1. Introduction
Stroke remains the second-leading and third-leading long-term cause of death death and disability worldwide, with approximately 12.2 million new strokes annually [
1]. The resulting neurological damage leads to significant impairments in motor control [
2], sensory perception [
3], and cognitive function [
4], severely compromising patients’ quality of life and independence [
5]. Among the numerous functional limitations faced by stroke survivors, reduced gait speed emerges as a particularly critical indicator of overall recovery, often termed the “sixth vital sign” due to its strong predictive value for mortality, hospital length of stay, discharge disposition, health status, and community reintegration [
6,
7]. Walking speed serves as a discriminative clinical measure that reflects the complex interplay of multiple physiological systems, including muscle strength, motor control, balance, and cardiovascular fitness [
8]. Despite significant advances in rehabilitation strategies that have shifted from compensatory approaches to evidence-based interventions targeting neurophysiological and muscular mechanisms [
9], up to 80% of chronic stroke patients continue to exhibit gait abnormalities and reduced walking speeds even after conventional rehabilitation [
10]. These gait deficits significantly impact community participation, increase fall risk, and contribute to secondary complications such as cardiovascular deconditioning and metabolic disorders [
11]. As the estimate global cost of stroke is expected to increase further from
$721 billion (2019 estimate [
1]), there is an urgent need for evidence-based interventions that effectively address the underlying biomechanical and neuromuscular impairments limiting functional recovery in this population.
Recent evidence from our laboratory demonstrated that a 12-week moderate-to-high intensity strength training program can significantly improve gait parameters in chronic stroke survivors, even without direct gait-specific training [
12]. Following the intervention, participants achieved bilateral improvements in walking speed (paretic limb: 0.61 to 0.69 m/s; non-paretic limb: 0.62 to 0.69 m/s), with the most notable changes occurring in spatial rather than temporal gait parameters. Correlation analyses revealed that walking speed improvements were strongly associated with increases in stride length on both sides, indicating that strength training primarily influenced force production capabilities that enabled longer steps.
Despite the demonstrated efficacy of strength training interventions in improving gait function post-stroke [
13,
14,
15], particularly when high intensity is applied [
16], the underlying neuromuscular mechanisms driving these improvements remain poorly understood. Traditional gait analysis approaches focus primarily on kinematic and spatiotemporal outcomes, providing limited insight into the muscle-level adaptations that enable functional recovery [
17,
18]. While increased muscle strength and joint power has been identified as a significant predictor of gait improvements [
19,
20], previous research has shown that stroke survivors often maintain altered muscle activation patterns [
21,
22], reduced force generation capacity [
23], and impaired intermuscular coordination [
24] during walking. These deficits usually come along with reduced ankle plantar flexor power generation during push-off, hip flexor power during swing initiation and hip extensor power during early stance [
21,
22,
25] after training.
Few studies have directly examined how changes in individual muscle function and coordination patterns translate to enhanced walking performance [
26], mainly because traditional gait analysis lacks the capability to isolate the specific contributions of individual muscles to walking function. Musculoskeletal modeling offers a powerful computational framework for addressing this limitation by decomposing complex multi-joint movements into individual muscle contributions [
27]. Modelling studies have demonstrated that hemiparetic individuals show reduced paretic soleus and gastrocnemius contributions to forward propulsion and swing initiation, with limited compensation from the non-paretic limb during pre-swing [
28]. Hip and knee muscle deficits identified through musculoskeletal modeling include hip flexor and extensor weakness leading to reduced swing initiation and early stance power generation respectively [
28,
29], gluteus medius dysfunction causing hip circumduction [
30], hamstring weakness resulting in extended knee during early stance [
17], knee extensor weakness contributing to hyperextension [
31], and rectus femoris spasticity causing stiff-knee gait patterns [
32,
33]. Despite the potential of these methods, a recent scoping review revealed that only 19 published studies have utilized musculoskeletal modeling to explore stroke locomotion, with the majority focusing on movement deficit assessment rather than training-induced adaptations [
35].
Therefore, the primary objective of this study was to investigate the specific muscle force and power adaptations that underlie the functional gait improvements observed following our 12-week strength training intervention in chronic stroke survivors. We hypothesized that strength training would result in increased muscle force production capabilities, particularly in ankle plantar flexors and hip flexors/extensors, which would translate to enhanced walking performance through improved coordination and power generation patterns. The findings from this investigation will provide novel mechanistic insights into how strength training interventions modify individual muscle function during walking and advance our understanding of the neuromuscular basis of motor recovery in stroke survivors.
2. Materials and Methods
2.1. Study Design and Participants
This study represents a secondary biomechanical analysis of data collected from participants in our previously published 12-week strength training intervention [
12]. The original study was a non-randomized trial conducted at the Neurological Rehabilitation Unit, University Hospital of Alexandroupolis, Greece, between January-December 2022. Ten chronic stroke survivors (age: 61±7.4 years, 9 males, BMI: 28±4.24 kg/m²) completed the full intervention protocol. Inclusion criteria were: (1) chronic stroke (≥6 months post-stroke), (2) age >18 years, (3) walking speed >0.2 m/s, (4) independent ambulation, and (5) confirmed hemiparesis with observable motor impairment.
2.2. Intervention Protocol
The strength training intervention consisted of a 12-week progressive resistance program conducted twice weekly, with session durations ranging from 45 to 60 minutes. Five qualified instructors administered the intervention, with two instructors supervising each participant during every session. Exercise intensity was systematically monitored using the Borg 1-10 point rating scale, with initial sessions targeting moderate perceived exertion levels (5-6) that progressively increased to high intensity (7-8, corresponding to “really hard” effort) as participants adapted to the training stimulus. The intervention utilized diverse Pilates equipment including reformer towers, wunda chairs, armchairs, barrels, rings, elastic bands, exercise balls, soft weights, and Bosu platforms. Each session comprised three distinct phases: warm-up (5-10 minutes) involving breathing, posture, and mobility exercises; main program (35-50 minutes) consisting of personalized strength exercises targeting upper and lower body musculature; and cool-down (5 minutes) incorporating breathing exercises and stretching protocols.
2.3. Data Collection
Three-dimensional gait analysis was performed at two time points: pre-intervention (baseline) and post-intervention (following 12-week training completion). During each session, participants were instructed to walk across a 10-meter corridor at their self-selected comfortable pace for ten consecutive trials. Retroreflective markers were positioned on specific anatomical landmarks according to the full-body Conventional Gait Model protocol [
36]. Marker trajectories were recorded using a 10-camera Vicon motion analysis system (Vicon Motion Systems Ltd., Oxford, UK) operating at 100 Hz sampling frequency. All data processing was conducted using Vicon Nexus 2.12.1® software, with marker trajectories subjected to low-pass filtering at 6 Hz cutoff frequency to eliminate high-frequency noise artifacts.
