Submitted:
19 October 2025
Posted:
20 October 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
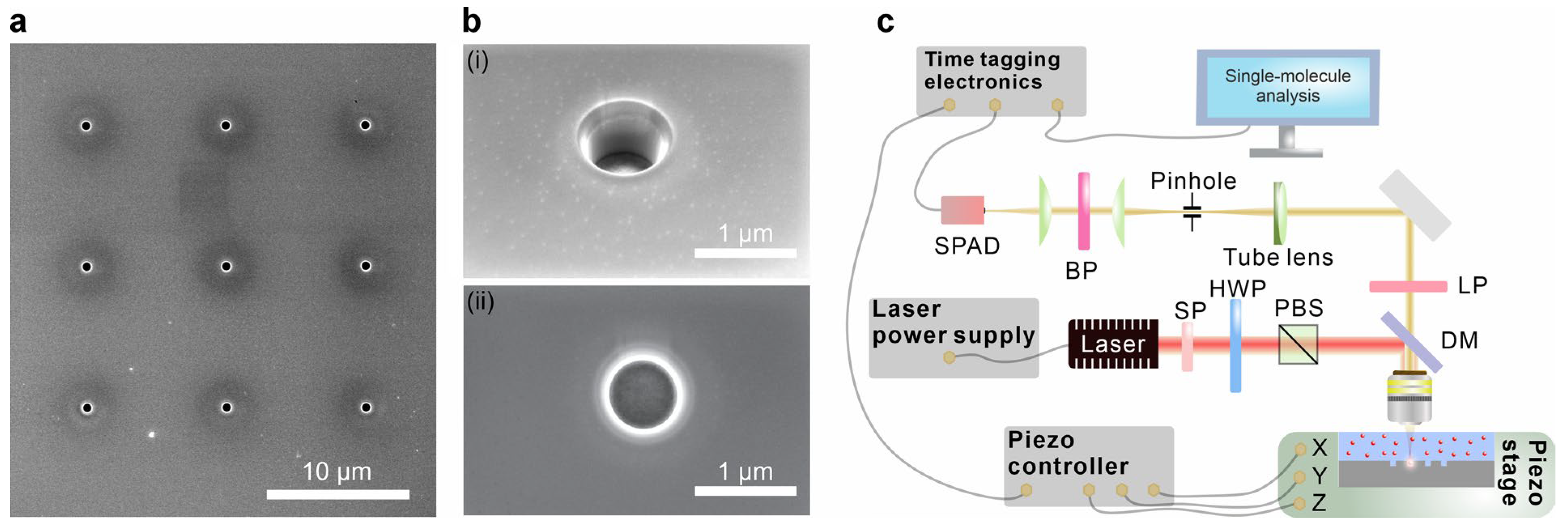
2.1. Design and Fabrication of Mie-Voids
2.2. Single-Molecule Detection Setup
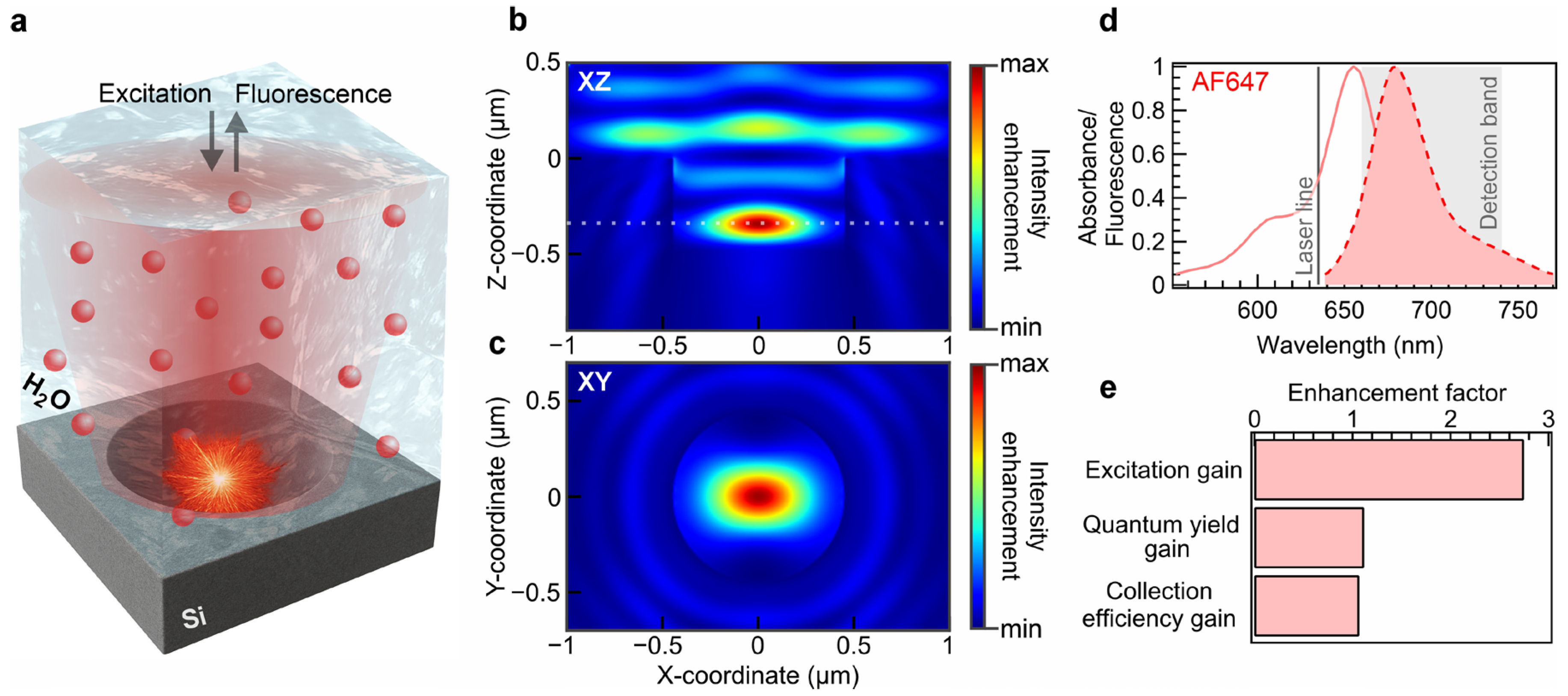
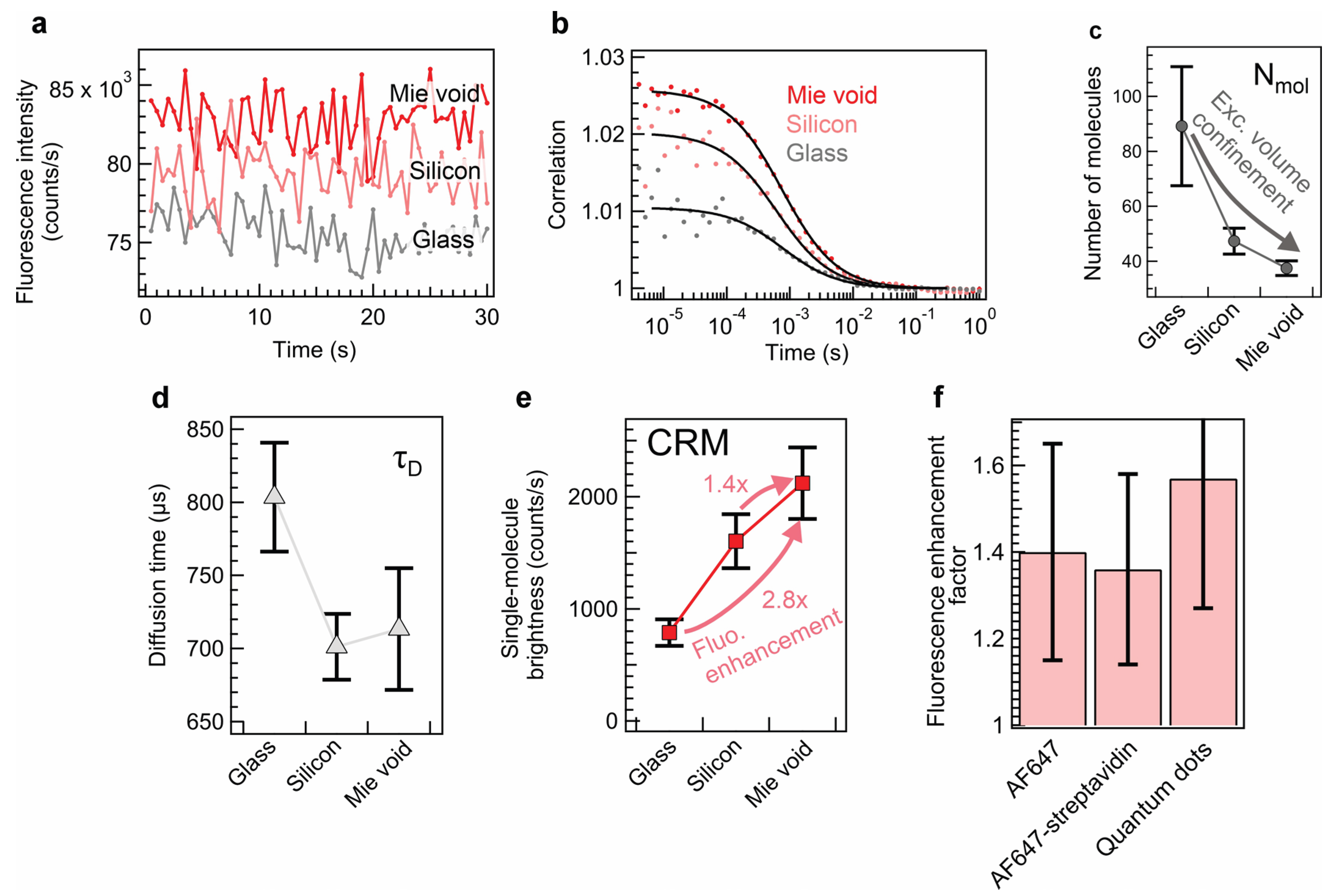
3. Results
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cordes, T.; Blum, S.A. Opportunities and Challenges in Single-Molecule and Single-Particle Fluorescence Microscopy for Mechanistic Studies of Chemical Reactions. Nature chemistry 2013, 5, 993–999.
