Submitted:
17 October 2025
Posted:
20 October 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction:
2. Methods
2.1. Procedural Details
Hernia Repair
Antibiotic Beads
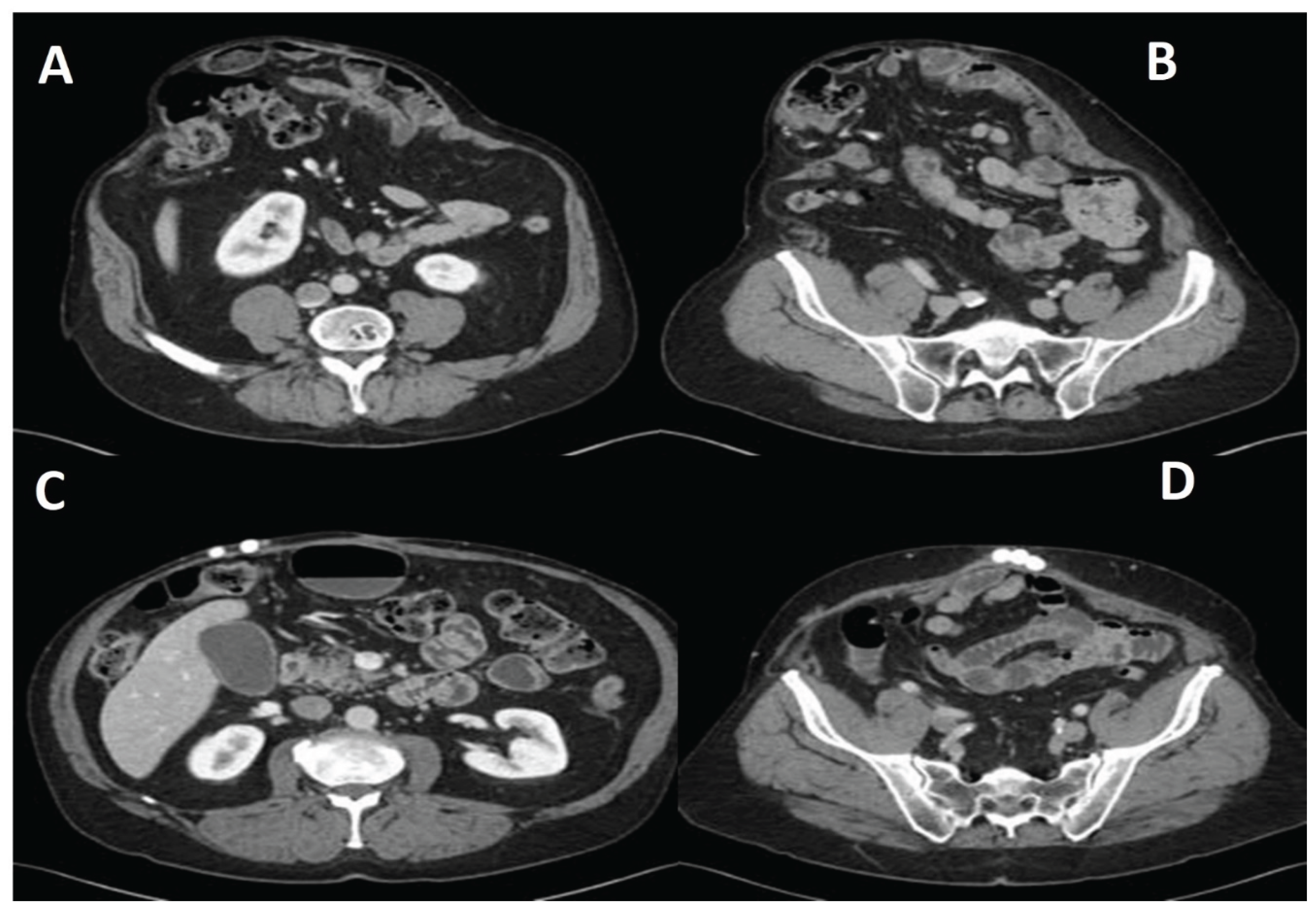
3. Results
4. Discussion
Limitations and Future Directions
5. Conclusions
References
- Poulose BK, Shelton J, Phillips S, Moore D, Nealon W, Penson D, et al. Epidemiology and cost of ventral hernia repair: making the case for hernia research. Hernia. 2012;16(2):179-83. [CrossRef]
- Mariette C, Wind P, Micelli Lupinacci R, Tresallet C, Adham M, Arvieux C, et al. Practice patterns in complex ventral hernia repair and place of biological grafts: a national survey among French digestive academic surgeons. J Visc Surg. 2014;151(1):9-16. [CrossRef]
- Arnold MR, Kao AM, Otero J, Marx JE, Augenstein VA, Sing RF, et al. Mesh fistula after ventral hernia repair: What is the optimal management? Surgery. 2020;167(3):590-7. [CrossRef]
- Holihan JL, Nguyen DH, Nguyen MT, Mo J, Kao LS, Liang MK. Mesh Location in Open Ventral Hernia Repair: A Systematic Review and Network Meta-analysis. World J Surg. 2016;40(1):89-99. [CrossRef]
- Holihan JL, Hannon C, Goodenough C, Flores-Gonzalez JR, Itani KM, Olavarria O, et al. Ventral Hernia Repair: A Meta-Analysis of Randomized Controlled Trials. Surg Infect (Larchmt). 2017;18(6):647-58. [CrossRef]
- Desai KA, Razavi SA, Hart AM, Thompson PW, Losken A. The Effect of BMI on Outcomes Following Complex Abdominal Wall Reconstructions. Ann Plast Surg. 2016;76 Suppl 4:S295-7. [CrossRef]
- Evans KK, Chim H, Patel KM, Salgado CJ, Mardini S. Survey on ventral hernias: surgeon indications, contraindications, and management of large ventral hernias. Am Surg. 2012;78(4):388-97. [CrossRef]
- Kockerling F. What Do We Know About the Chevrel Technique in Ventral Incisional Hernia Repair? Front Surg. 2019;6:15. [CrossRef]
- Ventral Hernia Working G, Breuing K, Butler CE, Ferzoco S, Franz M, Hultman CS, et al. Incisional ventral hernias: review of the literature and recommendations regarding the grading and technique of repair. Surgery. 2010;148(3):544-58. [CrossRef]
- Petro CC, O'Rourke CP, Posielski NM, Criss CN, Raigani S, Prabhu AS, et al. Designing a ventral hernia staging system. Hernia. 2016;20(1):111-7. [CrossRef]
- Krpata DM, Stein SL, Eston M, Ermlich B, Blatnik JA, Novitsky YW, et al. Outcomes of simultaneous large complex abdominal wall reconstruction and enterocutaneous fistula takedown. Am J Surg. 2013;205(3):354-8; discussion 8-9. [CrossRef]
- Trevino JM, Franklin ME, Jr., Berghoff KR, Glass JL, Jaramillo EJ. Preliminary results of a two-layered prosthetic repair for recurrent inguinal and ventral hernias combining open and laparoscopic techniques. Hernia. 2006;10(3):253-7. [CrossRef]
- Kugler NW, Bobbs M, Webb T, Carver TW, Milia D, Paul JS. A dual-stage approach to contaminated, high-risk ventral hernia repairs. J Surg Res. 2016;204(1):200-4. [CrossRef]
