Submitted:
20 October 2025
Posted:
21 October 2025
You are already at the latest version
Abstract
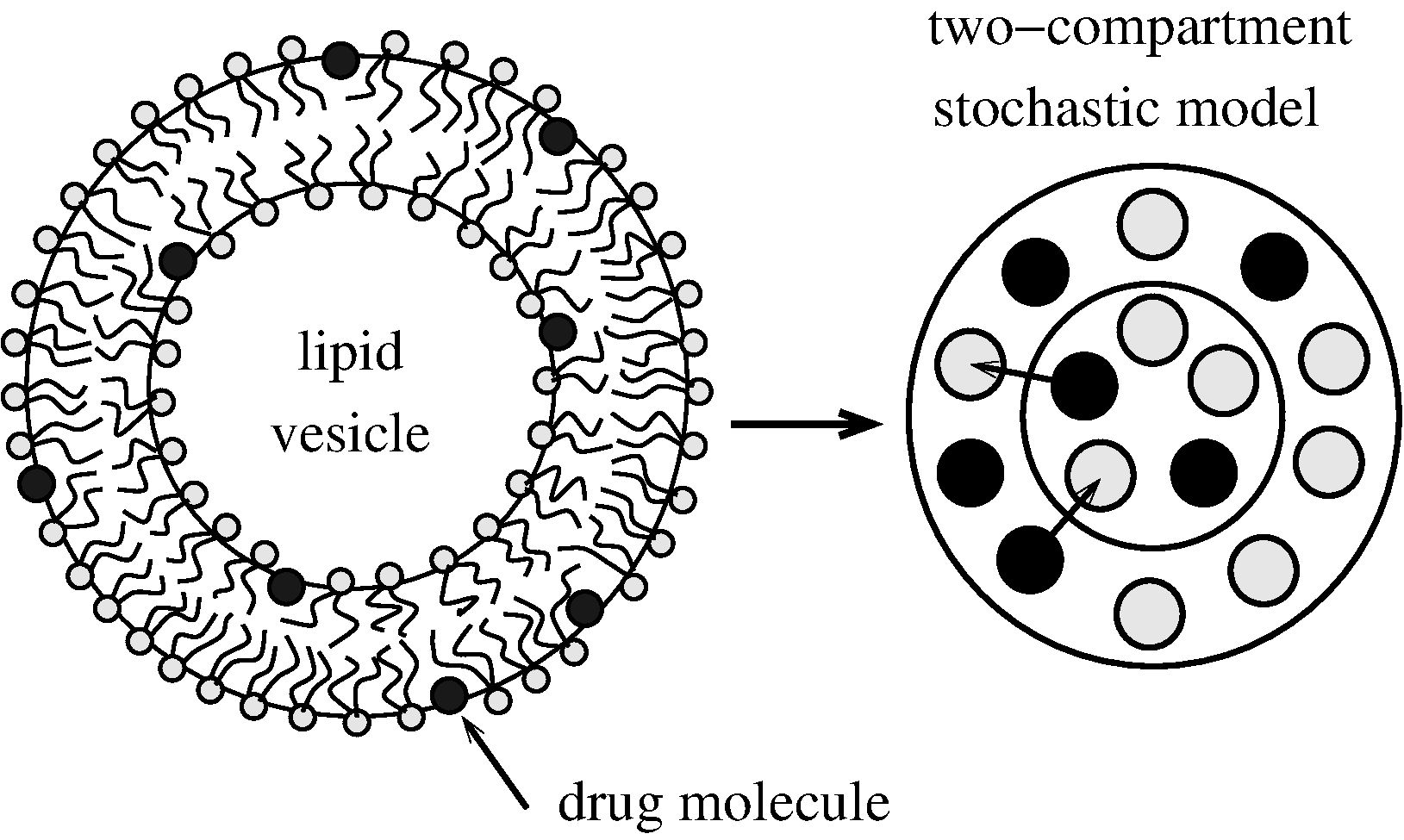
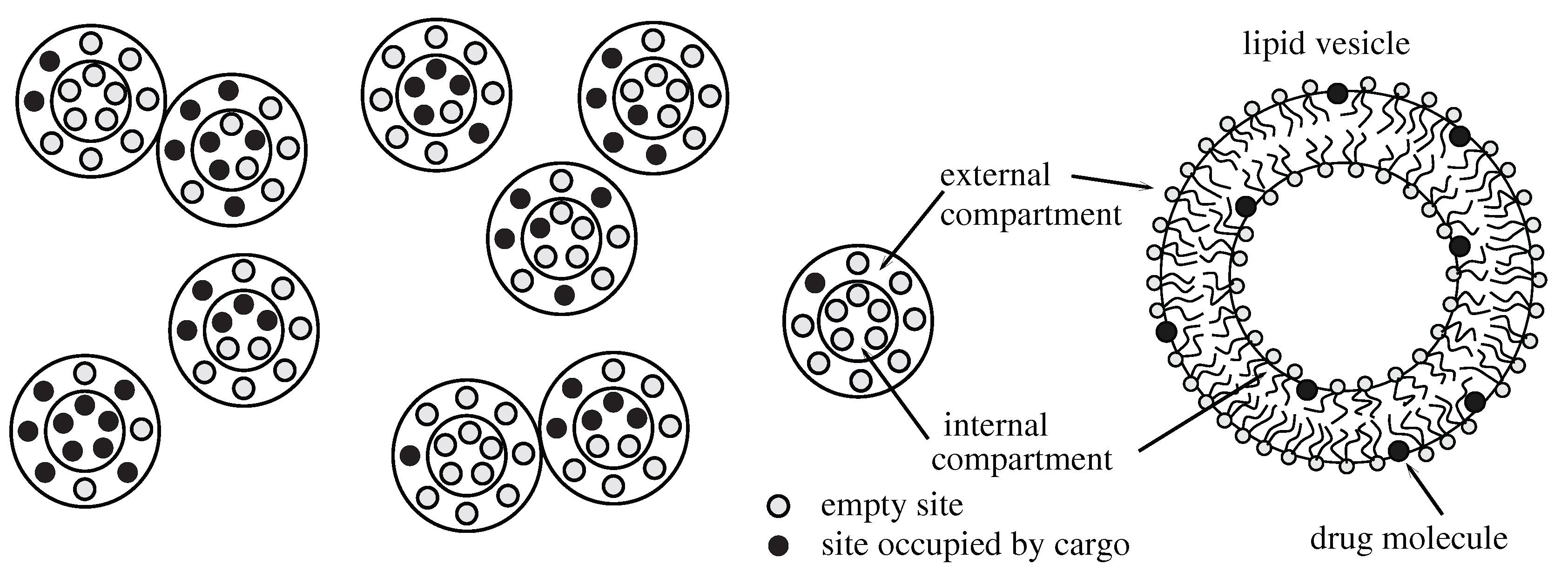
Keywords:
1. Introduction
2. Theoretical Model and Discussion
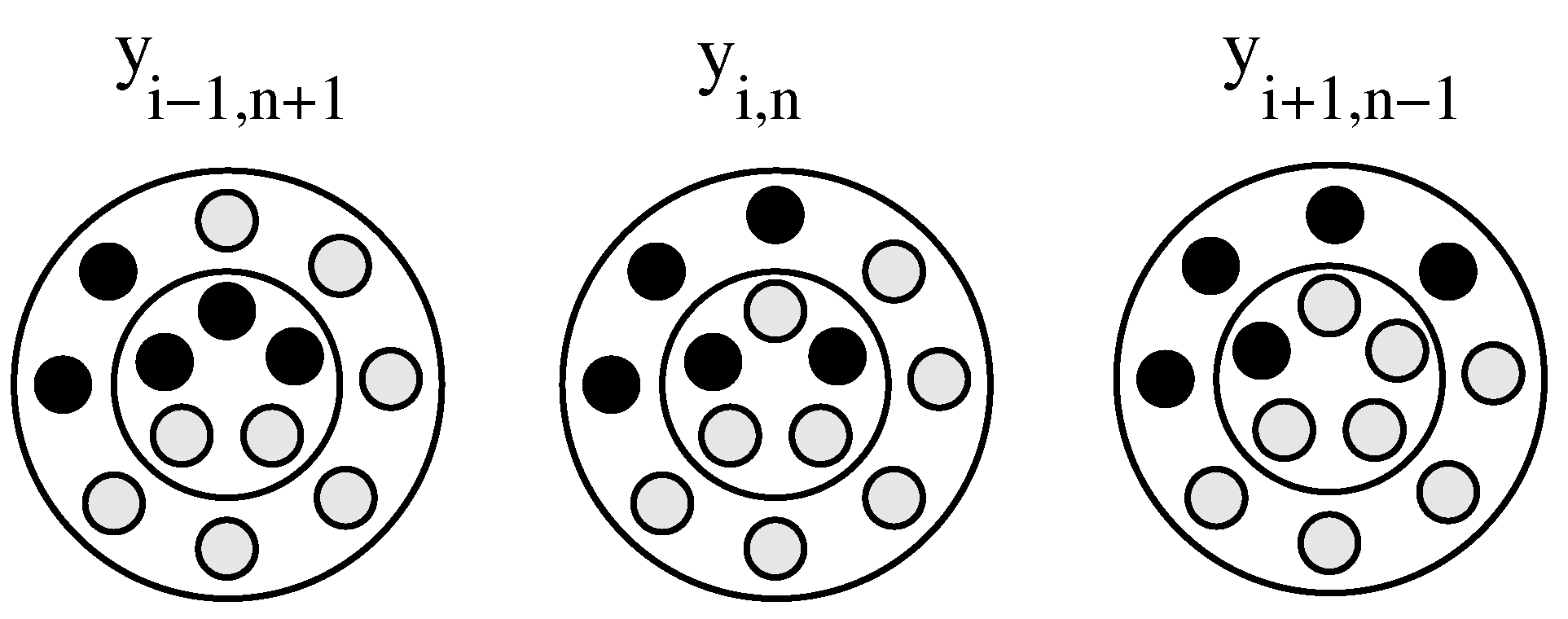
2.1. Rate Equations
2.2. Continuum Representation in the Gaussian Limit at low Occupation
2.3. Solution of the Fokker-Planck Equation
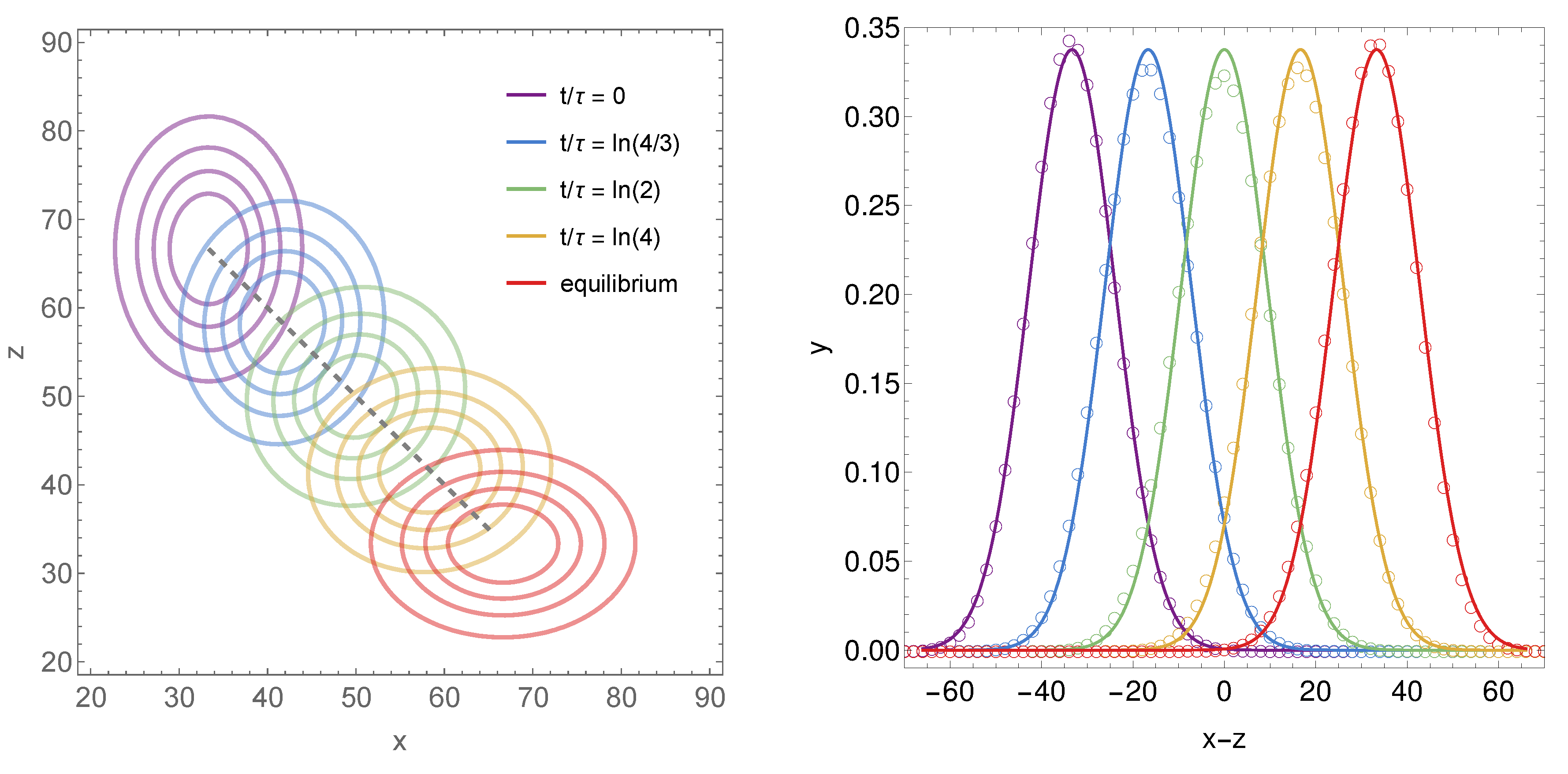
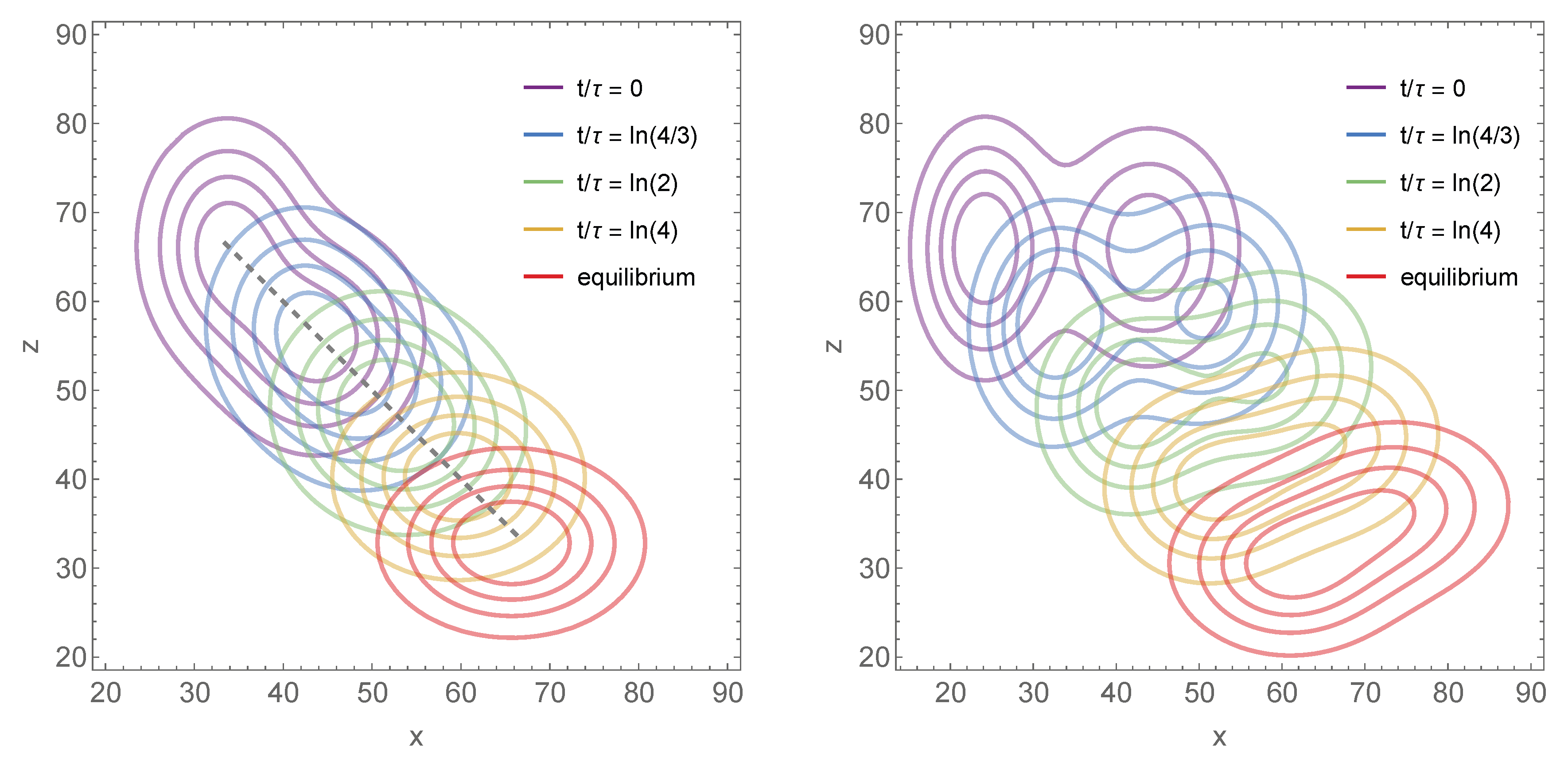
2.4. Three Specific Examples
2.4.1. First Example
2.4.2. Second Example
2.4.3. Third Example
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chamundeeswari, M.; Jeslin, J.; Verma, M.L. Nanocarriers for drug delivery applications. Environ. Chem. Lett. 2019, 17, 849–865. [Google Scholar] [CrossRef]
- Ramasamy, T.; Munusamy, S.; Ruttala, H.B.; Kim, J.O. Smart nanocarriers for the delivery of nucleic acid-based therapeutics: a comprehensive review. Biotechnol. J. 2021, 16, 1900408. [Google Scholar] [CrossRef]
- Silindir-Gunay, M.; Sarcan, E.T.; Ozer, A.Y. Near-infrared imaging of diseases: A nanocarrier approach. Drug Dev. Res. 2019, 80, 521–534. [Google Scholar] [CrossRef]
