, A. I.; Elkomy, M. M.; El-Missiry, M. A.; Dardor, M. Epigallocatechin-3-Gallate Prevents Cardiac Apoptosis by Modulating the Intrinsic Apoptotic Pathway in Isoproterenol-Induced Myocardial Infarction. Eur. J. Pharmacol. 2017, 794, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Nan, C.; Chen, Y.; Tian, J.; Jean-Charles, P.-Y.; Getfield, C.; Wang, X.; Huang, X. Calcium Desensitizer Catechin Reverses Diastolic Dysfunction in Mice with Restrictive Cardiomyopathy. Arch. Biochem. Biophys. 2015, 573, 69–76. [Google Scholar] [CrossRef]
- Pan, B.; Quan, J.; Liu, L.; Xu, Z.; Zhu, J.; Huang, X.; Tian, J. Epigallocatechin Gallate Reverses cTnI-Low Expression-Induced Age-Related Heart Diastolic Dysfunction through Histone Acetylation Modification. J. Cell. Mol. Med. 2017, 21, 2481–2490. [Google Scholar] [CrossRef]
- Oyama, J.-I.; Shiraki, A.; Nishikido, T.; Maeda, T.; Komoda, H.; Shimizu, T.; Makino, N.; Node, K. EGCG, a Green Tea Catechin, Attenuates the Progression of Heart Failure Induced by the Heart/Muscle-Specific Deletion of MnSOD in Mice. J. Cardiol. 2017, 69, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Potenza, M. A.; Marasciulo, F. L.; Tarquinio, M.; Tiravanti, E.; Colantuono, G.; Federici, A.; Kim, J.-A.; Quon, M. J.; Montagnani, M. EGCG, a Green Tea Polyphenol, Improves Endothelial Function and Insulin Sensitivity, Reduces Blood Pressure, and Protects against Myocardial I/R Injury in SHR. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E1378–1387. [Google Scholar] [CrossRef]
- Vilella, R.; Sgarbi, G.; Naponelli, V.; Savi, M.; Bocchi, L.; Liuzzi, F.; Righetti, R.; Quaini, F.; Frati, C.; Bettuzzi, S.; Solaini, G.; Stilli, D.; Rizzi, F.; Baracca, A. Effects of Standardized Green Tea Extract and Its Main Component, EGCG, on Mitochondrial Function and Contractile Performance of Healthy Rat Cardiomyocytes. Nutrients 2020, 12, 2949. [Google Scholar] [CrossRef] [PubMed]
- Jia, Q.; Yang, R.; Mehmood, S.; Li, Y. Epigallocatechin-3-Gallate Attenuates Myocardial Fibrosis in Diabetic Rats by Activating Autophagy. Exp. Biol. Med. Maywood NJ 2022, 247, 1591–1600. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, W.; Liu, J.; Gan, Y.; Liu, L.; Tian, J. Epigallocatechin-3 Gallate Prevents Pressure Overload-Induced Heart Failure by up-Regulating SERCA2a via Histone Acetylation Modification in Mice. PloS One 2018, 13, e0205123. [Google Scholar] [CrossRef]
- Zhang, Q.; Hu, L.; Chen, L.; Li, H.; Wu, J.; Liu, W.; Zhang, M.; Yan, G. (-)-Epigallocatechin-3-Gallate, the Major Green Tea Catechin, Regulates the Desensitization of Β1 Adrenoceptor via GRK2 in Experimental Heart Failure. Inflammopharmacology 2018, 26, 1081–1091. [Google Scholar] [CrossRef]
- Han, X.; Peng, C.; Huang, L.; Luo, X.; Mao, Q.; Wu, S.; Zhang, H. EGCG Prevents Pressure Overload--induced Myocardial Remodeling by Downregulating Overexpression of HDAC5 in Mice. Int. J. Mol. Med. 2022, 49, 11. [Google Scholar] [CrossRef]
- Mou, Q.; Jia, Z.; Luo, M.; Liu, L.; Huang, X.; Quan, J.; Tian, J. Epigallocatechin-3-Gallate Exerts Cardioprotective Effects Related to Energy Metabolism in Pressure Overload-Induced Cardiac Dysfunction. Arch. Biochem. Biophys. 2022, 723, 109217. [Google Scholar] [CrossRef]
- Xuan, F.; Jian, J. Epigallocatechin Gallate Exerts Protective Effects against Myocardial Ischemia/Reperfusion Injury through the PI3K/Akt Pathway-Mediated Inhibition of Apoptosis and the Restoration of the Autophagic Flux. Int. J. Mol. Med. 2016, 38, 328–336. [Google Scholar] [CrossRef]