- Moerner, W.; Fromm, D.P. Methods of Single-Molecule Fluorescence Spectroscopy and Microscopy. Review of Scientific instruments 2003, 74, 3597–3619.
- Sahl, S.J.; Hell, S.W.; Jakobs, S. Fluorescence Nanoscopy in Cell Biology. Nature reviews Molecular cell biology 2017, 18, 685–701.
- Hwang, J.; Banerjee, M.; Venable, A.S.; Walden, Z.; Jolly, J.; Zimmerman, C.; Adkisson, E.; Xiao, Q. Quantitation of Low Abundant Soluble Biomarkers Using High Sensitivity Single Molecule Counting Technology. Methods 2019, 158, 69–76. [CrossRef]
- Patra, S.; Claude, J.-B.; Naubron, J.-V.; Wenger, J. Fast Interaction Dynamics of G-Quadruplex and RGG-Rich Peptides Unveiled in Zero-Mode Waveguides. Nucleic Acids Research 2021, 49, 12348–12357.
- Chowdhury, A.; Nettels, D.; Schuler, B. Interaction Dynamics of Intrinsically Disordered Proteins from Single-Molecule Spectroscopy. Annual review of biophysics 2023, 52, 433–462.
- Du Nguyen, D.; Shuklin, F.; Barulina, E.; Albitskaya, H.; Novikov, S.; Chernov, A.I.; Kim, I.; Barulin, A. Recent Advances in Dynamic Single-Molecule Analysis Platforms for Diagnostics: Advantages over Bulk Assays and Miniaturization Approaches. Biosensors and Bioelectronics 2025, 117361.