2.4. Musculoskeletal Modeling Pipeline
Subject-specific musculoskeletal models were generated using the Rajagopal full-body model [
37] implemented in OpenSim 4.0 (Stanford University, CA, USA). Individual anthropometric scaling was performed using body mass data and marker distances generated during one static calibration trial for each experimental session. The scaled models incorporated 37 degrees of freedom and 80 muscle-tendon units representing the major muscle groups of the lower extremities. Joint angles throughout the stance phase (heel-strike to ipsilateral toe-off) were computed using OpenSim’s inverse kinematics algorithm, which minimized the weighted least-squares difference between experimental and virtual marker positions. The optimization procedure solved for generalized coordinates that best reproduced the experimental marker trajectories while respecting the kinematic constraints of the musculoskeletal model. Net joint moments were calculated using OpenSim’s inverse dynamics tool, incorporating measured ground reaction forces, joint kinematics, and segment anthropometric properties. Joint moments were normalized by individual body weight to account for inter-subject anthropometric differences. Individual muscle forces during stance phase were estimated using OpenSim’s static optimization algorithm, which solved the muscle redundancy problem by minimizing the sum of squared muscle activations while satisfying equilibrium constraints at each time instant. The optimization assumed that muscle activations could change instantaneously and that co-contraction was minimized. Muscle forces were subsequently normalized by body weight to enable cross-subject and cross-session comparisons. Muscle fiber lengths and contraction velocities were computed using OpenSim’s muscle analysis tool, which evaluated muscle-tendon dynamics based on Hill-type muscle models.
2.5. Data Processing and Analysis
Individual muscle forces were both analyzed separately and systematically grouped into functional anatomical categories based on their primary biomechanical actions: hip flexors (iliacus, psoas, rectus femoris, sartorius), hip extensors (extensor head of adductor magnus, gluteus maximus, semimembranosus, semitendinosus), hip abductors (gluteus medius, tensor fasciae latae), hip adductors (adductor brevis, adductor longus, adductor magnus), hip internal rotators (gluteus minimus), hip external rotators (piriformis), knee flexors (biceps femoris long and short heads, gracilis), knee extensors (vastus intermedius, vastus lateralis, vastus medialis), ankle dorsiflexors (extensor digitorum longus, extensor hallucis longus, tibialis anterior), and ankle plantarflexors (flexor digitorum longus, flexor hallucis longus, gastrocnemius, peroneus brevis, peroneus longus, soleus, tibialis posterior).
Instantaneous muscle power was calculated as the scalar product of muscle force and muscle fiber contraction velocity, providing measures of mechanical power generation (positive values) and absorption (negative values) throughout the stance phase. Muscle group powers were derived by summating individual muscle powers within each functional category. All kinematic, kinetic, and muscle force data were interpolated to 100 equally-spaced data points representing percentages of stance phase duration using quadratic spline interpolation. This normalization procedure enabled ensemble averaging across trials and subjects while preserving temporal relationships within the stance phase cycle. Statistical Parametric Mapping (SPM) was used to compare the stance phase time series to understand how the sub-phases of gait change pre and post training conditions. Depending on the data distribution, parametric or non-parametric paired sample t-tests were performed to determine the biomechanical differences between these two conditions. Where applicable, force and power data from separate bundles of a muscle were summed.
Peak values analysis was performed by extracting maximum absolute values during early stance (0-50%) and late stance (50-100%) phases for each parameter. Muscle work was calculated by integrating muscle power over the stance phase, with positive work representing energy generation and negative work representing energy absorption. Statistical significance was determined using paired t-tests or Wilcoxon signed-rank tests based on normality testing (Shapiro-Wilk, p>0.05), with significance set at p<0.05.
3. Results
The 12-week strength training intervention produced comprehensive neuromuscular adaptations that enhanced locomotor capacity while preserving fundamental movement coordination patterns.
3.1. Joint Kinematics and Kinetics Adaptations
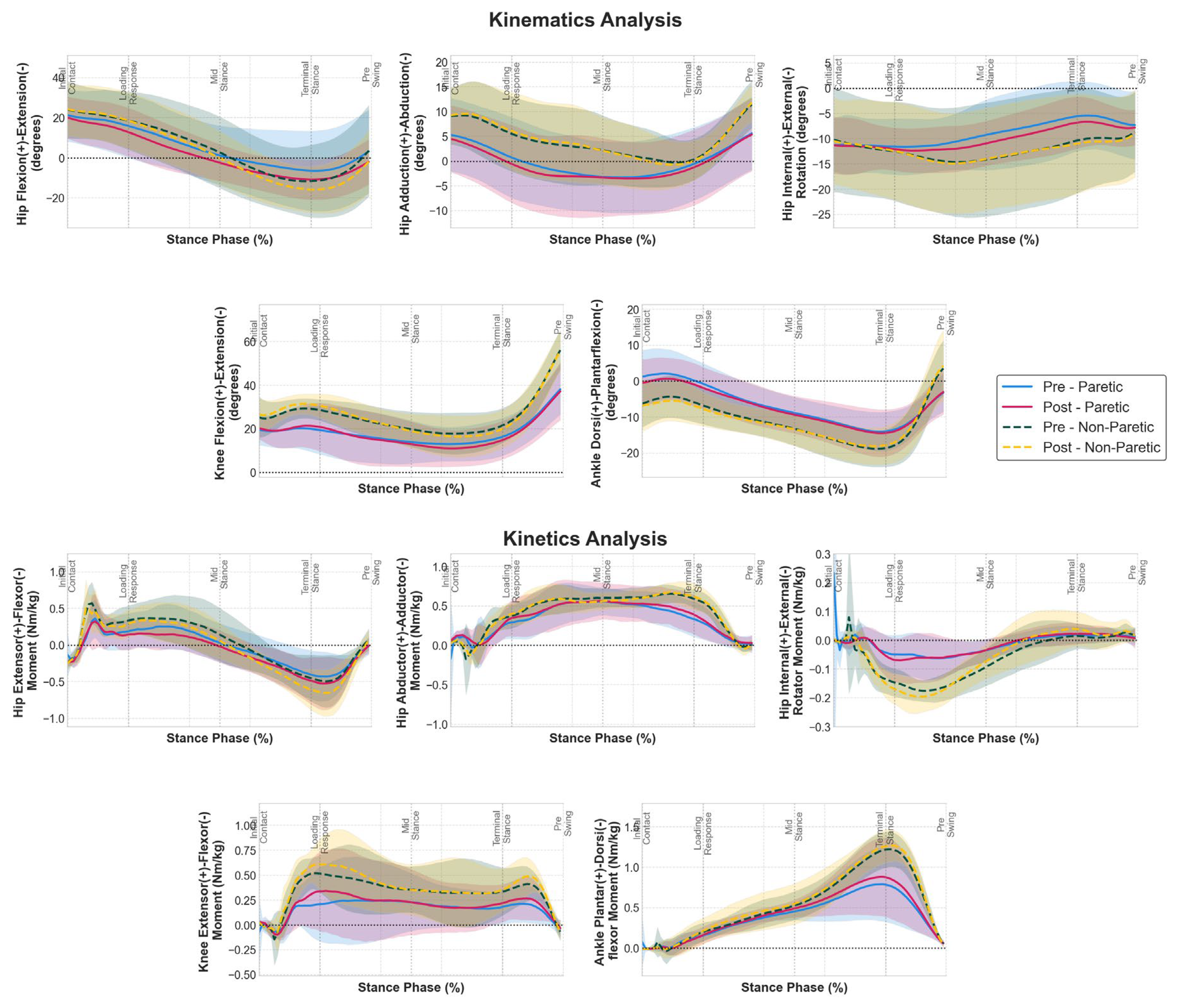
Analysis of joint angles and moments (
Figure 1) throughout the stance phase revealed that the 12-week strength training intervention preserved fundamental movement patterns while producing selective biomechanical adaptations, although SPM analysis did not reveal significant bilateral adaptations. Our results suggest that the stride length increase found in our previous work was mainly caused by increasing hip extension during late stance in both limbs, although statistically non-significant. Hip sagittal moments showed a similar pattern, where hip flexor moments were increased during late stance phase for both limbs.
