Submitted:
15 December 2023
Posted:
15 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study design and scenario
2.2. Ethical aspects of the study and registry
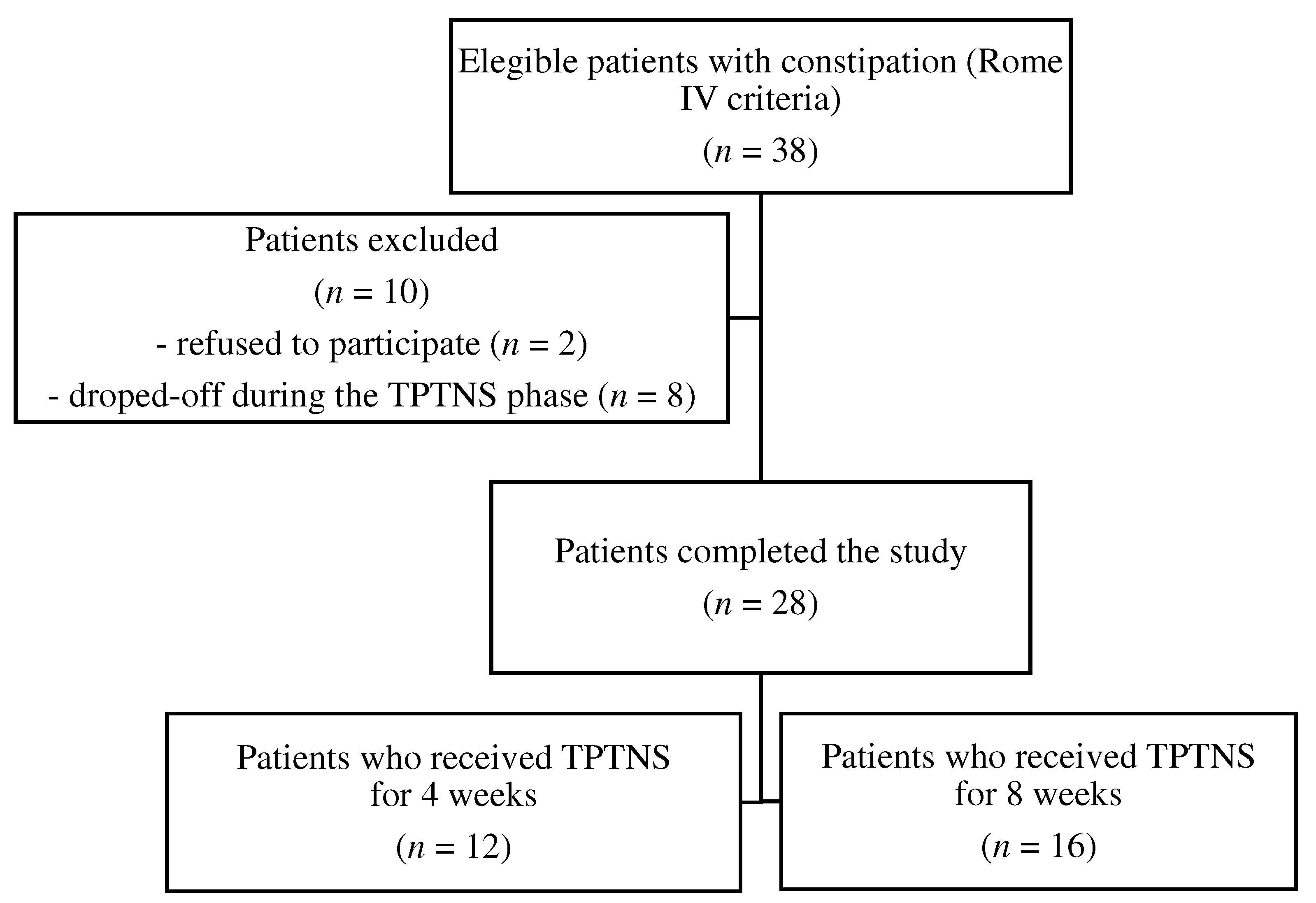
2.3. Eligibility criteria
2.4. Interventions
2.5. Treatment compliance and adverse events
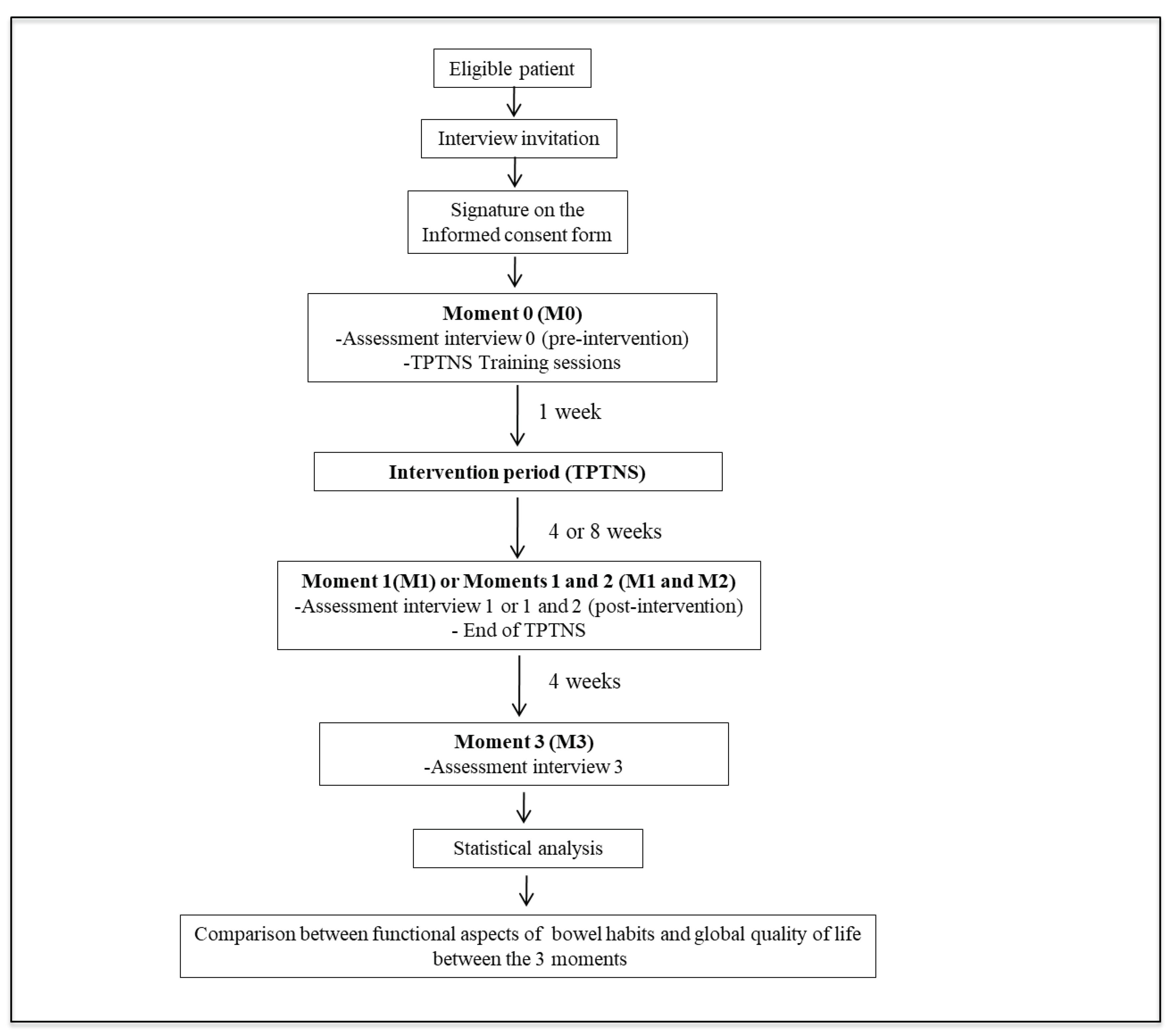
2.6. Periods of intervention and assessment moments
2.7. Assessment interviews
2.8. Analysis of results
2.9. Sample size calculation
2.10. Statistical analysis
3. Results
3.1. Demographic and clinical characteristics
3.2. Analysis of TPTNS effects on bowel function and quality of life
3.3. Analysis of TPTNS applicability
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
STROBE Statement
References
- Tabbers, M.M.; Boluyt, N.; Berger, M.Y.; Benninga, M.A. Clinical practice: diagnosis and treatment of functional constipation. Eur J Pediatr 2011, 170, 955–963. [Google Scholar] [CrossRef] [PubMed]
- Tabbers, M.M.; Di Lorenzo, C.; Berger, M.Y.; Faure, C.; Langedam, M.W.; Nurko, S.; Staiano Vandenplas, Y.; Benninga, M.A. Evaluation and treatment of functional constipation in infants and children: evidence-based recommendations from ESPGHAN and NASPGHAN. J Pediatr Gastroenterol Nutr 2014, 58, 258–274. [Google Scholar] [CrossRef] [PubMed]
- Liem, O.; Harman, J.; Benninga, M.; Kelleher, K.; Mousa, H.; Di Lorenzo, C. Health utilization and cost impact of childhood constipation in the United States. J Pediatr 2009, 154, 258–262. [Google Scholar] [CrossRef]
- Mugie, S.M.; Benninga, M.A.; Di Lorenzo, C. Epidemiology of constipation in children and adults: a systematic review. Best Pract Res Clin Gastroenterol 2011, 25, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Walia, R.; Mahajan, L.; Steffen, R. Recent advances in chronic constipation. Curr Opin Pediatr 2009, 21, 661–666. [Google Scholar] [CrossRef]
- Vriesman, M.H.; Rajindrajith, S.; Koppen, I.J.N.; van Etten-Jamaludin, F.S.; van Djik, M.; Devanarayana, N.M.; Tabbers, M.M.; Benninga, M.A. Quality of Life in Children with Functional Constipation: A Systematic Review and Meta-Analysis. J Pediatr 2019, 214, 141–150. [Google Scholar] [CrossRef]
- Vriesman, M.H.; Koppen, I.J.N.; Camilleri, M.; Di Lorenzo, C.; Benninga, M.A. Management of functional constipation in children and adults. Nat Rev Gastroenterol Hepatol 2020, 17, 21–39. [Google Scholar] [CrossRef]
