1. Introduction
In Japan, 2.8% of men and 4.4% of women are reported to experience constipation, which significantly impacts quality of life (QOL) [
1,
2,
3]. However, constipation is often managed based on patients’ subjective complaints, without objective assessment. This leads to poor communication, resulting in mismatched treatment and low patient satisfaction. This dissatisfaction often drives patients to attempt self-management, leading to treatment that increasingly deviates from appropriate care.
Ultrasonography (US) is a non-invasive examination that can be performed repeatedly. Consequently, US has been considered for evaluating chronic constipation, which often requires repeated assessment [
4,
5,
6]. Furthermore, the Study Group for US for Chronic Constipation proposed diagnosis and treatment algorithms based on rectal US findings and compiled them into a consensus document [
5,
7]. The consensus document classifies chronic constipation into three categories (“no fecal retention,” “fecal retention without hard stools,” and “fecal retention with hard stools”) based on rectal US findings and proposes appropriate management for each category.
Elobixibat, an ileal bile acid transporter (IBAT) inhibitor, blocks bile acid reabsorption in the ileum, thereby increasing the amount of bile acids entering the colon [
8]. These bile acids increase water and electrolyte influx into the colon, triggering strong bowel movements. In addition, elobixibat lowers rectal sensory thresholds and restores the urge to defecate in patients with chronic constipation [
9,
10]. Therefore, elobixibat is a promising treatment option for constipation because its mechanism differs from that of conventional laxatives [
7,
10].
The diagnosis and treatment algorithm for chronic constipation using rectal US findings recommends elobixibat, an IBAT inhibitor, as a first-line treatment for patients with “no fecal retention” whose condition does not improve with lifestyle modification or dietary therapy [
7]. This algorithm was developed based on expert opinion as well as drug characteristics. However, few studies have validated this three-category US classification and the corresponding management strategies, indicating the need for further evaluation. Moreover, in real-world practice, the presence of rectal gas cannot be ignored. Rectal evacuation disorder (RED) is a leading cause of intractable constipation not represented in the current three-category classification. Gas retention in the rectum and colon has been reported in patients with RED [
11]. In addition, gas retention in the descending colon has also been described among patients reporting incomplete evacuation [
12]. These patients may present with features not explained by the three-category classification, indicating that gas-dominant constipation is not well represented in the current system.
This retrospective observational study aimed to evaluate the efficacy and safety of elobixibat in patients with chronic constipation classified as “no fecal retention” by rectal US. Furthermore, the study addressed gas-dominant constipation by expanding the conventional three-category classification to four categories through the addition of “gas retention”: “no fecal retention,” “fecal retention without hard stools,” “fecal retention with hard stools,” and “gas retention.” We retrospectively analyzed patients with chronic constipation who underwent rectal US and received elobixibat and evaluated treatment outcomes according to this four-category classification. This study demonstrates that rectal US-based classification, especially the “no fecal retention” category, provides a practical framework for guiding elobixibat therapy in patients with chronic constipation.
2. Materials and Methods
2.1. Study Participants
This retrospective observational study utilized data collected between May 2019 and December 2024 at Hakodate Medical Center and Sapporo Cancer Screening Center. The study enrolled 32 patients aged ≥18 years with chronic constipation who underwent rectal US and received elobixibat. Patients who underwent rectal US on the day before the first elobixibat administration and received the drug at the recommended dose of 10 mg/day were included. The exclusion criteria were as follows: a history of hypersensitivity to elobixibat; confirmed or suspected intestinal obstruction due to tumors, hernia, or other causes; suspected constipation secondary to organic disease; or the use of intestinal cleansing agents, enemas, or bowel lavage from 2 days before to 3 days after rectal US.
2.2. Observation Period and Data Collection
Patient data were collected from the day before elobixibat initiation until 2 weeks after treatment. Information was collected from medical records and questionnaires, including age, sex, height, weight, comorbidities, prior medications, rectal US findings, colonic diameters, elobixibat dosing, spontaneous bowel movements (SBMs) during the first 3 days after elobixibat initiation, Bristol Stool Form Scale (BSFS), Constipation Scoring System (CSS), and adverse events [
13,
14].
2.3. Assessment of Bowel Movements
Bowel function was assessed by documenting the presence or absence of SBMs during the first 3 days after elobixibat initiation using patient questionnaires. SBMs were defined as bowel movements occurring without the use of bisacodyl suppositories, enemas, or digital disimpaction. Patients who experienced SBMs within this period were defined as responders.
2.4. Stool Form and Constipation Scoring
Stool consistency and constipation severity were evaluated using the BSFS and calculated modified CSS scores, respectively, based on entries in medical records and questionnaires. The modified CSS total score was calculated by subtracting the item “duration of constipation” from the CSS total score. Both BSFS and CSS were assessed at baseline and 2 weeks after treatment.
2.5. Ultrasonography (US)
Rectal ultrasound images were obtained using an Aplio i700® system (Canon Medical Systems, Japan) equipped with a convex probe (PVI-475BX). Imaging conditions were set as follows: depth, 14–21 cm; frequency, 4.0 MHz; gain, 73–80 dB; and dynamic range, 60 dB. Rectal US was performed in the supine position using a transabdominal approach with the probe placed on the suprapubic region.
2.6. Rectal US Classification
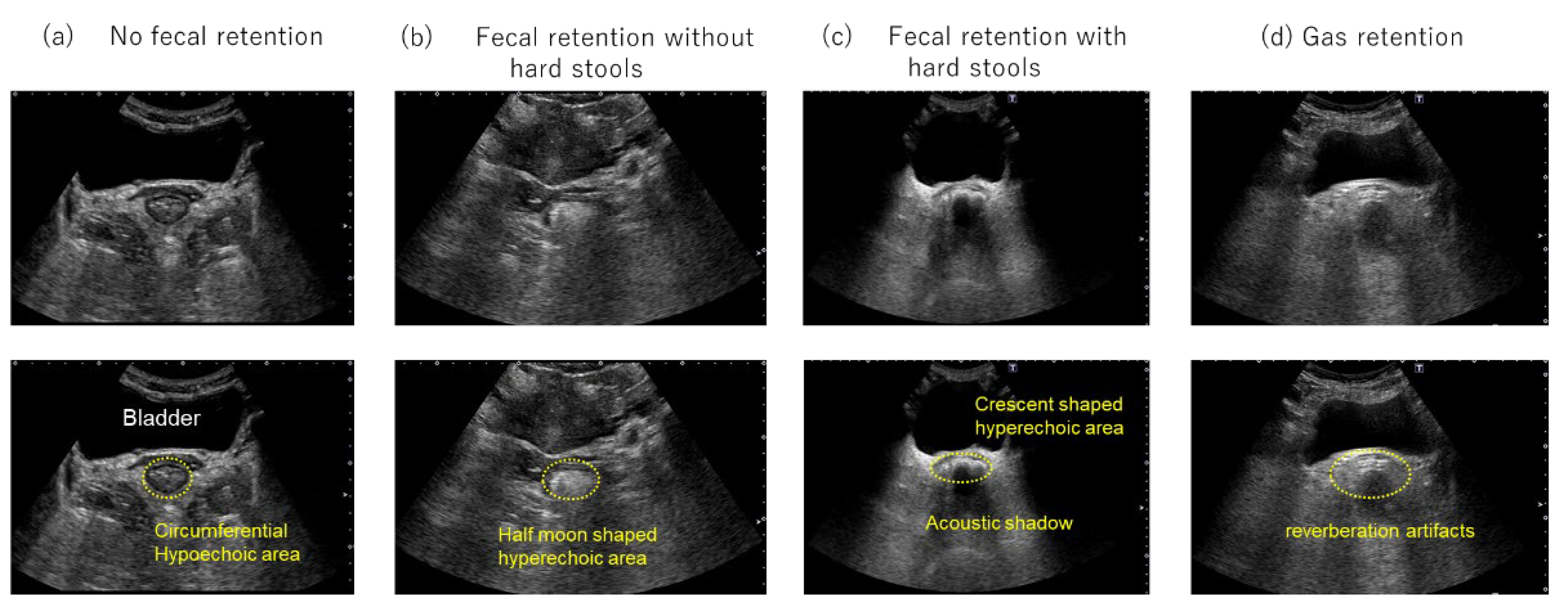
To classify rectal findings, the conventional three-category classification (no fecal retention, fecal retention without hard stools, and fecal retention with hard stools) was expanded by adding a fourth category, “gas retention.” The “gas retention” category was defined as the presence of multiple echoes with reverberation artifacts in the rectum on transabdominal US (
Figure 1).