- Srivastava, S.; Sharma, V.; Bhushan, B.; Malviya, R.; Awasthi, R.; Kulkarni, G.T. Nanocarriers for protein and peptide delivery: Recent advances and progress. J. Res. Pharm. 2021, 25, 99–116. [Google Scholar] [CrossRef]
- Machhi, J.; Shahjin, F.; Das, S.; Patel, M.; Abdelmoaty, M.M.; Cohen, J.D.; Singh, P.A.; Baldi, A.; Bajwa, N.; Kumar, R.; et al. Nanocarrier vaccines for SARS-CoV-2. Adv. Drug Deliv. Rev. 2021, 171, 215–239. [Google Scholar] [CrossRef]
- Saraiva, J.; Marotta-Oliveira, S.S.; Cicillini, S.A.; Eloy, J.d.O.; Marchetti, J.M. Nanocarriers for nitric oxide delivery. J. Drug Deliv. 2011, 2011, 936438. [Google Scholar] [CrossRef]
- Díaz-Saldívar, P.; Huidobro-Toro, J.P. ATP-loaded biomimetic nanoparticles as controlled release system for extracellular drugs in cancer applications. Int. J. Nanomed. 2019, 14, 2433–2447. [Google Scholar] [CrossRef] [PubMed]
- Gressler, S.; Hipfinger, C.; Part, F.; Pavlicek, A.; Zafiu, C.; Giese, B. A systematic review of nanocarriers used in medicine and beyond—definition and categorization framework. J. Nanobiotechnol. 2025, 23, 90. [Google Scholar] [CrossRef]