- Brown, J.W.P.; Bauer, A.; Polinkovsky, M.E.; Bhumkar, A.; Hunter, D.J.B.; Gaus, K.; Sierecki, E.; Gambin, Y. Single-Molecule Detection on a Portable 3D-Printed Microscope. Nature Communications 2019, 10, 5662. [CrossRef]
- Webb, W.W. Fluorescence Correlation Spectroscopy: Inception, Biophysical Experimentations, and Prospectus. Applied Optics 2001, 40, 3969–3983.
- Grabenhorst, L.; Sturzenegger, F.; Hasler, M.; Schuler, B.; Tinnefeld, P. Single-Molecule FRET at 10 MHz Count Rates. J. Am. Chem. Soc. 2024, 146, 3539–3544. [CrossRef]
- Tiwari, S.; Roy, P.; Claude, J.-B.; Wenger, J. Achieving High Temporal Resolution in Single-Molecule Fluorescence Techniques Using Plasmonic Nanoantennas. Advanced Optical Materials 2023, 11, 2300168. [CrossRef]
- Punj, D.; Mivelle, M.; Moparthi, S.B.; Van Zanten, T.S.; Rigneault, H.; Van Hulst, N.F.; García-Parajó, M.F.; Wenger, J. A Plasmonic ‘Antenna-in-Box’Platform for Enhanced Single-Molecule Analysis at Micromolar Concentrations. Nature nanotechnology 2013, 8, 512–516.
- Baibakov, M.; Barulin, A.; Roy, P.; Claude, J.-B.; Patra, S.; Wenger, J. Zero-Mode Waveguides Can Be Made Better: Fluorescence Enhancement with Rectangular Aluminum Nanoapertures from the Visible to the Deep Ultraviolet. Nanoscale Advances 2020, 2, 4153–4160.
- Bharadwaj, P.; Deutsch, B.; Novotny, L. Optical Antennas. Advances in Optics and Photonics 2009, 1, 438–483.
- Novotny, L. Effective Wavelength Scaling for Optical Antennas. Physical review letters 2007, 98, 266802.
- Novotny, L.; Van Hulst, N. Antennas for Light. Nature photonics 2011, 5, 83–90.
- Taminiau, T.; Stefani, F.; Segerink, F.B.; Van Hulst, N. Optical Antennas Direct Single-Molecule Emission. Nature photonics 2008, 2, 234–237.
- Akselrod, G.M.; Argyropoulos, C.; Hoang, T.B.; Ciracì, C.; Fang, C.; Huang, J.; Smith, D.R.; Mikkelsen, M.H. Probing the Mechanisms of Large Purcell Enhancement in Plasmonic Nanoantennas. Nature Photonics 2014, 8, 835–840.
- Flauraud, V.; Regmi, R.; Winkler, P.M.; Alexander, D.T.; Rigneault, H.; Van Hulst, N.F.; García-Parajo, M.F.; Wenger, J.; Brugger, J. In-Plane Plasmonic Antenna Arrays with Surface Nanogaps for Giant Fluorescence Enhancement. Nano letters 2017, 17, 1703–1710.
- Nüesch, M.F.; Ivanovic, M.T.; Claude, J.-B.; Nettels, D.; Best, R.B.; Wenger, J.; Schuler, B. Single-Molecule Detection of Ultrafast Biomolecular Dynamics with Nanophotonics. Journal of the American Chemical Society 2021, 144, 52–56.
- Barulin, A.; Claude, J.-B.; Patra, S.; Bonod, N.; Wenger, J. Deep Ultraviolet Plasmonic Enhancement of Single Protein Autofluorescence in Zero-Mode Waveguides. Nano Letters 2019, 19, 7434–7442.
- Baibakov, M.; Patra, S.; Claude, J.-B.; Moreau, A.; Lumeau, J.; Wenger, J. Extending Single-Molecule Forster Resonance Energy Transfer (FRET) Range beyond 10 Nanometers in Zero-Mode Waveguides. ACS nano 2019, 13, 8469–8480.