- Zhang, C.; Liao, P.; Liang, R.; Zheng, X.; Jian, J. Epigallocatechin Gallate Prevents Mitochondrial Impairment and Cell Apoptosis by Regulating miR-30a/P53 Axis. Phytomedicine Int. J. Phytother. Phytopharm. 2019, 61, 152845. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, M.; Hellige, N.; Rieder, P.; Kinkel, H.-T.; Trimpert, C.; Staudt, A.; Felix, S. B.; Baumann, G.; Stangl, K.; Stangl, V. Positive Inotropic Effects of Epigallocatechin-3-Gallate (EGCG) Involve Activation of Na+/H+ and Na+/Ca2+ Exchangers. Eur. J. Heart Fail. 2008, 10, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Salameh, A.; Schuster, R.; Dähnert, I.; Seeger, J.; Dhein, S. Epigallocatechin Gallate Reduces Ischemia/Reperfusion Injury in Isolated Perfused Rabbit Hearts. Int. J. Mol. Sci. 2018, 19, 628. [Google Scholar] [CrossRef] [PubMed]
- Hirai, M.; Hotta, Y.; Ishikawa, N.; Wakida, Y.; Fukuzawa, Y.; Isobe, F.; Nakano, A.; Chiba, T.; Kawamura, N. Protective Effects of EGCg or GCg, a Green Tea Catechin Epimer, against Postischemic Myocardial Dysfunction in Guinea-Pig Hearts. Life Sci. 2007, 80, 1020–1032. [Google Scholar] [CrossRef]
- Song, D.-K.; Jang, Y.; Kim, J. H.; Chun, K.-J.; Lee, D.; Xu, Z. Polyphenol (-)-Epigallocatechin Gallate during Ischemia Limits Infarct Size via Mitochondrial K(ATP) Channel Activation in Isolated Rat Hearts. J. Korean Med. Sci. 2010, 25, 380–386. [Google Scholar] [CrossRef]
- Kim, C. J.; Kim, J. M.; Lee, S. R.; Jang, Y. H.; Kim, J. H.; Chun, K. J. Polyphenol (-)-Epigallocatechin Gallate Targeting Myocardial Reperfusion Limits Infarct Size and Improves Cardiac Function. Korean J. Anesthesiol. 2010, 58, 169–175. [Google Scholar] [CrossRef]
- Piao, C. S.; Kim, D.-S.; Ha, K.-C.; Kim, H.-R.; Chae, H.-J.; Chae, S.-W. The Protective Effect of Epigallocatechin-3 Gallate on Ischemia/Reperfusion Injury in Isolated Rat Hearts: An Ex Vivo Approach. Korean J. Physiol. Pharmacol. Off. J. Korean Physiol. Soc. Korean Soc. Pharmacol. 2011, 15, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Hwang, H. S.; Kryshtal, D. O.; Yang, T.; Padilla, I. T.; Tiwary, A. K.; Puschner, B.; Pessah, I. N.; Knollmann, B. C. Coordinated Regulation of Murine Cardiomyocyte Contractility by Nanomolar (-)-Epigallocatechin-3-Gallate, the Major Green Tea Catechin. Mol. Pharmacol. 2012, 82, 993–1000. [Google Scholar] [CrossRef] [PubMed]
- Tadano, N.; Du, C.-K.; Yumoto, F.; Morimoto, S.; Ohta, M.; Xie, M.-F.; Nagata, K.; Zhan, D.-Y.; Lu, Q.-W.; Miwa, Y.; Takahashi-Yanaga, F.; Tanokura, M.; Ohtsuki, I.; Sasaguri, T. Biological Actions of Green Tea Catechins on Cardiac Troponin C. Br. J. Pharmacol. 2010, 161, 1034–1043. [Google Scholar] [CrossRef]
- Messer, A. E.; Bayliss, C. R.; El-Mezgueldi, M.; Redwood, C. S.; Ward, D. G.; Leung, M.-C.; Papadaki, M.; Dos Remedios, C.; Marston, S. B. Mutations in Troponin T Associated with Hypertrophic Cardiomyopathy Increase Ca(2+)-Sensitivity and Suppress the Modulation of Ca(2+)-Sensitivity by Troponin I Phosphorylation. Arch. Biochem. Biophys. 2016, 601, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Hassoun, R.; Budde, H.; Mannherz, H. G.; Lódi, M.; Fujita-Becker, S.; Laser, K. T.; Gärtner, A.; Klingel, K.; Möhner, D.; Stehle, R.; Sultana, I.; Schaaf, T.; Majchrzak, M.; Krause, V.; Herrmann, C.; Nowaczyk, M. M.; Mügge, A.; Pfitzer, G.; Schröder, R. R.; Hamdani, N.; Milting, H.; Jaquet, K.; Cimiotti, D. De Novo Missense Mutations in TNNC1 and TNNI3 Causing Severe Infantile Cardiomyopathy Affect Myofilament Structure and Function and Are Modulated by Troponin Targeting Agents. Int. J. Mol. Sci. 2021, 22, 9625. [Google Scholar] [CrossRef]