- Ding, C.; Li, Z. A review of drug release mechanisms from nanocarrier systems. Mater. Sci. Eng., C 2017, 76, 1440–1453. [Google Scholar] [CrossRef] [PubMed]
- Steck, T.; Kezdy, F.; Lange, Y. An activation-collision mechanism for cholesterol transfer between membranes. J. Biol. Chem. 1988, 263, 13023–13031. [Google Scholar] [CrossRef]
- Siepmann, J.; Siepmann, F. Mathematical modeling of drug delivery. Int. J. Pharm. 2008, 364, 328–343. [Google Scholar] [CrossRef] [PubMed]
- Loew, S.; Fahr, A.; May, S. Modeling the release kinetics of poorly water-soluble drug molecules from liposomal nanocarriers. J. Drug Delivery 2011, 2011. [Google Scholar] [CrossRef]
- Jain, A.; Jain, S.K. In vitro release kinetics model fitting of liposomes: An insight. Chem. Phys. Lipids 2016, 201, 28–40. [Google Scholar] [CrossRef]
- Abbasi, H.; Kouchak, M.; Mirveis, Z.; Hajipour, F.; Khodarahmi, M.; Rahbar, N.; Handali, S. What we need to know about liposomes as drug nanocarriers: an updated review. Adv. Pharm. Bull. 2022, 13, 7. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: from concept to clinical applications. Adv. Drug Delivery Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef]