- Regmi, R.; Berthelot, J.; Winkler, P.M.; Mivelle, M.; Proust, J.; Bedu, F.; Ozerov, I.; Begou, T.; Lumeau, J.; Rigneault, H. All-Dielectric Silicon Nanogap Antennas to Enhance the Fluorescence of Single Molecules. Nano letters 2016, 16, 5143–5151.
- Jiang, Q.; Roy, P.; Claude, J.-B.; Wenger, J. Single Photon Source from a Nanoantenna-Trapped Single Quantum Dot. Nano Letters 2021, 21, 7030–7036.
- Gao, Q.; Zang, P.; Li, J.; Zhang, W.; Zhang, Z.; Li, C.; Yao, J.; Li, C.; Yang, Q.; Li, S.; et al. Revealing the Binding Events of Single Proteins on Exosomes Using Nanocavity Antennas beyond Zero-Mode Waveguides. ACS Appl. Mater. Interfaces 2023, 15, 49511–49526. [CrossRef]
- Barulin, A.; Roy, P.; Claude, J.-B.; Wenger, J. Ultraviolet Optical Horn Antennas for Label-Free Detection of Single Proteins. Nature Communications 2022, 13, 1842. [CrossRef]
- Skolrood, L.; Wang, Y.; Zhang, S.; Wei, Q. Single-Molecule and Particle Detection on True Portable Microscopy Platforms. Sensors and Actuators Reports 2022, 4, 100063.
- Macchia, E.; Torricelli, F.; Caputo, M.; Sarcina, L.; Scandurra, C.; Bollella, P.; Catacchio, M.; Piscitelli, M.; Di Franco, C.; Scamarcio, G. Point-Of-Care Ultra-Portable Single-Molecule Bioassays for One-Health. Advanced Materials 2024, 36, 2309705.
- Macchia, E.; Torricelli, F.; Bollella, P.; Sarcina, L.; Tricase, A.; Di Franco, C.; Osterbacka, R.; Kovacs-Vajna, Z.M.; Scamarcio, G.; Torsi, L. Large-Area Interfaces for Single-Molecule Label-Free Bioelectronic Detection. Chemical Reviews 2022, 122, 4636–4699.
- Sanaee, M.; Sandberg, E.; Ronquist, K.G.; Morrell, J.M.; Widengren, J.; Gallo, K. Coincident Fluorescence-Burst Analysis of the Loading Yields of Exosome-Mimetic Nanovesicles with Fluorescently-Labeled Cargo Molecules. Small 2022, 18. [CrossRef]
- Silva, A.M.; Lázaro-Ibáñez, E.; Gunnarsson, A.; Dhande, A.; Daaboul, G.; Peacock, B.; Osteikoetxea, X.; Salmond, N.; Friis, K.P.; Shatnyeva, O.; et al. Quantification of Protein Cargo Loading into Engineered Extracellular Vesicles at Single-vesicle and Single-molecule Resolution. J of Extracellular Vesicle 2021, 10, e12130. [CrossRef]
- Singh, A.; de Roque, P.M.; Calbris, G.; Hugall, J.T.; van Hulst, N.F. Nanoscale Mapping and Control of Antenna-Coupling Strength for Bright Single Photon Sources. Nano letters 2018, 18, 2538–2544.
- Xiong, Y.; Huang, Q.; Canady, T.D.; Barya, P.; Liu, S.; Arogundade, O.H.; Race, C.M.; Che, C.; Wang, X.; Zhou, L.; et al. Photonic Crystal Enhanced Fluorescence Emission and Blinking Suppression for Single Quantum Dot Digital Resolution Biosensing. Nature Communications 2022, 13, 4647. [CrossRef]
- Sun, S.; Li, M.; Du, Q.; Png, C.E.; Bai, P. Metal–Dielectric Hybrid Dimer Nanoantenna: Coupling between Surface Plasmons and Dielectric Resonances for Fluorescence Enhancement. The Journal of Physical Chemistry C 2017, 121, 12871–12884.