- Koppen, I.J.N.; Lammers, L.A.; Benninga, M.A.; Tabbers, M.M. Management of Functional Constipation in Children: Therapy in Practice. Paediatr Drugs 2015, 17, 349–360. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence, (NICE). Constipation in children and young people: diagnosis and management. NICE Clinical Guideline 99. 26 May 2010. Available online: https://www.nice.org.uk/guidance/cg99.
- Bongers, M.E.J.; van Wijk, M.P.; Reitsma, J.B.; Benninga, M.A. Long-term prognosis for childhood constipation: clinical outcomes in adulthood. Pediatrics 2010, 126, e156–162. [Google Scholar] [CrossRef]
- Voskuijl, W.P.; van Ginkel, R.; Benninga, M.A.; Hart, G.A.; Taminiau, J.A.; Boeckxstaens, G.E. New insight into rectal function in pediatric defecation disorders: disturbed rectal compliance is an essential mechanism in pediatric constipation. J Pediatr 2006, 148, 62–67. [Google Scholar] [CrossRef]
- Sluka, K.A.; Walsh, D. Transcutaneous electrical nerve stimulation: basic science mechanisms and clinical effectiveness. J Pain 2003, 4, 109–121. [Google Scholar] [CrossRef]
- Lu, P.L.; Di Lorenzo, C. Neurostimulation of the gastrointestinal tract in children: is it time to shock the gut? Curr Opin Pediatr 2016, 28, 631–637. [Google Scholar] [CrossRef]
- Lecompte, J.F.; Hery, G.; Guys, J.M.; Louis-Borrione, C. Evaluation of transcutaneous electrical posterior tibial nerve stimulation for the treatment of fecal and urinary leaks in children: preliminary results. J Pediatr Surg 2015, 50, 630–633. [Google Scholar] [CrossRef]
- Iqbal, F.; Collins, B.; Thomas, G.P.; Askari, A.; Tan, E.; Nicholls, R.J.; Vaizey, C.J. Bilateral transcutaneous tibial nerve stimulation for chronic constipation. Colorectal Dis 2016, 18, 173–178. [Google Scholar] [CrossRef]
- Gokce, A.H.; Gokce, F.S.; Iliaz, R.; Gulaydin, N. Transcutaneous Tibial Nerve Stimulation as Therapy for Functional Constipation. Turk J Gastroenterol 2022, 33, 565–569. [Google Scholar] [CrossRef]
- Velasco-Benitez, C.; Villamarin, E.; Mendez, M.; Linero, A.; Hungria, G.; Saps, M. Efficacy of transcutaneous posterior tibial nerve stimulation in functional constipation. Eur J Pediatr 2023, 182, 1309–1315. [Google Scholar] [CrossRef]