Ankle sagittal moments exhibited notable adaptations during late stance, with both limbs showing slightly enhanced plantarflexor moment generation during the push-off phase (60-80% stance). Knee sagittal moments maintained their characteristic biphasic pattern throughout stance in the non-paretic limb, with enhanced extensor moment generation observed in both the loading response and late stance phases post-intervention. Notably, the paretic limb demonstrated restoration of the typical biphasic extensor moment pattern following training. Pre-intervention, the paretic knee moment profile exhibited an absent or severely diminished initial peak during the loading response phase; however, post-intervention analysis revealed emergence of this characteristic first peak alongside enhancement of the second peak during late stance, indicating normalization toward the physiological biphasic extensor moment pattern observed in the non-paretic limb and healthy gait.
3.2. Muscle Group Force Adaptations
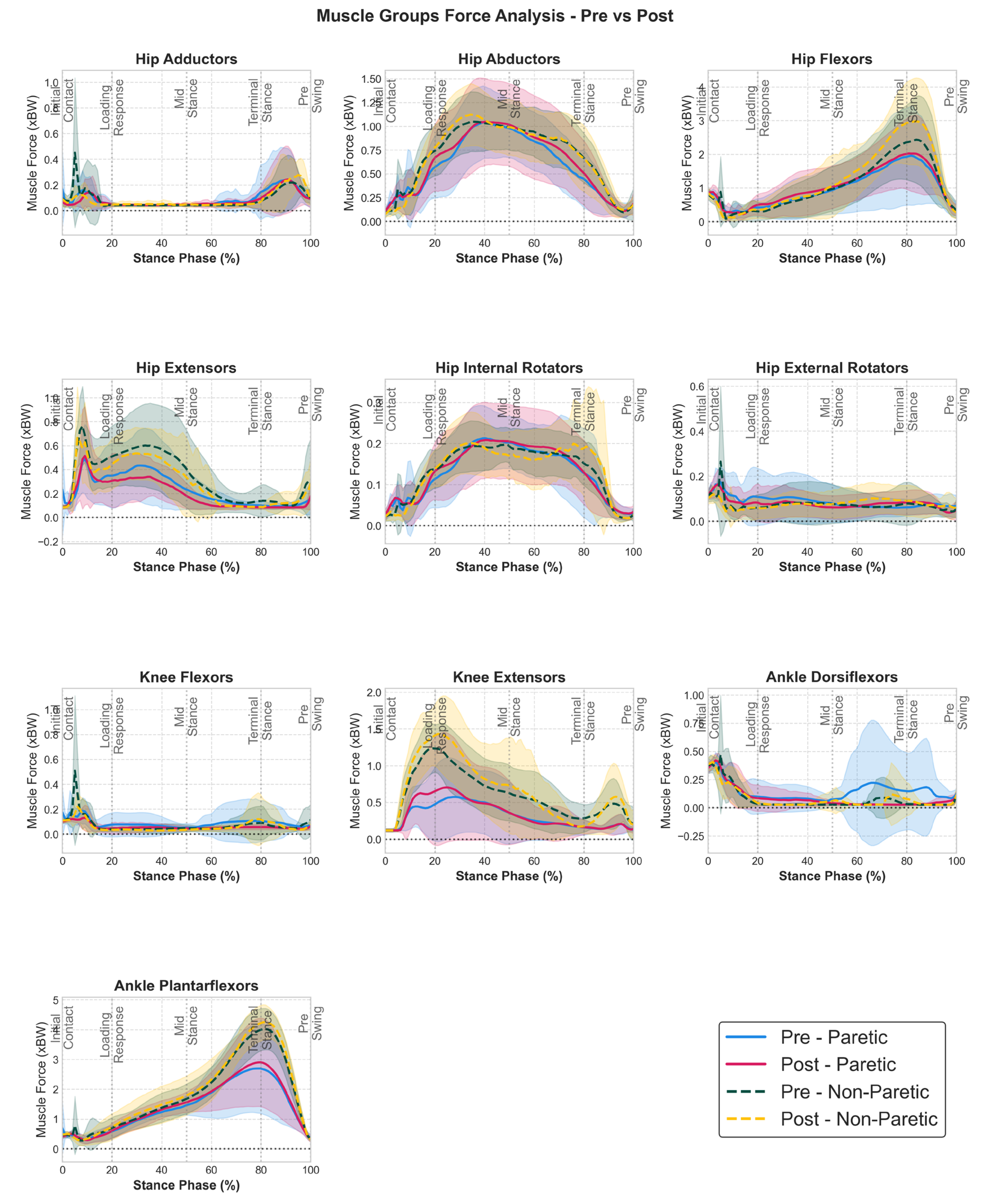
Time profiles of muscle group forces (
Figure 2) revealed bilateral neuromuscular adaptations, although SPM analysis did not find any statistically significant regions. Hip extensors showed decreases bilaterally post intervention. Knee extensor forces demonstrated enhanced force generation during the loading response phase (0-20% stance) for both limbs, while non-paretic hip flexor forces exhibited enhanced force generation during late stance (70-90%) preparing for swing phase initiation. Individual muscle analysis (see Supplementary material
Figure S1) confirmed these group-level findings, with all vasti muscles and non-paretic rectus femoris- iliopsoas muscles showing enhanced force generation throughout early and late stance respectively.