- Milichko, V.A.; Zuev, D.A.; Baranov, D.G.; Zograf, G.P.; Volodina, K.; Krasilin, A.A.; Mukhin, I.S.; Dmitriev, P.A.; Vinogradov, V.V.; Makarov, S.V. Metal-dielectric Nanocavity for Real-time Tracing Molecular Events with Temperature Feedback. Laser & Photonics Reviews 2018, 12, 1700227.
- Dmitriev, P.A.; Lassalle, E.; Ding, L.; Pan, Z.; Neo, D.C.; Valuckas, V.; Paniagua-Dominguez, R.; Yang, J.K.; Demir, H.V.; Kuznetsov, A.I. Hybrid Dielectric-Plasmonic Nanoantenna with Multiresonances for Subwavelength Photon Sources. ACS Photonics 2023, 10, 582–594.
- Mundy, W.; Roux, J.; Smith, A. Mie Scattering by Spheres in an Absorbing Medium. Journal of the Optical Society of America 1974, 64, 1593–1597.
- Vanecek, M.; Holoubek, J.; Shah, A. Optical Study of Microvoids, Voids, and Local Inhomogeneities in Amorphous Silicon. Applied physics letters 1991, 59, 2237–2239.
- Chen, C.-C. Electromagnetic Resonances of Immersed Dielectric Spheres. IEEE Transactions on Antennas and Propagation 1998, 46, 1074–1083.
- Hentschel, M.; Koshelev, K.; Sterl, F.; Both, S.; Karst, J.; Shamsafar, L.; Weiss, T.; Kivshar, Y.; Giessen, H. Dielectric Mie Voids: Confining Light in Air. Light: Science & Applications 2023, 12, 3. [CrossRef]
- Sarbajna, A.; Danielsen, D.R.; Casses, L.N.; Stenger, N.; Bøggild, P.; Raza, S. Encapsulated Void Resonators in Van Der Waals Heterostructures. Laser & Photonics Reviews 2025, 19, 2401215.
- Graydon, O. Mie Voids Generate Miniature Artworks. Nature Photonics 2023, 17, 133–133.
- Arslan, S.; Kappel, M.; Canós Valero, A.; Tran, T.T.H.; Karst, J.; Christ, P.; Hohenester, U.; Weiss, T.; Giessen, H.; Hentschel, M. Attoliter Mie Void Sensing. ACS Photonics 2025, 12, 3950–3958.
- Barulin, A.; Roy, P.; Claude, J.-B.; Wenger, J. Purcell Radiative Rate Enhancement of Label-Free Proteins with Ultraviolet Aluminum Plasmonics. J. Phys. D: Appl. Phys. 2021, 54, 425101. [CrossRef]
- Kayyil Veedu, M.; Wenger, J. Breaking the Low Concentration Barrier of Single-Molecule Fluorescence Quantification to the Sub-Picomolar Range. Small Methods 2025, n/a, 2401695. [CrossRef]
- Barulin, A.; Kim, I. Hyperlens for Capturing Sub-Diffraction Nanoscale Single Molecule Dynamics. Optics Express 2023, 31, 12162–12174.
- Punj, D.; Mivelle, M.; Moparthi, S.B.; Van Zanten, T.S.; Rigneault, H.; Van Hulst, N.F.; García-Parajó, M.F.; Wenger, J. A Plasmonic ‘Antenna-in-Box’Platform for Enhanced Single-Molecule Analysis at Micromolar Concentrations. Nature nanotechnology 2013, 8, 512–516.
- Kinkhabwala, A.; Yu, Z.; Fan, S.; Avlasevich, Y.; Müllen, K.; Moerner, W.E. Large Single-Molecule Fluorescence Enhancements Produced by a Bowtie Nanoantenna. Nature photonics 2009, 3, 654–657.
- Regmi, R.; Winkler, P.M.; Flauraud, V.; Borgman, K.J.; Manzo, C.; Brugger, J.; Rigneault, H.; Wenger, J.; García-Parajo, M.F. Planar Optical Nanoantennas Resolve Cholesterol-Dependent Nanoscale Heterogeneities in the Plasma Membrane of Living Cells. Nano letters 2017, 17, 6295–6302.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