- 18. Chan, A.-W.; Tetzlaff, J.M.; Altman, D.G.; Laupacis, A.; Gøtzsche, P.C.; Krleža-Jerić, K.; Hróbjartsson, A.; Mann, H.; Dickersin, K.; Berlin, J.; et al. SPIRIT 2013 Statement: defining standard protocol items for clinical trials. Ann Intern Med 2013, 158, 200–207. [Google Scholar] [CrossRef]
- Rego, R.M.P.; Machado, N.C.; Carvalho, M.A.; Graffunder, J.S.; Ortolan, E.V.P.; Lourenção, P.L.T.A. Transcutaneous posterior tibial nerve stimulation in children and adolescents with functional constipation: A protocol for an interventional study. Medicine (Baltimore) 2019, 98, e17755. [Google Scholar] [CrossRef]
- Hyams, J.S.; Di Lorenzo, C.; Saps, M.; Shulman, R.J.; Staiano, A.; van Tilburg, M. Functional disorders: children and adolescents. Gastroenterology 2016, 150, 1456–1468. [Google Scholar] [CrossRef]
- Benninga, M.A.; Faure, C.; Hyman, P.E.; Roberts, I.S.J.; Schechter, N.L.; Nurko, S. Childhood functional gastrointestinal disorders: neonate/toddler. Gastroenterology 2016, 150, 1443–1455. [Google Scholar] [CrossRef]
- Queralto, M.; Portier, G.; Cabarrot, P.H.; Bonnaud, G.; Chotard, J.P.; Nadrigny, M.; Lazorthes, F. Preliminary results of peripheral transcutaneous neuromodulation in the treatment of idiopathic fecal incontinence. Int J Colorectal Dis 2006, 21, 670–672. [Google Scholar] [CrossRef]
- Vitton, V.; Damon, H.; Roman, S.; Mion, F. Transcutaneous electrical posterior tibial nerve stimulation for faecal incontinence: effects on symptoms and quality of life. Int J Colorectal Dis 2010, 25, 1017–1020. [Google Scholar] [CrossRef]
- Arroyo Fernández, R.; Avendaño Coy, J.; Ando Lafuente, S.; Martín Correa, M.T.; Ferri Morales, A. Posterior tibial nerve stimulation in the treatment of fecal incontinence: a systematic review. Rev Esp Enferm Dig 2018, 110, 577–588. [Google Scholar] [CrossRef]
- Ramírez-García, I.; Blanco-Ratto, L.; Kauffmann, S.; Carralero-Martínez, A.; Sánchez, E. Efficacy of transcutaneous stimulation of the posterior tibial nerve compared to percutaneous stimulation in idiopathic overactive bladder syndrome: Randomized control trial. Neurourol Urodyn 2019, 38, 261–268. [Google Scholar] [CrossRef]
- George, A.T.; Maitra, R.K.; Maxwell-Armstrong, C. Posterior tibial nerve stimulation for fecal incontinence: Where are we? World J Gastroenterol 2013, 19, 9139–9145. [Google Scholar] [CrossRef] [PubMed]