- Buchholz HW, Engelbrecht H. [Depot effects of various antibiotics mixed with Palacos resins]. Chirurg. 1970;41(11):511-5.
- Huiras P, Logan JK, Papadopoulos S, Whitney D. Local antimicrobial administration for prophylaxis of surgical site infections. Pharmacotherapy. 2012;32(11):1006-19. [CrossRef]
- Morgenstern M, Vallejo A, McNally MA, Moriarty TF, Ferguson JY, Nijs S, et al. The effect of local antibiotic prophylaxis when treating open limb fractures: A systematic review and meta-analysis. Bone Joint Res. 2018;7(7):447-56.
- White TL, Culliford AT, Zomaya M, Freed G, Demas CP. Use of Antibiotic-Impregnated Absorbable Beads and Tissue Coverage of Complex Wounds. Am Surg. 2016;82(11):1068-72. [CrossRef]
- McConoughey SJ, Howlin RP, Wiseman J, Stoodley P, Calhoun JH. Comparing PMMA and calcium sulfate as carriers for the local delivery of antibiotics to infected surgical sites. J Biomed Mater Res B Appl Biomater. 2015;103(4):870-7. [CrossRef]
- Aiken SS, Cooper JJ, Florance H, Robinson MT, Michell S. Local release of antibiotics for surgical site infection management using high-purity calcium sulfate: an in vitro elution study. Surg Infect (Larchmt). 2015;16(1):54-61. [CrossRef]
- Junker MS, Kurjatko A, Hernandez MC, Heller SF, Kim BD, Schiller HJ. Salvage of rib stabilization hardware with antibiotic beads. Am J Surg. 2019;218(5):869-75. [CrossRef]
- Juhasz ES, Wolff BG, Meagher AP, Kluiber RM, Weaver AL, van Heerden JA. Incidental cholecystectomy during colorectal surgery. Ann Surg. 1994;219(5):467-72; discussion 72-4. [CrossRef]

| Preoperative characteristics | Values |
|---|---|
| Age, y, mean (IQR) | 61.5 (23.0 – 83.0) |
| Female sex, n (%) | 24 (58.6) |
| BMI, kg/m2, mean (IQR) | 38.1 (25.4 – 62.0) |
| Current smoker, n (%) | 4 (9.3) |
| Anticoagulation use, n (%) | 7 (16.2) |
| Hypertension, n (%) | 30 (69.7) |
| COPD, n (%) | 13 (30.2) |
| Diabetes mellitus, n (%) | 11 (25.5) |
| Steroid use, n (%) | 3 (6.9) |
| Prior wound/mesh infection, n (%) | 28 (65.1) |
| Methicillin resistant Staphylococcus (MRSA), n (%) | 3 (6.9) |
| Operative characteristics | Values |
|---|---|
| Nature of surgery Elective, n (%) Urgent, n (%) |
29 (67.4) 14 (32.5) |
| Types of mesh Polypropylene, n (%) Absorbable, n (%) |
26 (60.4) 17 (39.5) |
| Mean surface area of mesh, cm2 | 561 |
| Antibiotic beads Exchanged, n (%) Left in-situ, n (%) |
38 (88.3) 28 (65.1) |
| Postoperative characteristics | Values |
|---|---|
| Hernia recurrence, n (%) Central lightweight mesh rupture, n (%) |
4 (9.3) 3 (6.9) |
| Skin separation, n (%) Repeated skin separation, n (%) |
13 (30.2) 3 (6.9) |
| Hematoma, n (%) | 1 (2.3) |
| Seroma, n (%) | 22 (48.8) |
| Mesh infection, n (%) | 2 (4.65) |
| Mortality, n (%) | 1 (2.3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