Paretic ankle dorsiflexors showed a more similar decreased activation to the non-paretic side post intervention during mid- and terminal stance. Ankle plantarflexor forces also exhibited adaptations, with analysis revealing slightly enhanced force generation during the push-off phase (60-85% stance) in both limbs. Individual muscle analysis (see
Figure S1) revealed that gastrocnemius (both lateral and medial for non-paretic and only lateral for paretic) and soleus (paretic) contributed to these improvements, with enhanced force generation throughout their respective activation windows.
3.3. Muscle Group Power Adaptations
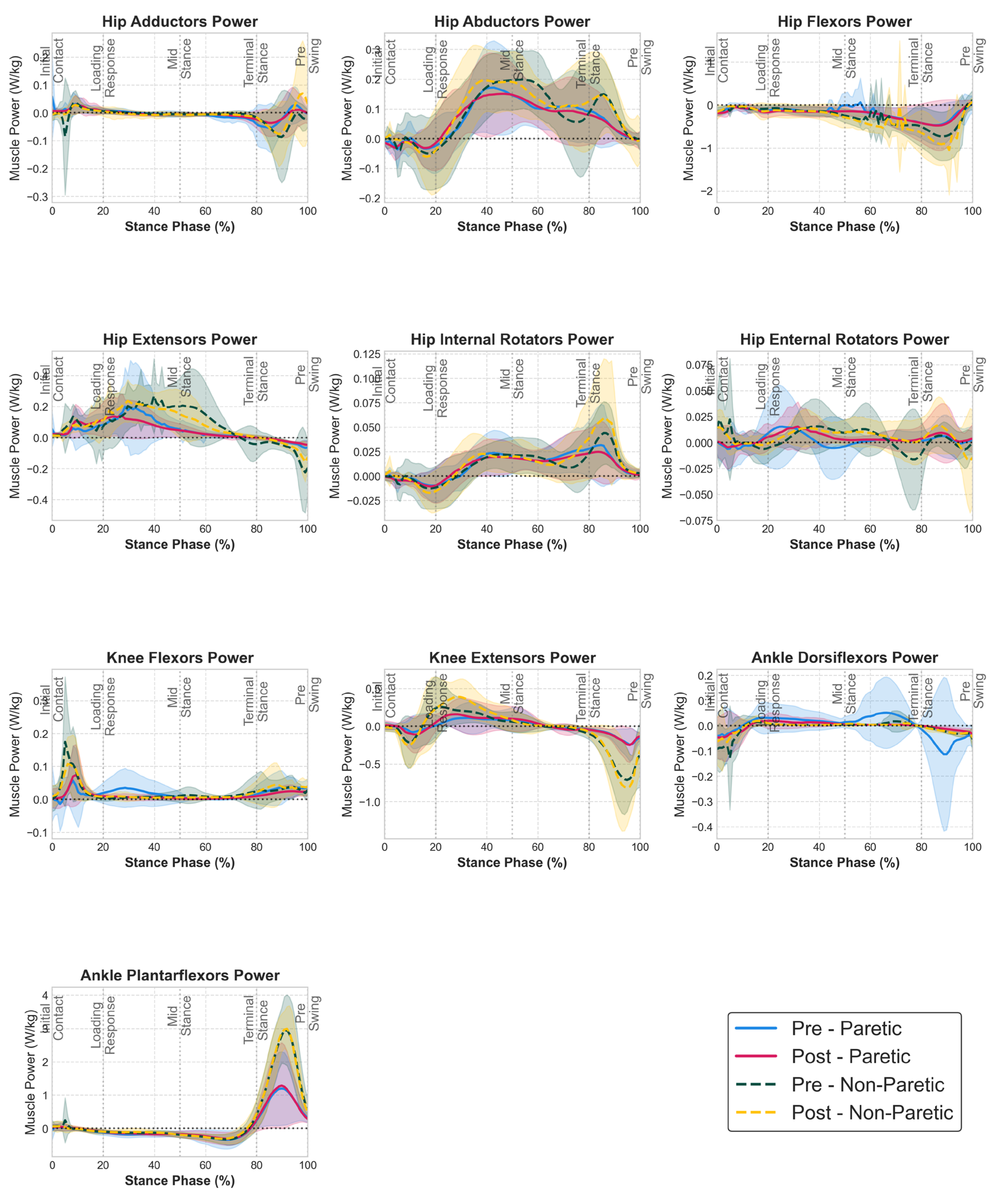
Time profiles of muscle group power (
Figure 3) revealed that the strength training intervention enhanced both concentric (positive) and eccentric (negative) power capacities while maintaining the temporal coordination of power production and absorption phases, although SPM analysis did not find any statistically significant regions.
One notable change is seen in non-paretic hip flexor power profiles, which showed an increase in power absorption during late stance, while the paretic limb hip flexor power profiles remained largely unchanged. The driving force behind this finding is the non-paretic rectus femoris power profile (see Supplementary material
Figure S2) which demonstrated statistically significant enhanced power absorption during late stance post-intervention. In addition, notable reductions in paretic limb muscle power were observed that resulted in more symmetrical bilateral power profiles. Specifically, paretic knee flexor and ankle dorsiflexor power showed reductions between 20-50 and 50-100% of the stance phase respectively post intervention. These power reductions brought the paretic limb power profiles closer to those of the non-paretic limb, suggesting improved inter-limb coordination and reduced compensatory power generation strategies that were present pre-intervention. Last, although non-paretic hip extensor power profile remained largely unchanged, paretic hip extensor power generation decreased during 20-50% of the stance phase, going below the levels of the non-paretic side.
3.4. Muscle Work Capacity Adaptations
Analysis of muscle work capacity revealed that the strength training intervention enhanced both positive (concentric) and negative (eccentric) work capabilities, with distinct adaptation patterns between work types.
Table 1 and
Table 2 show the results of the peak analysis.
The most substantial work capacity improvements occurred in negative work (eccentric contractions), indicating enhanced muscle control during lengthening contractions. Hip flexors demonstrated significant adaptation in the non-paretic limb, with negative work capacity increasing significantly from 0.28 to 0.34 J/kg (p = 0.033). On the contrary, hip extensor negative work decreased significantly bilaterally (paretic: from 0.010 to 0.006 J/kg, p=0.037; non-paretic: from 0.024 to 0.011 J/kg, p=0.014). Ankle dorsiflexors exhibited negative work reductions in both limbs, with the paretic side decreasing from 0.018 to 0.009 J/kg and the non-paretic limb showing a smaller decrease from 0.012 to 0.010 J/kg. Other muscle groups, including hip abductors, hip adductors, ankle plantarflexors, and knee flexors/extensors, maintained their baseline negative work capacity with minimal changes.