- Liu, P.; Chen, G.; Zhang, J. A review of liposomes as a drug delivery system: current status of approved products, regulatory environments, and future perspectives. Molecules 2022, 27, 1372. [Google Scholar] [CrossRef]
- Gabizon, A.; Shmeeda, H.; Barenholz, Y. Pharmacokinetics of pegylated liposomal Doxorubicin: review of animal and human studies. Clin. Pharmacokinet. 2003, 42, 419–436. [Google Scholar] [CrossRef]
- Federico, C.; Morittu, V.M.; Britti, D.; Trapasso, E.; Cosco, D. Gemcitabine-loaded liposomes: rationale, potentialities and future perspectives. Int. J. Nanomed. 2012, 5423–5436. [Google Scholar] [CrossRef]
- Grudzinski, W.; Sagan, J.; Welc, R.; Luchowski, R.; Gruszecki, W.I. Molecular organization, localization and orientation of antifungal antibiotic amphotericin B in a single lipid bilayer. Sci. Rep. 2016, 6, 32780. [Google Scholar] [CrossRef]
- Kuntsche, J.; Freisleben, I.; Steiniger, F.; Fahr, A. Temoporfin-loaded liposomes: physicochemical characterization. Eur. J. Pharm. Sci. 2010, 40, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Ditzinger, F.; Price, D.J.; Ilie, A.R.; Köhl, N.J.; Jankovic, S.; Tsakiridou, G.; Aleandri, S.; Kalantzi, L.; Holm, R.; Nair, A.; et al. Lipophilicity and hydrophobicity considerations in bio-enabling oral formulations approaches–a PEARRL review. J. Pharm. Pharmacol. 2019, 71, 464–482. [Google Scholar] [CrossRef]