2.7. Measurement of Colonic Diameter
To measure colonic diameter, US was performed at five sites: ascending colon, transverse colon, descending colon, sigmoid colon, and rectum [
4]. Up to three measurements (proximal, middle, and distal) were obtained for each site. The diameter for each site was calculated as the average of its measurements, and the mean colonic diameter was obtained by averaging across all sites. Colonic diameter was assessed at baseline only.
2.8. Statistical Analysis
All analyses were conducted using R (version 4.4.1; R Core Team, 2024). Continuous variables are summarized as means with standard deviations or medians with interquartile ranges and categorical variables as frequencies and percentages. The Wilson score method was used to calculate 95% confidence intervals (CIs). Before-and-after values were compared using the Wilcoxon signed-rank test. A two-sided p-value < 0.05 was considered statistically significant. A correlation analysis was conducted to examine the association between mean colonic diameter and rectal US classification. For this analysis, only patients with measurements available for all five colonic regions were included. We used linear regression models were used with “no fecal retention” as the reference group to estimate differences in transverse diameter.
3. Results
3.1. Patient Enrollment and Analysis Sets
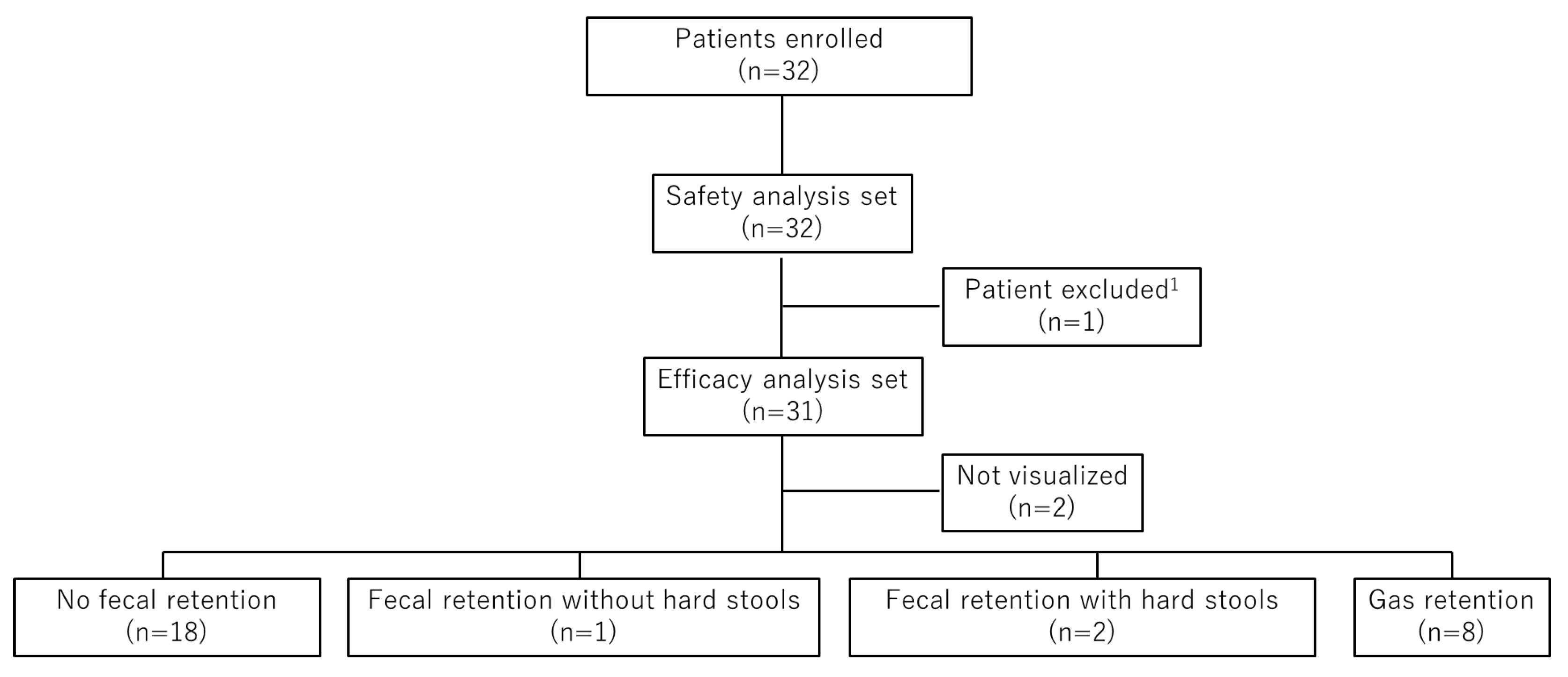
During the study period, 249 patients underwent rectal US, and 110 were prescribed elobixibat after the procedure. Among them, 32 patients met the eligibility criteria and were enrolled in the study. All 32 patients were included in the safety analysis set. One patient with a colostomy was excluded from the efficacy analysis set, leaving 31 patients for efficacy evaluation. Based on rectal US findings, 18 patients were classified as “no fecal retention,” 1 as “fecal retention without hard stools,” 2 as “fecal retention with hard stools,” and 8 as “gas retention.” Rectal US findings could not be visualized in two patients (
Figure 2).
3.2. Patient Characteristics
Patient characteristics are summarized in
Table 1. Most patients were female in both the efficacy and safety analysis sets. The mean age was 56.8 years in the efficacy set and 56.5 years in the safety set. Baseline bowel movement data (median) for the efficacy and safety sets, respectively, were as follows: BSFS score, 1.0 and 1.0; CSS total score, 13.5 and 13.5; and modified CSS total score, 10.0 and 10.0. CSS sub-scores were as follows: frequency of bowel movements, 1.0 and 1.0; painful evacuation effort, 2.0 and 2.0; feeling of incomplete evacuation, 3.0 and 3.0; abdominal pain, 1.0 and 1.0; minutes in lavatory per attempt, 1.0 and 1.0; type of assistance, 1.0 and 1.0; unsuccessful evacuation attempts per 24 hours, 1.0 and 1.0; and duration of constipation, 3.0 and 3.0 (
Table 1).