Positive work adaptations showed important selective enhancements in key locomotor muscles (
Table 2). Knee extensor positive work showed an almost significant improvement in the non-paretic limb (from 0.080 to 0.108 J/kg, p=0.057), while remaining unchanged in the paretic limb (from 0.044 to 0.052 J/kg, p=0.375). All other muscle groups showed no significant changes in positive work capacity. Hip extensor positive work remained unchanged bilaterally (non-paretic: from 0.102 to 0.088 J/kg, p=0.241; paretic: from 0.056 to 0.049 J/kg, p=1.000). Hip flexor positive work showed no changes in non-paretic limb from 0.020 to 0.019 J/kg, however in paretic there was a meaningful reduction from 0.025 to 0.008 J/kg, p=0.105. Ankle plantarflexors/dorsiflexors, knee flexors, hip abductors and adductors’ positive work showed no significant changes in either limb.
4. Discussion
The present study provides novel mechanistic insights into the neuromuscular adaptations underlying functional gait improvements following strength training in chronic stroke survivors. Through comprehensive musculoskeletal modeling analysis, we demonstrate that a 12-week moderate-to-high intensity strength training intervention produced selective muscle-level adaptations that directly explain the previously reported bilateral improvements in walking speed and stride length [
12]. The findings reveal that functional recovery was achieved through enhanced force generation capacity in key locomotor muscles—particularly knee extensors during loading response, ankle plantarflexors and hip flexors during push-off —while preserving fundamental temporal coordination patterns throughout the stance phase. Importantly, the mechanism of energy transfer at the lower limb to propel the body forwards, as described by Neptune et al [
34,
38], was improved in both legs, and will be discussed in detail below.
The enhancement of knee extensor force generation in both limbs during the critical loading response phase (0-20% stance) could serve as a one of the mechanisms underlying the observed speed increase. Our modeling analysis revealed that strength training enhanced knee extensor force production capacity bilaterally, with both paretic and non-paretic limbs demonstrating increased force generation during the weight acceptance phase when all three vastii muscles were critically activated for maintaining knee extension, trunk deceleration and stability [
39], as seen
Figure S1. Vastii power profiles indicate an increase during early stance only for the non-paretic limb, with respective significant increases in positive work. Simultaneously, hip extensors - primarily gluteus maximus - decreased their force and power generation to keep the knee extended, possibly as compensation for the enhanced function of knee extensors.
Ankle plantarflexor force generation during the push-off phase (60-85% stance) demonstrated slight improvements bilaterally, partially explaining the enhanced propulsion capacity underlying increased stride length and walking speed when these muscles generate the propulsive forces necessary for forward progression. Force increases primarily in soleus were responsible for paretic propulsion (see
Figure S1), in line with previous findings demonstrating soleus as the main contributor to forward progression [
34,
38]. Still, power and positive work of the muscle group during late stance did not change post intervention for both limbs, due to coinciding force/power increase on soleus and decrease in lateral gastrocnemius. Speed improvement in this patient cohort did not result from ankle plantarflexor power generation change, which contradicts literature findings relating increased ankle plantaflexor power with speed increase in low functioning elderly [
41] and stroke populations [
26,
42,
43]. However, no substantial ankle plantaflexor power increase was found for different stoke patient groups when increased their walking speed either volitionally [
44,
45] or as a result of training program [
46], suggesting increased hip flexor power generation [
19] during pull-off as the main compensatory mechanism. The apparent contradiction between enhanced force generation and unchanged power output suggests that contraction velocity decreased. Despite the strength training intervention, the study cohort maintained the velocity-dependent muscular deficits known to occur during ageing and stroke, due to greater declines in the velocity of contraction rather than the force generating component of muscle power production [
47,
48]. Conversely, paretic ankle dorsiflexors exhibited reductions in muscle force, power absorption during late stance (50-100% stance phase) and negative work capacity (from 0.018 to 0.009 J/kg paretic; from 0.012 to 0.010 J/kg non-paretic), resembling the non-paretic side post intervention. These reductions likely reflect improved gait efficiency, given the slight increase in the application of force by the plantar flexors, combined with the decrease in the application of force by the dorsiflexors during this specific period of the stance phase, improved the ability to control the ankle joint, which was manifested by a reduction in the dorsiflexors’ power that is not necessary for walking.
Hip flexor force improvements, particularly in the non-paretic limb during late stance, revealed compensatory mechanisms that contributed to overall gait speed increase. The predominance of non-paretic hip flexor force adaptations supports the bilateral compensation hypothesis, whereby the less-affected limb assumes greater responsibility for locomotor function to overcome paretic limb limitations [
49]. Hip flexors power profiles and negative work values indicate a larger energy absorption post intervention for the non-paretic side during late stance. High hip flexor energy absorption has been correlated with high walking speeds in literature [
42,
43,
50], however very few have explained this finding due to its counterintuitive nature to increasing speed. We found that the main hip flexor to increase both in force and power during late stance was found to be rectus femoris (see
Figures S1 and S2), a biarticular muscle mainly responsible for energy absorption through eccentric contraction during late stance. How this finding relates to speed increase was addressed by Neptune et al [
34], who used forward dynamics simulations to explore individual muscle function in walking sub-tasks. They reported that when rectus femoris contracts eccentrically during late stance, it accelerates the trunk by decelerating the leg through leg energy absorption, thus helping in forward trunk progression. Such a mechanism could explain the speed increase without concurrent ankle plantarflexion power increase which was discussed above.
The current study has several limitations that should be considered when interpreting the results. First, the absence of a control group limits our ability to determine whether the observed improvements were specifically attributable to the strength training intervention or could be partially explained by time-related recovery effects, although the chronic nature of the participant population (≥6 months post-stroke) makes spontaneous recovery unlikely. Second, the small sample size (n=10) may limit the generalizability of our findings to the broader stroke population and constitute the basic confounding factor for lack of statistically significant differences in most variables. Third, the small although significant speed increase as a result of the training program could also be insufficient to be elicited by significant changes in the muscle function. Fourth, the musculoskeletal modeling approach relied on static optimization algorithms that assume instantaneous muscle activation changes and minimize co-contraction, which may not accurately represent the complex neural control strategies employed by stroke survivors during walking. Finally, individual muscle forces were estimated rather than directly measured, however preliminary data on validation against electromyographic data have been performed in subgroup of the same group used in this study [
51], enhancing our confidence in the profile of estimated muscle forces.