- Wenk, M.R.; Fahr, A.; Reszka, R.; Seelig, J. Paclitaxel partitioning into lipid bilayers. J. Pharm. Sci. 1996, 85, 228–231. [Google Scholar] [CrossRef] [PubMed]
- Hossain, F.; Bossa, G.V.; May, S. Collision-mediated transfer kinetics of cargo among mobile nanocarriers. Phys. Rev. E 2025, 111, L042102. [Google Scholar] [CrossRef]
- Darvey, I.; Staff, P. Stochastic approach to first-order chemical reaction kinetics. J. Chem. Phys. 1966, 44, 990–997. [Google Scholar] [CrossRef]
- McQuarrie, D.A. Stochastic approach to chemical kinetics. J. Appl. Probab. 1967, 4, 413–478. [Google Scholar] [CrossRef]
- Sharifian Gh, M. Recent experimental developments in studying passive membrane transport of drug molecules. Mol. Pharm. 2021, 18, 2122–2141. [Google Scholar] [CrossRef]
- Venable, R.M.; Krämer, A.; Pastor, R.W. Molecular Dynamics Simulations of Membrane Permeability. Chem. Rev. 2019, 119, 5954–5997. [Google Scholar] [CrossRef]
- Carrer, M.; Eilsø Nielsen, J.; Cezar, H.M.; Lund, R.; Cascella, M.; Soares, T.A. Accelerating lipid flip-flop at low concentrations: A general mechanism for membrane binding peptides. J. Phys. Chem. Lett. 2023, 14, 7014–7019. [Google Scholar] [CrossRef]
- Gu, R.X.; Baoukina, S.; Tieleman, D.P. Cholesterol Flip-Flop in Heterogeneous Membranes. J. Chem. Theory Comput. 2019, 15, 2064–2070. [Google Scholar] [CrossRef] [PubMed]
- Schwindt, N.S.; Avidar, M.; Epsztein, R.; Straub, A.P.; Shirts, M.R. Interpreting effective energy barriers to membrane permeation in terms of a heterogeneous energy landscape. J. Membr. Sci. 2024, 712, 123233. [Google Scholar] [CrossRef]
- Alonso, M.; Satoh, M.; Miyanami, K. Kinetics of fines transfer among carriers in powder coating. Powder Technol. 1989, 59, 217–224. [Google Scholar] [CrossRef]
- Gardiner, C.W. Handbook of stochastic methods; springer: Berlin, 2004; Vol. 3. [Google Scholar]
- Vallance, C. An Introduction to chemical kinetics; Morgan & Claypool Publishers, 2017. [Google Scholar]
- Riley, K.F.; Hobson, M.P.; Bence, S.J. Mathematical methods for physics and engineering: a comprehensive guide; Cambridge university press, 2006. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