3.3. Proportion of Responders with SBMs Within 3 Days After the First Dose of Elobixibat
The primary endpoint was the proportion of responders in the “no fecal retention” category who had SBMs within 3 days after the first dose of elobixibat. In this category, 94.4% (95% CI, 74.2%–99.0%) achieved SBMs. Response rates were also 100% in the “fecal retention without hard stools” (95% CI, 20.7%–100.0%), “fecal retention with hard stools” (95% CI, 34.2%–100.0%), and “gas retention” (95% CI, 67.6%–100.0%) categories. Overall, the response rate across all four categories was 96.6% (95% CI, 82.8%–99.4%) (
Table 2). Both patients whose rectal US findings were not visualized also responded (data not shown).
3.4. Proportion of Responders with SBMs on Day 1 Following the First Dose of Elobixibat
On day 1, 83.3% (95% CI, 60.8%–94.2%) of patients in the “no fecal retention” category achieved SBMs. Response rates were 100% in the “fecal retention with hard stools” (95% CI, 34.2%–100.0%) and “gas retention” (95% CI, 67.6%–100.0%) categories. Overall, the response rate across all four categories was 89.3% (95% CI, 72.8%–96.3%) (
Table 3).
3.5. Stool Consistency Outcomes (BSFS)
Changes in stool consistency were evaluated using the BSFS. In patients classified as “no fecal retention,” the median BSFS scores were 1.5 (1.0–5.0) at baseline and 4.0 (2.5–5.0) at week 2, showing no significant difference. In the “fecal retention with hard stools” category, the BSFS scores were 1.5 (1.0–2.0) at baseline and 1.0 (1.0–1.0) at week 2, showing no improvement. In contrast, patients in the “gas retention” category demonstrated a significant improvement in BSFS scores from 1.0 (1.0–3.0) at baseline to 4.0 (2.5–4.5) at week 2 (
p = 0.0350). When analyzed across all four categories, BSFS scores significantly improved from 1.0 (1.0–3.5) at baseline to 4.0 (2.0–5.0) at week 2 (
p = 0.0104) (
Table 4).
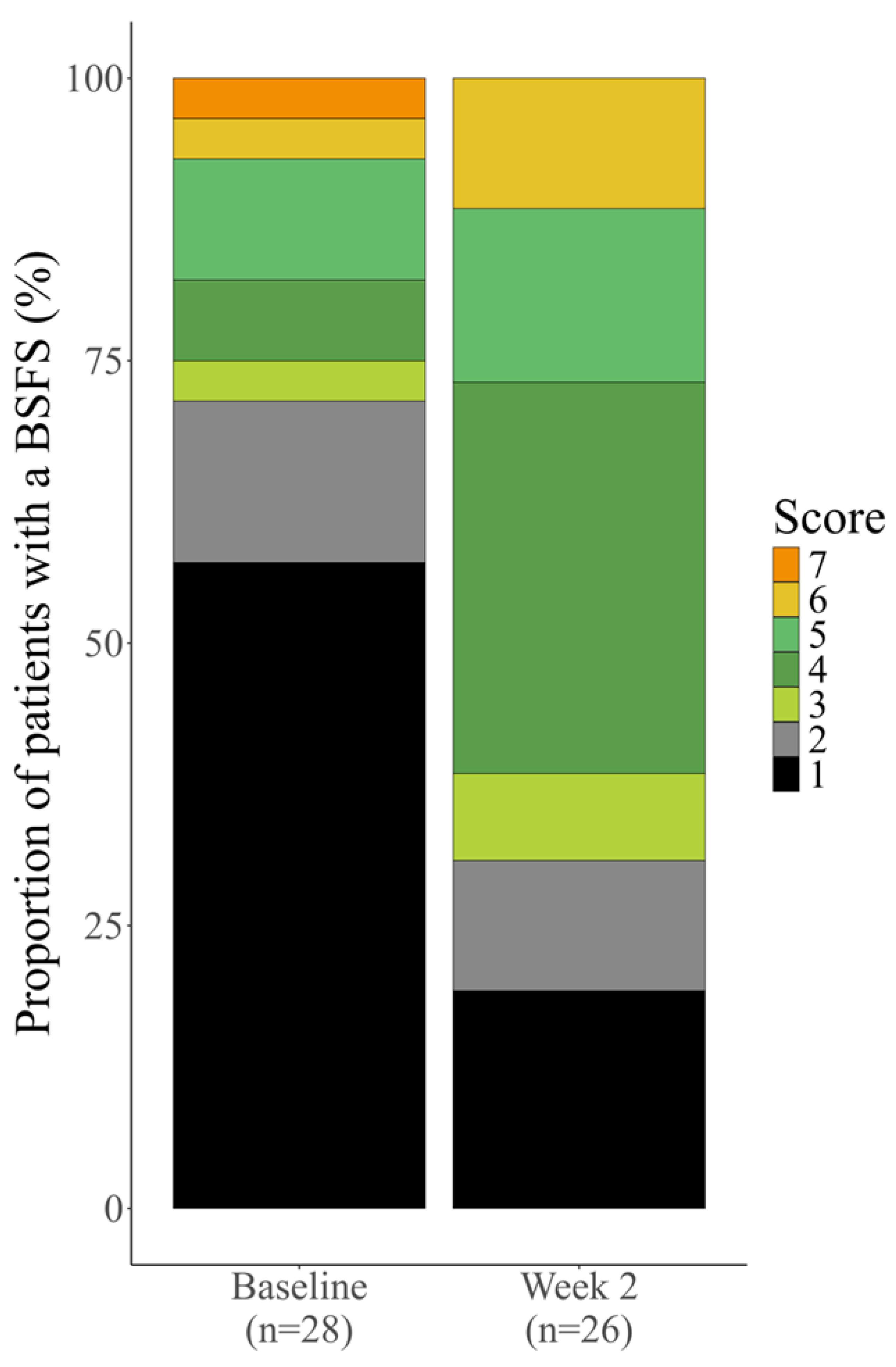
We also analyzed the distribution of stool consistency in the total cohort. The proportion of patients with a BSFS score of 1 or 2 decreased from 71.4% (20/28 patients) at baseline to 30.8% (8/26 patients) after elobixibat treatment. The proportion of patients with a BSFS score of 3–5 increased from 21.4% (6/28 patients) to 57.7% (15/26 patients), and that of patients with a BSFS score of 6 or 7 increased from 7.1% (2/28 patients) to 11.5% (3/26 patients). These findings indicate that elobixibat shifted stool consistency toward normal or softer stools (
Figure 3).