- Isbrucker, R. A.; Bausch, J.; Edwards, J. A.; Wolz, E. Safety Studies on Epigallocatechin Gallate (EGCG) Preparations. Part 1: Genotoxicity. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2006, 44, 626–635. [Google Scholar] [CrossRef]
- Isbrucker, R. A.; Edwards, J. A.; Wolz, E.; Davidovich, A.; Bausch, J. Safety Studies on Epigallocatechin Gallate (EGCG) Preparations. Part 2: Dermal, Acute and Short-Term Toxicity Studies. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2006, 44, 636–650. [Google Scholar] [CrossRef] [PubMed]
- Isbrucker, R. A.; Edwards, J. A.; Wolz, E.; Davidovich, A.; Bausch, J. Safety Studies on Epigallocatechin Gallate (EGCG) Preparations. Part 3: Teratogenicity and Reproductive Toxicity Studies in Rats. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2006, 44, 651–661. [Google Scholar] [CrossRef]
- Kim, E.-Y.; Ham, S.-K.; Shigenaga, M. K.; Han, O. Bioactive Dietary Polyphenolic Compounds Reduce Nonheme Iron Transport across Human Intestinal Cell Monolayers. J. Nutr. 2008, 138, 1647–1651. [Google Scholar] [CrossRef]
- Duda-Chodak, A.; Tarko, T. Possible Side Effects of Polyphenols and Their Interactions with Medicines. Mol. Basel Switz. 2023, 28, 2536. [Google Scholar] [CrossRef]
- Kiss, T.; Timár, Z.; Szabó, A.; Lukács, A.; Velky, V.; Oszlánczi, G.; Horváth, E.; Takács, I.; Zupkó, I.; Csupor, D. Effect of Green Tea on the Gastrointestinal Absorption of Amoxicillin in Rats. BMC Pharmacol. Toxicol. 2019, 20, 54. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, N.; Oda, O.; Nagao, S. Green Tea Catechins Such as (-)-Epicatechin and (-)-Epigallocatechin Accelerate Cu2+-Induced Low Density Lipoprotein Oxidation in Propagation Phase. FEBS Lett. 1997, 401, (2–3). [Google Scholar] [CrossRef] [PubMed]
- Caro, A. A.; Davis, A.; Fobare, S.; Horan, N.; Ryan, C.; Schwab, C. Antioxidant and Pro-Oxidant Mechanisms of (+) Catechin in Microsomal CYP2E1-Dependent Oxidative Stress. Toxicol. In Vitro 2019, 54, 1–9. [Google Scholar] [CrossRef]
- Galati, G.; Lin, A.; Sultan, A. M.; O’Brien, P. J. Cellular and in Vivo Hepatotoxicity Caused by Green Tea Phenolic Acids and Catechins. Free Radic. Biol. Med. 2006, 40, 570–580. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; He, S.-Q.; Hong, H.-Q.; Cai, Y.-P.; Zhao, L.; Zhang, M. High Doses of (-)-Epigallocatechin-3-Gallate from Green Tea Induces Cardiac Fibrosis in Mice. Biotechnol. Lett. 2015, 37, 2371–2377. [Google Scholar] [CrossRef]
- Rasheed, N. O. A.; Ahmed, L. A.; Abdallah, D. M.; El-Sayeh, B. M. Paradoxical Cardiotoxicity of Intraperitoneally-Injected Epigallocatechin Gallate Preparation in Diabetic Mice. Sci. Rep. 2018, 8, 7880. [Google Scholar] [CrossRef]
- Bao, L.; Lu, F.; Chen, H.; Min, Q.; Chen, X.; Song, Y.; Zhao, B.; Bu, H.; Sun, H. High Concentration of Epigallocatechin-3-Gallate Increased the Incidences of Arrhythmia and Diastolic Dysfunction via Β2-Adrenoceptor. J. Food Sci. 2015, 80, T659–663. [Google Scholar] [CrossRef]
- Bedrood, Z.; Rameshrad, M.; Hosseinzadeh, H. Toxicological Effects of Camellia Sinensis (Green Tea): A Review. Phytother. Res. PTR 2018, 32, 1163–1180. [Google Scholar] [CrossRef]
- Hu, J.; Webster, D.; Cao, J.; Shao, A. The Safety of Green Tea and Green Tea Extract Consumption in Adults—Results of a Systematic Review. Regul. Toxicol. Pharmacol. RTP 2018, 95, 412–433. [Google Scholar] [CrossRef]