- Chumpitazi, B.P.; Lane, M.M.; Czyzewski, D.I.; Weidler, E.M.; Swank, P.R.; Shulman, R.J. Creation and initial evaluation of a stool form scale for children. J Pediatr 2010, 157, 594–597. [Google Scholar] [CrossRef] [PubMed]
- Lane, M.M.; Czyzewski, D.I.; Chumpitazi, B.P.; Shulman, R.J. Reliability and validity of a modified Bristol Stool Form Scale for children. J Pediatr 2011, 159, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Jozala, D.R.; Oliveira, I.S.F.; Ortolan, E.V.P.; de Oliveira Jr, W.E.; Comes, G.T.; Cassettari, V.M.G.; Self, M.M.; Lourenção, P.L.T.A. Brazilian Portuguese translation, cross-cultural adaptation and reproducibility assessment of the modified Bristol Stool Form Scale for children. J Pediatr (Rio J) 2019, 95, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Jarvi, K.; Laitakari, E.M.; Koivusalo, A.; Rintala, R.J.; Pakarinen, M.P. Bowel function and gastrointestinal quality of life among adults operated for Hirschsprung disease during childhood: a population-based study. Ann Surg 2010, 252, 977–981. [Google Scholar] [CrossRef]
- Klatchoian, D.A.; Len, C.A.; Terreri, M.T.; Silva, M.; Itamoto, C.; Ciconelli, R.M.; Varni, J.W.; Hilário, M.O.E. Quality of life of children and adolescents from São Paulo: reliability and validity of the Brazilian version of the Pediatric Quality of Life Inventory version 4.0 Generic Core Scales. J Pediatr (Rio J) 2008, 84, 308–315. [Google Scholar] [CrossRef]
- Varni, J.W.; Burwinkle, T.M.; Seid, M.; Skarr, D. The PedsQL4.0 as a pediatric population health measure: feasibility, reliability, and validity. Ambul Pediatr 2003, 3, 329–341. [Google Scholar] [CrossRef]
- Mathias, A.L.; Tannuri, A.C.A.; Ferreira, M.A.E.; Santos, M.M.; Tannuri, U. Validation of questionnaires to assess quality of life related to fecal incontinence in children with anorectal malformations and Hirschsprung's disease. Rev Paul Pediatr 2016, 34, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Tannuri, A.C.A.; Ferreira, M.A.E.; Mathias, A.L.; Tannuri, U. Long-term results of the Duhamel technique are superior to those of the transanal pullthrough: A study of fecal continence and quality of life. J Pediatr Surg 2017, 52, 449–453. [Google Scholar] [CrossRef] [PubMed]