5. Conclusions
This study provides the first comprehensive mechanistic analysis of muscle-level adaptations underlying functional gait improvements following strength training in chronic stroke survivors. Through advanced musculoskeletal modeling, we demonstrated that a 12-week moderate-to-high intensity strength training program produced selective bilateral neuromuscular adaptations that directly explain previously reported improvements in walking speed and stride length. The key finding reveals that walking performance in both sides was improved, with subtle differences. Knee extensors in both sides increased their output to generate force during early stance, while an increase in power and positive work was found only for the non-paretic side. During push-off, soleus showed an increase in force and power generation for the paretic side, whereas enhanced power absorption capacity in non-paretic hip flexor muscles—particularly rectus femoris during push-off, leading to leg deceleration and concurrent trunk acceleration. These findings improved the walking mechanism towards increased walking speed and can have important implications for rehabilitation practice. Strength training programs can produce meaningful muscle-level adaptations that translate to functional improvements even in the absence of task-specific gait training, thereby supporting the integration of progressive resistance training into comprehensive stroke rehabilitation protocols.
Supplementary Materials
The following supporting information can be downloaded at website of this paper posted on Preprints.org, Figure S1: Individual muscle force profiles during stance phase before and after 12-week strength training intervention in chronic stroke patients. Each panel represents normalized muscle force (N/BW) across stance phase (0-100%) for paretic (solid lines) and non-paretic (dashed lines) limbs at pre-intervention (blue/teal) and post-intervention (pink/amber). Shaded regions represent standard deviation. Gait phase markers indicate initial contact (0%), loading response (~20%), mid-stance (~50%), terminal stance (~80%), and pre-swing (100%); Figure S2: Figure S2. Individual muscle power profiles during stance phase before and after 12-week strength training intervention in chronic stroke patients. Each panel represents normalized muscle power (J/kg) across stance phase (0-100%) for paretic (solid lines) and non-paretic (dashed lines) limbs at pre-intervention (blue/teal) and post-intervention (pink/amber). Shaded regions represent standard deviation. Gait phase markers indicate initial contact (0%), loading response (~20%), mid-stance (~50%), terminal stance (~80%), and pre-swing (100%).
Author Contributions
For research articles with several authors, a short paragraph specifying their individual contributions must be provided. The following statements should be used “Conceptualization, N.A., K.V.. A.T. and G.G. .; methodology, G.G., A.T., E.G., I.K. ; software, G.G., F.C.; validation, G.G., P.M., I.K. and N.A.; formal analysis, G.G. and P.M.; investigation, E.G. and E.M.; resources, N.A., P.M. and K.V.; data curation, G.G. and E.G.; writing—original draft preparation, G.G. and A.T.; writing—review and editing, G.G., N.A. and E.G.; visualization, G.G. and E.M.; supervision, N.A., F.C. and K.V.; project administration, P.M. and I.K.; funding acquisition, N.A. and K.V. All authors have read and agreed to the published version of the manuscript.” Please turn to the CRediT taxonomy for the term explanation. Authorship must be limited to those who have contributed substantially to the work reported.
Funding
We acknowledge the support of the work at hand by the project “Study of the interrelationships between neuroimaging, neurophysiological and biomechanical biomarkers in stroke rehabilitation (NEURO-BIO-MECH in stroke rehab)” (MIS 5047286), which is implemented under the Action “Support for Regional Excellence”- Operational Program “Competitiveness, Entrepreneurship and Innovation” (NSRFm2014-2020), under the auspices of Greece and the European Union (European Regional Development Fund).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Ethics Committee of Democritus University of Thrace.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data and results are available upon request to interested researchers.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- V. L. Feigin et al., “World Stroke Organization (WSO): Global Stroke Fact Sheet 2022,” International Journal of Stroke, vol. 17, no. 1. SAGE Publications Inc., pp. 18–29, Jan. 01, 2022, doi: 10.1177/17474930211065917.
- P. E. Gilmore and S. J. Spaulding, “Motor Control and Motor Learning: Implications for Treatment of Individuals Post Stroke,” Phys. Occup. Ther. Geriatr., vol. 20, no. 1, pp. 1–15, Nov. 2001, doi: 10.1080/J148V20N01_01.
- C. Hazelton et al., “Perceptual Disorders After Stroke: A Scoping Review of Interventions,” Stroke, vol. 53, no. 5, pp. 1772–1787, May 2022, doi: 10.1161/STROKEAHA.121.035671/SUPPL_FILE/STR_STROKE-2021-035671_SUPP1.PDF.
- J. H. Sun, L. Tan, and J. T. Yu, “Post-stroke cognitive impairment: epidemiology, mechanisms and management,” Ann. Transl. Med., vol. 2, no. 8, p. 80, Aug. 2014, doi: 10.3978/J.ISSN.2305-5839.2014.08.05.
- S. Parikh, S. Parekh, and N. Vaghela, “Impact of stroke on quality of life and functional independence,” Natl. J. Physiol. Pharm. Pharmacol., p. 12, 2018, doi: 10.5455/njppp.2018.8.0723807092018.
- C. M. Stinear, C. E. Lang, S. Zeiler, and W. D. Byblow, “Advances and challenges in stroke rehabilitation,” Lancet Neurol., vol. 19, no. 4, pp. 348–360, Nov. 2020, doi: 10.1016/S1474-4422(19)30415-6.
- S. Wesselhoff, T. A. Hanke, and C. C. Evans, “Community mobility after stroke: a systematic review,” Top. Stroke Rehabil., vol. 25, no. 3, pp. 224–238, Apr. 2018, doi: 10.1080/10749357.2017.1419617.
- S. Li, G. E. Francisco, and P. Zhou, “Post-stroke hemiplegic gait: New perspective and insights,” Front. Physiol., vol. 9, no. AUG, p. 389766, Aug. 2018, doi: 10.3389/FPHYS.2018.01021/BIBTEX.
- M. Abdollahi et al., “A Systematic Review of Fall Risk Factors in Stroke Survivors: Towards Improved Assessment Platforms and Protocols,” Front. Bioeng. Biotechnol., vol. 10, p. 910698, Aug. 2022, doi: 10.3389/FBIOE.2022.910698/BIBTEX.
- F. M. Ivey, R. F. Macko, A. S. Ryan, and C. E. Hafer-Macko, “Cardiovascular Health and Fitness After Stroke,” Top. Stroke Rehabil., vol. 12, no. 1, pp. 1–16, Dec. 2005, doi: 10.1310/GEEU-YRUY-VJ72-LEAR.
- M. D. Peterson, S. Al Snih, J. Stoddard, A. Shekar, and E. A. Hurvitz, “Obesity misclassification and the metabolic syndrome in adults with functional mobility impairments: Nutrition Examination Survey 2003–2006,” Prev. Med. (Baltim)., vol. 60, pp. 71–76, Mar. 2014, doi: 10.1016/J.YPMED.2013.12.014.