- Mazzanti, G.; Menniti-Ippolito, F.; Moro, P. A.; Cassetti, F.; Raschetti, R.; Santuccio, C.; Mastrangelo, S. Hepatotoxicity from Green Tea: A Review of the Literature and Two Unpublished Cases. Eur. J. Clin. Pharmacol. 2009, 65, 331–341. [Google Scholar] [CrossRef]
- Alemdaroglu, N. C.; Dietz, U.; Wolffram, S.; Spahn-Langguth, H.; Langguth, P. Influence of Green and Black Tea on Folic Acid Pharmacokinetics in Healthy Volunteers: Potential Risk of Diminished Folic Acid Bioavailability. Biopharm. Drug Dispos. 2008, 29, 335–348. [Google Scholar] [CrossRef]
- Chow, H.-H. S.; Cai, Y.; Hakim, I. A.; Crowell, J. A.; Shahi, F.; Brooks, C. A.; Dorr, R. T.; Hara, Y.; Alberts, D. S. Pharmacokinetics and Safety of Green Tea Polyphenols after Multiple-Dose Administration of Epigallocatechin Gallate and Polyphenon E in Healthy Individuals. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2003, 9, 3312–3319. [Google Scholar]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS); Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Filipič, M.; Frutos, M. J.; Galtier, P.; Gundert-Remy, U.; Kuhnle, G. G.; Lambré, C.; Leblanc, J.-C.; Lillegaard, I. T.; Moldeus, P.; Mortensen, A.; Oskarsson, A.; Stankovic, I.; Waalkens-Berendsen, I.; Woutersen, R. A.; Wright, M.; Di Domenico, A.; Fairweather-Tait, S.; McArdle, H. J.; Smeraldi, C.; Gott, D. Guidance on Safety Evaluation of Sources of Nutrients and Bioavailability of Nutrient from the Sources (Revision 1). EFSA J. Eur. Food Saf. Auth. 2021, 19, e06552. [Google Scholar] [CrossRef]
- Lu, J.-H.; He, J.-R.; Shen, S.-Y.; Wei, X.-L.; Chen, N.-N.; Yuan, M.-Y.; Qiu, L.; Li, W.-D.; Chen, Q.-Z.; Hu, C.-Y.; Xia, H.-M.; Bartington, S.; Cheng, K. K.; Lam, K. B. H.; Qiu, X.; Born in Guangzhou Cohort Study Group. Does Tea Consumption during Early Pregnancy Have an Adverse Effect on Birth Outcomes? Birth Berkeley Calif 2017, 44, 281–289. [Google Scholar] [CrossRef]
- Xie, J.; Jia, L.; Xie, P.; Yin, X.; Zhu, W.; Zhao, H.; Wang, X.; Meng, X.; Xing, L.; Zhao, H.; Li, X. Efficacy and Safety of Epigallocatechin-3-Gallate in Treatment Acute Severe Dermatitis in Patients with Cancer Receiving Radiotherapy: A Phase I Clinical Trial. Sci. Rep. 2023, 13, 13865. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhu, Q.; Chen, Z.; Wang, X.; Ma, B.; Wang, Y.; Xiao, Y.; Luo, R.; Zhang, W.; Wang, Y.; Fu, G. Self-Adaptive Covalent Coating for Vascular Stents: Coordinated Coagulation-Inflammation Regulation to Support Re-Endothelialization for Atherosclerosis Control. Biomaterials 2025, 325, 123600. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Jiang, C.; Song, J.; Bi, Y.; Chen, W.; Lu, C.; Chen, M.; Lv, L.; Yu, R.; Zou, J.; Zhao, Z.; Chen, X.; Ji, J. Microenvironment Responsive Nanoplatform for Targeted Removal of Cholesterol and Reshaping Inflammatory Microenvironment in Atherosclerotic Plaques. J. Control. Release Off. J. Control. Release Soc. 2025, 385, 114000. [Google Scholar] [CrossRef]
- Liu, T.; Hao, Y.; Zhang, Z.; Zhou, H.; Peng, S.; Zhang, D.; Li, K.; Chen, Y.; Chen, M. Advanced Cardiac Patches for the Treatment of Myocardial Infarction. Circulation 2024, 149, 2002–2020. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Wang, H.; Guo, L.; Cui, Y.; Zou, C.; Hu, J.; Zhang, H.; Yang, G.; Zhou, W. Multifunctional Nanomedicine for Targeted Atherosclerosis Therapy: Activating Plaque Clearance Cascade and Suppressing Inflammation. ACS Nano 2025, 19, 3339–3361. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