- Collins, B.; Norton, C.; Maeda, Y. Percutaneous tibial nerve stimulation for slow transit constipation: a pilot study. Colorectal Dis 2012, 14, e165–e170. [Google Scholar] [CrossRef] [PubMed]
- Iacona, R.; Ramage, L.; Malakounides, G. Current State of Neuromodulation for Constipation and Fecal Incontinence in Children: A Systematic Review. Eur J Pediatr Surg 2019, 29, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Correia, R.R.; Gameiro, L.F.O.; Trevisane, N.G.; Bertanha, M.; Ortolan, E.V.P.; Lourenção, P.L.T.A. Transcutaneous Neuromodulation for Constipation and Fecal Incontinence in Children: A Systematic Review and Meta-Analysis. Life (Basel) 2023, 13, 430. [Google Scholar] [CrossRef] [PubMed]
- van der Wilt, A.A.; van Wunnik, B.P.W.; Sturkenboom, R.; Han-Geurts, I.J.; Melenhorst, J.; Benninga, M.A.; Baeten, C.G.M.I.; Breukink, S.O. Sacral neuromodulation in children and adolescents with chronic constipation refractory to conservative treatment. Int J Colorectal Dis 2016, 31, 1459–1466. [Google Scholar] [CrossRef] [PubMed]
- Gaziev, G.; Topazio, L.; Iacovelli, V.; Asimakopoulos, A.; Di Santo, A.; De Nunzio, C.; Finazzi-Agrò, E. Percutaneous Tibial Nerve Stimulation (TPTNS) efficacy in the treatment of lower urinary tract dysfunctions: a systematic review. BMC Urol 2013, 13, 61. [Google Scholar] [CrossRef]
- Zhang, N.; Huang, Z.; Xu, F.; Xu, Y.; Chen, J.; Yin, J.; Lin, L.; Chen, J.D. Transcutaneous Neuromodulation at Posterior Tibial Nerve and ST36 for Chronic Constipation. Evid Based Complement Alternat Med 2014, 2014, 560802. [Google Scholar] [CrossRef]
- Russo, M.; Martinelli, M.; Sciorio, E.; Botta, C.; Miele, E.; Vallone, G.; Staiano, A. Stool consistency, but not frequency, correlates with total gastrointestinal transit time in children. J Pediatr 2012, 162, 1188–1192. [Google Scholar] [CrossRef]
- Rintala, R.J.; Lindahl, H. Is normal bowel function possible after repair of intermediate and high anorectal malformations? J Pediatr Surg 1995, 30, 491–494. [Google Scholar] [CrossRef]
- Baumgartner, P.C.; Haynes, R.B.; Hersberger, K.E.; Arnet, I. A Systematic Review of Medication Adherence Thresholds Dependent of Clinical Outcomes. Front Pharmacol 2018, 9, 1290. [Google Scholar] [CrossRef]
| Variable | |
|---|---|
| Children’s characteristics | |
| Gender | |
| Female | 16 (57.2) |
| Male | 12 (42.8) |