- G. Giarmatzis et al., “Effects of a 12-Week Moderate-to-High Intensity Strength Training Program on the Gait Parameters and Their Variability of Stroke Survivors,” Brain Sci., vol. 15, no. 4, pp. 1–14, 2025, doi: 10.3390/brainsci15040354.
- U. B. Flansbjer, M. Miller, D. Downham, and J. Lexell, “Progressive resistance training after stroke: effects on muscle strength, muscle tone, gait performance and perceived participation.,” J. Rehabil. Med., vol. 40, no. 1, pp. 42–48, Jan. 2008, doi: 10.2340/16501977-0129.
- M. R. Mwansa, S. Himalowa, and R. Kunda, “Functional Gait of Patients with Stroke after Strength Training: A Systematic Review of Randomised Controlled Trials,” Int. J. Heal. Sci. Res., vol. 11, no. 7, p. 144, 2021, doi: 10.52403/ijhsr.20210721.
- S. S. Pontes et al., “Effects of isokinetic muscle strengthening on muscle strength, mobility, and gait in post-stroke patients: a systematic review and meta-analysis,” Clin. Rehabil., vol. 33, no. 3, pp. 381–394, Mar. 2019, doi: 10.1177/0269215518815220/ASSET/IMAGES/LARGE/10.1177_0269215518815220-FIG4.JPEG.
- J. Veldema and P. Jansen, “Resistance training in stroke rehabilitation: systematic review and meta-analysis,” Clin. Rehabil., vol. 34, no. 9, pp. 1173–1197, Sep. 2020, doi: 10.1177/0269215520932964/ASSET/IMAGES/LARGE/10.1177_0269215520932964-FIG2.JPEG.
- B. A. Knarr, D. S. Reisman, S. A. Binder-Macleod, and J. S. Higginson, “Understanding compensatory strategies for muscle weakness during gait by simulating activation deficits seen post-stroke,” Gait Posture, vol. 38, no. 2, pp. 270–275, 2013, doi: 10.1016/j.gaitpost.2012.11.027.
- G. Chen, C. Patten, D. H. Kothari, and F. E. Zajac, “Gait differences between individuals with post-stroke hemiparesis and non-disabled controls at matched speeds,” Gait Posture, vol. 22, no. 1, pp. 51–56, Aug. 2005, doi: 10.1016/J.GAITPOST.2004.06.009.
- S. Nadeau, D. Gravel, A. B. Arsenault, and D. Bourbonnais, “Plantarflexor weakness as a limiting factor of gait speed in stroke subjects and the compensating role of hip flexors,” Clin. Biomech., vol. 14, no. 2, pp. 125–135, Feb. 1999, doi: 10.1016/S0268-0033(98)00062-X.
- B. F. Mentiplay, B. Adair, K. J. Bower, G. Williams, G. Tole, and R. A. Clark, “Associations between lower limb strength and gait velocity following stroke: A systematic review,” Brain Inj., vol. 29, no. 4, pp. 409–422, Apr. 2015, doi: 10.3109/02699052.2014.995231.
- D. J. Clark, L. H. Ting, F. E. Zajac, R. R. Neptune, and S. A. Kautz, “Merging of healthy motor modules predicts reduced locomotor performance and muscle coordination complexity post-stroke,” J. Neurophysiol., vol. 103, no. 2, pp. 844–857, 2010, doi: 10.1152/jn.00825.2009.
- S. J. Garland, V. L. Gray, and S. Knorr, “Muscle activation patterns and postural control following stroke,” Motor Control, vol. 13, no. 4. Human Kinetics, Inc., pp. 387–411, Oct. 01, 2009, doi: 10.1123/mcj.13.4.387.
- S. Dorsch, L. Ada, and C. G. Canning, “Lower Limb Strength Is Significantly Impaired in All Muscle Groups in Ambulatory People With Chronic Stroke: A Cross-Sectional Study,” Arch. Phys. Med. Rehabil., vol. 97, no. 4, pp. 522–527, Apr. 2016, doi: 10.1016/J.APMR.2015.10.106.
- J. L. Allen, S. A. Kautz, and R. R. Neptune, “The influence of merged muscle excitation modules on post-stroke hemiparetic walking performance,” Clin. Biomech., vol. 28, no. 6, pp. 697–704, 2013, doi: 10.1016/j.clinbiomech.2013.06.003.
- B. F. Mentiplay et al., “Gait Velocity and Joint Power Generation after Stroke: Contribution of Strength and Balance,” Am. J. Phys. Med. Rehabil., vol. 98, no. 10, pp. 841–849, Oct. 2019, doi: 10.1097/PHM.0000000000001122.
- E. C. Wonsetler and M. G. Bowden, “A systematic review of mechanisms of gait speed change Post-Stroke. Part 2: Exercise capacity, muscle activation, kinetics, and kinematics,” Topics in Stroke Rehabilitation, vol. 24, no. 5. Taylor and Francis Ltd., pp. 394–403, 2017, doi: 10.1080/10749357.2017.1282413.
- F. E. Zajac, R. R. Neptune, and S. A. Kautz, “Biomechanics and muscle coordination of human walking: Part I: Introduction to concepts, power transfer, dynamics and simulations,” Gait Posture, vol. 16, no. 3, pp. 215–232, Dec. 2002, doi: 10.1016/S0966-6362(02)00068-1.
- C. L. Peterson, A. L. Hall, S. A. Kautz, and R. R. Neptune, “Pre-swing deficits in forward propulsion, swing initiation and power generation by individual muscles during hemiparetic walking,” J. Biomech., vol. 43, no. 12, pp. 2348–2355, 2010, doi: 10.1016/j.jbiomech.2010.04.027.
- A. L. Hall, C. L. Peterson, S. A. Kautz, and R. R. Neptune, “Relationships between muscle contributions to walking subtasks and functional walking status in persons with post-stroke hemiparesis,” Clin. Biomech., vol. 26, no. 5, pp. 509–515, 2011, doi: 10.1016/j.clinbiomech.2010.12.010.
- T. Akbas, S. Prajapati, D. Ziemnicki, P. Tamma, S. Gross, and J. Sulzer, “Hip circumduction is not a compensation for reduced knee flexion angle during gait,” J. Biomech., vol. 87, pp. 150–156, 2019, doi: 10.1016/j.jbiomech.2019.02.026.
- G. F. Santos, E. Jakubowitz, N. Pronost, T. Bonis, and C. Hurschler, “Predictive simulation of post-stroke gait with functional electrical stimulation,” Sci. Rep., vol. 11, no. 1, pp. 1–12, Nov. 2021, doi: 10.1038/s41598-021-00658-z.