3.6. Constipation Scoring System (CSS) Outcomes
3.6.1. Modified CSS Total Score
Changes in the modified CSS total score were evaluated. In patients classified as “no fecal retention,” the median score decreased from 10.0 (7.0–14.0) at baseline to 8.0 (6.0–12.0) at week 2, with no significant difference. In the “fecal retention without hard stools” and “fecal retention with hard stools” categories, scores showed no consistent trend. In patients classified as “gas retention,” the score decreased from 10.0 (6.0–10.0) at baseline to 6.0 (3.0–11.5) at week 2, but the change was not statistically significant (
p = 0.0502). When analyzed across all four categories, the modified CSS total score significantly decreased from 10.0 (7.5–13.5) at baseline to 8.5 (5.0–12.0) at week 2 (
p = 0.0231) (
Table 5).
3.6.2. Modified CSS Sub-Scores
Changes in individual CSS sub-scores were also examined. No significant differences were observed in any sub-score when patients were classified by rectal US classification (data not shown).
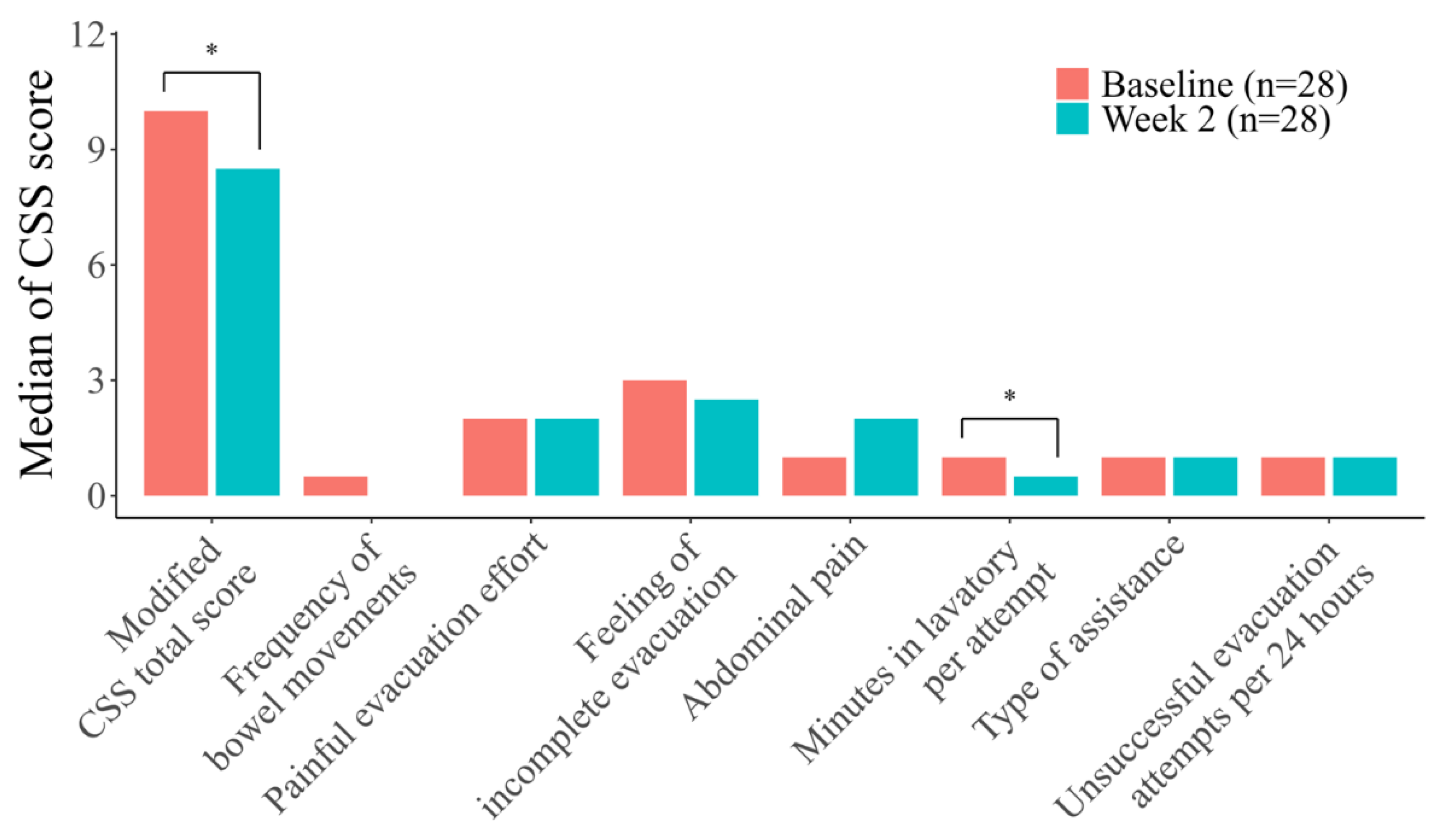
However, when analyzed across all four categories, the sub-score “minutes in lavatory per attempt” showed significant improvement after elobixibat treatment (
p = 0.0046). No other sub-scores demonstrated significant changes (
Table 6,
Figure 4).
3.7. Relationship Between Colonic Diameter and Rectal US Classification
The relationship between the mean transverse colonic diameter and rectal US classification was evaluated. The mean diameter was 25.00 ± 1.23 mm in patients with “no fecal retention,” 33.09 ± 5.88 mm in those with “fecal retention with hard stools,” and 28.86 ± 1.43 mm in those with “gas retention.” The overall mean diameter across all four categories was 27.31 ± 3.41 mm. In the “fecal retention without hard stools” category, no measurements were available (
Table 7). A correlation ratio of 0.694 was observed between the total classification and mean transverse diameter, indicating a positive association (
Table S1).
Linear regression analysis showed that the mean transverse diameter was significantly greater in patients with “fecal retention with hard stools” (
p = 0.0002) or “gas retention” (
p = 0.0027) than in those without fecal retention (
Table 8).
3.8. Safety
Adverse events occurred in two patients (6.3%) in the safety analysis set (
Table 9). All events were mild and resolved without intervention. No serious adverse events occurred, and no patients discontinued elobixibat during the 2-week observation period.
4. Discussion
This study demonstrated that elobixibat was highly effective and well tolerated in patients with chronic constipation, particularly in those categorized as “no fecal retention” on rectal US. The primary endpoint, defined as the proportion of responders achieving SBMs within 3 days of the first dose, was 94.4% in this group, underscoring the rapid therapeutic benefit of elobixibat. Early symptom relief is clinically relevant because it improves treatment adherence and patient satisfaction. The day 1 response rate (83.3%) was also consistent with previous Phase 3 data, supporting the reproducibility of our findings [
15].
The therapeutic benefit in the “no fecal retention” group can be explained by the unique pharmacological properties of elobixibat. Unlike conventional laxatives, elobixibat promotes bowel movements through multiple mechanisms: inhibition of IBAT to increase bile acid flow and fluid secretion, acceleration of large intestinal motility, and restoration of the urge to defecate [
9,
10]. Kessoku et al. recently reported that, in patients with “no fecal retention,” elobixibat significantly improved complete SBMs, colonic transit time, and the urge to defecate compared with magnesium oxide [
16]. Other studies have also shown that elobixibat enables switching from, or reducing the dose of, stimulant laxatives [
17,
18]. Together, these findings highlight elobixibat’s clinical value as a first-line therapy for patients without fecal retention.