| Age (mo) | 134 (105-145) |
| Firstborn child | 15 (53.6) |
| Parents’ characteristics | |
| Maternal age (yr) | 36 (32-40) |
| Paternal age (yr) | 40 (33.5-45.5) |
| Maternal schooling (yr) | 11 (9.5-11) |
| Paternal schooling (yr) | 11 (8-11) |
| Number of children | 2 (1-3) |
| Caregiver / Respondent | |
| Mother | 22(78.6) |
| Grandmother | 4 (14.3) |
| Other | 2 (7.1) |
| Housing characteristics | |
| Number of rooms | 5 (5-6) |
| Number of people | 4 (3-5) |
| Agglomeration index | 1 |
| Variable | |
|---|---|
| Symptoms duration (mo) | 84 (36-120) |
| Characteristics of defecation | |
| Hard bowel movements | 23 (82.1) |
| Painful bowel movements | 21 (75.0) |
| Stools characteristics | |
| mBSFS-C | 2 (2-2) |
| Number of defecations per week | 2 (1-3) |
| Bloody stools | 8 (28.5) |
| Fecal incontinence | 5 (17.9) |
| Associated symptoms | |
| Vomiting | 4 (14.3) |
| Anorexia | 5 (17.9) |
| Abdominal pain | 12 (42.8) |
| Variable | n (%) |
|---|---|
| Maintenance of the medication dose | 17 (60.7) |
| Interruption of all laxatives | 3 (10.7) |
| Decrease in laxative dose | 4 (14.3) |
| Increase in laxative dose | 4 (14.3) |
| Variable | M0 X M1 | P-value | M1 x M2 | P-value | M0 x M3 | P-value | M1 x M3 | P-value |
|---|---|---|---|---|---|---|---|---|
| mBSFS-C1 | 2 (2/2) x 3 (2/3) | 0.001 | 3 (2/3) x 3 (2/3) | 0.680 | 2 (2/2) x 3 (2/3) | 0.016 | 3 (2/3) x 3 (2/3) | 0.506 |
| Number of defecations/ week1 | 2 (2/3) x 6 (3.5/7) | 0.0002 | 7 (4/7) x 7 (4/7) | 1.000 | 2 (2/3) x 7 (3.25/7) | 0.0009 | 7 (3.5/7) x 7 (3/7) | 0.414 |
| BF-S1 | 16 (14/17) x 18 (17/19.5) | < 0.0001 | 18 (17/20) x 19 (17/19.2) | 0.313 | 16 (14.25/17) x 18 (17/19) | 0.0001 | 18 (17/20) x 18 (17/19) | 0.893 |
| Hard bowel movements2 | 81.4% x 22.2% | <0.001 | 18.7 % x 12.5% | 1.000 | 77.3% x 4.5% | <0.001 | 21.7% x 4.3% | 0.125 |
| Painful bowel movements2 | 74.0% x 7.4% | <0.001 | 0 x 6.25% | 1.000 | 68.2% x 9.1% | <0.001 | 4.3% x 8.7% | 1.000 |
| Incontinence2 | 18.5% x 11.1% | 0.625 | 12.5% x 6.2% | 1.000 | 13.6% x 9.1% | 1.000 | 8.7% x8.7% | 1.000 |
| Abdominal pain2 | 44.4% x 14.8% | 0.021 | 12.5% x 12.5% | 1.000 | 45.4% x 22.7% | 0.179 | 17.4% x 26.0% | 0.500 |
| Variable | M0 X M1 | P-value | M1 x M2 | P-value | M0 x M3 | P-value | M1 x M3 | P-value |
|---|---|---|---|---|---|---|---|---|