- N. Lampire, N. Roche, P. Carne, L. Cheze, and D. Pradon, “Effect of botulinum toxin injection on length and lengthening velocity of rectus femoris during gait in hemiparetic patients,” Clin. Biomech., vol. 28, no. 2, pp. 164–170, 2013, doi: 10.1016/j.clinbiomech.2012.12.006.
- T. Akbas, R. R. Neptune, and J. Sulzer, “Neuromusculoskeletal simulation reveals abnormal rectus femoris-gluteus medius coupling in post-stroke gait,” Front. Neurol., vol. 10, no. APR, pp. 1–10, 2019, doi: 10.3389/fneur.2019.00301.
- R. R. Neptune, F. E. Zajac, and S. A. Kautz, “Muscle force redistributes segmental power for body progression during walking,” Gait Posture, vol. 19, no. 2, pp. 194–205, Apr. 2004, doi: 10.1016/S0966-6362(03)00062-6.
- G. Giarmatzis et al., “Understanding Post-Stroke Movement by Means of Motion Capture and Musculoskeletal Modeling: A Scoping Review of Methods and Practices,” BioMed 2022, Vol. 2, Pages 409-421, vol. 2, no. 4, pp. 409–421, Nov. 2022, doi: 10.3390/BIOMED2040032.
- F. Leboeuf, R. Baker, A. Barré, J. Reay, R. Jones, and M. Sangeux, “The conventional gait model, an open-source implementation that reproduces the past but prepares for the future,” Gait Posture, vol. 69, pp. 235–241, Mar. 2019, doi: 10.1016/j.gaitpost.2019.04.015.
- A. Rajagopal, C. L. Dembia, M. S. DeMers, D. D. Delp, J. L. Hicks, and S. L. Delp, “Full-Body Musculoskeletal Model for Muscle-Driven Simulation of Human Gait,” IEEE Trans. Biomed. Eng., vol. 63, no. 10, pp. 2068–2079, 2016, doi: 10.1109/TBME.2016.2586891.
- R. R. Neptune, S. A. Kautz, and F. E. Zajac, “Contributions of the individual ankle plantar flexors to support, forward progression and swing initiation during walking,” J. Biomech., vol. 34, no. 11, pp. 1387–1398, Nov. 2001, doi: 10.1016/S0021-9290(01)00105-1.
- D. A. Winter, “Biomechanics and Motor Control of Human Movement: Fourth Edition,” Biomech. Mot. Control Hum. Mov. Fourth Ed., pp. 1–370, Sep. 2009, doi: 10.1002/9780470549148.
- B. A. Knarr, T. M. Kesar, D. S. Reisman, S. A. Binder-Macleod, and J. S. Higginson, “Changes in the activation and function of the ankle plantar flexor muscles due to gait retraining in chronic stroke survivors.,” J. Neuroeng. Rehabil., vol. 10, p. 12, 2013, doi: 10.1186/1743-0003-10-12.
- A. Graf, J. O. Judge, S. Õunpuu, and D. G. Thelen, “The Effect of Walking Speed on Lower-Extremity Joint Powers Among Elderly Adults Who Exhibit Low Physical Performance,” Arch. Phys. Med. Rehabil., vol. 86, no. 11, pp. 2177–2183, Nov. 2005, doi: 10.1016/J.APMR.2005.06.007.
- K. Parvataneni, S. J. Olney, and B. Brouwer, “Changes in muscle group work associated with changes in gait speed of persons with stroke,” Clin. Biomech., vol. 22, no. 7, pp. 813–820, Aug. 2007, doi: 10.1016/j.clinbiomech.2007.03.006.
- J. Brincks and J. F. Nielsen, “Increased power generation in impaired lower extremities correlated with changes in walking speeds in sub-acute stroke patients,” Clin. Biomech., vol. 27, no. 2, pp. 138–144, Feb. 2012, doi: 10.1016/j.clinbiomech.2011.08.007.
- I. Jonkers, S. Delp, and C. Patten, “Capacity to increase walking speed is limited by impaired hip and ankle power generation in lower functioning persons post-stroke,” Gait Posture, vol. 29, no. 1, pp. 129–137, 2009, doi: 10.1016/j.gaitpost.2008.07.010.
- J. Jonsdottir, M. Recalcati, M. Rabuffetti, A. Casiraghi, S. Boccardi, and M. Ferrarin, “Functional resources to increase gait speed in people with stroke: Strategies adopted compared to healthy controls,” Gait Posture, vol. 29, no. 3, pp. 355–359, Apr. 2009, doi: 10.1016/J.GAITPOST.2009.01.008.
- J. F. Alingh, B. E. Groen, E. H. F. Van Asseldonk, A. C. H. Geurts, and V. Weerdesteyn, “Effectiveness of rehabilitation interventions to improve paretic propulsion in individuals with stroke – A systematic review,” Clin. Biomech., vol. 71, pp. 176–188, Jan. 2020, doi: 10.1016/J.CLINBIOMECH.2019.10.021.
- P. Morgan, A. Embry, L. Perry, K. Holthaus, and C. M. Gregory, “Feasibility of lower-limb muscle power training to enhance locomotor function poststroke,” J. Rehabil. Res. Dev., vol. 52, no. 1, pp. 77–84, 2015, doi: 10.1682/JRRD.2014.04.0109,.
- J. Kostka, M. Niwald, A. Guligowska, T. Kostka, and E. Miller, “Muscle power, contraction velocity and functional performance after stroke,” Brain Behav., vol. 9, no. 4, p. e01243, Apr. 2019, doi: 10.1002/BRB3.1243.
- C. L. Richards, F. Malouin, and C. Dean, “Gait in Stroke: Assessment and Rehabilitation,” Clin. Geriatr. Med., vol. 15, no. 4, pp. 833–856, Nov. 1999, doi: 10.1016/S0749-0690(18)30034-X.
- L. F. Teixeira-Salmela, S. Nadeau, I. McBride, and S. J. Olney, “Effects of muscle strengthening and physical conditioning training on temporal, kinematic and kinetic variables during gait in chronic stroke survivors,” J. Rehabil. Med., vol. 33, no. 2, pp. 53–60, 2001, doi: 10.1080/165019701750098867.
- G. Giarmatzis, E. Giannakou, A. Gkrekidis, Κ. Vadikolias, and N. Aggelousis, “Clinical Validation Of Static Optimization During Post Stroke Gait,” 28th Congress of European Society of Biomechanics; 9-12 July 2023; Maastricht, Nederlands. Maastricht, Nederlands, 2023, [Online]. Available: https://esbiomech.org/conference/wp-content/uploads/2023/ESB2023_Book_of_Abstracts.pdf.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).