Patients with rectal gas retention also responded favorably to elobixibat, suggesting its potential role in gas-dominant constipation. In this category, all patients achieved SBMs within 3 days, and stool form improved significantly to BSFS type 4 by week 2. Since gas retention has been associated with rectal evacuation disorder and incomplete evacuation, these results suggest that elobixibat may be effective in a subset of patients not fully represented in the current three-category algorithm [
11,
12]. For such conditions, decreased rectal sensation has been proposed as an underlying mechanism [
19]. Elobixibat has been reported to improve rectal sensory thresholds and may thus be particularly effective for constipation accompanied by gas retention [
9]. However, structural defecatory disorders, such as rectocele or megacolon, cannot be excluded by rectal US alone, and complementary diagnostic modalities remain necessary [
6].
The impact of rectal US classification was further reflected in colonic diameter measurements. Patients in the “fecal retention with hard stools” and “gas retention” categories had significantly larger transverse colonic diameters than those in the “no fecal retention” group, indicating that both hard stool and gas retention are physiologically linked with upstream colonic dilatation. This supports the clinical utility of rectal US as a noninvasive tool for stratifying constipation phenotypes.
Elobixibat was generally safe and well tolerated in this cohort. Only two patients (6.3%) reported mild gastrointestinal adverse events (abdominal distension and abdominal pain), both resolving spontaneously without discontinuation. This profile aligns with previous clinical trials, underscoring the suitability of elobixibat for the long-term management of chronic constipation [
15,
20].
Several limitations of this study should be noted. The retrospective design and small sample size limit the strength of causal inference. The observation period was short, focusing only on early outcomes; thus, long-term improvements in stool consistency, symptom severity and QOL could not be assessed. Prior studies have demonstrated the sustained efficacy of elobixibat over longer follow-up periods [
21,
22]. Moreover, as this study was conducted exclusively in Japan, the generalizability of the findings to other populations remains uncertain. Future studies will need to validate these findings in larger, prospective, multicenter cohorts and should explore long-term outcomes, including stool consistency, symptom severity, and QOL. In addition, further research will clarify the role of gas-dominant constipation and refine rectal US–based treatment algorithms for broader clinical application.
Elobixibat showed clear and rapid efficacy in patients with chronic constipation, particularly in those with “no fecal retention” on rectal US. Patients with rectal gas retention also benefited, highlighting the value of incorporating this category into diagnostic algorithms. These findings support rectal US-based classification as a practical tool for guiding treatment and confirm elobixibat as an effective first-line option for patients without fecal retention
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org. Table S1: Correlation analysis between mean transverse colonic diameter Ultrasonography (US) diagnostic classification.
Author Contributions
Conceptualization and methodology, M.T.; formal analysis, M.T.; investigation, M.T., T.O., K.K. (Kanako Konishi) and M.M.; resources, S.K. and Y.H.; writing – original draft, M.T.; writing – review and editing, S.K., Y.H. and M.K.; supervision, N.M. and K.K. (Kimitoshi Kubo). All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the co-researchers, EA Pharma and Mochida Pharmaceutical Co., Ltd. EA Pharma and Mochida Pharmaceutical Co., Ltd. consulted with the study representative/investigators regarding the design, summary and contents of the study to be published but were not involved in the preparation of case reports, monitoring, data management or statistical analyses.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki. In addition, prior to study initiation, the protocol was approved by the Ethics Committees of the National Hospital Organization, Hakodate Medical Center (approval code, R6-1206001; date of approval, December 6, 2024) and the Hokkaido Cancer Society, Sapporo Cancer Screening Center (approval code, 2024-008; date of approval, December 27, 2024). The study was registered with UMIN-CTR on January 6, 2025 [UMIN000056635].
Informed Consent Statement
No informed consent was required since this was a retrospective study performed using existing information [Ethical Guidelines for Medical and Biological Research Involving Human Subjects]. Information on the study was posted on the websites of the participating medical institutions for a period of 3 months to ensure that potentially eligible patients had the opportunity to opt out of participation in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy and ethical restrictions.
Acknowledgments
This study was conducted with funding from co-researchers, EA Pharma and Mochida Pharmaceutical Co., Ltd. Some operations necessary for carrying out the study were outsourced to SRD Co., Ltd.
Conflicts of Interest
M.T. has received honoraria from EA Pharma Co., Ltd. S. K. and Y. H. are employees of EA Pharma Co., Ltd. The other authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| BSFS |
Bristol stool form scale |
| CSS |
Constipation scoring system |
| CI |
Confidence Interval |
| IBAT |
Ileal bile acid transporter |
| MedDRA/J |
Medical Dictionary for Regulatory Activities/J |
| QOL |
Quality of life |
| RED |
Rectal evacuation disorder |
| SBM |
Spontaneous bowel movement |
| US |
Ultrasonography |
References
- Summary Report of Comprehensive Survey of Living Conditions 2022. Available online: https://www.mhlw.go.jp/english/database/db-hss/dl/report_gaikyo_2022.pdf (accessed on 05 Sep 2025).