| PedsQL 4.0 Physical |
87.5 (81.2/98.4) x 93.7 (87.5/100)a | 0.029c | 93.7 (81.2/100) x 96.8 (85/100)a | 0.635c | 87.5 (81.2/96.8) x 96.8 (93.7/100)a | <0.001c | 93.7 (84.3/100) x 96.8 (93.7/100)a | 0.059c |
| PedsQL 4.0 Psychosocial |
74.4±12.9 x 81.8±10.8b | <0.001# | 85.8 (80/95) x 90 (83.3/93.3)a | 0.484c | 75.3±13.7 x 85.2±10.2b | 0.003d | 83.3 (70/93.5) x 88.3 (77.7/92.5)a | 0.180c |
| PedsQL 4.0 Total |
78.3±12.0 x 85.0±7.6b | <0.001# | 86.4 (83.9/90.2) x 89.2 (86.3/92.9)a | 0.278c | 79.3 (73/88.5) x 89.6 (79.3/94)a | 0.001c | 85.8(79.3/90.2) x 90.2(80.9/94.5)a | 0.085c |
| AQLCAFI Lifestyle |
3.7 (3.4/4) x 4 (3.7/4)a | 0.021c | 3.9 (3.5/4) x 4 (3.8/4)a | 0.260c | 3.7 (3.3/4) x 4 (3.8/4)a | <0.001c | 4 (3.7/4) x 4 (3.8/4) a | 0.239c |
| AQLCAFI Behavior |
3.2 (3.1/3.5) x 3.5 (3.3/3.9)a | <0.001c | 3.5 (3.2/3.8) x 3.7 (3.5 /4)a | 0.169c | 3.2 (3.1/3.5) x 3.7 (3.6 /4)a | <0.001c | 3.5 (3.3/3.8) x 3.7(3.5/4)a | 0.034c |
| AQLCAFI Depression |
2.7 (2.5/3) x 2.8 (8.7/3)a | 0.394c | 3 (2.6/3.1) x 3.5 (2.9/3.6)a | 0.001c | 2.8 (2.6/3) x 3.4 (3.4/3.7)a | <0.001c | 2.8 (2.7/3.1) x 3.4 (3.4/3.7)a | <0.001c |
| AQLCAFI Embarrassment |
2.2 (2.2/3) x 3.3 (3/4)a | <0.001c | 3.3 (3/4) x 3.8 (3/4)a | 0.380c | 2.3 (2.2/2.9) x 3.6 (3/4)a | <0.001c | 3.3 (3/4) x 3.6 (3/4)a | 0.687c |
| AQLCAFI Final |
12.2 (11.5/13.2) x 13.4 (13/14)a | <0.001c | 13.1 (12.7/14.1) x 14.6 (14/15.1)a | 0.001c | 12.4 ± 0.9 x 14.6 ± 0.7b | <0.001d | 13.5 (13/14) x 14.9 (14.2/15.2)a | <0.001c |
| Assessment of applicability | n (%) | 95% CI |
|---|---|---|
| Assessment of the experience | ||
| Excellent | 5 (17.9) | 1.8-35.6 |
| Good | 18 (64.2) | 45.2-79.3 |
| Regular | 5 (17.9) | 1.8-35.6 |
| Bad | 0 (0) | 0-1.2 |
| Awful | 0 (0) | 0-1.2 |
| Is the procedure considered difficult? | ||
| Yes | 2 (7.1) | 1.9-22.6 |
| No | 26 (92.9) | 77.3-98.2 |
| The most important difficulty pointed | ||
| Electrodes placing | 0 (0) | 0-1.2 |
| Turning the device on and off | 2 (7.1) | 1.9-22.6 |
| Procedure acceptance by the child | 2 (7.1) | 1.9-22.6 |
| Finding time for it | 7 (25.0) | 12.7-43.3 |
| Session duration | 0 (0) | 0-1.2 |
| None | 17 (60.7) | 42.4- 76.4 |
| Was there pain during the procedure? | ||
| Yes | 0 (0) | 0-1.2 |
| No | 28 (100) | 87.9-100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