- Chang, J.Y.; Locke, G.R. 3rd; McNally, M.A.; Halder, S.L.; Schleck, C.D.; Zinsmeister, A.R.; Talley, N.J. Impact of functional gastrointestinal disorders on survival in the community. Am J Gastroenterol 2010, 105, 822–832. [Google Scholar] [CrossRef] [PubMed]
- Tomita, T.; Kazumori, K.; Baba, K.; Zhao, X.; Chen, Y.; Miwa, H. Impact of chronic constipation on health-related quality of life and work productivity in Japan. J Gastroenterol Hepatol 2021, 36, 1529–1537. [Google Scholar] [CrossRef] [PubMed]
- Manabe, N.; Kamada, T.; Kusunoki, H.; Hata, J.; Haruma, K. Usefulness of ultrasonographic evaluation of stool and/or gas distribution for the treatment strategy of chronic constipation. JGH Open 2019, 3, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Misawa, N.; Tsuda, M.; Manabe, N.; Kessoku, T.; Tamai, N.; Kawamoto, A.; Sugama, J.; Tanaka, H.; Kato, M.; et al. Expert consensus document: diagnosis for chronic constipation with faecal retention in the rectum using ultrasonography. Diagnostics (Basel) 2022, 12, 300. [Google Scholar] [CrossRef]
- Ihara, E.; Manabe, N.; Ohkubo, H.; Ogasawara, N.; Ogino, H.; Kakimoto, K.; Kanazawa, M.; Kawahara, H.; Kusano, C.; Kuribayashi, S.; et al. Evidence-based clinical guidelines for chronic constipation 2023. Digestion 2025, 106, 62–89. [Google Scholar] [CrossRef]
- Kessoku, T.; Matsumoto, M.; Misawa, N.; Tsuda, M.; Miura, Y.; Uchida, A.; Toriumi, Y.; Onodera, T.; Arima, H.; Kawamoto, A.; et al. Expert consensus document: an algorithm for the care and treatment of patients with constipation based on ultrasonographic findings in the rectum. Diagnostics (Basel) 2024, 14, 1510. [Google Scholar] [CrossRef]
- Acosta, A.; Camilleri, M. Elobixibat and its potential role in chronic idiopathic constipation. Ther Adv Gastroenterol 2014, 7, 167–175. [Google Scholar] [CrossRef]
- Manabe, N.; Umeyama, M.; Ishizaki, S.; Ota, T.; Kuratani, S.; Katsumata, R.; Fujita, M.; Haruma, K.; Camilleri, M. Elobixibat improves rectal sensation in patients with chronic constipation aged ≥60 years: A randomised placebo-controlled study. BMJ Open Gastroenterol 2023, 10, e001257. [Google Scholar] [CrossRef]
- Ishikawa, T.; Fukuzawa, M. Efficacy of elobixibat on defecation desire in patients with chronic constipation: a single center, retrospective, observational study [in Japanese]. Shinryo Shinyaku 2021, 58, 865–872. [Google Scholar]
- Park, S.Y.; Khemani, D.; Nelson, A.D.; Eckert, D.; Camilleri, M. Rectal gas volume measured by computerized tomography identifies evacuation disorders in patients with constipation. Clin Gastroenterol Hepatol 2017, 15, 543–552.e4. [Google Scholar] [CrossRef]
- Seike, K.; Koda, K.; Takiguchi, N.; Oda, K.; Miyazaki, M. Gas volume analysis and postoperative bowel functional disorders in patients who received anterior resection for rectal cancer. Dis Colon Rectum 2003, 46, 661–666. [Google Scholar] [CrossRef]
- Lewis, S.J.; Heaton, K.W. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol 1997, 32, 920–924. [Google Scholar] [CrossRef]
- Agachan, F.; Chen, T.; Pfeifer, J.; Reissman, P.; Wexner, S.D. A constipation scoring system to simplify evaluation and management of constipated patients. Dis Colon Rectum 1996, 39, 681–685. [Google Scholar] [CrossRef]
- Nakajima, A.; Seki, M.; Taniguchi, S.; Ohta, A.; Gillberg, P.G.; Mattsson, J.P.; Camilleri, M. Safety and efficacy of elobixibat for chronic constipation: results from a randomised, double-blind, placebo-controlled, phase 3 trial and an open-label, single-arm, phase 3 trial. Lancet Gastroenterol Hepatol 2018, 3, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Kessoku, T.; Ishihara, Y.; Takahashi, K.; Dang, T.H.; Ohira, M.; Horiuchi, M.; Shibata, C.; Uchida, A.; Toriumi, Y.; Misawa, N.; et al. Comparison of the effectiveness of magnesium oxide and elobixibat on improving rectal sensitivity and colonic transit time in patients with chronic constipation: an observational study. Neurogastroenterol Motil 2025, e70106. [Google Scholar] [CrossRef] [PubMed]
- Uno, J.; Tsuboi, S.; Fujita, K. The utilization of elobixibat against Stimulant Laxatives administration to psychiatric disordered patients with chronic constipation: a single-center, retrospective observational study[in Japanese]. Jpn J Clin Psychopharmacol 2023, 26, 197–206. [Google Scholar]
- Eguchi, T.; Inatomi, O.; Shintani, S.; Momose, K.; Sako, T.; Takagi, M.; Fumihara, D.; Inoue, K.; Katayama, N.; Morisawa, T.; et al. Efficacy and safety of elobixibat in combination with or switched from conventional treatments of chronic constipation: A retrospective observational study. JGH Open 2024, 8, e70019. [Google Scholar] [CrossRef]
- Vollebregt, P.F.; Burgell, R.E.; Hooper, R.L.; Knowles, C.H.; Scott, S.M. Clinical impact of rectal hyposensitivity: a cross-sectional study of 2,876 patients with refractory functional constipation. Am J Gastroenterol 2021, 116, 758–768. [Google Scholar] [CrossRef]
- Kohno, T.; Okanobu, H.; Boda, K. Efficacy and safety of elobixibat for chronic constipation: a single-center prospective study [in Japanese]. Journal of Japanese Gastroenteroogical Association 2022, 6, 36–42. [Google Scholar]
- Nakajima, A.; Umeyama, M.; Higashikawa, M.; Shimada, Y.; Arai, Y. A multicenter, postmarketing surveillance of elobixibat in patients with chronic constipation in Japan: A final analysis report. Sage Open Med 2025, 13, 20503121251321659. [Google Scholar] [CrossRef]
- Shono, T.; Hyakutake, H. Efficacy and safety of long-term treatment with elobixibat in hemodialysis patients with chronic constipation: an observational study. Ren Replace Ther 2024, 10, 53. [Google Scholar] [CrossRef]
Figure 1.
Transverse rectal ultrasound images. (a) No fecal retention, with no hyperechoic area observed. A circumferential hypoechoic area is observed in the lower part of the bladder. (b) Fecal retention without hard stools, revealing a half-moon-shaped hyperechoic area in the lower part of the bladder. (c) Fecal retention with hard stools, revealing a crescent-shaped hyperechoic area with an acoustic shadow in the lower part of the bladder. (d) Gas retention, multiple echoes with reverberation artifacts are observed in the lower part of the bladder.
Figure 1.
Transverse rectal ultrasound images. (a) No fecal retention, with no hyperechoic area observed. A circumferential hypoechoic area is observed in the lower part of the bladder. (b) Fecal retention without hard stools, revealing a half-moon-shaped hyperechoic area in the lower part of the bladder. (c) Fecal retention with hard stools, revealing a crescent-shaped hyperechoic area with an acoustic shadow in the lower part of the bladder. (d) Gas retention, multiple echoes with reverberation artifacts are observed in the lower part of the bladder.
Figure 2.
Patient flow diagram. Thirty-two patients were enrolled, of whom 32 were included in the safety analysis set and 31 in the efficacy analysis set. Patients were classified into four rectal US categories: no fecal retention (n = 18), fecal retention without hard stools (n = 1), fecal retention with hard stools (n = 2), and gas retention (n = 8). 1 One patient with a colostomy was excluded because rectal US classification could not be performed.
Figure 2.
Patient flow diagram. Thirty-two patients were enrolled, of whom 32 were included in the safety analysis set and 31 in the efficacy analysis set. Patients were classified into four rectal US categories: no fecal retention (n = 18), fecal retention without hard stools (n = 1), fecal retention with hard stools (n = 2), and gas retention (n = 8). 1 One patient with a colostomy was excluded because rectal US classification could not be performed.
Figure 3.
Distribution of stool consistency in all four categories combined. BSFS score definitions:. 1 = separate hard lumps, like nuts; 2 = sausage-shaped but lumpy; 3 = like a sausage or snake but with cracks on its surface; 4 = like a sausage or snake, smooth and soft; 5 = soft blobs with clear-cut edges; 6 = fluffy pieces with ragged edges, a mushy stool; 7 = watery, no solid pieces. Data were compiled excluding patients with “Not evaluated” BSFS scores (baseline, n = 1; week 2, n = 3). Abbreviation: BSFS, Bristol Stool Form Scale.
Figure 3.
Distribution of stool consistency in all four categories combined. BSFS score definitions:. 1 = separate hard lumps, like nuts; 2 = sausage-shaped but lumpy; 3 = like a sausage or snake but with cracks on its surface; 4 = like a sausage or snake, smooth and soft; 5 = soft blobs with clear-cut edges; 6 = fluffy pieces with ragged edges, a mushy stool; 7 = watery, no solid pieces. Data were compiled excluding patients with “Not evaluated” BSFS scores (baseline, n = 1; week 2, n = 3). Abbreviation: BSFS, Bristol Stool Form Scale.
Figure 4.
Modified Constipation Scoring System (CSS) sub-scores in all four categories combined. Statistical analyses were performed using the Wilcoxon signed-rank test. Bars indicate items with statistically significant changes. * p < 0.05 Abbreviations: CSS, Constipation Scoring System.
Figure 4.
Modified Constipation Scoring System (CSS) sub-scores in all four categories combined. Statistical analyses were performed using the Wilcoxon signed-rank test. Bars indicate items with statistically significant changes. * p < 0.05 Abbreviations: CSS, Constipation Scoring System.
Table 1.
Patient characteristics.
Table 1.
Patient characteristics.
| Data |
Efficacy Analysis Set |
Safety Analysis Set |
| N |
Variable |
N |
Variable |
| Subjects |
31 |
|
32 |
|
| Male, n (%)1
|
|
6 (19.4) |
|
6 (18.8) |
| Female, n (%)1
|
|
25 (80.6) |
|
26 (81.3) |
| Age (years), mean ± SD |
31 |
56.8 ± 14.7 |
32 |
56.5 ± 14.6 |
| Height (cm), mean ± SD |
31 |
157.68 ± 7.19 |
32 |
157.56 ± 7.10 |
| Weight (kg), mean ± SD |
31 |
55.51 ± 11.96 |
32 |
55.18 ± 11.91 |
| Rectal US classification |
31 |
|
32 |
|
| No fecal retention, n (%)1
|
|
18 (58.1) |
|
18 (56.3) |
| Fecal retention without hard stools, n (%)1
|
|
1 (3.2) |
|
1 (3.1) |
| Fecal retention with hard stools, n (%)1
|
|
2 (6.5) |
|
2 (6.3) |
| Gas retention, n (%)1
|
|
8 (25.8) |
|
8 (25.0) |
| Not visualized, n (%)1, 2
|
|
2 (6.5) |
|
3 (9.4) |
| BSFS, median (IQR) |
30 |
1.0 (1.0–3.0) |
31 |
1.0 (1.0–4.0) |
| CSS |
|
|
|
|
| Frequency of bowel movements, median (IQR) |
30 |
1.0 (0.0–2.0) |
31 |
1.0 (0.0–2.0) |
| Painful evacuation effort, median (IQR) |
30 |
2.0 (0.0–3.0) |
31 |
2.0 (0.0–3.0) |
| Feeling of incomplete evacuation, median (IQR) |
30 |
3.0 (2.0–4.0) |
31 |
3.0 (2.0–4.0) |
| Abdominal pain, median (IQR) |
30 |
1.0 (0.0–2.0) |
31 |
1.0 (0.0–2.0) |
| Minutes in lavatory per attempt, median (IQR) |
30 |
1.0 (1.0–2.0) |
30 |
1.0 (1.0–2.0) |
| Type of assistance, median (IQR) |
30 |
1.0 (1.0–2.0) |
31 |
1.0 (1.0–2.0) |
| Unsuccessful evacuation attempts per 24 hours, median (IQR) |
30 |
1.0 (1.0–1.0) |
30 |
1.0 (1.0–1.0) |
| Duration of constipation, median (IQR) |
30 |
3.0 (2.0–4.0) |
31 |
3.0 (2.0–4.0) |
| CSS total score, median (IQR) |
30 |
13.5 (10.0–17.0) |
30 |
13.5 (10.0–17.0) |
| Modified CSS total score, median (IQR) |
30 |
10.0 (8.0–13.0) |
30 |
10.0 (8.0–13.0) |
| Colonic diameter |
|
|
|
|
| Rectum (mm), mean ± SD |
20 |
34.15 ± 13.18 |
20 |
34.15 ± 13.18 |
| Ascending colon (mm), mean ± SD |
24 |
35.70 ± 6.83 |
25 |
35.58 ± 6.72 |
| Transverse colon (mm), mean ± SD |
20 |
24.29 ± 3.34 |
21 |
24.35 ± 3.26 |
| Descending colon (mm), mean ± SD |
22 |
22.49 ± 4.02 |
23 |
22.26 ± 4.08 |
| Sigmoid colon (mm), mean ± SD |
22 |
21.80 ± 3.49 |
23 |
21.71 ± 3.43 |
| Mean colonic diameter (mm), mean ± SD |
24 |
28.32 ± 6.36 |
25 |
28.14 ± 6.29 |
| Comorbidities3
|
31 |
|
32 |
|
| Yes, n (%)1
|
|
13 (41.9) |
|
14 (43.8) |
| Mental disorder, n (%)1
|
|
3 (9.7) |
|
3 (9.4) |
| After cancer surgery, n (%)1
|
|
2 (6.5) |
|
3 (9.4) |
| Cholecystectomy, n (%)1
|
|
1 (3.2) |
|
1 (3.1) |
| Others, n (%)1, 4
|
|
8 (25.8) |
|
8 (25.0) |
| No, n (%)1
|
|
18 (58.1) |
|
18 (56.3) |
| Prior medications for constipation3, 5
|
31 |
|
32 |
|
| Yes, n (%)1
|
|
22 (71.0) |
|
23 (71.9) |
| Osmotic laxatives, n (%)1
|
|
12 (38.7) |
|
12 (37.5) |
| Stimulant laxatives, n (%)1
|
|
13 (41.9) |
|
14 (43.8) |
| Intestinal secretagogues, n (%)1, 6
|
|
2 (6.5) |
|
2 (6.3) |
| Others, n (%)1, 7
|
|
4 (12.9) |
|
4 (12.5) |
| No, n (%)1
|
|
9 (29.0) |
|
9 (28.1) |
| Prior medications for other comorbidities5
|
31 |
|
32 |
|
| Yes, n (%)1
|
|
17 (54.8) |
|
18 (56.3) |
| No, n (%)1
|
|
14 (45.2) |
|
14 (43.8) |
Table 2.
Proportion of responders with spontaneous bowel movements (SBMs) within 3 days after the first dose of elobixibat.
Table 2.
Proportion of responders with spontaneous bowel movements (SBMs) within 3 days after the first dose of elobixibat.
| Rectal US Classification |
N |
Responder, (n) |
Non-Responder, (n) |
Proportion of
Responders1, 2 (%) |
95% CI3 (%) |
| No fecal retention |
18 |
17 |
1 |
94.4 |
74.2–99.0 |
| Fecal retention without hard stools |
1 |
1 |
0 |
100.0 |
20.7–100.0 |
| Fecal retention with hard stools |
2 |
2 |
0 |
100.0 |
34.2–100.0 |
| Gas retention |
8 |
8 |
0 |
100.0 |
67.6–100.0 |
| Total of 4 categories |
29 |
28 |
1 |
96.6 |
82.8– 99.4 |
Table 3.
Proportion of responders with spontaneous bowel movements (SBMs) on day 1 following the first dose of elobixibat.
Table 3.
Proportion of responders with spontaneous bowel movements (SBMs) on day 1 following the first dose of elobixibat.
| Rectal US Classification |
N |
Responder, (n) |
Non-Responder, (n) |
Proportion of
Responders1, 2 (%) |
95% CI3 (%) |
| No fecal retention |
18 |
15 |
3 |
83.3 |
60.8–94.2 |
| Fecal retention without hard stools |
0 |
0 |
0 |
- |
- |
| Fecal retention with hard stools |
2 |
2 |
0 |
100.0 |
34.2–100.0 |
| Gas retention |
8 |
8 |
0 |
100.0 |
67.6–100.0 |
| Total of 4 categories |
28 |
25 |
3 |
89.3 |
72.8–96.3 |
Table 4.
Stool consistency outcomes (BSFS).
Table 4.
Stool consistency outcomes (BSFS).
| Rectal US Classification |
Baseline |
Week 2 |
| N |
Variable |
N |
Variable |
p1 |
| No fecal retention, median (IQR) |
18 |
1.5 (1.0–5.0) |
16 |
4.0 (2.5–5.0) |
0.1078 |
| Fecal retention without hard stools, median (IQR) |
1 |
1.0 (1.0–1.0) |
0 |
- |
- |
| Fecal retention with hard stools, median (IQR) |
2 |
1.5 (1.0–2.0) |
2 |
1.0 (1.0–1.0) |
1.0000 |
| Gas retention, median (IQR) |
7 |
1.0 (1.0–3.0) |
8 |
4.0 (2.5–4.5) |
0.0350*
|
| Total of 4 categories, median (IQR) |
28 |
1.0 (1.0–3.5) |
26 |
4.0 (2.0–5.0) |
0.0104*
|
Table 5.
Modified Constipation Scoring System (CSS) total score.
Table 5.
Modified Constipation Scoring System (CSS) total score.
| Rectal US Classification |
Baseline |
Week 2 |
| N |
Variable |
N |
Variable |
p1 |
| No fecal retention, median (IQR) |
18 |
10.0 (7.0–14.0) |
17 |
8.0 (6.0–12.0) |
0.2206 |
| Fecal retention without hard stools, median (IQR) |
1 |
10.0 (10.0–10.0) |
1 |
11.0 (11.0–11.0) |
1.0000 |
| Fecal retention with hard stools, median (IQR) |
2 |
16.5 (13.0–20.0) |
2 |
11.5 (11.0–12.0) |
0.3711 |
| Gas retention, median (IQR) |
7 |
10.0 (6.0–10.0) |
8 |
6.0 (3.0–11.5) |
0.0502 |
| Total of 4 categories, median (IQR) |
28 |
10.0 (7.5–13.5) |
28 |
8.5 (5.0–12.0) |
0.0231*
|
Table 6.
Modified Constipation Scoring System (CSS) sub-scores.
Table 6.
Modified Constipation Scoring System (CSS) sub-scores.
Rectal US
Classification |
CSS Sub-Score |
Timepoint |
N |
Variable, Median (IQR) |
p1 |
| Total of 4 categories |
Total score (excluding the duration of constipation)2
|
Baseline |
28 |
10.0 (7.5–13.5) |
0.0231*
|
| |
Week 2 |
28 |
8.5 (5.0–12.0) |
|
| |
Frequency of bowel movements |
Baseline |
28 |
0.5 (0.0–1.5) |
0.3543 |
| |
Week 2 |
28 |
0.0 (0.0–1.0) |
|
| |
Painful evacuation effort |
Baseline |
28 |
2.0 (0.0–3.0) |
0.6806 |
| |
Week 2 |
28 |
2.0 (0.0–3.0) |
|
| |
Feeling of incomplete evacuation |
Baseline |
28 |
3.0 (2.0–4.0) |
0.0615 |
| |
Week 2 |
28 |
2.5 (1.0–3.0) |
|
| |
Abdominal pain |
Baseline |
28 |
1.0 (0.0–2.0) |
0.1078 |
| |
Week 2 |
28 |
2.0 (0.5–2.0) |
|
| |
Minutes in lavatory per attempt |
Baseline |
28 |
1.0 (0.5–2.0) |
0.0046*
|
| |
Week 2 |
28 |
0.5 (0.0–1.0) |
|
| |
Type of assistance |
Baseline |
28 |
1.0 (1.0–2.0) |
0.0782 |
| |
Week 2 |
28 |
1.0 (0.0–1.0) |
|
| |
Unsuccessful evacuation attempts per 24 hours |
Baseline |
28 |
1.0 (1.0–1.0) |
0.0519 |
| |
Week 2 |
28 |
1.0 (0.5–1.0) |
|
Table 7.
Mean transverse colonic diameter stratified by rectal ultrasonography (US) classification.
Table 7.
Mean transverse colonic diameter stratified by rectal ultrasonography (US) classification.
| Rectal US Classification |
Number of Patients |
Mean Transverse Diameter of the Colon and Rectum1 (mm) |
| |
N2
|
Variable |
| No fecal retention, Mean ± SD |
9 |
25.00 ± 1.23 |
| Fecal retention without hard stools, Mean ± SD |
- |
- |
| Fecal retention with hard stools, Mean ± SD |
2 |
33.09 ± 5.88 |
| Gas retention, Mean ± SD |
6 |
28.86 ± 1.43 |
| Total of 4 categories, Mean ± SD |
17 |
27.31 ± 3.41 |
Table 8.
Prediction of transverse colonic diameter by rectal US classification (linear regression analysis).
Table 8.
Prediction of transverse colonic diameter by rectal US classification (linear regression analysis).
| Factor |
Estimated Difference1
|
95% CI |
p |
| Rectal US classification: fecal retention without hard stools |
- |
- |
- |
| Rectal US classification: fecal retention with hard stools |
8.09 |
[4.705–11.468] |
0.0002*
|
| Rectal US classification: gas retention |
3.86 |
[1.579–6.139] |
0.0027*
|
Table 9.
Adverse events in the safety analysis set.
Table 9.
Adverse events in the safety analysis set.
| |
Total |
Severity |
| |
Mild |
Moderate |
Severe |
| n (%) |
n (%) |
n (%) |
n (%) |
| Number |
|
|
32 |
32 |
32 |
32 |
| Adverse event |
|
|
2 (6.3) |
2 (6.3) |
0 (0.0) |
0 (0.0) |
| |
Gastrointestinal disorders |
|
2 (6.3) |
2 (6.3) |
0 (0.0) |
0 (0.0) |
| |
|
Abdominal distension |
1 (3.1) |
1 (3.1) |
0 (0.0) |
0 (0.0) |
| |
|
Abdominal pain |
1 (3.1) |
1 (3.1) |
0 (0.0) |
0 (0.0) |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).